Key Points
Question
Are there polymorphisms in the human leukocyte antigen (HLA) class I genes associated with Stevens-Johnson syndrome and toxic epidermal necrolysis and severe ocular complications following cold medicine use?
Findings
In this case-control study, among 39 Brazilian patients with cold medicine–associated Stevens-Johnson syndrome or toxic epidermal necrolysis and severe ocular complications of 74 patients with Stevens-Johnson syndrome or toxic epidermal necrolysis, HLA-A*66:01, HLA-B*44:03, and HLA-C*12:03 were associated with and HLA-A*11:01, HLA-B*08:01, and HLA-B*51:01 were inversely associated with Stevens-Johnson syndrome and toxic epidermal necrolysis with severe ocular complications. HLA-B*44:03 and HLA-C*12:03 were associated only among individuals with European ancestry.
Meaning
Describing the association of these alleles might facilitate the understanding of increased risk factors for developing Stevens-Johnson syndrome and toxic epidermal necrolysis with severe ocular complications.
Abstract
Importance
Describing the association with human leukocyte antigen (HLA) alleles could facilitate the understanding of increased risk factors for development of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) in patients with severe ocular complications (SOCs).
Objective
To investigate the association between HLA class I genes and cold medicine (CM)–associated SJS/TEN with SOCs.
Design, Setting, and Participants
This case-control study was conducted between February 8, 2013, and August 29, 2014. Thirty-nine Brazilian patients with CM-SJS/TEN of 74 patients with SJS/TEN with SOCs and 133 healthy Brazilian volunteers were enrolled. Human leukocyte antigen class I genes (HLA-A, HLA-B, and HLA-C) were examined to determine whether there was a genetic predisposition for CM-SJS/TEN with SOC. Patients were interviewed to identify possible etiologic factors. Data analysis was performed from April 14, 2013, to August 29, 2014.
Main Outcomes and Measures
Genetic predisposition for CM-SJS/TEN with SOCs by analysis of HLA class I genes.
Results
Of 74 patients included in the analysis, 32 (43%) were male; mean (SD) age was 36.01 [15.42] years. HLA-A*66:01 (odds ratio [OR], 24.0; 95% CI, 2.79-206.0; P < .001), HLA-B*44:03 (OR, 2.71; 95% CI, 1.11-6.65; P = .04), and HLA-C*12:03 (OR, 5.6; 95% CI, 1.67-18.80; P = .006) were associated with Brazilian CM-SJS/TEN with SOCs, and HLA-A*11:01 (OR, 0.074; 95% CI, 0.004-1.26; P = .008), HLA-B*08:01 (OR, 0.15; 95% CI, 0.02-1.15; P = .048), and HLA-B*51:01 (OR, 0.23; 95% CI, 0.05-1.03; P = .045) were inversely associated with Brazilian CM-SJS/TEN with SOCs (39 cases: 19 Pardo and 16 European ancestry; 14 males and 25 females; age, 35.2 [14.4] years; and 133 controls: 66 Pardo and 61 European ancestry; 55 males and 78 females; age, 41.2 [12.9] years). When multiple test correction within the HLA locus, HLA-A*66:01 and HLA-C*12:03 demonstrated associations. When participants were segregated into Pardo and locus is considered, HLA-A*66:01 was associated with CM-SJS/TEN with SOC among individuals of both ethnic groups (Pardo: OR, 12.2; 95% CI, 1.19-125.0; P = .03; and European: OR, 21.2; 95% CI, 0.97-465.0; P = .04). An association was observed only in the European cohort for HLA-B*44:03 (OR, 5.50; 95% CI, 1.47-20.50; P = .01) and HLA-C*12:03 (OR, 8.79; 95% CI, 1.83-42.20; P = .008).
Conclusions and Relevance
This study suggests that HLA-A*66:01 might be a marker for CM-SJS/TEN with SOCs in Brazilian individuals of Pardo and European ancestry and that HLA-B*44:03 and HLA-C*12:03 might be markers only in those of European ancestry. Moreover, HLA-A*11:01 might be a marker of resistance to CM-SJS/TEN with SOCs.
This case-control study examines class I human leukocyte antigens in patients with Stevens-Johnson syndrome and toxic epidermal necrolysis with severe ocular complications who were using cold medicines near the time of the disease onset.
