Key Points
Question
When implementing resuscitative endovascular balloon occlusion of the aorta (REBOA), is the distance from the point of entry of the catheter to the balloon position in zones I and III the same for the entire general population?
Findings
In this cohort study performed using 280 computed tomographic scans, the same distances were recorded in 97% of the sample population. These distances are expected to exist in more than 94% of the general population.
Meaning
By marking these 2 distances on the catheter, we found that REBOA could be implemented without fluoroscopy, in emergency prehospital and hospital settings and for every patient regardless of morphometric and medical background data.
Abstract
Importance
Resuscitative endovascular balloon occlusion of the aorta (REBOA) is an innovative procedure in the treatment of noncompressible truncal hemorrhage. However, readily available fluoroscopy remains a limiting factor in its widespread implementation. Several methods have been proposed to perform REBOA without fluoroscopic guidance, and these methods were adapted predominantly from the military theater.
Objective
To develop a method for performing REBOA in a civilian population using a standardized distance from a set point of entry.
Design, Setting, and Participants
A retrospective study of whole-body computed tomographic (CT) scans from a cohort of 280 consecutive civilian trauma patients from University Hospitals of Lyon, France, was used to calculate the endovascular distances from both femoral arteries at the level of the upper border of the symphysis pubis to aortic zone I (descending thoracic aorta) and zone III (infrarenal aorta). These whole-body CT scans were performed between 2013 and 2015. Data were analyzed from July 16 to December 7, 2015.
Main Outcomes and Measures
Two segments (1 per zone) common to all CT scans were isolated, and their location, length, prevalence in the cohort, and predicted prevalence in the general population were calculated by inverting 99% certainty tolerance limits.
Results
Among the 280 trauma patients (140 men and 140 women) in this study, the mean (SD) height was 170.7 (8.7) cm, and the mean (SD) age was 38.8 (16.5) years. The common segment in zone I (414-474 mm) existed in all CT scans. The common segment in zone III (236-256 mm) existed in 99.6% and 97.9% of CT scans from the right and left femoral arteries, respectively. These segments are expected to exist in 98.7% (zone I) and 94.9% (zone III) of the general population.
Conclusions and Relevance
Target distances for blind placement of REBOA exist with more than 94% prevalence in a civilian population. These findings support the expanded use of REBOA in emergency department and prehospital settings. Validation for safety and efficacy on cadaveric and clinical models is necessary.
This study describes a method for performing resuscitative endovascular balloon occlusion of the aorta (REBOA) using a standardized distance from a set point of entry.
Introduction
Hemorrhage is the most frequent cause of preventable deaths for both civilian and military trauma patients. During the recent conflicts in Iraq and Afghanistan, more than 80% of preventable deaths were attributed to hemorrhage. Similarly, in the civilian setting, hemorrhage is responsible for 15% to 40% of preventable deaths.
The most common source of bleeding leading to preventable deaths reported in the literature is noncompressible truncal hemorrhage, a recent concept rigorously defined as “hemorrhage arising from trauma to the torso vessels, pulmonary parenchyma, solid abdominal organs and disruption of the bony pelvis resulting in hypotension or shock.”(p122) This injury is extremely lethal, with mortality ranging from 18% to 45%.
Noncompressible truncal hemorrhage can be managed via external aortic clamping during resuscitative thoracotomy or laparotomy. However, these procedures are difficult to perform in the field, and they require significant equipment and trained physicians. They also expose the patient to the various complications of open surgery. Despite recent skepticism, another emerging technique in controlling noncompressible truncal hemorrhage is resuscitative endovascular balloon occlusion of the aorta (REBOA), which allows for endovascular occlusion of the aorta by inflating a balloon proximal to the focus of hemorrhage. Stannard et al divided the aorta into 3 zones: zone I (extending from the left subclavian artery to the celiac trunk), zone II (from the celiac trunk to the lower renal artery), and zone III (from the lower renal artery to the aortic bifurcation). The balloon is inflated in either zone I or zone III depending on the suspected focus of hemorrhage. Zone II is never occluded because it exposes the patient to the risks of zone I occlusion (visceral ischemia) without providing significant benefits compared with a zone III occlusion. Animal and human studies suggest that REBOA may be superior to aortic-cross clamping by thoracotomy. However, the need for fluoroscopic confirmation of balloon placement during REBOA remains a limiting factor in its use in the emergency prehospital setting.
