Abstract
Clinical chicken coccidiosis is mostly caused by simultaneous infection of several Eimeria species, and host immunity against Eimeria is species-specific. It is urgent to identify common immunodominant antigen of Eimeria for developing multivalent anticoccidial vaccines. In this study, sporozoite proteins of Eimeria tenella, Eimeria acervulina and Eimeria maxima were analyzed by two-dimensional electrophoresis (2DE). Western bot analysis was performed on the yielded 2DE gel using antisera of E. tenella E. acervulina and E. maxima respectively. Next, the detected immunodominant spots were identified by comparing the data from MALDI-TOF-MS/MS with available databases. Finally, Eimeria common antigens were identified by comparing amino acid sequence between the three Eimeria species. The results showed that analysis by 2DE of sporozoite proteins detected 629, 626 and 632 protein spots from E. tenella, E. acervulina and E. maxima respectively. Western bot analysis revealed 50 (E. tenella), 64 (E. acervulina) and 57 (E. maxima) immunodominant spots from the sporozoite 2DE gels of the three Eimeria species. The immunodominant spots were identified as 33, 27 and 25 immunodominant antigens of E. tenella, E. acervulina and E. maxima respectively. Fifty-four immunodominant proteins were identified as 18 ortholog proteins among the three Eimeria species. Finally, 5 of the 18 ortholog proteins were identified as common immunodominant antigens including elongation factor 2 (EF-2), 14-3-3 protein, ubiquitin-conjugating enzyme domain-containing protein (UCE) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH). In conclusion, our results not only provide Eimeria sporozoite immunodominant antigen map and additional immunodominant antigens, but also common immunodominant antigens for developing multivalent anticoccidial vaccines.
Keywords: Eimeria, sporozoites, common immunodominant antigens, immunoproteomics
INTRODUCTION
Avian coccidiosis, a major parasitic disease of chickens worldwide, was caused by intestinal infection of Eimeria spp. [1]. It causes reduction in weight gain and poor feed-conversion, and death of the chickens, leading to an estimated annual economic loss of more than US$3 billion to the global poultry [2, 3]. The species of E. tenella, E. acervulina and E. maxima are the most important in terms of global disease burden and economic impact [2, 4].
Present control strategy against this disease relies on anticoccidial drugs and live vaccines containing virulent or attenuated strains of Eimeria [5]. However, chemical residues, emergence of drug-resistant parasites and the high cost associated with the development of new drugs results in serious problems. Moreover, the live vaccines have inherent production limitations, risk of vaccinal pathogenicity as well as the potential reversion to a pathogenic form, and cost issues [2, 6, 7]. Thus, new vaccines containing either defined immunodominant antigens or based on recombinant DNA technology have been or are being developed [2, 8, 9]. Clinical coccidiosis is mainly caused by co-infection with multiple species of Eimeria [10, 11], hence, a practical novel anticoccidial vaccine should contain the common antigens among Eimeria or antigens from multiple Eimeria species. Therefore, exploring immunodominant antigens, especially common antigens of Eimeria, is essential for developing novel vaccine against the simultaneous infection clinically.
Here, we described immunoproteomic analysis of Eimeria tenella, Eimeria acervulina and Eimeria maxima. A batch of immunodominant antigens was identified, with 33, 27 and 25 found in E. tenella, E. acervulina and E. maxima, respectively. Eighteen ortholog proteins and 5 common immunodominant antigens across the three Eimeria species were identified. Our results provide additional immunodominant antigens and common antigens for the development of multivalent vaccines against Eimeria.
RESULTS
Sporozoite 2DE gel profile of E. tenella, E. acervulina and E. maxima
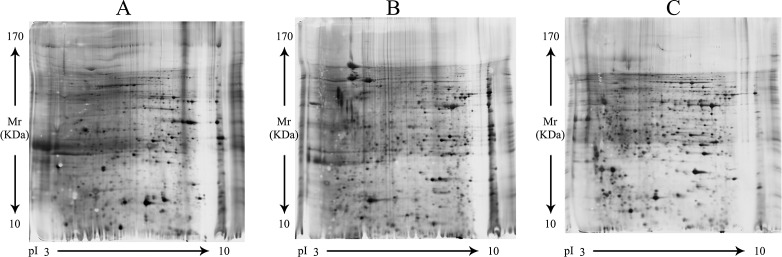
The separation by 2-DE of 400 μg solubilized sporozoite proteins detected 629, 626 and 632 spots of E. tenella, E. acervulina and E. maxima, respectively. Most spots were located between 13 and 140 kDa (Figure 1). Analysis with ImageMaster 2D Platinum (Version 5.0, GE Amersham) revealed 22 spots shared among all these species.
Figure 1. Sporozoite 2-DE gel profile of E. tenella, E. acervulina and E. maxima.
(A) E. tenella, (B) E. acervulina, (C) E. maxima. Soluble proteins (400 μg) from sporozoite of the three species were resolved by IEF over a broad, non-linear pH 3, 10 range followed by molecular mass on a 12.5% w/v acrylamide gel under denaturing conditions. Protein spots are visualized using silver stain.
Detection of immunodominant spots by Western blot
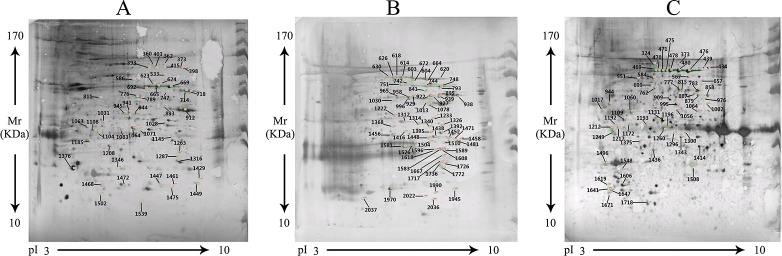
Sporozoite 2DE gels of E. tenella, E. acervulina and E. maxima were analyzed by western blot using the corresponding antisera of these Eimeria species separately. Western blot profiles of the 2DE gel were shown in Figure 2. Immunodominant spots were observed on the western blot profiles of the three Eimeria species. Comparison with ImageMaster 2D Platinum revealed that 50 (E. tenella), 64 (E. acervulina) and 57 (E. maxima) immunodominant spots had high similarity between the 2DE gel profile and western blot profile. When the same western blot was probed with sera from negative control chickens, no proteins were detected (Figure 3).
Figure 2. Western blot analysis of the sporozoite 2DE gels of E. tenella, E. acervulina and E. maxima with anti-E. tenella, anti-E. acervulina and anti-E. maxima sera.
(A) E. tenella, (B) E. acervulina, (C) E. maxima.
Figure 3. Western blot analysis of the sporozoite 2DE gels of E. tenella, E. acervulina and E. maxima with sera from negative control chickens.
(A) E. tenella, (B) E. acervulina, (C) E. maxima.