Introduction
Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening, acute inflammatory vesiculobullous reactions of the skin and mucous membranes. These diseases are commonly associated with a drug or infectious agent, and the worldwide estimated annual incidence is 1.9 to 10 per million persons. In severe acute cases, there is extensive membranous conjunctivitis with epithelial defects of the eyelids, conjunctiva, and cornea. Severe ocular involvement during the acute phase is seen in approximately 40% of patients with SJS and TEN. Chronic sequelae, such as symblepharon, entropion, trichiasis, tear film abnormalities, corneal opacity, keratinization, and corneal neovascularization, characterize the severe ocular complications (SOCs) and occur in approximately 35% of the patients.
Human leukocyte antigens (HLAs) are highly polymorphic proteins that initiate immunity by presenting pathogen-derived peptides to T-cells. Typing of HLA in large groups of patients with various autoimmune diseases has shown that some HLA alleles occur at a higher frequency in patients with particular diseases than in the general population, and individuals with a specific HLA allele are at increased risk for developing SJS and TEN. In Taiwanese Han Chinese, HLA-B*15:02 exhibited an association with carbamazepine-induced SJS and TEN and HLA-A*31:01 was associated with carbamazepine-induced severe cutaneous adverse reactions, including SJS and TEN in European and Japanese patients; in addition, HLA-B*58:01 was strongly associated with allopurinol-induced SJS and TEN in Han Chinese, European, and Japanese patients.
Ueta et al found that, in Japanese patients, HLA-A*02:06 was strongly associated and HLA-A*11:01 was inversely associated with SJS and TEN with SOCs. They later reported that approximately 80% of the reactions were associated with cold medicine (CM) and demonstrated an independent, strong association of HLA-A*02:06 and HLA-B*44:03 with CM-related SJS and TEN (CM-SJS/TEN) with severe mucosal involvement, including SOCs. The association of theses alleles was confirmed in a study that characterized groups of different ethnicities (eg, Indian, Brazilian, and Korean). These findings suggest genetic predispositions for the development of CM-SJS/TEN with SOCs. Cold medicines are a group of remedies that might help to relieve the symptoms of common cold. They are represented by analgesics and antipyretics (eg, dipyrone and acetaminophen) and nonsteroidal anti-inflammatory drugs (NSAIDs) (eg, salicylates, propionic acid, acetic acid, enolic acid, anthranilic acid derivatives, selective cyclooxygenase-2 inhibitors, and sulfonanilides).
In the present study, we examined associations between SJS/TEN with SOCs, with a focus on CM-SJS/TEN with SOCs. In addition, we examined HLA class I genes (HLA-A [Gene ID: 3105], HLA-B [Gene ID: 3106], and HLA-C [Gene ID: 3107]) to determine whether there was a genetic predisposition for CM-SJS/TEN with SOCs.
Methods
Patients and Controls
Between February 8, 2013, and August 29, 2014, 74 Brazilian patients with SJS/TEN (32 [43%] males and 42 [57%] females; age, 7 months to 70 years; mean [SD] age, 36.01 [15.42]) were independently recruited at the Federal University of São Paulo, Faculty of Medicine (main referral hospital for the treatment of SJS ocular). Their age at onset of SJS/TEN ranged from 3 to 69 years (mean [SD], 23.1 [15.9] years). Of the 74 patients 38 (51%) were of Pardo, 30 (41%) of European, 4 (5%) of African, and 2 (3%) of American Indian plus European ancestry. Pardo is a commonly used term to refer to Brazilians of mixed ethnic ancestries, typically white Brazilians and Afro-Brazilians. The diagnosis of SJS/TEN with SOCs was based on a confirmed history of acute-onset high fever, serious mucocutaneous illness with skin eruptions, and the involvement of at least 2 mucosal sites, including the oral cavity and ocular surface.
Our study was approved by the institutional review board of the Federal University of São Paulo and Kyoto Prefectural University of Medicine. All experimental procedures were conducted in accordance with the principles set forth in the Helsinki Declaration. The purpose of the experimental protocols was explained to all participants, and their written informed consent was obtained. There was no financial compensation.
In this study, we focused on CM-SJS/TEN, which might be induced by cold medicines such as dipyrone and NSAIDs. Patients included in this study had used such cold medicines for treatment of symptoms of common cold and 1 to 14 days before disease onset; they were classified as CM-SJS/TEN patients.
A total of 133 age, sex, and race/ethnicity frequency matching Brazilian healthy volunteers served as controls. This sample included 55 males and 78 females (age range, 10-70 years; mean, 41.2 [12.9] years) without any known or previously diagnosed dermatologic, allergic, or systemic disease similar to SJS or TEN who were independently recruited at the Federal University of São Paulo. Healthy volunteers, including university employees and students, and patients’ (except those with SJS and TEN) companions, were requested to answer the same questionnaire as those with SJS and TEN. This group of volunteers did not have any symptoms and signs similar to those of the study patients. Sixty-six volunteers (50%) were of Pardo, 61 (46%) of European, 4 (3%) of African, and 2 (1%) of Indian plus European ancestry.
