Abstract
Greatwall (Gwl) kinase plays an essential role in the regulation of mitotic entry and progression. Mitotic activation of Gwl requires both cyclin-dependent kinase 1 (CDK1)-dependent phosphorylation and its autophosphorylation at an evolutionarily conserved serine residue near the carboxyl terminus (Ser-883 in Xenopus). In this study we show that Gwl associates with protein phosphatase 1 (PP1), particularly PP1γ, which mediates the dephosphorylation of Gwl Ser-883. Consistent with the mitotic activation of Gwl, its association with PP1 is disrupted in mitotic cells and egg extracts. During mitotic exit, PP1-dependent dephosphorylation of Gwl Ser-883 occurs prior to dephosphorylation of other mitotic substrates; replacing endogenous Gwl with a phosphomimetic S883E mutant blocks mitotic exit. Moreover, we identified PP1 regulatory subunit 3B (PPP1R3B) as a targeting subunit that can direct PP1 activity toward Gwl. PPP1R3B bridges PP1 and Gwl association and promotes Gwl Ser-883 dephosphorylation. Consistent with the cell cycle-dependent association of Gwl and PP1, Gwl and PPP1R3B dissociate in M phase. Interestingly, up-regulation of PPP1R3B facilitates mitotic exit and blocks mitotic entry. Thus, our study suggests PPP1R3B as a new cell cycle regulator that functions by governing Gwl dephosphorylation.
Keywords: cell cycle, mitosis, phosphatase, phosphoprotein phosphatase 1 (PP1), Xenopus
Introduction
It has been well established that cell cycle progression through mitosis is controlled by mitotic kinases, especially CDK1/Cyclin B.3 Regulated activation and inactivation of these kinases are defining events of mitotic entry and exit (1–3). Interestingly, recent studies characterized a new Ser/Thr kinase, Gwl (also known as microtubule-associated serine/threonine kinase-like (MASTL)), that plays an essential role in mitotic entry and maintenance (4–7). The underlying mechanism of Gwl-dependent mitotic regulation is distinct from that of other mitotic kinases (8, 9). CDK1 and most other mitotic kinases directly phosphorylate numerous substrates that regulate spindle formation, chromatin condensation, nuclear envelope breakdown, and other mitotic processes (1–3). In contrast, Gwl phosphorylates two related proteins, α-endosulfine (ENSA) and cAMP-regulated phosphoprotein 19 (ARPP19), which then bind and inhibit PP2A/B55 (8, 9). Because PP2A/B55 is the principal phosphatase to dephosphorylate CDK1 substrates, its inhibition via the Gwl-ENSA/ARPP19 pathway allows mitotic phosphorylation of CDK1 substrates and warrants robust induction of mitotic entry (10–12). Consistent with the important function of Gwl, evidence obtained in various experimental systems demonstrated that Gwl deficiency led to severe defects of mitotic entry and progression (13–16). Interestingly, emerging evidence revealed a strong connection between Gwl up-regulation and cancer progression (17, 18). Several studies also characterized Gwl as a highly valuable drug target for cancer therapy (17, 19–21).
Compared with the function of Gwl, the regulation of Gwl is less understood. It has been shown that CDK1 phosphorylates multiple sites within the kinase domain of Gwl, leading to Gwl activation (22–24). In addition, a C terminus site, Ser-883 in Xenopus or Ser-875 in humans, is autophosphorylated by Gwl itself as an essential step of Gwl activation (22–24). Obviously, these phosphorylation events need to be tightly modulated by counteracting phosphatases in accordance with the transition of cell cycle stages. In fact, several recent studies suggested that both PP2A and PP1 are involved in the dephosphorylation of Gwl (25–30).