Immunodominant proteins analysis and identification using NCBI and Uniport database
All the immunodominant spots (171) detected by western blot were analyzed by MALDI-TOF-MS/MS. The obtained peptide mass fingerprint dates were submitted to MASCOT Sequence Query server (http://www.matrixscience.com) for identification against nonredundant NCBI database (http://www.ncbi.nlm.nih.gov/BLAST) and the uniprot database (http://www.uniprot.org/). Identification required a MASCOT confidence interval of 95%. As shown in Table 1, 112 spots were identified in the databases as corresponding to 85 Eimeria proteins, including 33 of E. tenella, 27 of E. acervulina and 25 of E. maxima. Fifty-four out of the 85 immunodominant proteins were 18 kinds of ortholog proteins among the three Eimeria species. Table 2 showed amino acids similarity of the 18 ortholog proteins between the three Eimeria species. All the ortholog proteins shared sequence similarity of more than 63% between the three Eimeria species except peroxiredoxin. Five of the ortholog proteins even shared sequence similarity of more than 93% between the three Eimeria species, namely, elongation factor 2 (EF-2), 14-3-3 protein, ubiquitin-conjugating enzyme domain-containing protein (UCE), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and transhydrogenase. Therefore, the five proteins were identified as common immunodominant antigens among the three Eimeria species. Since there were no matched proteins in the database, 59 spots were not identified successfully.
Table 1. Identification of E. tenella>, E. acervulina and E. maxima sporozoite proteins in NCBI and Uniprot database using data from MALDI-TOF-MS/MS analyses.
| Spot IDa | Identified protein | Database ID |
|---|---|---|
| A929 | 14 kDa phosphohistidine phosphatase, putative (E. acervulina) | CDI76382.1 |
| M1436 | 14 kDa phosphohistidine phosphatase, putative (E. maxima) | CDJ61236.1 |
| T1468, T669 | 14 kDa phosphohistidine phosphatase, putative (E. tenella) | CDJ40270.1, U6KXP8|U6KXP8 |
| T1475 | 14-3-3 protein (E. tenella) | gi|21541950 |
| A1222, A1392, A1395 | 14-3-3 protein, putative (E. acervulina) | XP_013250285 |
| M567 | 14-3-3 protein, putative (E. maxima) | XP_013335589 |
| M1056, M1064, M1414 | 56 kDa gametocyte antigen (E. maxima) | gi|25989587 |
| T1064 | 56 kDa gametocyte antigen, related (E. tenella) | CDJ41536.1 |
| A620 | 56 kDa gametocyte antigen, related OS= E. acervulina | CDI78697.1 |
| M1131 | 82 kDa gametocyte antigen OS= E. maxima | Q86LH7|Q86LH7 |
| T1472 | Actin depolymerizing factor (E. tenella) | ABM89551.1 |
| A1510 | Actin depolymerizing factor, putative (E. acervulina) | CDI83856.1 |
| M1647, M1343 | Actin depolymerizing factor, putative (E. maxima) | CDJ60866.1 |
| M469 | Actin, putative OS=E. maxima | U6M655|U6M655_ |
| T941 | Alanine dehydrogenase, putative (E. tenella) | CDJ43978.1 |
| A604 | Alanine dehydrogenase, putative OS=E. acervulina | U6GM75|U6GM75 |
| A1448 | Aldo/keto reductase family oxidoreductase, putative (E. acervulina) | CDI80286.1 |
| T1145 | Aldo/keto reductase family oxidoreductase, putative (E. tenella) | CDJ45355.1 |
| M584 | Aldo/keto reductase family oxidoreductase, putative OS=E. maxima | CDJ57423.1 |
| T393 | Aspartyl proteinase (Eimepsin) OS=E. tenella | Q9GN67|Q9GN67 |
| A1458 | Cytosol aminopeptidase, putative (E. acervulina) | CDI77668.1 |
| T362 | Cytosol aminopeptidase, putative OS=E. tenella | U6KUY7|U6KUY7 |
| M1619, M1641 | Dihydrolipoyl dehydrogenase OS=E. maxima | CDJ55972.1 |
| A1368 | Dihydrolipoyl dehydrogenase, putative (E. acervulina) | CDI82910.1 |
| T1376 | Dihydrolipoyl dehydrogenase, putative (E. tenella) | CDJ38931.1 |
| T415 | Dynein heavy chain protein, related OS=E. tenella | U6KWQ6|U6KWQ |
| A1970 | Elongation factor 1-alpha OS=E. acervulina | U6GWZ2|U6GWZ |
| M480, M470 | Elongation factor 1-alpha, putative (E. maxima) | gi|557198794 |
| M478 | Elongation factor 2, putative (E. maxima) | gi|557157066 |
| T811 | Elongation factor 2, putative (E. tenella) | gi|557140071 |
| A939, A965 | Elongation factor 2, putative OS=E. acervulina | U6GRG2|U6GRG2 |
| T747 | Enolase 2, putative (E. tenella) | CDJ38513.1 |
| A1456 | Enolase 2, putative OS=E. acervulina | CDI82390.1 |
| M1548 | Enolase 2, putative OS=E. maxima | CDJ56218.1 |
| A744, A742 | Fructose-bisphosphate aldolase OS=E. acervulina | CDI80998.1 |
| T398 | Fructose-bisphosphate aldolase OS=E. tenella | CDJ43237.1 |
| M1060 | Fructose-bisphosphate aldolase, related (E. maxima) | CDJ59017.1 |
| A751, A748 | Glyceraldehyde-3-phosphate dehydrogenase OS=E. acervulina | CDI78463.1 |
| T403 | Glyceraldehyde-3-phosphate dehydrogenase OS=E. tenella | CDJ43289.1 |
| M1249 | Glyceraldehyde-3-phosphate dehydrogenase, putative (E. maxima) | CDJ57266.1 |
| A1581, A1340 | Haloacid dehalogenase-like hydrolase domain-containing protein, putative (E. acervulina) | CDI76904.1 |
| T1287 | Haloacid dehalogenase-like hydrolase domain-containing protein, putative (E. tenella) | CDJ37571.1 |
| M471 | Haloacid dehalogenase-like hydrolase OS=E. maxima | CDJ61159.1 |
| T1185 | Hypothetical protein (E. tenella) | gi|357017711 |
| T1316 | Hypothetical protein (E. tenella) | AET50460.1 |
| T1447 | Hypothetical protein (E. tenella) | AET50635.1 |
| T1208 | Hypothetical protein (E. tenella) | gi|357017711 |
| A841 | Hypothetical protein, conserved (E. acervulina) | CDI78255.1 |
| T1263 | Hypothetical protein, conserved (E. tenella) | CDJ37254.1 |