Even though there have been reported uses of REBOA without radiographic guidance, the search for developing an effective fluoroscopy-free REBOA method is ongoing. Several methods have already been proposed. However, they were either performed on a male combatant population (which is not representative of the general population) or needed morphometric and medical background data, information that is usually unavailable in the acute setting. Thus, a simple model proposing the same distance for all patients in a civilian population is needed to make REBOA accessible to emergency prehospital and hospital settings where fluoroscopy and/or medical records are not immediately available. The aim of this study was to develop a fixed-distance model by determining whether there are specific portions within zone I and zone III of the aorta that are at a reliable and reproducible distance from a standardized point of entry and that could be used as targets for the positioning of the balloon during REBOA in a civilian population.
Methods
Study Population
A series of 280 anonymized contrast-enhanced whole-body computed tomographic (CT) scans of trauma patients (140 men and 140 women) hospitalized between January 1, 2013, and October 7, 2015, were selected at random from the Picture Archiving and Communication System of the University Hospitals of Lyon, France. Demographic data of the study population are presented in Table 1. We then performed a retrospective study of the distance from 2 standardized points of entry (the right and left femoral arteries) to zones I and III of the aorta. No approval from an ethics committee was necessary because this study was noninterventional, and the data were anonymized.
Table 1. Clinical Characteristics of the Study Population.
| Characteristic | Mean (SD) | Minimum | 25th Percentile | Median | 75th Percentile | Maximum | P Valuea |
|---|---|---|---|---|---|---|---|
| Men (n = 140) | |||||||
| Age, y | 37.8 (15.5) | 17 | 24 | 36 | 48 | 88 | NA |
| Height, cm | 176.6 (6.9) | 160 | 172 | 177 | 180 | 198 | NA |
| BMI | 24.47 (4.1) | 16.0 | 22.0 | 23.7 | 26.2 | 36.7 | NA |
| Women (n = 140) | |||||||
| Age, y | 39.8 (17.3) | 16 | 25 | 37 | 51 | 90 | NA |
| Height, cm | 164.9 (5.9) | 150 | 160 | 165 | 169 | 183 | NA |
| BMI | 23.50 (4.9) | 15.6 | 20.4 | 21.9 | 25.9 | 45.2 | NA |
| Total (N = 280) | |||||||
| Age, y | 38.8 (16.5) | 16 | 24 | 36 | 50 | 90 | .15 |
| Height, cm | 170.7 (8.7) | 150 | 165 | 170 | 177 | 198 | <.001 |
| BMI | 23.98 (4.5) | 15.6 | 21.0 | 23.0 | 26.1 | 45.2 | .04 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable.
Determined by use of the t test (comparing men and women); significance was set at P = .05.
Study Protocol
Reconstructed images of each CT scan (OsiriX; Pixmeo) were used to measure the distances between the vessel origins within 0.1 mm. The superior border of the symphysis pubis, an easily identified external landmark, was chosen as a reference point (RP) from which all distances were measured. Entry RPs were defined as the position within the femoral arteries at the level of the superior border of the symphysis pubis. The central axis of the femoral arteries, the iliac arteries, and the aorta were then plotted using the CT scans (eFigure in the Supplement). All distances were calculated along this central axis relative to the RPs. The aortic bifurcation (inferior boundary of zone III), the lower renal artery (superior boundary of zone III and inferior boundary of zone II), the celiac trunk (superior boundary of zone II and inferior boundary of zone I), and the left subclavian artery (superior boundary of zone I) were identified, and their distances from both RPs were calculated to determine the boundaries and lengths of the 3 aortic zones. Finally, the depth of the femoral artery, defined as the vertical distance from the skin to the femoral artery at the left RP, was measured on each CT scan, and its correlation with body mass index was calculated.
Data Analysis
All measurements were collected in an Excel spreadsheet (Microsoft), and data were analyzed with R version 3.1.3 for Windows. Analysis was performed for the entire cohort, as well as for male and female subgroups. All data regarding lengths are presented as mean (standard deviation) values.
Locating the Common Segments
Once this protocol had been performed on all CT scans, the following algorithm was used to find a common segment of at least 2 cm in each of zones I and III:
Find the minimum of the superior boundary of the zone (ie, the upper boundary of the common segment).
Find the maximum of the inferior boundary of the zone (ie, the lower boundary of the common segment).