PP1 and PP2A are the two most abundant forms of Ser/Thr phosphatases in the cell, and both of them have been shown to regulate the cell cycle (31, 32). PP1 in particular plays an essential role in the regulation of mitotic entry, progression, and exit by dephosphorylating many mitotic substrates, including Aurora A/B and H3 (32–34). Vertebrate cells encode several isoforms of PP1, including PP1α, β, and γ. These isoforms of PP1 share an extraordinarily high level of similarity in sequence and function, although previous studies noted their differences in tissue-specific expression and subcellular localization (35–37). As a major cellular Ser/Thr phosphatase, PP1 relies on additional regulatory/targeting subunits to achieve specific functions. Taking into account the vast array of regulatory/targeting subunits, it is now recognized that PP1 and other protein phosphatases exhibit similar complexity and specificity as kinases (34, 38–40). However, the identity of targeting subunits that direct PP1 to specific mitotic substrates and the mechanisms underlying cell cycle-dependent regulation of PP1 actions are largely unknown. In this study, we show that PP1 associates with Gwl and mediates Gwl dephosphorylation at Ser-883. Our results directly prove the role of PP1 in Gwl regulation using a phospho-specific antibody against Xenopus Gwl Ser-883. Gwl and PP1γ dissociate during mitosis, consistent with the mitotic activation of Gwl. Importantly, we identified PPP1R3B as a specific targeting subunit that may at least partially mediate PP1/Gwl association and Gwl Ser-883 dephosphorylation.
Results
Gwl associates with specific isoforms of PP1
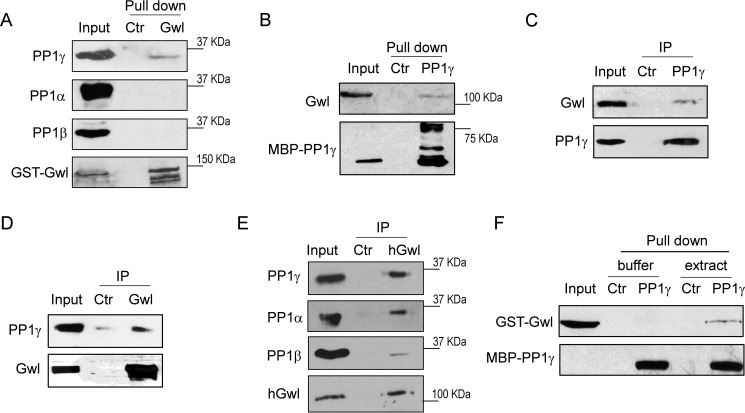
Reversible phosphorylation of Gwl at multiple Ser/Thr residues plays a central role in the regulation of Gwl kinase activation (22–24). To reveal Gwl regulation by protein phosphatases, we pulled down recombinant Gwl from interphase Xenopus egg extracts and probed for the presence of various PP1 catalytic subunits. Interestingly, we observed that PP1γ, but not PP1α or PP1β, associated with Gwl (Fig. 1A). Consistently, pulldown of recombinant PP1γ from interphase egg extracts also recovered Gwl (Fig. 1B). Endogenous Gwl was detected in the immunoprecipitation of PP1γ from interphase egg extracts (Fig. 1C), and, reciprocally, PP1γ co-immunoprecipitated with Gwl (Fig. 1D). Moreover, Gwl immunoprecipitation in human cell lysates confirmed its association with PP1γ as well as smaller portions of PP1β and PP1α (Fig. 1E), suggesting the involvement of PP1 isoforms in the regulation of mammalian Gwl.
Figure 1.
Gwl associates with PP1. A, a pulldown assay was performed using GST-tagged full-length Gwl in interphase Xenopus egg extracts as described under “Experimental Procedures.” The pulldown product, extract input, and a control (Ctr) pulldown (using empty beads) were analyzed by immunoblotting for PP1α, PP1β, PP1γ, and Gwl. B, a pulldown assay was performed using MBP-tagged PP1γ in Xenopus egg extracts. The pulldown product, extract input, and a control pulldown were analyzed by immunoblotting for Gwl and PP1γ. C, immunoprecipitation (IP) of endogenous PP1γ was performed in Xenopus egg extracts as described under “Experimental Procedures.” The IP product, extract input, and a control IP were analyzed by immunoblotting for Gwl and PP1γ. D, IP of endogenous Gwl was performed in Xenopus egg extracts. The IP product, extract input, and a control IP were analyzed by immunoblotting for Gwl and PP1γ. E, IP of endogenous Gwl was performed in HeLa cell lysates. The IP product, lysate input, and a control IP were analyzed by immunoblotting for PP1α, PP1β, PP1γ, and human Gwl (hGwl). F, purified GST-Gwl (in elution) and MBP-PP1γ (on beads) were incubated in either Xenopus egg extract or buffer. MBP-PP1γ or control bead pulldown was performed as in B. The pulldown products were analyzed by immunoblotting for Gwl and PP1γ. The data in this figure are representative of three or more independent experiments.
