Abstract
IL-2 is a pleiotropic cytokine that promotes the differentiation of T helper (Th) cell subsets including Th1, Th2, and Th9 cells, but impairs development of Th17 and Tfh cells. Although IL-2 is produced by all polarized Th subsets to some level, how it impacts cytokine production when effector T cells are restimulated is unknown. We show here that golgi transport inhibitors (GTI) blocked IL-9 production. Mechanistically, GTIs blocked secretion of IL-2 that normally feeds back in a paracrine manner to promote STAT5 activation and IL-9 production. IL-2 feedback had no effect on Th1 or Th17-signature cytokine production, but promoted Th2- and Th9-associated cytokine expression. These data suggest that use of GTI result in an underestimation of the presence of type 2 cytokine secreting cells, and highlight IL-2 as a critical component in optimal cytokine production by Th2 and Th9 cells in vitro and in vivo.
Introduction
Allergic inflammation is orchestrated by a CD4 T cell response that is dominated by interleukin (IL)-4, -5, and -13-secreting Th2 cells and IL-9-secreting Th9 cells. In combination, these cytokines act to promote mucus production by goblet cells, IgE class switching in B cells, and the recruitment of innate immune cells (eosinophils, mast cells) that further augment inflammation. Th2/Th9-associated cytokines also play a critical role in parasite immunity and clearance (1, 2).
IL-2, through STAT5 activation, promotes the differentiation of Th subsets including Th1, Th2, and Th9 cells, but impairs development of Th17 and Tfh cells (3-8). IL-2 alters differentiation both by direct effects on cytokine loci and by activating transcription factors that promote differentiation (4, 6). However, differentiated Th subsets all produce IL-2 to varying levels and how IL-2 secreted by stimulated T cells impacts the production of other Th cytokines is not clear.
The concept of cytokine-autonomy following differentiation of Th subsets suggests that once a T helper subset differentiates, it no longer requires the differentiating cytokine to produce cytokine following antigen receptor stimulation. In classic reports from the Paul laboratory, Th1 cells were shown to be cytokine-dependent as IL-12 could enhance IFN-γ production. In contrast, addition of IL-4 to Th2 cells did not enhance the production of Th2 cytokines, and so were thought to be cytokine-autonomous (9). This concept was extended by the demonstration of IL-23 promoting IL-17 production from Th17 cells (10). This highlights that cytokines other than the differentiating cytokine can impact acute cytokine production. Yet, little is understood about how the cytokine environment impacts Th cytokine production during antigen receptor stimulation.
We show here that golgi transport inhibitors (GTIs) such as monensin and brefeldin A (BFA) that are commonly used for detection of intracellular cytokines actually inhibit the production of IL-9, and transcription of the Il9 gene, during Th9 restimulation. GTIs act to limit IL-2 secretion that normally feeds back and activates Il9 transcription and protein production. The effect of IL-2 feedback was restricted to Th2 and Th9 cytokines, as there was no effect on Th1 or Th17 cells, demonstrating another level of cytokine control that is non-autonomous.
Methods
Mice and isolation of naïve CD4 T cells and in vitro culture
C57BL/6 mice and CD45.1+ C57BL/6 mice were bred in house at the IU School of Medicine, Indianapolis, IN, USA. IL-2-deficient mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA) and BCL6-conditional mutant mice were previously described (11). All mice were used with the approval of the Indiana University Institutional Animal Care and Use Committee.
Naïve CD44- or CD62L+ CD4+ T cells were isolated from C57BL/6 or Il2-/- mice using magnetic enrichment via the supplier's instructions (Miltenyi Biotec, Auburn, CA, USA). Naïve CD4 T cells (106 per ml) were cultured in flat bottom plates coated overnight at 4°C with anti-CD3 (2C11, 2μg/ml, BioXcell) and soluble anti-CD28 (37.51, 5μg/ml, BioXcell) as previously described (12). Briefly, Th1 cells were cultured in the presence of IL-12 (2ng/ml, Peprotech) and anti-IL-4 (11B11, 20μg/ml, BioXcell). Th2 polarization conditions contained IL-4 (20ng/ml, Peprotech), anti-IFN-γ (XMG, 20μg/ml, BioXcell) and anti-IL-10R (1B1.3A, 20μg/ml, BioXcell). Th9 cells were cultured in the presence of IL-4 (20ng/ml), anti-IFN-γ (20μg/ml), anti-IL-10R (20μg/ml) and human TGFβ-1 (2ng/ml, Miltenyi Biotec). Th17 cells were cultured with IL-6 (20ng/ml, Peprotech), human TGFβ-1 (2ng/ml), IL-23 (2ng/ml, Peprotech), anti-IFN-γ (20μg/ml), anti-IL-4 (20μg/ml) and anti-IL-2 (JES6-1A12, 20μg/ml, BioXcell). After 3 days of culture, cells were removed from plates containing plate-bound anti-CD3 and placed in 3 volumes of fresh media supplement with ½ of cytokine and antibody concentrations listed above, except for Th9 cultures that received full amounts of cytokines and antibodies at day 3. Additionally Th17 cells did not receive TGFβ-1 at day 3 of culture. In experiments where Th9 cells were derived from Il2-/- mice, cultures were supplemented with 50U/ml of recombinant human IL-2 (rhIL-2).
Stimulation of polarized Th cells and cytokine analysis
Cells (106/ml) were incubated in 96 well round-bottom plates in media alone (unstimulated) or stimulated in media containing PMA (50ng/ml, Sigma) and ionomycin (1μg/ml, Sigma) with monensin (2μM, Sigma) added at various time points during the 5.5–6 h stimulation at 37°C before analysis as previous described (12). In experiments where Th9 cells derived from WT and IL-2-deficient mice were co-cultured, PMA/ionomycin stimulation occurred in Eppindorf tubes under rotation at 37°C. Blocking antibodies to CD25 (3C7, 1μg/ml, Biolegend) were added 15 min prior to PMA/ionomycin stimulation and blocking antibodies to IL-2 (JES6-1A12 or S4B6-1, Biolegend or BioXcell), TNFα (MP6-XT22, Biolegend) and IL-4 were added (all antibodies used at 20 μg/ml) during stimulation as indicated. Real time PCR for analysis of gene expression, pSTAT protein staining, and retroviral transduction of primary cells was performed using standard methods as previously described (12).
