Abstract
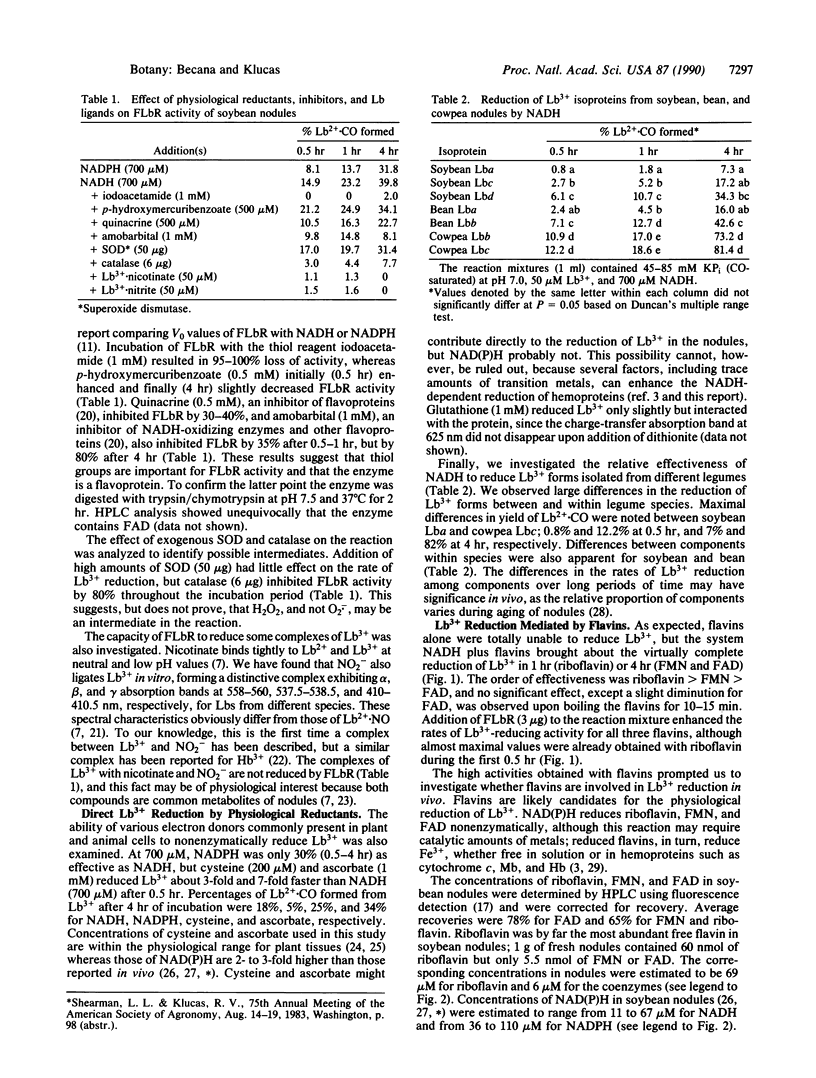
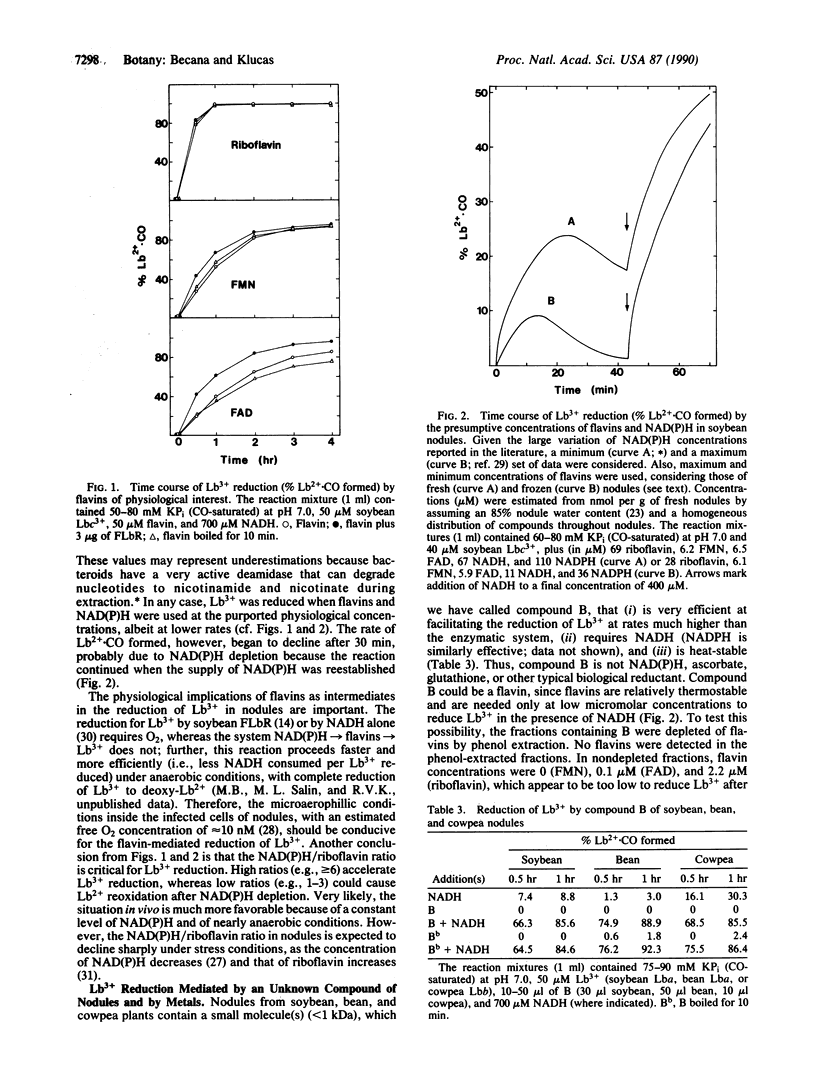
Evidence is presented for the operation in nodules of at least four systems for restoring functional ferrous leghemoglobin (LB2+) from its inactive, ferric form. (i) Reduction of ferric leghemoglobin (LB3+) by a reductase. The enzyme is a flavoprotein of 100 kDa with two equally sized subunits andexhibits a Km of 9 microM for soybean LB3+ component a and a Km of 51 microM for NADH. NADPH is only 30% (initial velocities) as effective as NADH. LB3+ reductase converts 215 nmol of LB3+ to LB2+.CO (or Lb2+.O2) per mg of protein per min and does not require an exogenous electron carrier. The enzyme shows similar affinity for soybean, bean, and cowpea LB3+, but different Vmax values. The reductase is inactive with LB3+ is bound to nicotinate or NO2-. (ii) Direct reduction of LB3+ by NAD(P)H, ascorbate, and cysteine. Reduction by NAD(P)H is greatly stimulated by trace amounts of metals such as Mn2+. (iii) Reduction of Lb3+ by the flow of electrons from NAD(P)H to free flavins to LB3+. The reaction does not occur via O2.- or H2O2, and thus NAD(P)H-reduced flavins can directly reduce LB3+. The efficiency of the reaction follows the order riboflavin > FMN > FAD. (iv) Reduction of LB3+ by an unknown compound, B, of nodules. B has a molecular mass < 1 kDa and is heat-stable. The reaction mediated by B differs from those mediated by flavins and metals in several ways, requires NAD(P)H, and generates O2.-.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dalton D. A., Russell S. A., Hanus F. J., Pascoe G. A., Evans H. J. Enzymatic reactions of ascorbate and glutathione that prevent peroxide damage in soybean root nodules. Proc Natl Acad Sci U S A. 1986 Jun;83(11):3811–3815. doi: 10.1073/pnas.83.11.3811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Parkhurst L. J., Geraci G. The reaction of methemoglobin with some ligands. J Biol Chem. 1969 Sep 10;244(17):4668–4676. [PubMed] [Google Scholar]
- Gruca M., Grigg G. C. Methemoglobin reduction in crocodile blood: are high levels of MetHb typical of healthy reptiles? J Exp Zool. 1980 Aug;213(2):305–308. doi: 10.1002/jez.1402130221. [DOI] [PubMed] [Google Scholar]
- Hagler L., Coppes R. I., Jr, Herman R. H. Metmyoglobin reductase. Identification and purification of a reduced nicotinamide adenine dinucleotide-dependent enzyme from bovine heart which reduces metmyoglobin. J Biol Chem. 1979 Jul 25;254(14):6505–6514. [PubMed] [Google Scholar]
