ABSTRACT
Delivery of recombinant proteins to vegetative tissue vacuoles was considered inconvenient since this compartment was expected to be hydrolytic; nevertheless there is growing evidence that certain foreign proteins accumulate at high yields in vacuoles. For example avidin, cellulolytic enzymes, endolysin, and transglutaminases were produced at high yields when were sorted to leaf central vacuole avoiding the detrimental effect of these proteins on plant growth. Also, several secretory mammalian proteins such as collagen, α1-proteinase inhibitor, complement-5a, interleukin-6 and immunoglobulins accumulated at higher yields in leaf vacuoles than in the apoplast or cytosol. To reach this final destination, fusions to sequence specific vacuolar sorting signals (ssVSS) typical of proteases or proteinase inhibitors and/or Ct-VSS representative of storage proteins or plant lectins were used and both types of motifs were capable to increase accumulation. Importantly, the type of VSSs or position, either the N or C-terminus, did not alter protein stability, levels or pos-translational modifications. Vacuolar sorted glycoproteins had different type of oligosaccharides indicating that foreign proteins reached the vacuole by 2 different pathways: direct transport from the ER, bypassing the Golgi (high mannose oligosaccharides decorated proteins) or trafficking through the Golgi (Complex oligosaccharide containing proteins). In addition, some glycoproteins lacked of paucimannosidic oligosaccharides suggesting that vacuolar trimming of glycans did not occur. Enhanced accumulation of foreign proteins fused to VSS occurred in several plant species such as tobacco, Nicotiana benthamiana, sugarcane, tomato and in carrot and the obtained results were influenced by plant physiological state. Ten different foreign proteins fused to vacuolar sorting accumulated at higher levels than their apoplastic or cytosolic counterparts. For proteins with cytotoxic effects vacuolar sorted forms yields were superior than ER retained variants, but for other proteins the results were the opposite an there were also examples of similar levels for ER and vacuolar variants. In conclusion vacuolar sorting in vegetative tissues is a satisfactory strategy to enhance protein yields that can be used in several plant species.
KEYWORDS: Foreign proteins, leaf central vacuole, lytic vacuoles, proteolysis, plant molecular farming, secretory pathway, storage vacuoles, vegetative tissue, vacuolar sorting signals, vacuolar deposition
The production of high-value proteins in plant has become a reality with numerous products on the market.1 Plant based platforms have several advantages such as low upstream costs, no risk of contamination with human or animal pathogens, absence of bacterial toxins, easy and rapidly scale up with low investment cost, availability of different technology to reduce downstream process cost, etc. Foreign proteins can be produced by using either transient expression in leaves or transgenic expression systems in whole plants or plant cell culture.1 Both transient and stable systems are fully scalable and several large scale manufacture facilities are available, including those that satisfy good manufacturing practice (GMP).1 Recombinant protein yields are widely variable and depend on numerous factors such as plant species, promoter, enhancers, incorporation of intron sequences, mRNA stability, 5′ and 3′ untranslated regions, codon usage, protein folding and stability, etc.2,3 Different technologies have been developed to increase transcription efficiency, mRNA stability and translation effectiveness and to improve protein folding and stability.3,4 Among the post-translational factors, subcellular localization is of particular interest as it has a profound impact on protein yields.2 In leaves, some complex proteins are usually targeted to the apoplastic space where proteolysis can occur5-7; alternatively if Golgi post-translational modifications are not necessary to obtain a fully active molecule, they can be retained on the endoplasmic reticulum (ER).8 In leaves, an alternative destination is to target the recombinant protein to vacuoles. Plant vacuoles are multifunctional organelles, essential to plant life, which share some of their properties with the lysosomes in animal cells. Although plant vacuoles are lytic compartments, they also have unique functions such as reservoirs for ions and metabolites, plant defense, detoxification processes, general cell homeostasis, etc.9 In seeds and specialized tissues that evolve to store high amounts of proteins, special storage compartments called protein storage vacuole (PSV) or ER derived protein bodies (PB) are found.10 Foreign proteins sorted to these special compartments accumulate in large amounts and in stable forms for long periods of time.3,11 Deposition of recombinant proteins in central vacuoles of vegetative tissues has initially been considered inadequate since this compartment was expected to be hostile. For example, green fluorescent protein is unstable in the central vacuole of Arabidopsis thaliana leaves or cultured cells since light triggers vacuolar acidification and proteolysis by cysteine proteases.12 Despite the lytic characteristics of central vacuole, when seed storage proteins are ectopically expressed in leaf tissue, they are located in neutral vacuoles that resemble seed PSV.13 In addition, different types of vacuoles can be generated from existing vacuoles as a consequence of environmental changes14 or stage of development.15 Taking all these facts into consideration, vacuoles of vegetative tissue are highly dynamic structures whose characteristics are affected by environmental conditions, development programs and even ectopic deposition of proteins. Herein we discuss the current status of the employment of vacuolar delivery in vegetative tissues as a strategy to enhance heterologous protein yields. Accumulation of foreign protein in reproductive seed storage compartments has been reviewed elsewhere.3,11 Selected examples of vacuolar sorted recombinant proteins in vegetative tissue are presented in Table 1, and we shall highlight their particularities.
Table 1.