Intracellular cytokine staining and analysis
After stimulation, as per above, T cells were in some cases stained with anti-CD3 (145-2C11, eBioscience) and anti-CD4 (RM4-5, eBioscience), fixed with 4% formaldehyde for 20 min at 4°C and washed subsequently with FACS buffer. Cells were then permeabilized with permeabilization buffer (eBioscience) for 10 min at room temperature and subsequently stained with antibodies for intracellular cytokines (IL-2: JES6-5H4, eBioscience, TNF: MP6-XT22, eBioscience, IL-9: RM9A4, Biolegend, IL-10: JES5-16E3, eBioscience) for 30 min at room temperature (all antibodies were used at 1μg/ml, except TNF which was used at 0.4μg/ml). Cells were washed twice in FACS buffer and run on an Attune Flow Cytometer (Thermo Fisher). Flow cytometry data was analyzed on FlowJo version 8.87.
Aspergillus fumigatus extract exposure
C57BL/6 mice were exposed to 25 μg of Aspergillus fumigatus (AF) extract (GREER, NC, USA) in PBS 3 days a week with one day of rest between each exposure for 6 weeks. After the 6th week of exposure, mice were rested for 2 days prior to harvest of lung mononuclear cells. In brief, lungs from AF-exposed mice were digested with 1mg/ml of type 2 collagenase (Worthington, NJ, USA) for 45 min at 37°C followed by pressing digested lungs through a wire mesh sieve (Bellco Glass, NJ, USA) to make a single cell suspension. CD4+ cells from this lung suspension were enriched by magnetic enrichment (CD4 microbeads, Miltenyi Biotec) to purities greater than 90% following the manufacturer's directions. Enriched CD4+ T cells were stimulated at 106 cells/ml with PMA and ionomycin in the presence or absence of monensin for 6 hours in U-bottom 96 well plates (Corning).
ChIP-seq analysis
ChIP-seq datasets for STAT5A and STAT5B in Th2 cells with the accession number: GSE12346 (13) (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE12346) were retrieved from NCBI. BED records were converted into FASTA sequence files using the BED tools getfasta utility (14) and the mm8 reference genome. The FASTA sequence files were aligned back to the current mouse reference genome using bowtie2 (15) with default parameters. The resulting SAM files were converted into BAM format using SAM-BAM conversion utility from SAM tools (16). Peak calling was performed using MACS (Model-based analysis of Chip-Seq) algorithm (16) with default parameters. The peaks identified in each sample in the form of wig files resulting from running MACS, were visualized using UCSC genome browser.
Results
IL-9 production is inhibited by the use of golgi transport inhibitors
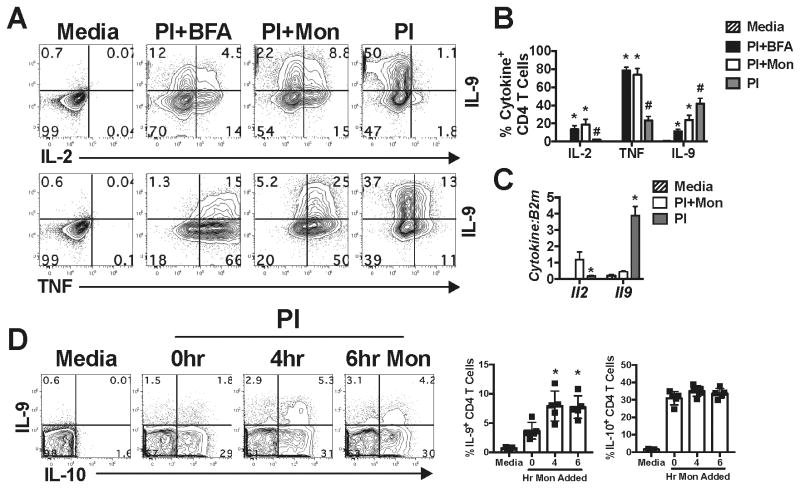
Traditionally, intracellular cytokine staining (ICS) is done in the presence of golgi transport inhibitors (GTIs) (i.e. monensin or brefeldin A, BFA) to retain cytokines within the cell during stimulation. Using this approach, cytokine-positive cells are subsequently identified using flow cytometry. However, the timing of GTI administration and type of GTI used during stimulation has been shown to be important for the optimal detection of a number of cytokines (17). While optimizing the ICS protocol for IL-9 detection by polarized Th9 cells, we observed that stimulation of Th9 cells in the presence of monensin or BFA resulted in an increase in the frequency of cells staining positive for intracellular TNFα, IL-2 and IL-9 compared with unstimulated (Media) cells (p<0.05, Fig. 1A, B), where monensin was slightly better for intracellular detection of IL-9 (Fig. 1A, B). As a control, we also stimulated the same cells in the absence of monensin or BFA. Predictably, we observed a diminished frequency of cells staining positive for intracellular TNF and IL-2, but surprisingly saw a ∼2-4-fold increase (p<0.05) in the frequency of cells staining for intracellular IL-9 as compared to GTI-treated cells (Fig. 1A, B). This result was not due to cross reaction by the IL-9 detection antibody used in these experiments, as Th9 cells from IL-9-deficient mice showed no intracellular IL-9 staining (data not shown). Similarly, Il2 mRNA was most highly expressed in monensin-treated cells, whereas Il9 mRNA levels were significantly increased (p<0.05) when stimulated in the absence of monensin (Fig. 1C). These data indicate that GTIs inhibit the induction of Il9 at the transcriptional level.
Figure 1.
IL-9 production is inhibited by GTIs. (A) Th9 cells were cultured for five days, harvested and stimulated with media, or PMA/ionomycin (PI) in the presence or absence of brefeldin A (BFA) or monensin (Mon) for 5.5 hours. Representative contour plots are shown for intracellular IL-2, TNF and IL-9 staining. (B) The frequency of intracellular IL-2+, TNF+, and IL-9+ cells at day 5 of culture. (C) Il2 and Il9 mRNA levels of day 5 Th9 cells after stimulation with media, PI, or PI + monensin (PIM). These data represent >3 individual experiments and error bars represent standard deviation of at least 3 animals per group. *, p<0.05, reduced as compared to PIM stimulated controls. #, p<0.05 increased as compared to PIM-treated controls. (D) Intracellular IL-9 and IL-10 in viable, pulmonary CD3+ CD4+ T cells from mice chronically exposed to Aspergillus fumigatus extract for 6 weeks. Purified CD4 T cells were stimulated with PI and monensin added at 0, 4, or 6 hours of stimulation. These studies represent 2 individual experiments with 4-5 mice per group. *, p<0.05 greater as compared to 0 hour monensin-treated cells.