| M1375 | KH domain-containing protein, putative (E. maxima) | CDJ59518.1 |
| T776 | KH domain-containing protein, putative (E. tenella) | CDJ38027.1 |
| A895 | KH domain-containing protein, putative OS=E. acervulina | CDI84033.1 |
| A1618 | Lactate dehydrogenase (E. acervulina) | ACM77785.1 |
| M783, M762 | Lactate dehydrogenase OS=E. maxima GN=LDH | Q8I8U3|Q8I8U3_E |
| T718 | Lactate dehydrogenase OS=E. tenella | CDJ37067.1 |
| A1736 | Microneme 2 (E. acervulina) | KR063282.1 |
| M1002 | Microneme protein 7 OS=E. maxima GN=mic7 | G0LEU8|G0LEU8 |
| T1502 | Microneme protein MIC3, partial (E. tenella) | gi|40549149 |
| T1449 | Mitochondrial branched-chain alpha-keto acid dehydrogenase E1, putative (E. tenella) | CDJ43491.1 |
| A626 | Mitochondrial branched-chain alpha-keto acid dehydrogenase E1, putative, partial (E. acervulina) | CDI81447.1 |
| T692 | Nucleoside diphosphate kinase OS=E. tenella | U6KIW7|U6KIW7 |
| M373 | Nucleoside diphosphate kinase, putative (E. maxima) | CDJ59193.1 |
| A927 | Peroxiredoxin, putative (E. acervulina) | CDI84011.1 |
| M1109 | Peroxiredoxin, putative OS=E. maxima | CDJ59087.1 |
| T360 | Peroxisomal catalase, putative OS=E. tenella | CDJ43752.1 |
| A1233, A922 | Proteasome subunit alpha type 7, putative (E. acervulina) | CDI79975.1, U6GIG8|U6GIG8_ |
| T893, T624 | Proteasome subunit alpha type 7, putative (E. tenella) | CDJ41908, U6KYA5|U6KYA5 |
| M475, M476, M434, M875 | Purine nucleoside phosphorylase, putative (E. maxima) | CDJ57289.1 |
| T1083 | Purine nucleoside phosphorylase, putative (E. tenella) | CDJ44020.1 |
| A996 | Purine nucleoside phosphorylase, putative OS=E. acervulina | CDI78710.1 |
| T533 | Putative uncharacterized protein OS=E. tenella | H9B9×1|H9B9×1_ |
| A603, A614, A618 | Pyruvate kinase, putative (E. acervulina) | CDI78351.1 |
| A1326 | Sporozoite antigen, partial (E. acervulina) | CAA33905.1 |
| T623 | Transhydrogenase (Fragment) OS=E. tenella | Q24937|Q24937 |
| T714 | Transhydrogenase OS=E. tenella GN=7B2 PE=4 | Q07600|Q07600 |
| A1078, A1717 | Transhydrogenase, putative (E. acervulina) | CDI76761.1 |
| M1718 | Transhydrogenase, putative (E. maxima) | CDJ61620.1 |
| T1028, T665 | Triosephosphate isomerase (E. tenella) | CDJ37485.1, H9BA04|H9BA04 |
| A958, A1772 | Triosephosphate isomerase OS=E. acervulina | U6GH90|U6GH90, CDI79606.1 |
| M1606, M1212 | Triosephosphate isomerase, putative (E. maxima) | CDJ60494.1 |
| A1481, A1438, A1452 | Ubiquitin-conjugating enzyme domain-containing protein, putative (E. acervulina) | gi|557117367 |
| M699 | Ubiquitin-conjugating enzyme domain-containing protein, putative (E. maxima) | CDJ61561 |
| T1063 | Ubiquitin-conjugating enzyme domain-containing protein, putative (E. tenella) | XP_013236351 |
| M995, M909 | Uncharacterized protein OS=E. maxima | U6M744|U6M744_ |
| M858 | Uncharacterized protein OS=E. maxima | U6LYQ2|U6LYQ2 |
Note: 1.a Number of the spot in the 2-DE gel and the western blot membrane; 2. A: E. acervulina, T: E. tenella, M: E. maxima.
Table 2. Amino acid sequence similarity of the 18 immunodominant ortholog proteins among E. tenella E. acervulina and E. acervulina.
| Immunodominant ortholog proteins | Amino acid sequence similarity between | ||
|---|---|---|---|
| E. tenella and E. acervulina | E. acervulina and E. maxima | E. tenella and E. maxima | |
| Elongation factor 2, putative | 99% | 99% | 99% |
| 14-3-3 protein | 97.5% | 99.6% | 97.1% |
| Ubiquitin-conjugating enzyme domain-containing protein, putative | 96.6% | 97.4% | 98.3% |
| Glyceraldehyde-3-phosphate dehydrogenase | 94.1% | 95.9% | 92.6% |
| Transhydrogenase | 93.4% | 94.5% | 92.9% |
| Actin depolymerizing factor | 89.8% | 93.2% | 88.1% |
| Triosephosphate isomerase | 82.1% | 92.0% | 78.9% |
| Fructose-bisphosphate aldolase | 77.8% | 99.6% | 80.4% |
| Purine nucleoside phosphorylase, putative | 79.3% | 81.2% | 81.2% |
| Aldo/keto reductase family oxidoreductase, putative | 78.5% | 79.3% | 85.7% |
| Dihydrolipoyl dehydrogenase | 75.7% | 81.0% | 84.1% |
| Enolase 2 | 76.4% | 86.2% | 72.7% |
| Haloacid dehalogenase-like hydrolase domain-containing protein | 74.5% | 82.0% | 76.2% |
| KH domain-containing protein, putative | 71.5% | 77.9% | 69.3% |
| Lactate dehydrogenase | 68.2% | 80.6% | 63.6% |
| 14 kDa phosphohistidine phosphatase, putative | 64.0% | 76.5% | 64.2% |
| 56 kDa gametocyte antigen | 68.0% | 77.3% | 65.9% |
| Peroxiredoxin | 13.8% | 89.3% | 16.2% |
DISCUSSION
The immunity elicited by infections with Eimeria is species specific and an effective recombinant vaccine should include common protective antigens among several Eimeria species [12–14]. Some researchers have reported several Eimeria common antigens. Talebi reported a conserved immunodominant protein band (45 kDa) among sporulated oocysts of five Eimeria species (E. acervulina, E. maxima, E. necatrix, E. praecox and E. tenella) recognized by chicken anti-E. maxima serum. Sasai and colleagues reported a common antigen present on the apical complex of all chicken Eimeria sporozoites [15]. Constantinoiui and colleagues reported a highly conserved apical antigen among Eimeria species [13]. However, the reported common antigens are not specific. In the present study, we identified at least 5 specific Eimeria common immunodominant antigens by immunoproteomic analysis. Our research provided additional candidate common antigens for developing multivalent vaccines against simultaneous infection by several Eimeria species.