If the upper boundary minus the lower boundary is 2 cm or more, then this is the common segment to all CT scans. If it is less than 2 cm, then for each CT scan, begin with the segment [superior boundary – 2 cm; superior boundary] and decrease the distance from each boundary to the RPs by 0.1 mm until reaching the segment [inferior boundary; inferior boundary + 2 cm]. The common segment is the one that is present in the maximum number of CT scans.
A length of 2 cm was arbitrarily chosen as the minimum length for the common segments, given the size of the balloons used for REBOA and the diameter of the aorta.
Normality Testing
For both zone I and zone III, separate distributions were generated for the superior and inferior boundaries. These 4 distributions were assessed for normality using quantile-quantile plots. Normality was quantified with the Shapiro-Wilk test. Statistical significance was set at P = .05.
Calculating the Probability of the Segments’ Existence in the General Population
For each segment, each of its 2 boundaries was set as a 99% certainty 1-sided tolerance limit for the corresponding zone boundary distribution (ie, the upper boundary of the segment for the upper boundary of the zone). One-sided tolerance limits are single cutoff values above which (or below which) x% of the general population will fall with y% certainty. Tolerance limits are calculated for a given proportion of the population. However, the equation can be solved to calculate the proportion of the population for a given tolerance limit. The proportion zu% of the general population lying inferior to the superior boundary was calculated. Similarly, the proportion zl% lying superior to the inferior boundary was calculated. The proportion of the population for which the segment is valid is the complement of the union of zu% and zl%.
Results
Normality Testing
The quantile-quantile plots for the 4 distributions of the zone boundaries closely follow the normal distribution, on both sides. The Shapiro-Wilk test P value was calculated for all distributions before and after the removal of the maximum value of each distribution (Table 2). Before the removal, on the right side, only the superior boundary of zone I and the inferior boundary of zone III follow a normal distribution. On the left side, only the superior boundary of zone I follows a normal distribution. After the removal, all distributions become normal, on both sides. The maximum values were removed as outliers, as evidenced by the quantile-quantile plots.
Table 2. Data on Zone Boundary Distributions on Both Sides in the Study Population.
| Distribution | Zone Boundary, mm | P Valuea | |
|---|---|---|---|
| Range | Mean (SD) | ||
| Right side | |||
| Superior boundary of zone I | 474.1-652.6 | 549.8 (29) | .06 |
| After removing maximum value | 474.1-635.6 | 549.4 (28.5) | .23 |
| Inferior boundary of zone I | 284.0-414.1 | 329.4 (18.4) | .005 |
| After removing maximum value | 284.0-391.3 | 329.1 (17.7) | .76 |
| Superior boundary of zone III | 259.6-362.5 | 297.0 (17) | .03 |
| After removing maximum value | 259.6-354.0 | 296.7 (16.6) | .25 |
| Inferior boundary of zone III | 160.1-246.8 | 200.4 (16) | .40 |
| After removing maximum value | 160.1-235.6 | 200.2 (15.7) | .10 |
| Left side | |||
| Superior boundary of zone I | 474.0-649.1 | 546.3 (29) | .12 |
| After removing maximum value | 474.0-628.9 | 546.0 (28.4) | .39 |
| Inferior boundary of zone I | 274.3-410.6 | 326.0 (18.6) | .003 |
| After removing maximum value | 274.3-394.0 | 325.6 (18) | .65 |
| Superior boundary of zone III | 249.0-366.5 | 293.5 (17.7) | .02 |
| After removing maximum value | 249.0-359.0 | 293.3 (17.2) | .61 |
| Inferior boundary of zone III | 151.6-279.0 | 197.0 (17) | .002 |
| After removing maximum value | 151.6-247.9 | 196.7 (16.3) | .69 |
Shapiro-Wilk test results. Normality was accepted if P > .05.
Common Segments
On the right side, zone I extended from 284 to 653 mm from the RP. On the left side, it extended from 274 to 649 mm. The mean (SD) length of zone I was 220 (21) mm.
On the right side, zone III extended from 160 to 363 mm from the RP. On the left side, it extended from 152 to 367 mm. The mean (SD) length of zone III was 97 (12) mm.
