The immune system in cancer
Ehrlich hypothesized that the immune system would be critical in preventing the growth of an “overwhelming frequency” of cancers [1]. Coley’s success in occasionally inducing tumor regression following the injection of bacteria into patients provided early hints in support of this view [2,3]. Nearly 50 years after Ehrlich’s observations, following the development of inbred mouse strains and studies on mice immunized with syngeneic tumors [4], Macfarlane Burnet and Lewis Thomas articulated their theories on “cancer immunosurveillance”. These theories posited the evolutionary necessity for the immune system to control and eliminate neoplastic cells before they become malignant [5,6][5,6].
More recently, clinical trials using chimeric antigen receptor T cell therapy, adoptive T cell transfers, and checkpoint blockade (particularly with PD-1) have demonstrated that the immune response can be harnessed to eliminate tumors, creating a critical inflection point in cancer immunotherapy. The success of checkpoint blockade, in particular, has demonstrated that tumor-infiltrating lymphocytes (TILs) are indeed cancer-specific immune cells, but they are induced to become exhausted or dysfunctional in the tumor microenvironment, resulting in the abrogation of the antitumor immune response. While much of the focus in tumor immunology has been on CD8+ cytolytic cells whose activity is closely linked to patient survival [7], T cells do not work in a vacuum. B cells account for up to 25% of all cells in some tumors. Furthermore, 40% of TILs in some breast cancer subjects are B cells [8–10]. Consistent with a strong immunomodulatory role for these cells, 40% of high-grade serous ovarian cancers have also been shown to contain infiltrating CD20+ B cells. [11] In some mouse models of cancer, about a third of tumor-draining lymph nodes cells are B cells [12], suggesting that these cells may have critical roles in modulating tumor responses. Furthermore, therapeutic immune checkpoint blockade may also target activated B cells, in addition to activated T cells, sincePD-1, PD-L1, CTLA-4, and the B7 molecules are expressed on B cells. Additionally, both CTLA-4 and PD-1 inhibit B cell activity, and blockade of either molecule enhances the proliferation of memory B cells and the production of antibody, either by directly or indirectly acting on B cells [13–23].
Antibodies, all made by B cells, can alter the function of their antigenic targets on cancer cells, opsonize tumor cells for the presentation and cross-presentation of tumor antigens by dendritic cells, activate the complement cascade, or contribute to NK cell mediated tumor killing via antibody-dependent cell-mediated cytotoxicity. While antibodies against tumor antigens have been frequently found in the serum of cancer patients [24], the role of humoral immune responses against cancer remains controversial. Furthermore, many of the antibodies in cancer patients are directed against autoantigens -- molecules that are present in both the tumor cells as well as in unmutated host cells.
In this review, we will primarily examine the immunological mechanisms by which B cells promote, as well as inhibit, anti-tumor immunity in the context of a range of malignancies. This review will not address how aberrant VDJ recombination events, or unique events in the B lineage, such as somatic hypermutation and isotype switching, contribute to malignancies of the B lineage. We will also not discuss how antibodies generated in an anti-tumor context can mediate paraneoplastic syndromes as these have been covered in detail in other reviews [25–27].
B cell suppression of the antitumor response
Since the 1970’s, it had been appreciated that B cells could facilitate the growth of certain experimental tumors in mice. In early studies from Brodt and Gordon, mice depleted of B cells from birth (by the injection of anti-IgM antibodies) exhibited an increased resistance to an injected syngeneic fibrosarcoma, as evidenced by slower tumor growth and a decreased incidence of spontaneous pulmonary metastasis [28]. In this section, we will describe how antibodies and B cells may contribute to cancer growth and progression.
Antibody-mediated immune suppression
Some of the antibodies observed in the cancer context are against tumor-specific neo-antigens, such as mutated p53 [29], while others are against non-mutated host proteins. [30] Cloning and sequencing of autoantibody genes from tumor subjects have revealed the existence of IgG antibodies with a high degree of somatic hypermutation [31]. Apoptotic and necrosed tumor cells and endogenous adjuvant moieties may contribute to an inflamed tumor environment, releasing more self-antigens, resulting in a break in immunological tolerance reminiscent to that observed in autoimmune diseases. Despite the presence of antibodies against cytosolic and nuclear proteins derived from tumors, these antibodies may actually represent an epiphenomenon with no real significance for tumor growth. However, as will be discussed in a subsequent section, some of the antibodies against tumor antigens may enhance anti-tumor immunity. In this sub-section, we will discuss the opposite phenomenon -- how some antibodies might contribute to the progression of tumors.
What makes certain anti-tumor antibodies drivers of tumor progression? The correlations between anti-tumor antibodies and disease outcome may be linked to the ability of these antibodies to generate circulating immune complexes (CICs). While CICs are generally thought of in the context of diseases like systemic lupus erythematosus and serum sickness, they also have a role in the setting of cancer [32]. In human cancers, CICs in the circulation or in tumor tissue do not generally correlate with protection against the tumor, but instead reflect poor clinical outcome [33,34]. This pro-tumorigenic role of B cells and CIC has been supported by studies using a genetic mouse model of squamous cell carcinoma [35], wherein CICs are deposited in premalignant tissue and activate Fcγ receptors on resident and infiltrating myeloid cells (particularly macrophages and mast cells) [36]; they also trigger the complement pathway, thus feeding into a pro-angiogenic program of tissue remodeling that ultimately resulted in keratinocyte hyperproliferation and malignant progression [32,35].
In a recent study examining B16-F10 melanoma tumor cells, tumor-derived extracellular vesicles induced antibodies that promoted tumorigenesis, but this tumor-promoting activity could be abrogated by subcapsular sinus macrophages that captured antigens before they activated B cells in draining lymph nodes [37]. Extracellular vesicles derived from tumors contain membrane and lumenal proteins that are capable of stimulating adaptive immune responses [38]. We hypothesize that when the major tumor antigens that induce antibodies are either surface or intracellular proteins that do not contain potential antigenic, tumor-derived, MHC class I-binding peptides, they can nonetheless be incorporated into immune complexes, and this set of CICs do not have the potential to facilitate CD8+ CTL activation. These CICs may, however, have the ability to bind to myeloid cells within tumors, activate Fcγ receptors on these cells and thus induce myeloid suppressor cell activity that promotes tumorigenesis (Figure 1).