To determine if GTI usage also resulted in diminished detection of IL-9 in ex vivo-derived cells, we utilized a model of chronic Aspergillus extract exposure to induce IL-9-secreting cells in the inflamed lung (18). Enriched CD4 T cells isolated from the lungs of Aspergillus-exposed mice were left unstimulated (Media) or stimulated with PMA and ionomycin (PI) with monensin added in culture at 0, 4 or 6 (just prior to cell harvest) hours post stimulation. After a total of 6 hours of stimulation, cells were stained for cell surface CD3 and CD4, fixed and stained for intracellular IL-9 and IL-10. Similar to our in vitro studies, we detected ∼2-fold more IL-9+ cells (p<0.05) when cells were treated at 4 or 6 hours with monensin as compared to cells treated at 0 hrs (Fig. 1D). We did not observe any significant increase (p>0.05) in the frequency of IL-10+ CD4+ T cells with monensin added at later time points. However, detection of IL-10 did not require the use of GTI from ex vivo stimulated cells. Taken together, these data suggest that use of GTIs limits IL-9 production from in vitro-derived cells and cells analyzed ex vivo. Thus, standard ICS protocols might lead to technical limitations in measuring the numbers of IL-9-secreting cells.
IL-2 feedback is required for optimal IL-9 expression after stimulation
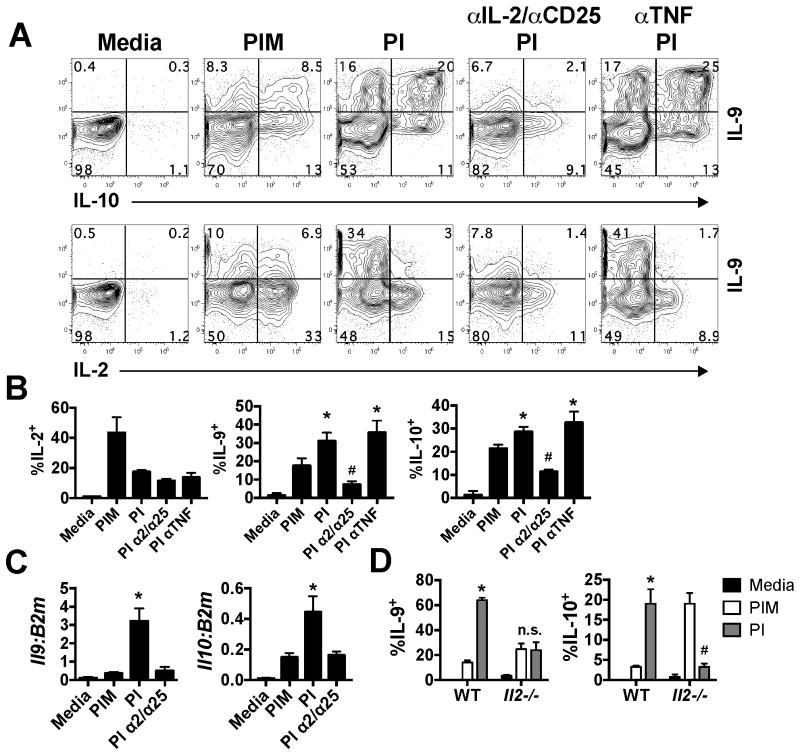
As monensin and BFA act to inhibit cytokine secretion, we hypothesized that a secreted cytokine, induced after stimulation, played a role in amplifying IL-9 production. We observed that cells stimulated in the absence of monensin had significant loss (p<0.05) of intracellular IL-2 and TNF (Fig. 1A), likely indicating secretion. We therefore neutralized these cytokines by using blocking antibodies to IL-2 and its receptor, CD25, or to TNF during stimulation of Th9 cells with PI and examined at IL-9, IL-10 and IL-2 production by intracellular cytokine staining. Similar to what we observed with IL-9 in Fig. 1, IL-10 was also significantly increased in both protein level and mRNA level (Fig. 2A-C) when stimulated in the absence of monensin. Interestingly, blockade of IL-2 and CD25 (α2/α25) significantly reduced levels of intracellular IL-9 and IL-10, similar to levels observed in PIM-treated cells (p<0.05, Fig. 2A, B). In contrast, neutralization of TNF had no effect on levels of intracellular IL-9 or IL-10 (p>0.05, Fig. 2A, B). Further, blockade of IL-2 and CD25 significantly reduced Il9 and Il10 transcription (Fig. 2C) and secretion after 24 hours of stimulation with plate-bound αCD3 (data not shown).
Figure 2.
IL-2 secretion and feedback is required for optimal IL-9 production. (A) Differentiated Th9 cells were left unstimulated (media) or stimulated with PI in the presence of monensin or blocking antibodies to IL-2/CD25 or to TNF for 5.5 hours. Shown are representative contour plots of intracellular IL-9, IL-10 and IL-2. (B) The frequency of intracellular IL-2+, IL-9+ and IL-10+ CD4 T cells quantified from A. (C) Il9 and Il10 mRNA levels from differentiated Th9 cells cultured and stimulated as described in A. (D) WT and Il2-/- cells were cultured under Th9 conditions, as per the materials and methods, and stimulated for 5.5 hours with PI in the presence or absence of monensin. Shown is the frequency of IL-9+ and IL-10+ cells after stimulation. * p<0.05, significantly decreased in comparison to Nil-treated cells. # p<0.05, significantly increased as compared to Nil-treated cells. These data represent 2-5 individual experiments with cultures being derived from 3 individual mice per experiment.
In parallel, we also examined the capacity of IL-2-deficient cells to produce IL-9 after stimulation. In these experiments, WT cells were polarized under standard Th9 conditions, whereas IL-2-deficient cells were supplemented with rhIL-2, as IL-2 is critical for their differentiation (6, 7). At day 5 of culture, cells were rigorously washed to remove any residual IL-2 and stimulated with PI in the presence or absence of monensin. While a greater frequency of WT cells stimulated in the absence of monensin had intracellular IL-9 and IL-10 as compared to monensin-treated controls (p<0.05), this was not the observed in Il2-/- cultures (p>0.05, Fig. 2D). Together, these data indicate a critical role for IL-2 feedback in amplifying IL-9 and IL-10 transcription, protein production and secretion by Th9 cells during re-stimulation.
