This population-based, cross-sectional study examines the association between patient ethnicity and reduction in vision-specific functioning in Chinese, Malay, and Indian patients with age-related macular degeneration.
Key Points
Question
Does ethnicity have a role in the association between age-related macular degeneration and vision-specific functioning?
Findings
In this cross-sectional, population-based study that included 9962 Chinese, Malay, and Indian adults living in Singapore, early and late age-related macular degeneration reduced vision-specific functioning in Chinese but not in Indian and Malay study participants.
Meaning
Ethnic-specific strategies may be of value in planning health services and interventions as well as informing public policy decisions that can delay the onset or progression of age-related macular degeneration.
Abstract
Importance
Understanding the link between ethnicity and health is critical to making appropriate public policy decisions. Few population-level data are available about this connection, however, including the influence of ethnicity on the association between age-related macular degeneration (AMD) and vision-specific functioning (VSF).
Objective
To identify the influence of ethnicity on VSF among Chinese, Malay, and Indian patients with AMD.
Design, Setting, and Participants
This cross-sectional, population-based study relied on patients and their data from 3 population-based studies in 3 ethnic groups: Chinese, Malay and Indian. Of 10 033 Chinese, Malay, and Indian adults who participated in the study, 9962 (99.3%) who had gradable fundus images and Visual Function Index (VF-11) data available were included in the analyses for the present study. Uniocular presenting distance visual acuity was measured using the logMAR chart. Separate multiple linear regression models examined the association between AMD and VSF in the 3 ethnic groups, adjusting for age, sex, presenting visual acuity in the better-seeing eye, educational level, income, smoking status, hypertension, diabetes, cardiovascular disease, total cholesterol level, and other eye conditions. Data were collected between January 20, 2004, and December 19, 2011; data analysis was conducted between November 12, 2015, and December 28, 2016.
Exposures
Age-related macular degeneration according to fundus photographs graded using a modified Wisconsin Age-Related Maculopathy Grading System.
Main Outcomes and Measures
Rasch analysis was used to convert VF-11 questionnaire scores to estimated interval measures of VSF.
Results
Of the 9962 participants, the mean (SD) age was 58.8 (10.4) years; 4909 (49.3%) were male; 590 (5.9%) had early AMD (241 Chinese, 161 Malays, and 188 Indians) and 60 (0.6%) had late AMD (25 Chinese, 21 Malays, and 14 Indians). In the adjusted models, compared with no AMD, early AMD was associated with a small reduction in VSF (2.9%; β = −0.12; 95% CI, −0.23 to −0.00; P = .046) in the Chinese group but not in the Indian and Malay groups. Moreover, Chinese participants with late AMD had a clinically significant 19.1% loss of VSF (β = −0.78; 95% CI, −1.13 to −0.43, P < .001). In the Malay group, those with late AMD had a 13.5% drop in VSF (β = −0.49; 95% CI, −1.01 to 0.04; P = .07) compared with their counterparts without AMD. Similarly, late AMD was not associated with VSF in the Indian group.
Conclusions and Relevance
Early and late AMD negatively affected VSF in Chinese but not in Indian and Malay participants. This finding suggests that there is an independent ethnic influence in the association of the disease with VSF in multiethnic Asian populations, thus warranting ethnicity-based strategies to delay the onset or progression of AMD.
Introduction
Age-related macular degeneration (AMD) is a chronic, irreversible eye condition that results in loss of central vision because of damage to the macula. It accounts for approximately 9% of blindness among older adults in developed countries. Early AMD is characterized by large soft drusen, and late AMD is characterized by geographic atrophy and/or exudative macular degeneration. Because of the rapid aging of the world’s population, the global prevalence of AMD is expected to reach 288 million by 2040. The prevalence of AMD in Asia is increasing because of population aging, urbanization, and adoption of Western dietary habits. By 2050, half of all patients with AMD will reside in Asia.
Studies of populations in the West and in Asia have consistently shown AMD to be associated with poorer vision-related quality of life. For example, in a recent clinical study in Singapore, patients with neovascular AMD had independent reductions in the reading (by 21%), mobility (by 14%), and emotional well-being (by 44%) subscales of the Impact of Vision Impairment Questionnaire compared with control patients without AMD. However, whether there are ethnic differences in the effect of AMD on quality of life is unknown. A qualitative study by Xie and colleagues involving Malay, Chinese, and Indian patients in Singapore with knee osteoarthritis found important ethnic differences in themes associated with mental and social health. Similarly, ethnic variations in well-being have been found among Hispanic, non-Hispanic white, African American, and Asian Indian individuals with type 2 diabetes and among black, Hispanic, and white patients who are cancer survivors.
Understanding the link between ethnicity and health in population-based studies is important in identifying eye health disparities, promoting legislation associated with community eye health, and more effectively allocating vision-specific resources. Few data, however, are available on the effect of AMD at a multiethnic level from a population perspective, and none, to our knowledge, that compare these corrrelations in the 3 major Asian ethnic groups of Chinese, Malay, and Indian individuals. Because rates of eye diseases, use of eye care services, illness perception, help-seeking attitudes, and cultural or religious beliefs and habits vary across ethnic groups, we hypothesized that there may be ethnic differences in the association between AMD and vision-specific functioning (VSF). Using data from the Singapore Epidemiology of Eye Diseases (SEED) study, we investigated whether the association between AMD and VSF varied across Chinese, Malay, and Indian ethnicities.