HLA Genotyping
Samples of DNA were extracted from whole peripheral blood (PAXgene blood DNA kit; Qiagen) or from saliva (Oragene DNA kit; Kyodo International). We analyzed HLA-A, HLA-B, and HLA-C in all 74 SJS/TEN patients and the 133 controls. Polymerase chain reaction assays were followed by hybridization with sequence-specific oligonucleotide probes using commercially available bead-based typing kits (Wakunaga Pharmaceutical). Briefly, target DNA was polymerase chain reaction–amplified with biotinylated primers specifically designed for amplified exons 2 and 3 of HLA-A, HLA-B, and HLA-C genes. The polymerase chain reaction amplicon was then denatured and hybridized to complementary oligonucleotide probes immobilized on fluorescent-coded microsphere beads. At the same time, the biotinylated polymerase chain reaction product was labeled with phycoerytrin-conjugated streptavidin and immediately examined (Luminex 100; Luminex). Genotype determination and data analysis were performed automatically (WAKFlow typing software; Wakunaga) according to the manufacturer’s instructions.
Statistical Analysis
We compared the carrier frequency and gene frequency of individual HLA alleles in our patients and controls based on the dominant model. Each allele was assessed as an independent variable, and separated P values were calculated with the Fisher exact test. The odds ratio (OR) and 95% CI were calculated using JMP, version 11, software (SAS Institute). In HLA genotypes with no samples in either group of cases and controls, ORs were calculated using Woolf’s correction. Considering that distributions of HLA alleles are correlated, we empirically conducted a permutation test to assess multiple testing corrections within the HLA locus. We randomly shuffled the case-control phenotype label to each person and each person’s HLA genotypes (×20 000 iterations, α = .05).
Results
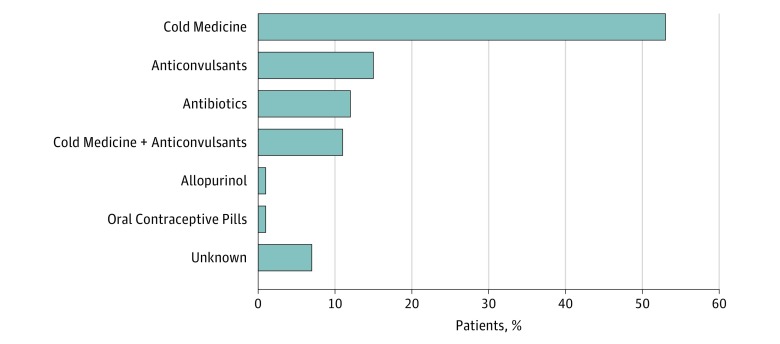
Of the 74 patients with SJS/TEN, 39 (53%) had used CMs, such as dipyrone (n = 37), acetaminophen (n = 4), nimesulide (n = 1), aspirin (n = 4), diclofenac (n = 7), piroxicam (n = 1), and codeine (n = 1) for a few or several days before SJS/TEN onset for common cold symptom. The second-most frequently used group of medications was anticonvulsants (11 [15%]: phenobarbital, 5; carbamazepine, 3; and phenytoin, 3). The source of SJS in 5 patients was unknown (Figure). We also examined HLA class I (HLA-A, HLA-B, and HLA-C) using the 74 patients with SJS/TEN SOCs and 133 controls (eTable 1 in the Supplement).
Figure. Medicines That Could Induce Stevens-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis With Severe Ocular Complications in a Brazilian Population.
The first most frequently used group of medications was cold medicines followed by anticonvulsants. The source of SJS in 5 patients was unknown.
