Abstract
Introduction
Phylogenetic analysis determines similarities among HIV genetic sequences from persons infected with HIV, identifying clusters of transmission. We determined characteristics associated with both membership in an HIV transmission cluster and the number of clustered sequences among a cohort of young black men who have sex with men (YBMSM) in Chicago.
Methods
Pairwise genetic distances of HIV-1 pol sequences were collected during 2013–2016. Potential transmission ties were identified among HIV-infected persons whose sequences were ≤1.5% genetically distant. Putative transmission pairs were defined as ≥1 tie to another sequence. We then determined demographic and risk attributes associated with both membership in an HIV transmission cluster and the number of ties to the sequences from other persons in the cluster.
Results
Of 86 available sequences, 31 (36.0%) were tied to ≥1 other sequence. Through multivariable analyses, we determined that those who reported symptoms of depression and those who had a higher number of confidants in their network had significantly decreased odds of membership in transmission clusters. We found that those who had unstable housing and who reported lower marijuana use had significantly more ties to other individuals within transmission clusters, while those identifying as bisexual, those participating in group sex and those with higher numbers of sexual partners had significantly fewer ties.
Conclusion
This study demonstrates the potential for combining phylogenetic and individual and network attributes to target HIV control efforts to persons with potentially higher transmission risk, as well as suggesting some unappreciated specific predictors of transmission risk among YBSM in Chicago for future study.
INTRODUCTION
Phylogenetic analysis can be used to determine similarities between genetic sequences of persons infected with HIV 1. Highly similar viral strains can then be combined with epidemiologic data to identify an inferred transmission network for a particular population.2,3 Results from these analyses can be used to assess demographic or risk characteristics associated with membership in a transmission cluster4 and transmission cluster size.5 Results from these analyses can also highlight differences in transmission by geographic location,5,6 infection subtype,7 and drug type resistance.8 Phylogenetic analysis and transmission network analyses are key to targeted interventions and controlling the outbreak of infectious diseases,3,9 including HIV.
Previous studies have examined characteristics that are associated with having highly similar HIV strains, termed membership in a transmission cluster.4,5,10,11 These studies, however, have focused primarily on differences by geography 4,5,10 or basic demographic characteristics.11 Findings have demonstrated that young, Black, MSM (YBMSM) are those most likely to be members of an HIV transmission cluster and are also more likely to be in larger HIV transmission clusters. To appropriately target and disrupt transmission of HIV among YBMSM, especially given the high incidence of HIV among YBMSM,12 it is necessary to examine specific demographic and risk characteristics which are associated with transmission network membership and size. Understanding these specific characteristics associated with HIV transmission among YBMSM will inform intervention strategies and guide public health department HIV prevention policy.
We examined HIV Type 1 (hereafter referred to simply as HIV) pol sequences collected between 2013–2016 from study participants in Chicago, Illinois. From those data, we inferred the molecular transmission network, identified transmission clusters, and determined the network degree among these clusters. We then determined characteristics associated with both membership in a transmission cluster and the number of persons within a transmission cluster.
METHODS
The setting and population have been described in detail previously.13 Briefly, uConnect is a longitudinal population-based cohort study14,15 which was designed to assess factors associated with HIV risk and transmission among a sample of YBMSM. uConnect participants resided mainly in South Chicago and the adjacent southern suburbs, which represents the largest contiguous Black community area in the United States.16
Eligibility Criteria
Study respondents were eligible to participate if they 1) self-identified as African American or Black, 2) were assigned male at birth, 3) were between 16 and 29 years of age (inclusive), 4) reported oral or anal sex with a male within the past 24 months, 5) spent the majority of their time on the South side of Chicago, and 6) were willing and able to provide informed consent at the time of the study visit.
Interview
Recruitment utilizing respondent driven sampling and survey follow-up occurred between August 2013 and January of 2016. Surveys were conducted across three waves of study, each separated by nine months. Interviews were conducted using Computer Aided Personal Interviewing (CAPI) with some portions self-administered. The interview itself involved different types of questions and activities: background and socio-demographic questions, self-administered scales of substance use, and HIV care continuum measures.
Participants were asked a series of questions which would allow for the construction of both a sexual and confidant network. Participants were asked “Thinking back over the past six months, that is since MONTH, how many people, including men, women, and transgender women have you had sexual activity with, even if only one time” and “So I can ask some follow-up question, please list the names of the people with whom you discuss things that are important to you”, to elicit number of sexual partners and confidants, respectively. For sexual partners, participants were asked identifying information and a series of questions regarding their relationship and risk behaviors with each partner. For confidants, participants were asked identifying information and a series of questions regarding their relationship with up to five confidants.
