Abstract
Importance
Several surrogates for prostate cancer–specific mortality satisfying the Prentice criteria exist, but whether these are surrogates for all-cause mortality, and how their performance compares, is unknown.
Objective
To ascertain and compare the performance of 4 candidate surrogates (prostate-specific antigen [PSA] failure, PSA nadir >0.5 ng/mL, PSA doubling time <9 months, and interval to PSA failure <30 months) for all-cause mortality using the proportion of treatment-effect metric.
Design, Setting, and Participants
For this randomized clinical trial, 206 men with unfavorable-risk prostate cancer who were seen at a Harvard-affiliated academic hospital or an associated community hospital between December 1, 1995, to April 15, 2001, were identified, randomized to radiation therapy alone or radiation therapy followed by 6 months of androgen deprivation therapy, and followed for a median 16.62 years. This analysis looks at the subgroup of 157 men with minimal comorbidities or no comorbidity (median follow-up, 16.49 months).
Interventions
Patients were previously randomized to receive radiation therapy or radiation and 6 months of androgen deprivation therapy.
Main Outcomes and Measures
Risk of all-cause mortality.
Results
Overall, a cohort of 157 men (median [interquartile range] age, 72.43 [68.75-75.53]) with unfavorable-risk prostate cancer and minimal or no comorbidities were selected for this study. Three tested metrics met all 4 Prentice criteria for surrogacy for the surrogate covariate in the adjusted model for all-cause mortality: PSA nadir greater than 0.5 ng/mL (adjusted hazard ratio [aHR], 1.72; 95% CI, 1.17-2.52; P = .01), PSA doubling time less than 9 months (aHR, 2.06; 95% CI, 1.29-3.28; P = .003), and interval to PSA failure less than 30 months (aHR, 1.76; 95% CI, 1.06-2.92; P = .03); while PSA failure did not. For the 3 successful surrogates, the proportion of treatment effect values were 103.86%, 43.09%, and 41.26%, respectively.
Conclusions and Relevance
A PSA nadir value of greater than 0.5 ng/mL following radiation and androgen deprivation therapy appears to identify men prior to PSA failure who are at high-risk for death. This could be used to select men for entry at the time of PSA nadir onto randomized trials evaluating the impact on survival of salvage androgen deprivation therapy with or without agents shown to prolong survival in men with castrate-resistant metastatic prostate cancer.
Trial Registration
clinicaltrials.gov Identifier: NCT00116220
This subgroup analysis of a randomized clinical trial of radiation therapy vs radiation therapy plus androgen deprivation therapy for prostate cancer compares the performance of 4 candidate surrogates for all-cause mortality in men with localized unfavorable-risk prostate cancer using the proportion of treatment-effect metric.
Key Points
Question
How do surrogate end points for death in men treated with radiation therapy (RT) and androgen deprivation therapy (ADT) for localized prostate cancer compare?
Findings
In this subgroup analysis of data from a randomized clinical trial comparing effect of RT alone vs RT plus 6 months of ADT in all-cause mortality in men with localized prostate cancer, a prostate-specific antigen (PSA) nadir of greater than 0.5 ng/mL met the Prentice criteria for death and had the highest proportion of treatment-effect value among surrogates evaluated.
Meaning
A PSA nadir value of greater than 0.5 ng/mL following RT and ADT could be used to select men at high risk for death prior to PSA recurrence for early entry into randomized trials evaluating ADT with or without novel agents.
Introduction
Prostate cancer (PC) remains the second leading cause of male cancer death in the United States with 26 000 estimated deaths projected in 2016. Of the 180 000 new cases of prostate cancer diagnosed each year, roughly 95% will have localized PC. Some of these men with localized disease will eventually develop metastatic disease and die; these account for two-thirds of all PC deaths observed in the United States. Clearly, there is a need to expedite improved therapy in patients diagnosed with localized and unfavorable-risk PC.
Biochemical recurrence (BCR), or an increasing prostate-specific antigen (PSA) after definitive treatment for localized PC indicates the potential for local recurrence and/or metastatic PC. While BCR is often managed with salvage androgen deprivation therapy (ADT), there are no randomized studies to our knowledge to show that ADT in this setting prolongs survival. Also, BCR is associated with but does not necessarily mean a patient will experience prostate cancer–specific mortality (PCSM) because many men will die from competing risks, which are prevalent in men who are likely to develop prostate cancer. Therefore, surrogate end points for PCSM that occur prior to clinical recurrence, that are not only prognostic but also predict for a very high risk of PCSM, can help us identify men earlier who are candidates for entry on to randomized clinical trials (RCTs) evaluating the standard of care with or without novel agents shown to prolong survival in men with more advanced or metastatic PC. Moreover, surrogate end points could shorten the necessary follow-up time of RCTs before a new treatment regimen could be adopted, thereby expediting results for patient care and decreasing the needed resources to run clinical trials.