Next, we focused on 39 patients with CM-SJS/TEN with SOCs who were of Pardo (n = 19) and European (n = 16) ancestry (14 males, 25 females; age, 15-63 years; mean [SD], 36.6 [14.3] years) and compared them with 133 members of the control group who were of Pardo (n = 66) and European (n = 61) ancestry (55 males, 78 females; age, 10-70 years; mean, 41.2 [12.9] years). Analysis showed that HLA-A*66:01 was associated (carrier frequency P < .001; OR, 24.0; 95% CI, 2.79-206.0) and HLA-A*11:01 was inversely associated (carrier frequency P = .008; OR, 0.074; 95% CI, 0.004-1.26) with CM-SJS/TEN with SOCs (Table; eTable 2 in the Supplement). Regarding HLA-B, HLA-B*44:03 (carrier frequency P = .04; OR, 2.71; 95% CI, 1.11-6.65) was associated. The HLA-B*08:01 (carrier frequency P = .048; OR, 0.15; 95% CI, 0.02-1.15) and HLA-B*51:01 (carrier frequency P = .045; OR, 0.23; 95% CI, 0.05-1.03) alleles were inversely associated (Table; eTable 3 in the Supplement). This study showed that HLA-C*12:03 was associated (carrier frequency P = .006; OR, 5.60; 95% CI, 1.67-18.80) (Table; eTable 4 in the Supplement). When multiple-test correction within the HLA locus is considered, HLA-A*66:01 and HLA-C*12:03 demonstrated associations (permutation P < .05) (Table; eTable 5 in the Supplement).
Table. HLA Class I Genes Associated With CM-SJS/TEN With SOCs.
| HLA | Carrier Frequency | Gene Frequency | ||||||
|---|---|---|---|---|---|---|---|---|
| No. (%) | OR (95% CI) | P Valuea | No. (%) | OR (95% CI) | P Valuea | |||
| Cases (n = 39) |
Controls (n = 133) |
Cases (n = 78) |
Controls (n = 266) |
|||||
| All CM-SJS/TEN | ||||||||
| A*11:01 | 0 | 19 (14) | 0.074 (0.004-1.26)b | .008 | 0 | 19 (7) | 0.081 (0.0053-1.35)b | .01 |
| A*66:01 | 6 (15) | 1 (0.8) | 24.0 (2.79-206.0) | <.001 | 6 (8) | 1 (0.4) | 22.1 (2.6-186.0) | .0007 |
| B*08:01 | 1 (3) | 20 (15) | 0.15 (0.02-1.15) | .048 | 1 (1) | 21 (8) | 0.15 (0.02-1.14) | .04 |
| B*44:03 | 10 (26) | 15 (11) | 2.71 (1.11-6.65) | .04 | 11 (14) | 15 (6) | 2.75 (1.21-6.26) | .03 |
| B*51:01 | 2 (5) | 25 (19) | 0.23 (0.05-1.03) | .045 | 2 (3) | 26 (10) | 0.24 (0.06-1.05) | .06 |
| C*12:03 | 7 (18) | 5 (4) | 5.60 (1.67-18.80) | .006 | 8 (10) | 5 (2) | 5.97 (1.89-18.8) | .002 |
| Pardo CM-SJS/TEN | (n = 19) | (n = 66) | (n = 38) | (n = 132) | ||||
| A*11:01 | 0 | 6 (9) | 0.24 (0.01-4.43)b | .33 | 0 | 6 (5) | 0.25 (0.01-4.59) | .34 |
| A*66:01 | 3 (16) | 1 (2) | 12.2 (1.19-125.0) | .03 | 3 (8) | 1 (0.8) | 11.2 (1.13-111.0) | .04 |
| B*08:01 | 0 | 5 (8) | 0.29 (0.02-5.42)b | .58 | 0 | 5 (4) | 0.30 (0.02-5.57) | .59 |
| B*44:03 | 3 (16) | 8 (12) | 1.36 (0.32-5.72) | .70 | 3 (8) | 8 (6) | 1.33 (0.335-5.28) | .71 |
| B*51:01 | 1 (5) | 9 (14) | 0.35 (0.04-2.97) | .45 | 1 (3) | 10 (8) | 0.33 (0.04-2.66) | .46 |
| C*12:03 | 2 (11) | 2 (3) | 3.8 (0.49-28.7) | .22 | 2 (5) | 2 (2) | 3.61 (0.49-26.50) | .22 |
| European CM-SJS/TEN | (n = 16) | (n = 61) | (n = 32) | (n = 122) | ||||
| A*11:01 | 0 | 12 (20) | 0.12 (0.007-2.14) | .06 | 0 | 12 (10) | 0.14 (0.008-2.36) | .07 |
| A*66:01 | 2 (13) | 0 | 21.2 (0.97-465.0) | .04 | 2 (6) | 0 | 20.1 (0.94-429.0) | .04 |
| B*08:01 | 1 (6) | 14 (23) | 0.224 (0.03-1.85) | .17 | 1 (3) | 15 (12) | 0.23 (0.03-1.81) | .20 |
| B*44:03 | 6 (38) | 6 (10) | 5.50 (1.47-20.50) | .01 | 7 (22) | 6 (5) | 5.41 (1.68-17.5) | .006c |
| B*51:01 | 1 (6) | 14 (23) | 0.22 (0.03-1.85) | .17 | 1 (3) | 14 (12) | 0.25 (0.03-1.97) | .20 |
| C*12:03 | 5 (31) | 3 (5) | 8.79 (1.83-42.20) | .008 | 6 (19) | 3 (3) | 9.15 (2.15-39.0) | .003c |
Abbreviations: CM-SJS/TEN, cold medicine–associated Stevens-Johnson syndrome/toxic epidermal necrolysis; HLA, human leukocyte antigen; OR, odds ratio; SOCs, severe ocular complications.