Because Cdc25C, retinoblastoma, and several other cell cycle regulators are themselves direct interactors of PP1, we asked whether Gwl is a new PP1-interacting protein. However, we were unable to detect direct protein interaction using purified Gwl and PP1γ, whereas these proteins bound upon addition into interphase Xenopus egg extracts (Fig. 1F). Therefore, PP1γ and Gwl association may be mediated by a third protein, possibly a targeting subunit of PP1.
PP1γ mediates Gwl dephosphorylation at Ser-883
Existing evidence revealed three classes of reversible phosphorylation of Gwl. First, CDK1-dependent phosphorylation of Gwl within the kinase domain initiates Gwl kinase activation (22–24). Second, phosphorylation at the C-terminal Ser-883 catalyzed by Gwl itself is an indispensable step toward Gwl activation (22). Third, multiple phosphorylation sites within the less conserved middle segment of Gwl by CDK1 and Plk1 may regulate the subcellular localization of Gwl (16, 41). It has been indicated that dephosphorylation of CDK1 substrate sites is primarily mediated by PP2A/B55 (11). Therefore, we hypothesized that PP1 may regulate the dephosphorylation of Gwl at Ser-883.
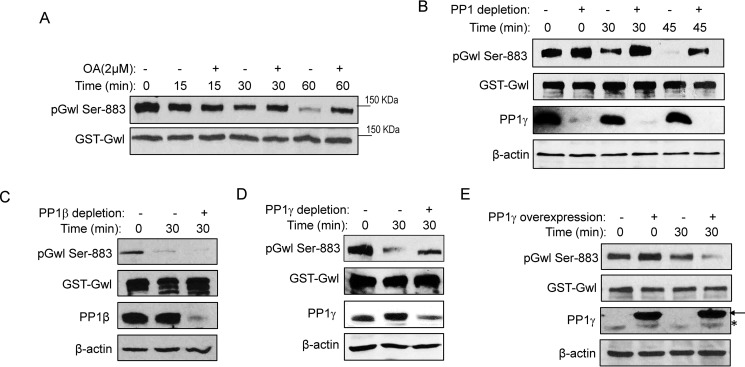
To study Gwl dephosphorylation, prephosphorylated Gwl was supplemented in interphase egg extracts. Gwl Ser-883 phosphorylation was examined using a phospho-specific antibody we recently generated and validated (supplemental Fig. S1). In Xenopus egg extracts, the phosphatase activity toward Gwl Ser-883 was suppressed by okadaic acid, an inhibitor of PP2A and PP1 (Fig. 2A). Moreover, depletion of PP1 from extracts using a specific PP1-binding motif derived from the phosphatase 1 nuclear targeting subunit (Pnuts) prevented the dephosphorylation of Gwl Ser-883 (Fig. 2B and supplemental Fig. S2).
Figure 2.
PP1 mediates Gwl dephosphorylation at Ser-883. A, to prephosphorylate Gwl, GST-Gwl beads were incubated in metaphase-arrested CSF extracts for 30 min and reisolated. GST-Gwl beads were then incubated in interphase egg extracts with or without okadaic acid (OA) for 0, 15, 30, and 60 min. GST-Gwl beads were reisolated and analyzed by immunoblotting for phospho-Gwl Ser-883 and Gwl. B, GST-Gwl was prephosphorylated as in A and then incubated in interphase extracts with or without PP1 depletion for 0, 30, and 45 min. To deplete PP1 from interphase egg extracts, the PP1-binding domain of Pnuts was purified on beads, incubated in extracts for 30 min, and then removed. A mock depletion was performed as a control. Depletion of PP1 was confirmed by immunoblotting for PP1γ and β-actin. Dephosphorylation of Gwl Ser-883 in extracts with or without PP1 depletion was measured by immunoblotting for phospho-Gwl Ser-883 and Gwl. C, GST-Gwl was prephosphorylated as in A and then incubated in interphase extracts with or without PP1β immunodepletion for 30 min. Dephosphorylation of Gwl Ser-883 was measured by immunoblotting for phospho-Gwl Ser-883, Gwl, PP1β, and β-actin. D, GST-Gwl was prephosphorylated as in A and then incubated in interphase extracts with or without PP1γ immunodepletion for 30 min. Dephosphorylation of Gwl Ser-883 was measured by immunoblotting for phospho-Gwl Ser-883, Gwl, PP1γ, and β-actin. E, GST-Gwl was prephosphorylated as in A and then incubated in interphase extracts with or without supplementation of purified His-PP1γ as in Ref. 51. Dephosphorylation of Gwl Ser-883 was measured by immunoblotting for phospho-Gwl Ser-883, Gwl, PP1γ, and β-actin. The arrow points to His-PP1γ, and the asterisk marks the endogenous PP1γ. The data in this figure are representative of three or more independent experiments.