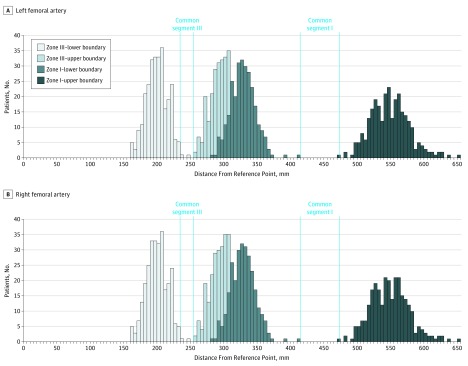
A segment common to 100% of CT scans on both sides exists in zone I extending from 414 to 474 mm from the RP (60 mm long). A segment common to 99.6% of CT scans on the right side and to 97.9% of CT scans on the left side exists in zone III extending from 236 to 256 mm from the RP (20 mm long) (Figure).
Figure. Distributions of the Distance of Zone I and Zone III Boundaries From the Reference Point in the Study Population.
Zero corresponds to the reference point on each side. The blue dotted lines delineate the common segments. Bins are 5 mm in width.
Predictably, on both sides, segment III was inferior to the inferior boundary of zone I in 100% of CT scans, and segment I was superior to the superior boundary of zone III in 100% of CT scans. This means that a balloon placed in the common segment in zone I will never appear in zone III and that a balloon placed in the common segment in zone III will never appear in zone I.
Frequency of Common Segments in the Study Population
Right-sided common segments I and III were calculated to exist in 98.99% and 95.97%, respectively, of the general population. From the left RP, common segments I and III exist in 98.67% and 94.90%, respectively, of the general population (Table 3).
Table 3. Projection of Existence of the Common Segments in the General Population.
| Common Segment Boundary | Right Side | Left Side | ||
|---|---|---|---|---|
| % of Population Above Upper or Below Lower Boundarya | % of Population With Free Common Segmentb | % of Population Above Upper or Below Lower Boundarya | % of Population With Free Common Segmentb | |
| Zone I | ||||
| Upper boundary (474 mm) |
1.01 | 1.33 | ||
| Lower boundary (414 mm) |
0.002 | 0.001 | ||
| Sum | 1.01 | 98.99 | 1.33 | 98.67 |
| Zone III | ||||
| Upper boundary (256 mm) |
1.65 | 3.03 | ||
| Lower boundary (236 mm) |
2.38 | 2.07 | ||
| Sum | 4.03 | 95.97 | 5.10 | 94.90 |
Calculated from 99% certainty 1-sided tolerance limit.
Calculated as the complement of the population lying outside of the segment.
Depth of the Femoral Artery
The mean (SD) depth of the femoral artery was 24 (10) mm and was directly proportional to the body mass index (R2 = 0.41, P < .001).
Comparison of the Male and Female Subgroups
The differences between men and women regarding zone boundaries are presented in Table 4. The common segment in zone I was present in 100% of men and women in our sample, and there was no statistically significant difference between men and women for the common segment in zone III (men = 100% and women = 99.3% for the right side [P = .16]; men = 97.1% and women = 98.6% [P = .21] for the left side). Regarding the mean (SD) depth of the femoral artery, there was no difference between men (23 [9] mm) and women (24 [10] mm) (P = .09).
Table 4. Comparison of the Distances From the Reference Points to Zone I and Zone III Boundaries Between Men and Women.
| Boundary | Mean (SD) Distance, mm | P Valuea | |
|---|---|---|---|
| Men | Women | ||
| Zone I | 231 (18) | 210 (17) | <.001 |
| Upper boundary | |||
| Right | 560 (28) | 540 (27) | <.001 |
| Left | 556 (28) | 537 (27) | <.001 |
| Lower boundary | |||
| Right | 329 (19) | 330 (18) | .31 |
| Left | 325 (19) | 327 (18) | .16 |
| Zone II | 33 (9) | 31 (9) | .03 |
| Zone III | 98 (11) | 96 (13) | .08 |
| Upper boundary | |||
| Right | 295 (17) | 299 (17) | .06 |
| Left | 291 (17) | 296 (18) | .02 |
| Lower boundary | |||
| Right | 198 (16) | 203 (15) | .003 |
| Left | 194 (17) | 200 (17) | .001 |
Determined by use of the t test (significance set at P = .05).