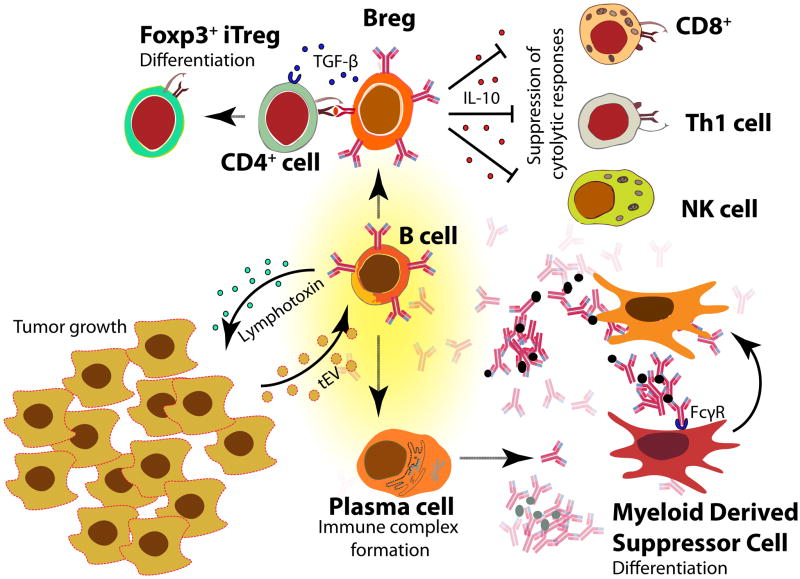
Figure 1. B cell suppression of the antitumor response.
B cells can produce lymphotoxin, which induces angiogenesis and thus promotes tumor growth. Tumor-derived extracellular vesicles (tEVs) can activate B cells to produce antibodies, which can bind antigen and form immune complexes. These circulating immune complexes can activate Fcγ receptors on myeloid cells, inducing them to become myeloid-derived suppressor cells, which suppress anti-tumor CD4+ and CD8+ T cell responses. A subset of B cells called regulatory B cells (Bregs) can also secrete immunoregulatory cytokines, including TGFβ, which induces CD4+ T cells to become Foxp3+ CD4+ T regulatory cells (Tregs), and IL-10, which suppresses CD4+ Th1 cells, natural killer (NK) cells, and CD8+ cytotoxic T cells.
Pro-tumorigenic factors secreted by B cells
One important feature of cancer is the induction of lymphangiogenesis to promote growth and metastasis of neoplastic cells throughout the body [39], pointing to the important role of lymphatic vessels in tumor progression. Lymphangiogenesis can be promoted by inflammation and thus facilitate immune cell trafficking into the lymphatics. The crosstalk that occurs between tumor cells, stromal cells, and immune cells may be crucial to the induction of lymphangiogenesis.
It is now appreciated that tumor-infiltrating B cells can provide lymphotoxin, a survival factor that can induce angiogenesis, to tumors. Androgen ablation by castration in a mouse prostate cancer model, followed by cell death of the cancer cells, causes damage to the stromal cells of the tumor microenvironment, promoting leukocyte infiltration into the tumor. Following androgen ablation, the production of the chemokine CXCL13 -- potentially by intratumor T follicular helper cells, whose presence has been associated with tumor CXCL13 expression and the progression of a number of tumor types – recruit B cells into the tumor [40–43]. These tumor-infiltrating B cells then secrete lymphotoxin, which activates non-canonical and canonical NF-κB signaling and STAT3 in the remaining cancer cells, resulting in androgen-refractory growth and tumor progression [44–46]. Additionally, B cells can also enhance metastasis of bladder cancer cells by upregulating IL-8, which can modulate androgen receptor and matrix metalloproteinase signaling [47]. Lymphotoxin, however, may also contribute to tumor regression in certain tumor settings, and this is discussed in a subsequent section.
Inhibition of antitumor immunity via cytokine production by regulatory B cells
In addition to their ability to secrete antibodies that can directly inflame the tumor parenchyma and molecules that can activate angiogenesis in the vasculature, B cells that produce a variety of immunoregulatory cytokines are also capable of suppressing the anti-tumor immune response by inhibiting effector cells such as CD8+CTLs and NK cells [48]. These B cells, termed regulatory B cells (Bregs) are a heterogeneous population that suppress inflammatory responses, either directly or indirectly, and their role in cancer has been well-described [49–54]. Bregs are operationally described as cells that secrete IL-10, but some Bregs can also secrete TGFβ while others make IL-35 [55–58]. Furthermore, it also appears that there are phenotypic and functional differences between many of these Bregs: there are IL-10 producing Bregs that exacerbate inflammation and support cancer growth [35,44], B cells that inhibit CD4+ T cell responses [59], and distinct tumor-evoked B regulatory cells.[56,60] While B cell suppression of Th1-type responses during chronic inflammation may be beneficial in autoimmunity, this same immune-modulatory activity may thwart the ability of the host to detect and eliminate mutated neoplastic cells, allowing tumor cells to survive and proliferate in the chronically inflamed environment.
How do these B cells gain regulatory potential? One mechanism by which B cells may become regulatory is intrinsic to the neoplasm itself: the tumor may exert long-range control to induce the recruitment of cells and mediators to promote its growth. Bregs can be generated from the actions of the metabolites of 5-lipoxygenase (such as leukotriene B4), produced by the tumor that activate PPARα in B cells and initiate their differentiation into Bregs [60]. TGFβ produced by Bregs can convert naïve CD4+ T cells into Foxp3+ Tregs, which inhibit NK cells and effector CD8+ CTLs, which would otherwise thwart the metastasis of neoplastic cells [56,57,61]; this finding may explain studies in B cell-deficient μMT mice implicating B cells in the inhibition of the induction of CTL-mediated tumor immunity by disabling CD4 T cell help [59]. As these tumor-evoked Bregs trigger a chain of events culminating in immune suppression, it is likely that they are continually induced as long as the cancer cells persist [62], thus underscoring the value of finding ways to control and deplete these cells in the course of therapeutic intervention.