Methods
Study Population
The population-based, cross-sectional SEED study conducted in Singapore includes the Singapore Malay Eye Study (SiMES) (2004-2006), the Singapore Indian Eye Study (SINDI) (2007-2009), and the Singapore Chinese Eye Study (SCES) (2009-2011). Baseline data collection is complete for all 3 studies; 6-year follow-up data collection is complete for SiMES and SINDI and is still under way for SCES. For the present study, an age-stratified random sampling strategy was used to select adults aged 40 to 80 years in each ethnic group (Malay, Indian, and Chinese) who were part of the baseline phase of the SEED studies. Overall, 4168 Malay, 4497 Indian, and 4605 Chinese individuals were identified and invited to participate in the study, of whom 3280 Malay (78.7%), 3400 Indian (75.6%), and 3353 Chinese (72.8%) individuals participated (N = 10 033). Data were collected between January 20, 2004, and December 19, 2011; data analysis was conducted between November 12, 2015, and December 28, 2016. The study was approved by the Singapore Eye Research Institute Institutional Review Board. All participants gave written informed consent, and the study adhered to the Declaration of Helsinki.
Assessment and Definitions of AMD
Fundus photographs were taken of each participant using a digital retinal camera (Canon CR-DGi with digital 10D SLR camera backing; Canon) following pupil dilation. Two-field color photographs were taken for each eye—one centered on the optic disc and the other on the fovea—according to the Early Treatment for Diabetic Retinopathy Study guidelines. Of the 10 033 participants in SEED, 9962 (99.3%) had gradable fundus photographs for AMD signs and had Visual Function Index (VF-11) questionnaire data. Participants’ AMD was graded at the University of Sydney Westmead Hospital by the same graders from the Blue Mountains Eye Study and using the modified Wisconsin Age-Related Maculopathy Grading System. Early AMD was defined as the presence of any soft drusen and increased or decreased retinal pigment or as the presence of large soft drusen (≥125 μm in diameter) with a large drusen area greater than 500 μm in diameter or large (≥125 μm) indistinct soft drusen in the absence of signs of late AMD. Late AMD was defined as the presence of geographic atrophy or exudative macular degeneration or both.
Vision and Other Assessments
Visual acuity (VA) testing and a detailed clinical slitlamp examination were performed according to a standardized protocol. Visual acuity was measured uniocularly using a logMAR vision chart (Lighthouse International) at a distance of 4 m. If the participant could not read any numbers at 4 m, the participant was moved to 3, 2, and then 1 m. If the participant could not identify any numbers on the chart, the participant’s VA was assessed as counting fingers, hand movements, perception of light, or no perception of light. Presenting VA (PVA) was measured with participants wearing their habitual optical correction (spectacles or contact lenses).
Diabetic retinopathy was graded from retinal photographs by using the modified Airlie House classification system from the Early Treatment for Diabetic Retinopathy Study. Glaucoma was diagnosed and classified using the International Society of Geographic and Epidemiologic Ophthalmology scheme based on gonioscopy, optic disc characteristics, and visual fields results. Clinical assessment of lens status and the presence of aphakia or pseudophakia were determined with a slitlamp.
Trained interviewers fluent in Malay, Tamil, and Mandarin administered questionnaires to collect sociodemographic characteristics, family and medical history, and lifestyle factors. Two measurements of systolic blood pressure and diastolic blood pressure were taken using a digital automatic blood pressure monitor (Dinamap Pro Series DP110X-RW; GE Medical Systems Information Technologies, Inc), and a third measurement was obtained if the 2 previous systolic blood pressure or diastolic blood pressure readings differed by more than 10 mm Hg or 5 mm Hg, respectively. The mean of these measurements was used in analyses. Height was measured using a wall-mounted, adjustable measuring scale, and weight was measured with a calibrated scientific weight scale. Body mass index was calculated as weight in kilogras divided by height in meters squared. Blood samples were collected for hemoglobin A1c, random glucose, and total and low-density lipoprotein and high-density lipoprotein cholesterol measurements.
Psychometric Assessment of VSF
Vision-specific functioning was measured using the VF-11 questionnaire, a modified version of the VF-14 that has been culturally validated for a Singapore population. The questionnaire was translated into Malay, Tamil, and Mandarin and then back-translated into English by bilingual interpreters. Participants were given the choice to be interviewed in Malay, Tamil, Mandarin, or English. For Chinese participants who spoke nonwritten dialects, such as Cantonese, Hokkein, Hakka, and Teochew, the VF-11 was verbally translated by each interviewer using identical translations to avoid contaminating the results. Items 1 to 9 were rated on a 5-point Likert scale that ranged from 1 (no difficulty) to 5 (unable to do because of my vision). The remaining 2 driving items had 3 response options: 1 (no difficulty), 2 (a little difficulty), and 3 (a great difficulty).