Discussion
Resuscitative endovascular balloon occlusion of the aorta is a promising technique for the control of noncompressible truncal hemorrhage. However, it cannot be currently used in the prehospital setting because of the need for fluoroscopic confirmation of balloon inflation in the correct aortic zone and because accidental inflation inside aortic branches, such as the renal arteries, must be avoided. Six different ways have been proposed to determine the correct wire length, so that the balloon will be inflated in the right position without fluoroscopy: (1) for zone III only, blind introduction of the balloon up to 50 cm, slow inflation with saline solution, withdrawal until wedged in the aortic bifurcation, and advancement of 5 cm upward; (2) measuring the distance from the point of insertion to external landmarks corresponding to zones I and III; (3) a fixed-distance model (calculated on a male combatant population); (4) a linear model calculating the expected locations of zones I and III given the torso height of the patient; (5) ultrasonographic guidance; and (6) a multivariate model using morphological and medical background data, recently proposed by MacTaggart et al.
If REBOA is to be used in the prehospital setting, it is important to develop a very simple method that requires as little information as possible and is applicable to every patient. Our study reports a fixed-distance model in a mixed civilian population that does not depend on any patient information. This model works in 97% of our cohort and is projected to succeed in 94% of the general population. The “general population” is defined as the population from which the sample is drawn, in our case the population of the Lyon area in France. However, this group may not be representative of broader populations. Fortunately, aortic morphometry seems to be more influenced by height and/or torso length than ethnic background. This means that we can interpret the general population from a height and/or torso length point of view, and given our cohort’s diversity (height ranged from 150 to 198 cm), it is reasonable to believe that our results are applicable to patients with the same demographic characteristics as our cohort, regardless of their ethnic background.
Our study also presents data on the comparison of zone boundaries between men and women for the implementation of REBOA. Despite some statistically significant differences, it is important to note that the common segments apply to everybody, regardless of sex.
The fixed-distance model herein proposed is designed to be applied as a blind placement method. An important point concerning the applicability of such a method is its safety. In 3 small series, in which a blind method was successfully applied, the complications reported included inflation inside the iliac artery (2 of 13 cases) and aortic rupture requiring operative repair (1 of 6 cases). Both of these complications were prospectively addressed (feeling for the disappearance of the femoral artery pulse and repositioning and cautiously inflating the balloon, respectively). Owing to the possibility of such complications in any blind model, our results must be validated for safety and efficacy on cadaveric and clinical models, with the use of balloons that will already have landmarks for zone I and zone III marked on the catheter (at 444 and 246 mm, respectively [common segment centers], from the middle of the balloon).
One issue that has to be taken into account is the percentage of the general population for whom the model does not work. Unfortunately, there seems to be no way of detecting these patients before the model is actually implemented. For zone I, the model is predicted to fail in 1% of the general population on the right and 1.3% of the general population on the left side (Table 3), with the upper boundary (474 mm from the RP) being more prone to fallibility. Therefore, the closer the balloon is to the lower boundary, herein proposed at 414 mm, the more negligible chances of failure become.
When occluding zone III, however, the probability of incorrect balloon positioning (zone II or iliac artery occlusion) is 5.1% (Table 3). Zone II occlusion (3%) is not an issue in an exsanguinating patient, because the priority is to stop the bleeding, and the balloon can be repositioned under fluoroscopic guidance when available. An iliac artery occlusion (2.4%) is suspected if the patient does not improve despite adequate resuscitation and is confirmed if a contralateral femoral artery pulse remains present. In that case, the balloon is deflated, pushed upstream, and reinflated, if fluoroscopy is not immediately available.
Ultrasonographic devices are portable and, indeed, easy to use in a prehospital setting, and potentially attractive tools in performing REBOA without fluoroscopy while minimizing complications of a blind technique. However, the inability to visualize the abdominal aorta in certain patients (eg, obese patients or patients with a lot of air in the bowel) can necessitate a completely blind model, such as the one we propose. Specifically, 25% of our population had a body mass index ranging from 26 to 45 (Table 1); ultrasonography might not be an option for these patients.
When applying our model, some practical issues must be taken into account. First, our model was calculated to a level of accuracy that cannot be achieved in a true prehospital trauma setting. However, given the potential error of a few millimeters, the common segments are large enough that any such error should be of little consequence. Second, the zone boundaries are calculated relative to the center of the lumen of the femoral arteries, thus ignoring the distance from the skin to the center of the vessel. This distance depends on the identification of external landmarks, the needle’s angle, and the distance of the femoral artery from the skin, which in turn depends on subcutaneous fat thickness, but ultimately amounts to a variance of just a few millimeters, which we believe is mitigated by the comparatively large common segments. Finally, REBOA has to be implemented through the femoral artery proximal to the origin of the profunda owing to the large diameter of current devices. While it is conceivable that the needle cannot be inserted at the standardized point of entry that we propose, the model can still be applied by positioning the marking on the catheter or wire at the level of the superior border of the symphysis pubis.
