Abstract
There is substantial evidence for behavioral sex differences in risk trajectories for alcohol and substance use, with internalizing factors such as negative affectivity contributing more to female risk. Because the neural development of emotion circuitry varies between males and females across adolescence, it represents a potential mechanism by which underlying neurobiology contributes to risk for substance use. Longitudinal functional magnetic resonance imaging was conducted in males and females (n = 18 each) with a family history of alcohol use disorders starting at ages 8–13 years. Participants performed an affective word task during functional magnetic resonance imaging at 1- to 2-year intervals, covering the age range of 8.5–17.6 years (3–4 scans per participant). Significant age-related sex differences were found in the right amygdala and right precentral gyrus for the negative vs neutral word condition. Males showed a significant decrease in both amygdala and precentral gyrus activation with age, whereas the response in females persisted. The subjective experience of internalizing symptomatology significantly increased with age for females but not for males. Taken together, these results reveal sex differences in negative affect processing in at-risk adolescents, and offer longitudinal neural evidence for female substance use risk through internalizing pathways.
Keywords: fMRI, emotion, amygdala, substance use, sex differences
Introduction
Adolescence is the developmental period during which many psychopathologies begin to emerge, and they do so in a sex-specific manner. Emotional development in particular is sexually divergent; female adolescents are twice as likely as males to experience depression (Angold et al., 1998; Hankin et al., 1998; Twenge and Nolen-Hoeksema, 2002; Hilt et al., 2011) and anxiety (for review, see Albano and Krain, 2005), which are highly comorbid with one another (Cummings et al., 2014). Both depression and anxiety are characterized by the same underlying core psychopathological process called internalizing, or the tendency to direct feelings or emotional states inward (Achenbach and Edelbrock, 1981; Caspi et al., 2014). Factors or vulnerabilities contributing to internalizing disorders in females, such as negative affectivity, emerge or intensify during adolescence (Hyde et al., 2008; Cummings et al., 2014).
Adolescence is also a time when alcohol and drug use typically begins, making it an important interval in the etiology of alcohol (AUD) and other substance use disorders (SUD). Adolescent SUD and internalizing disorders are comorbid in 9–48% of findings from community and clinical samples, but tend to be higher for females than males (O’Neil et al., 2011, for review). Several psychosocial risk factors associated with gender differences in AUD vulnerability, such as coping styles and drinking motives/expectancies, demonstrate how this comorbidity may arise (Nolen-Hoeksema, 2004, 2012). For example, women are more likely than men to drink heavily when experiencing psychological distress, unpleasant emotions, conflict with others, or to alleviate internal stress or tension (Mooney et al., 1987; Lex, 1991; Rubonis et al., 1994; Annis and Graham, 1995; Lau-Barraco et al., 2009; Choi and DiNitto, 2011). Females with AUD report using alcohol as a way to change negative mood compared to female social drinkers (Olenick and Chalmers, 1991), whereas males expect more positive effects from drinking (Mooney et al., 1987; Berger and Adesso, 1991; Satre and Knight, 2001). Therefore, it is not surprising that women more often report having developed depression before they developed an AUD, whereas men more often report developing an AUD prior to depression (Sannibale and Hall, 2001). Together, this evidence suggests a sexually divergent behavioral profile regarding risk for substance use and later AUD and SUD, one that emerges in adolescence and emphasizes internalizing in females.
The link between internalizing behaviors and substance use in adolescence has received less attention than externalizing, despite evidence of a strong comorbidity in youth and adults (Kessler et al., 1996; O’Neil et al., 2010). In particular, the neural mechanisms underlying the contribution of internalizing to risk for later substance use and AUD/SUD is not well understood. Several theories suggest that females are more emotionally reactive and report greater emotional intensity to negative events as they appraise them as being more stressful than males do (Rudolph and Hammen, 1999; Ge et al., 2001; Hyde et al., 2008). A number of neuroimaging studies of healthy subjects have identified key brain structures involved in affect processing, specifically the amygdala (Lindquist et al., 2012, for review). The amygdala plays a central role in processing stimuli that are salient and/or emotionally impactful, such as negative emotional stimuli. In individuals with internalizing disorders, such as anxiety (Duval et al., 2015, for review) and depression (Rive et al., 2013, for review), amygdala hyper-activation has been consistently observed during tasks that involve emotion reactivity or the modulation of affect. This stems from decreased inhibitory control of the amygdala and other emotion processing areas by prefrontal regions in response to negative affect (Johnstone et al., 2007; Milad and Rauch, 2007; Motzkin et al., 2015). The prefrontal cortex is one of the last brain regions to fully mature, demonstrating functional and anatomical changes that continue into early adulthood (Giedd et al., 1999; Gogtay et al., 2004). In contrast, subcortical regions such as the amygdala reach maturity much earlier, usually by early adolescence (Giedd et al., 1996; Ostby et al., 2009; Wierenga et al., 2014). This maturational difference creates an imbalance between subcortical and cortical circuitry proposed to underlie a number of stereotypical adolescent behaviors (Casey et al., 2008; Steinberg, 2008; Somerville et al., 2010; Mills et al., 2014), including heightened emotional reactivity (Hare et al., 2008; Perlman and Pelphrey, 2011; McRae et al., 2012; Gee et al., 2013; Spear, 2013). In healthy adolescents, development of the amygdala impacts affective processing in a sex-specific manner (Blanton et al., 2010; Alarcón et al., 2015), and sex differences in the vulnerability to anxiety and depression can also arise from the maturational imbalance between subcortical and cortical regions (Zahn-Waxler et al., 2008; Blanton et al., 2010; Burghy et al., 2012). Together, these factors potentially leave females more vulnerable for substance use and later SUD. Further, the development of emotion circuitry, specifically the amygdala, represents a potential mechanism by which underlying neurobiology contributes to risk for substance use in females.
Here we focus on brain responses to emotional stimuli, specifically the passive viewing of affective words (Heitzeg et al., 2008; Glaser et al., 2014), using functional magnetic resonance imaging (fMRI). Our prior work demonstrates that brain activation during this task in 17- to 21-year-olds differentiates resilient and vulnerable adolescents who are at risk (Heitzeg et al., 2008) and is associated with later alcohol consumption, an effect mediated by negative affectivity (Glaser et al., 2014). In this study, we investigate the developmental trajectory of this circuitry using longitudinal data collected at 1- to 2-year intervals starting at ages 8–13 and covering the range of 8–17 years. Participants were male and female youth with a family history of AUD, which is a significant risk factor for later AUD and SUD. We hypothesize that, across development, at-risk females, but not males, will show increasing activation in neural regions known to be associated with affective processing, such as the amygdala. They will also score higher on subjective measures of internalizing behavior with increasing age, compared to at-risk males.
Methods
Participants
Thirty-six right-handed subjects (18 females) aged 8–13 years at baseline participated in the study. Participants were recruited from the Michigan Longitudinal Study, an ongoing, prospective study of families with high levels of parental AUD and a contrast sample of non-alcoholic families (Zucker et al., 1996, 2000). Parental AUD diagnosis was based on DSM-V criteria, and assessed by way of the Diagnostic Interview Schedule–Version 4 (Robins et al., 1981, 2000), supplemented with the Drinking and Drug History (Zucker and Fitzgerald, 1994). All participants in this study came from families where at least one parent had an AUD diagnosis during the child’s lifetime. Exclusionary criteria for this study included: neurological, acute, uncorrected, or chronic medical illness; current or recent (within 6 months) treatment with centrally active medications; and history of psychosis or schizophrenia in first-degree relatives. The presence of Axis I psychiatric or developmental disorders that would interfere with the interpretation of the data was also exclusionary; this did not include past history of mood disorder or current unmedicated mood disorder, or current or past history of conduct disorders or attention deficit disorder. Diagnosis was determined using the Diagnostic Interview Schedule–Child (Costello et al., 1984). As part of the Michigan Longitudinal Study, all offspring were assessed annually on substance use and related problems (Zucker et al., 1996; also see Supplementary Data). All participants were told to abstain from alcohol and illicit substances/recreational drugs for 48 h prior to the fMRI scan. For participants aged 15 and older, urine drug screens were conducted immediately prior to the fMRI scan; positive results were exclusionary. In participants aged 14 and younger, we relied on verbal confirmation of drug and alcohol abstinence on the day of the scan. No participants had to be excluded from this study due to a positive drug screen or affirmative self-report of alcohol or drug use. All participants gave written consent/assent after explanation of the experimental protocol, as approved by the local institutional review board. As participants were under the age of 18, at least one parent gave written informed consent. Table 1 contains subject information.
