Key Points
Question
Does the neuroanatomical male brain phenotype carry a higher intrinsic risk for autism spectrum disorder than the female neurophenotype, which could explain the male preponderant prevalence of autism spectrum disorder?
Findings
In this case-control study of 98 adults with autism spectrum disorder and 98 matched neurotypical control individuals, the neurobiological male phenotype was associated with a higher risk for autism spectrum disorder than the female phenotype across the binary categories dictated by biological sex.
Meaning
In addition to genetic and environmental factors, normative sex-related phenotypic diversity should be considered when determining an individual’s risk for autism spectrum disorder.
Abstract
Importance
Autism spectrum disorder (ASD) is 2 to 5 times more common in male individuals than in female individuals. While the male preponderant prevalence of ASD might partially be explained by sex differences in clinical symptoms, etiological models suggest that the biological male phenotype carries a higher intrinsic risk for ASD than the female phenotype. To our knowledge, this hypothesis has never been tested directly, and the neurobiological mechanisms that modulate ASD risk in male individuals and female individuals remain elusive.
Objectives
To examine the probability of ASD as a function of normative sex-related phenotypic diversity in brain structure and to identify the patterns of sex-related neuroanatomical variability associated with low or high probability of ASD.
Design, Setting, and Participants
This study examined a cross-sectional sample of 98 right-handed, high-functioning adults with ASD and 98 matched neurotypical control individuals aged 18 to 42 years. A multivariate probabilistic classification approach was used to develop a predictive model of biological sex based on cortical thickness measures assessed via magnetic resonance imaging in neurotypical controls. This normative model was subsequently applied to individuals with ASD. The study dates were June 2005 to October 2009, and this analysis was conducted between June 2015 and July 2016.
Main Outcomes and Measures
Sample and population ASD probability estimates as a function of normative sex-related diversity in brain structure, as well as neuroanatomical patterns associated with low or high ASD probability in male individuals and female individuals.
Results
Among the 98 individuals with ASD, 49 were male and 49 female, with a mean (SD) age of 26.88 (7.18) years. Among the 98 controls, 51 were male and 47 female, with a mean (SD) age of 27.39 (6.44) years. The sample probability of ASD increased significantly with predictive probabilities for the male neuroanatomical brain phenotype. For example, biological female individuals with a more male-typic pattern of brain anatomy were significantly (ie, 3 times) more likely to have ASD than biological female individuals with a characteristically female brain phenotype (P = .72 vs .24, respectively; χ21 = 20.26; P < .001; difference in P values, 0.48; 95% CI, 0.29-0.68). This finding translates to an estimated variability in population prevalence from 0.2% to 1.3%, respectively. Moreover, the patterns of neuroanatomical variability carrying low or high ASD probability were sex specific (eg, in inferior temporal regions, where ASD has different neurobiological underpinnings in male individuals and female individuals).
Conclusions and Relevance
These findings highlight the need for considering normative sex-related phenotypic diversity when determining an individual’s risk for ASD and provide important novel insights into the neurobiological mechanisms mediating sex differences in ASD prevalence.
This case-control study examines the probability of autism spectrum disorder as a function of normative sex-related phenotypic diversity in brain structure in a sample of adults with autism spectrum disorder and matched neurotypical control individuals.
Introduction
Autism spectrum disorder (ASD) is a complex neurodevelopmental condition that is 2 to 5 times more common in male individuals than in female individuals. While the male preponderant prevalence of ASD might partially be explained by sex differences in clinical symptoms, etiological models suggest that the biological male phenotype itself (ie, in general) carries a higher risk for ASD than the female phenotype. However, despite the growing number of studies examining sex differences in the brain in ASD (eg, those by Lai et al and by Schaer et al), this hypothesis has never been tested directly, to our knowledge, and the neurobiological mechanisms that underpin the male preponderant prevalence of ASD remain elusive.
This study examined the probability of ASD as a function of normative sex-related phenotypic diversity in brain structure. To do so, we initially developed a predictive model of biological sex based on multivariate differences in brain structure in a sample of typically developing (TD) male and female control individuals. This normative model was subsequently applied to male individuals and female individuals with ASD. Unlike recent studies examining sexual dimorphism of the brain (reviewed by Ruigrok et al), we used a probabilistic pattern classification approach, which allowed us to accommodate interindividual phenotypic diversity within and across the binary categories dictated by biological sex. As a result, we were able (1) to examine the probability of ASD along a normative phenotypic axis ranging from the characteristic female to male brain phenotype and (2) to identify the patterns of sex-related neuroanatomical variability associated with low or high probability of ASD.
We based our analysis on measures of cortical thickness (CT) because these measurements have previously been shown to be highly variable between neurotypical male individuals and female individuals and tend to be significantly altered in individuals with ASD (eg, as shown by Ecker et al and by Wallace et al). Moreover, measures of CT are less sensitive to global confounders associated with biological sex (eg, differences in total brain volume) than other morphometric features (eg, surface area). Last, we investigated whether the cortical underpinnings of ASD are significantly modulated by biological sex and whether ASD has common or distinct neuroanatomical underpinnings in male individuals and female individuals.
Methods
Participants
Ninety-eight right-handed adults with ASD (49 male and 49 female; mean [SD] age, 26.88 [7.18] years) and 98 matched neurotypical controls (51 male and 47 female; mean [SD] age, 27.39 [6.44] years) aged 18 to 42 years were recruited locally by advertisement and assessed at the Institute of Psychiatry, Psychology and Neuroscience (IoPPN), London, England, and the Autism Research Centre, Cambridge, England (Table). Approximately equal ratios of cases to controls and male individuals to female individuals were recruited within sites (eTable 1 in the Supplement). Exclusion criteria included a history of major psychiatric disorder (eg, psychosis), head injury, ASD-associated genetic disorders (eg, fragile X syndrome and tuberous sclerosis), or any other medical condition affecting brain function (eg, epilepsy). We also excluded individuals taking antipsychotic medication, mood stabilizers, or benzodiazepines.