Paracrine IL-2 responsiveness plays a dominant role in IL-9 production
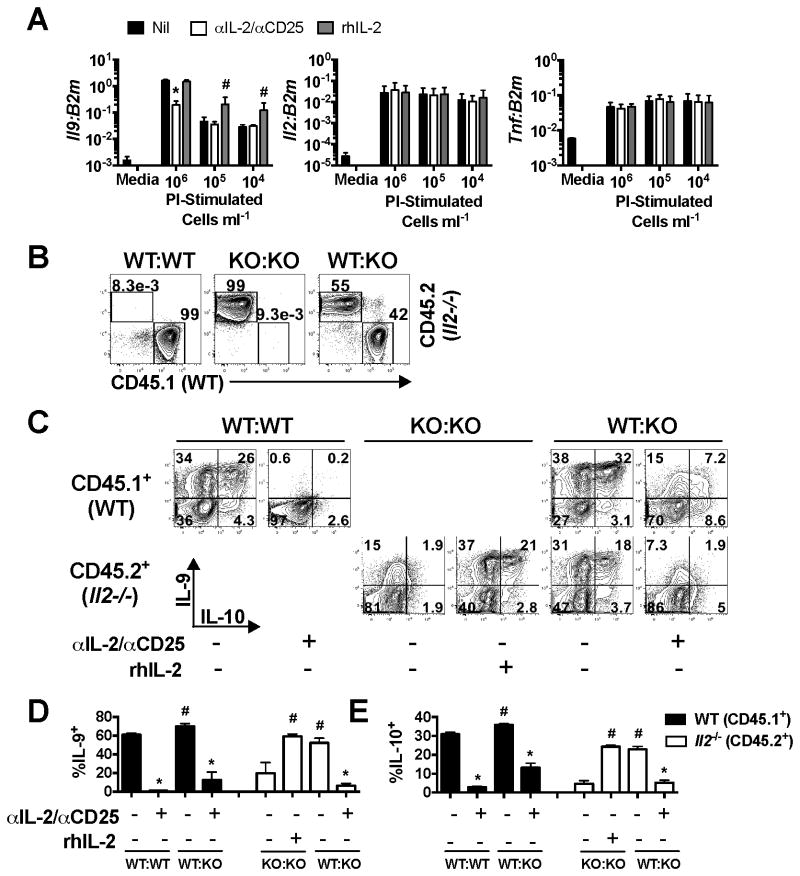
As both IL-2-producing and non-producing cells have the capacity to produce IL-9 (Fig. 1A) we next asked whether the effect of IL-2 in these cultures was predominantly autocrine or paracrine. To address this, we initially stimulated Th9 cells at different cell densities with the expectation that at lower cell densities, an autocrine effect of IL-2 would be retained but that cells spaced further apart would be less exposed to paracrine IL-2. In these experiments, we observed a dramatic decrease (p<0.05) in Il9 mRNA as cells were diluted, while Il2 and Tnf transcript levels were not altered by cell dilution (Fig. 3A), suggesting a specific dominant role of paracrine IL-2 signaling for Il9 production. This effect was further supported by a significant increase (p<0.05) in Il9 mRNA levels when exogenous rhIL-2 was added to cells stimulated at lower cell densities (105 and 104 cells/ml, Fig. 3A). However, our data also show that while blockade of IL-2 was effective at decreasing Il9 at high cell densities, it had no significant effects at lower cell densities (p>0.05), possibly supporting a modest role for autocrine signaling that could potentially be difficult to block with anti-IL-2 antibodies. In contrast, Il2 and Tnf mRNA levels were completely unaltered by either cell density or blockade/addition of IL-2, suggesting that they are not regulated by paracrine or autocrine signaling. These data suggest that induction of Il9 is distinct from that of some other cytokines in that it is subject to environmental cues.
Figure 3.
Paracrine and autocrine production responsiveness to IL-2 promote IL-9 production. (A) Th9 cells were cultured as before and stimulated at 106, 105 and 104 cells per ml for 5.5 hours with PI in the presence or absence of IL-2/CD25 blocking antibodies or rhIL-2 (50U/ml) and Il9, Il2, and Tnf mRNA levels were assessed by RT-PCR. (B) Th9 cells were cultured from WT mice (CD45.1+) or Il2-/- mice (CD45.2+) for 5 days. Differentiated Th9 cells from WT and Il2-/- mice were cultured alone or mixed at a 1:1 ratio. (C) Cells were subsequently stimulated for 5.5 hours with PI in the presence or absence of IL-2/CD25 blocking antibodies or rhIL-2. Representative contour plots of intracellular IL-9 and IL-10 staining are shown for each condition. The frequency of IL-9+ (D) and IL-10+ (E) cells was determined based off of plots shown in panel C. * p<0.05, significantly decreased in comparison to Nil-treated cells. # p<0.05, significantly increased as compared to Nil-treated cells. These data represent 2 individual experiments with cultures being derived from 3 individual mice per experiment.
To address the question of autocrine versus paracrine IL-2 signaling using a parallel approach, we cultured WT (CD45.1+) and Il2-/- (CD45.2+) Th9 cells separately for 5 days. Immediately prior to stimulation, cells were washed, mixed at an approximate 1:1 ratio (Fig. 3B), and stimulated with PI in the presence or absence of IL-2/CD25 blocking antibodies or rhIL-2. IL-2/CD25 blockade or IL-2-deficiency inhibited production of IL-9, and demonstrated similar effects on the production of IL-10 (p<0.05, Fig. 3C, E). However, addition of either rhIL-2 or WT Th9 cells to Il2-/- cells rescued IL-9/10 production to percentages that were similar to WT cells (p>0.05), suggesting a dominant role for paracrine IL-2 signaling in promoting Th9 cytokine production.