We developed a matching algorithm to create the social network among respondents in the uConnect network. At each uConnect wave, respondents were asked detailed information regarding their sexual partners and confidants, including name, age, geographic residence and other sociodemographics, if known. These data provided by respondents were then matched across all waves to identify unobserved ties which may exist as a result of different respondents naming the same individual as a network member. The algorithm used to complete the matching process was verified by two separate analysts with all matches confirmed manually, and has been described in detail elsewhere.17
Transmission Network Inference
Dried blood spots were collected as a portion of each participant’s survey at each wave. Each participant’s HIV infection status (including acute infection) was determined by 4th generation HIV immunoassay (Abbott ARCHITECT HIV Ag/Ab Combo assay), HIV-1/-2 Ab differentiation (Bio-Rad Multispot HIV-1/-2 Rapid Test) and viral load testing (Abbott ReaLTime HIV-1 assay) applied to samples eluted from dry blood spots (DBS).18 HIV pol sequences were obtained from all persons whose viral load was ≥2000 copies/mL, the allowable limit for elution from dried blood spots. Specific procedures describing extraction of cell-associated HIV DNA from dried blood spots and HIV pol amplicon sequencing, including number of base pairs analyzed and primers used, have been previously described.19 For participants whose viral sequences were unable to be determined, we obtained sequences (if available) collected through routine surveillance by the Chicago Department of Public Health (CDPH). All participants whose data was accessed through CDPH provided a release of information to obtain any available sequences or HIV related test results.
All available genetic sequences were aligned to the HXB2 reference sequence using MUSCLE (MUltiple Sequence Comparison by Log-Expectation, European Bioinformatics Institute) multiple sequence alignment20 in the MEGA v7.0 (Molecular Evolutionary Genetics Analysis) software package.21 Phylogenetic tree analyses were performed by using the neighbor-joining method,22 with distance calculated by TN9323 analysis. One sequence was obtained from each participant. Each individual is referred to as a node. An inferred potential transmission event was defined as having a genetic distance ≤1.5% between pol sequences, and referred to as a tie. It should be noted that phylogenetic analyses cannot define which of those with similar sequences was transmitter versus recipient, or if transmission was indirect via an unidentified individual rather than directly between those with similar sequences. A cluster was defined as ≥2 persons linked by ≥1 tie. All cluster visualizations were performed by using NodeXL v1.0.1.340 (Social Media Research Foundation).24
Dependent variables
We utilized two main outcomes in our analysis: 1) membership in a transmission cluster and 2) number of connections within the transmission cluster, referred to as transmission network degree. Cluster membership was a dichotomous measure indicating membership in an HIV transmission cluster. The network degree, or number of connections, within the transmission cluster was defined as the number of ties each individual had to other individuals the transmission cluster (e.g. one individual tied to three other individuals would have a value of three).
Independent variables
Sexual identity was categorized as gay, bisexual, or other. The following variables were utilized as dichotomous measures: 1) self-reported possession of health coverage at the time of interview, 2) currently a student, 3) housing instability at any point in the previous 12 months, 4) presence of depressive symptoms (measured using the Brief Symptom Inventory 18-question survey25), 5) group sex, and 6) condomless sex. Condomless sex and group sex were defined as at least once instance of each in the past 12 months. Drug use was defined as any use in the past 12 months; due to the high usage of marijuana in this population, use of marijuana was separately categorized as never, intermittent use (up to and including several times per week), and heavy use (at least once per day). All other drugs were combined into a single variable (including ecstasy/molly/E, poppers, crack/cocaine, heroin, psychedelics, methamphetamines, or prescription drugs).
Sexual network degree, confidant network degree, and total (combined sexual and social) network degree were determined using the full matched data across all three waves. In each type of network, network degree was utilized as a continuous variable. Confidants were asked as, “Please list the names of the people with whom you discuss things that are important to you.” Number of sexual partners was asked as “How many people, including men, women, and transgender women have you had sexual activity with, even if only one time?”
Statistical Analyses
Association of all variables with both membership in a cluster and transmission network degree within the cluster were first analyzed by using unadjusted logistic and Poisson regressions, respectively. RDS-weighted multivariable logistic and Poisson regressions were then used to estimate the association between all variables with membership in a transmission cluster and the transmission network degree as the dependent variables. All covariates identified as statistically significant at the p ≤ .05 level, using Wald test statistic, were included in the multivariable regression model. Effect modification was assessed by using cross-products individually between sexual and social network degree and each of the covariates. All analyses were performed in Stata v14.0.26
Results
Sample characteristics
The final analytic sample included 266 HIV-diagnosed participants. Of these, 86 (32.3%) participants had an available sequence. There were no differences in sociodemographics or risk behaviors between participants who did, and did not, have a sequence available (not shown). Characteristics of those with sequence data are presented in Table 1, and are stratified by presence in a transmission cluster. The majority of participants with sequence data did not have health insurance (42, 48.8%), had low income (72, 83.7%), self-identified as gay (59, 68.7%), and reported condomless sex in the past 12 months (49, 57.0%).