Impact of vacuolar targeting on vegetative tissue on recombinant protein yields.
| Protein |
Production Host |
Transformation Method |
Plant Organ |
Vacuolar Signal |
Yields of proteins sorted to different subcellular Localizations |
Level, stability or other relevant information |
References |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| ER | Vacuole | Apoplast | Cytosol | |||||||
| Egg white Avidin Streptavidin | Nicotiana tabacum (tobacco) | Stable | Leaves | Nt VSS (MESKFAHIIVFFLLATPF ETLLARKESDGPE) potato proteinase inhibitor I (PPI-I) | 1.5% | Avidin was found in protein body-like structures within the vacuole | Murray et al., 2002 16 | |||
| Egg white Avidin | Saccharum officinarum (sugarcane) | Stable | Stems (cane) | Nt ssVSS s legumain (LV) Nt VSS (PPI-I) (ΔV) | NR | NR | ΔV - avidin yield was higher than ER-avidin and LV-avidin ones. | Jackson et al., 2010 17 | ||
| Cellobiohydrolase I CBH I Cellobiohydrolase II CBH II Endoglucanase (EG) | Saccharum officinarum (sugarcane) | Stable | Green leaves | Ct VSS (DELKAEAK) barley polyamine oxidase | 2.38 AU/mg 5.92 AU/mg 210.1 ng/mg | 11.18 AU/mg 7.33 AU/mg 281.36 ng/mg | vacCBH II yield was 2- fold higher than ER-CBH II.CBH I and CBH II yields were reduced in senescent leaves and EG was not detected | Harrison et al., 2011 20Harrison et al., 2014 21 | ||
| Bacteriophage CP933 endolysin (EL) | Nicotiana benthamiana | Transient (PVX) | Leaves | Nt VSS potato proteinase inhibitor I (PPI-I) (ΔV) | NR | 0.6mg/g | NR | Cyto- EL produced severe growth detriment. Vac-EL had no toxic effect | Kovalskaya et al., 2016 22 | |
| Human tissue trans- glutaminase (TG2) | Nicotiana benthamiana | Transient | Leaves | Ct VSS (KISIA) Amaranth 11S globulin | 9.5 µg/g | 9.9 µg/g | 0.6 µg/g | 1.1 µg/g | ER-TG2 and vac-TG2 yields were 9- to 16-fold higher cyto-TG2 and sec-TG2 | Marin Viegas et al., 2015 23 |
| Glucocerebrosidase | Daucus carota subsp. sativus (carrot) | Stable | suspension culture cells | Ct VSS (DLLVDTM) Tobacco chitinase A | — | NR | — | Vacuolar glucocerebrosidase was stable | Shaaltiel et al., 2007 24 | |
| Collagen (rhCOL1) | Nicotiana tabacum (tobacco) | Stable | Leaves | Nt ssVSS (NPIRL) barley aleurain | — | 2% | NS | NS | vac- rhCOL1 yield was 10-fold higher than apo- and cyto- rhCOL1 ones. | Stein et al., 2009 25 |
| Silk-like protein (DP1B) | Arabidopsis thaliana | Stable | Leaves | Nt ssVSS (NPIRL) sweet potato sporamin | 6.7% | NS | 8.5% | Vacuolar DP1B was unstable | Yang et al 200526 | |
| human α1-proteinase inhibitor (α1-PI) | Solanum lycopersicum (tomato) | Stable | Leaves | Nt ssVSS (NPIRL) sweet potato sporamin | 3.05% | 1.89% | 1.40% | 0.458% | ER- α1-PtdIns yield was 1.6-fold- higher than vac- α1-PI yield | Jha et al., 2012 27 |
| Human complement 5a C5a | Nicotiana tabacum (tobacco) | Stable | Leaves | Ct VSS (AFVY) Phaseolin 7S globulin | 0.0003% | 0.001% | 0.0002% | Vac C5a yield was 3 to 5-fold higher in ER-C5a and Apo-C5a | Nausch et al., 2012 28 | |
| Human Complement 5a C5a | Nicotiana benthamiana | Transient | Leaves | Ct VSS (AFVY) Phaseolin 7S globulin | 0.2% | 0.7% 558 µg/g | Vac C5a yield was 3.5-fold higher than ER-C5a yield | Nausch et al., 2012 28 | ||
| Interleukin 6 (IL6) | Nicotiana tabacum (tobacco) | Stable | Leaves | Ct VSS (AFVY) Phaseolin 7S globulin | 0.005% | 0.0008% | 0.0005% | ER-IL6 yield was 625 fold higher than vac-IL6 yield | Nausch et al., 2012 29 | |
| Human Ig G1 and G4 | Nicotiana tabacum (tobacco) | Stable | BY2 cells | Ct VSS (DLLVDTM) Tobacco chitinase A | NR | NR | NR | Apo-IgG yield was higher than ER- and vac-IgG ones. | Shaaltiel et al., 2012 30Tekoah et al.2015 31 | |
| Mouse IgG | Nicotiana tabacum (tobacco) | Stable | BY2 cells | Nt ssVSS (NPIRL) sweet potato sporamin | — | 28.5-80 ng/g | — | — | IgG exhibit paucimannose glycan structure | Misaki et al., 2011 32 |
| Mouse Ig G1 | Nicotiana benthamiana | Transient | Leaves | Ct VSS (KISIA) and ssVSS (NIFRGF) Amaranth 11S globulin | 1.7% | 1.6% | 0.13% | NR | ER- and vac-Abs yields were 10-15-fold higher than sec-Ab | Ocampo et al., 2016 33 |
Foreign proteins were grouped based on their characteristics in: I) cytotoxic or with detrimental effect on plant growth (light blue), II) fibrous proteins (yellow). III) secretory mammalian protein (pink). Ct, COOH terminus, Nt, NH2 terminus, VSS: vacuolar sorting signal, LV: lytic vacuole, ΔV: delta vacuole, %: g. protein of interest per 100 g total soluble protein, NR no reporter, NS: no stable, AU: arbitrary units.