Immune cells at the site of the tumor or tumor-draining lymph node can also induce a Breg phenotype. It has also been shown that the production of IL-21 by Tregs may also make B cells become more “regulatory” in nature, as IL-21 promotes the upregulation of IL-10, IDO, and granzyme B in B cells [63]. While granzyme B is normally thought to be an important effector molecule in cytotoxic NK cells, CD8+CTLs and CD4+ CTLs as well, this serine protease apparently also has an immunomodulatory function. It has been reported that Bregs can transfer granzyme B to T cells, degrading the T-cell receptor zeta chain without inducing T-cell apoptosis and thus suppressing antitumor T cell activity [63].
Phenotypic characterization of B cells that preferentially accumulated in the tumor-draining lymph nodes in one B16-F10 melanoma mouse model elucidated the specific subset responsible for driving remodeling of the tumor-draining lymph node and promoting metastasis. These cells were T2-MZP B cells, which neither increased IL-10 production nor promoted the generation of Tregs, unlike their Breg counterparts in the spleen [64]. These T2-MZP Bregs appeared to have been recruited to the draining lymph nodes by the tumor via the lymphatics early in tumor development to develop the immunosuppressive environment required initially for growth and ultimately for metastasis to the lymph node and other organs. We originally described “T2-MZP” cells later known as MZP cells (for marginal zone precursors) as cells that are IgMhiIgDhiCD1dhi CD21hi B cells that are exclusively seen in the spleen [65,66]. While the original description of regulatory B cells by Mizoguchi et al. [67,68] identified cells in the intestine that are CD1dhi, cells with the complete MZP phenotype have not been described outside the spleen in the mouse, and certainly not in lymph nodes, making this finding in the melanoma model all the more intriguing.
In a study that sought to identify B cell-dependent factors that inhibit anti-tumor immunity, irradiated tumor cells were co-cultured in vitro with splenocytes from either wild-type or B cell-deficient mice [69]. While B cell-deficient splenocytes were able to eliminate the implanted tumors, wild-type splenocytes were unable to control tumor growth, owing to the fact that the wild-type B cells co-cultured with tumor cells produced IL-10, which decreased the IFNγ production from CD8 T cells and NK cells.
A single injection of a tumor vaccine was able to inhibit tumor growth in B cell-deficient μMT mice, but not in wild-type mice [70]. In this model it has been suggested that Bregs inhibit NK cell activation. Similarly, tumors in B cell-deficient mice, but not in wild type mice could be rejected with only one vaccination with the gp96-chaperone protein [71]. Similarly, B cell depletion facilitated the clearance of squamous cell carcinomas by activated CD8+ CTLs after cis-platinum- and Taxol-based chemotherapy [72]. Studies examining the growth of murine mammary tumors implanted in mice have demonstrated the association of tumor-associated B cells with the recruitment and proliferation of Treg cells within the tumor microenvironment, as well as the reduced recruitment of CD49+ NK and CD8+ T cells into the tumor, though this was independent of IL-10 secretion by B cells [73]. Together, these findings demonstrate the important influence of B cells in controlling cytotoxic NK and CD8+ CTL responses in the tumor context.
Cancer-induced B cells are also required to induce the full regulatory activity of myeloid-derived suppressor cells (MDSCs), allowing them to suppress CD4+ and CD8+ T cells. Tumor Bregs produce TGFβ, which acts on the monocyte and granulocyte subpopulations of MDSCs to upregulate ROS and NO production, both of which are required for the complete and efficient suppression of anti-tumor CD4+ and CD8+ T cells [57]. B cells can also blunt the immunogenic effects of low-dose oxaliplatin, a chemotherapeutic that promotes cell death and CD8+ CTL killing: in one study, three different mouse prostate cancer models showed no response to oxaliplatin unless B cells were depleted [74]. Oxaliplatin induced the expression of TGFβ in the tumor, converting tumor-infiltrating B cells into IgA-secreting plasmacytes that also produced IL-10 and expressed PD-L1 and Fas-L, activities that inhibited CTL function and induced local immunosuppression.
Regulatory B cells therefore represent an important cell type involved in the suppression of anti-tumor immune responses. These B cells for the most part either directly attenuate anti-tumor immunity through the secretion of IL-10 [69,75] or indirectly compromise immune responses to tumors through the production of TGFβ and the conversion of resting CD4+ T cells into Tregs [56,76] (Figure 1). There is growing evidence in some human tumors for the presence of Bregs, but although more global B cell depletion can be achieved using anti-CD20 and other reagents, there are at present no therapeutic approaches to selectively remove Bregs alone. Additionally, the success of B cell depletion in impeding tumor growth in mouse models has been mixed: in one study, B cell depletion using an anti-CD20 antibody was able to impede tumor growth in a number of solid tumor models, as well as bolster the response to a tumor vaccine [77] in mice. However, other studies have seen no additional effect of rituximab on IL-2 therapy for renal cell carcinoma and melanoma patients [78]. Furthermore, evidence from mouse studies suggest that anti-CD20 depletion can impair T cell-mediated antitumor immunity [79] and even promote cancer escape, as Bregs evoked by the tumor often express CD20 at low levels [80].
B cells as positive mediators of the anti-tumor response
B cells play critical roles in autoimmunity and transplant rejection. In fact, antibody-mediated B cell depletion is an effective therapeutic strategy for restraining aberrant T cell responses in the clinic [81]. Consistent with this, B cell depletion in adult mice has been shown to blunt auto-antigen specific CD4+ T cell responses [82]. Thus, it should come as no surprise that these cells are capable of driving anti-tumor responses, which have significant parallels with chronic “anti-self” immune responses. Highlighting this idea, B cells and follicular dendritic cells at the invasive margins of colorectal cancer are described as a “Crohn’s like reaction” [83]. However, B cells, as the “other” tumor-infiltrating lymphocytes (TILs) [11], have been overshadowed by cytolytic T cells despite evidence showing that TIL B cells also exhibit antigen-driven clonal expansion, class switching and affinity maturation in the cancer environment [84–86].