Table 1.
Participant characteristics
| All Participants | Female | Male | |
|---|---|---|---|
| Total participant, N | 36 | 18 | 18 |
| Participants with two scans | 36 | 18 | 18 |
| Participants with three scans | 24 | 12 | 12 |
| Participants with four scans | 16 | 8 | 8 |
| Total scans | 112 | 56 | 56 |
| Age at scan (mean ± s.d.) in years | |||
| All scans | 12.8 ±2.3 | 12.8 ±2.5 | 12.8 ±2.1 |
| Time 1 | 10.5 ±1.1 | 10.4 ±1.2 | 10.7 ±1.0 |
| Time 2 | 12.7 ±1.3 | 12.7 ±1.5 | 12.7 ±1.1 |
| Time 3 | 14.4 ±1.4 | 14.4 ±1.8 | 14.4 ±1.0 |
| Time 4 | 15.9 ±1.2 | 16.1 ±1.3 | 16.0 ±1.1 |
| Age range | 8.5–17.6 | 8.5–17.6 | 9.1–17.0 |
| IQ (mean ± s.d.) | 104.6 ±14.4 | 105.8 ±16.4 | 103.4 ±12.7 |
| ADHD diagnosis, Na | 2 | 1 | 1 |
| Conduct disorder diagnosis, N | 0 | 0 | 0 |
| Generalized anxiety diagnosis, Nb | 2 | 1 | 1 |
| Depression diagnosis, Nb | 1 | 1 | 0 |
| Alcohol/drug use, N | |||
| Baseline (Time 1) | |||
| Alcohol use | 2 | 0 | 2 |
| Marijuana use | 3 | 0 | 3 |
| Illicit drug use | 5 | 1 | 4 |
| Total subjects reporting any use at Time 1 | 5 | 1 | 4 |
| Follow-up scans (Times 2, 3 or 4) | |||
| Alcohol use | 8 | 4 | 4 |
| Marijuana use | 6 | 2 | 4 |
| Illicit drug use | 1 | 0 | 1 |
| Total subjects reporting any use at Times 2, 3 or 4 | 8 | 4 | 4 |
Of the two participants with ADHD diagnoses, neither were on medication for ADHD during study participation.
Diagnosis for generalized anxiety and depression is missing for one subject.
All participants had at least two fMRI scans (n = 36); 24 participants had three fMRI scans (n = 24); 16 participants had four fMRI scans (n = 16) (Table 1). Thus, each participant had either 2, 3 or 4 observations; each subsequent set of observations was always a subset of the participants from the previous time point. These will be referred to as Time 1 (first fMRI scan), Time 2 (second fMRI scan), Time 3 (third fMRI scan) and Time 4 (fourth fMRI scan) for the remainder of this manuscript; all scans together will be referred to as time points.
Measures
Youth Self-Report
Emotional and behavioral problems were assessed annually as part of the MLS protocol, using the Youth Self-Report (YSR), designed to be utilized in adolescents (Achenbach, 1991). The YSR assesses problem behaviors along two broadband scales of internalizing and externalizing that include anxiety/depression, withdrawal and somatic complaints (internalizing), as well as aggression and rule-breaking behaviors (externalizing). YSR assessments closest to each participant’s fMRI scan were used. Externalizing was included as it has previously been linked to alcohol use problems in males (Kendler et al., 2015).
fMRI task
Emotion arousal while in the scanner was probed using an affective word task (Heitzeg et al., 2008; Hsu et al., 2010; Mickey et al., 2011; Glaser et al., 2014). Previous neuroimaging studies have demonstrated that emotional words are able to elicit comparable neural responses, particularly in the amygdala, to pictures and faces (Kissler et al., 2006; Citron, 2012). Words were selected from the Affective Norms for English Words (Bradley and Lang, 1999) which provides norm ratings for two separate scales, emotional valence and arousal, on a scale of 1 (negative valence, low arousal) to 9 (positive valence, high arousal). The following criteria were used to select 36 words (12 words for each emotional category): negative (valence rating <3), neutral (4.5< valence rating >5.5) and positive (valence rating >7). Negative and positive words had an arousal rating >5, whereas the arousal rating for neutral words was <2.
Words were presented one at a time in a blocked design. Each block consisted of six trials. Each trial started with a 3 s word presentation followed by a crosshair that lasted 1 s. Participants were instructed to press a button while the word was on the screen indicating that they understood the word. Following each task block, participants viewed a blank screen for 18 s. Six task blocks and six rest blocks were included in each run, and each condition was presented twice. The order of presented conditions was counterbalanced using the Latin Squares Design. The entire experiment consisted of three runs in total, which lasted 12 min and 36 s.
Following the scan, participants completed a questionnaire containing 54 words, of which 36 were from the task. Equal numbers of words were included across conditions (negative, neutral and positive). For each word, participants were asked to identify whether they remembered seeing it in the scanner and rate how pleasant (valence) on a 9-point scale they found the word to be (0 = not pleasant; 9 = pleasant). In order to avoid confounding brain activation, valence ratings were collected after—as opposed to during—the scan. Evidence suggests that evaluation of stimuli with affective qualities alters emotional reactions and brain activation when compared to passive viewing (Taylor et al., 2003).
MRI data acquisition
Whole-brain blood oxygenated level-dependent (BOLD) images were acquired on a 3.0 Tesla GE Signa scanner (Milwaukee, WI, USA) using a T2*-weighted single-shot combined spiral in-out sequence (Glover and Law, 2001) with the following parameters: repetition time = 2000 ms; time to echo = 30 ms; flip angle = 90°; field of view = 200 mm; 64×64 matrix; in-plane resolution = 3.12×3.12 mm; and slice thickness = 4 mm. The entire volume of 29 axial slices was acquired every 2 s. A high-resolution anatomical T1 scan was obtained for spatial normalization (three-dimensional spoiled gradient-recalled echo, repetition time = 25 ms; minimum time to echo; field of view = 25 cm; 256×256 matrix, slice thickness = 1.4 mm). Participant motion was minimized using foam pads placed around the head along with a forehead strap.
Data analysis
Behavioral data
For post-scan questionnaire performance, values that fell ±3 s.d. outside of the mean were removed (see Supplementary Data for details). Mean hit rate for each condition (positive, negative, neutral words) was calculated across all time points, and then for each time point individually. This was used to calculate recognition memory performance (below). In SPSS (Version 19.0, IBM Corp., Armonk, NY), linear mixed model analyses were used to investigate sex differences across age for recognition memory performance and memory bias. Recognition memory is the ability to correctly recognize a recently encountered item, and accounts for the number of incorrect words participants say they saw. Recognition memory performance (p′) was calculated using the formula p′ = (p−fp)/ (1−fp) where (p) is the percentage of correct responses to target stimuli and (fp) is the percentage of incorrect responses to distractor stimuli (Epstein et al., 2006). Memory bias takes neutral word performance into account, and can compare recognition memory for positive relative to negative words. Negative memory bias was calculated by subtracting recognition memory performance to neutral words from that of negative words (Glaser et al., 2014); a lower score indicates that negative words did not differ much from neutral words. Positive memory bias was calculated by subtracting recognition memory performance to neutral words from that of positive words (Glaser et al., 2014), and a lower score indicates positive words did not differ much from neutral words. Research indicates that emotional memories are stronger for women (Hamann and Canli, 2004), and emotional memory is correlated with amygdala activity in females and males (Stevens and Hamann, 2012). Linear mixed models were run on the following variables to investigate sex differences across age: (i) positive, negative and neutral performance measures for recognition memory performance, and (ii) positive and negative memory bias. Each measure was entered individually as a dependent variable, with sex as a factor and age mean-centered as a fixed-effect covariate. Time point (1, 2,…, 4) was included as a repeated effect and subject was entered as a random factor. Schwarz’s Bayesian information criteria (BIC; Schwarz, 1978) was used to determine the best-fitting covariance structure (autoregressive–first order; all longitudinal models used BIC to determine best fit), and models were fit using maximum likelihood estimation method. Models were also run for each sex separately to determine individual group contributions to significance. Independent samples t-tests were run at each time point for each performance variable to determine whether differences existed between males and females. For analysis details regarding valence ratings, see Supplementary Data.