Fisher exact test.
Woolf correction.
Analysis showed that HLA-A*66:01, HLA-B*44:03, and HLA-C*12:03 were associated with Brazilian CM-SJS/TEN with SOCs and HLA-A*11:01, HLA-B*08:01, and HLA-B*51:01 were inversely associated with Brazilian CM-SJS/TEN with SOCs.
The segregation of individuals with Pardo and European ancestry showed that there were 19 patients and 66 controls with Pardo and 16 patients and 61 controls with European ancestry. Although the numbers were low, some associations with CM-SJS/TEN with SOCs persisted. The HLA-A*66:01 allele was associated in both the Pardo (carrier frequency P = .03; OR, 12.2; 95% CI, 1.19-125.0) with CM-SJS/TEN with SOCs and European ancestry (carrier frequency P = .04; OR, 21.2; 95% CI, 0.97-465.0) with CM-SJS/TEN with SOCs cohorts (Table).
Because of the low number of samples, the inverse association with HLA-A*11:01 disappeared. No patients of Pardo and European ancestry manifested HLA-A*11:01, although it was present in 9% of the Pardo and 20% of the European ancestry controls (Table). Association with HLA-B*44:03 was noted among individuals of European ancestry (carrier frequency P = .01; OR, 5.50; 95% CI, 1.47-20.50) but not with the Pardo group (carrier frequency P = .70; OR, 1.36; 95% CI, 0.32-5.72) (Table). The inverse association with HLA-B*08:01 and HLA-B*51:01 also disappeared owing to small sample numbers. However, among individuals of Pardo ancestry, no patients and 5 controls (8%) manifested HLA-B*08:01, and among participants with European ancestry, 1 patient (6%) and 14 controls (23%) manifested HLA-B*08:01. Among participants of Pardo ancestry, 1 patient (5%) and 9 controls (14%) and, among those of European ancestry, 1 patient (6%) and 14 controls (23%) manifested HLA-B*51:01 (Table). An association was noted for HLA-C*12:03 among individuals with European ancestry (carrier frequency P = .008; OR, 8.79; 95% CI, 1.83-42.20) but not with those of Pardo ancestry (carrier frequency P = .22; OR, 3.61; 95% CI, 0.49-26.50) (Table).
Discussion
In this study we focused on Brazilian patients with CM-SJS/TEN and SOCs (39 cases and 133 controls) and examined HLA class I genes (HLA-A, HLA-B, and HLA-C) and divided them into groups according to the main ethnicities, Pardo (19 cases and 66 controls) and European (16 cases and 61 controls) ancestry to evaluate their possible genetic predisposition for CM-SJS/TEN with SOCs. Most of our patients developed SJS/TEN with SOCs after taking certain medicines, mainly cold medicines (53%), followed by anticonvulsants (15%). Our investigation of HLA class I genes (HLA-A, HLA-B, and HLA-C) in CM-SJS/TEN with SOCs showed that HLA-A*66:01, HLA-B*44:03, and HLA-C*12:03 were associated and HLA-A*11:01, HLA-B*08:01, and HLA-B*51:01 were inversely associated with CM-SJS/TEN with SOCs in our Brazilian study population. Moreover, even when multiple-test correction within the HLA locus is considered, HLA-A*66:01 and HLA-C*12:03 demonstrated strong associations (permutation P < .05). After the patients and controls were segregated by ancestry, HLA-A*66:01 continued to be associated with both Pardo and European ancestry; however, HLA-B*44:03 and HLA-C*12:03 were associated with only patients and controls of European ancestry. The association with HLA-B*44:03 in participants of European ancestry confirmed earlier findings. In the present study we document that HLA-A*66:01 and HLA-C*12:03 render individuals genetically predisposed for CM-SJS/TEN. Japanese populations do not manifest HLA-A*66:01 or HLA-C*12:03, and HLA-A*11:01 was inversely associated with CM-SJS/TEN with SOCs in our study population of Brazilians of diverse ethnicity. None of our patients and 9% of the Pardo controls and 20% of the European ancestry controls manifested HLA-A*11.01. Because Ueta et al documented the inverse association with HLA-A*11:01 in Japanese patients with SJS/TEN with SOCs, it may represent a universal marker for the resistance to CM-SJS/TEN with SOCs. Nevertheless, further validations of the associated results with additional individuals would be desirable, as would assessment of potential population stratifications.