STAT5 is activated rapidly after stimulation and is sufficient for IL-2-mediated IL-9 production
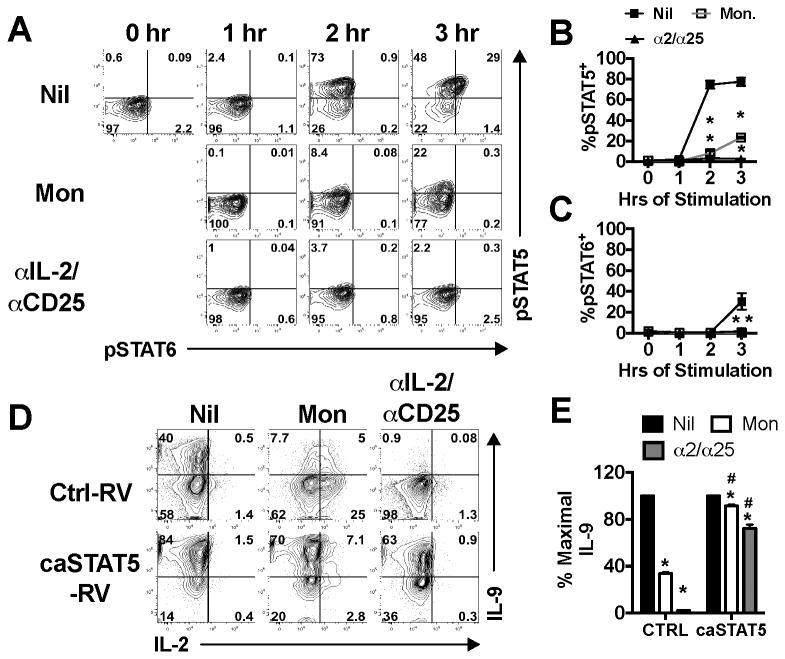
As IL-2 primarily signals through STAT5, we examined the kinetics of STAT5 activation after stimulation of Th9 cells with PI in the presence or absence of monensin or IL-2/CD25 blocking antibodies. STAT5 is potently phosphorylated after as little as 2 hours post PI-stimulation and is maintained at 3 hours of stimulation (Fig. 4A, B). Either monensin or IL-2/CD25 blocking antibodies significantly inhibited the induction of pSTAT5 at 2 hours of stimulation and STAT6 at 3 hours of stimulation (p<0.05, Fig. 4A-C). Interestingly, only pSTAT5+ cells were positive for phosphorylated STAT6 (Fig. 3A), suggesting that competence for STAT6 activation is a cell-intrinsic effect of STAT5 signaling. Together, these data indicate that monensin and IL-2/CD25 blocking antibodies inhibit the induction of pSTAT5 during stimulation and reduce the capacity of Th9 cells to also respond to STAT6-activating cytokines.
Figure 4.
STAT5 is activated rapidly after stimulation and is sufficient for optimal IL-9 production. (A) Th9 cells were cultured under standard conditions and stimulated with PI in the presence or absence of monensin or IL-2/CD25 blocking antibodies. At 0, 1, 2, or 3 hours cells were harvested and stained for intracellular pSTAT5 and pSTAT6. The frequency of pSTAT5 (B) and pSTAT6 (C) Th9 cells were enumerated at each time point based on the plots shown in panel A. (D) Th9 cells were transduced with an empty retroviral vector (Ctrl-RV) that expresses hNGFR or a RV that expresses both hNGFR and constitutively active STAT5 (caSTAT5-RV) on day 3 of culture. Cells were harvested on day 5 of culture and stimulated with PI in the presence or absence of monensin or IL-2/CD25 blocking antibodies for 5.5 hours. Shown are representative contour plots of intracellular IL-2 and IL-9 staining of hNGFR+ T cells. (E) The percentage of maximal IL-9 production, with 100% represented by Th9 cells stimulated with PI alone. These data represent 2 individual experiments with cultures being derived from 3 individual mice per experiment. *, p<0.05, significantly reduced as compared to PI-stimulated controls. #, p<0.05 significantly greater than Ctrl-RV-transduced cells.
We next asked if a constitutively active STAT5 (caSTAT5) could rescue IL-9 production when cells were stimulated in the presence of monensin or IL-2/CD25 blocking antibodies. In these experiments, Th9 cells were transduced with either a control retrovirus (hNGFR Ctrl-RV) or a RV expressing hNGFR-IRES-caSTAT5 (caSTAT5-RV) and stimulated with PI in the presence or absence of monensin or IL-2/CD25 blocking antibodies. Ctrl-RV-transduced cells showed a dramatic loss (p<0.05) of IL-9 protein production in the presence of monensin or blocking antibodies (Fig. 4D, E). Although caSTAT5-transduced cells exhibited some loss of IL-9 production in the presence of monensin and blocking antibodies as compared to untreated cells (p<0.05), this loss was significantly (p<0.05) mitigated by expression of caSTAT5 (Fig. 4D, E). As a whole, these data suggest that IL-2 signaling through STAT5 amplifies IL-9 production.
IL-2 has a critical role in the differentiation of Th2 and Th9 cells. In Th2 cells, IL-2-induced STAT5 is required for maintenance of Gata3 expression as well as Il4 production by directly binding these loci (8). STAT5 in Th9 cells similarly binds directly to Il9 (6, 19), inhibits induction of the transcriptional repressor BCL6, and competes with BCL6 for binding to the Il9 locus (3, 6). We show here that IL-2 also is required for IL-9 production by fully differentiated cells, independent of its presence during differentiation. Although blockade of IL-2 during stimulation did result in increased BCL6 expression (data not shown), IL-2 blockade in BCL6-deficient Th9 cells was as effective in repressing IL-9 production as it was in BCL6-sufficient cells (data not shown). These data indicate that IL-2-induced STAT5 during stimulation likely acts to directly bind the IL-9 locus rather than acting via repressing BCL6.
IL-2 specifically enhances Th2/Th9-associated cytokine production
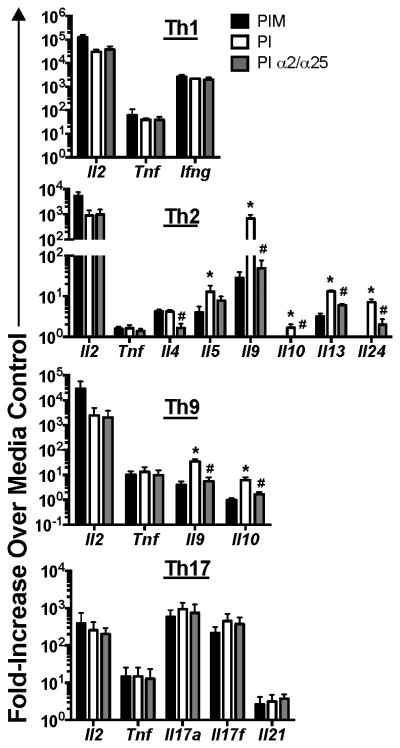
Since IL-2 enhanced not only IL-9, but also IL-10 production by Th9 cells (Fig. 2-3) we questioned if IL-2 acted to acutely amplify production of other lineage- or non-lineage-specific cytokines. For these studies, we cultured Th1, Th2, Th9 and Th17 cells that were left unstimulated or stimulated with PI in the presence or absence of monensin or IL-2/CD25 blocking antibodies. After stimulation we examined cytokine mRNA levels after stimulation. Interestingly, the expression of non-lineage-specific cytokines (i.e. Il2 and Tnf) was not negatively impacted by the addition of monensin or IL-2/CD25 blocking antibodies in any Th subset (Fig. 5). Further, neither the expression of the Th1 signature cytokine gene (Ifng) nor the Th17-associated cytokine genes (i.e. Il17a, Il17f, Il21) were altered by monensin or IL-2/CD25 blocking antibodies (p>0.05, Fig. 5). In stark contrast, virtually all Th2/Th9-associated cytokine genes, with the exception of Il4 and Il5, were decreased (p<0.05) with the addition of monensin or IL-2/CD25 blocking antibodies during stimulation (Fig. 5). Il4 expression by Th2 cells, although unaffected by monensin treatment, was diminished by IL-2/CD25 blockade (p<0.05, Fig. 5). Il5 mRNA levels were decreased by addition of monensin (p<0.05, Fig. 5), but not consistently by IL-2/CD25 blockade (p>0.05, Fig. 5). These data clearly indicate that IL-2 specifically amplifies production of many of the Th2/Th9-associated cytokine genes.