Production of egg white avidin or streptavidin in plants is of interest since they are efficient biocontrol agents.16 Avidin binds with very high affinity to biotin, which impairs the activity of carboxylases, enzymes that are essential for cellular metabolism in different organisms including insects and plants. Taking into account that 80% of biotin pools of plant cells are located in the cytoplasm and the rest in the mitochondria and chloroplast, delivery of avidin to vacuole was hypothesized as a safe strategy to avoid detrimental effects caused by biotin sequestration. Avidin and streptavidin were expressed in transgenic Nicotiana tabacum fused to the NH2 terminus (Nt) vacuolar sorting signal (VSS) MESKFAHIIVFFLLATPFETLLARKESDGPE of potato proteinase inhibitor I (PPI-I) that is sufficient to target to vacuoles that have δ-TIP on their tonoplast defined as ΔV.14 ΔV–sorted avidin yields in leaves were around 1.5 % TSP and remained relatively constant throughout leaf lifetime. Avidin was detected in protein body-like structures within the vacuole. Plants had a normal phenotype and produced fertile pollen and seeds.16 Furthermore, avidin was also fused to a different type of VSS: sugarcane legumain sequence specific (ssVSS) that targets to lytic vacuole (LV). The expression was analyzed in transgenic sugarcane.17 The highest avidin levels in leaves, stem and roots were found for the ΔV sorted version, compared to the LV, ER, apoplast or cytosol targeted variants, but these plants developed a biotin deficient phenotype. In contrast, sugarcane plants that expressed LV-avidin had a normal phenotype but avidin suffered a site-specific limited proteolysis.17 Therefore, sugarcane ΔV was shown to be a stable environment for recombinant protein accumulation. It is worth noticing that the co-existence of 2 different types of vacuoles in the same cells has been described in a limited number of cell types18; sugarcane has the unusual capacity to accumulate sucrose in stem cell vacuoles and contains several types of vacuoles that differ in their pH and capacity to hydrolyze different substrates.19 Unlike sugarcane, N. tabacum leaves does not specialize in storage. However, they were able to accumulate ΔV–sorted avidin in a stable form.
A further example of stable deposition of proteins in sugarcane vacuoles is the cellulolytic enzyme that also needs to be compartmentalized to avoid interference with the cell wall structure. The production of this enzyme is of interest to make cost-competitive cellulosic ethanol. Fungal cellobiohydrolase (CBH) I and II and bacterial endoglucanase (EG) accumulated to higher levels when fused to the barley polyamine oxidase Ct VSS compared to the fusion to an ER-retention signal.20 Yields of CBH I, CBH II and EG were reduced in senescent leaves probably due to endo- and exo-peptidases released during leaf senescence.20,21 These results emphasize the importance of the development stage for a foreign protein deposition in leaves.
Another toxic protein that was successfully expressed in Nicotiana benthamiana leaves is the bacteriophage CP933 endolysin (EL), an enzyme that hydrolyzes peptidoglycan. This feature makes EL a promising antimicrobial agent for antibiotic-resistant microorganism. EL was targeted to ΔV by fusion to Nt VSS of PPI-I. Plants producing the ΔV-EL did not exhibit the severe detrimental effects on growth found in cytosolic-EL plants. This result suggests that sequestration of EL in the vacuole reduces its toxicity.22
Transglutaminases 2 (TG2) are also challenging proteins for the different expression systems since their cross-linking activity has toxic effects on cell growth and development.34 Attempts to produce transgenic BY-2 expressing cytosolic-TG2 were unsuccessful, probably due to the toxic effect of this enzyme.35 We have recently shown, by using transient expression in Nicotiana benthamiana leaves, that ER-TG2 and vac-TG2 yields are 9 to 16-fold higher than cytosolic and secretory versions.23 Therefore, compartmentalization of TG within the endomembrane systems avoids cytosolic toxicity and also apoplastic proteolysis.
Glucocerebrosidase is an acid-β-glucosidase used in enzyme replacement therapy for Gaucher's disease, a rare lysosomal storage disorder. The manufacture cost of this enzyme in other expression systems was very high; therefore, Protalix Biotherapeutics developed a technology to produce it in carrot suspension culture. Two variants were produced by fusion to the Ct-VSS from tobacco chitinase A (DLLVDTM) and also to an ER retention sequence. Vacuolar glucocerebrosidase yields were higher than ER variants. In addition, paucimannose glycan structures in vacuolar glucocerebrosidase favored mannose receptor-mediated uptake by macrophages which made this variant more effective therapeutically than the ER version.24
Deposition of proteins with the ability to produce fibers on vacuoles of vegetative tissues has also been assayed. For example, human collagen type I (rhCOL1) is a heterotrimeric protein that requires essential posttranslational modifications to self-organize into fibers. These modifications are performed by human prolyl-4-hydroxylases (P4H) and lysyl hydroxylase 3 (LH3). The genes encoding for rhPCOL1 α 1 and α 2 chains, P4H α, P4H β, and LH3 were expressed in transgenic tobacco plants using different targeting signals to sort to vacuoles (barley aleurain Nt ssVSS MAHARVLLLALAVLATAAVAVASSSSFADSNPIRPVTDRAASTLA), apoplast or cytosol.25 Cytoplasm sorted rhCOL1 was not detectable, while apoplast-targeted rhCOL1 yields were very low. Vac-rhCol1 yields were the highest, and molecules were able to form stable triple helical structures that were fully functional in inducing proliferation of human cells.25 These results highlight that leaf vacuoles are a suitable compartment to store rhCOL1. Another fibrous protein: the spider dragkine silk (DP18) was also fused to a ssVSS: the NPIRL from sporamine. Different sorted version were expressed in transgenic A. thaliana. However, in this case only the ER variant accumulated at high levels while vac-DP18 was not stable.26