Rasch analysis assessed the psychometric properties of the VF-11 by using the Andrich rating scale model with Winsteps software, version 3.92 (Winsteps). Rasch analysis converts raw questionnaire scores into data that approximate interval-level measurement expressed in log of the odds units (logits). Because scores were reversed during Rasch analysis, a high person measure (in logits) indicated that a person possessed a high level of VSF. Following the iterative removal of 3 items because of misfit, a good fit to the Rasch model was obtained.
Statistical Analyses
Patients’ demographics and baseline characteristics were summarized by mean and SD for normally distributed continuous data, by the median and interquartile range for skewed distributed data, and by counts and percentages for categorical data. Key covariates included age (in years); sex; ethnicity (Malay, Indian, or Chinese); educational level (primary or lower [≤6 years], secondary [7-10 years], postsecondary [>10 years]); income (<SGD1000, SGD1000 to <SGD2000, and ≥SGD2000 per month [<US $710, US $710 to <US $1420, and ≥US $1420 per month]); smoking status (never, current smoker, or past smoker); self-reported cardiovascular disease (angina, myocardial infarction, or stroke); hypertension; diabetes (yes or no); body mass index; hemoglobin A1c level, systolic blood pressure and diastolic blood pressure measurements; total cholesterol level; high-density lipoprotein level, triglyceride level; PVA (better and worse eye); and presence of diabetic retinopathy, glaucoma, or cataract (yes or no).
We used χ2 statistics and analysis of variance to compare patient characteristics in 3 AMD severity groups (none, early AMD, and late AMD), and linear regression models were then used to determine univariable associations between AMD and VSF. Initially, we conducted multiple linear regression analyses to explore the association between AMD and VSF in the full SEED sample (N = 9962). Because we observed an independent ethnic effect on VSF (eTable 1 in the Supplement), we conducted a stratified analysis for ethnicity to determine if the β coefficients for the association between AMD and VSF differed by ethnicity, and we conducted multiple linear regression analysis in each of the 3 ethnic groups separately. All 3 models were adjusted for clinically important variables, including age, sex, PVA (better eye and worse eye), educational level, income, smoking status, hypertension, diabetes, cardiovascular disease, total cholesterol level, and the presence of diabetic retinopathy, glaucoma, and cataract. To facilitate interpretation of the β coefficients in a clinically meaningful way, the coefficients were converted into adjusted means to obtain the relative changes and are presented in percentage form. Associations were considered statistically significant at P < .05; all values were 2-sided. The P value for trend was estimated by considering AMD severity (none, early AMD, or late AMD) as an ordinal variable in a linear regression model. Statistical analyses were performed using Stata, version 12.0 (StataCorp LLC).
Results
Among the 9962 participants, mean (SD) age was 58.8 (10.4) years and 4909 (49.3%) were male. There were ethnic differences in most sociodemographic and clinical variables (Table 1). Chinese participants reported the highest mean (SD) VSF scores (4.05 [0.91]; range, −1.68 to 4.70), followed by the Indian (3.67 [1.25]; range, −4.97 to 4.70) and Malay (3.63 [1.30]; range, −3.70 to 4.70) participants, and these differences were statistically significant (Table 1). Of the 9962 participants, 590 (5.9%) had early AMD (241 Chinese, 161 Malay, and 188 Indian participants) and 60 (0.6%) had late AMD (25 Chinese, 21 Malay, and 14 Indian participants; eTable 2 in the Supplement). People with no or early AMD had better VSF (mean [SD], 3.69 [1.24] logits for both) than those with late AMD (2.92 [1.86 logits] (P < .001; eTable 2 in the Supplement).
Table 1. Sociodemographic and Clinical Characteristics by Ethnicity.