Figure 5.
IL-2 feed back specifically enhances Th2/Th9-associated cytokine production. Th1, Th2, Th9 and Th17 cells were cultured for 5 days and left unstimulated or stimulated with PI in the presence or absence of monensin or IL-2/CD25 blocking antibodies (α2/α25) for 5.5 hours. mRNA was collected from these cells and increases lineage-specific and non-specific cytokine transcript levels were determined by RT-PCR. *p<0.05, significantly increased as compared to monensin-treated controls. #p<0.05, significantly different from PI only-stimulated cells. These data represent 2-3 individual experiments with cultures being derived from 3 individual mice per experiment.
As STAT6 was also phosphorylated after stimulation in the absence of monensin (Fig. 4), it was possible that secreted IL-4 might also enhance type II cytokine production. To determine if this was the case, we blocked IL-2, IL-4 and IL-4/IL-2/CD25 together to assess the relative contributions of IL-2 and IL-4 in the observed cytokine feedback. IL-2/CD25 blockade efficiently suppressed phosphorylation of STAT5 (p<0.05) and to a lesser extent STAT6 (p<0.05) as shown in Fig. 4, and was not further suppressed by co-blockade of IL-4 (Fig. 6A). Similarly, blockade of IL-4 significantly reduced STAT6 phosphorylation (p<0.05) to a similar level to that of monensin treatment, indicating efficient IL-4 neutralization (Fig. 6A). After 6 hours of PI stimulation in the presence or absence of blocking antibodies we also assessed type II-associated cytokine gene expression. While IL-2/CD25 blockade suppressed Il9, Il10, Il13, and Il24 expression (p<0.05) to similar levels as observed in monensin-treated controls, only Il24 was suppressed by blockade of IL-4 (p<0.05, Fig. 6B). Interestingly, combination blockade of IL-2/CD25 and IL-4 further reduced expression of all type II cytokine genes as compared to IL-2/CD25 or IL-4 blockade alone (p<0.05); even for Il5 that was not suppressed by either IL-2/CD25 or IL-4 single blockade (Fig. 6B). These data suggest that while IL-2/STAT5 signaling is dominant over IL-4/STAT6 signaling, both cytokines can indeed work in combination to drive type II cytokine production.
Figure 6.
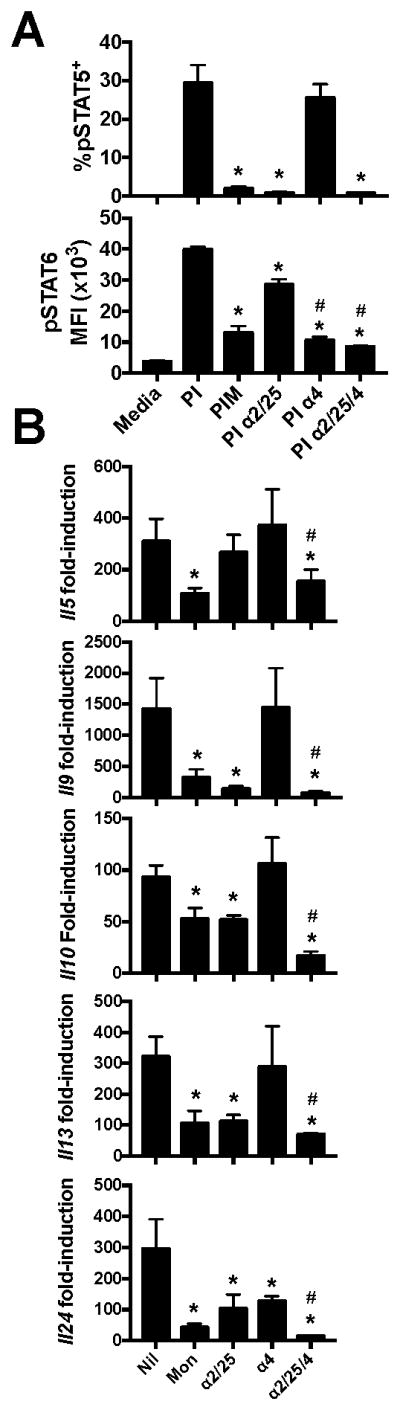
The relative roles of IL-2 and IL-4 signaling in type II cytokine production. Polarized Th2 cells were stimulated with PMA and ionomycin in the presence or absence of monensin, IL-2/CD25 blocking antibodies, IL-4 blocking antibodies or both IL-2/25 and IL-4 blocking antibodies. Cells were harvested 4 hours after stimulation for pSTAT5 and pSTAT6 analysis (A) and 6 hours after stimulation for analysis of cytokine gene expression (B). These data represent 3 independent experiments with cultures derived from 3 mice per experiment. *p<0.05, significantly decreased as compared to PI-stimulated controls. #p<0.05, significantly decreased from cells stimulated in the presence of IL-2/CD25 blocking antibodies.
Discussion
IL-2 and STAT5 signaling play a myriad of roles during Th cell differentiation. During Th1 and Th2 differentiation, IL-2 and STAT5 induce polarizing cytokine receptor expression and drive the expression of lineage-specific master transcription factors and cytokines (4, 8, 20). Similarly, IL-2 and STAT5 are required for differentiation of Th9 cells (3, 6, 7). During this process, STAT5 directly binds the Il9 gene and promotes gene expression. Further, STAT5 suppresses expression of the repressive transcription factor BCL6 and potentially competes with BCL6 for binding in the Il9 promoter (3, 6). In contrast to its positive role in Th1, Th2 and Th9 differentiation, IL-2 and STAT5 signaling inhibit the differentiation of Th17 and TFH cells. STAT5 suppresses expression of both components of the IL-6 receptor that is required for Th17 and TFH differentiation (20), and limits the expression of lineage-specific master transcription factors (BCL6 and RORγt) that are respectively required for TFH and Th17 development (21-25).