Solid tumors in humans often contain significant B cell populations, suggesting a role for these cells in cooperating with other resident cells to influence the tumor microenvironment [87]. Adoptive transfer studies in mice and correlative studies in human cancer have also ascribed a protective and sometimes antigen-specific role to TIL B cells. In one study of metastatic melanoma, tumor-infiltrating B cells were identified as the second-best predictor of positive disease outcome, after CD8+ TILs [88]. Similar studies have shown that the presence of peritumoral B cells in cervical cancer [89] and TIL B cells in lung cancer [90,91] correlate with lower relapse rates and increased survival, respectively. Along the same lines, Milne et al. performed a detailed study in ovarian cancer showing that, not only does the presence of CD20+ cells alone correlate with better outcomes, the presence of B cells together with T cells is associated with higher survival than the presence of either one alone [92]. The potential for cooperative interplay between B and T cells was also highlighted by studies where co-transfer of tumor draining lymph node B and T cells was much more effective at limiting metastasis of a murine carcinoma line, 4T1, than transfer of a single lymphocyte subset [93]. As will be discussed in greater detail below, B cells can play critical roles in anti-tumor immunity by producing antibodies, acting as antigen presenting cells, and making inflammatory molecules that regulate other immune cells.
Antibodies that promote tumor clearance
Antibodies directed against intracellular tumor antigens are frequently observed in human cancer patients [24]. Antibodies directed against aberrantly exposed β-actin in apoptotic tumor cells have been frequently found in medullary breast cancer patients [31,94]. Interestingly, several independent studies suggest that the favorable clinical outcomes characteristic of this breast cancer subtype correlate with the presence of lymphoplasmacytic infiltrates in the tumor [95,96]. Although there is some discordance in the literature, a few studies have also shown that the presence of anti-p53 antibodies correlates with favorable outcomes in lung cancer [34]. Furthermore, regression of human lung cancer tissue xenotransplanted into SCID mice was associated with increased tumor-resident B cell derived IgG in the serum of recipients [97]. Consistent with this, TIL B cell derived antibodies have been shown to bind mouse tumors in an antigen-specific manner and mediate complement-dependent lysis [12]. Interestingly, a recent elegant study, leveraging single cell transcriptomics together with bulk gene expression data from The Cancer Genome Atlas (TCGA), has shown that the expression of complement genes correlated with “inferred” T cell abundance in numerous tumors [98]. Although experimental proof in the context of cancer is lacking, it is possible that antigen, antibody and complement containing immune complexes contribute to the inflammation that facilitates T cell infiltration into tumors. In addition, the induction of autoantibodies in mice via vaccination with immunogenic epitopes identified by serological analysis of tumor derived cDNA libraries enhances CD8 T cell responses against the tumor [99]. Direct injection of tumor-binding allogeneic natural IgG antibodies with concomitant dendritic cell activation has also been shown to drive tumor regression. In this context, antibodies facilitated the opsonization and subsequent uptake of tumor cells by dendritic cells via Fcγ receptors. Antigen-bearing dendritic cells then efficiently activated tumor-specific CD8+ T cells, which proceeded to mount a highly effective response against the tumor (Figure 2). Recapitulating this finding, the authors also showed that human tumor cells coated with allogeneic natural IgG (and not those with autologous IgG) induced robust activation of BMDCs from mesothelioma patients, which could then drive the proliferation of the patient’s own CD4+ T-cells [100].
Figure 2. B cells as positive mediators of the antitumor response.
B cells can produce lymphotoxin, which has an additional role of promoting the formation of tertiary lymphoid organs, which is positively correlated with disease outcome and patient survival. The production of antibodies by plasma cells has many roles in contributing to the antitumor response: antitumor antibodies promote antibody- and complement-mediated killing of the tumor cells, Fc-mediated phagocytosis by macrophages, and antibody-dependent cell-mediated cytotoxicity (ADCC) by natural killer (NK) cells. Importantly, antibody-coated tumor cells can also be taken up and processed by dendritic cells, which present tumor antigens to CD4+ T cells and cross-present antigens to CD8+ T cells. If the tumor antigen contains an MHC-I epitope, anti-tumor CD8+ T cells could be activated; these effector CD8+ cytotoxic T cells will then traffic to the site of the tumor, killing the tumor cells. In some cases, B cells can also take up and process tumor antigens, which can then be presented to CD4+ T cells.
B cells as antigen-presenting cells that promote anti-tumor immunity
B cells, consistent with their ability to take up low concentrations of antigen using their BCRs, are efficient antigen-presenting cells. Memory B cells are long-lived and could present antigens driving T cell expansion and subsequent memory formation, once the initial wave of dendritic cells wanes [101,102]. In fact, in ovarian cancer tissues that have very few dendritic cells, B cells are in close proximity to T cells, suggesting an ongoing and close collaboration [92].
In Friend murine leukemia virus (F-MuLV)-induced leukemia, B cells were required for the induction of tumor-specific CD4+ and CD8+ T cells following priming with the virus or with a recombinant virus containing the F-MuLV antigens [103]. In another mouse model using transfer of syngeneic B16 melanoma cells, anti-CD20 mediated depletion of B cells also led to blunted CD4+ and CD8+ T cell responses accompanied by a two-fold increase in tumor size and lung metastasis [104]. In addition, in vivo administration as well as in vitro activation with anti-CD40 antibodies complementing T cell targeting anti-CD3 antibodies has been shown to drive potent T cell responses. Not surprisingly, these effects were dependent on the presence of B cells [12].
B cells as immunomodulatory cells that promote anti-tumor immunity
Although lymphotoxin can facilitate tumorigenesis [44], this B cell produced cytokine can also facilitate the formation of ectopic tertiary lymphoid organs (TLOs) [58,105,106]. The numbers of TLOs correlate directly with positive outcomes in both human disease and mouse models [106–108]. In fact, the presence of B cells in high density within TLOs is a prognostic marker predicting longer patient survival in lung cancer [109]. Complementing their ability to make cytokines, B cells also make several chemokines and recruit other immune cells to secondary lymphoid organs, TLOs and effector sites [102,110–113]. The anti-tumor B cell arsenal is further bolstered by their ability to provide antigen independent help to anti-tumor cytolytic T cells through CD27-CD70 interactions [114].