It is important to note that all blind placement methods, including ours, have different drawbacks. However, the aggregation of these approaches may result in a method that could make a blind implementation of REBOA in the prehospital setting a reality.
Conclusions
The current study, performed on a mixed civilian population, proposes a more than 94% accurate model for the correct positioning of a REBOA catheter for the management of noncompressible truncal hemorrhage without fluoroscopic guidance. This model allows for a standardized method using a simple external landmark (symphysis pubis) to facilitate the use of REBOA in the trauma bay, as well as in the prehospital setting.
eFigure. Route of the central axis of the aorta, plotted with OsiriX
References
- 1.Holcomb JB, McMullin NR, Pearse L, et al. . Causes of death in U.S. Special Operations Forces in the global war on terrorism: 2001-2004. Ann Surg. 2007;245(6):986-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kelly JF, Ritenour AE, McLaughlin DF, et al. . Injury severity and causes of death from Operation Iraqi Freedom and Operation Enduring Freedom: 2003-2004 versus 2006. J Trauma. 2008;64(2 suppl):S21-S26. [DOI] [PubMed] [Google Scholar]
- 3.Eastridge BJ, Hardin M, Cantrell J, et al. . Died of wounds on the battlefield: causation and implications for improving combat casualty care. J Trauma. 2011;71(1 suppl):S4-S8. [DOI] [PubMed] [Google Scholar]
- 4.Eastridge BJ, Mabry RL, Seguin P, et al. . Death on the battlefield (2001-2011): implications for the future of combat casualty care [published correction appears in J Trauma Acute Care Surg. 2013;74(2):706]. J Trauma Acute Care Surg. 2012;73(6)(suppl 5):S431-S437. [DOI] [PubMed] [Google Scholar]
- 5.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: an overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60(6 suppl):S3-S11. [DOI] [PubMed] [Google Scholar]
- 6.Tien HC, Spencer F, Tremblay LN, Rizoli SB, Brenneman FD. Preventable deaths from hemorrhage at a level I Canadian trauma center. J Trauma. 2007;62(1):142-146. [DOI] [PubMed] [Google Scholar]
- 7.Teixeira PGR, Inaba K, Hadjizacharia P, et al. . Preventable or potentially preventable mortality at a mature trauma center. J Trauma. 2007;63(6):1338-1346. [DOI] [PubMed] [Google Scholar]
- 8.Morrison JJ, Rasmussen TE. Noncompressible torso hemorrhage: a review with contemporary definitions and management strategies. Surg Clin North Am. 2012;92(4):843-858. [DOI] [PubMed] [Google Scholar]
- 9.Stannard A, Morrison JJ, Scott DJ, Ivatury RA, Ross JD, Rasmussen TE. The epidemiology of noncompressible torso hemorrhage in the wars in Iraq and Afghanistan. J Trauma Acute Care Surg. 2013;74(3):830-834. [DOI] [PubMed] [Google Scholar]
- 10.Scott DJ, Eliason JL, Villamaria C, et al. . A novel fluoroscopy-free, resuscitative endovascular aortic balloon occlusion system in a model of hemorrhagic shock. J Trauma Acute Care Surg. 2013;75(1):122-128. [DOI] [PubMed] [Google Scholar]
- 11.Morrison JJ, Stannard A, Rasmussen TE, Jansen JO, Tai NRM, Midwinter MJ. Injury pattern and mortality of noncompressible torso hemorrhage in UK combat casualties. J Trauma Acute Care Surg. 2013;75(2)(suppl 2):S263-S268. [DOI] [PubMed] [Google Scholar]
