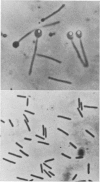
Abstract
A reasonable degree of synchrony in the sporulation of Clostridium thermosaccharolyticum 3814 was obtained by using three 10% transfers of 8-hr cultures in a medium containing 0.5% L-arabinose, 0.5% peptone, 0.5% yeast extract, and Gc minerals. Sporulation was stimulated by L-arabinose and L-xylose, but was repressed by glucose, mannose, fructose, and D-pentoses. Sporulating cells were long and thin, whereas repressed cells were shorter and thicker. The optimal pH for sporulation was in the range of pH 5.0 to 5.5. As sporulation continued, the accumulated acetate decreased. Label studies indicated that a significant amount of acetate-2-C14 was incorporated into the spore lipid. The calcium, phosphorus, and dipicolinic acid (DPA) concentrations on a dry weight basis were 2.55, 2.60, and 7.25%, respectively. The molar ratio of Ca-DPA was 1.47.
Full text
PDF

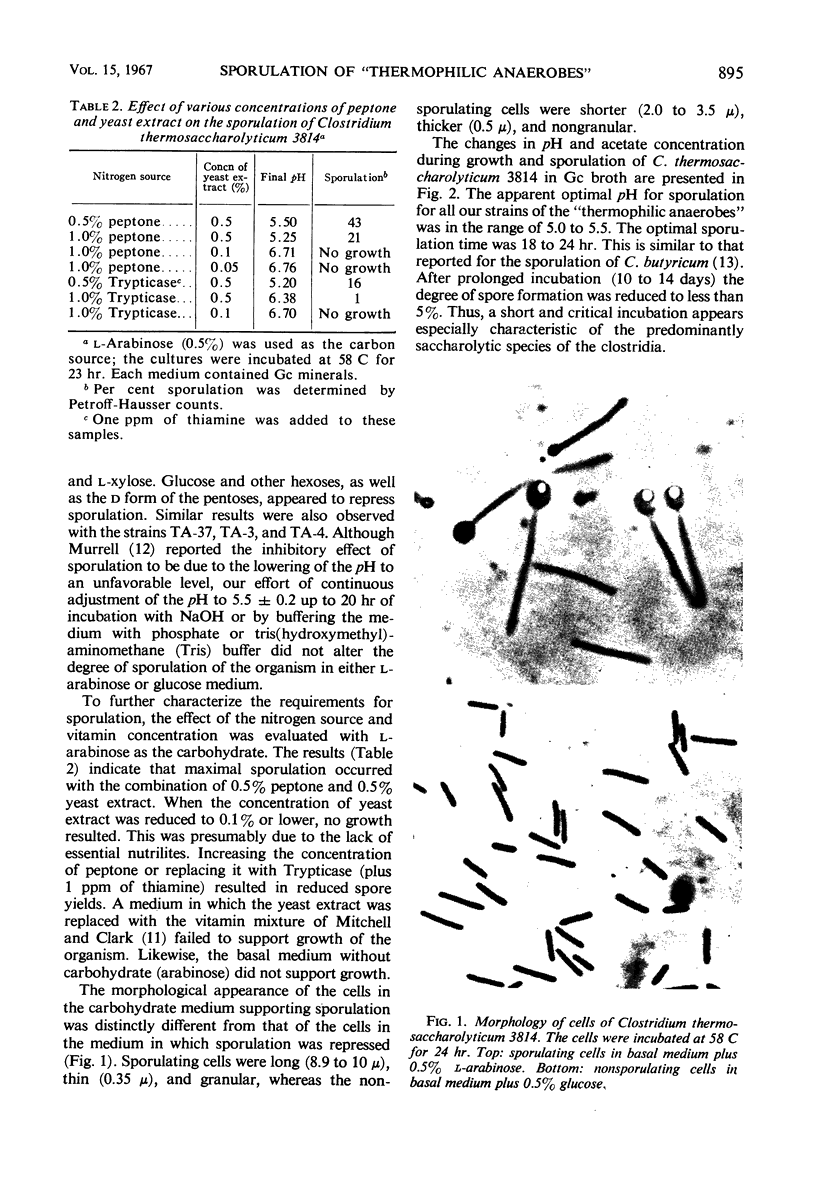
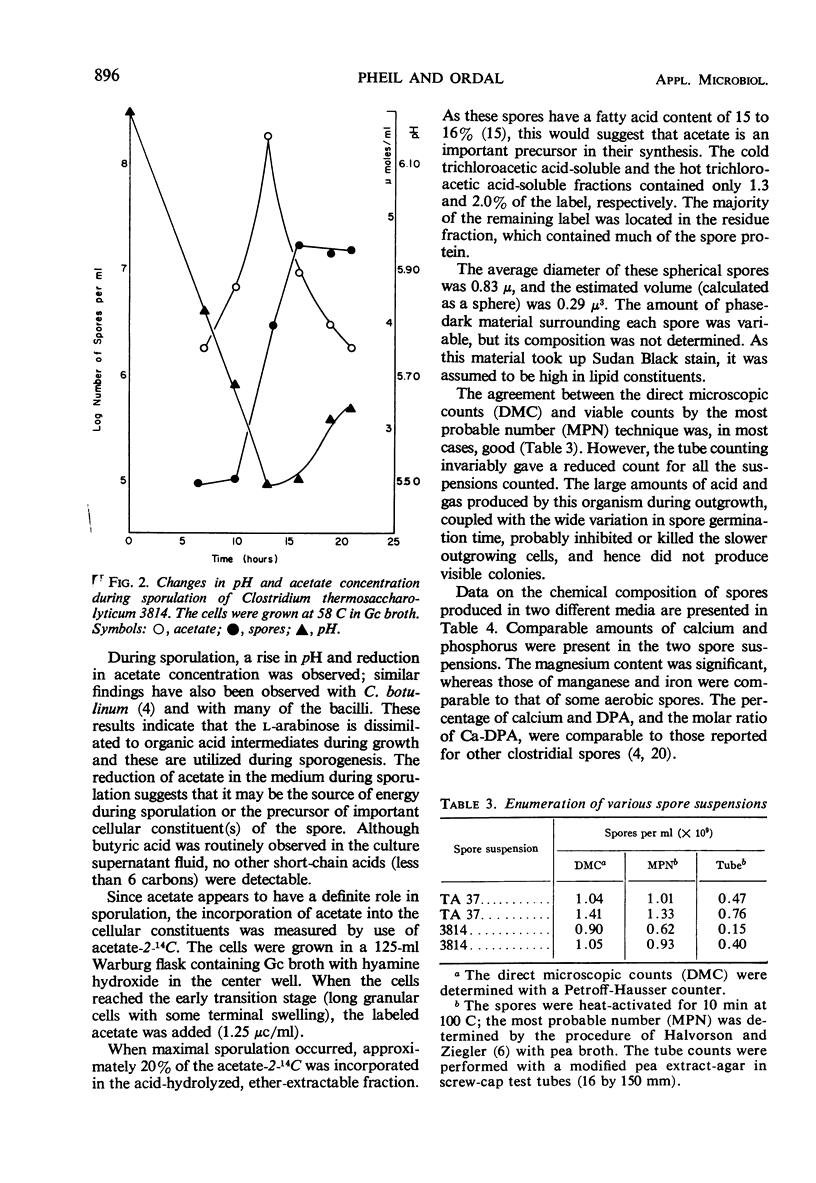


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergère J. L., Hermier J. Etude des facteurs contrôlant la croissance et la sporulation de Clostridium butyricum. I. Composition du milieu de culture. Ann Inst Pasteur (Paris) 1965 Jul;109(1):80–89. [PubMed] [Google Scholar]