Table 1.
Sample characteristics stratified by presence in a cluster1 among young Black MSM in Chicago, uConnect (N = 86)
| Characteristic | Total | Not in a cluster1 | In a cluster1 | p-value2 | |||
|---|---|---|---|---|---|---|---|
|
| |||||||
| No. | % | No. | % | No. | % | ||
| Total | 86 | 100 | 55 | 64.0 | 31 | 36.0 | – |
| Demographics, n(%) | |||||||
| Currently insured | 42 | 48.8 | 29 | 52.7 | 13 | 41.9 | 0.336 |
| Currently a student | 24 | 27.9 | 15 | 27.3 | 9 | 29.0 | 0.956 |
| Housing instability4 | 28 | 32.6 | 16 | 29.1 | 12 | 40.0 | 0.306 |
| Low income3 | 72 | 83.7 | 45 | 83.3 | 27 | 90.0 | 0.403 |
| Sexual identity | 0.660 | ||||||
| Gay | 66 | 77.7 | 42 | 77.8 | 24 | 77.4 | |
| Bisexual | 14 | 16.5 | 8 | 14.8 | 6 | 19.4 | |
| Straight or other | 5 | 5.9 | 4 | 7.4 | 1 | 3.2 | |
| Mental Health5, n(%) | |||||||
| Depressive Symptoms | 11 | 12.8 | 9 | 16.4 | 2 | 6.5 | 0.186 |
| Risk behaviors4 | |||||||
| Condomless sex | 49 | 57.0 | 33 | 60.0 | 16 | 51.6 | 0.451 |
| Group sex | 22 | 25.6 | 16 | 29.1 | 6 | 20.0 | 0.360 |
| Drug use4, n(%) | |||||||
| Marijuana6 | 0.956 | ||||||
| Never | 21 | 24.4 | 13 | 23.6 | 8 | 25.8 | |
| Intermittent | 30 | 34.9 | 19 | 34.6 | 11 | 35.5 | |
| Heavy | 35 | 40.7 | 23 | 41.8 | 12 | 38.7 | |
| Other Substance Use4,7 | 23 | 26.7 | 16 | 29.1 | 7 | 22.6 | 0.513 |
| Network measures,8 mean (range) | |||||||
| Confidant network degree9 | 4.0 | 1–9 | 4.2 | 1–9 | 3.6 | 1–7 | 0.084 |
| Sexual network degree | 6.4 | 1–15 | 6.6 | 1–15 | 6.1 | 1–14 | 0.238 |
Abbreviations: MSM = men who have sex with men
A cluster was defined as ≥2 persons whose pol sequences were <1.5% genetically distant
Using chi-square analysis
Defined as <$20,000 per year
In the past 12 months
Using the Brief Symptom Inventory 18 (BSI-18)
Intermittent use is defined marijuana use less than and including weekly use; heavy use is defined as at least once per day.
Includes the use of ecstasy/molly/E, poppers, cocaine/crack, heroin, psychedelics, methamphetamines, prescription drugs
Degree is defined as the number of ties each individual had to other individuals in the network (e.g. one individual tied to three other individuals would have a value of three)
Confidants were defined as a close social contact, someone with whom the respondent would discuss things that are important to them
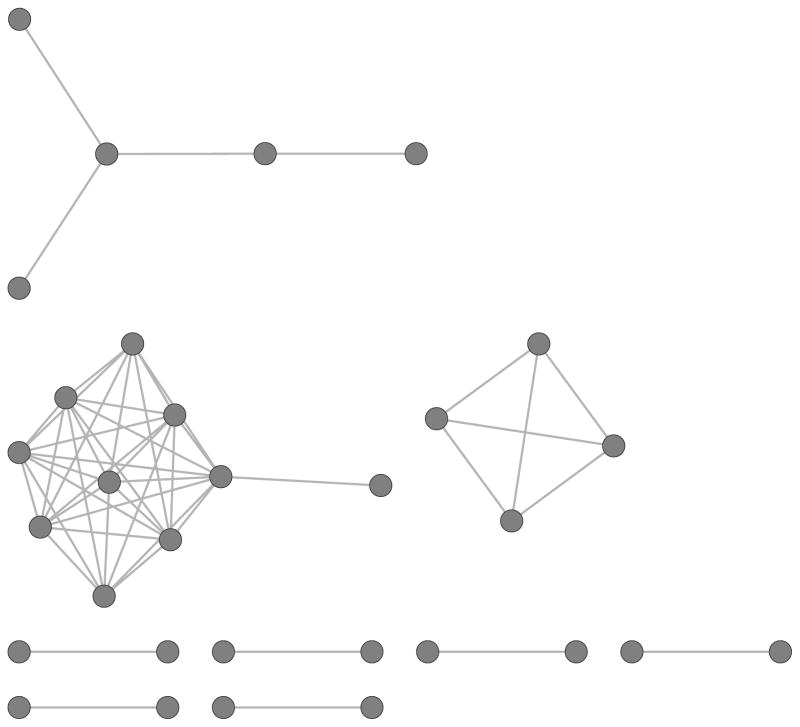
Figure 1 depicts the inferred HIV transmission clusters identified by phylogenetic analysis in the study sample. Among participants with available viral sequences, 31 (36.0%) were determined to be members of a transmission cluster. Connections between nodes represent an inferred HIV transmission as determined by phylogenetic analysis of the pol region with a maximum genetic distance of 0.015 nucleotide substitutions per site. There were a total of 9 observed HIV transmission clusters with a total of 51 ties among all clustered individuals. The majority of members of transmission clusters were more likely to have low household income (27, 90.0%), self-identify as gay (24, 77.4%), and report at least once instance of condomless sex in the past 12 months (16, 51.6%). We also find that confidant network degree and HIV prevalence are significantly positively correlated (r = 0.12; p = 0.004). None of these characteristics of cluster members, however, differed significantly in unadjusted analyses from those who were not in clusters based on HIV sequence similarity (Table 1).