| Characteristic | All (N = 9962) |
Chinese (n = 3338) |
Malay (n = 3253) |
Indian (n = 3371) |
P Value |
|---|---|---|---|---|---|
| Categorical Variables, No. (%) | |||||
| Males | 4909 (49.3) | 1657 (49.6) | 1564 (48.1) | 1688 (50.1) | .23 |
| Age, y | |||||
| 40-49 | 2464 (24.7) | 705 (21.1) | 811 (24.9) | 948 (28.1) | <.001a |
| 50-59 | 3133 (31.4) | 1111 (33.3) | 951 (29.2) | 1071 (31.8) | |
| 60-69 | 2543 (25.5) | 891 (26.7) | 775 (23.8) | 877 (26.0) | |
| 70-80 | 1822 (18.3) | 631 (18.9) | 716 (22.0) | 475 (14.1) | |
| AMD severityb | |||||
| No AMD | 9312 (93.5) | 3072 (92.0) | 3071 (94.4) | 3169 (94.0) | <.001a |
| Early AMD | 590 (5.92) | 241 (7.22) | 161 (4.95) | 188 (5.58) | |
| Late AMD | 60 (0.60) | 25 (0.75) | 21 (0.65) | 14 (0.42) | |
| AMD laterality | |||||
| Unilateral | 473 (72.8) | 203 (76.3) | 131 (72.0) | 139 (68.8) | .19 |
| Bilateralc | 177 (27.2) | 63 (23.7) | 51 (28.0) | 63 (31.2) | |
| Educational level | |||||
| Primary or lower | 6017 (60.5) | 1780 (53.3) | 2365 (72.8) | 1872 (55.7) | <.001a |
| Secondary | 2333 (23.5) | 845 (25.3) | 673 (20.7) | 815 (24.2) | |
| Postsecondary | 1595 (16.0) | 712 (21.3) | 209 (6.44) | 674 (20.1) | |
| Income, SGD/mo (US $/mo) | |||||
| <1000 (US $710) | 5383 (55.2) | 1536 (47.4) | 2230 (69.0) | 1617 (49.2) | <.001a |
| 1000 to <2000 (US $710 to <US $1420) | 2197 (22.5) | 735 (22.7) | 654 (20.2) | 808 (24.6) | |
| ≥2000 (US $1420) | 2180 (22.3) | 969 (29.9) | 347 (10.7) | 864 (26.3) | |
| Smoking status | |||||
| Never | 6923 (69.6) | 2454 (73.5) | 1996 (61.5) | 2473 (73.4) | <.001a |
| Current | 1593 (16.0) | 440 (13.2) | 658 (20.3) | 495 (14.7) | |
| Past | 1437 (14.4) | 444 (13.3) | 593 (18.3) | 400 (11.9) | |
| Cardiovascular diseased | 1074 (10.8) | 233 (6.98) | 361 (11.1) | 480 (14.3) | <.001a |
| Hypertension | 6119 (61.6) | 1972 (59.1) | 2237 (69.1) | 1910 (56.8) | <.001a |
| Diabetes | |||||
| Yes | 2364 (24.7) | 483 (15.1) | 762 (24.4) | 1119 (34.6) | <.001a |
| No | 7191 (75.2) | 2709 (84.9) | 2363 (75.5) | 2119 (65.4) | <.001a |
| Diabetes without retinopathy | 1550 (16.2) | 339 (10.6) | 495 (15.8) | 716 (22.1) | <.001a |
| Diabetes with retinopathy | 818 (8.56) | 144 (4.51) | 271 (8.66) | 403 (12.5) | |
| Cataract | 4229 (44.0) | 1379 (42.1) | 1519 (48.9) | 1331 (41.2) | <.001a |
| Glaucoma | 359 (3.60) | 133 (3.98) | 149 (4.58) | 77 (2.28) | <.001a |
| Continuous Variables, Mean (SD) | |||||
| PVA—better eye, logMAR | 0.20 (0.23) | 0.20 (0.20) | 0.22 (0.27) | 0.19 (0.22) | <.001a |
| PVA—worse eye, logMAR | 0.39 (0.46) | 0.38 (0.42) | 0.42 (0.51) | 0.37 (0.46) | <.001a |
| Blood pressure, mean (SD) | |||||
| Systolic, mm Hg | 139.9 (21.9) | 136.6 (19.5) | 147.5 (24.0) | 135.9 (20.1) | <.001a |
| Diastolic, mm Hg | 78.3 (10.6) | 77.6 (9.90) | 79.8 (11.3) | 77.6 (10.3) | <.001a |
| BMI | 25.4 (4.71) | 23.7 (3.65) | 26.4 (5.12) | 26.2 (4.75) | <.001a |
| Hemoglobin A1c, % | 6.32 (1.32) | 6.06 (0.91) | 6.45 (1.55) | 6.43 (1.39) | <.001a |
| Total cholesterol level, mg/dL | 97.66 (20.36) | 98.20 (19.28) | 101.44 (21.08) | 23.51 (20.00) | <.001 |
| VSF score, mean (SD) [range] | 3.78 (1.18) [−4.97 to 4.70] |
4.05 (0.91) [−1.68 to 4.70] |
3.63 (1.30) [−3.70 to 4.70] |
3.67 (1.25) [−4.97 to 4.70] |
<.001a |
Abbreviations: AMD, age-related macular degeneration; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); PVA, presenting visual acuity; SGD, Singapore dollars; VSF, vision-specific functioning.
SI conversion factors: To convert total cholesterol to millimoles per liter, multiply by 0.0259; hemoglobin A1c to a proportion of total hemoglobin, multiply by 0.01.
Indicates statistical significance.
For definitions of early and late AMD used in this study, see the Assessment and Definitions of AMD subsection in the Methods section.
Both eyes have at least early AMD.
Includes myocardial infarction, angina, or stroke.