Several candidate surrogates meeting the Prentice criteria for surrogacy, a well-defined set of 4 requirements necessary to establish a surrogate end point, for PCSM have been identified, including PSA greater than 0.5 ng/mL (to convert to μg/L, multiply by 1.0) 6 months after randomization; PSA doubling time (DT) of less than 3 months, less than 12 months, or less than 15 months; and time to biochemical failure of less than 1.5 years, less than 2 years, or less than 2.5 years following radical prostatectomy or radiation therapy (RT) with or without 6 months of ADT. However, to our knowledge, none of these candidate surrogates have been compared for their ability to predict all-cause mortality (ACM) using a metric such as the proportion of the treatment effect explained (PTE) by the surrogate within the context of a single RCT that found a survival benefit in the investigational arm as compared with the conventional randomized treatment arm. Therefore, the purpose of this study was to use data from such a mature, prospective randomized clinical trial to ascertain and compare the performance of 4 candidate surrogates (PSA failure, PSA nadir >0.5 ng/mL, PSA DT<9 months, and interval to PSA failure <30 months) for ACM using the PTE metric for potential use as an entrance criteria in future randomized trials that evaluate the current standard of care vs the standard of care with agents shown to prolong survival in metastatic and castrate-resistant PC.
Methods
Patient Population and Treatment
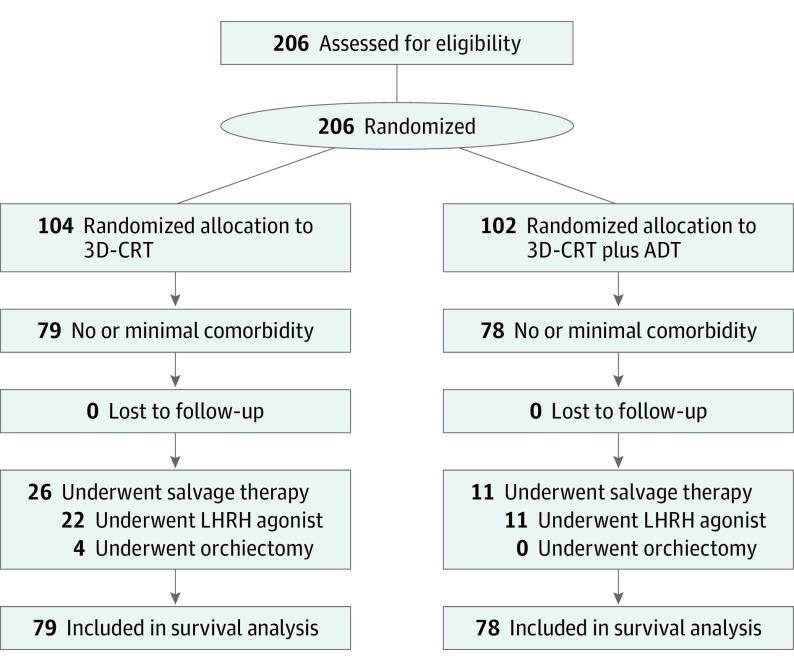
Two hundred and six men with unfavorable-risk PC (PSA>10 but <40 ng/mL or Gleason score ≥7; or endorectal magnetic resonance imaging evidence of extracapsular extension and/or seminal vesicle invasion) were randomized to receive 70.2 Gy (to convert to rad, multiply by 100) in 39 1.8 Gy fractions of 3-dimensional conformal RT alone or the same RT and 6 months of ADT at 3 community hospitals (St Anne’s Hospital, Metrowest Medical Center, and Suburban Oncology Center) and 3 academic-based centers (Dana-Farber Cancer Institute, Brigham and Women’s Hospital, and Beth Israel Deaconess Medical Center) in Massachusetts between December 1, 1995, and April 15, 2001. All pathology underwent centralized review by a dedicated genitourinary pathologist (A.R.). Given that complete data on patient comorbid conditions was available for review at randomization, a comorbidity score was assigned for each patient by the principal investigator (A.V.D.) using the Adult Comorbidity Evaluation 27 (ACE-27), a 27-item validated comorbidity index for use in patients with cancer. Of the 206 men, 157 (76%) were categorized as having no or minimal comorbidity per ACE-27 and, given that PSA failure has been shown to be associated with an increased risk of ACM in men with no or minimal comorbidity, and there was an association with prolonged survival in men randomized to RT and ADT compared with RT-alone, this population defined the study cohort as shown in the CONSORT diagram (Figure 1).