Youth Self-Report
An independent samples t-test was used to determine whether group differences existed at baseline (Time 1) and at Time 4. To investigate sex differences across age, each YSR scale was entered individually as a dependent variable into the linear mixed model, with sex as a factor and age mean-centered as a covariate. Restricted maximum likelihood (REML) was the estimation method, and the covariance structure was ante-dependence first order. Models were also run separately for each sex.
fMRI preprocessing
An iterative algorithm was used to reconstruct functional images (Sutton et al., 2003; Noll et al., 2005). Subject head motion was corrected using FSL 5.0.2.2 (Analysis Group, FMRIB, Oxford, UK) (Jenkinson et al., 2002). Analysis of estimated motion parameters confirmed that overall head motion within each run did not exceed 3 mm translation or 3° rotation in any direction (Table S9). All remaining image processing (including slice timing correction) and statistical analysis were completed using statistical parametric mapping (SPM8; Wellcome Institute of Cognitive Neurology, London, UK). Functional images were spatially normalized to a standard stereotaxic space as defined by the Montreal Neurological Institute. A 6 mm full-width half-maximum Gaussian spatial smoothing kernel was applied to improve signal-to-noise ratio and to account for differences in anatomy.
Individual analysis was completed using a general linear model. Four regressors (positive, negative, and neutral words, rest) were convolved with the canonical hemodynamic response function, with event durations of 4 s from stimulus presentation. Motion parameters were modeled as nuisance regressors to remove residual motion artifacts. The main contrasts of interest were: (i) Positive vs Neutral words; and (ii) Negative vs Neutral words. These two contrasts will be referred to as POSvsNEU and NEGvsNEU, respectively. These were calculated by linearly combining parameter estimates over all three runs of the task.
Task effect
Task effect was determined for all participants across all scans using a one-sample t-test. Areas of activation were deemed significant if they reached a cluster-level false discovery rate (FDR)-corrected threshold of P < 0.05 in the NEGvsNEU and POSvsNEU contrasts.
Longitudinal analysis
Differences in linear developmental trajectories between groups were tested using a second-level multiple regression analysis in Matlab (version R2010a, The MathWorks, Inc., Natick, MA, USA) and SPM. The participant-specific intercept terms and age regressors (linear age, quadratic age, and cubic age) were specified by a script written in Matlab. Age regressors were first constructed across all participants, and then Gram-Schmidt orthogonalization was performed. Participants were then split into two groups: males and females. The multiple regression model was then built in SPM with these calculated subject-specific intercept terms and the group-specific predictors as a second-level analysis, as in Hardee et al. (2014). Contrasts were calculated to examine sex differences as a function of age, with linear age effect differences being the primary focus.
Missing data were handled by utilizing all observations in the calculation of each age effect by group, such that individuals with more time points contributed more data to the estimation of the group-age slope. Notably, the missing data were distributed equally across groups so there was no number of observations by group bias (Laird, 1988). As we did not note that the (unobserved) fMRI response in missing subjects predicted their chance of not having four data points, we are operating with ‘Missing Completely at Random’ or at most ‘Missing at Random’ assumptions, and are so justified in using all available data.
Differences between groups for linear age effects were viewed at P = 0.005, and regions were deemed significant if whole-brain activation reached a cluster-level FDR-corrected statistical threshold of P < 0.05. The amygdala was selected as an a priori region of interest (ROI) due to extensive literature demonstrating its role in affect processing; therefore, amygdala activation was deemed significant if it reached a whole-brain statistical threshold of P < 0.001, uncorrected. Values from significant clusters were extracted using MarsBaR Region of Interest toolbox (Brett et al., 2002).
Extracted values from the amygdala and precentral gyrus for contrasts of interest were imported into SPSS to quantify interaction effects, posthoc. Linear mixed model analyses were conducted for each sex (male, female), using time point as a repeated measure, subject as a random factor and mean-centered age as a fixed-effect covariate. REML estimation was used, and the covariance structure was first-order autoregressive. Next, correlations between the amygdala and YSR internalizing broadband and subscale scores and the amygdala and negative memory bias were examined to determine whether brain activation was linked to internalizing measures and/or played a role in remembering negative words. Each measure (YSR variables, negative memory bias) was investigated independently using separate mixed model analyses with these measures as the dependent variable, extracted amygdala values as a fixed-effect covariate, compound symmetry as a covariance structure, and REML as an estimation method. These analyses were repeated to investigate the effect of sex and brain on YSR measures, this time using each measure (YSR, negative memory bias) as the dependent variable, and compound symmetry as the covariance structure.
Results
Participant characteristics
There were no significant differences between groups for IQ, t(31)=0.47, P = 0.64; overall age, t(110)= −0.13, P = 0.90; or age at Time 1, t(34)= −0.81, P = 0.42; Time 2, t(34)= −0.05, P = 0.96; Time 3, t(22)= −0.08, P = 0.93; or Time 4, t(14)=0.70, P = 0.50. Five participants reported substance use (alcohol, marijuana or any illicit drug) at Time 1. In the follow-up scans (Times 2, 3 or 4), an additional eight participants reported substance use at one time point minimally. Table 1 contains subject details (number of scans, age, IQ, diagnoses, substance use information). For detailed information regarding substance use, see Supplementary Data (Tables S1 and S2).
Behavioral data
Recognition memory. There were no significant age-by-gender interaction effects for recognition memory performance; there was a significant main effect of age for negative words [F(1, 37.39)=5.50, P = 0.02], and a trend toward significance for positive words for age [F(1, 84.56)=3.72, P = 0.06]; there was no significant effect for age or gender for neutral recognition memory performance. See Table S3 for recognition memory performance means broken down by sex and time point.
Memory bias. For negative memory bias, a significant interaction was found [F(1, 82.43)=4.28, P = 0.04]; males significantly increased with age [F(1, 39.54)=5.92, P = 0.02] whereas females did not [F(1, 38.33)=0.12, P = 0.74]. However, there was a significant group difference for negative memory bias at Time 1, t(32)=2.69, P = 0.01, with females (mean at Time 1= 0.15 ±0.20) having a greater bias for remembering negative words than males (mean at Time 1= −0.02 ±0.17) (Table S4). For positive memory bias, there was a main effect of age [F(1, 83.91)=6.07, P = 0.02], where bias increased with age (see Table S4 for means). Valence rating results can be found in Supplementary Data (Table S5).
