Abstract
Platelets play a multifaceted role in cancer progression and metastasis. Mean platelet volume (MPV) and platelet distribution width (PDW) are commonly used platelet parameters from routine blood test. The aim of the present study was to investigate the correlation between platelet indices and prognosis in patients with non-small cell lung cancer (NSCLC). A total of 270 patients who were diagnosed with NSCLC between January 2009 and December 2009 were retrospectively analyzed. Patients’ characteristics and hematologic tests data at initial diagnosis were collected. The overall survival rate was estimated using Kaplan-Meier method. The prognostic analysis was carried out with univariate and multivariate Cox regressions model. Reduced PDW was significantly correlated with T stage, N stage, TNM stage, and histological type of the disease. Moreover, survival analysis showed that the overall survival of patients with PDW ≥ 16.3% was significantly longer than that of those with PDW < 16.3% (P < 0.001). In multivariate Cox regression model, age, sex, TNM stage, and PDW were identified as independent prognostic factors for overall survival (for PDW, P < 0.001). In conclusion, reduced PDW is an unfavorable predictive factor of NSCLC patient survival. Further studies are warranted.
Introduction
Lung cancer is one of the most common cancers worldwide, with non-small cell lung cancer (NSCLC) accounting for 80% of all diagnosed lung cancer cases. Current treatments, including surgery, radiotherapy, and chemotherapy, exhibit limited effectiveness for NSCLC. Moreover, long-term survival after surgical resection remains poor owing to the high rate of recurrence and metastasis1. Therefore, to improve treatment strategies, investigation of novel biomarkers is clinically urgently needed.
Platelets play a multifaceted role in cancer progression and metastases. Complex interactions between platelets and tumor cells result in tumor growth, aberrant angiogenesis, invasion, and metastasis2, 3. Several studies have reported that elevated platelets are associated with a poor prognosis in various types of cancer, including pancreatic cancer, gastric cancer, colorectal cancer, endometrial cancer, and ovarian cancer4–8. However, total platelet count is determined by the balance between the rate of production and consumption of platelets. A normal platelet count could conceal the presence of highly hypercoagulative and pro-inflammatory cancer phenotypes in the presence of efficient compensatory mechanisms9.
Mean platelet volume (MPV) is an indicator of activated platelets and is associated with different inflammatory conditions10. Platelet distribution width (PDW), another platelet index, indicates variation in platelet size and differentially diagnoses thrombocytopenia11. In addition, both MPV and PDW are easily detected with routinely used hemocytometers. A study found that reduced MPV predicted an unfavorable prognosis in patients with NSCLC12. However, PDW has not been studied completely.
The purpose of this study was to investigate the prognostic impact of the preoperative platelet indices on the survival in NSCLC patients.
Results
The characteristics of the patients are summarized in Table 1. Overall, there were 177 (65.6%) male and 93 (34.4%) female patients, and the median age was 57.3 ± 9.3 years (range 32–80). The numbers of patients with adenocarcinoma, squamous cell carcinoma, and other carcinomas were 159, 103, and 8, respectively. In terms of the staging system, 84 cases were categorized as stage I, 146 as stage II, 30 as stage III and 10 as stage IV. Median follow-up time was 60.0 months. Throughout the follow-up period there were 51 deaths. The estimated cumulative 5-year survival for this patient population was 20.4% for OS.
Table 1.
Baseline characteristics of the patients with lung cancer according to PDW levels.