Figure 1. CONSORT Diagram.
ADT indicates androgen deprivation therapy; 3D-CRT, 3-dimensional conformal radiation therapy; LHRH, luteinizing hormone–releasing hormone.
Follow-up and Determination of the Cause of Death
The day of randomization was defined as time 0 and initiated the follow-up period. The date the patient was last observed or the date of death through February 21, 2015, whichever came first, concluded follow-up. No patients were lost to follow-up.
Men were seen in follow-up every 3 months for 2 years, every 6 months for the subsequent 3 years, and every year thereafter. Each follow-up visit included a history and physical exam with a serum PSA level obtained before the digital rectal examination. In addition to routine follow-up assessment, a bone scan and computed tomography or magnetic resonance imaging of the pelvis were obtained at the time of PSA failure. When PSA levels rose to approximately 10 ng/mL, salvage ADT, generally using a luteinizing hormone–releasing hormone agonist, was recommended.
The oncologist following the patient determined the cause of death. For PC to be the cause of death the following criteria had to be met: castrate–resistant metastatic PC, a rising PSA despite multiple salvage ADT regiments, and usually chemotherapy before death. Institutional review boards at St Anne’s Hospital and the Dana Farber Harvard Cancer Center approved the informed consent form that was signed by all participants. For long-term follow-up, a waiver of consent was obtained.
Statistical Methods
Distribution and Comparison of Clinical Characteristics and Treatment Stratification
The Table illustrates the distribution of clinical characteristics and treatment among men who did or did not achieve 1 of the 4 candidate surrogates (PSA failure, PSA nadir >0.5 ng/mL, PSA DT<9 months, and interval to PSA failure <30 months). The PSA DT was calculated using PSA values following PSA failure (nadir +2) by assuming first-order kinetics and by using a minimum of 3 PSA measurements, each separated by a minimum of 3 months and each with a PSA increase of more than 0.2 ng/mL. The distribution of these characteristics is compared for each candidate surrogate using a Mantel-Haenszel χ2 test for all categorical characteristics including the American Joint Committee on Cancer (AJCC) T category, highest Gleason score, and randomized treatment arm, whereas the Wilcoxon rank-sum test is used for the continuous clinical factors of age and PSA.
Table. Distribution and Comparison of Clinical Characteristics and Treatment Stratified by Whether the Candidate Surrogate Was Observed or Not.
| Characteristics | Candidate Surrogate, No. (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PSA Failure | P Value | PSA Nadir (>0.5 ng/mL) | P Value | PSA DT (<9 mo) | P Value | Int to PSA Failure (<30 mo) | P Value | |||||
| Yes (n = 85) |
No (n = 72) |
Yes (n = 58) |
No (n = 99) |
Yes (n = 35) |
No (n = 122) |
Yes (n = 29) |
No (n = 128) |
|||||
| Age, median (range), y | 72.12 (67.43-75.01) | 73.09 (69.96-76.29) | .25 | 72.04 (66.81-75.01) | 72.81 (69.97-75.61) | .26 | 71.97 (66.74-76.45) | 72.48 (68.86-75.04) | .86 | 72.57 (68.94-75.71) | 72.40 (68.64-75.46) | .70 |
| PSA, median (range), ng/mL | 12.20 (8.40-17.79) | 9.95 (5.97-13.45) | .004 | 11.92 (9.00-17.93) | 11.00 (6.86-14.84) | .04 | 16.15 (9.10-26.90) | 10.95 (7.21-40.12) | .002 | 16.50 (10.96-21.90) | 10.85 (7.26-14.52) | .001 |
| 1992 AJCC T stage | ||||||||||||
| T2 (n = 76) | 51 (67.11) | 25 (32.89) | .002 | 32 (42.11) | 44 (57.89) | .20 | 25 (12.35) | 71 (87.65) | .002 | 22 (28.95) | 54 (71.05) | .001 |
| T1 (n = 81) | 34 (41.98) | 47 (58.02) | 26 (32.10) | 55 (67.90) | 10 (32.89) | 51 (67.11) | 7 (8.64) | 74 (91.36) | ||||
| Highest Gleason Score | ||||||||||||
| 8-10 (n = 18) | 12 (66.67) | 6 (33.33) | .02 | 8 (44.44) | 10 (55.56) | .41 | 8 (44.44) | 10 (55.56) | .001 | 7 (38.89) | 11 (61.11) | .002 |
| 7 (n = 91) | 54 (59.34) | 37 (40.66) | 34 (37.36) | 57 (62.64) | 23 (25.27) | 68 (74.73) | 19 (20.88) | 72 (79.12) | ||||
| ≤6 (n = 48) | 19 (39.58) | 29 (60.42) | 16 (33.33) | 32 (66.67) | 4 (8.33) | 44 (91.67) | 3 (6.25) | 45 (93.75) | ||||
| Randomized treatment | ||||||||||||