The association between HLA genotypes and SJS/TEN was known 3 decades ago. Studies from the United States and France showed that the level of HLA-B12 (HLA-Bw44) antigen was increased in patients of European ancestry with SJS. Because the HLA-B12 antigen is mainly coded by HLA-B*44:02 or HLA-B*44:03, the association of HLA-B12 with SJS and TEN in patients of European ancestry may be attributable to the association with the HLA-B*44:03 genotype. Ueta et al also demonstrated the association between the HLA-B*44:03 antigen and CM-SJS/TEN with SOCs in Indian populations that are genetically close to individuals of European ancestry as well as in the Japanese population.
Cold medicines such as dipyrone, NSAIDs, and cold-medicine ingredients (eg, acetaminophen) down-regulate the production of prostanoid, including prostaglandin E2 (PGE2). Because the PGE2-prostaglandin EP3 pathway suppresses inflammation of the ocular surface and skin, we suggest that the down-regulation of PGE2 by dipyrone, NSAIDs, or acetaminophen is involved in the onset of CM-SJS/TEN with SOCs.
We suspect that, in addition to CM, some viral or microbial infections might be important in the development of SJS and TEN. We reported that patients with SJS or TEN using acetaminophen showed a higher rate of experiencing SOCs than did those using other drugs that often cause SJS and TEN, such as carbamazepine, allopurinol, and quinolones. In addition, antipyretic-analgesic drugs, including acetaminophen and NSAIDs, used for the treatment of common cold showed a high frequency of patients with SJS/TEN experiencing SOCs compared with those using antipyretic-analgesic agents for the treatment of other diseases. These findings might show the importance of the interaction between cold medicine use and infection in SJS/TEN with SOCs.
We hypothesize that, when individuals with a genetic background containing SJS/TEN with SOC susceptibility factors acquire a viral or microbial infection, they develop abnormal immune responses. Administration of cold medicine at that time, which down-regulates PGE2-suppressing inflammation, might augment abnormal immune response, resulting in the induction of SJS/TEN with SOCs. In contrast, individuals with no SJS/TEN with SOC genetic susceptibility factors develop a normal immune response upon microbial infection, and the administration of cold medicine has no untoward effect.
HLA-A, a component of HLA class I, alerts the immune system that the cell may be infected with a virus. Based on previous findings about disordered innate immune response in SJS/TEN with SOCs, we suggest that, in addition to microbial infections and cold medicines, the combination of multiple gene polymorphisms and their interactions contribute strongly to the onset of CM-SJS/TEN with SOCs. A more comprehensive study of the molecular mechanisms underlying these diseases would be warranted.
Limitations
A potential limitation of the study is the confounding between use of cold medicine and the presence of infection. Because we could not observe any patients with infections who did not use cold medicines, we cannot determine whether it was the infection, rather than the cold medicine, that was responsible for the association with the cold medicine.
Conclusions
We demonstrate an association between different alleles associated with CM-SJS/TEN with SOCs in Brazilian individuals of Pardo and European ancestry. The HLA-A*66:01 allele may be a marker for CM-SJS/TEN with SOCs in individuals of Pardo and European ancestry, and HLA-B*44:03 and HLA-C*12:03 are markers only in those of European ancestry. We suggest that HLA-A*11:01 is a universal marker of resistance to CM-SJS/TEN with SOCs.