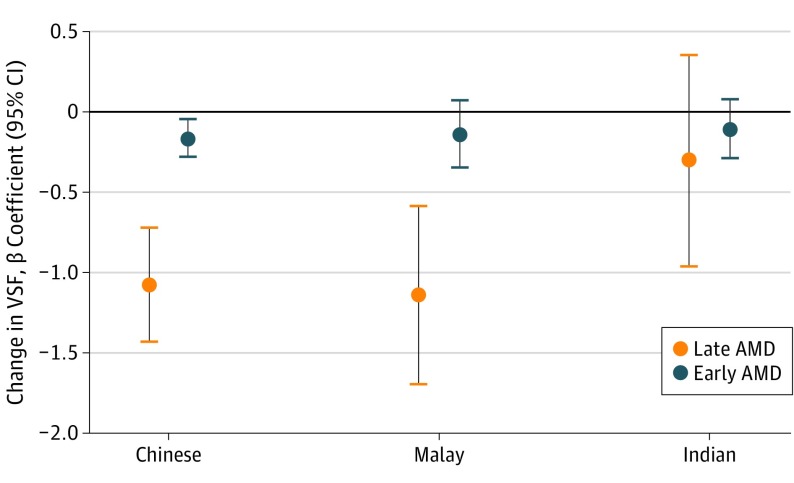
When stratified by ethnicity, VSF worsened as the severity of AMD increased for all 3 ethnic groups (Table 2). For example, in the Chinese group, mean (SD) VSF scores were 4.07 (0.88) for no AMD, 3.91 (1.00) for early AMD, and 3.00 (1.91) for late AMD (P < .001). Similarly, in subanalyses (Figure), Chinese participants with early AMD and late AMD had lower VSF than those with no AMD (β = −0.16; 95% CI, −0.28 to −0.04; P = .007 vs β = −1.07; 95% CI, −1.42 to −0.71; P < .001). Malay participants with late (but not early) AMD also had worse VSF (β = −1.14; 95% CI, −1.69 to −0.58; P < .001) than their counterparts with no AMD; however, there was no association between VSF and AMD severity among the Indian participants.
Table 2. Association Between Severity of AMD and VSF by Ethnicitya.
| Ethnic Group | VSF Score, Mean (SD) | P Value | |||
|---|---|---|---|---|---|
| All Participants | No AMD | Early AMD | Late AMD | ||
| Chinese | 4.05 (0.91) | 4.07 (0.88) | 3.91 (1.00) | 3.00 (1.91) | <.001b |
| Malay | 3.63 (1.30) | 3.64 (1.28) | 3.51 (1.51) | 2.51 (2.01) | <.001b |
| Indian | 3.67 (1.25) | 3.68 (1.25) | 3.57 (1.24) | 3.38 (1.48) | .37 |
Abbreviations: AMD, age-related macular degeneration; VSF, vision-specific functioning.
For definitions of early and late AMD used in this study, see the Assessment and Definitions of AMD subsection in the Methods section.
Indicates statistical significance.
Figure. Effect of Early and Late Age-Related Macular Degeneration (AMD) on Vision-Specific Functioning (VSF) in Chinese, Malay, and Indian Study Participants.
Late AMD is associated with significant reductions in VSF in Chinese and Malay participants but not in Indian participants. For definitions of early and late AMD used in this study, see the Assessment and Definitions of AMD subsection in the Methods section.
In multiple regression models, compared with no AMD, early AMD was associated with a small reduction in VSF (2.9%; β = −0.12; 95% CI, −0.23 to −0.00; P = .046) in the Chinese group but not in the Indian and Malay groups (Table 3). Moreover, Chinese participants with late AMD had a clinically significant 19.1% loss of VSF (β = −0.78; 95% CI, −1.13 to −0.43; P < .001). In Malay participants, those with late AMD had a 13.5% reduction in VSF (β = −0.49; 95% CI, −1.01 to 0.04; P = .07) compared with their counterparts without AMD, and there was a linear decrement in VSF as AMD severity increased (P for trend = .001). In contrast, late AMD was not associated with VSF (β = 0.12; 95% CI −0.47 to 0.72; P = .68) among Indian participants. When we included worse-eye PVA instead of better-eye PVA in the multiple regression models (eTable 3 in the Supplement), the results were similar, with the exception that late AMD was now independently associated with lower VSF in Malay participants (β = −0.55; 95% CI, −1.08 to −0.03; P = .04) and early AMD was no longer independently associated with VSF in Chinese participants (β = −0.11; 95% CI, −0.22 to 0.01; P = .07).
Table 3. Association Between Severity of AMD and VSF by Ethnicity in Multiple Linear Regression Modelsa.
| AMD Category by Ethnic Group |
VSF Score, Mean (SD) or β Coefficient (95% CI) |
P Value | Change From Reference Category, % |
|---|---|---|---|
| Chinese | |||
| No AMDb | 4.08 (0.02) | <.001c | |
| Early AMDd | −0.12 (−0.23 to −0.00)e | .046e | 2.9e |
| Late AMDd | −0.78 (−1.13 to −0.43)f | <.001e | 19.1e |
| Malay | |||
| No AMDb | 3.63 (0.02) | .001 | |
| Early AMDd | −0.02 (−0.21 to 0.18) | .88 | 0.4 |
| Late AMDd | −0.49 (−1.01 to 0.04) | .07 | 13.5 |
| Indian | |||
| No AMDb | 3.69 (0.02) | .12c | |
| Early AMDd | 0.01 (−0.17 to 0.20) | .89 | 0.4 |
| Late AMDd | 0.12 (−0.47 to 0.72) | .68 | 3.2 |
Abbreviations: AMD, age-related macular degeneration; VSF, vision-specific functioning.