Table. Participant Demographics and Global Brain Measuresa.
| Variable | Mean (SD) [Range] | |
|---|---|---|
| ASD (n = 98) |
TD Control (n = 98) |
|
| Male Individuals (n = 100) | ||
| No. | 49 | 51 |
| Age, y | 26.16 (7.20) [18-41] | 27.22 (5.58) [18-42] |
| IQ | ||
| Full-scale WASI | 112.61 (12.27) [89-135] | 114.67 (10.88) [93-137] |
| Verbal | 110.51 (13.01) [83-137] | 109.86 (11.03) [88-137] |
| Performance | 112.00 (13.65) [85-138] | 116.74 (10.90) [93-133] |
| ADI-R | ||
| Social | 17.57 (5.50) [10-28] | NA |
| Communication | 13.86 (4.16) [8-24] | NA |
| Repetitive behavior | 4.96 (2.37) [1-10] | NA |
| ADOS | ||
| Social plus communication | 9.43 (4.39) [1-21] | NA |
| Stereotypic behavior | 1.27 (1.27) [0-5]b | NA |
| Cortical thickness, mm | 2.35 (0.10) | 2.33 (0.10) |
| Volume, L | ||
| Total gray matter | 0.76 (0.08) | 0.76 (0.05) |
| Total intracranial | 1.59 (0.21) | 1.58 (0.17) |
| Female Individuals (n = 96) | ||
| No. | 49 | 47 |
| Age, y | 27.60 (7.12) [18-48] | 27.60 (7.31) [19-52] |
| IQ | ||
| Full-scale WASI | 114.88 (12.36) [84-136] | 118.38 (7.32) [99-129] |
| Verbalc | 116.60 (12.15) [76-144] | 117.83 (8.81) [96-135] |
| Performancec | 110.56 (14.50) [67-138] | 114.38 (8.26) [96-128] |
| ADI-R | ||
| Social | 16.32 (4.24) [10-26] | NA |
| Communication | 12.43 (3.96) [7-22] | NA |
| Repetitive behavior | 4.36 (1.98) [1-9] | NA |
| ADOS | ||
| Social plus communication | 7.71 (5.28) [0-19] | NA |
| Stereotypic behavior | 0.75 (0.97) [0-3]b | NA |
| Cortical thickness, mm | 2.32 (0.11) | 2.34 (0.10) |
| Volume, L | ||
| Total gray matter | 0.67 (0.06) | 0.68 (0.06) |
| Total intracranial | 1.32 (0.18) | 1.32 (0.17) |
Abbreviations: ADI-R, Autism Diagnostic Interview–Revised; ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; NA, not applicable; TD, typically developing; WASI, Wechsler Abbreviated Scale of Intelligence.
There were no significant between-group differences in age, full-scale IQ, mean cortical thickness, total gray matter volume, or total intracranial volume (2-tailed P < .05). The ADI-R values were based on 49 male individuals and 44 female individuals with ASD. The ADOS values were based on 49 male individuals and 48 female individuals with ASD.
Statistically significant between male individuals and female individuals based on 2-tailed P < .05 (t95 = 2.23, P = .03).
Verbal and performance IQ data were not available for 1 female individual with ASD.
All participants with ASD were diagnosed according to the International Statistical Classification of Diseases, Tenth Revision research criteria. The clinical diagnosis was confirmed using the Autism Diagnostic Interview–Revised (ADI-R) or the Autism Diagnostic Observation Schedule (ADOS). All participants diagnosed with the ADI-R reached algorithm cutoffs in the 3 domains of (1) communication (cutoff of 8), (2) reciprocal social interaction (cutoff of 10), and (3) repetitive behaviors and stereotyped patterns of interest (cutoff of 3), although failure to reach the cutoff in the repetitive domain by maximally 2 points was permitted. Because reliable informants were unavailable, we were unable to obtain ADI-R diagnostic data from 5 female individuals with ASD and confirmed diagnostic status using ADOS cutoffs. In all other participants, the ADOS scores were used to assess the severity of current symptoms. One female individual with ASD fell short by 1 point on the ADI-R communication and repetitive behavior domains but met ADOS criteria. All participants had a full-scale IQ greater than 70 assessed using the Wechsler Abbreviated Scale of Intelligence. Participants gave informed written consent in accord with ethics approval by the National Research Ethics Committee, Suffolk, England. The study dates were June 2005 to October 2009, and this analysis was conducted between June 2015 and July 2016.
Magnetic Resonance Imaging Data Acquisition
Imaging took place at the IoPPN and the Addenbrooke’s Hospital, Cambridge, using a 3-T system (Signa; GE Medical Systems). A specialized acquisition protocol using quantitative, T1-weighted mapping was used to ensure standardization of structural magnetic resonance imaging scans across sites, which resulted in high-resolution structural T1-weighted inversion recovery images, with 1 × 1 × 1–mm resolution, a 256 × 256 × 176–pixel matrix, repetition time of 1800 milliseconds, inversion time of 50 milliseconds, flip angle of 20°, and field of view of 25 cm.