Youth Self-Report
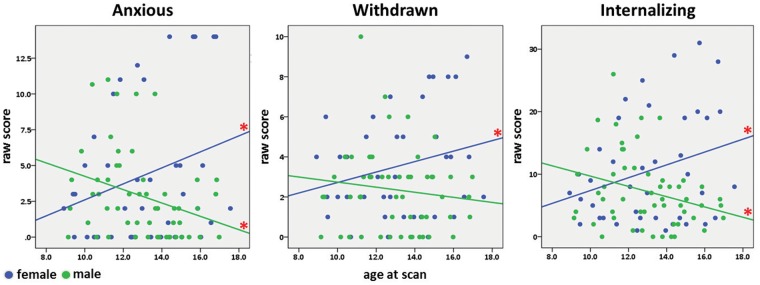
There were no significant differences in YSR scores between groups at baseline (Time 1). By Time 4, however, females had higher internalizing scores, t(14)=2.14, P = 0.05. Subscale scores, disaggregated components of the internalizing broadband score, showed trend level differences on the anxious, t(14)=1.94, P = 0.07 and withdrawn subscales, t(14)=1.90, P = 0.08 (Supplementary Data, Table S6). Linear mixed model analyses revealed significant interactions between sex and age for internalizing; again subscale analyses indicated a significant interaction for anxious and a trend toward significance for withdrawn subscales (Table 2A). Posthoc analyses (Figure 1) revealed a significant increase with age in females for internalizing, driven by anxious and withdrawn subscales (Table 2B). Males, on the other hand, showed a significant decrease in internalizing, driven by a decrease in anxious scores with age (Table 2B). There were no significant differences between groups for externalizing scores with age (Table 2A) or at Times 1 or 4 (Table S6).
Table 2.
YSR linear mixed model results
| YSR measure | A |
B |
||||
|---|---|---|---|---|---|---|
| Interaction |
Male |
Female |
||||
| F | P-value | F | P-value | F | P-value | |
| Internalizing problems* | 8.84 | 0.01* | 5.25 | 0.03* | 8.17 | 0.01* |
| Anxious behavior* | 6.66 | 0.01* | 6.70 | 0.01* | 4.86 | 0.04* |
| Somatic complaints | 2.85 | 0.10 | ||||
| Withdrawn† | 3.65 | 0.06† | 0.95 | 0.34 | 5.62 | 0.03† |
| Externalizing problems | 1.26 | 0.27 | ||||
| Aggressive behavior | 1.25 | 0.27 | ||||
| Rule-breaking behavior | 1.97 | 0.17 | ||||
In A, * indicates significance at P < 0.05; † indicates a trend toward significance.
In B, * indicates which group(s) drove significance in A, † indicates which group drove trend in A.
Fig. 1.
Significant YSR scores. Longitudinal linear age effects are depicted in the scatterplots for anxious and withdrawn raw scores, subscales which make up the broader internalizing raw score. Female scores (blue) significantly increase across age for anxious, withdrawn, and internalizing. Male scores (green) significantly decrease across age for anxious and internalizing. Red asterisks indicate P < 0.05.
fMRI results
Task effect results can be found in Supplementary Data.
Age-by-sex longitudinal effects
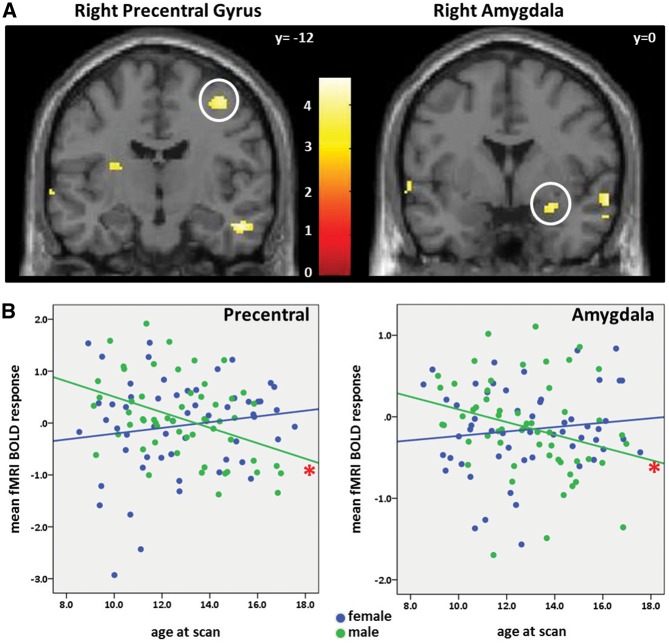
For the POSvsNEU contrast, no regions passed the corrected level of significance for the whole-brain analysis. For the NEGvsNEU contrast, a significant age-by-sex interaction was found in the right precentral gyrus (centered at x = 38, y= −12, z = 56; k = 966, t = 3.94), encompassing portions of the right postcentral gyrus and the right superior parietal lobule (Figure 2A, left). For the a priori amygdala, a significant group-by-age interaction was centered at x = 26, y = 0, z= −16; k = 67, t = 3.94 in the whole-brain analysis (Figure 2A, right) for NEGvsNEU. A small volume correction was applied to the amygdala using an anatomical ROI [defined using the AAL set of templates in PickAtlas (Maldjian et al., 2003)] to determine whether the interaction passed voxel-wise statistics at P < 0.05, corrected. Small volume correction revealed a voxel-wise significance of P = 0.004 (corrected).
Fig. 2.
Negative vs neutral words. (A) This contrast revealed a significant group difference for linear age in the whole-brain longitudinal analysis in the right precentral gyrus at a statistical threshold of P < 0.05, FDR-corrected (top left, white circle). The amygdala was chosen as an a priori region of interest, and passed a pre-selected threshold of P < 0.001, uncorrected (top right, white circle). Color bar represents t-values and the y-coordinate is in MNI space. (B) Extracted values from the precentral gyrus and amygdala, depicted in scatterplots with mean fMRI activation on the y-axis and age at scan on the x-axis. All time points are depicted. Males (green) show a significant decrease in right precentral and right amygdala activation with age, whereas females (blue) show a persistence of activation with age. Red asterisks indicate P < 0.05.
When quantifying the significance of this effect for each sex in SPSS, posthoc linear mixed model analyses revealed a significant decrease in the right precentral gyrus activation with age in males [F(1, 43.56)=4.53, P = 0.04], with females showing a non-significant increase in activation with age [F(1, 42.04)=2.43, P = 0.13] (Figure 2B, left). In the right amygdala, there was also a significant decrease in activation with age in males [F(1, 43.38)=9.64, P = 0.003], again with females showing a non-significant increase in activation with age [F(1, 40.24)=1.35, P = 0.25] (Figure 2B, right). The contribution of each component (Negative vs Rest, Neutral vs Rest) by sex can be found in Supplementary Data.
This linear mixed model was repeated after the 13 participants reporting any substance use were removed (eight males, five females; Table S1). The significant interaction remained for both the amygdala [F(1, 47.09)=4.84, P = 0.03] and the precentral gyrus [F(1, 50.25)=5.64, P = 0.02]. Posthoc analyses showed trends toward significance in decreased activation with age for males in the amygdala [F(1, 23.47)=3.88, P = 0.06] and precentral gyrus [F(1, 24.80)=3.72, P = 0.06], and non-significant increases with age for females in both the amygdala [F(1, 23.58)=1.54, P = 0.23] and precentral gyrus [F(1, 25.62)=2.84, P = 0.13].
Group differences at Time 1
In SPSS, an independent samples t-test revealed a significant group difference for NEGvsNEU at Time 1 for both the amygdala, t(34)= −2.16, P = 0.038, and the precentral gyrus, t(34)= −2.55, P = 0.02. For each ROI, males had greater mean activation at Time 1 (amygdala: 0.37 ±0.75; precentral: 0.05 ±0.32) than females (amygdala: −0.35 ±1.20; precentral: −0.43 ±0.72). At Time 4, there was a trend toward significance for the amygdala, t(14)=1.72, P = 0.07, and the precentral gyrus, t(14)= 1.88, P = 0.08, with females having greater mean activation at Time 4 (amygdala: 0.12 ±0.49; precentral: 0.08 ±0.35) than males (amygdala: −0.45 ±0.67; precentral: −0.42 ±0.66). This was repeated after the five participants reporting any substance use prior to Time 1 were removed, and the significant group difference still remained [amygdala, t(21)= −2.18, P = 0.04; precentral gyrus, t(21)= −2.64, P = 0.02], with males having greater mean activation at Time 1 for each ROI (amygdala: 0.53 ±0.79; precentral: 0.12 ±0.33) than females (amygdala: −0.33 ±1.02; precentral: −0.52 ±0.72).