Moreover, different biopharmaceuticals proteins have been produced successfully in vacuoles of vegetative tissues. Human α 1-proteinase inhibitor (α1-PI), also known as α1- antitrypsin, is a serine protease inhibitor essential to keep lung elasticity. The production of a glycosylated biologically active α1-PI has been assayed in different systems, but none of them could fulfill the requirements of cost-effective production, clinical safety and biological activity. Consequently, this protein was expressed in Solanum lycopersicum (tomato) by using different sorting signal to target to cytosol, apoplast, ER and vacuole [Nt ssVSS (NPIRL) sweet potato sporamine]. The highest average yields in T1 progeny were 3.05 % TSP for ER, 1.89% for vacuolar, 1.40% for apoplast and 0.08 % for cytosolic forms. Although vacuolar α1-PI was produced in tomato leaves with comparable yields respect to the ER form, the enzyme exhibit lower specific activity.27
Another example of therapeutic protein sorted to vacuoles is human complement factor 5a (C5a) that was expressed in leaves and seeds of transgenic N. tabacum. The Ct VSS AFVY of phaseolin 7S storage protein was used to target C5a to the vacuole. Vac-C5a yields were 3 to 5-fold higher than ER- or apo-C5a. These C5a versions were also transiently expressed in Nicotiana benthamiana leaves using an hybrid binary vector (MagnICON) based on tobacco mosaic virus (TMV) that contains viral sequences required for RNA replication leading to amplification of RNA transcript, and also the highest yields were detected for vac-C5a variants (3.5-fold higher than ER).28 Therefore vacuoles were found as the most suitable compartment to produce C5a, and the higher yields were attributed to the selection of the AFVY Ct, which is considered a PSV-specific targeting signal.28 The authors argue that although lytic vacuoles are expected to be prevalent in vegetative tissues, the expression of storage protein derived sequence could induce the formation of storage organelles in vegetative tissue.28 Unexpected transient overexpression of ER-C5a and vac-C5a in Nicotiana benthamiana was accompanied by cytotoxic effects and a rapid decrease of recombinant C5a even though it is not anticipated that this protein could interfere with plant metabolism.28 Due to toxic effect, yields for vac-C5a (0,7%TSP) were higher than ER-C5a (0.2% TSP) but for non toxic protein using MagnICON system around 10% TSP were expected. Using the same sorting strategy, human interleukin (IL) 6 was expressed in stable transgenic N. tabacum and temporally in Nicotiana benthamiana, but ER sorted-IL6 produced yields 6.25 higher than vac-IL6, although IL6 was also fused to AFVY Ct VSS.29 ER-targeted IL6 in leaves using the MagnICON system resulted in yields of up to 7% TSP and none cytotoxic effect were observed.29
Deposition of antibodies (Abs) in vacuoles of vegetative tissues had also been studied and information about trafficking and modifications in different compartments was obtained based on its N-glycosylation pattern. The N-glycosylation of proteins starts in the ER with the transfer of the Glc3Man9GlcNAc2 oligosaccharide to a specific Asn residues on the nascent polypeptide followed of a limited trimming in both the ER and Golgi and sequential addition of monosaccharides, as the protein travel through the Golgi complex, to yield complex N-glycans, typically GlcNAc2Man3FucXylGlcNAc2 structures (Fig. 1).36 Secretory plant N-glycans contain galactose β 1,3 and fucose α 1,4 linked to the terminal GlcNAc forming the called Lewis A oligosaccharide structure.36 In addition, paucimannosidic, that derives from the removal of terminal GlcNAc residues from complex N-glycans, are present in vacuolar and secreted proteins.36-38 Humans IgG1 and IgG4 were expressed, in transgenic suspension-cultured of tobacco BY2, sorted to different compartments, resulting in secretory versions producing higher yields than ER and vacuolar versions.30,31 In addition, a mouse IgG fused to the sporamin Nt ssVSS (NPIRL) was produced also in transgenic BY2 cells, in intact form at levels of 8.5-80 ng/g and paucimannose Man3FucXylGlcNAc2 as main N-glycan structure.32 We had also produced a vacuolar mouse IgG1 by transient expression in Nicotiana benthamiana leaves. To target the Ab to vacuoles, the heavy chain was fused to 2 sequences derived from amaranth 11S storage protein: KISIA Ct VSS (vac1-Ab) and NIFRGF ssVSS (vac2-Ab), and as control ER-Ab and sec-Ab variants were produced. ER-Ab and vac-Abs accumulations levels were 10-15-fold higher than sec-Ab.33 Although NPIRL motif is typical of lytic vacuole proteins and the short and hydrophobic C terminus are distinctive of storage proteins39, no significant differences were found between vac1-Ab and vac2-Abs yields. Another important finding of our work, was the presence of oligomannosidic (Man 7-9) as the major glycoform in vac-Abs (75%), what suggests a direct transport from the ER to vacuoles bypassing the Golgi apparatus.33 Furthermore vac-Abs have 25% of GlcNAc2Man3XylFucGlcNAc2 therefore removal of terminal GlcNAc residues in the vacuole did not occur.33
Figure 1.