Figure 1.
Inferred HIV transmission network among persons in clusters (n = 31, 40.7% of total persons with sequences) in the uConnect cohort. Connection between nodes represents an inferred transmission between persons, assessed through phylogenetic analysis of the pol region with a maximum genetic distance of 0.015 nucleotide substitutions per site.
In contrast, RDS-weighted adjusted logistic and Poisson regression analyses revealed that compared to those who do not report symptoms of depression, those who do report symptoms of depression are significantly less likely to be members of a transmission cluster (AOR = 0.13; 95% CI: 0.02–0.69). Additionally, we found that each additional member of a participant’s confidant network significantly decreases the odds of membership in a transmission cluster (AOR = 0.70; 95% CI: 0.50–0.98). Compared to those with stable housing, those without stable housing were significantly more likely to be members of a transmission cluster (AOR = 3.71; 95% CI: 1.08—12.78).
The RDS-weighted multivariable analyses also found associations with cluster size (Table 2). Those who reported housing instability had significantly more ties to other individuals in clusters (ARR = 1.95; 95% CI: 1.36–2.81), compared to those who have stable housing (Table 2) Compared to those who do not report low income, those with low income have significantly fewer connections to other individuals in HIV transmission clusters (ARR = 0.56; 95% CI: 0.37—0.86). Participants who reported using marijuana heavily, compared to those who reported never using marijuana, had significantly more connections to other individuals in transmission clusters (ARR = 1.96; 95% CI: 1.20–3.19). We also found that each additional member of a participant’s sexual network significantly decreases the number of connects to other individuals (ARR = 0.91; 95% CI: 0.86—0.97)
Table 2.
RDS-weighted adjusted logistic and Poisson regression models of association of selected characteristics with both membership in a cluster1 and transmission network degree 1 among HIV diagnosed YBMSM with a known viral genetic sequence, uConnect (N = 86)
| Characteristic | Membership in a cluster2 | Size of cluster3 | ||
|---|---|---|---|---|
|
| ||||
| AOR | 95% CI | ARR | 95% CI | |
| Sexual identity | ||||
| Gay | Ref | – | Ref | – |
| Bisexual | 0.48 | 0.10—2.18 | 0.41* | 0.21—0.83 |
| Straight or Other | Empty | — | Empty | — |
| Currently insured | ||||
| No | Ref | – | Ref | – |
| Yes | 0.79 | 0.23—2.70 | 1.02 | 0.49—2.11 |
| Currently a student | ||||
| No | Ref | – | Ref | – |
| Yes | 1.10 | 0.50—2.40 | 0.84 | 0.59—1.21 |
| Housing instability4 | ||||
| No | Ref | – | Ref | – |
| Yes | 3.71* | 1.08—12.78 | 1.95** | 1.36—2.81 |
| Low income | ||||
| ≥$20,000 per year | Ref | – | Ref | – |
| <$20,000 per year | 1.39 | 0.21—8.98 | 0.56** | 0.37—0.86 |
| Depressive Symptoms5 | ||||
| No | Ref | – | Ref | – |
| Yes | 0.13* | 0.02—0.69 | 0.49 | 0.13—1.80 |
| Group sex4 | ||||
| None | Ref | – | Ref | – |
| ≥1 | 1.91 | 0.33—11.06 | 0.54* | 0.31—0.95 |
| Marijuana4,6 | ||||
| Never | Ref | – | Ref | – |
| Intermittent | 1.86 | 0.41—8.44 | 1.20 | 0.59—2.44 |
| Heavy | 1.95 | 0.42—9.00 | 1.96** | 1.20—3.19 |
| Other Substance Use4,7 | ||||
| None | Ref | – | Ref | – |
| ≥1 other drug | 0.69 | 0.15—3.30 | 0.45 | 0.17—1.15 |
| Network measures | ||||
| Confidant network degree8 | 0.70* | 0.50—0.98 | 0.95 | 0.87—1.04 |
| Sexual network degree | 0.97 | 0.81—1.17 | 0.91** | 0.86—0.97 |
Abbreviations: HIV = human immunodeficiency virus, YBMSM = young black men who have sex with men, AOR = adjusted odds ratio, ARR = adjusted rate ratio, CI = confidence interval
A cluster was defined as having ≥2 connected persons whose pol sequences were <1.5% genetically distant. Cluster size was defined as the number of ties each individual had others in the cluster.