Brain and behavior associations
YSR. There was a trend towards a significant association between amygdala activation and subjective experience of anxiety problems [F(1, 94.03)=3.45, P = 0.07], where increased amygdala activation was associated with higher anxiety scores. This was the only YSR scale to have a significant association with the amygdala.
Negative memory bias. There was no significant association between amygdala activation and negative memory bias [F(1, 23.39)=0.05, P = 0.82].
Discussion
This longitudinal study examined sex differences in the neural development of children and adolescents at risk for problem substance use in response to a negative word task. Here we found that activation in the right amygdala and right precentral gyrus significantly decreased across age in males, whereas in females activation remained steady.
The amygdala is involved in the detection and processing of emotion, particularly negative emotion (for review, see Costafreda et al., 2008; Garcia-Garcia et al., 2016), and augments the neural representation of emotional stimuli based on current needs, goals and values (Cunningham and Brosch, 2012). The amygdala also evaluates the subjective salience and relevance of stimuli (Adolphs, 2010; Pessoa and Adolphs, 2010), particularly social and emotional stimuli. Throughout adolescence, the salience and relevance of these stimuli can change, reflected in amygdala activation increases and decreases (Scherf et al., 2013). The precentral gyrus, on the other hand, is primarily a motor region but consistently activates during emotion perception and experience (de Gelder et al., 2004; Hajcak et al., 2007); damage to this area can cause emotion recognition deficits (Adolphs et al., 2000). Both the precentral and postcentral gyrus are part of the core brain network involved in discriminating between the basic emotions (Saarimaki et al., 2015). The pre-supplementary motor area, located on a portion of the precentral gyrus, in particular is involved in processing emotional words presented both visually and verbally (Beauregard et al., 1997; Isenberg et al., 1999; Crosson et al., 2002; Kensinger and Schacter, 2006; Warren et al., 2006; Hinojosa et al., 2014). In general, the motor cortices in the precentral gyrus seemingly play a significant role in motor preparation related to emotion (Frijda 1986; Mazzola et al., 2013).
Although previous studies have demonstrated co-activation and functional connectivity between amygdala and cortical motor-related regions during the perception of emotional expressions (de Gelder et al., 2004; Grosbras and Paus, 2006; Pichon et al., 2008, 2009; Van den Stock et al., 2011; Conty et al., 2012; Grezes et al., 2013), a recent diffusion tensor imaging study provides evidence for a direct pathway between the amygdala and motor cortices, including the precentral gyrus, formerly illustrated only in cats, monkeys and rats (Grezes et al., 2014). The authors suggest that ipsilateral connections from the amygdala to these motor regions provide a direct mechanism by which the amygdala could influence, rather than trigger, complex and subtle motor behaviors. Here, the amygdala and precentral gyrus exhibit similar BOLD patterns to one another across development in at-risk males (significant decrease in activation) and in females (sustained activation), suggesting this limbic-motor interaction is sex dependent in the context of affect processing. These differences in affective processing relative to family history could be one factor that leads to sex differences in substance use risk in adolescents.
Amygdala responsiveness is greater in healthy adolescents than in healthy adults, indicating that amygdala activation should decrease with age (Baird et al., 1999; Monk et al., 2003; Guyer et al., 2008; Pfeifer et al., 2011; Somerville et al., 2011), in agreement with what at-risk adolescent males exhibit here. The sustained activation in at-risk females across adolescence, on the other hand, must be interpreted with caution; one possible explanation is that this reflects hyper-vigilance to negative stimuli. This theory builds on previous research showing a greater amygdala response in females compared to males for negative stimuli in adulthood (Stevens and Hamann, 2012; Andreano et al., 2014). It is also possible that the findings reflect a sex difference in amygdala habituation, although this was not directly tested here. Prior reports suggest the degree to which there are sex differences in the habituation of the amygdala response to negative stimuli may vary based on valence, arousal and image novelty (for meta-analyses, see Wager et al., 2003; Sergerie et al., 2008; Stevens and Hamann, 2012; Garcia-Garcia et al., 2016). For example, adult females have been shown to have a more persistent amygdala response than males to negative stimuli that are familiar but not those that are novel, and persistence in amygdala response was further associated with greater negative affect (Andreano et al., 2014). To the extent that emotional words may be considered familiar stimuli, the present findings may be due to greater habituation of amygdala response in males across development compared with a lack of habituation in females.
Importantly, the amygdala may have a larger impact on affective behavior in females than in males. For example, Blanton et al. (2010) found that larger amygdala volume was correlated with poorer emotional control in females but not males, hypothesized to result in a greater risk for the development of anxiety and depression. Foster et al. (2015) posit a developmental cascade to explain how the early emergence of internalizing symptoms (i.e. anxiety, depression) in adolescent females could exacerbate alcohol use problems later in life. Alcohol use first begins as a way to cope with negative emotions stemming from depression and anxiety; in turn, alcohol use magnifies internalizing problems by negatively impacting psychosocial development, which may then increase alcohol problems even more (Foster et al., 2015). In this study, the YSR scores anxious and withdrawn, components of the broader internalizing score, significantly increase across age for females but not males, consistent with the view that internalizing and negative affect may mediate substance use risk in females. Moreover, Kuntsche and Muller (2012) showed adolescent females were twice as likely as males to report trying alcohol for the first time in order to cope with negative emotions. Together, these results lend evidence to the theory that females may be more vulnerable to SUD risk through an emotion processing pathway that includes the amygdala.
The exact role of the precentral gyrus in this pathway is less clear. Even though the right amygdala and right precentral gyrus show similar patterns of activation across development in males and females, it is unknown how the precentral gyrus contributes to SUD risk. However, it is likely that amygdala hyper-vigilance is involved, as previous studies have demonstrated that the amygdala can trigger and inhibit motor responses and/or modulate motor programs in emotionally specific contexts (LeDoux, 2000; Sagaspe et al., 2011). Additionally, El Zein et al. (2015) demonstrated that healthy individuals with high anxiety show hyper-vigilance to potentially threatening stimuli by way of earlier electroencephalogram signals in the motor cortex compared to low anxiety individuals. Thus, it is possible that emotion processing in at-risk adolescent females influences both the amygdala and motor-related regions. Future research is needed to specifically examine the role of the precentral gyrus in affect processing during the developmental period—both late childhood and early adolescence—particularly via connectivity analyses.
There are several limitations to note. First, results should be interpreted carefully, as a significant decrease across age was found in the amygdala and precentral gyrus only for at-risk males, yet results are discussed from the framework of at-risk females. There is evidence for this framework from both the YSR results and the broader literature, but given the small sample size, only tentative conclusions can be drawn. Furthermore, these analyses focused on average amygdala activation to valenced stimuli across each scan session and therefore no conclusions can be drawn regarding a role of habituation. This will be an important area for future work. Second, this study focused on at-risk individuals only, which precludes these results from generalizing to males and females who have no family history of substance use. Future studies would need a non-high risk control group in order to interpret a gender comparison between high-risk and control participants. Third, the maximum age here is 17.6 years; it is unknown what neural and behavioral changes would occur with respect to both groups as they continue to develop. Potential follow-up studies should capture these subsequent outcomes, particularly as these participants move into substance use and abuse. Finally, the greater amount of substance use reported in males than females throughout the study could be a confound; however, the supplementary analyses run without participants reporting substance use suggests that this is not likely.
To conclude, this study revealed developmental differences in the functional brain response of at-risk males and females to negative emotional stimuli. These neural distinctions may underlie sex differences in behavioral risk trajectories for SUDs, such as negative emotionality and vulnerability to internalizing disorders in adolescent females. This study suggests there may be sex-specific neural and behavioral patterns visible in females at-risk for developing SUDs, which could potentially be useful for predicting future problem use.