| Variables | Total n (%) | PDW ≤ 16.3 n (%) | PDW > 16.3n (%) | P value |
|---|---|---|---|---|
| Age (years) | 0.495 | |||
| ≤60 | 174 (64.4) | 27 (60.0) | 147 (65.3) | |
| >60 | 96 (35.6) | 18 (40.0) | 78 (34.7) | |
| Gender | 0.390 | |||
| Male | 177 (65.6) | 32 (71.1) | 145 (64.4) | |
| Female | 93 (34.4) | 13 (28.9) | 80 (35.6) | |
| Smoking | 0.476 | |||
| Yes | 151 (55.9) | 23 (51.1) | 128 (30.8) | |
| No | 119 (44.1) | 22 (48.9) | 97 (43.1) | |
| Tumor size | 0.078 | |||
| >4 cm | 84 (31.1) | 9 (20.0) | 75 (33.3) | |
| ≤4 cm | 186 (68.9) | 36 (80.0) | 150 (66.7) | |
| Differentiation | 0.362 | |||
| Well/moderate | 195 (72.2) | 30 (66.7) | 165 (73.3) | |
| Poor | 75 (27.8) | 15 (33.3) | 60 (26.7) | |
| Histological type | <0.001 | |||
| Adenocarcinoma | 159 (58.9) | 26 (57.8) | 133 (59.1) | |
| Squamous cell carcinoma | 103 (38.1) | 17 (37.8) | 86 (38.2) | |
| Others | 8 (3.0) | 2 (4.4) | 6 (2.7) | |
| T stage | <0.001 | |||
| T1 | 84 (31.1) | 19 (42.2) | 65 (28.9) | |
| T2 | 146 (54.1) | 18 (40.0) | 128 (56.9) | |
| T3 | 30 (11.1) | 8 (17.8) | 22 (9.8) | |
| T4 | 10 (3.7) | 0 (0) | 10 (4.4) | |
| N stage | 0.013 | |||
| N0 | 180 (66.7) | 31 (68.9) | 149 (66.2) | |
| N1 | 34 (12.6) | 3 (6.7) | 31 (13.8) | |
| N2 | 51 (18.9) | 10 (22.2) | 41 (18.2) | |
| N3 | 5 (1.9) | 1 (2.2) | 4 (1.8) | |
| Cancer Stage | <0.001 | |||
| I | 143 (53.0) | 18 (40.0) | 125 (55.5) | |
| II | 53 (19.6) | 11 (24.4) | 42 (18.7) | |
| III | 74 (27.4) | 16 (35.6) | 58 (25.8) | |
PDW, platelet distribution width.
The median value of PDW was 17.0 (range, 9.7–20.6). ROC curve analysis was used to determine the optimal cutoff value for PDW. ROC analysis showed that if the chosen cut-off point for PDW was 16.3, the specificity and sensitivity were 51.9% and 96.3%, respectively (AUC = 0.785, 95% CI: 0.732–0.833, P < 0.0001). Of the total of 270 patients, 45 patients (16.7%) were detected with PDW of less than or equal to 16.3, while there were 225 patients (83.3%) whose PDW was greater than 16.3.
Correlations between PDW and clinicopatholotic parameters are shown in Table 2. There were no significant differences in age, sex, BMI, smoking history, hemoglobin, platelet count, MPV, NLR, PLR, tumor size, and differentiation between the two groups. However, T stage, N stage, TNM stage, and histological type in two groups show significant difference.
Table 2.
Baseline characteristics of the patients with lung cancer according to PDW levels.
| Variables | PDW ≤ 16.3 | PDW > 16.3 | P value |
|---|---|---|---|
| Age (years) | 57.6 (9.7) | 57.2 (9.3) | 0.786 |
| BMI (kg/m2) | 22.5 (2.8) | 23.5 (3.1) | 0.071 |
| Hemoglobin (g/dl) | 133.8 (18.7) | 138.1 (18.6) | 0.163 |
| Platelet count (×109/L) | 267.6 (83.2) | 241.5 (68.3) | 0.053 |
| MPV (fL) | 8.8 (1.7) | 8.6 (1.3) | 0.435 |
| NLR | 2.97 (1.69) | 2.49 (2.11) | 0.152 |
| PLR | 151.5 (71.4) | 138.2 (63.8) | 0.214 |
PDW, platelet distribution width; BMI, body mass index; MPV, mean platelet volume; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
The 5-year overall survival rate in this cohort was 20.4%. The 5-year overall survival rates in patients with PDW ≤ 16.3 and PDW > 16.3 were 35.6% and 88.9%, respectively. The overall survival of patients with PDW less than or equal to 16.3 was significantly shorter than those with PDW greater than 16.3 (P < 0.001) (Fig. 1).
Figure 1.