Adjusted for age, sex, presenting visual acuity (better-seeing eye), educational level, income, smoking status, hypertension, diabetes, diabetic retinopathy, cataract, glaucoma, cardiovascular disease (myocardial infarction, angina, or stroke), and total cholesterol level. For definitions of early and late AMD used in this study, see the Assessment and Definitions of AMD subsection in the Methods section.
Reference category; data for VSF score are given as mean (SD).
P value for trend by the χ2 test.
Data for VSF score are given as β coefficient (95% CI).
Indicates a statistically significant association (P < .05).
Indicates a statistically significant and clinically meaningful association.
Discussion
In our population-based study of nearly 10 000 adult Chinese, Malay, and Indian participants, we found ethnic differences in the association between AMD and VSF that are independent of factors traditionally associated with vision-related quality of life, such as VA and socioeconomic status. Both early AMD and late AMD were associated with poorer VSF in Chinese participants, and there was a trend toward worse VSF with increasing AMD severity in Malay participants; however, there was no association between AMD severity and VSF in Indian participants. This information is important for clinicians, researchers, and policy planners in designing culturally sensitive interventions to improve participation in daily living activities for different ethnic groups with AMD. Longitudinal research to identify the underlying factors contributing to these observed ethnic differences in VSF is needed. Preventive strategies to stop or slow the progression of AMD are also warranted given that even early AMD was associated with reduced VSF in Chinese participants.
Although our findings support other population-based studies in Singapore that have reported ethnic differences in quality-of-life outcomes, they indicate some interesting differences as well. In our study, AMD had the least effect on VSF in Indian participants and the greatest effect on VSF in Chinese people. In contrast, Thumboo and associates found that, compared with Indian ethnicity, Chinese and Malay ethnicities were associated with higher scores in the physical functioning, role-physical, bodily pain, social functioning, role-emotional, and mental health domains of the 36-Item Short Form Health Survey (Rand Corp). Indian individuals reported higher scores than the Chinese only in the general health domain. Conversely, a study examining ethnic differences in psychosocial factors, knowledge, and adherence behaviors in a multiethnic population of people with diabetes in the United States found that Asian Indian individuals had the most positive outlook for their disease, were the least worried about the future, and reported higher social support compared with their non-Hispanic white, Hispanic, and black counterparts.
Conceptual models suggest that the influence of ethnicity on health is mediated through socioeconomic factors and sex. Indeed, studies in the United States have found that apparent ethnic differences in health-related quality of life between black, white, and Hispanic American individuals are driven by socioeconomic and life-burden factors. However, the association between AMD and VSF in Chinese participants in our study was independent of sex as well as educational level and income, both proxy indicators of socioeconomic status. This finding is consistent with the multiethnic, population-based studies in Singapore described previously, in which the influence of ethnicity on health-related quality of life was also independent of socioeconomic status. However, many factors may mediate the association between AMD and VSF, such as coping skills, adaptation to vision loss, social support, illness perceptions, use of alternative medicine, identity, and minority status, all of which vary according to religious and cultural backgrounds. There are also genetic and ethnic factors underlying the prevalence, incidence, and pathogenesis of AMD and its subtypes. More research is needed to elucidate the underlying pathophysiological, behavioral, psychosocial, and cultural reasons behind the ethnic differences reported in our study. If modifiable factors such as health beliefs or barriers to accessing eye care are found, these can be targeted to improve VSF in the different ethnic groups in Singapore.
It is possible that the independent effect of ethnicity on VSF in our Chinese sample results from ethnic differences in the psychometric properties of the VF-11. However, although the psychometric properties of the VF-14 have not been directly compared across the 3 ethnic groups, the instrument has shown good agreement in Chinese populations. Similarly, we did not find any item bias for ethnicity during Rasch analysis, suggesting that psychometric issues are unlikely to be driving the ethnic difference. However, researchers using the VF-11 should adjust for ethnicity in analyses to reduce risk of type I error.
Strengths and Limitations
Strengths of our study include its large sample size and population-based study design, meaning that the results are likely to be generalizable to the Singapore population with AMD, as well as our use of Rasch analysis to generate interval scores for VSF. Because the 3 ethnic groups share a similar environmental background and access to resources, the potential confounding influence of differences in language and environment was minimized. Although our study may be less generalizable to Chinese, Malay, and Indian individuals outside of Singapore because of differences in access to health care systems, our findings may be of widespread interest given that the 3 ethnic groups comprise a combined world population of at least 2 billion and populations around the world are becoming increasingly multicultural.