- 12.Kisat M, Morrison JJ, Hashmi ZG, Efron DT, Rasmussen TE, Haider AH. Epidemiology and outcomes of non-compressible torso hemorrhage. J Surg Res. 2013;184(1):414-421. [DOI] [PubMed] [Google Scholar]
- 13.Ledgerwood AM, Kazmers M, Lucas CE. The role of thoracic aortic occlusion for massive hemoperitoneum. J Trauma. 1976;16(08):610-615. [DOI] [PubMed] [Google Scholar]
- 14.Millikan JS, Moore EE. Outcome of resuscitative thoracotomy and descending aortic occlusion performed in the operating room. J Trauma. 1984;24(5):387-392. [DOI] [PubMed] [Google Scholar]
- 15.Wiencek RG Jr, Wilson RF. Injuries to the abdominal vascular system: how much does aggressive resuscitation and prelaparotomy thoracotomy really help? Surgery. 1987;102(4):731-736. [PubMed] [Google Scholar]
- 16.Seamon MJ, Pathak AS, Bradley KM, et al. . Emergency department thoracotomy: still useful after abdominal exsanguination? J Trauma. 2008;64(1):1-7. [DOI] [PubMed] [Google Scholar]
- 17.Burlew CC, Moore EE, Moore FA, et al. . Western Trauma Association critical decisions in trauma: resuscitative thoracotomy. J Trauma Acute Care Surg. 2012;73(6):1359-1363. [DOI] [PubMed] [Google Scholar]
- 18.Biffl WL, Fox CJ, Moore EE. The role of REBOA in the control of exsanguinating torso hemorrhage. J Trauma Acute Care Surg. 2015;78(5):1054-1058. [DOI] [PubMed] [Google Scholar]
- 19.Seamon MJ, Haut ER, Van Arendonk K, et al. . An evidence-based approach to patient selection for emergency department thoracotomy: a practice management guideline from the Eastern Association for the Surgery of Trauma. J Trauma Acute Care Surg. 2015;79(1):159-173. [DOI] [PubMed] [Google Scholar]
- 20.Britt LD, Peitzman AB. Acute Care Surgery. Philadelphia, PA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. [Google Scholar]
- 21.Qasim Z, Brenner M, Menaker J, Scalea T. Resuscitative endovascular balloon occlusion of the aorta. Resuscitation. 2015;96:275-279. [DOI] [PubMed] [Google Scholar]
- 22.Stannard A, Eliason JL, Rasmussen TE. Resuscitative endovascular balloon occlusion of the aorta (REBOA) as an adjunct for hemorrhagic shock. J Trauma. 2011;71(6):1869-1872. [DOI] [PubMed] [Google Scholar]
- 23.Wise D, Davies G, Coats T, Lockey D, Hyde J, Good A. Emergency thoracotomy: “how to do it”. Emerg Med J. 2005;22(1):22-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mollberg NM, Glenn C, John J, et al. . Appropriate use of emergency department thoracotomy: implications for the thoracic surgeon. Ann Thorac Surg. 2011;92(2):455-461. [DOI] [PubMed] [Google Scholar]
- 25.Puchwein P, Sommerauer F, Clement HG, et al. . Clamshell thoracotomy and open heart massage—a potential life-saving procedure can be taught to emergency physicians: an educational cadaveric pilot study. Injury. 2015;46(9):1738-1742. [DOI] [PubMed] [Google Scholar]
- 26.Joint Theater Trauma System Clinical Practice Guidelines: resuscitative endovascular balloon occlusion of the aorta (REBOA) for hemorrhagic shock. http://www.usaisr.amedd.army.mil/cpgs/REBOA_for_Hemorrhagic_Shock_16Jun2014.pdf. Released June 16, 2014. Accessed November 1, 2015.