Since antibodies could confer protective immunity against Eimeria [17–20], in addition, the induced antibodies are relatively long lasting and easy for collection [13, 17, 21]. Therefore, in some previous reports, Eimeria antisera were used to identify immunodominant Eimeria antigens. For example, Réfega et al. obtained a total of 119 cDNA clones by immunoscreening E. tenella libraries using intestinal antibodies [22]. Laurent et al. screened a 19-kilodalton antigen present in several Eimeria species using sera raised to E. acervulina or E. tenella [23]. Some immunodominant antigens were screened from cDNA libraries by corresponding Eimeria antisera, such as TA4 [24], LPMC-61 [25], rhomboid proteins ETRH01 of E. tenella [26] and so on, and these identified immunodominant antigen were further demonstrated to be able to confer protection against Eimeria challenge [27, 28]. Therefore, we also used the Eimeria antisera to screen the immunodominant antigens of Eimeria species and obtained 85 immunodominant proteins. Part of the identified antigens were also identified as immunodominant antigens in previous studies, including MIC3, pyruvate kinase, enolase, actin, aspartyl proteinase, 14-3-3 protein, lactate dehydrogenase and so on [29–31]. Some of the identified antigens have been demonstrated to be able to confer protection against Eimeria challenge, such as lactate dehydrogenase [32], microneme 2 [33], microneme 7 [34] and so on. Therefore, the immunodominant antigens identified in this study have the potential for conferring protection against Eimeria challenge.
In this study, most of the identified immunodominant antigens shared orthologous relationships across the three Eimeria species. Nearly all the ortholog proteins shared amid acid sequence similarity of more than 63%, furthermore, five proteins shared sequence similarity of more than 93% and were identified as common immunodominant proteins among the three Eimeria species. In addition, we compared the available amid acid sequence of the five identified common immunodominant proteins among 7 chicken Eimeria species, and found that nearly all the sequence similarity among the 7 Eimeria species were more than 90% except E. mitis UCE, indicating the 5 protein were highly conserved among 7 chicken Eimeria. Our further studies demonstrated the identified common antigen 14-3-3 protein and GAPDH could confer effective protection against challenge by several Eimeria species (unpublished data). Taken together, our data demonstrated that immunoproteomics screening could be an efficient approach for identifying common immunodominant proteins of Eimeria species.
Biological functions of the five common antigens have been generally described in some protozoa. Eukaryotic elongation factor 2 plays crucial role in the elongation stage of mRNA translation in eukaryotes, by mediating the translocation of the ribosome relative to the mRNA after addition of each amino acid to the nascent chain [35, 36]. The 14-3-3 proteins are a family of conserved regulatory molecules expressed in all eukaryotic cells. And play important roles in extensively regulatory processes, such as mitogenic signal transduction, apoptotic cell death, cell cycle control, and protein localization [37, 38]. E. tenella 14-3-3 protein could interact with the telomerase RNA-binding domain of telomerase reverse transcriptase [39]. UCE is a member of the family of ubiquitin-conjugating (E2) enzymes characterized by the presence of a highly conserved ubiquitin-conjugating (UBC) domain. E2 enzymes are well-conserved in eukaryotes and involved in Ub/UBL-modification pathways, and play central roles in processes like regulating protein degradation, function, and localization, thereby controlling the biology of the eukaryotic cell [40]. GAPDH is a key glycolytic enzyme in the process of metabolism of coccidian, as several pathogenic protozoa entirely depend on glycolysis as the source of ATP in the host [41, 42]. Transhydrogenase catalyses transhydrogenation between analogues of NAD(H) and NADP(H). A transhydrogenase was found to be located in the Eimeria refractile body and might function in relation with the ATP hydrolysis and respiration in the process of oocysts sporulation [43]. However, their specific biological functions in Eimeria need further studies.
In previous studies, the similarities of conserved or common antigens ranged from 70% to 99% [44–46]. In theory, the higher similarity a protein among several species is, the more conserved the protein is. Thus, we used a high threshold of 93% to define the common antigens. Certainly, we can use a lower threshold less than 93%. If so, more antigens would be identified as common antigens.
It has been reported that some of the five common immunodominant antigens were protective in protozoa and other parasites. Toxoplasma gondii 14-3-3 protein was proved to be a potential vaccine candidate against toxoplasmosis [47]. Leishmania elongation factor 2 was identified as T cell-stimulating antigen and might constitute potential vaccine candidates for leishmaniasis [48]. Elongation factor 1-Alpha was reported as protective antigen in Toxoplasma gondii and Cryptosporidium parvum [49, 50]. GAPDH was proved to confer protection against Haemonchus contortus and Schistosoma mansoni [51, 52]. Protozoal GAPDHs were suggested as a potential antiparasitic targets in Plasmodium falciparum [41, 42], Leishmania mexicana [53], Trypanosoma brucei and Trypanosoma cruzi [54, 55]. Chen et al. [56] reported that immunization with recombinant UCE induced protection against Taenia pisiformis. Our subsequent studies demonstrated that 14-3-3 protein and GAPDH could confer protection against coinfection of E. tenella, E. acervulina and E. maxima (unpublished data). Taken together, the five common immunodominant antigens could be selected as vaccine candidates against Eimeria.
We provided reference maps of sporozoite immunodominant proteins for E. tenella, E. acervulina and E. maxima. In some previous studies, sporozoites protein 2DE profiles of Eimeria have been reported [29, 57–60]. However, sporozoites immunodominant protein 2DE profiles were seldom reported. de Venevelles et al. analyzed the sporozoite 2DE map of E. tenella and detected approximately 50 immunodominant protein spots. However, they only identified a few of the immunodominant spots by mass spectrometry [29]. In this study, 50, 64 and 57 sporozoite immunodominant protein spots of E. tenella, E. acervulina and E. maxima were detected and identified as corresponding to 33 immunodominant antigens of E. tenella, 27 of E. acervulina and 25 of E. maxima respectively. Our results provided additional sporozoite immunodominant antigens and sporozoite immunodominant proteins reference maps for E. tenella, E. acervulina and E. maxima.
MATERIALS AND METHODS
Ethics statement
Animal experiments were conducted following the guidelines of the Animal Ethics Committee, Nanjing Agricultural University, China. All animal experiments were evaluated and approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (approval number: 2012CB120750).
Chickens and parasites
New-hatched Hy-Line layer chickens (commercial breed W-36) were reared in sterilized wire cages under coccidian-free conditions and provided daily with coccidiostat-free feed and water until the end of experiment. Oocysts of E. tenella, E. acervulina and E. maxima were propagated, harvested and sporulated using a previously described protocol [61], and then stored in 2% (w/v) potassium dichromate solution at 4°C no longer than 2 weeks. Purity of the parasites was determined by PCR based on the internal transcribed spacer-1 (ITS-1) as previously described [62, 63]. Sporozoites of the parasites were harvested from sporulated oocysts by in vitro excystation and purified over nylon wool and DE-52 cellulose columns [61].
Antisera preparation
Three antisera were prepared by inoculation of two-week-old chickens with pure coccidian. Chickens were orally inoculated 6 times at 3-day intervals with 1 × 104 sporulated oocysts of E. tenella, E. acervulina or E. maxima per chicken. Negative control birds were maintained under the same conditions and inoculated with distilled water. One week post the last inoculation, blood was collected from wing vein of the chickens. Subsequently, the sera were collected and determined by ELISA. A seventh even eighth dose would be given unless titers of the sera were beyond 1: 64. Sera were stored at −20°C for Western blot analysis. Meanwhile, serum was collected from uninfected chickens as negative control [21, 64].