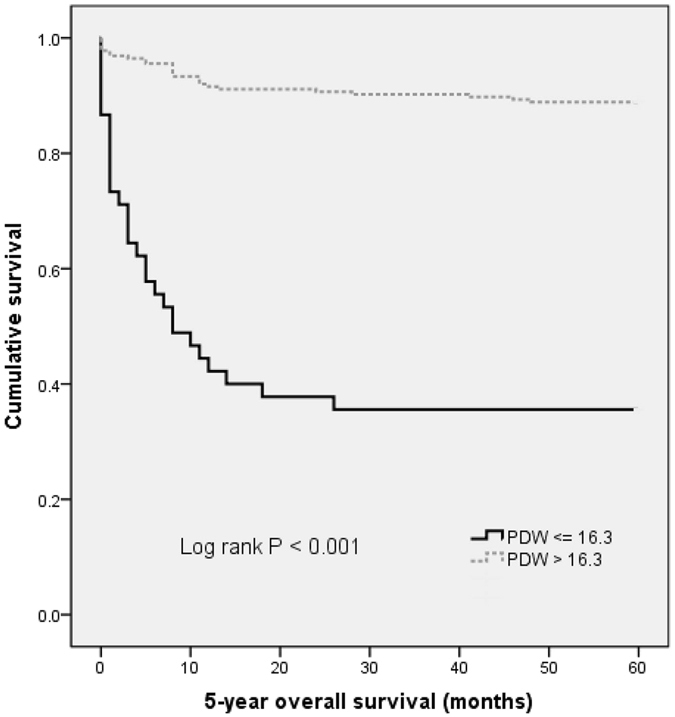
Kaplan–Meier analysis of overall survival in NSCLC patients.
The results of univariate and multivariate analysis were shown in Tables 3 and 4. Univariate analysis showed that age, sex, TNM stage, platelet count, PDW, NLR and PLR were significantly related to overall survival. Then, Cox regression analyzed models was constructed to compare the prognostic significance of PDW. Age, sex, TNM stage, and PDW were independent prognostic markers for overall survival. Notably, PDW before treatment was an independent factor for overall survival, with HR of 0.102 (95% CI: 0.058–0.180, P < 0.001).
Table 3.
Univariate analysis of overall survival in patients with lung cancer.
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| Age (years) (≤60 versus >60) | 1.725 | 1.010–2.946 | 0.046 |
| Gender (male versus female) | 2.470 | 1.243–4.909 | 0.010 |
| BMI (kg/m2) | 0.994 | 0.959–1.030 | 0.733 |
| Smoking (yes versus no) | 0.830 | 0.486–1.415 | 0.493 |
| T stage | 0.754 | 0.510–1.115 | 0.157 |
| N stage | 1.087 | 0.805–1.470 | 0.585 |
| Cancer Stage (III versus I+II) | 2.435 | 1.423–4.167 | 0.001 |
| Tumor Size (cm) (≤4 versus >4) | 0.934 | 0.521–1.676 | 0.820 |
| Differentiation (well/moderate versus poor) | 1.571 | 0.904–2.729 | 0.109 |
| Hemoglobin (g/dl) | 0.992 | 0.980–1.005 | 0.233 |
| Platelet count (×109/L) | 1.004 | 1.001–1.008 | 0.020 |
| MPV (fL) | 1.140 | 0.949–1.370 | 0.162 |
| PDW (%) (≤16.3 versus >16.3) | 0.113 | 0.066–0.195 | <0.001 |
| NLR | 1.117 | 1.041–1.200 | 0.002 |
| PLR | 1.004 | 1.002–1.007 | 0.003 |
PDW, platelet distribution width; BMI, body mass index; MPV, mean platelet volume; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio.
Table 4.
Multivariate analysis of overall survival in patients with lung cancer.
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| Age (years) (≤60 versus >60) | 2.123 | 1.220–3.695 | 0.008 |
| Gender (male versus female) | 2.626 | 1.294–5.326 | 0.007 |
| Cancer Stage (III versus I+II) | 2.770 | 1.576–4.871 | <0.001 |
| Platelet count (×109/L) | 1.002 | 0.998–1.007 | 0.356 |
| PDW (≤16.3 versus >16.3) | 0.102 | 0.058–0.180 | <0.001 |
| NLR | 1.096 | 0.929–1.294 | 0.277 |
| PLR | 1.000 | 0.994–1.007 | 0.988 |
Variables that showed a P value < 0.10 in univariate analysis were included in a multivariate Cox proportional hazards regression model.