Cortical Reconstruction Using FreeSurfer
A software program (FreeSurfer, version 5.3.0; http://surfer.nmr.mgh.harvard.edu) was used to derive cortical surface models for each T1-weighted image. These well-validated and fully automated procedures have been extensively described elsewhere. All surface models were visually inspected for errors, and scans with visible reconstruction inaccuracies were excluded from the statistical analysis (dropout <10%). We based our analysis on measures of CT (ie, the closest distance from the gray matter (GM) or white matter boundary to the GM or cerebrospinal fluid boundary at each cerebral vertex). Cortical thickness maps were smoothed with a 15-mm surface-based gaussian kernel.
Gaussian Process Classification
Gaussian process classification (GPC) was used for the probabilistic prediction of biological sex based on neuroanatomical variability in CT (details are available in the eMethods in the Supplement). In brief, we initially developed a normative model of biological sex by predicting the binary class labels yi ε {−1, +1} for female and male TD controls, respectively, using CT measures estimated at approximately 320 000 vertices on the cortical surface (Gaussian Processes for Machine Learning Matlab toolbox; http://www.gaussianprocess.org/gpml/code/matlab/doc). In addition to the overall model accuracy (ie, proportion of biological male individuals or female individuals correctly classified as phenotypic male individuals or female individuals), GPC returned a set of predictive class probabilities indicating the probability of an individual belonging to the male or female category. Class probabilities ranged from 0 to 1 for the characteristic female to male brain phenotype, respectively, so that a class probability of 0.5 represents a binary cutoff separating both classes. Classifier performance was validated by cross-validation and tested for statistical significance via 1000 permutations of class labels. The normative model was subsequently applied to male individuals and female individuals with ASD.
A predictive mapping approach was used to identify (1) the spatially distributed patterns of neuroanatomical variability in CT characteristics for the neurotypical female or male brain phenotype and (2) the set of brain regions associated with low or high probability of ASD. Normative or risk (ie, low or high probability) patterns were derived by quantifying the extent to which the individual’s neuroanatomy interacts with the spatial representation of the decision function (ie, the weight vector w) separating neurotypical male individuals from female individuals phenotypically (ie, the product of w and CT). The high ASD probability pattern included all male individuals (or female individuals) with a male neuroanatomical phenotype (ie, class probability >0.5). The low ASD probability pattern included all male individuals (or female individuals) with a female neuroanatomical phenotype (ie, class probability <0.5). We also identified these patterns across all individuals along the normative axis of predictive class probabilities. At each vertex, the resulting maps were summarized by the mean value across all individuals within examined groups.
Estimation of ASD Probability
To examine the probability of ASD as a function of normative phenotypic variability in brain structure, we converted the continuous axis of class probabilities into a set of discrete bins (eg, from 0 to 1 in steps of 0.125). Within each bin, the sample probability of ASD was determined as the ratio of individuals with ASD relative to the total number of individuals per bin. Moreover, these data allowed us to estimate the population prevalence of ASD given that an individual exhibits a male or female neuroanatomical phenotype in addition to being biologically male or female (details are available in the eMethods in the Supplement). In brief, using Bayes theorem, we combined our sample prevalence estimates with previously published population prevalence rates of ASD for biological male individuals and female individuals (ie, 1:42 for male individuals and 1:189 for female individuals). In this way, we were able to estimate the population prevalence of ASD (D = 1) given a male (or female) neuroanatomical brain phenotype (M) for biological male individuals (or female individuals) (S), that is P̂ (D = 1 | M, S). Confidence intervals were determined using an exact binomial test implemented in a software package (R Project for Statistical Computing; https://www.r-project.org).
Vertexwise Between-Group Comparison of CT
Vertexwise statistical analysis of CT measures was conducted using the R2014a Matlab toolbox (SurfStat; http://www.math.mcgill.ca/keith/surfstat). For the comparison between male and female controls, parameter estimates were obtained by regression of a general linear model at each vertex i, with biological sex and center as categorical fixed-effects factors and total GM volume as a continuous covariate. To examine whether the neuroanatomy of ASD is significantly modulated by biological sex, we also included a main effect of diagnostic group and biological sex and a group × sex interaction as follows:
| yi = β0 + β1 Group + β2 Sex + β3 (Group × Sex) + β4 GMtotal + β5 Center + εi, |
where y refers to the variable that is predicted by the model (ie, the CT at voxel i) and εi is the residual error. Effects were estimated from the coefficients β1-3 normalized by the corresponding standard error. Corrections for multiple comparisons were performed using a random field theory–based cluster analysis for nonisotropic images at a cluster threshold of 2-tailed P < .05. We also confirmed our findings using a nonparametric clustering approach (2-tailed P < .05) using 10 000 permutations of the original data.
Results
Prediction of Biological Sex Based on Normative Variability in CT
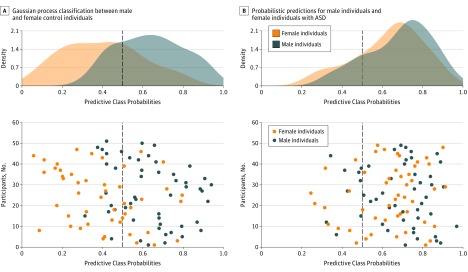
Overall, our CT-driven probabilistic classifier predicted biological sex at an accuracy of 71.4% under cross-validation in neurotypical controls, which was significantly higher than would be expected by chance (P < .001 obtained via permutation testing). In total, 68.1% (32 of 47) of all biological female individuals were correctly allocated to the category of phenotypic female individuals and 74.5% (38 of 51) of all biological male individuals to the category of phenotypic male individuals (Figure 1A, lower panel). Across individuals, class probabilities ranged continuously from 0 to 1 for the characteristic female to male brain phenotype, respectively (Figure 1A, upper panel). Moreover, the patterns of CT variability associated with the phenotypic shift of the brain from a female to male presentation resembled the set of brain regions where female individuals significantly differed from male individuals (eFigure 1 and eFigure 2 in the Supplement). Therefore, while a perfect phenotypic differentiation between biological sexes could not be achieved, we were able to separate male individuals from female individuals in most cases based on multivariate differences in brain structure.