| RT+ADT (n = 78) | 25 (32.05) | 53 (67.95) | <.001 | 5 (6.41) | 73 (93.59) | <.001 | 11 (14.10) | 67 (85.90) | .01 | 6 (7.69) | 72 (92.31) | <.001 |
| RT (n = 79) | 60 (75.95) | 19 (24.05) | 53 (67.09) | 26 (32.91) | 24 (30.38) | 55 (69.62) | 23 (29.11) | 56 (70.89) | ||||
| Odds ratio (95% CI)a | 6.70 (3.32-13.50) | <.001 | 29.76 (10.73-82.55) | <.001 | 2.66 (1.20-5.90) | .02 | 4.93 (1.88-12.92) | .001 | ||||
Abbreviations: ADT, androgen deprivation therapy; AJCC, American Joint Commission on Cancer; DT, doubling time; Int, interval; PSA, prostate-specific antigen; RT, radiation therapy.
Unadjusted odds ratio represents the likelihood that the candidate surrogate was observed in men randomized to receive RT compared with RT and ADT. Mantel-Haenszel χ2 is used to compare the distributions of categorical variables. Wilcoxon 2-sample test is used to compare the distributions of continuous variable.
Evaluation and Selection of the Optimal Surrogate
To show that a candidate surrogate satisfies the Prentice criteria there are 4 requirements. First, treatment with RT and ADT must be significantly associated with a reduction in the risk of ACM in an unadjusted Cox model. Second, the occurrence of the surrogate must be significantly less likely in men randomized to RT and ADT as measured in an unadjusted logistic regression analysis (Table). Third, the candidate surrogate should be significantly associated with an increased risk of ACM in an unadjusted Cox model, and finally, once the surrogate is included in the adjusted Cox model then treatment with RT and ADT is no longer significantly associated with a reduced risk of ACM, whereas the surrogate remains significantly associated with an increased risk of ACM. However, because men were not stratified by comorbidity prior to randomization, we also included age and known PC prognostic factors—specifically PSA, Gleason score, and T stage—in the adjusted model because we only evaluated the subset of 157 men with no or minimal comorbidity, given that PSA failure has been shown to be associated with an increased risk of ACM only in men with no or minimal comorbidity. All candidate surrogates were treated as time-dependent covariates given that time 0 was the date of randomization. Each adjusted and unadjusted hazard ratio is accompanied by a 95% CI and P value. The additional covariates evaluated include PSA and age at randomization as continuous covariates, and treatment (RT and ADT vs RT), AJCC clinical T category (T2 vs T1), and Gleason score (8-10, 7 vs ≤6).
Further assessment of each proposed surrogate end point was performed by determining the PTE that was explained by the proposed surrogate end point. Perfect evidence to support the Prentice criteria corresponds to a PTE of 100%, signifying that 100% of the treatment effect can be explained by the proposed surrogate end point. The optimal surrogate was defined as that with the corresponding PTE value closest to 100%.
Estimates of All-Cause Mortality
Estimates of ACM (1 minus the Kaplan-Meier estimates of OS) following randomization stratified by men who were observed to achieve the candidate surrogate or not were calculated using the extended Kaplan-Meier methodology with time-dependent covariates and adjusting for age. These estimates were compared using a Cox time-dependent covariates P value. A 2-sided P value less than .0125 was considered statistically significant after adjusting for the analysis evaluating 4 candidate surrogates using a Bonferroni correction. We used Power Analysis and Sample Size Software (PASS) 13 (NCSS Statistical Software) to calculate the power to measure a statistically significant difference (P < .0125 after the Bonferroni correction) in the 8-year actuarial survival differences observed for men who experienced each of the 4 candidate surrogates or not. The calculation provided power of 98.51%, 99.99%, 97.25%, and 90.50% for the candidate surrogates of PSA failure, PSA nadir greater than 0.5 ng/mL, PSA DT less than 9 months, and interval to PSA failure less than 30 months, respectively. SAS version 9.4 (SAS Institute) was used for all statistical analyses except the age-adjusted ACM estimates and 95% CIs with time-dependent covariates, for which R version 3.2.3 (R Foundation) was used.