The relatively ubiquitous use of golgi transport inhibitors in analysis of cytokine production represent their utility, but as this report highlights, might also bring cautionary notes. Previously, monensin had some effects on limiting IL-10 production from human PBMCs, but did not have effects on IFN–γ or IL-4 detection (17). Other cytokines had not been investigated. In this report we demonstrated that IL-9 is the most profoundly affected cytokine analyzed thus far, which includes all of the standard T helper cell cytokines. The sensitivity of IL-9 to IL-2 feedback suggests that it is exquisitely responsive to STAT5 activation. It remains unclear how prevalent the cytokine-feedback paradigm is in other cytokine-secreting cells, but this will be interesting to explore, and might point towards some important changes in data interpretation.
Our report demonstrates that IL-2 has a novel and specific role in promoting Th2 and Th9 cytokine production outside of the differentiation process and identifies another level of control of type II inflammation. Recent reports have also highlighted the impact of IL-2 on the migration and differentiation of Th2 cells generated in vivo. In a mouse model of chronic allergic airway disease, IL-2 receptor (CD25) deficiency inhibited the formation of lung-resident memory Th2 cells that were vital for induction of allergic inflammation (26), although the cytokine production by CD25-deficient Th cells was not examined. In similar studies, treatment of mice exposed to house dust mite extract with recombinant IL-2 enhanced proliferation of CD4 T cells in the lung, enhanced Th2-associated cytokine production, and increased pulmonary eosinophilia (27). Moreover, in clinical studies, CD25 blocking antibodies (Daclizumab) were effective in improving airway function in patients with corticosteroid-resistant chronic asthma (28). Thus, the biological effects of IL-2 production are not restricted to an early point in the generation of the type II response and therapeutic targeting might still remain viable even at an effector stage.
IL-2 secretion and STAT5 activation after secondary stimulation specifically enhances production of type II cytokines potentially through a direct effect of STAT5 binding to multiple cytokine loci. STAT5 binds the Il9 promoter and can directly activate gene expression (3, 6, 19). Additionally, IL-2-induced STAT5 binds the Il4 locus and Th2 locus control region to induce the expression of the Th2-associated cytokines IL-4, IL-5 and IL-13 (4, 8, 13). Our work demonstrates that expression of Il10 and Il24 is also diminished in the presence of monensin or IL-2/CD25 blocking antibodies. Using available data from Liao et al (13), there was enhanced STAT5A and STAT5B binding to the Il10 and Il24 loci upon IL-2 treatment as compared to controls (Supplementary Figure 1). This supports a direct link between STAT5 and induction of multiple type II cytokine loci. Importantly, the effect of STAT5 is even broader than IL-4 during acute restimulation as neutralization of IL-4 impaired only Il24 expression.
The availability of cytokine in the extracellular milieu and appropriate expression of the cytokine receptor must be temporally matched to mediate these responses. IL-2 has effects on the differentiation of all Th cells because IL-2R complex is expressed rapidly after activation. Th2 cells maintain expression of CD25 through differentiation (29), and as shown here, Th9 cells maintain a high level of IL-2 responsiveness. It was interesting that Th17 were refractory to the effects of IL-2 blockade or monensin during restimulation, and we observed no changes in the production of Th17 lineage cytokines. It is possible that the lack of effect is due to low levels of CD25 expression on Th17 cells at late stages of differentiation (30). It is likely that IL-2 availability is also tightly regulated by the immune system in vivo. T regulatory cells that express high levels of CD25 have been shown to compete for bioavailable IL-2 with effector T cells (31). Thus, the activation of paracrine feedback in Th2 and Th9 cells in vivo will be limited by a balance with other competing cells in the microenvironment.
The ability of IL-2 to modulate Th9 cytokine production might also make it a viable supplement for cellular anti-tumor therapy. Th9 cells have also been shown to be highly effective in tumor immunotherapy (32, 33). In adoptive immunotherapy, pulsing Th9 cells prior to transfer with recombinant IL-2 might boost anti-tumor efficacy. Thus, the identification of this effector paracrine effect of IL-2 might also impact potential cellular therapies.
Supplementary Material
Acknowledgments
This work was supported by NIH Public Health Service grants R01 AI057459 and R03 AI101628 to MHK, and MRO was supported by PHS T32 AR062495. Additional support was provided by U54 DK106846. Support provided by the Herman B Wells Center was in part from the Riley Children's Foundation.
Abbreviations
- BFA
brefeldin A
- GTI
golgi transport inhibitors
- PI
PMA + ionomycin
- α2/α25
anti-IL-2/anti-CD25
References
- 1.Kaplan MH, Hufford MM, Olson MR. The development and in vivo function of T helper 9 cells. Nat Rev Immunol. 2015;15:295–307. doi: 10.1038/nri3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Licona-Limon P, Kim LK, Palm NW, Flavell RA. TH2, allergy and group 2 innate lymphoid cells. Nat Immunol. 2013;14:536–542. doi: 10.1038/ni.2617. [DOI] [PubMed] [Google Scholar]
- 3.Bassil R, Orent W, Olah M, Kurdi AT, Frangieh M, Buttrick T, Khoury SJ, Elyaman W. BCL6 controls Th9 cell development by repressing Il9 transcription. J Immunol. 2014;193:198–207. doi: 10.4049/jimmunol.1303184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci U S A. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kagami S, Nakajima H, Suto A, Hirose K, Suzuki K, Morita S, Kato I, Saito Y, Kitamura T, Iwamoto I. Stat5a regulates T helper cell differentiation by several distinct mechanisms. Blood. 2001;97:2358–2365. doi: 10.1182/blood.v97.8.2358. [DOI] [PubMed] [Google Scholar]
- 6.Liao W, Spolski R, Li P, Du N, West EE, Ren M, Mitra S, Leonard WJ. Opposing actions of IL-2 and IL-21 on Th9 differentiation correlate with their differential regulation of BCL6 expression. Proc Natl Acad Sci U S A. 2014;111:3508–3513. doi: 10.1073/pnas.1301138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmitt E, Germann T, Goedert S, Hoehn P, Huels C, Koelsch S, Kuhn R, Muller W, Palm N, Rude E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J Immunol. 1994;153:3989–3996. [PubMed] [Google Scholar]