Discussion
With the present study, we demonstrated that reduced PDW was independently associated with the prognosis of NSCLC.
Despite best current medical and surgical treatment, the overall prognosis of patients with lung cancer remains poor. An increasing body of evidence point to the key roles of platelets in tumor progression13. Thrombocytosis is linked to with reduced survival in patients with various tumor types, including cancer of lung, ovary, endometrium, rectum, kidney, stomach, pancreas, brain, and breast. Recent studies revealed that cancer-associated thrombocytosis was a paraneoplastic phenomenon. Tumors could promote platelet production and activation through the interleukin (IL)-6 pathway14. In lung cancer, platelet-derived growth factor (PDGF) beta-receptor expression was found to be significantly higher in rare sarcomatoid NSCLC versus non-sarcomatoid NSCLC controls15.
A specific mechanism to explain reduced PDW in lung cancer remains to be determined. Cancer-associated inflammation may be a common mechanism for decreased PDW. PDW is a measure of platelet volume heterogeneity. Platelet volume is determined both during megakaryopoiesis and during thrombopoiesis. Megakaryocytic maturation, platelet production and platelet size could be modulated by cytokines, such as interleukin-6 (IL-6), granulocytes colony stimulating factor (G-CSF) and macrophage colony stimulating factor (M-CSF)16. Elevated interleukin-6 (IL-6) has been observed in almost all types of tumors acting as a major pro-inflammatory mediator in tumor microenvironment. Numerous studies suggests that IL-6 promotes tumorigenesis by regulating apoptosis, survival, proliferation, angiogenesis, metastasis and metabolism17. Moreover, megakaryopoiesis and subsequent thrombopoiesis in cancer may be stimulated by pro-inflammatory cytokines G-CSF and M-CSF, which could be secreted by tumor cells18. In accord with previous results, we found that reduced PDW is negatively correlated with white blood cell count (see Supplementary Table 1).
However, the change of PDW in different types of cancers is inconsistent. PDW has been noted to be increased in gastric cancer and lung cancer19, 20. In contrast, PDW is decreased in thyroid cancer and breast cancer21, 22. The conflicting data maybe due to small sample sizes, failure to rule out confounding factors, different tumor type, and selected populations. Therefore, further research on PDW levels in cancer is warranted.
The present study has some limitations. Firstly, this was a single-center retrospective study and multicentric prospective studies are needed to reduce selection bias. Secondly, a mechanistic explanation for our findings is not provided by our data and further study is needed to identify the precise mechanism. Thirdly, because the study sample includes only Chinese participants, caution is needed in extrapolating our results to different ethnic groups.
In conclusion, our study indicates that a preoperative reduced PDW is an independent prognostic biomarker for overall survival in NSCLC that is superior to NLR and PLR. The results suggest that platelets may play a greater role in promoting NSCLC progression. Additional investigations are needed to fully understand the potential mechanism.
Materials and Methods
Study population
The records of patients with lung cancer who were admitted to Harbin Medical University Cancer Hospital, Harbin Medical University between January 2009 and December 2009 were retrospectively reviewed. Patients meeting all of the following requirements were eligible for enrollment: (1) undergone complete surgical resection and diagnosis of lung cancer was confirmed by histology; (2) without distant metastasis at diagnosis; (3) untreated before diagnosis. Exclusion criteria included: hematological disorders, autoimmune diseases, systemic inflammatory diseases, coronary artery disease, hypertension, diabetes mellitus, thyroid disease, renal disease, hepatic disorder and other cancer, and medical treatment with anticoagulant, statins, and acetylic salicylic acid. The pathological stages of patients were determined according to the international TNM classification system for lung cancer23.
Written informed consents were obtained from all patients. This study was approved by the Institutional Review Board of Harbin Medical University Cancer Hospital of Harbin Medical University. All studies were conducted according to guidelines (Declaration of Helsinki) for biomedical research. The last follow-up date was December 31, 2014. Overall survival (OS) was calculated from the date of surgery to the date of death or last follow-up.