Results
As shown in the Table, men randomized to receive treatment with RT vs RT and ADT were significantly more likely (P ≤ .02) to have the candidate surrogate observed. The median PSA was higher for PSA failure (12.20 ng/mL vs 9.95 ng/mL; P = .004), PSA nadir greater than 0.5 ng/mL (11.92 vs 11.00 ng/mL; P = .04), PSA DT less than 9 months (16.15 ng/mL vs 10.95 ng/mL; P = .002), and interval to PSA failure less than 30 months (16.50 ng/mL vs 10.85 ng/mL; P = .001). The distribution of median age and other PC prognostic factors were not noted to be significantly different (P ≥ .25 for median age) among men who were observed to achieve the candidate surrogate compared with those who did not (Table).
After a median follow-up of 16.49 years, among the 157 men in the study cohort, 110 died (70%). Among the 4 candidate surrogates, only 3 (PSA nadir >0.5 ng/mL, PSA DT<9 months, and interval to PSA failure <30 months) met all 4 Prentice criteria for surrogacy including that the candidate surrogate remained significant in the adjusted analysis (PSA nadir >0.5 ng/mL: adjusted hazard ratio [aHR], 1.72; 95% CI, 1.17-2.52; P = .01; PSA DT <9 months: aHR, 2.06; 95% CI, 1.29-3.28; P = .003; and interval to PSA failure <30 months: aHR, 1.76; 95% CI, 1.06-2.92; P = .03) while treatment was no longer significant (P ≥ .27), whereas PSA failure did not meet this criteria (aHR, 1.47; 95% CI, 0.93-2.32; P = .10) (eTable in the Supplement).
For the 3 PSA metrics that met the Prentice criteria for surrogacy, the PTE values were 103.86% for PSA nadir greater than 0.5 ng/mL; 43.09% for PSA DT less than 9 months; and 41.26% for PSA failure less than 30 months. Given that a PSA nadir of greater than 0.5 ng/mL had the PTE value closest to 100%, it was selected as the optimal surrogate.
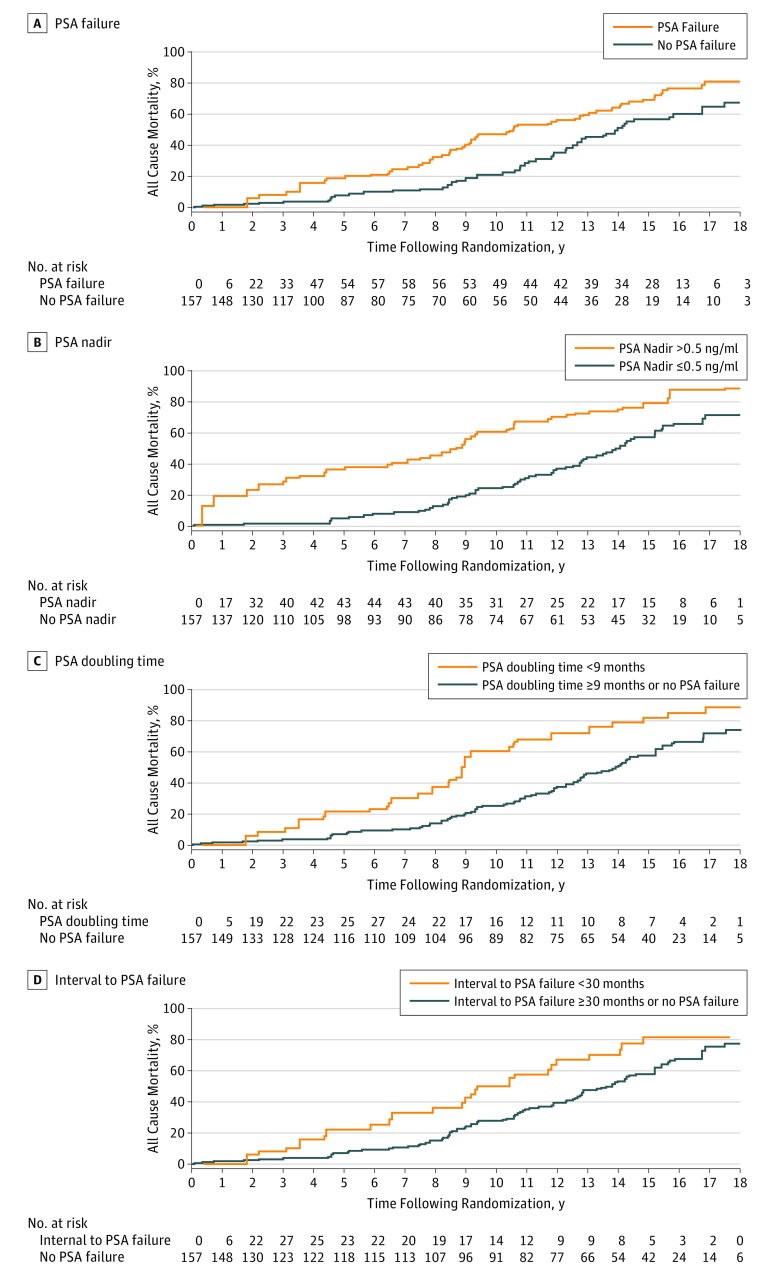
Estimates of age-adjusted all-cause mortality were significantly greater among men in whom the candidate surrogates were observed vs not as shown in Figure 2. Specifically, for PSA failure, PSA nadir greater than 0.5 ng/mL, PSA DT less than 9 months, and interval to PSA failure less than 30 months, the respective P values were .0083, <.001, <.001, and .0018. Specifically, 8-year point estimates and 95% CI for all-cause mortality for each of these respective candidate surrogates in the setting where the surrogate was observed vs not were 32.5% (21.30%-47.52%) vs 11.83% (7.23%-19.02%); 47.44% (36.81%-59.39%) vs 13.63% (8.53%-21.41%); 40.70% (27.44%-57.33%) vs 14.12% (9.30%-21.15%); and 39.43% (25.52%-57.39%) vs 15.26% (10.31%-22.28%).