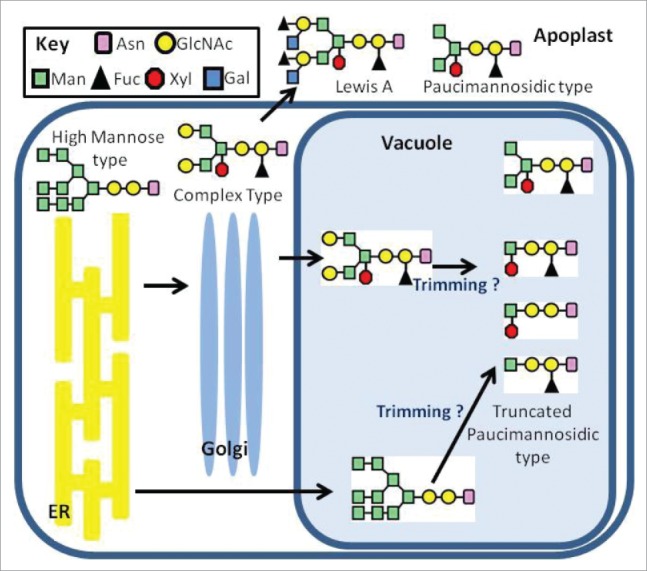
Schematic representation of the plant N-glycans processing pathway. The arrows indicate the trafficking pathways. N-glycosylation of vacuolar proteins suggests a direct ER-vacuole transport route bypassing the Golgi apparatus, and also the classical Golgi-dependent pathway. ER, endoplasmic reticulum; Asn, asparagine; GlcNAc, N-acetylglucosamine Man, mannose; Fuc, fucose; Xyl, xylose ; Gal, galactose. High-mannose type: Man 9 Man9GlcNAc2; Man 8: Man8GlcNAc2. Man 7: Man7GlcNAc2 oligosaccharides. Complex type: GlcNAc2Man3GlcNAc2 and GlcNAc2Man3XylFucGlcNAc2 oligosaccharides; Lewis (A) GalFucGlcNAc2Man3XylFucGlcNAc2 oligosaccharide. Paucimannosidic type: Man3XylFucGlcNAc2; ManXylFucGlcNAc2, ManXylGlcNAc2, ManFucGlcNAc2 oligosaccharides.
Ability of plants cells to accumulate toxic proteins in vacuoles is not surprising since variety of natural and synthetic chemicals are inactivated and transported to vacuole by different detoxification mechanisms.40 For example, some xenobiotic compounds are conjugated to glutathione in the cytosol and then transported to vacuole by an ATP-dependent tonoplast transporter.40 Plant secondary metabolites, such as flavonoids are also delivery to vacuoles using tonoplast transporters, but for anthocyanins a transport mediated by vesicle trafficking has also been described.41 Anthocyanins are uploaded into the ER compartment by membrane translocators, followed by an ER to vacuole transport either by a direct route (bypassing Golgi) or by Golgi dependent pathway.41
From the 15 vegetative vacuole-sorted proteins listed in Table 1, accumulation levels of variants fused to different targeting signals, were informed only for 8 and the results are summarized in Fig. 2. Vacuolar sorted variants had yields 3,0-9,0 and 1,2-16,5-fold-times higher than their cytosolic or apoplastic counterparts, respectively. Although these values are based on a reduced number of proteins (5), other proteins in Table 1 such as rhCol1, EL, CBH I, CBH II and EG had the same behavior, but apoplast or cytosolic yields were not reported due to instability or low levels (Table 1). Therefore for 10 proteins the fusion to vacuolar sorting signals enhanced the production of recombinant proteins. The impact of vacuolar versus ER location on foreign protein accumulation was variable. For proteins that produced detrimental effect in cellular metabolism, such as CBH II, EG, C5a and CBH I, vacuolar sorted forms yields were 1,2-4,7 higher than ER retained variants. In contrast, ER-IL6- and ER-α1-PI had higher accumulation levels than their vacuolar counterparts. Vacuolar- and ER- sorted forms of mouse IgG1- and TG2 had equal protein yields. These results indicate that a vacuolar sorting strategy is superior to apoplastic or cytosolic targeting, and could be also better that ER retention.
Figure 2.
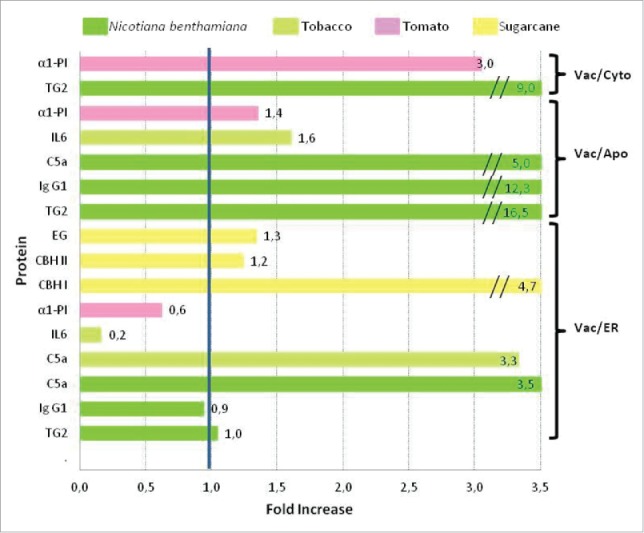
Comparison of yields obtained when proteins were sorted to different compartments in the secretory pathway. X axis represents the ratio of yields obtained for vacuolar-/ER-versions (Vac/ER), vacuolar-/secreted-forms (Vac/apo) or vacuolar-/cytosolic-variants (Vac/cyto). The obtained value are shown in each bar. The X axis has a maximum value of 3.5 and higher values are not to at scale. The bar color represents the plant species used to express the different proteins showed in the Y axis. α1-PI: human α1-proteinase inhibitor, TG2: Human tissue transglutaminase, IL6: Interleukin 6, C5a: Human complement 5a, IgG1: Immunoglobulin G1, EG: Endoglucanase, CBH I: Cellobiohydrolase I , CBH II: Cellobiohydrolase II.