Two-dimensional electrophoresis (2DE)
Purified sporozoites of the three species were smashed in lysis buffer (7 M urea, 2 M thiourea, 4% CHAPS, 40 mM dithiothreitol (DTT), 0.2% Bio-Lyte 3–10 ampholytes and 1 mM PMSF) by ultrasonic in ice bath (200 W, work time 5 s, intervaltime10 s, 50 cycles). Soluble proteins were obtained after centrifugation for 1 min (15,000 rpm) at 4°C. Then the soluble proteins were treated with 2D clean up kit and quantified using PlusOne™ 2-D Quant Kit (Amersham Pharmacia) [65].
For 2DE, 400μg of sporozoite proteins were loaded onto analytical and preparative gels. The Ettan IPGphor Isoelectric Focusing System (GE Amersham) and pH 3–10 immobilized pH gradient (IPG) strips (13 cm, nonlinear; GE Healthcare) were used for isoelectric focusing (IEF). The IPG strips were rehydrated for 12 h in 250 μl of rehydration buffer containing the protein samples. IEF was performed in four steps: 30V for 12 h, 500 V for 1 h, 1000 V for 1 h, and 8000 V for 8 h. The gel strips were equilibrated for 15 min in equilibration buffer (50 mM Tris-HCl (pH 8.8), 6 M urea, 2% SDS, 30% glycerol, and 1% DTT). This step was repeated using the same buffer with 4% iodoacetamide in place of 1% DTT. The strips were then subjected to the second-dimensional electrophoresis after transfer onto 12.5% SDS-polyacrylamide gels. Electrophoresis was performed using the Hofer SE 600 system (GE Amersham). The 2DE was performed twice for each sample simultaneously, and one of obtained gels was used for immunoblot analysis, while the other one prepared for silver staining.
Western blot and image analysis
Proteins in the 2DE gel were transferred electrophoretically onto a 0.45 μm pore size polyvinylidene fluoride (PVDF) membrane (GE Healthcare, USA) for 2 h at 100V using a TE62 Tank Transfer Unit system (GE Healthcare, USA). Membranes were then blocked in 5% skim milk in PBS containing 0.05% Tween 20 (PBST) for 1h at room temperature with gently swinging. And then was incubated with anti-E. tenella, anti-E. acervulina and anti-E. maxima sera (1:100) for 2 h at 37°C. The uninfected chicken serum was used to test another membrane as a negative control. After frequent washing with PBST, the membrane were incubated with secondary antibody of Goat anti-chicken IgG (1:2000, PTG Inc., USA) for 2 h at 37°C. Finally, 3, 3′-diaminobenzidine (DAB, Sigma) was added to visualize the immunodominant protein spots, according to the manufacturer's instructions.
Blots were scanned using TyphoonTMFLA 9500 (GE Amersham, USA). Through ImageMaster 2D Platinum (Version 5.0, GE Amersham, USA), the spots on the membranes were matched to their orthologs in 2DE gels stained using a modified silver staining methods compatible with subsequent mass spectrometric analysis [66].
Two-dimensional gel excision, Tryptic Digestion, and Desalting
All the the immunodominant spots on the PVDF membranes were excised from 2D gels from the preparative gels. Subsequently, the protein spots were destained for 20 min in 30 mM potassium ferricyanide/100mM sodium thiosulfate (1:1 v/v) and washed with Milli-Q waters. The spots were incubated in 0.2 M NH4HCO3 for 20 min and then lyophilized. Each spot was digested overnight in 12.5 ng/μl trypsin in 25 mM NH4HCO3. The peptides were extracted three times with 60% acetonitrile (ACN)/0.1% trifluoroacetic acid (TFA). The extracts were pooled and dried completely by a vacuum centrifuge.
MS analysis of protein spot and database searches
MS analysis of protein spot was performed by APT (Applied ProteinTechnology co. ltd, Shanghai, China). MS and MS/MS data for protein identification were obtained by using a MALDI-TOF-TOF instrument (5800 proteomics analyzer; Applied Biosystems). Instrument parameters were set using the 4000 Series Explorer software (Applied Biosystems). The MS spectra were recorded in reflector mode in a mass range from 800 to 4000 with a focus mass of 2000. MS was used a CalMix5 standard to calibrate the instrument (ABI 4700 Calibration Mixture). For one main MS spectrum 25 subspectra with 125 shots per subspectrum were accumulated using a random search pattern. For MS calibration, autolysis peaks of trypsin ([M+H]+842.5100 and 2,211.1046) were used as internal calibrates, and up to 10 of the most intense ion signals were selected as precursors for MS/MS acquisition, excluding the trypsin autolysis peaks and the matrix ion signals. In MS/MS positive ion mode, for one main MS spectrum 50 subspectra with 50 shots per subspectrum were accumulated using a random search pattern. Collision energy was 2 kV, collision gas was air, and default calibration was set by using the Glu1-Fibrino-peptide B ([M+H] + 1,570.6696) spotted onto Cal 7 positions of the MALDI target. Combined peptide mass fingerprinting PMF and MS/MS queries were performed by using the MASCOT search engine 2.2 (Matrix Science, Ltd.) embedded into GPS-Explorer Software 3.6 (Applied Biosystems) on the database of uniprot Eimeria or NCBI with the following parameter settings: 100 ppm mass accuracy, trypsin cleavage one missed cleavage allowed, carbamidomethylation set as fixed modification, oxidation of methionine was allowed as variable modification, MS/MS fragment tolerance was set to 0.4 Da. a GPS Explorer protein confidence index ≥ 95% were used for further manual validation.
Acknowledgments
We gratefully thank Menghui Li, ZhenChao Zhang and JingWei Huang for sample collection and valuable suggestions. We gratefully thank Ehsan for his careful polish with the manuscript.
Abbreviations
- E. tenella
Eimeria tenella
- E. acervulina
Eimeria acervulina
- E. maxima
Eimeria maxima
- E. mitis
Eimeria mitis
- 2DE
two-dimensional electrophoresis
- MALDI-TOF-MS/MS
Matrix-Assisted Laser Desorption/ Ionization Time of Flight Mass Spectrometry/ Mass Spectrometry
- NCBI
The National Center for Biotechnology Information
- spp.