eTable 1. Association Analysis Between HLA Class 1 Types and SJS/TEN With SOC
eTable 2. HLA-A Analysis With Brazilian CM-SJS/TEN With SOC
eTable 3. HLA-B Analysis With Brazilian CM-SJS/TEN With SOC
eTable 4. HLA-C Analysis With Brazilian CM-SJS/TEN With SOC
eTable 5. Significant Thresholds of the HLA Alleles in the Permutation Test
References
- 1.Ueta M, Sotozono C, Inatomi T, et al. Toll-like receptor 3 gene polymorphisms in Japanese patients with Stevens-Johnson syndrome. Br J Ophthalmol. 2007;91(7):962-965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ueta M, Sotozono C, Nakano M, et al. Association between prostaglandin E receptor 3 polymorphisms and Stevens-Johnson syndrome identified by means of a genome-wide association study. J Allergy Clin Immunol. 2010;126(6):1218-25.e10. [DOI] [PubMed] [Google Scholar]
- 3.Yamane Y, Aihara M, Ikezawa Z. Analysis of Stevens-Johnson syndrome and toxic epidermal necrolysis in Japan from 2000 to 2006. Allergol Int. 2007;56(4):419-425. [DOI] [PubMed] [Google Scholar]
- 4.Yetiv JZ, Bianchine JR, Owen JA Jr. Etiologic factors of the Stevens-Johnson syndrome. South Med J. 1980;73(5):599-602. [DOI] [PubMed] [Google Scholar]
- 5.Böttiger LE, Strandberg I, Westerholm B. Drug-induced febrile mucocutaneous syndrome with a survey of the literature. Acta Med Scand. 1975;198(3):229-233. [PubMed] [Google Scholar]
- 6.Chan HL, Stern RS, Arndt KA, et al. The incidence of erythema multiforme, Stevens-Johnson syndrome, and toxic epidermal necrolysis: a population-based study with particular reference to reactions caused by drugs among outpatients. Arch Dermatol. 1990;126(1):43-47. [PubMed] [Google Scholar]
- 7.Rzany B, Mockenhaupt M, Baur S, et al. Epidemiology of erythema exsudativum multiforme majus, Stevens-Johnson syndrome, and toxic epidermal necrolysis in Germany (1990-1992): structure and results of a population-based registry. J Clin Epidemiol. 1996;49(7):769-773. [DOI] [PubMed] [Google Scholar]
- 8.Sotozono C, Ueta M, Nakatani E, et al. ; Japanese Research Committee on Severe Cutaneous Adverse Reaction . Predictive factors associated with acute ocular involvement in Stevens-Johnson syndrome and toxic epidermal necrolysis. Am J Ophthalmol. 2015;160(2):228-237.e2. [DOI] [PubMed] [Google Scholar]
- 9.McCluskey J, Peh CA. The human leucocyte antigens and clinical medicine: an overview. Rev Immunogenet. 1999;1(1):3-20. [PubMed] [Google Scholar]
- 10.Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428(6982):486. [DOI] [PubMed] [Google Scholar]
- 11.McCormack M, Alfirevic A, Bourgeois S, et al. HLA-A*3101 and carbamazepine-induced hypersensitivity reactions in Europeans. N Engl J Med. 2011;364(12):1134-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ozeki T, Mushiroda T, Yowang A, et al. Genome-wide association study identifies HLA-A*3101 allele as a genetic risk factor for carbamazepine-induced cutaneous adverse drug reactions in Japanese population. Hum Mol Genet. 2011;20(5):1034-1041. [DOI] [PubMed] [Google Scholar]
- 13.Hung SI, Chung WH, Liou LB, et al. HLA-B*5801 allele as a genetic marker for severe cutaneous adverse reactions caused by allopurinol. Proc Natl Acad Sci U S A. 2005;102(11):4134-4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lonjou C, Borot N, Sekula P, et al. ; RegiSCAR study group . A European study of HLA-B in Stevens-Johnson syndrome and toxic epidermal necrolysis related to five high-risk drugs. Pharmacogenet Genomics. 2008;18(2):99-107. [DOI] [PubMed] [Google Scholar]
- 15.Tohkin M, Kaniwa N, Saito Y, et al. ; Japan Pharmacogenomics Data Science Consortium . A whole-genome association study of major determinants for allopurinol-related Stevens-Johnson syndrome and toxic epidermal necrolysis in Japanese patients. Pharmacogenomics J. 2013;13(1):60-69. [DOI] [PubMed] [Google Scholar]