Our study has several limitations. First, the number of people with late AMD in each ethnic group was small, which could have reduced our power to detect associations. Indeed, although the association between late AMD and VSF was of borderline significance in Malay participants, the P for trend (.001) indicated that there was insufficient power to detect the true association in this group. However, in Indians, the P for trend (.12) suggested that lack of power was unlikely to be masking a true association between AMD and VSF. Second, we have a limited understanding of why there are ethnic differences in the effect of AMD on VSF because we did not measure potentially important clinical, cultural, psychosocial, behavioral, and cognitive factors. For example, it is possible that different ethnicities have different visual demands that can influence how they report visual function, and there may be a strong social desirability bias in some ethnic groups but less in others. Third, the observed ethnic differences in our study may be related to differences in subtypes of late AMD, as polypoidal choroidal vasculopathy is a common subtype of exudative AMD in Asians; however, evidence suggests that deficits in vision-related quality of life resulting from polypoidal choroidal vasculopathy and typical neovascular AMD are similar. Finally, there were statistically significant differences in sociodemographic and health-related factors across the ethnic groups, which could have been driving the observed differences in VSF. However, differences were not systematic and are therefore not likely to be indicative of bias. Moreover, when these variables were included in regression models, the magnitude of the associations between AMD and VSF remained unchanged (data not shown).
Conclusions
Late AMD was associated with a clinically meaningful 19% deficit in VSF in Chinese participants even when accounting for presenting visual acuity and socioeconomic status. A similar trend was observed for Malay participants but not for Indian participants. Culturally sensitive interventions to improve VSF for Chinese and Malay people with AMD may be warranted. More research is needed to untangle the factors influencing the observed ethnic differences and inform communication strategies to help understand the impact of disease in different populations. Screening for early detection and management of AMD is needed to curb the progression of the disease and minimize its effect on VSF.
eTable 1. Association Between Age-Related Macular Degeneration (AMD) and Vision-Specific Functioning (VSF) in Multiple Linear Regression Models
eTable 2. Sociodemographic and Clinical Characteristics of Participants, Stratified by Age-Related Macular Degeneration
eTable 3. Association Between Severity of AMD and VSF by Ethnicity in Multiple Linear Regression Models Using Worse-Seeing Presenting Visual Acuity
References
- 1.Lim LS, Mitchell P, Seddon JM, Holz FG, Wong TY. Age-related macular degeneration. Lancet. 2012;379(9827):1728-1738. [DOI] [PubMed] [Google Scholar]
- 2.Wong WL, Su X, Li X, et al. . Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-e116. [DOI] [PubMed] [Google Scholar]
- 3.Mackenzie PJ, Chang TS, Scott IU, et al. . Assessment of vision-related function in patients with age-related macular degeneration. Ophthalmology. 2002;109(4):720-729. [DOI] [PubMed] [Google Scholar]
- 4.Cahill MT, Banks AD, Stinnett SS, Toth CA. Vision-related quality of life in patients with bilateral severe age-related macular degeneration. Ophthalmology. 2005;112(1):152-158. [DOI] [PubMed] [Google Scholar]
- 5.Fenwick EK, Cheung CM, Ong PG, et al. . The impact of typical neovascular age-related macular degeneration and polypoidal choroidal vasculopathy on vision-related quality of life in Asian patients [published online August 2, 2016]. Br J Ophthalmol. [DOI] [PubMed] [Google Scholar]
- 6.Lamoureux EL, Mitchell P, Rees G, et al. . Impact of early and late age-related macular degeneration on vision-specific functioning. Br J Ophthalmol. 2011;95(5):666-670. [DOI] [PubMed] [Google Scholar]
- 7.Xie F, Li SC, Fong KY, et al. . What health domains and items are important to patients with knee osteoarthritis? a focus group study in a multiethnic urban Asian population. Osteoarthritis Cartilage. 2006;14(3):224-230. [DOI] [PubMed] [Google Scholar]
- 8.Misra R, Lager J. Ethnic and gender differences in psychosocial factors, glycemic control, and quality of life among adult type 2 diabetic patients. J Diabetes Complications. 2009;23(1):54-64. [DOI] [PubMed] [Google Scholar]
- 9.Canada AL, Fitchett G, Murphy PE, et al. . Racial/ethnic differences in spiritual well-being among cancer survivors. J Behav Med. 2013;36(5):441-453. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Hyman L. Population-based studies in ophthalmology. Am J Ophthalmol. 2008;146(5):656-663. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention Measuring Healthy Days: Population Assessment of Health-Related Quality of Life. Atlanta, GA: CDC; 2000. [Google Scholar]
- 12.Wong TY. Cataract extraction rates among Chinese, Malays, and Indians in Singapore: a population-based analysis. Arch Ophthalmol. 2001;119(5):727-732. [DOI] [PubMed] [Google Scholar]