We showed in Fig. 1 that PP1γ associated with Gwl in Xenopus egg extracts. To specifically investigate the role of PP1β and PP1γ in the dephosphorylation of Gwl Ser-883, we immunodepleted either PP1β or PP1γ from egg extracts. Interestingly, depletion of PP1γ, but not PP1β, significantly reduced Ser-883 dephosphorylation (Fig. 2, C and D). Conversely, supplementation of recombinant PP1γ accelerated Ser-883 dephosphorylation (Fig. 2E). Notably, addition of exogenous PP1γ severalfold over the endogenous level did not fully dephosphorylate Gwl Ser-883, suggesting a need for an additional, titratable factor that mediates Gwl dephosphorylation by PP1.
Gwl and PP1γ associate in a cell cycle-dependent manner
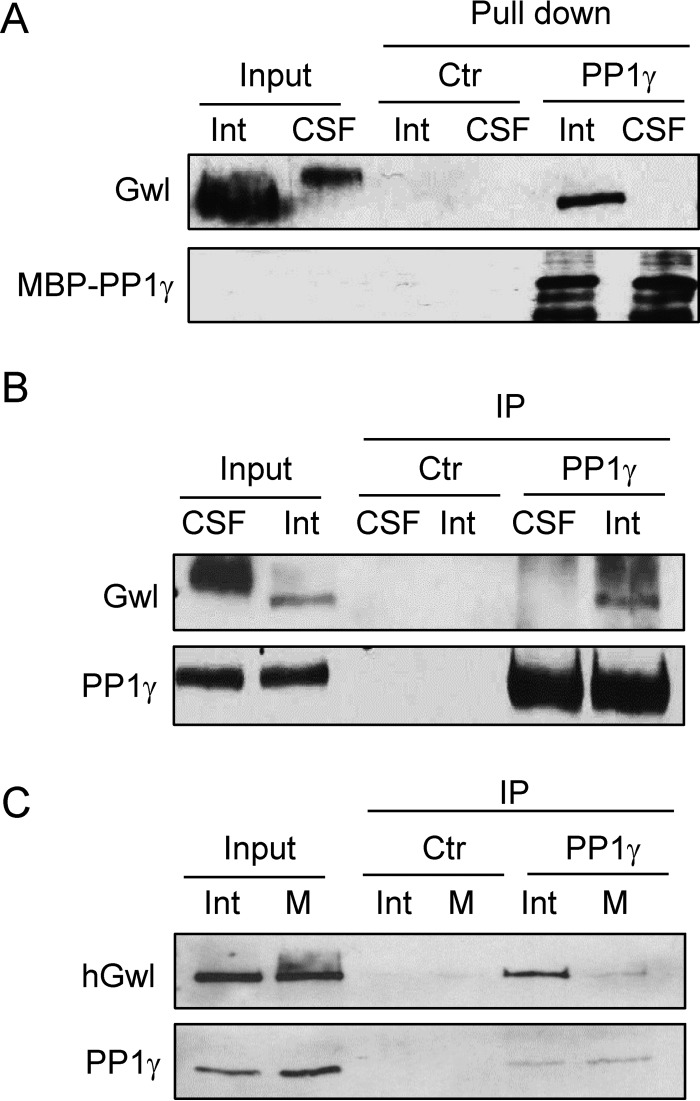
As Gwl plays an essential role in mitosis, its dephosphorylation at Ser-883 needs to be prevented during mitosis to allow Gwl activation. We reasoned that a possible mechanism to account for the mitotic phosphorylation of Gwl Ser-883 is disruption of its PP1-association during M phase. To this end, we analyzed the association between Gwl and PP1γ in both interphase and CSF (M phase) extracts. Interestingly, PP1γ pulldown recovered Gwl in interphase but not CSF extracts (Fig. 3A). The same conclusion was also reached by immunoprecipitation of endogenous PP1γ (Fig. 3B). Finally, PP1γ immunoprecipitation was performed in human cells synchronized in interphase or M phase, and only in interphase did Gwl exhibit PP1 association (Fig. 3C).