Figure 1. Gaussian Process Classification of Biological Sex.
A, Gaussian process classification between male and female typically developing (TD) control individuals based on normative (ie, neurotypical) variability in cortical thickness. The x-axis indicates predictive class probabilities. Therefore, a class probability of 0.5 served as a binary cutoff separating male individuals from female individuals. The y-axis indicates the position of each individual on the normative axis of sex-related phenotypic diversity in brain structure (lower panel). The upper panel shows the density (ie, frequency) of male individuals and female individuals along the normative axis of class probabilities. B, Probabilistic predictions for male individuals and female individuals with autism spectrum disorder (ASD) using the normative model for biological sex. The density functions for male individuals and female individuals with ASD (upper panel) show the phenotypic shift of the brain in female individuals with ASD toward a more male phenotypic presentation. Of 49 female individuals with ASD, 39 (79.6%) fell within the category of phenotypic male individuals (lower panel).
Phenotypic Prediction of Biological Sex for Individuals With ASD
When applying our normative predictive model of biological sex to the independent sample of individuals with ASD, we found that, in female individuals with ASD, predictive class probabilities for the male neuroanatomical brain phenotype were significantly increased relative to female controls (t94 = 6.13, P < .001) (Figure 1B). Overall, 39 of 49 female individuals (79.6%) with ASD were allocated to the category of phenotypic male individuals, which was significantly more frequent than expected based on the typical rate of misclassification for female controls (79.6% [39 of 49] vs 31.9% [15 of 47], respectively; χ21 = 20.26, P < .001). No such differences were observed in male individuals with ASD, who were correctly allocated to the male category in 81.6% (40 of 49) of all cases and also did not differ significantly from male controls in predictive class probabilities (t98 = 1.35, 2-tailed P > .17). Therefore, when representing individuals along a single axis of normative sex-related phenotypic diversity in brain structure, female individuals with ASD displayed a CT pattern that resembled more closely the neurotypical male rather than female neurophenotype. This phenotypic shift in female individuals is likely to influence the probability of ASD.
ASD Probability as a Function of Normative Sex-Related Phenotypic Variability in Brain Structure
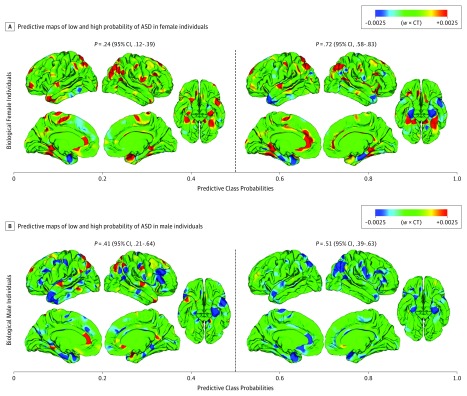
In female individuals with ASD, the sample probability of ASD increased with increasing predictive probabilities for the male neuroanatomical brain phenotype (eFigure 3A in the Supplement). More specifically, female individuals with class probabilities exceeding the binary sex cutoff of 0.5 (ie, biological female individuals falling into the category of phenotypic male individuals) were significantly (ie, 3 times) more likely to have a diagnosis of ASD than biological female individuals with a class probability lower than 0.5 (P = .72 vs .24, respectively; χ21 = 20.26; P < .001; difference in P values, 0.48; 95% CI, 0.29-0.68). Reciprocally, male individuals with class probabilities lower than 0.5 (ie, biological male individuals falling into the category of phenotypic female individuals) were 1.2 times less likely to have ASD than male individuals allocated to the category of phenotypic male individuals (P = .41 vs .51, respectively; χ21 = 0.38; P > .53; difference in P values, −0.10; 95% CI, −0.36 to 0.15) (eFigure 3B in the Supplement). If one combines these sample probabilities with previously published prevalence rates of ASD in the general population (eg, 1:42 for male individuals and 1:189 for female individuals), our study shows that biological female individuals with male neuroanatomical features were 6.5 times more likely to have ASD than biological female individuals with a characteristically female neuroanatomy, which translates to an estimated variability in population prevalence between 0.2% to 1.3%, respectively (eTable 2 in the Supplement). Therefore, normative sex-related phenotypic diversity in brain structure significantly affected the probability of ASD in addition to biological sex alone, with male neuroanatomical characteristics carrying a higher intrinsic risk for ASD than female characteristics.
The particular patterns of neuroanatomical variability associated with low or high ASD probability differed between men and women in sign and regional composition (Figure 2 and eTable 3 and eFigure 4 in the Supplement). For example, CT variability in the left and right inferior temporal lobe differentiated between low or high risk in women but not in men (tmax = 3.78, cluster P = 6.29 × 10−6 for the left; tmax = 3.47, cluster P = 1.09 × 10−2 for the right). Therefore, while it is possible to link the probability of ASD to particular patterns of neuroanatomical variability in CT, our findings suggest that these patterns are sex specific.
Figure 2. Neuroanatomical Patterns Associated With Low and High Autism Spectrum Disorder (ASD) Probability.