To target foreign proteins to vacuoles, different signals have been used located either in the N or C terminus, including a NPIR/NPIXL sequence specific motif typical of protease inhibitors or vacuolar proteases [Nt-ssVSS of barley aleurain, legumain, and sweet potato sporamin] or short-hydrophobic Ct characteristic of chitinases, cereal lectins or storage proteins (Ct-VSS of phaseolin 7S globulin, amaranth 11S protein, barley polyamine oxidase and tobacco chitinase A).42 Both types of VSSs were demonstrated to be useful to maximize recombinant protein levels. Although Nt-VSS and Ct-VSS were supposed to target proteins to lytic and storage vacuoles, respectively, both type of motif targeted proteins to central vacuole of vegetative tissue by a molecular mechanism that is currently unclear.43 The N-glycosylation pattern of the foreign exemplified differences in vacuolar sorting mechanism, for example glucocerebrosidase-Ct-VSS exhibited paucimannose structures and complex glycan added in the trans Golgi; supporting a Golgi dependent transport24 while a mouse IgG1 fused to a ssVSS and Ct-VSS of amaranth storage proteins is decorated with Man 7 and Man 8 glycans supporting a direct transport bypassing the Golgi33 (Fig. 1) These glycosylation patterns maybe adequate for some foreign proteins such as glucocerebrosidase whose vacuolar variant is easily internalized by human cells, but it is no convenient for therapeutic antibodies since effectors' functions are dependent of heavy chain glycosylation. Nevertheless vacuolar sorted antibodies could be useful for diagnostic, purification and other research applications.
Table 1 and Fig. 2 also showed also that vacuolar targeting is an effective strategy to produce high yields of intact and fully active proteins in several plant species such as Nicotiana benthamiana, tobacco, tomato, sugarcane and carrot. The only species that showed unsatisfactory results was arabidopsis. Other important conclusion is that the accumulation levels of vacuolar sorted foreign proteins were dependent of the developmental stage and physiological condition of leaves, therefore to achieve high yields samples should be collected prior senescence.20,21
In conclusion, vacuolar sorting in vegetative plant tissues is a satisfactory strategy to enhance protein yields and the obtained results are superior than targeting to cytosol or to apoplast an could be also better than ER retention for cytotoxic proteins. For recombinant glycosylated proteins will be desirable to have a better understanding of the mechanism that control vacuolar delivery by the different targeting routes in order to predict glycosylation pattern.
Funding Statement
This research was supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) through the grants PICT Start Up- 2015-0010 and by Universidad Nacional de La Plata (grant 11X/630). VSMV and CGO are fellows at CONICET. SP is researcher from CONICET and Professor of the Facultad de Ciencias Exactas-UNLP.
Abbreviations
- Ab
monoclonal antibody
- CBH I
Cellobiohydrolase I
- CBH II
Cellobiohydrolase II
- Ct
COOH terminus
- C5a
human complement 5a
- DP1B
silk-like protein
- EG
endoglucanase
- EL
bacteriophage CP933 endolysin
- ER
endoplasmic reticulum
- GMP
good manufacturing practice
- IL6
interleukin 6
- IgG
immunoglobulin
- LV
lytic vacuole
- Nt
NH2 terminus
- PB
protein bodies
- PSV
protein storage vacuole
- PPI-I
potato proteinase inhibitor I
- rhCOL1
human collagen type I
- ssVSS
sequence specific VSS
- TG2
human tissue transglutaminase
- TIP
tonoplast intrinsic proteins
- TSP
total soluble protein
- VSS
vacuolar sorting signal
- α1-PI
human α1-proteinase inhibitor
- ΔV
delta vacuole
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Author Contributions
VSMV; CGO and SP wrote the paper. Authors contributed equally to this work.
References
- [1].Sack M, Hofbauer A, Fischer R, Stoger E. The increasing value of plant-made proteins. Curr Opin Biotechnol 2015; 32:163-70; PMID:25578557; https://doi.org/ 10.1016/j.copbio.2014.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Egelkrout E, Rajan V, Howard JA. Overproduction of recombinant proteins in plants. Plant Sci 2012; 184:83-101; PMID:22284713; https://doi.org/ 10.1016/j.plantsci.2011.12.005 [DOI] [PubMed] [Google Scholar]
- [3].Yao J, Weng Y, Dickey A, Wang KY. Plants as factories for human pharmaceuticals: Applications and challenges. Int J Mol Sci 2015; 16:28549-65; PMID:26633378; https://doi.org/ 10.3390/ijms161226122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Gleba YY, Tusé D, Giritch A. Plant viral vectors for delivery by agrobacterium. Curr Top Microbiol Immunol. 2014;375:155-92. doi: 10.1007/82_2013_352. Review. PubMed PMID: 23949286.21895943 [DOI] [PubMed] [Google Scholar]
- [5].Goulet C, Khalf M, Sainsbury F, D'Aoust MA, Michaud D. A protease activity-depleted environment for heterologous proteins migrating towards the leaf cell apoplast. Plant Biotechnol J 2012: 10:83-94; PMID:21895943; https://doi.org/ 10.1111/j.1467-7652.2011.00643.x [DOI] [PubMed] [Google Scholar]