Figure 3.
Gwl associates with PP1 in a cell cycle-dependent manner. A, MBP-PP1γ pulldown was performed in interphase (Int) or CSF egg extracts. The pulldown product, extract input, and a control (Ctr) pulldown were immunoblotted for Gwl and MBP. B, immunoprecipitation of PP1γ was performed in interphase or CSF egg extracts and immunoblotted for Gwl and PP1γ. C, immunoprecipitation of PP1γ was performed in interphase or M phase HeLa cell lysates and immunoblotted for human Gwl and PP1γ. HeLa cells were synchronized in interphase or M phase by thymidine block or nocodazole arrest, respectively. The data in this figure are representative of three or more independent experiments.
Gwl Ser-883 dephosphorylation is an early and essential event of mitotic exit
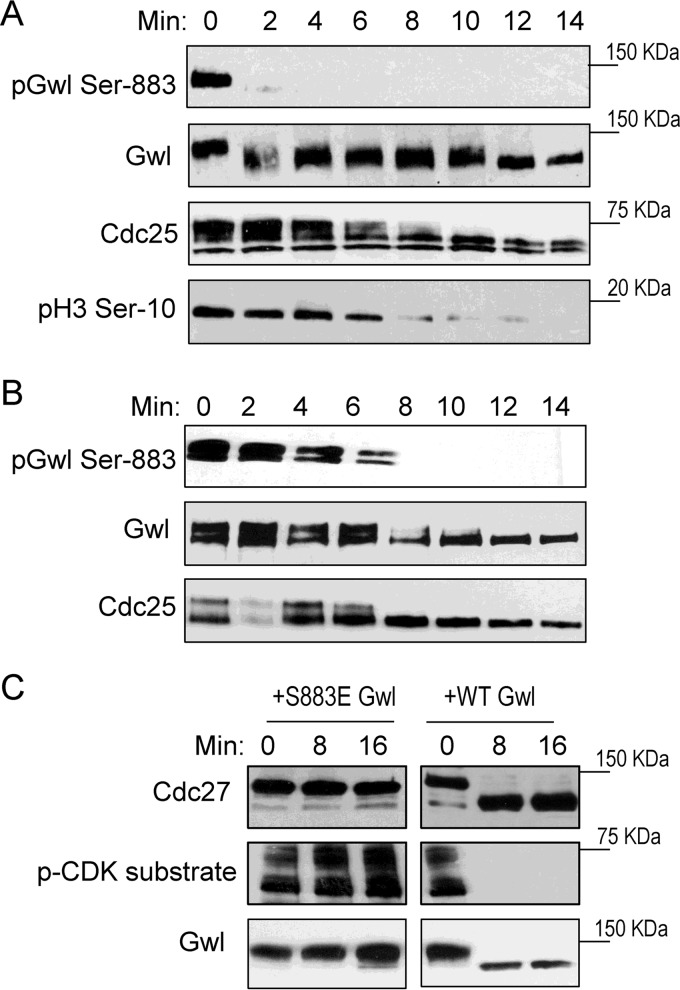
Mitotic exit is triggered by deactivation of CDK1 and reactivation of anti-mitotic phosphatases. These events together lead to dephosphorylation of mitotic phosphoproteins and reorganization of cellular structures to the interphase state. Given the role of Gwl in the regulation of PP2A/B55, it has been suggested that deactivation of Gwl kinase is an essential event of mitotic exit (27, 28, 30). To delineate the involvement of PP1-dependent dephosphorylation of Gwl Ser-883 in mitotic exit, we investigated the fine kinetics of Ser-883 dephosphorylation in CSF egg extracts that undergo synchronized mitotic exit following addition of calcium. Interestingly, Gwl Ser-883 was efficiently dephosphorylated within 2 min, in correlation with partially increased gel mobility of Gwl (Fig. 4A). Complete loss of Gwl gel retardation was observed at 10 min, presumably reflecting its dephosphorylation at CDK1-targeted sites. In comparison, dephosphorylation of Cdc25, which is primarily phosphorylated by CDK1, was evident in 8–10 min (Fig. 4A). Histone H3 Ser-10, a known substrate of PP1, was not dephosphorylated until 8 min, suggesting that only a portion of PP1 is initially activated to dephosphorylate Gwl Ser-883 and trigger mitotic exit (Fig. 4A).
Figure 4.