Figure 2. Estimates of Age-Adjusted All-Cause Mortality in 157 Men With No or Minimal Comorbidity.
All data are stratified by the occurrence of the observation of: A, prostate-specific antigen (PSA) failure (age-adjusted Cox P = .01); B, PSA of a nadir greater than 0.5 ng/mL (age-adjusted Cox P < .001); C, PSA doubling time less than 9 months (age-adjusted Cox P < .001); D, interval to PSA failure less than 30 months (age-adjusted Cox P = .002).
Discussion
In this study, we found that a PSA nadir greater than 0.5 ng/mL after radiation with or without 6 months of combined ADT was a surrogate for ACM using the Prentice criteria. When compared with the other candidate surrogates evaluated including PSA DT<9 months and interval to PSA failure less than 30 months, PSA nadir greater than 0.5 ng/mL performed better as measured by a PTE of 103.86% vs 43.09% and 41.26%, respectively. Moreover, we did not find, despite selecting men with minimal or no comorbidities for this study, that PSA failure was a surrogate for ACM, despite being a prognostic factor as shown in eTable in the Supplement (P = .03).
The clinical importance of this finding is that it provides an opportunity for patient selection for clinical trial entry at a time when the patient is still responding to but has not yet declared as having failed primary therapy. Specifically, men undergoing conventional dose radiation (70 Gy) and 6 months of combined ADT whose PSA nadir does not fall below 0.5 ng/mL could be considered for entry on to clinical trials at the time of PSA nadir investigating standard of care (conventional ADT with an luteinizing hormone–releasing hormone agonist) with or without novel forms of hormonal therapy (eg, enzalutamide or abiraterone) or cytotoxic chemotherapy (eg, docetaxel) that have been shown to prolong survival in men with metastatic and castrate–resistant prostate cancer. By enriching the study cohort with men who have achieved a surrogate endpoint for ACM, one can enhance the likelihood that the study will be able to answer the question of whether survival is prolonged when novel treatment is added to standard of care as compared with standard of care and over a shorter time period.