Clinical examination and biochemical measurements
All the subjects underwent physical examination. Body mass index (BMI) was calculated as the ratio of weight (kg) to height squared (m2). Clinical data including smoking status, medical history and medication use were recorded for each subject. Venous blood samples after a 10-hour overnight fasting were collected from the individuals within 1 week prior to surgery. White blood cell (WBC), haemoglobin, and platelet indices were measured by an autoanalyzer (Sysmex XE-2100, Kobe, Japan). The whole blood samples were collected in EDTA-containing tubes, and all samples were processed within 30 minutes after blood collection. The inter- and intra-assays coefficients of variation (CVs) of all these assays were below 5%. The platelet-to-lymphocyte ratio (PLR) was calculated as the absolute platelet count measured in ×109/L divided by the absolute lymphocyte count measured in ×109/L. The neutrophil-to-lymphocyte ratio (NLR) was calculated as the absolute neutrophil count measured in ×109/L divided by the absolute lymphocyte count measured in ×109/L. The ideal cutoff value for PDW was determined applying receiver operating curve analysis.
Statistical analysis
All statistical analyses were performed using SPSS Statistics version 22.0 (SPSS Inc., Chicago, IL, USA). The descriptive statistics are presented as means ± SD or medians (interquartile range) for continuous variables and percentages of the number for categorical variables. When baseline characteristics between two groups were compared, normally distributed continuous variables were compared with the Student t test and skewed-distributed with the Mann-Whitney U test. The Chi-square test was used for categorical variables. Survival curves were visualized by the Kaplan–Meier method and examined by a log-rank test. Univariate and multivariate analyses of the significance of demographic and clinico–pathological parameters associated with lung cancer were assessed by a Cox proportional hazards regression model. Variables that showed a P value < 0.1 in univariate analysis were included in a multivariate Cox proportional hazards regression model. The significance level was set at P < 0.05.
Electronic supplementary material
Acknowledgements
This work was supported financially by grants from the Harbin Medical University Cancer Hospital (JJZD2017-05).
Author Contributions
M.M.C., N.L., and X.L. participated in study design, data analysis and manuscript preparation. Z.Y.Y. and Y.N. participated in data collection and data analysis. Y.Z., B.N.G. and T.M.L. participated in data analysis. R.T.W. participated in study design, data analysis, manuscript preparation and editing. All authors read and approved the final manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Ming-ming Cui, Na Li and Xing Liu contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03772-z
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Peters S, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012;23(Suppl 7):vii56–64. doi: 10.1093/annonc/mds226. [DOI] [PubMed] [Google Scholar]
- 2.Bambace NM, Holmes CE. The platelet contribution to cancer progression. J. Thromb. Haemost. 2011;9(2):237–249. doi: 10.1111/j.1538-7836.2010.04131.x. [DOI] [PubMed] [Google Scholar]
- 3.Goubran HA, Stakiw J, Radosevic M, Burnouf T. Platelet-cancer interactions. Semin. Thromb. Hemost. 2014;40(3):296–305. doi: 10.1055/s-0034-1370767. [DOI] [PubMed] [Google Scholar]
- 4.Chadha AS, et al. Paraneoplastic thrombocytosis independently predicts poor prognosis in patients with locally advanced pancreatic cancer. Acta Oncol. 2015;54(7):971–978. doi: 10.3109/0284186X.2014.1000466. [DOI] [PubMed] [Google Scholar]