Gwl Ser-883 dephosphorylation is an early and essential event of mitotic exit. A, the CSF egg extract was released into interphase by addition of calcium. Extract samples at various time points were analyzed by immunoblotting for phospho-Gwl Ser-883, Gwl, Cdc25C, and phospho-H3 Ser-10. B, the CSF egg extract was added to the interphase egg extract at a 1:3 ratio. Samples at various time points were analyzed by immunoblotting for phospho-Gwl Ser-883, Gwl, and Cdc25C. C, CSF extracts were immunodepleted of endogenous Gwl and supplemented with WT or S883E Gwl as in Ref. 22. The resulting extracts were treated with calcium, harvested at the indicated time points, and analyzed by immunoblotting for Cdc27, phospho-CDK substrates, and Gwl. The data in this figure are representative of three or more independent experiments.
The differential kinetics of Gwl Ser-883 dephosphorylation in comparison with other mitotic phosphoproteins likely reflects the orderly reactivation of distinct phosphatases during mitotic exit. Indeed, such a pattern of dephosphorylation was not observed when the CSF extract was diluted in an interphase extract in which all antimitotic phosphatase activities are readily active (Fig. 4B). We then sought to directly assess the functional importance of Ser-883 dephosphorylation for mitotic exit. As shown in Fig. 4C, WT or phosphomimetic S883E Gwl was supplemented in CSF extracts immunodepleted of endogenous Gwl. Although the extract reconstituted with WT Gwl underwent normal mitotic exit after calcium treatment, the extract with S883E Gwl was not released into interphase (Fig. 4C).
PPP1R3B mediates PP1 and Gwl association
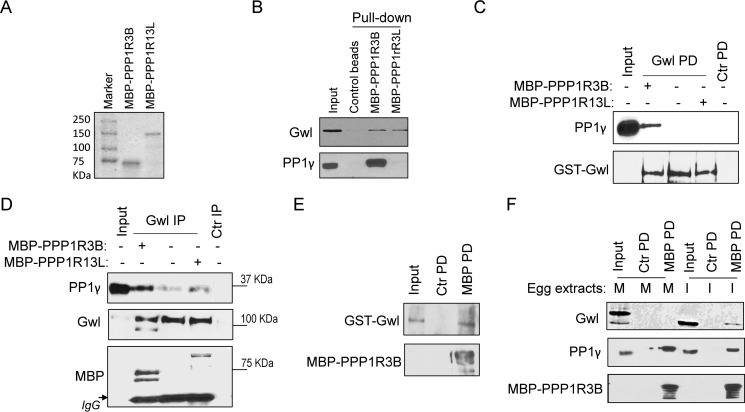
We noted that purified PP1 and Gwl do not directly interact, which led to the immediate possibility that a targeting subunit of PP1 is required to bridge their association. Gwl pulldown was performed in Xenopus egg extracts, and the pulldown product was subjected to mass spectrometric analysis. Interestingly, two potential targeting subunits of PP1, PPP1R3B and PPP1R13L, were identified. PPP1R3B was characterized previously as a glycogen-targeting subunit of PP1 (42), and PPP1R13L, also known as inhibitor of apoptosis-stimulating protein of p53 (iASPP), was implicated in the regulation of apoptosis and transcription via the p53 and NF-κB pathways (43). We cloned both PPP1R3B and PPP1R13L from a Xenopus oocyte cDNA library and purified recombinant proteins (Fig. 5A). Pulldown experiments confirmed that both PPP1R3B and PPP1R13L associate with Gwl (Fig. 5B).
Figure 5.
PPP1R3B and PPP1R13L associate with Gwl. A, MBP-PPP1R3B and MBP-PPP1R13L were expressed and purified as described under “Experimental Procedures.” The purified proteins were examined by Coomassie staining. B, MBP-PPP1R3B and MBP-PPP1R13L were purified and bound to amylose resin as in A. Beads were incubated in interphase extract for 30 min and then reisolated. The input and pulldown products were analyzed by immunoblotting for Gwl and PP1γ. C, GST-Gwl pulldown (PD) was performed in interphase extracts with or without supplementation of purified MBP-PPP1R3B and MBP-PPP1R13L. The input and pulldown products were analyzed by immunoblotting for Gwl and PP1γ. Ctr, control. D, Gwl IP was performed in interphase extracts with or without supplementation of purified MBP-PPP1R3B and MBP-PPP1R13L. The input and IP products were analyzed by immunoblotting for Gwl, PP1γ, and MBP. E, MBP-PPP1R3B was purified on beads, incubated with purified GST-Gwl, and reisolated. The input GST-Gwl protein, control pulldown, and MBP pulldown samples were analyzed by immunoblotting for GST and MBP. F, MBP-PPP1R3B pulldown was performed in CSF (M phase, M) or interphase (I) egg extracts. The input and pulldown products were analyzed by immunoblotting for Gwl, PP1γ, and MBP. The data in this figure are representative of three or more independent experiments.