Supplementary Material
Acknowledgments
The authors thank Dr Thomas Nichols for construction of and assistance with the longitudinal model.
Funding
This work was supported by the National Institute on Drug Abuse (R01 DA27261, T32s DA007267 and DA007268), the National Institute on Alcohol Abuse and Alcoholism (R01s AA12217 and AA007065), and the National Center for Advancing Translational Sciences (UL1 TR000433).
Supplementary data
Supplementary data are available at SCAN online.
Conflict of interest. None declared.
References
- Achenbach T. (1991). Manual for the Youth Self-Report and 1991 Profile. Burlington, VT: Department of Psychiatry, University of Vermont. [Google Scholar]
- Achenbach T.M., Edelbrock C.M. (1981). Behavioral problems and competencies reported by parents of normal and disturbed children aged four through sixteen. Monographs of the Society for Research in Child Development, 46(1), 1–82. [PubMed] [Google Scholar]
- Adolphs R. (2010). Emotion. Current Biology, 20(13), R549–52. [DOI] [PubMed] [Google Scholar]
- Adolphs R., Damasio H., Tranel D., Cooper G., Damasio A.R. (2000). A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. The Journal of Neuroscience, 20(7), 2683–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcón G., Cservenka A., Rudolph M.D., Fair D.A., Nagel B.J. (2015). Developmental sex differences in resting state functional connectivity of amygdala sub-regions. Neuroimage, 115, 235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albano A.M., Krain A. (2005). Anxiety and anxiety disorders in girls In: Bell D.J., Foster S.L., Mash E.J., editors. Handbook of Behavioral and Emotional Problems in Girls. New York: Kluwer Academic/Plenum Publishers, 79–116. [Google Scholar]
- Andreano J.M., Dickerson B.C., Barrett L.M. (2014). Sex differences in the persistence of the amygdala response to negative material. Social Cognitive and Affective Neuroscience, 9(9), 1388–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A., Costello E.J., Worthman C.M. (1998). Puberty and depression: the roles of age, pubertal status and pubertal timing. Psychological Medicine, 28(1), 51–61. [DOI] [PubMed] [Google Scholar]
- Annis H.M., Graham J.M. (1995). Profile types on the inventory of drinking situations—implications for relapse prevention counseling. Psychology of Addictive Behaviors, 9(3), 176–82. [Google Scholar]
- Baird A.A., Gruber S.A., Fein D.A., et al. (1999). Functional magnetic resonance imaging of facial affect recognition in children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 38(2), 195–9. [DOI] [PubMed] [Google Scholar]
- Beauregard M., Chertkow H., Bub D., Murtha S., Dixon R., Evans A. (1997). The neural substrate for concrete, abstract, and emotional word lexica a positron emission tomography study. Journal of Cognitive Neuroscience, 9(4), 441–61. [DOI] [PubMed] [Google Scholar]
- Berger B.D., Adesso V.J. (1991). Gender differences in using alcohol to cope with depression. Addictive Behaviors, 16(5), 315–27. [DOI] [PubMed] [Google Scholar]
- Blanton R., Chaplin T.M., Sinha R. (2010). Sex differences in the correlation of emotional control and amygdala volumes in adolescents. Neuroreport, 21, 953–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. (1999). Fearfulness and affective evaluations of pictures. Motivation and Emotion, 23(1), 1–13. [Google Scholar]
- Brett M., Anton J.-L., Valabregue R., Poline J.-B. (2002). Region of interest analysis using an SPM toolbox [abstract]. In: 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan, 2–6 June 2002. Available on CD-ROM in NeuroImage, Vol. 16, No 2.
- Burghy C.A., Stodola D.E., Ruttle P.L., et al. (2012). Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience, 15(12), 1736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. (2008). The adolescent brain. Annals of the New York Academy of Sciences, 1124, 111–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Houts H.M., Belsky D.W., et al. (2014). The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science, 2(2), 119–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi N.G., DiNitto D.M. (2011). Psychological distress, binge/heavy drinking, and gender differences among older adults. The American Journal on Addictions, 20(5), 420–8. [DOI] [PubMed] [Google Scholar]
- Citron F.M. (2012). Neural correlates of written emotion word processing: a review of recent electrophysiological and hemodynamic neuroimaging studies. Brain and Language, 122(3), 211–26. [DOI] [PubMed] [Google Scholar]
- Conty L., Dezecache G., Hugueville L., Grezes J. (2012). Early binding of gaze, gesture, and emotion: neural time course and correlates. The Journal of Neuroscience, 32(13), 4531–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costafreda S.G., Brammer M.J., David A.S., Fu C.H. (2008). Predictors of amygdala activation during the processing of emotional stimuli: a meta-analysis of 385 PET and fMRI studies. Brain Research Reviews, 58(1), 57–70. [DOI] [PubMed] [Google Scholar]
- Costello A.J., Edelbrock C.S., Dulcan M.K., et al. (1984). Development and Testing of the NIMH Diagnostic Interview Schedule for Children in a Clinic Population: Final Report. Rockville, MD: NIMH Center for Epidemiologic Studies. [Google Scholar]
- Crosson B., Cato M.A., Sadek J.R., et al. (2002). Semantic monitoring of words with emotional connotation during fMRI: contribution of anterior left frontal cortex. Journal of the International Neuropsychological Society, 8(5), 607–22. [DOI] [PubMed] [Google Scholar]
- Cummings C.M., Caporino N.E., Kendall P.C. (2014). Comorbidity of anxiety and depression in children and adolescents: 20 years after. Psychological Bulletin, 140(3), 816–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham W.A., Brosch T. (2012). Motivational salience: amygdala tuning from traits, needs, values, and goals. Current Directions in Psychological Science, 21(1), 54–9. [Google Scholar]
- de Gelder B., Snyder J., Greve D., Gerard G., Hadjikhani N. (2004). Fear fosters flight: a mechanism for fear contagion when perceiving emotion expressed by a whole body. Proceedings of the National Academy of Sciences of the United States of America, 101(47), 16701–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duval E.R., Javanbakht A., Liberzon I. (2015). Neural circuits in anxiety and stress disorders: a focused review. Therapeutics and Clinical Risk Management, 11, 115–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Zein M., Gamond L., Conty L., Grezes L. (2015). Selective attention effects on early integration of social signals: same timing, modulated neural sources. Neuroimage, 106, 182–8. [DOI] [PubMed] [Google Scholar]
- Epstein J., Pan H., Kocsis J.H., et al. (2006). Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. The American Journal of Psychiatry, 163(10), 1784–90. [DOI] [PubMed] [Google Scholar]
- Foster K.T., Hicks B.M., Iacono W.G., McGue M. (2015). Gender differences in the structure of risk for alcohol use disorder in adolescence and young adulthood. Psychological Medicine, 45(14), 3047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijda N.H. (1986). The current status of emotion theory. Bulletin of the British Psychological Society, 39, A75. [Google Scholar]
- Garcia-Garcia I., Kube J., Gaebler M., Horstmann A., Villringer A., Neumann J. (2016). Neural processing of negative emotional stimuli and the influence of age, sex and task-related characteristics. Neuroscience and Biobehavioral Reviews, 68, 773–93. [DOI] [PubMed] [Google Scholar]
- Ge X., Conger R.D., Elder G.H. (2001). Pubertal transition, stressful life events, and the emergence of gender differences in adolescent depressive symptoms. Developmental Psychology, 37(3), 404–17. [DOI] [PubMed] [Google Scholar]
- Gee D.G., Humphreys K.L., Flannery J., et al. (2013). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. The Journal of Neuroscience, 33, 4584–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Blumenthal J., Jeffries N.O., et al. (1999). Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience, 2(10), 861–3. [DOI] [PubMed] [Google Scholar]
- Giedd J.N., Snell J.W., Lange N., et al. (1996). Quantitative magnetic resonance imaging of human brain development: ages 4–18. Cerebral Cortex, 6(4), 551–60. [DOI] [PubMed] [Google Scholar]