species
- EF2
Elongation factor 2
- UCE
Ubiquitin-conjugating enzyme domain-containing protein
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- PBS
phosphate buffer saline
- ITS-1
internal transcribed spacer-1
Authors’ contributions
SXK designed the study and critically revised the manuscript. LXR, YRF and XLX helped in the study design and analyzed the data. LLR performed the laboratory tests. HXM contributed to the preparation and purity identification of Eimeria species and wrote the draft. LJH, LWY and JYH contributed to the separation of the Eimeria sporozoites antigen. TD, TL and YXC contributed to the preparation of the antisera and western blot analysis. All authors read and approved the final version of the manuscript.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
FUNDING
This work was supported by the National Natural Science Foundation of China (Grant No. 31672545; 31372428; 31661143017), the Natural Science Foundation of Jiangsu Province of China (Grant No. BK20161442), the Fundamental Research Funds for the Central Universities (Grant No. KYZ201631) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
REFERENCES
- 1.Dalloul RA, Lillehoj HS. Poultry coccidiosis: recent advancements in control measures and vaccine development. Expert Rev. Vaccines. 2006;5:143–163. doi: 10.1586/14760584.5.1.143. [DOI] [PubMed] [Google Scholar]
- 2.Blake DP, Tomley FM. Securing poultry production from the ever-present Eimeria challenge. Trends Parasitol. 2014;30:12–19. doi: 10.1016/j.pt.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Witcombe DM, Smith NC. Strategies for anti-coccidial prophylaxis. Parasitology. 2014;141:1379–1389. doi: 10.1017/S0031182014000195. [DOI] [PubMed] [Google Scholar]
- 4.Reid AJ, Blake DP, Ansari HR, Billington K, Browne HP, Bryant J, Dunn M, Hung SS, Kawahara F, Miranda-Saavedra D, Malas TB, Mourier T, Naghra H, et al. Genomic analysis of the causative agents of coccidiosis in domestic chickens. Genome Res. 2014;24:1676–1685. doi: 10.1101/gr.168955.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark EL, Tomley FM, Blake DP. Are Eimeria Genetically Diverse, and Does It Matter? Trends Parasitol. Trends Parasitol. 2017;33:231–241. doi: 10.1016/j.pt.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Clarke L, Fodey TL, Crooks SR, Moloney M, O'Mahony J, Delahaut P, O'Kennedy R, Danaher M. A review of coccidiostats and the analysis of their residues in meat and other food. Meat Sci. 2014;97:358–374. doi: 10.1016/j.meatsci.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Ahmad TA, El-Sayed BA, El-Sayed LH. Development of immunization trials against Eimeria spp. Trials Vaccinol. 2016;5:38–47. [Google Scholar]
- 8.Vermeulen AN. Progress in recombinant vaccine development against coccidiosis. A review and prospects into the next millennium. Int J Parasitol. 1998;28:1121–1130. doi: 10.1016/s0020-7519(98)00080-0. [DOI] [PubMed] [Google Scholar]
- 9.Meunier M, Chemaly M, Dory D. DNA vaccination of poultry: the current status in 2015. Vaccine. 2016;34:202–211. doi: 10.1016/j.vaccine.2015.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carvalho FS, Wenceslau AA, Teixeira M, Matos Carneiro JA, Melo AD, Albuquerque GR. Diagnosis of Eimeria species using traditional and molecular methods in field studies. Vet Parasitol. 2011;176:95–100. doi: 10.1016/j.vetpar.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 11.Ogedengbe JD, Hunter DB, Barta JR. Molecular identification of Eimeria species infecting market-age meat chickens in commercial flocks in Ontario. Vet Parasitol. 2011;178:350–354. doi: 10.1016/j.vetpar.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 12.del Cacho E, Gallego M, Lee SH, Lillehoj HS, Quilez J, Lillehoj EP, Sánchez-Acedo C. Induction of protective immunity against Eimeria tenella, Eimeria maxima, and Eimeria acervulina infections using dendritic cell-derived exosomes. Infect Immun. 2012;80:1909–1916. doi: 10.1128/IAI.06413-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Constantinoiu CC, Lillehoj HS, Matsubayashi M, Hosoda Y, Tani H, Matsuda H, Sasai K, Baba E. Analysis of cross-reactivity of five new chicken monoclonal antibodies which recognize the apical complex of Eimeria using confocal laser immunofluorescence assay. Vet Parasitol. 2003;118:29–35. doi: 10.1016/j.vetpar.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Chapman HD. Milestones in avian coccidiosis research: a review. Poult Sci. 2014;93:501–511. doi: 10.3382/ps.2013-03634. [DOI] [PubMed] [Google Scholar]
- 15.Talebi A. Protein profiles of five avian Eimeria species. Avian Pathol. 1995;24:731–735. doi: 10.1080/03079459508419112. [DOI] [PubMed] [Google Scholar]
- 16.Sasai K, Lillehoj HS, Matsuda H, Wergin WP. Characterization of a chicken monoclonal antibody that recognizes the apical complex of Eimeria acervulina sporozoites and partially inhibits sporozoite invasion of CD8+ T lymphocytes in vitro. J. Parasitol. 1996;82:82–87. [PubMed] [Google Scholar]
- 17.Guzman VB, Silva DA, Kawazoe U, Mineo JR. A comparison between IgG antibodies against Eimeria acervulina, E. maxima, and E. tenella and oocyst shedding in broiler-breeders vaccinated with live anticoccidial vaccines. Vaccine. 2003;21:4225–4233. doi: 10.1016/s0264-410x(03)00462-6. [DOI] [PubMed] [Google Scholar]
- 18.Wallach M, Pillemer G, Yarus S, Halabi A, Pugatsch T, Mencher D. Passive immunization of chickens against Eimeria maxima infection with a monoclonal antibody developed against a gametocyte antigen. Infect Immun. 1990;58:557–562. doi: 10.1128/iai.58.2.557-562.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crane MS, Murray PK, Gnozzio MJ, MacDonald TT. Passive protection of chickens against Eimeria tenella infection by monoclonal antibody. Infect Immun. 1988;56:972–976. doi: 10.1128/iai.56.4.972-976.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith NC, Wallach M, Miller CM, Braun R, Eckert J. Maternal transmission of immunity to Eimeria maxima: Western blot analysis of protective antibodies induced by infection. Infect Immun. 1994;62:4811–4817. doi: 10.1128/iai.62.11.4811-4817.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Constantinoiu CC, Molloy JB, Jorgensen WK, Coleman GT. Development and validation of an ELISA for detecting antibodies to Eimeria tenella in chickens. Vet Parasitol. 2007;150:306–313. doi: 10.1016/j.vetpar.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Réfega S, Girard-Misguich F, Bourdieu C, Péry P, Labbé M. Gene discovery in Eimeria tenella by immunoscreening cDNA expression libraries of sporozoites and schizonts with chicken intestinal antibodies. Vet Parasitol. 2003;113:19–33. doi: 10.1016/s0304-4017(03)00033-5. [DOI] [PubMed] [Google Scholar]