Predictive maps (ie, w × CT) associated with low and high probability of ASD in female individuals (A) and male individuals (B). Low ASD probability maps were computed across all male individuals (or female individuals) with predictive probabilities lower than 0.5 (ie, biological male individuals or female individuals falling into the category of phenotypic female individuals). High ASD probability maps were computed across all male individuals (or female individuals) with predictive probabilities larger than 0.5 (ie, biological male individuals or female individuals falling into the category of phenotypic male individuals). At each vertex, the color scale thus indicates the product of the weight vector w and cortical thickness (CT), averaged across all individuals within the 4 probability groups. The probability of ASD was determined as the number of male individuals (or female individuals) with ASD relative to the total number of individuals within predictive probability bins.
Biological Sex Significantly Modulates the Cortical Anatomy of ASD
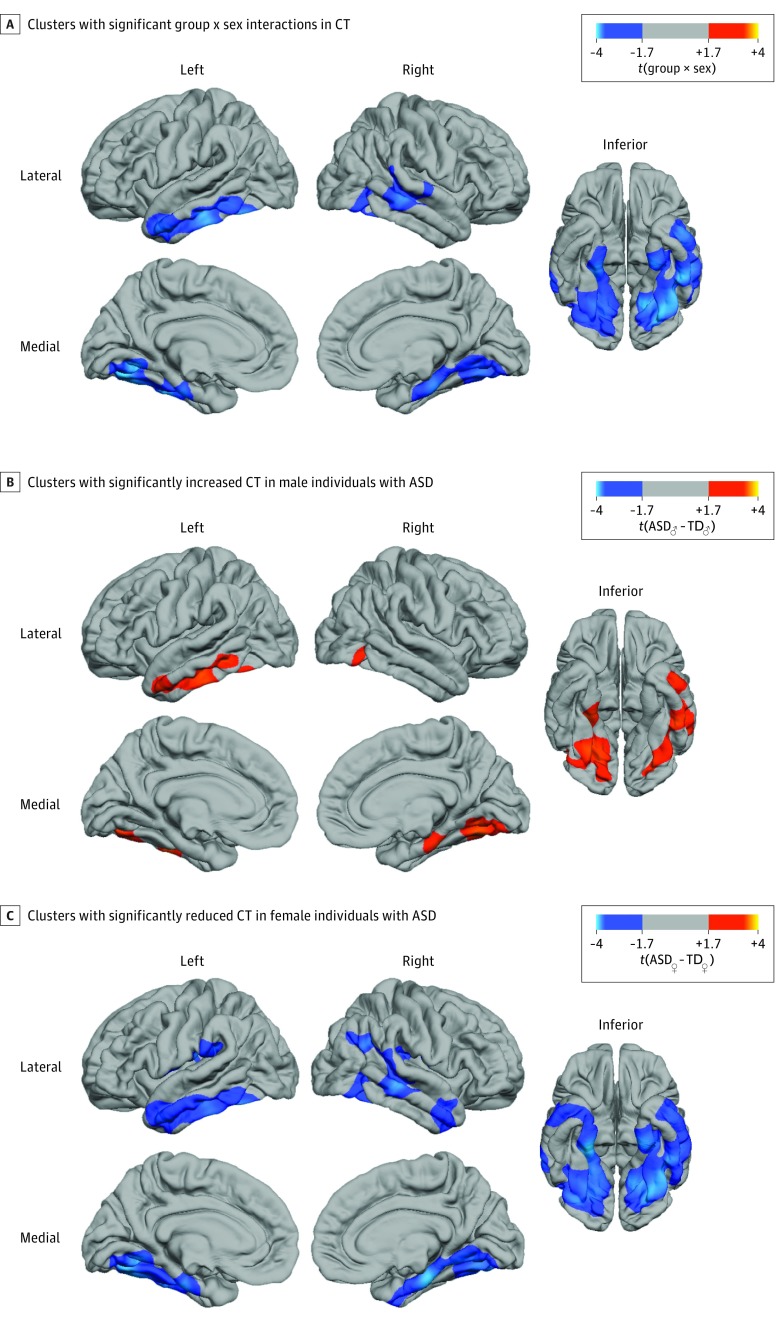
Last, using a conventional general linear model, we tested for significant CT differences between men and women with ASD and group × sex interactions. We found that the cortical neuroanatomy of ASD was significantly modulated by biological sex, particularly in the bilateral parahippocampal and entorhinal cortex (Brodmann area [BA] 28/34), the fusiform and lingual gyrus (BA 20/37/19), and the inferior or middle temporal lobe (BA 21/22) (Figure 3 and eTable 4 and eFigure 5 in the Supplement). In these regions, the degree of cortical abnormality in female individuals significantly exceeded the degree of abnormality observed in male individuals compared with their respective normative populations (t = 3.29, cluster P = 3.5 × 10−6 for the left; t = 3.42, cluster P = 5.49 × 10−4 for the right) (Figure 3B and C and eFigure 6 in the Supplement). However, female individuals with ASD were not more significantly impaired than male individuals in clinical symptom severity (eTable 5 in the Supplement). The clusters with significant group × sex interactions also remained significant when controlling for variability in verbal and performance IQ (eTable 5 and eFigure 7 in the Supplement) and thus do not seem to reflect a simple functional difference in these clinical or neurocognitive measures (further details are available in eFigure 8 and the eAppendix in the Supplement).
Figure 3. Significant Group × Sex Interactions.