Using logistic regression among those who are HIV seropositive
Using Poisson regression among those with an available viral genetic sequence
In the past 12 months
Self-reported via the Brief Symptom Inventory 18-question survey
Intermittent use is defined as anything less than and including weekly use; heavy use is defined as at least once per day.
Includes the use of ecstasy/molly/E, poppers, crack/cocaine, heroin, psychedelics, methamphetamines, prescription drugs
Defined as consuming five or more drinks in one sitting in the past 30 days
p <0.05;
p <0.001
Discussion
In this study, we present novel findings regarding characteristics which may influence the spread of HIV through networks of YBMSM. First, we find that nearly one-third of HIV-diagnosed individuals with an available viral sequence in our sample were members of an HIV transmission cluster. Second, we find that having a greater number of confidants in one’s network significantly reduces the odds of being in a transmission cluster. Third, we find that participants with symptoms of depression are significantly less likely to be members of transmission clusters. Finally, we find that both housing instability and heavy marijuana use significantly increase the number of connections to other individuals in a transmission cluster.
A higher number of confidants in one’s network may reduce the risk of HIV transmission among YBMSM. Past studies have shown that social networks of HIV-positive black MSM exhibit a preponderance of family members and relatives.27 Additional work has shown that personal networks consisting of greater family network proportion are associated with lower rates of both sex-drug – that is drugs with are used to make sex easier, more enjoyable, or last longer— use and group sex.28 BMSM with greater support networks have also been shown to participate less in high-risk sexual behavior and to have a greater number of HIV tests in the previous past two years.29 Further, greater social support has been associated with higher HIV care uptake and adherence to antiretroviral medications.30 The findings presented in this study support past work suggesting that greater social support in one’s network plays a protective role in the movement of HIV through transmission networks of YBMSM. Future work should be conducted to address factors which are more protective for persistently HIV-negative social and confidant network members.
Contrary to past findings, we found depression to be associated with a lower likelihood of being a member of a transmission cluster. Depression has previously been associated with an overall increase in participation in HIV risk behavior,31–35 even given knowledge of one’s HIV status.36 These behaviors include inconsistent condom use37 and a higher number of lifetime sexual partners38,39; depression has also been associated with poor adherence to antiretroviral medications.40,41 Given past research, our findings of depression playing a “protective” role are a bit surprising; it is possible that our association may only be found in relation to HIV transmission clusters and not overall HIV risk. Addtionally, our findings may be attributed to our use of the Brief Symptom Inventory 18-question survey (BSI-18), which only indicates the presence of depressive symptoms and does not identify level of depression. Further research is need to fully ascertain the relationship between HIV transmission clusters and depression.
Both housing instability and heavy marijuana are associated with larger HIV transmission clusters. Any marijuana use has previously been associated with participation in HIV risk behaviors including both condomless sex and group sex13 while heavy marijuana use has been associated with being HIV positive and unaware of one’s status.15 Our findings support this past research and suggest that engaging heavy marijuana users in HIV prevention efforts may reduce the overall size of transmission clusters and may serve to disrupt forward transmission of HIV through networks of YBMSM.
Similar to past studies we defined housing instability as one’s perception of being homeless and not one’s physical address.42 Early evidence has shown that HIV rates among homeless adults are higher for blacks than whites and for MSM compared to other risk groups.43 More recent research has shown that homelessness is also associated with HIV risk among both substance users44,45 and street-involved youth.46 Additionally, survival sex, or trading sex for basic goods in the face of extreme need, has been shown to be a strong predictor of HIV risk among lesbian, bisexual, and gay homeless youth.47 AWhile engaging those YBMSM who perceive themselves as homeless in HIV prevention services may aid in disrupting HIV transmission clusters, these individuals may be more impacted through structural interventions vis-à-vis stable housing.