Perspectives and Final Thoughts
As discussed in the aforementioned sections, B cells play widely varied roles in the context of tumor immunity, and in different cancers contribute to either tumor growth or anti-tumor immunity. Correlative studies on human tumor infiltrating B cells are somewhat limited by their inability to differentiate between functional B cells present in tumors and bystanders simply recruited as a result of the local cytokine milieu. On the other hand, mouse models of cancer, which are usually accompanied by rapid responses reminiscent of viral infections, might not provide an accurate representation of the chronic anti-cancer immune responses seen in humans [11][11].
Genetic and immunologic “sledgehammer” type approaches such as the use of mice lacking B cells or anti-CD20 mediated B cell depletion fail to take into account the functional heterogeneity of B cells. As one might expect, tumor-infiltrating CD8+ TIL responses are bolstered by activated B cells, but inhibited by resting B cells; however, B-cell deficient mice lack both resting and activated B cells, thus confounding the study of the role of B cells in cancer immunity. This is of critical significance, since B cells present in human cancer patients are typically activated [11], suggesting that in human disease, they may frequently facilitate antitumor immunity.
The activation status of B cells can also be used to bin them into subsets, particularly using cell surface markers, which has been a key way to study their functional role in immunity and disease. The importance of B cell subsets in cancer immunity was made apparent in a recent study demonstrating that IL-6 dependent activation of STAT3, which had long been associated with chronic inflammation in the tumor microenvironment [44,56,115], was confined to the subpopulation of CD5+, but not CD5− B cells [116]. CD5+ B cell numbers and STAT3 activation correlated with poor survival
There is a need to identify better markers for pro-tumorigenic B cells as well as for anti-tumor B cells. Some B cell subsets, including Bregs, are refractory to depletion by anti-CD20 antibody, underscoring the need for more specific therapeutics targeting B cell subsets. In the future, it may be possible to use immunotherapy to break tolerance against the tumor to promote the induction of neutralizing tumor-specific antibodies, as well as to use novel biologics that selectively deplete pro-tumorigenic B cells.
Future studies will be needed to identify the immunologic conditions that specifically promote the pro-tumorigenic effects of B cells, as well as to identify reagents that enhance the ability of B cells to facilitate anti-tumor immunity.
Outstanding Questions.
What conditions render B cells pro- or anti-tumorigenic?
Are anti-tumor antibodies “bystanders” or do they play a role in tumor immunity?
Is our understanding of B cells from mouse tumor models representative of human disease?
What is the role of regulatory B cells in human tumors?
What phenotypic markers can distinguish pro-tumorigenic B cells from anti-tumor B cells?
How can we target B cells as a form of cancer immunotherapy?
Trends Box.
B cells play an important role in modulating the immune response to cancer, consistent with being the second most abundant tumor-infiltrating lymphocyte.
B cells can inhibit tumor development through the production of tumor-reactive antibodies, promoting tumor killing by NK cells, phagocytosis by macrophages, and the priming of CD4+ and CD8+ T cells.
B cells can promote tumor development through the production of autoantibodies and tumor growth factors. Regulatory B cells can directly and indirectly suppress Th1 and CD8+ cytolytic T cell responses.
Targeting specific B cell subsets may be of therapeutic value in cancer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ehrlich P. Beitraege zur Experimentellen Pathologie und Chemotherapie. Akademische Verlagsgesellschaft; Leipzig: 1909. Ueber den jetzigen Stand der Karzinomforschung. Vortrag gehalten vor den Studenten der Amsterdamer Universitaet, Vereinigung fuer wissenschaftliche Arbeit 1 June 1908. Printed in: P. Ehrlich. [Google Scholar]
- 2.Coley WB. CONTRIBUTION TO THE KNOWLEDGE OF SARCOMA. Ann Surg. 1891;14:199–220. doi: 10.1097/00000658-189112000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coley WB. TREATMENT OF INOPERABLE MALIGNANT TUMORS WITH THE TOXINES OF ERYSIPELAS AND THE BACILLUS PRODIGIOSUS. Am J Med Sci. 1894;108:50–66. [Google Scholar]
- 4.Klein G. Tumor antigens. Annu Rev Microbiol. 1966;20:223–252. doi: 10.1146/annurev.mi.20.100166.001255. [DOI] [PubMed] [Google Scholar]
- 5.Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27. doi: 10.1159/000386035. [DOI] [PubMed] [Google Scholar]
- 6.Burnet M. IMMUNOLOGICAL FACTORS IN THE PROCESS OF CARCINOGENESIS. Br Med Bull. 1964;20:154–158. doi: 10.1093/oxfordjournals.bmb.a070310. [DOI] [PubMed] [Google Scholar]
- 7.Pagès F, et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–1102. doi: 10.1038/onc.2009.416. [DOI] [PubMed] [Google Scholar]
- 8.Coronella-Wood JA, Hersh EM. Naturally occurring B-cell responses to breast cancer. Cancer Immunol Immunother. 2003;52:715–738. doi: 10.1007/s00262-003-0409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chin Y, et al. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992;12:1463–1466. [PubMed] [Google Scholar]
- 10.Marsigliante S, et al. Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett. 1999;139:33–41. doi: 10.1016/s0304-3835(98)00379-6. [DOI] [PubMed] [Google Scholar]
- 11.Nelson BH. CD20+ B cells: the other tumor-infiltrating lymphocytes. J Immunol. 2010;185:4977–4982. doi: 10.4049/jimmunol.1001323. [DOI] [PubMed] [Google Scholar]
- 12.Li Q, et al. Simultaneous targeting of CD3 on T cells and CD40 on B or dendritic cells augments the antitumor reactivity of tumor-primed lymph node cells. J Immunol. 2005;175:1424–1432. doi: 10.4049/jimmunol.175.3.1424. [DOI] [PubMed] [Google Scholar]