- 16.Ueta M, Kaniwa N, Sotozono C, et al. Independent strong association of HLA-A*02:06 and HLA-B*44:03 with cold medicine-related Stevens-Johnson syndrome with severe mucosal involvement. Sci Rep. 2014;4:4862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ueta M, Kannabiran C, Wakamatsu TH, et al. Trans-ethnic study confirmed independent associations of HLA-A*02:06 and HLA-B*44:03 with cold medicine-related Stevens-Johnson syndrome with severe ocular surface complications. Sci Rep. 2014;4:5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ueta M, Sawai H, Sotozono C, et al. IKZF1, a new susceptibility gene for cold medicine-related Stevens-Johnson syndrome/toxic epidermal necrolysis with severe mucosal involvement. J Allergy Clin Immunol. 2015;135(6):1538-45.e17. [DOI] [PubMed] [Google Scholar]
- 19.Ueta M, Sotozono C, Inatomi T, Kojima K, Hamuro J, Kinoshita S. Association of IL4R polymorphisms with Stevens-Johnson syndrome. J Allergy Clin Immunol. 2007;120(6):1457-1459. [DOI] [PubMed] [Google Scholar]
- 20.Ueta M, Sotozono C, Inatomi T, Kojima K, Hamuro J, Kinoshita S. Association of combined IL-13/IL-4R signaling pathway gene polymorphism with Stevens-Johnson syndrome accompanied by ocular surface complications. Invest Ophthalmol Vis Sci. 2008;49(5):1809-1813. [DOI] [PubMed] [Google Scholar]
- 21.Ueta M, Sotozono C, Inatomi T, Kojima K, Hamuro J, Kinoshita S. Association of Fas ligand gene polymorphism with Stevens-Johnson syndrome. Br J Ophthalmol. 2008;92(7):989-991. [DOI] [PubMed] [Google Scholar]
- 22.Ueta M, Sotozono C, Tokunaga K, Yabe T, Kinoshita S. Strong association between HLA-A*0206 and Stevens-Johnson syndrome in the Japanese. Am J Ophthalmol. 2007;143(2):367-368. [DOI] [PubMed] [Google Scholar]
- 23.Ueta M, Tokunaga K, Sotozono C, et al. HLA class I and II gene polymorphisms in Stevens-Johnson syndrome with ocular complications in Japanese. Mol Vis. 2008;14:550-555. [PMC free article] [PubMed] [Google Scholar]
- 24.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 25.Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74(4):765-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nyholt DR. Evaluation of Nyholt’s procedure for multiple testing correction. Hum Hered. 2005;60(1):61-62. [DOI] [PubMed] [Google Scholar]
- 27.Salyakina D, Seaman SR, Browning BL, Dudbridge F, Muller-Myhsok B. Evaluation of Nyholt’s procedure for multiple testing correction. Hum Hered. 2005;60(1):19-25. [DOI] [PubMed] [Google Scholar]
- 28.Mondino BJ, Brown SI, Biglan AW. HLA antigens in Stevens-Johnson syndrome with ocular involvement. Arch Ophthalmol. 1982;100(9):1453-1454. [DOI] [PubMed] [Google Scholar]
- 29.Roujeau JC, Bracq C, Huyn NT, Chaussalet E, Raffin C, Duédari N. HLA phenotypes and bullous cutaneous reactions to drugs. Tissue Antigens. 1986;28(4):251-254. [DOI] [PubMed] [Google Scholar]
- 30.Roujeau JC, Huynh TN, Bracq C, Guillaume JC, Revuz J, Touraine R. Genetic susceptibility to toxic epidermal necrolysis. Arch Dermatol. 1987;123(9):1171-1173. [PubMed] [Google Scholar]
- 31.Matsuoka T, Narumiya S. Prostaglandin receptor signaling in disease. Scientific World Journal. 2007;7:1329-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ueta M, Matsuoka T, Narumiya S, Kinoshita S. Prostaglandin E receptor subtype EP3 in conjunctival epithelium regulates late-phase reaction of experimental allergic conjunctivitis. J Allergy Clin Immunol. 2009;123(2):466-471. [DOI] [PubMed] [Google Scholar]
- 33.Honda T, Matsuoka T, Ueta M, Kabashima K, Miyachi Y, Narumiya S. Prostaglandin E(2)-EP(3) signaling suppresses skin inflammation in murine contact hypersensitivity. J Allergy Clin Immunol. 2009;124(4):809-18.e2. [DOI] [PubMed] [Google Scholar]
- 34.Kaniwa N, Ueta M, Nakamura R, et al. Drugs causing severe ocular surface involvements in Japanese patients with Stevens-Johnson syndrome/toxic epidermal necrolysis. Allergol Int. 2015;64(4):379-381. [DOI] [PubMed] [Google Scholar]
- 35.Ueta M. Genetic predisposition to Stevens-Johnson syndrome with severe ocular surface complications. Cornea. 2015;34(suppl 11):S158-S165. [DOI] [PubMed] [Google Scholar]
- 36.Ueta M, Kinoshita S. Ocular surface inflammation is regulated by innate immunity. Prog Retin Eye Res. 2012;31(6):551-575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Association Analysis Between HLA Class 1 Types and SJS/TEN With SOC
eTable 2. HLA-A Analysis With Brazilian CM-SJS/TEN With SOC
eTable 3. HLA-B Analysis With Brazilian CM-SJS/TEN With SOC
eTable 4. HLA-C Analysis With Brazilian CM-SJS/TEN With SOC
eTable 5. Significant Thresholds of the HLA Alleles in the Permutation Test