- DAY L. E., COSTILOW R. N. PHYSIOLOGY OF THE SPORULATION PROCESS IN CLOSTRIDIUM BOTULINUM. I. CORRELATION OF MORPHOLOGICAL CHANGES WITH CATABOLIC ACTIVITIES, SYNTHESIS OF DIPICOLINIC ACID, AND DEVELOPMENT OF HEAT RESISTANCE. J Bacteriol. 1964 Sep;88:690–694. doi: 10.1128/jb.88.3.690-694.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. Biochemistry of sporulation. I. Metabolism of acetate by vegetative and sporulating cells. J Bacteriol. 1963 Feb;85:451–460. doi: 10.1128/jb.85.2.451-460.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halvorson H. O., Ziegler N. R. Application of Statistics to Problems in Bacteriology: I. A Means of Determining Bacterial Population by the Dilution Method. J Bacteriol. 1933 Feb;25(2):101–121. doi: 10.1128/jb.25.2.101-121.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- LONG S. K., WILLIAMS O. B. Method for removal of vegetative cells from Bacterial spore preparations. J Bacteriol. 1958 Sep;76(3):332–332. doi: 10.1128/jb.76.3.332-332.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAJUMDER S. K., PADMA M. C. Screening of carbohydrates for sporulation of bacilli in fluid medium. Can J Microbiol. 1957 Jun;3(4):639–642. doi: 10.1139/m57-069. [DOI] [PubMed] [Google Scholar]
- Pheil C. G., Ordal Z. J. Fatty acid composition of spores of the "thermophilic anaerobes". J Bacteriol. 1967 May;93(5):1727–1728. doi: 10.1128/jb.93.5.1727-1728.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOLEY B. C., COLLIER R. E. CHANGES IN THERMORESISTANCE OF CLOSTRIDIUM ROSEUM AS RELATED TO THE INTRACELLULAR CONTENT OF CALCIUM AND DIPICOLINIC ACID. Can J Microbiol. 1965 Apr;11:279–285. doi: 10.1139/m65-034. [DOI] [PubMed] [Google Scholar]