- 13.Postow MA, et al. Immune Checkpoint Blockade in Cancer Therapy. J Clin Oncol. 2015;33:1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong H, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8:1039–1039. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 15.Fanoni D, et al. New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett. 2011;134:157–160. doi: 10.1016/j.imlet.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 16.Korman AJ, et al. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishimura H, et al. Immunological studies on PD-1 deficient mice: implication of PD-1 as a negative regulator for B cell responses. Int Immunol. 1998;10:1563–1572. doi: 10.1093/intimm/10.10.1563. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura H, et al. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 20.Thibult M-L, et al. PD-1 is a novel regulator of human B-cell activation. Int Immunol. 2013;25:129–137. doi: 10.1093/intimm/dxs098. [DOI] [PubMed] [Google Scholar]
- 21.Pioli C, et al. Inhibition of IgG1 and IgE production by stimulation of the B cell CTLA-4 receptor. J Immunol. 2000;165:5530–5536. doi: 10.4049/jimmunol.165.10.5530. [DOI] [PubMed] [Google Scholar]
- 22.Intlekofer AM, Thompson CB. At the bench: preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J Leukoc Biol. 2013;94:25–39. doi: 10.1189/jlb.1212621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Okazaki T, et al. PD-1 immunoreceptor inhibits B cell receptor-mediated signaling by recruiting src homology 2-domain-containing tyrosine phosphatase 2 to phosphotyrosine. Proc Natl Acad Sci U S A. 2001;98:13866–13871. doi: 10.1073/pnas.231486598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reuschenbach M, et al. A systematic review of humoral immune responses against tumor antigens. Cancer Immunol Immunother. 2009;58:1535–1544. doi: 10.1007/s00262-009-0733-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–1554. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 26.Posner JB, Dalmau J. Paraneoplastic syndromes. Curr Opin Immunol. 1997;9:723–729. doi: 10.1016/s0952-7915(97)80055-6. [DOI] [PubMed] [Google Scholar]
- 27.Dropcho EJ. Update on paraneoplastic syndromes. Curr Opin Neurol. 2005;18:331–336. doi: 10.1097/01.wco.0000169754.38944.a4. [DOI] [PubMed] [Google Scholar]
- 28.Brodt P, Gordon J. Anti-tumor immunity in B lymphocyte-deprived mice. I Immunity to a chemically induced tumor. J Immunol. 1978;121:359–362. [PubMed] [Google Scholar]
- 29.Scanlan MJ, et al. Characterization of human colon cancer antigens recognized by autologous antibodies. Int J Cancer. 1998;76:652–658. doi: 10.1002/(sici)1097-0215(19980529)76:5<652::aid-ijc7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 30.Curiel DT, Douglas JT. Cancer Gene Therapy. Humana Press; 2007. [Google Scholar]
- 31.Hansen MH, et al. Translocation of an intracellular antigen to the surface of medullary breast cancer cells early in apoptosis allows for an antigen-driven antibody response elicited by tumor-infiltrating B cells. J Immunol. 2002;169:2701–2711. doi: 10.4049/jimmunol.169.5.2701. [DOI] [PubMed] [Google Scholar]
- 32.Gunderson AJ, Coussens LM. B cells and their mediators as targets for therapy in solid tumors. Exp Cell Res. 2013;319:1644–1649. doi: 10.1016/j.yexcr.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tan T-T, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Kumar S, et al. Prognostic implications of circulating anti-p53 antibodies in lung cancer--a review. Eur J Cancer Care. 2009;18:248–254. doi: 10.1111/j.1365-2354.2008.01019.x. [DOI] [PubMed] [Google Scholar]
- 35.de Visser KE, et al. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 36.Andreu P, et al. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pucci F, et al. SCS macrophages suppress melanoma by restricting tumor-derived vesicle-B cell interactions. Science. 2016 doi: 10.1126/science.aaf1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raposo G, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 40.Bindea G, et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Bindea G, et al. The immune landscape of human tumors: Implications for cancer immunotherapy. Oncoimmunology. 2014;3:e27456. doi: 10.4161/onci.27456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu-Trantien C, et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J Clin Invest. 2013;123:2873–2892. doi: 10.1172/JCI67428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teng MWL, et al. From mice to humans: developments in cancer immunoediting. J Clin Invest. 2015;125:3338–3346. doi: 10.1172/JCI80004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ammirante M, et al. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302–305. doi: 10.1038/nature08782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo J-L, et al. Nuclear cytokine-activated IKKα controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446:690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 46.Woo JR, et al. Tumor infiltrating B-cells are increased in prostate cancer tissue. J Transl Med. 2014;12:30. doi: 10.1186/1479-5876-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ou Z, et al. Tumor microenvironment B cells increase bladder cancer metastasis via modulation of the IL-8/androgen receptor (AR)/MMPs signals. Oncotarget. 2015;6:26065–26078. doi: 10.18632/oncotarget.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20:332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balkwill, et al. B regulatory cells in cancer. Trends Immunol. 2013;34:169–173. doi: 10.1016/j.it.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Y, et al. Regulatory B cells in anti-tumor immunity. Int Immunol. 2015;27:521–530. doi: 10.1093/intimm/dxv034. [DOI] [PubMed] [Google Scholar]
- 51.Gorosito Serrán M, et al. The regulatory role of B cells in autoimmunity, infections and cancer: Perspectives beyond IL10 production. FEBS Lett. 2015;589:3362–3369. doi: 10.1016/j.febslet.2015.08.048. [DOI] [PubMed] [Google Scholar]
- 52.Fremd C, et al. B cell-regulated immune responses in tumor models and cancer patients. Oncoimmunology. 2013;2:e25443. doi: 10.4161/onci.25443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Catalina Lee-Chang MBAAB. Tumor-Evoked Regulatory B Cells as Important Mediators of Cancer Escape. In: Shurin et M, editor. The Tumor Microenvironment. [Google Scholar]