A, Clusters with significant group × sex interactions in cortical thickness (CT) as examined by a conventional general linear model–type approach. In these regions, the difference in CT between female individuals with autism spectrum disorder (ASD) and female control individuals significantly exceeded the difference between male individuals with ASD and male controls (statistical details are available in eTable 4 in the Supplement). The group × sex interactions were driven by reduced CT in female individuals with ASD relative to female controls and increased CT in male individuals with ASD relative to male controls (eFigure 6 in the Supplement). B, Clusters with significantly increased CT in male individuals with ASD relative to male controls (random field theory–based, cluster-corrected P < .05). C, Clusters with significantly reduced CT in female individuals with ASD relative to female controls (random field theory–based, cluster-corrected P < .05). eFigure 5 in the Supplement shows results of the permutation-based cluster thresholding of the same contrasts. TD indicates typically developing.
Discussion
Our study demonstrates that normative sex-related phenotypic diversity in brain structure affects the prevalence of ASD in addition to biological sex alone, with male neuroanatomical characteristics carrying a higher intrinsic risk for ASD than female characteristics. This increase in risk was predominantly driven by female individuals with ASD, who displayed a pattern of neuroanatomical variability in CT that resembled more closely the neurotypical male rather than female neurophenotype overall. Moreover, our study links low and high risk (ie, probability) of ASD to particular patterns of sex-related neuroanatomical variability, thus providing important novel insights into the neurobiological mechanisms underpinning the male preponderant prevalence of ASD.
An important feature of GPC is that it is possible to summarize the highly complex and multivariate pattern of normative sex-related phenotypic diversity in brain structure to a single measure (ie, class probability) indicative of the individual’s neurophenotypic sex. This ability sets our approach apart from existing studies (eg, those by Ruigrok et al and by Escorial et al) examining sexual dimorphism of the brain based on univariate group differences between male individuals and female individuals, which precludes the investigation of interindividual phenotypic diversity across the binary categories dictated by biological sex. This study is of importance because it has recently been suggested that, on a phenotypic scale, the brain should be considered a “mosaic” of regions, each of relative maleness or femaleness, resulting in significant interindividual variability both within and between sexes. Therefore, the multivariate patterns derived from the classification between neurotypical male and female controls may be interpreted as being representative of such a mosaic, which drives the brain toward a female or male phenotypic end point overall (see also the study by Chekroud et al).
Furthermore, we demonstrated that the normative pattern of sex-related neuroanatomical variability is predictive of ASD probability, particularly in female individuals, and that low and high ASD probability can be linked to regional differences in CT. These patterns not only included many of the brain regions where women typically differ from men in CT but also highlighted brain areas that have previously been linked to the core behavioral deficits characteristic of ASD (reviewed by Amaral et al and by Ecker et al). Notably, some of these brain regions (eg, inferior temporal lobes) carried low or high probability of ASD in women but not in men. Herein, we found that the degree of cortical abnormality in female individuals with ASD significantly exceeded the degree of abnormality in male individuals compared with their respective normative population. Yet, female individuals with ASD were not more severely impaired than male individuals on the level of autistic symptoms. Therefore, our finding agrees with previous reports suggesting that (1) biological sex significantly modulates the neurobiological basis of ASD (eg, the studies by Lai et al and by Schaer et al) and (2) female individuals may have a higher threshold (ie, minimum liability sufficient to cause ASD) for reaching affection status than male individuals (reviewed by Werling and Geschwind) and may need to deviate more neurobiologically from their normative population to demonstrate a clinical ASD phenotype.
Limitations
While our approach holds promise for future investigations into the neurobiological mechanisms that underpin risk and resilience for conditions with a sex difference in prevalence, this study has several limitations. First, our study was designed to establish the statistical association between normative sex–related phenotypic diversity and ASD probability. Therefore, future studies are needed to examine the causal mechanisms for this association (eg, as shown by Lai et al and Baron-Cohen et al). Second, because of reasons outlined above, we based our study on measures of CT in a sample of high-functioning adults with ASD. While this scope is sufficient to provide proof of concept, it will be necessary in the future to extend our approach to other neurobiological features that have previously been linked to ASD (eg, cortical surface area and regional volumes) and to replicate our findings in other subgroups on the autism spectrum (eg, different age groups and individuals with learning disabilities). Third, in future research, it will be crucial to further explore the functional relevance of our findings and to examine how normative sex-related phenotypic diversity in brain structure relates to sex differences in general cognitive or behavioral profiles (eg, as shown by Miller and Halpern and by Hyde) and to different clinical ASD phenotypes.
Conclusions
Our findings suggest that the neurobiological male phenotype carries a higher intrinsic risk for ASD than the female phenotype across the binary categories dictated by biological sex. In addition to genetic and environmental factors, normative sex-related phenotypic diversity should thus be taken into account when determining an individual’s probability of ASD. Therefore, our approach to modeling normative sex-related phenotypic diversity may be more widely used in the future to elucidate the neurobiological mechanisms that underpin risk and resilience for mental health disorders.