- 13.Wong TY, Foster PJ, Seah SK, Chew PT. Rates of hospital admissions for primary angle closure glaucoma among Chinese, Malays, and Indians in Singapore. Br J Ophthalmol. 2000;84(9):990-992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim AS, Bishop GD. The role of attitudes and beliefs in differential health care utilisation among Chinese in Singapore. Psychol Health. 2000;14(6):965-977. [DOI] [PubMed] [Google Scholar]
- 15.Foong AW, Saw SM, Loo JL, et al. . Rationale and methodology for a population-based study of eye diseases in Malay people: The Singapore Malay eye study (SiMES). Ophthalmic Epidemiol. 2007;14(1):25-35. [DOI] [PubMed] [Google Scholar]
- 16.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 17.Mitchell P, Smith W, Attebo K, Wang JJ. Prevalence of age-related maculopathy in Australia. The Blue Mountains Eye Study. Ophthalmology. 1995;102(10):1450-1460. [DOI] [PubMed] [Google Scholar]
- 18.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin Age-Related Maculopathy Grading System. Ophthalmology. 1991;98(7):1128-1134. [DOI] [PubMed] [Google Scholar]
- 19.Fisher DE, Klein BE, Wong TY, et al. . Incidence of age-related macular degeneration in a multi-ethnic United States population: the multi-ethnic study of atherosclerosis. Ophthalmology. 2016;123(6):1297-1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Early Treatment Diabetic Retinopathy Study Research Group Grading diabetic retinopathy from stereoscopic color fundus photographs: an extension of the modified Airlie House classification: ETDRS report number 10. Ophthalmology. 1991;98(5)(suppl):786-806. [PubMed] [Google Scholar]
- 21.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. Br J Ophthalmol. 2002;86(2):238-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamoureux EL, Pesudovs K, Thumboo J, Saw SM, Wong TY. An evaluation of the reliability and validity of the visual functioning questionnaire (VF-11) using Rasch analysis in an Asian population. Invest Ophthalmol Vis Sci. 2009;50(6):2607-2613. [DOI] [PubMed] [Google Scholar]
- 23.Andrich D. Rating formulation for ordered response categories. Psychometrika. 1978;43:561-573. [Google Scholar]
- 24.Linacre JM. A User’s Guide to Winsteps/Ministeps Rasch-Model Computer Programs. Chicago, IL: MESA press; 2014. [Google Scholar]
- 25.Lamoureux E, Pesudovs K. Vision-specific quality-of-life research: a need to improve the quality. Am J Ophthalmol. 2011;151(2):195-197.e2. [DOI] [PubMed] [Google Scholar]
- 26.Thumboo J, Fong KY, Machin D, et al. . Quality of life in an urban Asian population: the impact of ethnicity and socio-economic status. Soc Sci Med. 2003;56(8):1761-1772. [DOI] [PubMed] [Google Scholar]
- 27.Wee HL, Li SC, Cheung YB, Fong KY, Thumboo J. The influence of ethnicity on health-related quality of life in diabetes mellitus: a population-based, multiethnic study. J Diabetes Complications. 2006;20(3):170-178. [DOI] [PubMed] [Google Scholar]
- 28.Schulman KA, Rubenstein LE, Chesley FD, Eisenberg JM. The roles of race and socioeconomic factors in health services research. Health Serv Res. 1995;30(1, pt 2):179-195. [PMC free article] [PubMed] [Google Scholar]
- 29.Ashing-Giwa K, Ganz PA, Petersen L. Quality of life of African-American and white long term breast carcinoma survivors. Cancer. 1999;85(2):418-426. [DOI] [PubMed] [Google Scholar]
- 30.Caldwell EM, Baxter J, Mitchell CM, Shetterly SM, Hamman RF. The association of non-insulin-dependent diabetes mellitus with perceived quality of life in a biethnic population: the San Luis Valley Diabetes Study. Am J Public Health. 1998;88(8):1225-1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bishop GD. Cognitive organization of disease concepts in Singapore. Psychol Health. 1998;13:121-133. [Google Scholar]
- 32.Liu K, Lai TY, Ma L, et al. . Ethnic differences in the association of SERPING1 with age-related macular degeneration and polypoidal choroidal vasculopathy. Sci Rep. 2015;5:9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robman LD, Islam FM, Chong EW, et al. . Age-related macular degeneration in ethnically diverse Australia: Melbourne Collaborative Cohort Study. Ophthalmic Epidemiol. 2015;22(2):75-84. [DOI] [PubMed] [Google Scholar]
- 34.Globe D, Varma R, Azen SP, Paz S, Yu E, Preston-Martin S; Los Angeles Latino Eye Study Group . Psychometric performance of the NEI VFQ-25 in visually normal Latinos: the Los Angeles Latino Eye Study. Invest Ophthalmol Vis Sci. 2003;44(4):1470-1478. [DOI] [PubMed] [Google Scholar]
- 35.Khadka J, Huang J, Mollazadegan K, et al. . Translation, cultural adaptation, and Rasch analysis of the visual function (VF-14) questionnaire. Invest Ophthalmol Vis Sci. 2014;55(7):4413-4420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Association Between Age-Related Macular Degeneration (AMD) and Vision-Specific Functioning (VSF) in Multiple Linear Regression Models
eTable 2. Sociodemographic and Clinical Characteristics of Participants, Stratified by Age-Related Macular Degeneration
eTable 3. Association Between Severity of AMD and VSF by Ethnicity in Multiple Linear Regression Models Using Worse-Seeing Presenting Visual Acuity