- 54.Biragyn A, Lee-Chang C. A new paradigm for an old story: the role of regulatory B cells in cancer. Front Immunol. 2012;3:206. doi: 10.3389/fimmu.2012.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shen P, et al. IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases. Nature. 2014;507:366–370. doi: 10.1038/nature12979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Olkhanud PB, et al. Tumor-evoked regulatory B cells promote breast cancer metastasis by converting resting CD4+ T cells to T-regulatory cells. Cancer Res. 2011;71:3505–3515. doi: 10.1158/0008-5472.CAN-10-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bodogai M, et al. Immunosuppressive and Prometastatic Functions of Myeloid-Derived Suppressive Cells Rely upon Education from Tumor-Associated B Cells. Cancer Res. 2015;75:3456–3465. doi: 10.1158/0008-5472.CAN-14-3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15:441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 59.Qin Z, Richter G, Schüler T, Ibe S, Cao X, Blankenstein T. B cells inhibit induction of T cell-dependent tumor immunity. Nature Medicine. doi: 10.1038/nm0598-627. at < http://www.ncbi.nlm.nih.gov/pubmed/9585241>. [DOI] [PubMed]
- 60.Wejksza K, et al. Cancer-produced metabolites of 5-lipoxygenase induce tumor-evoked regulatory B cells via peroxisome proliferator-activated receptor α. J Immunol. 2013;190:2575–2584. doi: 10.4049/jimmunol.1201920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Olkhanud PB, et al. Breast cancer lung metastasis requires expression of chemokine receptor CCR4 and regulatory T cells. Cancer Res. 2009;69:5996–6004. doi: 10.1158/0008-5472.CAN-08-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee-Chang C, et al. Inhibition of breast cancer metastasis by resveratrol-mediated inactivation of tumor-evoked regulatory B cells. J Immunol. 2013;191:4141–4151. doi: 10.4049/jimmunol.1300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lindner S, et al. Interleukin 21-induced granzyme B-expressing B cells infiltrate tumors and regulate T cells. Cancer Res. 2013;73:2468–2479. doi: 10.1158/0008-5472.CAN-12-3450. [DOI] [PubMed] [Google Scholar]
- 64.Ganti SN, et al. Regulatory B cells preferentially accumulate in tumor-draining lymph nodes and promote tumor growth. Sci Rep. 2015;5:12255. doi: 10.1038/srep12255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cariappa A, Pillai S. Antigen-dependent B-cell development. Curr Opin Immunol. 2002;14:241–249. doi: 10.1016/s0952-7915(02)00328-x. [DOI] [PubMed] [Google Scholar]
- 66.Pillai S, et al. Positive selection and lineage commitment during peripheral B-lymphocyte development. Immunol Rev. 2004;197:206–218. doi: 10.1111/j.0105-2896.2003.097.x. [DOI] [PubMed] [Google Scholar]
- 67.Mizoguchi E, et al. Regulatory role of mature B cells in a murine model of inflammatory bowel disease. Int Immunol. 2000;12:597–605. doi: 10.1093/intimm/12.5.597. [DOI] [PubMed] [Google Scholar]
- 68.Mizoguchi A, et al. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. doi: 10.1016/s1074-7613(02)00274-1. [DOI] [PubMed] [Google Scholar]
- 69.Inoue S, et al. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7747. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 70.Perricone MA, et al. Enhanced efficacy of melanoma vaccines in the absence of B lymphocytes. J Immunother. 2004;27:273–281. doi: 10.1097/00002371-200407000-00003. [DOI] [PubMed] [Google Scholar]
- 71.Oizumi S, et al. Surmounting tumor-induced immune suppression by frequent vaccination or immunization in the absence of B cells. J Immunother. 2008;31:394–401. doi: 10.1097/CJI.0b013e31816bc74d. [DOI] [PubMed] [Google Scholar]
- 72.Affara NI, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–821. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhang Y, et al. B lymphocyte inhibition of anti-tumor response depends on expansion of Treg but is independent of B-cell IL-10 secretion. Cancer Immunol Immunother. 2013;62:87–99. doi: 10.1007/s00262-012-1313-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shalapour S, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–98. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schioppa T, et al. B regulatory cells and the tumor-promoting actions of TNF-α during squamous carcinogenesis. Proc Natl Acad Sci U S A. 2011;108:10662–10667. doi: 10.1073/pnas.1100994108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tadmor T, et al. The absence of B lymphocytes reduces the number and function of T-regulatory cells and enhances the anti-tumor response in a murine tumor model. Cancer Immunol Immunother. 2011;60:609–619. doi: 10.1007/s00262-011-0972-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim S, et al. B-cell depletion using an anti-CD20 antibody augments antitumor immune responses and immunotherapy in nonhematopoetic murine tumor models. J Immunother. 2008;31:446–457. doi: 10.1097/CJI.0b013e31816d1d6a. [DOI] [PubMed] [Google Scholar]
- 78.Aklilu M, et al. Depletion of normal B cells with rituximab as an adjunct to IL-2 therapy for renal cell carcinoma and melanoma. Ann Oncol. 2004;15:1109–1114. doi: 10.1093/annonc/mdh280. [DOI] [PubMed] [Google Scholar]
- 79.Candolfi M, et al. B Cells Are Critical to T-cell—Mediated Antitumor Immunity Induced by a Combined Immune-Stimulatory/Conditionally Cytotoxic Therapy for Glioblastoma. Neoplasia. 2011;13:947–IN23. doi: 10.1593/neo.11024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bodogai M, et al. Anti-CD20 antibody promotes cancer escape via enrichment of tumor-evoked regulatory B cells expressing low levels of CD20 and CD137L. Cancer Res. 2013;73:2127–2138. doi: 10.1158/0008-5472.CAN-12-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pillai S, et al. B cells and autoimmunity. Curr Opin Immunol. 2011;23:721–731. doi: 10.1016/j.coi.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bouaziz J-D, et al. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci U S A. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ueno H, et al. Objective criteria for crohn-like lymphoid reaction in colorectal cancer. Am J Clin Pathol. 2013;139:434–441. doi: 10.1309/AJCPWHUEFTGBWKE4. [DOI] [PubMed] [Google Scholar]
- 84.Simsa P, et al. Tumor-infiltrating B cell immunoglobulin variable region gene usage in invasive ductal breast carcinoma. Pathol Oncol Res. 2005;11:92–97. doi: 10.1007/BF02893374. [DOI] [PubMed] [Google Scholar]
- 85.Coronella JA, et al. Evidence for an antigen-driven humoral immune response in medullary ductal breast cancer. Cancer Res. 2001;61:7889–7899. [PubMed] [Google Scholar]
- 86.Nzula S, et al. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res. 2003;63:3275–3280. [PubMed] [Google Scholar]