An additional point of clinical importance is that PSA failure was not a surrogate, even among men with no or minimal comorbidity. While this requires external validation, it suggests that, in men with moderate-to-severe comorbidity, PSA failure is unlikely to translate into a surrogate for ACM and therefore has potential implications on how such men should be managed at the time of PSA failure. Specifically, some of these men with moderate-to-severe comorbidity may be optimal candidates for surveillance. Therefore a more judicious approach to salvage ADT should be considered in these men. Particularly given the known cardiometabolic adverse effects of ADT, which can at the very least lessen quality of life and perhaps quantity of life in such men.
Limitations
Several points require further discussion. First, classically, to establish surrogacy, applying the Prentice criteria requires an adjusted model for the end point of interest (eg, ACM as in the current study) in which you include the candidate surrogate and treatment in the model using data from a randomized trial, where one treatment has been shown to be superior to the other with regards to the end point of interest. However, our results are based on a single, small clinical trial involving a subgroup of 157 men with no or minimal comorbidity analyzed in a postrandomization fashion and therefore requires validation. Another potential limitation is that the doses of RT (70.2 Gy) in the RCT evaluated was low by today’s standard; however, if PSA nadir greater than 0.5 ng/mL is a surrogate after 70 Gy and 6 months of ADT, it would be expected to also be a surrogate after 79.2 Gy (a higher, more conventional dose of radiation based on phase 3 data). A higher dose of radiation with a poor response in the PSA nadir value, one would expect that would also establish surrogacy given it was established when 70.0 Gy and not 79.2 Gy was delivered. We would expect men treated to a higher RT dose to achieve a lower median PSA nadir which was observed in the randomized dose escalation trial by the American College of Radiology evaluating RT doses of 79.2 Gy vs 70.2 Gy.
Conclusions
Despite these considerations, our study provides evidence to support that, in men undergoing conventional dose RT and 6 months of ADT, a PSA nadir value of greater than 0.5 ng/mL is a surrogate for ACM. Therefore, PSA nadir greater than 0.5 ng/mL could be considered an entrance criteria at the time of PSA nadir for prospective RCTs evaluating salvage with conventional ADT with or without agents found to prolong survival in men with metastatic castrate–resistant prostate cancer.
eTable 1. Unadjusted and Adjusted Hazard Ratios for Clinical Characteristics, Candidate Surrogates and Treatment for the Risk of All-Cause Mortality
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. [DOI] [PubMed] [Google Scholar]
- 2.Miller DC, Hafez KS, Stewart A, Montie JE, Wei JT. Prostate carcinoma presentation, diagnosis, and staging: an update form the National Cancer Data Base. Cancer. 2003;98(6):1169-1178. [DOI] [PubMed] [Google Scholar]
- 3.Sweeney C, Nakabayashi M, Regan M, et al. ; ICECaP Working Group . The Development of Intermediate Clinical Endpoints in Cancer of the Prostate (ICECaP). J Natl Cancer Inst. 2015;107(12):djv261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network Prostate Cancer (NCCN Guidelines Version 3.2016). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed December 6, 2016.
- 5.Paller CJ, Antonarakis ES. Management of biochemically recurrent prostate cancer after local therapy: evolving standards of care and new directions. Clin Adv Hematol Oncol. 2013;11(1):14-23. http://www.ncbi.nlm.nih.gov/pubmed/23416859. Accessed June 16, 2016. [PMC free article] [PubMed] [Google Scholar]
- 6.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591-1597. http://www.ncbi.nlm.nih.gov/pubmed/10235151. Accessed June 16, 2016. [DOI] [PubMed] [Google Scholar]
- 7.Arvold ND, Chen M-H, Moul JW, et al. Risk of death from prostate cancer after radical prostatectomy or brachytherapy in men with low or intermediate risk disease. J Urol. 2011;186(1):91-96. doi: 10.1016/j.juro.2011.03.003 [DOI] [PubMed] [Google Scholar]
- 8.Dicker AP, Kelly WK, Trabulsi EJ, Zaorsky NG. Prostate Cancer: A Multidisciplinary Approach to Diagnosis and Management. New York, NY: Demos Medical Publishing, LLC; 2015. [Google Scholar]