Our study should be viewed in the context of its limitations. First, our data are cross-sectional and thus do not allow for causal inference. Second, we were able to obtain HIV sequences for only 32.3% of persons who were HIV seropositive during the study period, and thus, the inferred transmission network is incomplete. Due to the small size of our study population, the results are limited in their scope and generalizability, our limited cohort may not necessarily be representative of the larger population of interest in Chicago. Additionally, we are unable to determine whether the observed ties between HIV-diagnosed individuals are indirect or direct transmission links, nor can we determine a direction of transmission from our data.
Even in light of these limitations were still able to draw meaningful conclusions from our data. We have shown that an increased number of confidants in one’s network may play a role in reducing the likelihood of being a member of a transmission cluster. We have also demonstrated that perception of being homeless and heavy marijuana use may increase the size of HIV transmission clusters. Future work should also be conducted to examine in greater depth the observed relationship between depression and transmission networks. Finally, the prospective use of phylogenetic analyses ought to be evaluated further for incorporation into HIV surveillance methods in local departments of public health. Our results suggest that determination of sequence clusters may aid in determining factors associated with spread in a population, which may vary in other locales from what we observed among YBMSM and provide a method for prioritizing limited public health resources to limit HIV spread.
Acknowledgments
We would like to thank the community advisory board and many study participants for recruiting their network members and involvement in uConnect. The sample development, analyses and phylogenetic work was supported by the University of Chicago Office of Diversity & Inclusion and NIH grants R21MH098768, R01DA033875, R01DA039934 and P30AI 117943. The dried blood spot assay development and validation was supported by NIH grants P30AI-027757 and UM1-AI-68636 and -06701. We would like to thank Audrey Wong, Jose Ortega, Eleanor Espinosa, Carol Gallardo, Randee Estes, Corey Scherrer, and Glenda Daza for laboratory technical support.
References
- 1.Leitner T, Escanilla D, Franzén C, Uhlén M, Albert J. Accurate reconstruction of a known HIV-1 transmission history by phylogenetic tree analysis. Proc Natl Acad Sci USA. 1996;93:10864–10869. doi: 10.1073/pnas.93.20.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wertheim JO, Leigh Brown A, Hepler N, et al. The Global Transmission Network of HIV-1. J Infect Dis. 2014;209(2):304–313. doi: 10.1093/infdis/jit524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Little SJ, Kosakovsky Pond SL, Anderson CM, et al. Using HIV networks to inform real time prevention interventions. PLoS One. 2014;9(6):e98443. doi: 10.1371/journal.pone.0098443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lubelchek RJ, Hoehnen SC, Hotton AL, Kincaid SL, Barker DE, French AL. Transmission clustering among newly diagnosed HIV patients in Chicago, 2008 to 2011: using phylogenetics to expand knowledge of regional HIV transmission patterns. J Acquir Immune Defic Syndr. 2015;68(1):46–54. doi: 10.1097/QAI.0000000000000404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan E, Oster A, Townsell S, Peace D, Benbow N, Schneider JA. Movement of HIV-1 Infection Through Transmission Networks of Younger Persons in Chicago, Illinois. Public Health Rep. 2016 doi: 10.1177/0033354916679988. in-press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oster AM, Wertheim JO, Hernandez AL, Ocfemia MCB, Saduvala N, Hall HI. Using Molecular HIV Surveillance Data to Understand Transmission Between Subpopulations in the United States. J Acquir Immune Defic Syndr. 2015;70(4):444–451. doi: 10.1097/QAI.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalmet K, Staelens D, Blot S, et al. Epidemiological study of phylogenetic transmission clusters in a local HIV-1 epidemic reveals distinct differences between subtype B and non-B infections. BMC Infect Dis. 2010;10:262. doi: 10.1186/1471-2334-10-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yerly S, Junier T, Gayet-Ageron A, et al. The impact of transmission clusters on primary drug resistance in newly diagnosed HIV-1 infection. AIDS. 2009;23(11):1415–1423. doi: 10.1097/QAD.0b013e32832d40ad. [DOI] [PubMed] [Google Scholar]