- 87.Schmidt M, et al. The humoral immune system has a key prognostic impact in node-negative breast cancer. Cancer Res. 2008;68:5405–5413. doi: 10.1158/0008-5472.CAN-07-5206. [DOI] [PubMed] [Google Scholar]
- 88.Erdag G, et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 2012;72:1070–1080. doi: 10.1158/0008-5472.CAN-11-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nedergaard BS, et al. A comparative study of the cellular immune response in patients with stage IB cervical squamous cell carcinoma. Low numbers of several immune cell subtypes are strongly associated with relapse of disease within 5 years. Gynecol Oncol. 2008;108:106–111. doi: 10.1016/j.ygyno.2007.08.089. [DOI] [PubMed] [Google Scholar]
- 90.Riemann D, et al. Phenotypic analysis of T lymphocytes isolated from non-small-cell lung cancer. Int Arch Allergy Immunol. 1997;114:38–45. doi: 10.1159/000237640. [DOI] [PubMed] [Google Scholar]
- 91.Al-Shibli KI, et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non-small cell lung cancer. Clin Cancer Res. 2008;14:5220–5227. doi: 10.1158/1078-0432.CCR-08-0133. [DOI] [PubMed] [Google Scholar]
- 92.Milne K, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One. 2009;4:e6412. doi: 10.1371/journal.pone.0006412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li Q, et al. Adoptive transfer of tumor reactive B cells confers host T-cell immunity and tumor regression. Clin Cancer Res. 2011;17:4987–4995. doi: 10.1158/1078-0432.CCR-11-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hansen MH, et al. The tumor-infiltrating B cell response in medullary breast cancer is oligoclonal and directed against the autoantigen actin exposed on the surface of apoptotic cancer cells. Proc Natl Acad Sci U S A. 2001;98:12659–12664. doi: 10.1073/pnas.171460798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bacus SS, et al. Medullary carcinoma is associated with expression of intercellular adhesion molecule-1. Implication to its morphology and its clinical behavior. Am J Pathol. 1994;145:1337–1348. [PMC free article] [PubMed] [Google Scholar]
- 96.Gaffey MJ, et al. Medullary carcinoma of the breast. Identification of lymphocyte subpopulations and their significance. Mod Pathol. 1993;6:721–728. [PubMed] [Google Scholar]
- 97.Mizukami M, et al. Effect of IgG produced by tumor-infiltrating B lymphocytes on lung tumor growth. Anticancer Res. 2006;26:1827–1831. [PubMed] [Google Scholar]
- 98.Tirosh I, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science. 2016;352:189–196. doi: 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nishikawa H, et al. Role of SEREX-defined immunogenic wild-type cellular molecules in the development of tumor-specific immunity. Proc Natl Acad Sci U S A. 2001;98:14571–14576. doi: 10.1073/pnas.251547298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Carmi Y, et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature. 2015;521:99–104. doi: 10.1038/nature14424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rodríguez-Pinto D. B cells as antigen presenting cells. Cell Immunol. 2005;238:67–75. doi: 10.1016/j.cellimm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 102.Yanaba K, et al. B-lymphocyte contributions to human autoimmune disease. Immunol Rev. 2008;223:284–299. doi: 10.1111/j.1600-065X.2008.00646.x. [DOI] [PubMed] [Google Scholar]
- 103.Schultz KR, et al. The role of B cells for in vivo T cell responses to a Friend virus-induced leukemia. Science. 1990;249:921–923. doi: 10.1126/science.2118273. [DOI] [PubMed] [Google Scholar]
- 104.Di Lillo DJ, et al. B Cells Are Required for Optimal CD4 and CD8 T Cell Tumor Immunity: Therapeutic B Cell Depletion Enhances B16 Melanoma Growth in Mice. doi: 10.4049/jimmunol.0903009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Luther SA, et al. BLC expression in pancreatic islets causes B cell recruitment and lymphotoxin-dependent lymphoid neogenesis. Immunity. 2000;12:471–481. doi: 10.1016/s1074-7613(00)80199-5. [DOI] [PubMed] [Google Scholar]
- 106.Schrama D, et al. Targeting of Lymphotoxin-α to the Tumor Elicits an Efficient Immune Response Associated with Induction of Peripheral Lymphoid-like Tissue. Immunity. 2001;14:111–121. doi: 10.1016/s1074-7613(01)00094-2. [DOI] [PubMed] [Google Scholar]
- 107.Dieu-Nosjean M-C, et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. 2008;26:4410–4417. doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 108.Pimenta EM, Barnes BJ. Role of Tertiary Lymphoid Structures (TLS) in Anti-Tumor Immunity: Potential Tumor-Induced Cytokines/Chemokines that Regulate TLS Formation in Epithelial-Derived Cancers. Cancers. 2014;6:969–997. doi: 10.3390/cancers6020969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Germain C, et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 2014;189:832–844. doi: 10.1164/rccm.201309-1611OC. [DOI] [PubMed] [Google Scholar]
- 110.Hoff ST, et al. Human B cells produce chemokine CXCL10 in the presence of Mycobacterium tuberculosis specific T cells. Tuberculosis. 2015;95:40–47. doi: 10.1016/j.tube.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 111.Bystry RS, et al. B cells and professional APCs recruit regulatory T cells via CCL4. Nat Immunol. 2001;2:1126–1132. doi: 10.1038/ni735. [DOI] [PubMed] [Google Scholar]
- 112.Menard LC, et al. B Cells Amplify IFN-γ Production By T Cells via a TNF-α-Mediated Mechanism. The Journal of Immunology. 2007;179:4857–4866. doi: 10.4049/jimmunol.179.7.4857. [DOI] [PubMed] [Google Scholar]
- 113.Schaniel C, et al. Activated Murine B Lymphocytes and Dendritic Cells Produce a Novel CC Chemokine which Acts Selectively on Activated T Cells. J Exp Med. 1998;188:451–463. doi: 10.1084/jem.188.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Deola S, et al. Helper B cells promote cytotoxic T cell survival and proliferation independently of antigen presentation through CD27/CD70 interactions. J Immunol. 2008;180:1362–1372. doi: 10.4049/jimmunol.180.3.1362. [DOI] [PubMed] [Google Scholar]
- 115.Yang C, et al. B cells promote tumor progression via STAT3 regulated-angiogenesis. PLoS One. 2013;8:e64159. doi: 10.1371/journal.pone.0064159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhang C, et al. CD5 Binds to Interleukin-6 and Induces a Feed-Forward Loop with the Transcription Factor STAT3 in B Cells to Promote Cancer. Immunity. 2016;44:913–923. doi: 10.1016/j.immuni.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]