- 9.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8(4):431-440. http://www.ncbi.nlm.nih.gov/pubmed/2727467. Accessed June 16, 2016. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico AV, Chen M-H, de Castro M, et al. Surrogate endpoints for prostate cancer-specific mortality after radiotherapy and androgen suppression therapy in men with localised or locally advanced prostate cancer: an analysis of two randomised trials. Lancet Oncol. 2012;13(2):189-195. [DOI] [PubMed] [Google Scholar]
- 11.D’Amico AV, Moul JW, Carroll PR, Sun L, Lubeck D, Chen M-H. Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst. 2003;95(18):1376-1383. Accessed June 16, 2016. [DOI] [PubMed] [Google Scholar]
- 12.D’Amico AV, Moul J, Carroll PR, Sun L, Lubeck D, Chen M-H. Prostate specific antigen doubling time as a surrogate end point for prostate cancer specific mortality following radical prostatectomy or radiation therapy. J Urol. 2004;172(5 Pt 2):S42-6-7. [DOI] [PubMed] [Google Scholar]
- 13.Denham JW, Steigler A, Wilcox C, et al. ; Trans-Tasman Radiation Oncology Group 96.01 Trialists . Time to biochemical failure and prostate-specific antigen doubling time as surrogates for prostate cancer-specific mortality: evidence from the TROG 96.01 randomised controlled trial. Lancet Oncol. 2008;9(11):1058-1068. [DOI] [PubMed] [Google Scholar]
- 14.Lin DY, Fleming TR, De Gruttola V. Estimating the proportion of treatment effect explained by a surrogate marker. Stat Med. 1997;16(13):1515-1527. [DOI] [PubMed] [Google Scholar]
- 15.D’Amico AV, Chen M-H, Renshaw A, Loffredo M, Kantoff PW. Long-term follow-up of a randomized trial of radiation with or without androgen deprivation therapy for localized prostate cancer. JAMA. 2015;314(12):1291-1293. [DOI] [PubMed] [Google Scholar]
- 16.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL Jr. Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291(20):2441-2447. [DOI] [PubMed] [Google Scholar]
- 17.Giacalone NJ, Wu J, Chen M-H, et al. Prostate-specific antigen failure and risk of death within comorbidity subgroups among men with unfavorable-risk prostate cancer treated in a randomized trial [published online September 6, 2016]. J Clin Oncol. doi: 10.1200/JCO.2016.68.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agresti A. Categorical Data Analysis. 3rd ed John Wiley & Sons, Inc; 2012. [Google Scholar]
- 19.Hollander M, Wolfe D, Chicken E. Nonparametric Statistical Methods. 3rd Edition. John Wiley & Sons, Inc; 2014. [Google Scholar]
- 20.Klein J, Moeschberger M. Survival Analysis: Techniques for Censored and Truncated Data. Springer; 2013. [Google Scholar]
- 21.Snapinn S, Jiang Q, Iglewicz B. Illustrating the impact of a time-varying covariate with an extended kaplan-meier estimator. Am Stat. 2005;59(4):301-307. [Google Scholar]
- 22.Cupples LA, Gagnon DR, Ramaswamy R, D’Agostino RB. Age-adjusted survival curves with application in the Framingham Study. Stat Med. 1995;14(16):1731-1744. [DOI] [PubMed] [Google Scholar]
- 23.Scher HI, Fizazi K, Saad F, et al. ; AFFIRM Investigators . Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187-1197. [DOI] [PubMed] [Google Scholar]
- 24.de Bono JS, Logothetis CJ, Molina A, et al. ; COU-AA-301 Investigators . Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tannock IF, de Wit R, Berry WR, et al. ; TAX 327 Investigators . Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512. [DOI] [PubMed] [Google Scholar]
- 26.Martin NE, Chen M-H, Beard CJ, et al. Natural history of untreated prostate specific antigen radiorecurrent prostate cancer in men with favorable prognostic indicators. Prostate Cancer. 2014;2014:912943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine GN, D’Amico AV, Berger P, et al. ; American Heart Association Council on Clinical Cardiology and Council on Epidemiology and Prevention, the American Cancer Society, and the American Urological Association . Androgen-deprivation therapy in prostate cancer and cardiovascular risk: a science advisory from the American Heart Association, American Cancer Society, and American Urological Association: endorsed by the American Society for Radiation Oncology. CA Cancer J Clin. 2010;60(3):194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bruner DW, Hunt D, Michalski JM, et al. Preliminary patient-reported outcomes analysis of 3-dimensional radiation therapy versus intensity-modulated radiation therapy on the high-dose arm of the Radiation Therapy Oncology Group (RTOG) 0126 prostate cancer trial. Cancer. 2015;121(14):2422-2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from Proton Radiation Oncology Group/American College of Radiology 95-09. J Clin Oncol. 2010;28(7):1106-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Unadjusted and Adjusted Hazard Ratios for Clinical Characteristics, Candidate Surrogates and Treatment for the Risk of All-Cause Mortality