- 5.Feng Z, et al. Thrombocytosis and hyperfibrinogenemia are predictive factors of clinical outcomes in high-grade serous ovarian cancer patients. BMC Cancer. 2016;16:43. doi: 10.1186/s12885-016-2070-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heng S, Benjapibal M. Preoperative thrombocytosis and poor prognostic factors in endometrial cancer. Asian Pac. J. Cancer Prev. 2014;15(23):10231–10236. doi: 10.7314/APJCP.2014.15.23.10231. [DOI] [PubMed] [Google Scholar]
- 7.Li FX, et al. Significance of thrombocytosis in clinicopathologic characteristics and prognosis of gastric cancer. Asian Pac. J. Cancer Prev. 2014;15(16):6511–6517. doi: 10.7314/APJCP.2014.15.16.6511. [DOI] [PubMed] [Google Scholar]
- 8.Josa V, et al. Relationship of postoperative thrombocytosis and survival of patients with colorectal cancer. Int J Surg. 2015;18:1–6. doi: 10.1016/j.ijsu.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Seretis C, Youssef H, Chapman M. Hypercoagulation in colorectal cancer: what can platelet indices tell us. Platelets. 2015;26(2):114–118. doi: 10.3109/09537104.2014.894969. [DOI] [PubMed] [Google Scholar]
- 10.Gasparyan AY, Ayvazyan L, Mikhailidis DP, Kitas GD. Mean platelet volume: a link between thrombosis and inflammation. Curr. Pharm. Des. 2011;17(1):47–58. doi: 10.2174/138161211795049804. [DOI] [PubMed] [Google Scholar]
- 11.Kaito K, et al. Platelet size deviation width, platelet large cell ratio, and mean platelet volume have sufficient sensitivity and specificity in the diagnosis of immune thrombocytopenia. Br. J. Haematol. 2005;128(5):698–702. doi: 10.1111/j.1365-2141.2004.05357.x. [DOI] [PubMed] [Google Scholar]
- 12.Kumagai S, et al. Prognostic significance of preoperative mean platelet volume in resected non-small-cell lung cancer. Mol Clin Oncol. 2015;3(1):197–201. doi: 10.3892/mco.2014.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li N. Platelets in cancer metastasis: To help the “villain” to do evil. Int. J. Cancer. 2016;138(9):2078–2087. doi: 10.1002/ijc.29847. [DOI] [PubMed] [Google Scholar]
- 14.Lin RJ, Auid-Oho, Afshar-Kharghan V, Schafer AI. Paraneoplastic thrombocytosis: the secrets of tumor self-promotion. Blood. 2014;124(2):184–187. doi: 10.1182/blood-2014-03-562538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsao AS, et al. Immunohistochemical overexpression of platelet-derived growth factor receptor-beta (PDGFR-beta) is associated with PDGFRB gene copy number gain in sarcomatoid non-small-cell lung cancer. Clin Lung Cancer. 2011;12(6):369–374. doi: 10.1016/j.cllc.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaushansky K. Growth factors and hematopoietic cell fate. A new feature: controversies in hematology. Blood. 1998;92(2):345–344. [PubMed] [Google Scholar]
- 17.Kumari, N., Dwarakanath, B. S., Das, A. & Bhatt, A. N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol (2016). [DOI] [PubMed]
- 18.Kowanetz M, et al. Granulocyte-colony stimulating factor promotes lung metastasis through mobilization of Ly6G + Ly6C + granulocytes. Proc. Natl. Acad. Sci. USA. 2010;107(50):21248–21255. doi: 10.1073/pnas.1015855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gunaldi, M. et al. Platelet Distribution Width as a Predictor of Metastasis in Gastric Cancer Patients. J Gastrointest Cancer. doi:10.1007/s12029-016-9886-5 (2016). [DOI] [PubMed]
- 20.Oncel M, et al. Evaluation of Platelet Indices in Lung Cancer Patients. Asian Pac J Cancer Prev. 2015;16(17):7599–7602. doi: 10.7314/APJCP.2015.16.17.7599. [DOI] [PubMed] [Google Scholar]
- 21.Okuturlar Y, et al. Utility of peripheral blood parameters in predicting breast cancer risk. Asian Pac J Cancer Prev. 2015;16(6):2409–2412. doi: 10.7314/APJCP.2015.16.6.2409. [DOI] [PubMed] [Google Scholar]
- 22.Yaylaci S, et al. Lack of Variation in Inflammatory Hematological Parameters between Benign Nodular Goiter and Papillary Thyroid Cancer. Asian Pac J Cancer Prev. 2016;17(4):2321–2323. doi: 10.7314/APJCP.2016.17.4.2321. [DOI] [PubMed] [Google Scholar]
- 23.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111(6):1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