eTable 1. Frequency Ratios of ASD Cases to TD Controls, and Males to Females, Acquired Within and Across the Different Acquisition Sites
eTable 2. Population Prevalence of ASD for Biological Males and Females With Male or Female Neuroanatomical Brain Phenotype
eTable 3. Differences in Neuroanatomical Patterns Associated With High Probability of ASD Between Men and Women
eTable 4. Results of the Vertex-wise Analysis of CT Utilizing a General Linear Model (GLM)
eTable 5. Differences in Demographic Measures Between Diagnostic Groups and Biological Sexes
eMethods. Supplementary Methods
eFigure 1. Sex-Related Pattern of Neuroanatomical Variability in Cortical Thickness
eFigure 2. Significant Differences in CT Between Male and Female TD Controls
eFigure 3. Sample Probability of ASD for Females (A) and Males (B) as a Function of Normative Sex-Related Phenotypic Variability in CT
eFigure 4. Clusters With Significant Differences in the Neuroanatomical Pattern of CT Associated With High Probability of ASD Between Males and Females (i.e., Product of w and CT)
eFigure 5. Clusters With Significant Group-by-Sex Interactions Following a Non-parametric Clustering Approach With n = 10,000 Permutations of Group (i.e., Sex) Labels (Permutation-Based Cluster-Corrected, P < .05)
eFigure 6. Group-by-Sex Interaction Graph Based on Mean CT in the Left-Hemisphere Cluster That Extended Into the Hippocampal/Entorhinal Cortex, the Fusiform and Lingual Gyrus, and the Inferior/Middle Temporal Lobe
eFigure 7. Differences in Full-Scale, Verbal and Non-verbal (i.e., Performance) IQ Between Individual Subgroups of ASD vs TD Individuals, and Males vs Females, Examined Using t Tests for Independent Samples
eFigure 8. Spatially Distributed Pattern of Negative (Blue) and Positive (Red) Correlations Between Verbal IQ Measures and CT Assessed Across Male and Female Neurotypical Controls
eAppendix. Supplementary Appendix
References
- 1.Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, Baron-Cohen S. Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry. 2015;54(1):11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fombonne E. Epidemiology of autistic disorder and other pervasive developmental disorders. J Clin Psychiatry. 2005;66(suppl 10):3-8. [PubMed] [Google Scholar]
- 3.Szatmari P, Liu XQ, Goldberg J, et al. Sex differences in repetitive stereotyped behaviors in autism: implications for genetic liability. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(1):5-12. [DOI] [PubMed] [Google Scholar]
- 4.Dworzynski K, Ronald A, Bolton P, Happé F. How different are girls and boys above and below the diagnostic threshold for autism spectrum disorders? J Am Acad Child Adolesc Psychiatry. 2012;51(8):788-797. [DOI] [PubMed] [Google Scholar]
- 5.Werling DM, Geschwind DH. Sex differences in autism spectrum disorders. Curr Opin Neurol. 2013;26(2):146-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai MC, Lombardo MV, Suckling J, et al. ; MRC AIMS Consortium . Biological sex affects the neurobiology of autism. Brain. 2013;136(pt 9):2799-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schaer M, Kochalka J, Padmanabhan A, Supekar K, Menon V. Sex differences in cortical volume and gyrification in autism. Mol Autism. 2015;6(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai MC, Lerch JP, Floris DL, et al. Imaging sex/gender and autism in the brain: etiological implications. J Neurosci Res. 2017;95(1-2):380-397. [DOI] [PubMed] [Google Scholar]
- 9.Ruigrok AN, Salimi-Khorshidi G, Lai MC, et al. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rasmussen CE, Williams CKI. Gaussian Processes for Machine Learning. Cambridge, MA: MIT Press; 2006. [Google Scholar]
- 11.McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677-683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sowell ER, Peterson BS, Kan E, et al. Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex. 2007;17(7):1550-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Im K, Lee JM, Lee J, et al. Gender difference analysis of cortical thickness in healthy young adults with surface-based methods. Neuroimage. 2006;31(1):31-38. [DOI] [PubMed] [Google Scholar]
- 14.Ecker C, Ginestet C, Feng Y, et al. ; MRC AIMS Consortium . Brain surface anatomy in adults with autism: the relationship between surface area, cortical thickness, and autistic symptoms. JAMA Psychiatry. 2013;70(1):59-70. [DOI] [PubMed] [Google Scholar]
- 15.Wallace GL, Dankner N, Kenworthy L, Giedd JN, Martin A. Age-related temporal and parietal cortical thinning in autism spectrum disorders. Brain. 2010;133(pt 12):3745-3754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lenroot RK, Giedd JN. Sex differences in the adolescent brain. Brain Cogn. 2010;72(1):46-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenroot RK, Gogtay N, Greenstein DK, et al. Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage. 2007;36(4):1065-1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Geschwind DH, Rakic P. Cortical evolution: judge the brain by its cover. Neuron. 2013;80(3):633-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zilles K, Palomero-Gallagher N, Amunts K. Development of cortical folding during evolution and ontogeny. Trends Neurosci. 2013;36(5):275-284. [DOI] [PubMed] [Google Scholar]
- 20.Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview–Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659-685. [DOI] [PubMed] [Google Scholar]
- 21.Lord C, Rutter M, Goode S, et al. Autism Diagnostic Observation Schedule: a standardized observation of communicative and social behavior. J Autism Dev Disord. 1989;19(2):185-212. [DOI] [PubMed] [Google Scholar]
- 22.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI). San Antonio, TX: Harcourt Assessment; 1999. [Google Scholar]
- 23.Deoni SC, Williams SC, Jezzard P, Suckling J, Murphy DG, Jones DK. Standardized structural magnetic resonance imaging in multicentre studies using quantitative T1 and T2 imaging at 1.5 T. Neuroimage. 2008;40(2):662-671. [DOI] [PubMed] [Google Scholar]