- [6].Pillay P, Schluter U, Van Wyk S, Kunert KJ, Vorster BJ. Proteolysis of recombinant proteins in bioengineered plant cells. Bioengineered 2014; 5:15-20; PMID:23778319; https://doi.org/ 10.4161/bioe.25158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Benchabane M, Goulet C, Rivard D, Faye L, Gomord V, Michaud D. Preventing unintended proteolysis in plant protein biofactories. Plant Biotechnol J 2008: 6:633-48; PMID:18452504; https://doi.org/ 10.1111/j.1467-7652.2008.00344.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hood E, Cramer C, Medrano G, Xu J. Protein targeting: Strategic planning for optimizing protein products through plant biotechnology. In A. Altman and P.M. Hasegawa (Eds.), Plant Biotechnology and Agriculture, pp. 35–54. San Diego, CA: Academic Press. ISBN 9780123814661; https://doi.org/ 10.1016/B978-0-12-381466-1.00003-1. [DOI] [Google Scholar]
- [9].Marty F. Plant vacuoles. Plant Mol Biol 1998: 11:587-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Herman EM, Larkins BA. Protein storage bodies and vacuoles. Plant Cell 1999; 11:601-14; PMID:10213781; https://doi.org/ 10.1105/tpc.11.4.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takaiwa F. Increasing the production yield of recombinant protein in transgenic seeds by expanding the deposition space within the intracellular compartment. Bioengineered 2013; 4:136-9; PMID:23563599; https://doi.org/ 10.4161/bioe.24187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tamura K, Shimada T, Ono E, Tanaka Y, Nagatani A, Higashi SI, Watanabe M, Nishimura M, Hara-Nishimura I. Why green fluorescent fusion proteins have not been observed in the vacuoles of higher plants. Plant J 2003: 35:545-55; PMID:12904216; https://doi.org/ 10.1046/j.1365-313X.2003.01822.x [DOI] [PubMed] [Google Scholar]
- [13].Park M, Kim SJ, Vitale A, Hwang I. Identification of the protein storage vacuole and protein targeting to the vacuole in leaf cells of three plant species. Plant Physiol 2004; 134:625-39; PMID:14730078; https://doi.org/ 10.1104/pp.103.030635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jauh GY, Fischer AM, Grimes HD, Ryan CA Jr., Rogers JC. Delta tonoplast intrinsic protein defines unique plant vacuole functions. Proc. Natl. Acad. Sci. USA 1998: 95:12995-9; https://doi.org/ 10.1073/pnas.95.22.12995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jauh G-Y, Phillips TE, Rogers JC. Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 1999; 11:1867-82; PMID:10521518; https://doi.org/ 10.1105/tpc.11.10.1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Murray C, Sutherland PW, Phung MM, Lester MT, Marshall RK, Christeller JT. Expression of biotin-binding proteins, avidin and streptavidin, in plant tissues using plant vacuolar targeting sequences. Transgenic Res 2002: 11:199-214; PMID:12054353; https://doi.org/ 10.1023/A:1015237610263 [DOI] [PubMed] [Google Scholar]
- [17].Jackson MA, Nutt KA, Hassall R, Rae AL. Comparative efficiency of subcellular targeting signals for expression of a toxic protein in sugarcane. Funct Plant Biol 2010; 37:785-93; https://doi.org/ 10.1071/FP09243 [DOI] [Google Scholar]
- [18].Frigerio L, Hinz G, Robinson DG. Multiple vacuoles in plant cells: Rule or exception? Traffic 2008: 9:1564-70; PMID:18537999; https://doi.org/ 10.1111/j.1600-0854.2008.00776.x [DOI] [PubMed] [Google Scholar]
- [19].Rae AL, Jackson MA, Nguyen CH, Bonnett GD. Functional specialization of vacuoles in sugarcane leaf and stem. Trop Plant Biol 2009; 2:13-22; https://doi.org/ 10.1007/s12042-008-9019-9 [DOI] [Google Scholar]
- [20].Harrison MD, Geijskes J, Coleman HD, Shand K, Kinkema M, Palupe A, Hassall R, Sainz M, Lloyd R, Miles S, et al.. Accumulation of recombinant cellobiohydrolase and endoglucanase in the leaves of mature transgenic sugar cane. Plant Biotechnol J 2011: 9:884-96; PMID:21356003; https://doi.org/ 10.1111/j.1467-7652.2011.00597.x [DOI] [PubMed] [Google Scholar]
- [21].Harrison MD, Geijskes RJ, Lloyd R, Miles S, Palupe A, Sainz MB, Dale JL. Recombinant cellulase accumulation in the leaves of mature, vegetatively propagated transgenic sugarcane. Mol Biotechnol 2014; 56:795-802; PMID:24793894; https://doi.org/ 10.1007/s12033-014-9758-9 [DOI] [PubMed] [Google Scholar]
- [22].Kovalskaya N, Foster-Frey J, Donovan DM, Bauchan G, Hammond RW. Antimicrobial activity of bacteriophage endolysin produced in nicotiana benthamiana plants. J Microbiol Biotechnol 2015: 26:160-70; https://doi.org/ 10.4014/jmb.1505.05060 [DOI] [PubMed] [Google Scholar]
- [23].Marin Viegas VS, Acevedo GR, Bayardo MP, Chirdo FG, Petruccelli S. Production of the main celiac disease autoantigen by transient expression in nicotiana benthamiana. Front Plant Sci 2015; 6:1067; PMID:26648956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shaaltiel Y, Bartfeld D, Hashmueli S, Baum G, Brill-Almon E, Galili G, Dym O, Boldin-Adamsky SA, Silman I, Sussman JL, et al.. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of gaucher's disease using a plant cell system. Plant Biotechnol J 2007: 5:579-90; PMID:17524049; https://doi.org/ 10.1111/j.1467-7652.2007.00263.x [DOI] [PubMed] [Google Scholar]
- [25].Stein H, Wilensky M, Tsafrir Y, Rosenthal M, Amir R, Avraham T, Ofir K, Dgany O, Yayon A, Shoseyov O. Production of bioactive, post-translationally modified, heterotrimeric, human recombinant type-i collagen in transgenic tobacco. Biomacromolecules 2009; 10:2640-5; PMID:19678700; https://doi.org/ 10.1021/bm900571b [DOI] [PubMed] [Google Scholar]