- Hultquist D. E. Methemoglobin reduction system of erythrocytes. Methods Enzymol. 1978;52:463–473. doi: 10.1016/s0076-6879(78)52051-x. [DOI] [PubMed] [Google Scholar]
- Jaffé E. R. Methaemoglobinaemia. Clin Haematol. 1981 Feb;10(1):99–122. [PubMed] [Google Scholar]
- Koizumi C., Brown W. D. A peroxidative mechanism for the nonenzymatic reduction of metmyoglobin. Biochim Biophys Acta. 1972 Mar 30;264(1):17–24. doi: 10.1016/0304-4165(72)90112-2. [DOI] [PubMed] [Google Scholar]
- Lee K. K., Klucas R. V. Reduction of ferric leghemoglobin in soybean root nodules. Plant Physiol. 1984 Apr;74(4):984–988. doi: 10.1104/pp.74.4.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light D. R., Walsh C., Marletta M. A. Analytical and preparative high-performance liquid chromatography separation of flavin and flavin analog coenzymes. Anal Biochem. 1980 Nov 15;109(1):87–93. doi: 10.1016/0003-2697(80)90014-7. [DOI] [PubMed] [Google Scholar]
- Maskall C. S., Gibson J. F., Dart P. J. Electron-paramagnetic-resonance studies of leghaemoglobins from soya-bean and cowpea root nodules. Identification of nitrosyl-leghaemoglobin in crude leghaemoglobin preparations. Biochem J. 1977 Nov 1;167(2):435–445. doi: 10.1042/bj1670435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs A. Preparation of blood hemoglobins of vertebrates. Methods Enzymol. 1981;76:5–29. doi: 10.1016/0076-6879(81)76111-1. [DOI] [PubMed] [Google Scholar]
- Saari L. L., Klucas R. V. Ferric leghemoglobin reductase from soybean root nodules. Arch Biochem Biophys. 1984 May 15;231(1):102–113. doi: 10.1016/0003-9861(84)90367-9. [DOI] [PubMed] [Google Scholar]
- Saari L. L., Klucas R. V. Nonenzymatic reduction of ferric leghemoglobin. Biochim Biophys Acta. 1987 Apr 8;912(2):198–202. doi: 10.1016/0167-4838(87)90089-6. [DOI] [PubMed] [Google Scholar]
- Saetre R., Rabenstein D. L. Determination of cysteine and glutathione in fruit by high-performance liquid chromatography. J Agric Food Chem. 1978 Jul-Aug;26(4):982–983. doi: 10.1021/jf60218a028. [DOI] [PubMed] [Google Scholar]
- Streeter J. G. Nitrate inhibition of legume nodule growth and activity : I. Long term studies with a continuous supply of nitrate. Plant Physiol. 1985 Feb;77(2):321–324. doi: 10.1104/pp.77.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. S., Duffy P., Brown W. D. Interaction of myoglobin and cytochrome C. J Biol Chem. 1972 Mar 25;247(6):1899–1903. [PubMed] [Google Scholar]