- 23.Laurent F, Bourdieu C, Kazanji M, Yvoré P, Péry P. The immunodominant Eimeria acervulina sporozoite antigen previously described as p160/p240 is a 19-kilodalton antigen present in several Eimeria species. Mol Biochem Parasitol. 1994;63:79–86. doi: 10.1016/0166-6851(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 24.Brothers VM, Kuhn I, Paul LS, Gabe JD, Andrews WH, Sias SR, McCaman MT, Dragon EA, Files JG. Characterization of a surface antigen of Eimeria tenella sporozoites and synthesis from a cloned cDNA in Escherichia coli. Mol Biochem Parasitol. 1988;28:235–247. doi: 10.1016/0166-6851(88)90008-4. [DOI] [PubMed] [Google Scholar]
- 25.Ko C, Smith CK, 2nd, McDonell M. Identification and characterization of a target antigen of a monoclonal antibody directed against Eimeria tenella merozoites. Mol Biochem Parasitol. 1990;41:53–63. doi: 10.1016/0166-6851(90)90096-5. [DOI] [PubMed] [Google Scholar]
- 26.Li J, Zhang X, Liu Q, Yin J, Yang J. Eimeria tenella: cloning of a novel Eimeria tenella cDNA encoding a protein related to rhomboid family from F2 hybrid strain. Exp Parasitol. 2006;113:215–220. doi: 10.1016/j.exppara.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Xu Q, Song X, Xu L, Yan R, Shah MA, Li X. Vaccination of chickens with a chimeric DNA vaccine encoding Eimeria tenella TA4 and chicken IL-2 induces protective immunity against coccidiosis. Vet Parasitol. 2008;156:319–323. doi: 10.1016/j.vetpar.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 28.Li J, Zheng J, Gong P, Zhang X. Efficacy of Eimeria tenella rhomboid-like protein as a subunit vaccine in protective immunity against homologous challenge. Parasitol Res. 2012;110:1139–1145. doi: 10.1007/s00436-011-2603-1. [DOI] [PubMed] [Google Scholar]
- 29.de Venevelles P, Chich JF, Faigle W, Loew D, Labbé M, Girard-Misguich F, Péry P. Towards a reference map of Eimeria tenella sporozoite proteins by two-dimensional electrophoresis and mass spectrometry. Int J Parasitol. 2004;34:1321–1331. doi: 10.1016/j.ijpara.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, Xu L, Yan F, Yan R, Song X, Li X. Immunoproteomic analysis of the second-generation merozoite proteins of Eimeria tenella. Vet Parasitol. 2009;164:173–82. doi: 10.1016/j.vetpar.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 31.de Venevelles P, François Chich J, Faigle W, Lombard B, Loew D, Péry P, Labbé M. Study of proteins associated with the Eimeria tenella refractile body by a proteomic approach. Int J Parasitol. 2006;36:1399–1407. doi: 10.1016/j.ijpara.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Schaap D, Arts G, Kroeze J, Niessen R, Roosmalen-Vos SV, Spreeuwenberg K, Kuiper CM, Beek-Verhoeven NV, Kok JJ, Knegtel RM, Vermeulen AN. An Eimeria vaccine candidate appears to be lactate dehydrogenase; characterization and comparative analysis. Parasitology. 2004;128:603–616. doi: 10.1017/s0031182004005104. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, Liu L, Huang J, Wang S, Lu M, Song X, Xu L, Yan R, Li X. The molecular characterization and immune protection of microneme 2 of Eimeria acervulina. Vet Parasitol. 2016;215:96–105. doi: 10.1016/j.vetpar.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Huang J, Zhang Z, Li M, Song X, Yan R, Xu L, Li X. Immune protection of microneme 7 (EmMIC7) against Eimeria maxima challenge in chickens. Avian Pathol. 2015;44:392–400. doi: 10.1080/03079457.2015.1071780. [DOI] [PubMed] [Google Scholar]
- 35.Browne GJ, Proud CG. Regulation of peptide-chain elongation in mammalian cells. Eur J Biochem. 2002;269:5360–5368. doi: 10.1046/j.1432-1033.2002.03290.x. [DOI] [PubMed] [Google Scholar]
- 36.Kaul G, Pattan G, Rafeequi T. Eukaryotic elongation factor-2 (eEF2): its regulation and peptide chain elongation. Cell Biochem Funct. 2011;29:227–234. doi: 10.1002/cbf.1740. [DOI] [PubMed] [Google Scholar]
- 37.Fu H, Subramanian RR, Masters SC. 14–3-3 proteins: structure, function, and regulation. Annu Rev Pharmacol Toxicol. 2000;40:617–647. doi: 10.1146/annurev.pharmtox.40.1.617. [DOI] [PubMed] [Google Scholar]
- 38.Mathew DE, Larsen K, Janeczek P, Lewohl JM. Expression of 14–3-3 transcript isoforms in response to ethanol exposure and their regulation by miRNAs. Mol Cell Neurosci. 2016;75:44–49. doi: 10.1016/j.mcn.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 39.Zhao N, Gong P, Cheng B, Li J, Yang Z, Li H, Yang J, Zhang G, Zhang X. Eimeria tenella: 14-3-3 protein interacts with telomerase. Parasitol Res. 2014;113:3885–3889. doi: 10.1007/s00436-014-4108-1. [DOI] [PubMed] [Google Scholar]
- 40.van Wijk SJ, Timmers HT. The family of ubiquitin-conjugating enzymes (E2s): deciding between life and death of proteins. FASEB J. 2010;24:981–993. doi: 10.1096/fj.09-136259. [DOI] [PubMed] [Google Scholar]
- 41.Bruno S, Uliassi E, Zaffagnini M, Prati F, Bergamini C, Amorati R, Paredi G, Margiotta M, Conti P, Costi MP, Kaiser M, Cavalli A, Fato R, et al. Molecular basis for covalent inhibition of glyceraldehyde-3-phosphate dehydrogenase by a 2-phenoxy-1,4-naphthoquinone small molecule. Chem Biol Drug Des. 2017. Jan 12, Epub ahead of print. [DOI] [PubMed]
- 42.Bruno S, Margiotta M, Pinto A, Cullia G, Conti P, De Micheli C, Mozzarelli A. Selectivity of 3-bromo-isoxazoline inhibitors between human and Plasmodium falciparum glyceraldehyde-3-phosphate dehydrogenases. Bioorg Med Chem. 2016;24:2654–2659. doi: 10.1016/j.bmc.2016.04.033. [DOI] [PubMed] [Google Scholar]
- 43.Vermeulen AN, Kok JJ, van den Boogaart P, Dijkema R, Claessens AJ. Eimeria refractile body proteins contain two potentially functional characteristics: Transhydrogenase and carbohydrate transport. FEMS Microbiol Lett. 1993;110:223–229. doi: 10.1111/j.1574-6968.1993.tb06324.x. [DOI] [PubMed] [Google Scholar]
- 44.Jaiswal V, Chauhan RS, Rout C. Common antigens prediction in bacterial bioweapons: A perspective for vaccine design. Infect Genet Evol. 2014;21:315–319. doi: 10.1016/j.meegid.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 45.Li K, Guidice GJ, Tamai K, Do HC, Sawamura D, Diaz LA, Uitto J. Cloning of partial cDNA for mouse 180-kDa bullous pemphigoid antigen (BPAG2), a highly conserved collagenous protein of the cutaneous basement membrane zone. J Invest Dermatol. 1992;99:258–263. doi: 10.1111/1523-1747.ep12616611. [DOI] [PubMed] [Google Scholar]