We speculated that, if PPP1R3B or PPP1R13L serves to mediate PP1 and Gwl association, then its up-regulation should augment PP1 and Gwl association. This notion was confirmed for PPP1R3B but not PPP1R13L: Gwl pulldown or immunoprecipitation recovered higher levels of PP1 in the presence of recombinant PPP1R3B (Fig. 5, C and D). We also showed that PPP1R3B associates with Gwl via direct protein-protein interaction (Fig. 5E). Finally, in light of our finding that Gwl associates with PP1 in interphase but not M phase, we investigated the cell cycle dependence of PPP1R3B association with Gwl and PP1. Of great interest to us was that PPP1R3B bound only interphase, unphosphorylated Gwl, whereas the interaction of this regulatory subunit with PP1 appeared to be constitutive (Fig. 5F).
PPP1R3B controls Gwl dephosphorylation and mitotic transitions
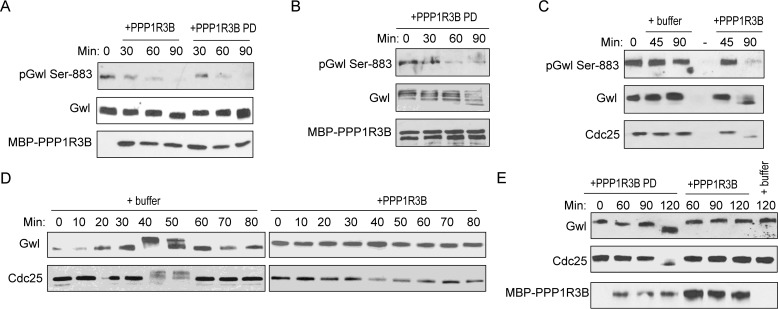
As PPP1R3B bridges PP1 and Gwl association, we sought to uncover its role in Gwl dephosphorylation. Interestingly, addition of PPP1R3B in diluted CSF extracts resulted in dephosphorylation of Gwl Ser-883 (Fig. 6A). A potential concern with overexpression of a phosphatase subunit is that this targeting subunit may compete with endogenous targeting subunits for PP1 binding. To alleviate this concern, PPP1R3B was preincubated in egg extracts so that the subsequent addition of PPP1R3B pulldown contains PP1 and other components of the holoenzyme. Addition of this PPP1R3B pulldown led to a similar effect of Gwl dephosphorylation (Fig. 6A). Moreover, PPP1R3B pulldown was able to dephosphorylate purified Gwl in an in vitro phosphatase assay (Fig. 6B). As we earlier characterized Gwl Ser-883 dephosphorylation as an early and essential event of mitotic exit, we then asked whether PPP1R3B regulates mitotic exit via Gwl dephosphorylation. Interestingly, supplementation of PPP1R3B to CSF extracts was sufficient to dephosphorylate Gwl and trigger mitotic exit (Fig. 6C), whereas addition of PPP1R3B to cycling extracts blocked mitotic entry (Fig. 6D). To again address the concern that exogenous PPP1R3B competes for endogenous PP1, we compared the effect of purified PPP1R3B with that of PPP1R3B pulldown in promoting mitotic exit. As shown in Fig. 6E, PPP1R3B pulldown was more efficient in causing mitotic exit, indicating that PPP1R3B does not influence mitotic exit indirectly by affecting endogenous PP1-containing complexes.
Figure 6.