- 27.Belenkiy SM, Batchinsky AI, Rasmussen TE, Cancio LC. Resuscitative endovascular balloon occlusion of the aorta for hemorrhage control: Past, present, and future. J Trauma Acute Care Surg. 2015;79(4)(suppl 2):S236-S242. [DOI] [PubMed] [Google Scholar]
- 28.Chaudery M, Clark J, Wilson MH, Bew D, Yang G-Z, Darzi A. Traumatic intra-abdominal hemorrhage control: has current technology tipped the balance toward a role for prehospital intervention? J Trauma Acute Care Surg. 2015;78(1):153-163. [DOI] [PubMed] [Google Scholar]
- 29.White JM, Cannon JW, Stannard A, Markov NP, Spencer JR, Rasmussen TE. Endovascular balloon occlusion of the aorta is superior to resuscitative thoracotomy with aortic clamping in a porcine model of hemorrhagic shock. Surgery. 2011;150(3):400-409. [DOI] [PubMed] [Google Scholar]
- 30.Moore LJ, Brenner M, Kozar RA, et al. . Implementation of resuscitative endovascular balloon occlusion of the aorta as an alternative to resuscitative thoracotomy for noncompressible truncal hemorrhage. J Trauma Acute Care Surg. 2015;79(4):523-530. [DOI] [PubMed] [Google Scholar]
- 31.Martinelli T, Thony F, Decléty P, et al. . Intra-aortic balloon occlusion to salvage patients with life-threatening hemorrhagic shocks from pelvic fractures. J Trauma. 2010;68(4):942-948. [DOI] [PubMed] [Google Scholar]
- 32.Søvik E, Stokkeland P, Storm BS, Asheim P, Bolås O. The use of aortic occlusion balloon catheter without fluoroscopy for life-threatening post-partum haemorrhage. Acta Anaesthesiol Scand. 2012;56(3):388-393. [DOI] [PubMed] [Google Scholar]
- 33.Manning JE. Feasibility of blind aortic catheter placement in the prehospital environment to guide resuscitation in cardiac arrest. J Trauma Acute Care Surg. 2013;75(2)(suppl 2):S173-S177. [DOI] [PubMed] [Google Scholar]
- 34.Irahara T, Sato N, Moroe Y, Fukuda R, Iwai Y, Unemoto K. Retrospective study of the effectiveness of intra-aortic balloon occlusion (IABO) for traumatic haemorrhagic shock. World J Emerg Surg. 2015;10(1):1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison JJ, Stannard A, Midwinter MJ, Sharon DJ, Eliason JL, Rasmussen TE. Prospective evaluation of the correlation between torso height and aortic anatomy in respect of a fluoroscopy free aortic balloon occlusion system. Surgery. 2014;155(6):1044-1051. [DOI] [PubMed] [Google Scholar]
- 36.Chaudery M, Clark J, Morrison JJ, Wilson MH, Bew D, Darzi A. Can contrast-enhanced ultrasonography improve Zone III REBOA placement for prehospital care? J Trauma Acute Care Surg. 2016;80(1):89-94. [DOI] [PubMed] [Google Scholar]
- 37.MacTaggart JN, Poulson WE, Akhter M, et al. . Morphometric roadmaps to improve accurate device delivery for fluoroscopy-free resuscitative endovascular balloon occlusion of the aorta. J Trauma Acute Care Surg. 2016;80(6):941-946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilmink AB, Pleumeekers HJ, Hoes AW, Hubbard CS, Grobbee DE, Quick CR. The infrarenal aortic diameter in relation to age: only part of the population in older age groups shows an increase. Eur J Vasc Endovasc Surg. 1998;16(5):431-437. [DOI] [PubMed] [Google Scholar]
- 39.Stannard A, Morrison JJ, Sharon DJ, Eliason JL, Rasmussen TE. Morphometric analysis of torso arterial anatomy with implications for resuscitative aortic occlusion. J Trauma Acute Care Surg. 2013;75(2)(suppl 2):S169-S172. [DOI] [PubMed] [Google Scholar]
- 40.Rogers IS, Massaro JM, Truong QA, et al. . Distribution, determinants, and normal reference values of thoracic and abdominal aortic diameters by computed tomography (from the Framingham Heart Study). Am J Cardiol. 2013;111(10):1510-1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jasper A, Harshe G, Keshava SN, Kulkarni G, Stephen E, Agarwal S. Evaluation of normal abdominal aortic diameters in the Indian population using computed tomography. J Postgrad Med. 2014;60(1):57-60. [DOI] [PubMed] [Google Scholar]
- 42.Natrella MG. Experimental Statistics. Mineola, NY: Dover Publications; 2005. [Google Scholar]
- 43.Guliani S, Amendola M, Strife B, et al. . Central aortic wire confirmation for emergent endovascular procedures: as fast as surgeon-performed ultrasound. J Trauma Acute Care Surg. 2015;79(4):549-554. [DOI] [PubMed] [Google Scholar]
- 44.Tsurukiri J, Akamine I, Sato T, et al. . Resuscitative endovascular balloon occlusion of the aorta for uncontrolled haemorrahgic shock as an adjunct to haemostatic procedures in the acute care setting. Scand J Trauma Resusc Emerg Med. 2016;24:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Igari T. The length of the aorta from the subclavian artery to the renal artery based on computed tomographic measurements in Japanese adults. J Artif Organs. 2006;9(4):267-270. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Route of the central axis of the aorta, plotted with OsiriX