- Glaser Y.G., Zubieta J.K., Hsu D.T., et al. (2014). Indirect effect of corticotropin-releasing hormone receptor 1 gene variation on negative emotionality and alcohol use via right ventrolateral prefrontal cortex. The Journal of Neuroscience, 34(11), 4099–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover G.H., Law C.S. (2001). Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine, 46(3), 515–22. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., et al. (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America, 101(21), 8174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J., Adenis M.S., Pouga L., Armony J.L. (2013). Self-relevance modulates brain responses to angry body expressions. Cortex, 49(8), 2210–20. [DOI] [PubMed] [Google Scholar]
- Grezes J., Valabregue R., Gholipour B., Chevallier C. (2014). A direct amygdala-motor pathway for emotional displays to influence action: a diffusion tensor imaging study. Human Brain Mapping, 35(12), 5974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosbras M.H., Paus T. (2006). Brain networks involved in viewing angry hands or faces. Cerebral Cortex, 16(8), 1087–96. [DOI] [PubMed] [Google Scholar]
- Guyer A.E., Monk C.S., McClure-Tone E.B., et al. (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20(9), 1565–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G., Molnar C., George M.S., Bolger K., Koola J., Nahas Z. (2007). Emotion facilitates action: a transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology, 44(1), 91–7. [DOI] [PubMed] [Google Scholar]
- Hamann S., Canli T. (2004). Individual differences in emotion processing. Current Opinion in Neurobiology, 14(2), 233–8. [DOI] [PubMed] [Google Scholar]
- Hankin B.L., Abramson L.Y., Moffitt T.E., Silva P.A., McGee R., Angell K.E. (1998). Development of depression from preadolescence to young adulthood: emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology, 107(1), 128–40. [DOI] [PubMed] [Google Scholar]
- Hardee J.E., Weiland B.J., Nichols T.E., et al. (2014). Development of impulse control circuitry in children of alcoholics. Biological Psychiatry, 76(9), 708–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare T.A., Tottenham N., Galvan A., Voss H.U., Glover G.H., Casey B.J. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63, 927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitzeg M.M., Nigg J.T., Yau W.Y., Zubieta J.K., Zucker R.A. (2008). Affective circuitry and risk for alcoholism in late adolescence. Alcoholism, Clinical and Experimental Research, 32(6), 414–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilt L., Hanson J., Pollak S. (2011). Emotion dysregulation In: Levesque E., editor. Encyclopedia of Adolescence. New York, NY: Springer-Verlag. [Google Scholar]
- Hinojosa J.A., Albert J., Lopez-Martin S., Carretie L. (2014). Temporospatial analysis of explicit and implicit processing of negative content during word comprehension. Brain and Cognition, 87, 109–21. [DOI] [PubMed] [Google Scholar]
- Hsu D.T., Langenecker S.A., Kennedy S.E., Zubieta J.K., Heitzeg M.M. (2010). fMRI BOLD responses to negative stimuli in the prefrontal cortex are dependent on levels of recent negative life stress in major depressive disorder. Psychiatry Research, 183(3), 202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde J.S., Mezulis A.H., Abramson L.Y. (2008). The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychological Review, 115(2), 291–313. [DOI] [PubMed] [Google Scholar]
- Isenberg N., Silbersweig D., Engelien A., et al. (1999). Linguistic threat activates the human amygdala. Proceedings of the National Academy of Sciences of the United States of America, 96(18), 10456–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17(2), 825–41. [DOI] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C.M., Urry H.L., Kalin N.H., Davidson R.J. (2007). Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience, 27(33), 8877–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K.S., Edwards A.C., Gardner C.O. (2015). Sex differences in the pathways to symptoms of alcohol use disorder: a study of opposite-sex twin pairs. Alcoholism, Clinical and Experimental Research, 39(6), 998–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger E.A., Schacter D.L. (2006). Processing emotional pictures and words: effects of valence and arousal. Cognitive, Affective & Behavioral Neuroscience, 6(2), 110–26. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Nelson C.B., McGonagle K.A. (1996). The epidemiology of co-occurring addictive and mental disorders: implications for prevention and service utilization. The American Journal of Orthopsychiatry, 66(1), 17–31. [DOI] [PubMed] [Google Scholar]
- Kissler J., Assadollahi R., Herbert C. (2006). Chapter 8 Emotional and semantic networks in visual word processing: insights from ERP studies. Progress in Brain Research, 156, 147–83. [DOI] [PubMed] [Google Scholar]
- Kuntsche E., Muller S. (2012). Why do young people start drinking? Motives for first-time alcohol consumption and links to risky drinking in early adolescence. European Addiction Research, 18(1), 34–9. [DOI] [PubMed] [Google Scholar]
- Laird N.M. (1988). Missing data in longitudinal studies. Statistics in Medicine, 7(1–2), 305–15. [DOI] [PubMed] [Google Scholar]
- Lau-Barraco C., Skewes M.C., Stasiewicz R. (2009). Gender differences in high-risk situations for drinking: are they mediated by depressive symptoms? Addictive Behaviors, 34, 68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E. (2000). Emotion circuits in the brain. Annual Review of Neuroscience, 23, 155–84. [DOI] [PubMed] [Google Scholar]
- Lex B.W. (1991). Gender differences and substance abuse In: Mello N.K., editor. Advances in Alcohol and Substance Abuse. London, England: Jessica Kingsley Publishers Ltd. [Google Scholar]
- Lindquist K.A., Wager T.D., Kober H., Bliss-Moreau E., Barrett L.F. (2012). The brain basis of emotion: a meta-analytic review. The Behavioral and Brain Sciences, 35, 121–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI datasets. NeuroImage, 19, 1233–9. [DOI] [PubMed] [Google Scholar]
- Mazzola V., Vuilleumier P., Latorre V., et al. (2013). Effects of emotional contexts on cerebello-thalamo-cortical activity during action observation. PLoS One, 8(9), e75912.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K., Gross J.J., Weber J., et al. (2012). The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Social Cognitive and Affective Neuroscience, 7, 11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mickey B.J., Zhou Z., Heitzeg M.M., et al. (2011). Emotion processing, major depression, and functional genetic variation of neuropeptide Y. Archives of General Psychiatry, 68(2), 158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad M.R., Rauch S.L. (2007). The role of the orbitofrontal cortex in anxiety disorders. Annals of the New York Academy of Sciences, 1121, 546–61. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Goddings A.L., Clasen L.S., Giedd J.N., Blakemore S.J. (2014). The developmental mismatch in structural brain maturation during adolescence. Developmental Neuroscience, 36, 147–60. [DOI] [PubMed] [Google Scholar]
- Mooney D.K., Fromme K., Kivlahan D.R., Marlatt G.R. (1987). Correlates of alcohol consumption: sex, age, and expectancies relate differentially to quantity and frequency. Addictive Behaviors, 12, 235–40. [DOI] [PubMed] [Google Scholar]
- Monk C.S., McClure E.B., Nelson E.E., et al. (2003). Adolescent immaturity in attention-related brain engagement to emotional facial expressions. Neuroimage, 20(1), 420–8. [DOI] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. (2015). Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biological Psychiatry, 77(3), 276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. (2004). Gender differences in risk factors and consequences for alcohol use and problems. Clinical Psychology Review, 24(8), 981–1010. [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. (2012). Emotion regulation and psychopathology: the role of gender. Annual Review of Clinical Psychology, 8, 161–87. [DOI] [PubMed] [Google Scholar]