- 8.Zhu J, Cote-Sierra J, Guo L, Paul WE. Stat5 activation plays a critical role in Th2 differentiation. Immunity. 2003;19:739–748. doi: 10.1016/s1074-7613(03)00292-9. [DOI] [PubMed] [Google Scholar]
- 9.Hu-Li J, Huang H, Ryan J, Paul WE. In differentiated CD4+ T cells, interleukin 4 production is cytokine-autonomous, whereas interferon gamma production is cytokine-dependent. Proc Natl Acad Sci U S A. 1997;94:3189–3194. doi: 10.1073/pnas.94.7.3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murphy CA, Langrish CL, Chen Y, Blumenschein W, McClanahan T, Kastelein RA, Sedgwick JD, Cua DJ. Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J Exp Med. 2003;198:1951–1957. doi: 10.1084/jem.20030896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hollister K, Kusam S, Wu H, Clegg N, Mondal A, Sawant DV, Dent AL. Insights into the role of Bcl6 in follicular Th cells using a new conditional mutant mouse model. J Immunol. 2013;191:3705–3711. doi: 10.4049/jimmunol.1300378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson MR, Verdan FF, Hufford MM, Dent AL, Kaplan MH. STAT3 Impairs STAT5 Activation in the Development of IL-9-Secreting T Cells. J Immunol. 2016;196:3297–3304. doi: 10.4049/jimmunol.1501801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liao W, Schones DE, Oh J, Cui Y, Cui K, Roh TY, Zhao K, Leonard WJ. Priming for T helper type 2 differentiation by interleukin 2-mediated induction of interleukin 4 receptor alpha-chain expression. Nat Immunol. 2008;9:1288–1296. doi: 10.1038/ni.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quinlan AR, I, Hall M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics. 2010;26:841–842. doi: 10.1093/bioinformatics/btq033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R S. Genome Project Data Processing. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muris AH, Damoiseaux J, Smolders J, Cohen Tervaert JW, Hupperts R, Thewissen M. Intracellular IL-10 detection in T cells by flowcytometry: the use of protein transport inhibitors revisited. J Immunol Methods. 2012;381:59–65. doi: 10.1016/j.jim.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 18.Kerzerho J, Maazi H, Speak AO, Szely N, Lombardi V, Khoo B, Geryak S, Lam J, Soroosh P, Van Snick J, Akbari O. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J Allergy Clin Immunol. 2013;131:1048–1057. 1057 e1041–1042. doi: 10.1016/j.jaci.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao W, Zhang Y, Jabeen R, Nguyen ET, Wilkes DS, Tepper RS, Kaplan MH, Zhou B. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013;38:360–372. doi: 10.1016/j.immuni.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao W, Lin JX, Wang L, Li P, Leonard WJ. Modulation of cytokine receptors by IL-2 broadly regulates differentiation into helper T cell lineages. Nat Immunol. 2011;12:551–559. doi: 10.1038/ni.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ballesteros-Tato A, Leon B, Graf BA, Moquin A, Adams PS, Lund FE, Randall TD. Interleukin-2 inhibits germinal center formation by limiting T follicular helper cell differentiation. Immunity. 2012;36:847–856. doi: 10.1016/j.immuni.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnston RJ, Choi YS, Diamond JA, Yang JA, Crotty S. STAT5 is a potent negative regulator of TFH cell differentiation. J Exp Med. 2012;209:243–250. doi: 10.1084/jem.20111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O'Shea J. Interleukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Nurieva RI, Podd A, Chen Y, Alekseev AM, Yu M, Qi X, Huang H, Wen R, Wang J, Li HS, Watowich SS, Qi H, Dong C, Wang D. STAT5 protein negatively regulates T follicular helper (Tfh) cell generation and function. J Biol Chem. 2012;287:11234–11239. doi: 10.1074/jbc.M111.324046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang XP, Ghoreschi K, Steward-Tharp SM, Rodriguez-Canales J, Zhu J, Grainger JR, Hirahara K, Sun HW, Wei L, Vahedi G, Kanno Y, O'Shea JJ, Laurence A. Opposing regulation of the locus encoding IL-17 through direct, reciprocal actions of STAT3 and STAT5. Nat Immunol. 2011;12:247–254. doi: 10.1038/ni.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hondowicz BD, An D, Schenkel JM, Kim KS, Steach HR, Krishnamurty AT, Keitany GJ, Garza EN, Fraser KA, Moon JJ, Altemeier WA, Masopust D, Pepper M. Interleukin-2-Dependent Allergen-Specific Tissue-Resident Memory Cells Drive Asthma. Immunity. 2016;44:155–166. doi: 10.1016/j.immuni.2015.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ballesteros-Tato A, Randall TD, Lund FE, Spolski R, Leonard WJ, Leon B. T Follicular Helper Cell Plasticity Shapes Pathogenic T Helper 2 Cell-Mediated Immunity to Inhaled House Dust Mite. Immunity. 2016;44:259–273. doi: 10.1016/j.immuni.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Busse WW, Israel E, Nelson HS, Baker JW, Charous BL, Young DY, Vexler V, Shames RS G. Daclizumab Asthma Study. Daclizumab improves asthma control in patients with moderate to severe persistent asthma: a randomized, controlled trial. Am J Respir Crit Care Med. 2008;178:1002–1008. doi: 10.1164/rccm.200708-1200OC. [DOI] [PubMed] [Google Scholar]
- 29.Hwang ES, I, White A, Ho IC. An IL-4-independent and CD25-mediated function of c-maf in promoting the production of Th2 cytokines. Proc Natl Acad Sci U S A. 2002;99:13026–13030. doi: 10.1073/pnas.202474499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quintana FJ, Jin H, Burns EJ, Nadeau M, Yeste A, Kumar D, Rangachari M, Zhu C, Xiao S, Seavitt J, Georgopoulos K, Kuchroo VK. Aiolos promotes TH17 differentiation by directly silencing Il2 expression. Nat Immunol. 2012;13:770–777. doi: 10.1038/ni.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leon B, Bradley JE, Lund FE, Randall TD, Ballesteros-Tato A. FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat Commun. 2014;5:3495. doi: 10.1038/ncomms4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu Y, Hong S, Li H, Park J, Hong B, Wang L, Zheng Y, Liu Z, Xu J, He J, Yang J, Qian J, Yi Q. Th9 cells promote antitumor immune responses in vivo. J Clin Invest. 2012;122:4160–4171. doi: 10.1172/JCI65459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purwar R, Schlapbach C, Xiao S, Kang HS, Elyaman W, Jiang X, Jetten AM, Khoury SJ, Fuhlbrigge RC, Kuchroo VK, Clark RA, Kupper TS. Robust tumor immunity to melanoma mediated by interleukin-9-producing T cells. Nat Med. 2012;18:1248–1253. doi: 10.1038/nm.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.