- 24.Ecker C, Suckling J, Deoni SC, et al. ; MRC AIMS Consortium . Brain anatomy and its relationship to behavior in adults with autism spectrum disorder: a multicenter magnetic resonance imaging study. Arch Gen Psychiatry. 2012;69(2):195-209. [DOI] [PubMed] [Google Scholar]
- 25.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis, II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195-207. [DOI] [PubMed] [Google Scholar]
- 26.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis, I: segmentation and surface reconstruction. Neuroimage. 1999;9(2):179-194. [DOI] [PubMed] [Google Scholar]
- 27.Ségonne F, Dale AM, Busa E, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060-1075. [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050-11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marquand A, Howard M, Brammer M, Chu C, Coen S, Mourão-Miranda J. Quantitative prediction of subjective pain intensity from whole-brain fMRI data using Gaussian processes. Neuroimage. 2010;49(3):2178-2189. [DOI] [PubMed] [Google Scholar]
- 30.Marquand AF, Brammer M, Williams SC, Doyle OM. Bayesian multi-task learning for decoding multi-subject neuroimaging data. Neuroimage. 2014;92:298-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baio J; Developmental Disabilities Monitoring Network Surveillance Year 2010 Principal Investigators; Centers for Disease Control and Prevention (CDC) . Prevalence of autism spectrum disorder among children aged 8 years: Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2010. MMWR Surveill Summ. 2014;63(2):1-21. [PubMed] [Google Scholar]
- 32.Worsley KJ, Andermann M, Koulis T, MacDonald D, Evans AC. Detecting changes in nonisotropic images. Hum Brain Mapp. 1999;8(2-3):98-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hagler DJ Jr, Saygin AP, Sereno MI. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33(4):1093-1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Escorial S, Román FJ, Martínez K, Burgaleta M, Karama S, Colom R. Sex differences in neocortical structure and cognitive performance: a surface-based morphometry study. Neuroimage. 2015;104:355-365. [DOI] [PubMed] [Google Scholar]
- 35.Joel D, McCarthy MM. Incorporating sex as a biological variable in neuropsychiatric research: where are we now and where should we be? Neuropsychopharmacology. 2017;42(2):379-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chekroud AM, Ward EJ, Rosenberg MD, Holmes AJ. Patterns in the human brain mosaic discriminate males from females. Proc Natl Acad Sci U S A. 2016;113(14):E1968. doi: 10.1073/pnas.1523888113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Amaral DG, Schumann CM, Nordahl CW. Neuroanatomy of autism. Trends Neurosci. 2008;31(3):137-145. [DOI] [PubMed] [Google Scholar]
- 38.Ecker C, Bookheimer SY, Murphy DG. Neuroimaging in autism spectrum disorder: brain structure and function across the lifespan. Lancet Neurol. 2015;14(11):1121-1134. [DOI] [PubMed] [Google Scholar]
- 39.Baron-Cohen S, Auyeung B, Nørgaard-Pedersen B, et al. Elevated fetal steroidogenic activity in autism. Mol Psychiatry. 2015;20(3):369-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller DI, Halpern DF. The new science of cognitive sex differences. Trends Cogn Sci. 2014;18(1):37-45. [DOI] [PubMed] [Google Scholar]
- 41.Hyde JS. Sex and cognition: gender and cognitive functions. Curr Opin Neurobiol. 2016;38:53-56. [DOI] [PubMed] [Google Scholar]
- 42.Bölte S, Duketis E, Poustka F, Holtmann M. Sex differences in cognitive domains and their clinical correlates in higher-functioning autism spectrum disorders. Autism. 2011;15(4):497-511. [DOI] [PubMed] [Google Scholar]
- 43.Mandy W, Chilvers R, Chowdhury U, Salter G, Seigal A, Skuse D. Sex differences in autism spectrum disorder: evidence from a large sample of children and adolescents. J Autism Dev Disord. 2012;42(7):1304-1313. [DOI] [PubMed] [Google Scholar]
- 44.Solomon M, Miller M, Taylor SL, Hinshaw SP, Carter CS. Autism symptoms and internalizing psychopathology in girls and boys with autism spectrum disorders. J Autism Dev Disord. 2012;42(1):48-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Frequency Ratios of ASD Cases to TD Controls, and Males to Females, Acquired Within and Across the Different Acquisition Sites
eTable 2. Population Prevalence of ASD for Biological Males and Females With Male or Female Neuroanatomical Brain Phenotype
eTable 3. Differences in Neuroanatomical Patterns Associated With High Probability of ASD Between Men and Women
eTable 4. Results of the Vertex-wise Analysis of CT Utilizing a General Linear Model (GLM)
eTable 5. Differences in Demographic Measures Between Diagnostic Groups and Biological Sexes
eMethods. Supplementary Methods
eFigure 1. Sex-Related Pattern of Neuroanatomical Variability in Cortical Thickness
eFigure 2. Significant Differences in CT Between Male and Female TD Controls
eFigure 3. Sample Probability of ASD for Females (A) and Males (B) as a Function of Normative Sex-Related Phenotypic Variability in CT
eFigure 4. Clusters With Significant Differences in the Neuroanatomical Pattern of CT Associated With High Probability of ASD Between Males and Females (i.e., Product of w and CT)
eFigure 5. Clusters With Significant Group-by-Sex Interactions Following a Non-parametric Clustering Approach With n = 10,000 Permutations of Group (i.e., Sex) Labels (Permutation-Based Cluster-Corrected, P < .05)
eFigure 6. Group-by-Sex Interaction Graph Based on Mean CT in the Left-Hemisphere Cluster That Extended Into the Hippocampal/Entorhinal Cortex, the Fusiform and Lingual Gyrus, and the Inferior/Middle Temporal Lobe
eFigure 7. Differences in Full-Scale, Verbal and Non-verbal (i.e., Performance) IQ Between Individual Subgroups of ASD vs TD Individuals, and Males vs Females, Examined Using t Tests for Independent Samples
eFigure 8. Spatially Distributed Pattern of Negative (Blue) and Positive (Red) Correlations Between Verbal IQ Measures and CT Assessed Across Male and Female Neurotypical Controls
eAppendix. Supplementary Appendix