- Noll D.C., Fessler J.A., Sutton B.P. (2005). Conjugate phase MRI reconstruction with spatially variant sample density correction. IEEE Transactions on Medical Imaging, 24(3), 325–36. [DOI] [PubMed] [Google Scholar]
- Olenick N.L., Chalmers D.K. (1991). Gender-specific drinking styles in alcoholics and nonalcoholics. Journal of Studies on Alcohol, 52(4), 325–30. [DOI] [PubMed] [Google Scholar]
- O’Neil K.A., Conner B.T., Kendall P.C. (2011). Internalizing disorders and substance use disorders in youth: comorbidity, risk, temporal order, and implications for intervention. Clinical Psychology Review, 31(1), 104–12. [DOI] [PubMed] [Google Scholar]
- O’Neil K.A., Podell J.L., Benjamin C.L., Kendall P.C. (2010). Comorbid depressive disorders in anxiety-disordered youth: demographic, clinical, and family characteristics. Child Psychiatry and Human Development, 41(3), 330–41. [DOI] [PubMed] [Google Scholar]
- Ostby Y., Tamnes C.K., Fjell A.M., Westlye L.T., Due-Tonnessen P., Walhovd K.B. (2009). Heterogeneity in subcortical brain development: a structural magnetic resonance imaging study of brain maturation from 8 to 30 years. The Journal of Neuroscience, 29(38), 11772–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman S.B., Pelphrey K.A. (2011). Developing connections for affective regulation: age-related changes in emotional brain connectivity. Journal of Experimental Child Psychology, 108(3), 607–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L., Adolphs R. (2010). Emotion processing and the amygdala: from a ‘low road’ to ‘many roads’ of evaluating biological significance. Nature Reviews. Neuroscience, 11(11), 773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer J.H., Masten C.L., Moore W.E. III, et al. (2011). Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron, 69(5), 1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichon S., de Gelder B., Grezes J. (2008). Emotional modulation of visual and motor areas by dynamic body expressions of anger. Social Neuroscience, 3(3–4), 199–212. [DOI] [PubMed] [Google Scholar]
- Pichon S., de Gelder B., Grezes J. (2009). Two different faces of threat. Comparing the neural systems for recognizing fear and anger in dynamic body expressions. Neuroimage, 47(4), 1873–83. [DOI] [PubMed] [Google Scholar]
- Rive M.M., van Rooijen G., Veltman D.J., Phillips M.L., Schene A.H., Ruhe H.G. (2013). Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37, 2529–53. [DOI] [PubMed] [Google Scholar]
- Robins L.N., Helzer J.E., Croughan J., Ratcliff K.S. (1981). National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Archives of General Psychiatry, 38(4), 381–9. [DOI] [PubMed] [Google Scholar]
- Robins L.N., Cottler L.B., Bucholz K.K., Compton W.M., North C.S., Rourke K.M. (2000). Diagnostic Interview Schedule for the DSM-IV (DIS-IV). St. Louis, MO: Washington University School of Medicine. [Google Scholar]
- Rubonis A.V., Colby S.M., Monti P.M., Rohsenow D.J., Gulliver S.B., Sirota A.D. (1994). Alcohol cue reactivity and mood induction in male and female alcoholics. Journal of Studies on Alcohol, 55(4), 487–94. [DOI] [PubMed] [Google Scholar]
- Rudolph K.D., Hammen C. (1999). Age and gender as determinants of stress exposure, generation, and reactions in youngsters: a transactional perspective. Child Development, 70, 660–77. [DOI] [PubMed] [Google Scholar]
- Saarimaki H., Gotsopoulos A., Jaaskelainen I.P., et al. (2015). Discrete neural signatures of basic emotions. Cerebral Cortex, 26(6), 2563–73. [DOI] [PubMed] [Google Scholar]
- Sagaspe P., Schwartz S., Vuilleumier P. (2011). Fear and stop: a role for the amygdala in motor inhibition by emotional signals. Neuroimage, 55(4), 1825–35. [DOI] [PubMed] [Google Scholar]
- Sannibale C., Hall W. (2001). Gender-related symptoms and correlates of alcohol dependence among men and women with a lifetime diagnosis of alcohol use disorders. Drug and Alcohol Review, 20, 369–83. [Google Scholar]
- Satre D.D., Knight B.G. (2001). Alcohol expectancies and their relationship to alcohol use: age and sex differences. Aging & Mental Health, 5(1), 73–83. [DOI] [PubMed] [Google Scholar]
- Scherf K.S., Smyth J.M., Delgado M.R. (2013). The amygdala: an agent of change in adolescent neural networks. Hormones and Behavior, 64(2), 298–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. (1978). Estimating the dimension of a model.Annals of Statistics, 2, 461–4. [Google Scholar]
- Sergerie K., Chochol C., Armony J.L. (2008). The role of the amygdala in emotional processing: a quantitative meta-analysis of functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 32(4), 811–30. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Fani N., McClure-Tone E.B. (2011). Behavioral and neural representation of emotional facial expressions across the lifespan.Developmental Neuropsychology, 36(4), 408–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville L.H., Jones R.M., Casey B.J. (2010). A time of change: behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition, 72(1), 124–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear L.P. (2013). Adolescent neurodevelopment.The Journal of Adolescent Health, 52, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. (2008). Neuroscience perspective on adolescent risk taking. Developmental Review, 28, 1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J.S., Hamann S. (2012). Sex differences in brain activation to emotional stimuli: a meta-analysis of neuroimaging studies. Neuropsychologia, 50(7), 1578–93. [DOI] [PubMed] [Google Scholar]
- Sutton B.P., Noll D.C., Fessler J.A. (2003). Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities. IEEE Transactions on Medical Imaging, 22(2), 178–88. [DOI] [PubMed] [Google Scholar]
- Taylor S.F., Phan K.L., Decker L.R., Liberzon I. (2003). Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage, 18(3), 650–9. [DOI] [PubMed] [Google Scholar]
- Twenge J.M., Nolen-Hoeksema S. (2002). Age, gender, race, socioeconomic status, and birth control differences on the children’s depression inventory: a meta-analysis.Journal of Abnormal Psychology, 111(4), 578–88. [DOI] [PubMed] [Google Scholar]
- Van den Stock J., Tamietto M., Sorger B., Pichon S., Grezes J., de Gelder B. (2011). Cortico-subcortical visual, somatosensory, and motor activations for perceiving dynamic whole-body emotional expressions with and without striate cortex (V1). Proceedings of the National Academy of Sciences of the United States of America, 108(39), 16188–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J.E., Sauter D.A., Eisner F., et al. (2006). Positive emotions preferentially engage an auditory-motor “mirror” system. The Journal of Neuroscience, 26(50), 13067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager T.D., Phan K.L., Liberzon I., Taylor S.F. (2003). Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage, 19(3), 513–31. [DOI] [PubMed] [Google Scholar]
- Wierenga L., Langen M., Ambrosino S., van Dijk S., Oranje B., Durston S. (2014). Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. Neuroimage, 96, 67–72. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C., Shirtcliff E.A., Marceau K. (2008). Disorders of childhood and adolescence: gender and psychopathology. Annual Review of Clinical Psychology, 4, 275–303. [DOI] [PubMed] [Google Scholar]
- Zucker R.A., Fitzgerald H.E. (1994). Drinking and Drug History Form for Children. Ann Arbor, MI: Department of Psychiatry, Addiction Research Center, University of Michigan. [Google Scholar]
- Zucker R.A., Ellis D.A., Fitzgerald H.E., Bingham C.R., Sanford K.P. (1996). Other evidence for at least two alcoholisms II: life course variation in antisociality and heterogeneity of alcoholic outcome. Development and Psychopathology, 8, 831–48. [Google Scholar]
- Zucker R.A., Fitzgerald H.E., Refior S.K., Puttler L.I., Pallas D.M., Ellis D.A. (2000). The clinical and social ecology of childhood for children of alcoholics: description of a study and implications for a differentiated social policy In: Fitzgerald H.E., Lester B.M., Zucker R.A., editors. Children of Addiction: Research, Health and Policy Issues. New York: RoutledgeFalmer Publishers, 109–41. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.