- [26].Yang J, Barr LA, Fahnestock SR, Liu ZB. High yield recombinant silk-like protein production in transgenic plants through protein targeting. Transgenic Res 2005: 14:313-24; PMID:16145839; https://doi.org/ 10.1007/s11248-005-0272-5 [DOI] [PubMed] [Google Scholar]
- [27].Jha S, Agarwal S, Sanyal I, Jain GK, Amla DV. Differential subcellular targeting of recombinant human alpha 1-proteinase inhibitor influences yield, biological activity and in planta stability of the protein in transgenic tomato plants. Plant Sci 2012; 196:53-66; PMID:23017899; https://doi.org/ 10.1016/j.plantsci.2012.07.004 [DOI] [PubMed] [Google Scholar]
- [28].Nausch H, Mischofsky H, Koslowski R, Meyer U, Broer I, Huckauf J. Expression and subcellular targeting of human complement factor c5a in nicotiana species. PLoS One 2012; 7:e53023; PMID:23285250; https://doi.org/ 10.1371/journal.pone.0053023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Nausch H, Mikschofsky H, Koslowski R, Meyer U, Broer I, Huckauf J. High-level transient expression of er-targeted human interleukin 6 in nicotiana benthamiana. PLoS One 2012; 7:e48938; PMID:23152824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shaaltiel Y, Hashmueli S, Bartfeld D, Baum G, Ratz T, Mizrachi E, Forster Y. System and method for production of antibodies in plant cell culture. Patent no.: US 8119406 B2/2012. [Google Scholar]
- [31].Tekoah Y, Shulman A, Kizhner T, Ruderfer I, Fux L, Nataf Y, Bartfeld D, Ariel T, Gingis–Velitski S, Hanania U, et al.. Large-scale production of pharmaceutical proteins in plant cell culture—the protalix experience. Plant Biotechnol J 2015; 13:1199-208; PMID:26102075; https://doi.org/ 10.1111/pbi.12428 [DOI] [PubMed] [Google Scholar]
- [32].Misaki R, Sakai Y, Omasa T, Fujiyama K, Seki T. N-terminal vacuolar sorting signal at the mouse antibody alters the n-linked glycosylation pattern in suspension-cultured tobacco by2 cells. J Biosci Bioeng 2011; 112:476-84; PMID:21802986; https://doi.org/ 10.1016/j.jbiosc.2011.07.002 [DOI] [PubMed] [Google Scholar]
- [33].Ocampo CG, Lareu F, Marin Viegas VS, Mangano S, Loos A, Steinkellner H, Petruccelli S. Vacuolar targeting of recombinant antibodies in nicotiana benthamiana. Plant Biotechnol J 2016. E-Pub ahead of print. https://doi.org/ 10.1111/pbi.12580; PubMed PMID: 27159528.12366374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Griffin M, Casadio R, Bergamini CM. Transglutaminases: Nature's biological glues. Biochem J 2002; 368:377-96; PMID:12366374; https://doi.org/ 10.1042/bj20021234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sorrentino A, Schillberg S, Fischer R, Rao R, Porta R, Mariniello L. Recombinant human tissue transglutaminase produced into tobacco suspension cell cultures is active and recognizes autoantibodies in the serum of coeliac patients. Int J Biochem Cell Biol 2005; 37:842-51; PMID:15694843; https://doi.org/ 10.1016/j.biocel.2004.11.001 [DOI] [PubMed] [Google Scholar]
- [36].Gomord V, Fitchette A, Menu-Bouaouiche L, Saint-Jore-Dupas C, Plasson C, Michaud D, Faye L. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol J 2010; 8:564-87; PMID:20233335; https://doi.org/ 10.1111/j.1467-7652.2009.00497.x [DOI] [PubMed] [Google Scholar]
- [37].Schneider J, Castilho A, Pabst M, Altmann F, Gruber C, Strasser R, Gattinger P, Seifert GJ, Steinkellner H. Characterization of plants expressing the human beta 1,4-galactosyltrasferase gene. Plant Physiol Biochem 2015; 92:39-47; PMID:25900423; https://doi.org/ 10.1016/j.plaphy.2015.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Liebminger E, Veit C, Pabst M, Batoux M, Zipfel C, Altmann F, Mach L, Strasser R. Beta-n-acetylhexosaminidases hexo1 and hexo3 are responsible for the formation of paucimannosidic n-glycans in arabidopsis thaliana. J Biol Chem 2011; 286:10793-802; PMID:21252225; https://doi.org/ 10.1074/jbc.M110.178020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Matsuoka K, Neuhaus J. Cis-elements of protein transport to the plant vacuoles. J Exp Bot 1999; 50:165-74; https://doi.org/ 10.1093/jxb/50.331.165 [DOI] [Google Scholar]
- [40].Coleman J, Blake-Kalff M, Davies E. Detoxification of xenobiotics by plants: Chemical modification and vacuolar compartmentation. Trend Plant Sci 1997; 2:144-51; https://doi.org/ 10.1016/S1360-1385(97)01019-4 [DOI] [Google Scholar]
- [41].Petrussa E, Braidot E, Zancani M, Peresson C, Bertolini A, Patui S, Vianello A. Plant flavonoids- biosynthesis, transport and involvement in stress responses. Int J Mol Sci 2013; 14:14950-73; PMID:23867610; https://doi.org/ 10.3390/ijms140714950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Matsuoka K, Neuhaus JM. Cis-elements of protein transport to the plant vacuoles. J Exp Bot 1999; 50:165-74; https://doi.org/ 10.1093/jxb/50.331.165 [DOI] [Google Scholar]
- [43].Robinson DG, Pimpl P. Receptor-mediated transport of vacuolar proteins: A critical analysis and a new model. Protoplasma 2014; 251:247-64; PMID:24019013; https://doi.org/ 10.1007/s00709-013-0542-7 [DOI] [PubMed] [Google Scholar]