- 9.Lee S, Rocha LEC, Liljeros F, Holme P. Exploiting temporal network structures of human interaction to effectively immunize populations. PLoS One. 2012;7(5):e36439. doi: 10.1371/journal.pone.0036439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oster AM, Pieniazek D, Zhang X, et al. Demographic but not geographic insularity in HIV transmission among young black MSM. AIDS. 2011;25(17):2157–2165. doi: 10.1097/QAD.0b013e32834bfde9. [DOI] [PubMed] [Google Scholar]
- 11.Whiteside YO, Song R, Wertheim JO, Oster AM. Molecular analysis allows inference into HIV transmission among young men who have sex with men in the United States. AIDS. 2015;29(18):2517–2522. doi: 10.1097/QAD.0000000000000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention. Lifetime risk of HIV diagnosis in the United States. 2016 Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2016. [Accessed March 7, 2016]. http://www.cdc.gov/nchhstp/newsroom/2016/croi-2016.html#Graphics2. [Google Scholar]
- 13.Morgan E, Skaathun B, Michaels S, et al. Marijuana Use as a Sex-Drug is Associated with HIV Risk Among Black MSM and Their Network. AIDS Behav. 2015 doi: 10.1007/s10461-015-1195-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanna AS, Michaels S, Skaathun B, et al. Preexposure Prophylaxis Awareness and Use in a Population-Based Sample of Young Black Men Who Have Sex With Men. JAMA Intern Med. 2015:1–3. doi: 10.1001/jamainternmed.2015.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan E, Khanna AS, Skaathun B, et al. Marijuana use among young black men who have sex with men and the HIV Care Continuum: Findings from the uConnect Cohort. Subst Use Misuse. 2016 doi: 10.1080/10826084.2016.1197265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.United States Census Bureau. 2005–2009 American Community Survey 5-Year Estimates. 2011. [Google Scholar]
- 17.Skaathun B, Voisin D, Lauderdale DS, Schneider JA. Environmental Factors and Network Dynamics among YBMSM in Chicago. 2016. [Google Scholar]
- 18.Chang M, Daza G, Dragavon J, Hart S, Seilie M, Murphy S. Application of a 4th generation HIV diagnostic testing algorithm using finger-prick dried blood spot cards. Annual Clinical Virology Symposium; Daytona Beach. 2014. [Google Scholar]
- 19.Nyaku AN, Morgan E, D’Aquila R, Schneider JA. Molecular Epidemiology of HIV Infection in a Chicago Cohort of Young Black Men Who Have Sex with Men. 2016. [Google Scholar]
- 20.Edgar R. Nucleic Acids Res. 5. Vol. 32. Oxford Univ Press; 2004. MUSCLE: multiple sequence alignment with high accuracy and high throughput; pp. 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 23.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. [Accessed June 2, 2016];Mol Biol Evol. 1993 10(3):512–526. doi: 10.1093/oxfordjournals.molbev.a040023. http://www.ncbi.nlm.nih.gov/pubmed/8336541. [DOI] [PubMed] [Google Scholar]
- 24.Smith M, Ceni A, Milic-Frayling N, et al. NodeXL: a free and open network overview, discovery and exploration add-in for Excel 2007/2010/2013/2016. 2010 http://nodexl.codeplex.com/ from the Social Media Research Foundation. http://www.smrfoundation.org.
- 25.Derogatis LR. BSI 18, Brief Symptom Inventory 18: Administration, Scoring and Procedures Manual. Minneapolis, MN: MCS Pearson, Inc; 2001. [Google Scholar]
- 26.StataCorp. Stata Statistical Software: Release 14. 2015. [Google Scholar]
- 27.Wohl AR, Galvan FH, Myers HF, et al. Social support, stress and social network characteristics among HIV-positive Latino and African American women and men who have sex with men. AIDS Behav. 2010;14(5):1149–1158. doi: 10.1007/s10461-010-9666-3. [DOI] [PubMed] [Google Scholar]
- 28.Schneider J, Michaels S, Bouris A. Family network proportion and HIV risk among Black men who have sex with men. J Acquir Immune Defic Syndr J Acquir Immune Defic Syndr. 2012 Dec;61(5):627–635. doi: 10.1097/QAI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauby JL, Marks G, Bingham T, et al. Having supportive social relationships is associated with reduced risk of unrecognized HIV infection among black and Latino men who have sex with men. AIDS Behav. 2012;16(3):508–515. doi: 10.1007/s10461-011-0002-3. [DOI] [PubMed] [Google Scholar]
- 30.Amirkhanian YA. Social Networks, Sexual Networks and HIV Risk in Men Who Have Sex with Men. Curr HIV/AIDS Rep. 2014;11(1):81–92. doi: 10.1007/s11904-013-0194-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hutton HE, Lyketsos CG, Zenilman JM, Thompson RE, Erbelding EJ. Depression and HIV risk behaviors among patients in a sexually transmitted disease clinic. Am J Psychiatry. 2004;161(5):912–914. doi: 10.1176/appi.ajp.161.5.912. [DOI] [PubMed] [Google Scholar]