- 46.Ishibashi M, Noda AO, Sakate R, Imanishi T. Evolutionary growth process of highly conserved sequences in vertebrate genomes. Gene. 2012;504:1–5. doi: 10.1016/j.gene.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 47.Meng M, He S, Zhao G, Bai Y, Zhou H, Cong H, Lu G, Zhao Q, Zhu XQ. Evaluation of protective immune responses induced by DNA vaccines encoding Toxoplasma gondii surface antigen 1 (SAG1) and 14-3-3 protein in BALB/c mice. Parasite Vectors. 2012;5:273. doi: 10.1186/1756-3305-5-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Probst P, Stromberg E, Ghalib HW, Mozel M, Badaro R, Reed SG, Webb JR. Identification and characterization of T cell-stimulating antigens from Leishmania by CD4 T cell expression cloning. J Immunol. 2001;166:498–505. doi: 10.4049/jimmunol.166.1.498. [DOI] [PubMed] [Google Scholar]
- 49.Wang S, Zhang Z, Wang Y, Gadahi JA, Xu L, Yan R, Song X, Li X. Toxoplasma gondii elongation factor 1-alpha is a novel vaccine candidate antigen against toxoplasmosis. Front Microbiol. 2017;8:168. doi: 10.3389/fmicb.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsubayashi M, Teramoto-Kimata I, Uni S, Lillehoj HS, Matsuda H, Furuya M, Tani H, Sasai K. Elongation factor-1alpha is a novel protein associated with host cell invasion and a potential protective antigen of Cryptosporidium parvum. J Biol Chem. 2013;288:34111–34120. doi: 10.1074/jbc.M113.515544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Han K, Xu L, Yan R, Song X, Li X. Vaccination of goats with glyceraldehyde-3-phosphate dehydrogenase DNA vaccine induced partial protection against Haemonchus contortus. Vet Immunol Immunopathol. 2012;149:177–185. doi: 10.1016/j.vetimm.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Tallima H, Montash M, Veprek P, Velek J, Jezek J, El Ridi R. Differences in immunogenicity and vaccine potential of peptides from Schistosoma mansoni glyceraldehyde 3-phosphate dehydrogenase. Vaccine. 2003;21:3290–3300. doi: 10.1016/s0264-410x(03)00180-4. [DOI] [PubMed] [Google Scholar]
- 53.Suresh S, Bressi JC, Kennedy KJ, Verlinde CL, Gelb MH, Hol WG. Conformational changes in Leishmania mexicana glyceraldehyde-3-phosphate dehydrogenase induced by designed inhibitors. J Mol Biol. 2001;309:423–435. doi: 10.1006/jmbi.2001.4588. [DOI] [PubMed] [Google Scholar]
- 54.Pereira JM, Severino RP, Vieira PC, Fernandes JB, da Silva MF, Zottis A, Andricopulo AD, Oliva G, Corrêa AG. Anacardic acid derivatives as inhibitors of glyceraldehyde-3-phosphate dehydrogenase from Trypanosoma cruzi. Bioorg Med Chem. 2008;16:8889–8895. doi: 10.1016/j.bmc.2008.08.057. [DOI] [PubMed] [Google Scholar]
- 55.Belluti F, Uliassi E, Veronesi G, Bergamini C, Kaiser M, Brun R, Viola A, Fato R, Michels PA, Krauth-Siegel RL, Cavalli A, Bolognesi ML. Toward the development of dual-targeted glyceraldehyde-3-phosphate dehydrogenase/trypanothione reductase inhibitors against Trypanosoma brucei and Trypanosoma cruzi. ChemMedChem. 2014;9:371–382. doi: 10.1002/cmdc.201300399. [DOI] [PubMed] [Google Scholar]
- 56.Chen L, Yang D, Xie Y, Nong X, Huang X, Fu Y, Gu X, Wang S, Peng X, Yang G. Protection against Taenia pisiformis larval infection induced by a recombinant oncosphere antigen vaccine. Genet Mol Res. 2014;13:6148–6159. doi: 10.4238/2014.February.13.14. [DOI] [PubMed] [Google Scholar]
- 57.Bromley E, Leeds N, Clark J, McGregor E, Ward M, Dunn MJ, Tomley F. Defining the protein repertoire of microneme secretory organelles in the apicomplexan parasite Eimeria tenella. Proteomics. 2003;3:1553–1561. doi: 10.1002/pmic.200300479. [DOI] [PubMed] [Google Scholar]
- 58.Lal K, Bromley E, Oakes R, Prieto JH, Sanderson SJ, Kurian D, Hunt L, Yates JR, 3rd, Wastling JM, Sinden RE, Tomley FM. Proteomic comparison of four Eimeria tenella life-cycle stages: unsporulated oocyst, sporulated oocyst, sporozoite and second-generation merozoite. Proteomics. 2009;9:4566–4576. doi: 10.1002/pmic.200900305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Oakes RD, Kurian D, Bromley E, Ward C, Lal K, Blake DP, Reid AJ, Pain A, Sinden RE, Wastling JM, Tomley FM. The rhoptry proteome of Eimeria tenella sporozoites. Int J Parasitol. 2013;43:181–188. doi: 10.1016/j.ijpara.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 60.El-Ashram S, Yin Q, Liu H, Al Nasr I, Liu X, Suo X, Barta J. From the Macro to the Micro: Gel Mapping to Differentiate between Sporozoites of Two Immunologically Distinct Strains of Eimeria maxima (Strains M6 and Guelph) PLoS One. 2015;10:e0143232. doi: 10.1371/journal.pone.0143232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomley F. Techniques for isolation and characterization of apical organelles from Eimeria tenella sporozoites. Methods. 1997;13:171–176. doi: 10.1006/meth.1997.0509. [DOI] [PubMed] [Google Scholar]
- 62.Jenkins MC, Miska K, Klopp S. Application of polymerase chain reaction based on ITS1 rDNA to speciate Eimeria. Avian Dis. 2006;50:110–114. doi: 10.1637/7439-091305R.1. [DOI] [PubMed] [Google Scholar]
- 63.Haug A, Thebo P, Mattsson JG. A simplified protocol for molecular identification of Eimeria species in field samples. Vet. Parasitol. 2007;146:35–45. doi: 10.1016/j.vetpar.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 64.Xie M, Gilbert JM, McDougald LR. Electrophoretic and immunologic characterization of proteins of merozoites of Eimeria acervulina. E. maxima, E. necatrix, and E. tenella. J Parasitol. 1992;78:82–86. [PubMed] [Google Scholar]
- 65.Zhang Z, Huang J, Li M, Sui Y, Wang S, Liu L, Xu L, Yan R, Song X, Li X. Identification and molecular characterization of microneme 5 of Eimeria acervulina. PLoS One. 2014;9:e115411. doi: 10.1371/journal.pone.0115411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yan JX, Wait R, Berkelman T, Harry RA, Westbrook JA, Wheeler CH, Dunn MJ. A modified silver staining protocol for visualization of proteins compatible with matrix-assisted laser desorption/ionization and electrospray ionization-mass spectrometry. Electrophoresis. 2000;21:3666–3672. doi: 10.1002/1522-2683(200011)21:17<3666::AID-ELPS3666>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]