- 32.Perdue T, Hagan H, Thiede H, Valleroy L. Depression and HIV Risk Behavior among Seattle-Area Injection Drug Users and Young Men Who Have Sex With Men. AIDS Educ Prev. 2003;15(1):81–92. doi: 10.1521/aeap.15.1.81.23842. [DOI] [PubMed] [Google Scholar]
- 33.Houston E, Sandfort T, Dolezal C, Carballo-Diéguez A. Depressive Symptoms Among MSM Who Engage in Bareback Sex: Does Mood Matter? AIDS Behav. 2012;16(8):2209–2215. doi: 10.1007/s10461-012-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvy LM, McKirnan DJ, Mansergh G, et al. Depression is Associated with Sexual Risk Among Men Who Have Sex with Men, but is Mediated by Cognitive Escape and Self-Efficacy. AIDS Behav. 2011;15(6):1171–1179. doi: 10.1007/s10461-010-9678-z. [DOI] [PubMed] [Google Scholar]
- 35.Reisner SL, Mimiaga MJ, Skeer M, et al. Clinically Significant Depressive Symptoms as a Risk Factor for HIV Infection Among Black MSM in Massachusetts. AIDS Behav. 2009;13(4):798–810. doi: 10.1007/s10461-009-9571-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Valverde EE, Cassetti I, Metsch LR, et al. Sex risk practices among HIV-positive individuals in Buenos Aires, Argentina. AIDS Patient Care STDS. 2009;23(7):551–556. doi: 10.1089/apc.2008.0094. [DOI] [PubMed] [Google Scholar]
- 37.Mazzaferro KE, Murray PJ, Ness RB, Bass DC, Tyus N, Cook RL. Depression, stress, and social support as predictors of high-risk sexual behaviors and STIs in young women. J Adolesc Health. 2006;39(4):601–603. doi: 10.1016/j.jadohealth.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Rubin AG, Gold MA, Primack BA. Associations between depressive symptoms and sexual risk behavior in a diverse sample of female adolescents. J Pediatr Adolesc Gynecol. 2009;22(5):306–312. doi: 10.1016/j.jpag.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turner AK, Latkin C, Sonenstein F, Tandon SD. Psychiatric disorder symptoms, substance use, and sexual risk behavior among African-American out of school youth. Drug Alcohol Depend. 2011;115(1–2):67–73. doi: 10.1016/j.drugalcdep.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mugavero M, Ostermann J, Whetten K, et al. Barriers to antiretroviral adherence: the importance of depression, abuse, and other traumatic events. AIDS Patient Care STDS. 2006;20(6):418–428. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- 41.Gordillo V, del Amo J, Soriano V, González-Lahoz J. Sociodemographic and psychological variables influencing adherence to antiretroviral therapy. [Accessed September 27, 2016];AIDS. 1999 13(13):1763–1769. doi: 10.1097/00002030-199909100-00021. http://www.ncbi.nlm.nih.gov/pubmed/10509579. [DOI] [PubMed] [Google Scholar]
- 42.Surratt HL, Inciardi JA. HIV risk, seropositivity and predictors of infection among homeless and non-homeless women sex workers in Miami, Florida, USA. AIDS Care. 2004;16(5):594–604. doi: 10.1080/09540120410001716397. [DOI] [PubMed] [Google Scholar]
- 43.Allen DM, Lehman JS, Green TA, Lindegren ML, Onorato IM, Forrester W. HIV infection among homeless adults and runaway youth, United States, 1989–1992. Field Services Branch. [Accessed September 27, 2016];AIDS. 1994 8(11):1593–1598. http://www.ncbi.nlm.nih.gov/pubmed/7848596. [PubMed] [Google Scholar]
- 44.Wechsberg WM, Lam WKK, Zule W, Hall G, Middlesteadt R, Edwards J. Violence, Homelessness, and HIV Risk Among Crack-Using African-American Women. Subst Use Misuse. 2003;38(3–6):669–700. doi: 10.1081/JA-120017389. [DOI] [PubMed] [Google Scholar]
- 45.Kilbourne AM, Herndon B, Andersen RM, Wenzel SL, Gelberg L. Psychiatric symptoms, health services, and HIV risk factors among homeless women. [Accessed September 27, 2016];J Health Care Poor Underserved. 2002 13(1):49–65. doi: 10.1353/hpu.2010.0189. http://www.ncbi.nlm.nih.gov/pubmed/11836913. [DOI] [PubMed] [Google Scholar]
- 46.Marshall BDL, Kerr T, Shoveller JA, Patterson TL, Buxton JA, Wood E. Homelessness and unstable housing associated with an increased risk of HIV and STI transmission among street-involved youth. Health Place. 2009;15(3):753–760. doi: 10.1016/j.healthplace.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gangamma R, Slesnick N, Toviessi P, Serovich J. Comparison of HIV Risks among Gay, Lesbian, Bisexual and Heterosexual Homeless Youth. J Youth Adolesc. 2008;37(4):456–464. doi: 10.1007/s10964-007-9171-9. [DOI] [PMC free article] [PubMed] [Google Scholar]