PPP1R3B regulates mitotic entry and exit in Xenopus egg extracts. A, CSF extracts were diluted in a phosphatase buffer (50 mm HEPES, 100 mm NaCl, 2 mm DTT, 0.01% Brij 35, and 1 mm MnCl2) and incubated with MBP-PPP1R3B beads that were mock-treated or pulled down (PD) from interphase egg extracts. Extract samples at various time points were analyzed by immunoblotting for phospho-Gwl Ser-883, Gwl, and MBP. B, the MBP-PPP1R3B pulldown as in A was incubated with purified active Gwl for 30, 60, and 90 min. Dephosphorylation of Gwl Ser-883 was monitored by immunoblotting for phospho-Gwl Ser-883, Gwl, and MBP. C, CSF extracts were incubated with buffer or purified MBP-PPP1R3B. Extract samples at various time points were analyzed by immunoblotting for phospho-Gwl Ser-883, Gwl, and Cdc25C. D, cycling egg extracts were supplemented with buffer or purified MBP-PPP1R3B. Extract samples at various time points were analyzed by immunoblotting for Gwl and Cdc25C. E, CSF extracts were incubated with buffer (last lane) or MBP-PPP1R3B beads that were mock-treated or pulled down from interphase egg extracts. Extract samples at various time points were analyzed by immunoblotting for Gwl, Cdc25C, and MBP. The data in this figure are representative of three or more independent experiments.
Discussion
Multiple phosphatases coordinately prevent the premature activation of Gwl
Studies in multiple experimental systems characterized an important role of Gwl kinase in mitotic progression via phosphorylation of ENSA/ARPP19 and the subsequent inhibition of PP2A/B55 (8, 9). Like CDK1 and other mitotic kinases, the activity of Gwl is regulated in a cell cycle-dependent manner and peaks in mitosis (24). It is therefore of great importance to reveal the detailed mechanisms that account for activation of Gwl during mitotic entry and inactivation of Gwl during mitotic exit. The activation of Gwl during mitotic entry requires at least two classes of phosphorylation events. First, CDK1 phosphorylates several sites within the activation loop of the Gwl kinase domain (22–24), and second, the autophosphorylation of Gwl at Ser-883 is indispensable for the kinase activity of Gwl (22). Accordingly, to inactivate Gwl during mitotic exit, phosphorylation at these sites should be removed by phosphatases, and several such enzymes may be involved.
Because PP2A/B55 acts as a principal phosphatase for many CDK1 substrates and is reactivated at M phase exit (11, 12, 44), it is plausible that PP2A/B55 mediates the dephosphorylation of Gwl at the CDK1-targeted sites. Consistently, a previous study showed that active PP2A/B55 associated with Gwl in interphase but not M phase oocytes (26), and more recent evidence established that PP2A can dephosphorylate Gwl at residues targeted by CDK1 (25, 45). Other phosphatases may also be involved in removing this class of phosphorylation of Gwl: RNA polymerase II carboxyl-terminal domain phosphatase Fcp1 bound Gwl and dephosphorylated Gwl at CDK1-targeted sites (25, 29).
In this study, we show that PP1, particularly PP1γ, binds Gwl and is responsible for the dephosphorylation of Gwl at its Ser-883 autophosphorylation site. Our results validate and extend the findings of several recent studies concluding that the dephosphorylation and inactivation of Gwl during mitotic exit is mediated by PP1 (27, 28, 30). In this study, we were able to generate a Gwl Ser-883 phospho-specific antibody and use it to directly demonstrate the role of PP1 in Gwl Ser-883 dephosphorylation in Xenopus egg extracts. Interestingly, we report here that the PP1 and Gwl association is disrupted in mitotic extracts. This finding suggests that PP1 may prevent the premature activation of Gwl by suppressing its autophosphorylation in interphase and that dissociation of PP1 from Gwl in mitosis allows Gwl activation via autophosphorylation. We showed also, in human cells, that PP1 associated with Gwl in interphase but not in M phase, thus confirming the evolutionarily conserved nature of Gwl regulation by PP1. Given that the association of PP2A/B55 with Gwl is similarly limited to interphase (26), a picture emerges of several phosphatases patrolling interphase Gwl to ensure that the kinase does not activate prematurely.
PPP1R3B directs PP1 to dephosphorylate Gwl Ser-883 in Xenopus egg extracts
PP1 is a major form of serine/threonine phosphatase that accounts for ∼50% of all Ser/Thr phosphatase activities in the cell (40). Recent studies have convincingly shown that PP1 relies on additional targeting subunits to achieve specific functions. It has been estimated that over 100 targeting subunits of PP1 exist to direct the specific action of PP1 toward its numerous cellular substrates (34, 38–40). Thus, identification and characterization of specific targeting subunits of PP1 represent a major challenge in understanding the cellular function and regulation of PP1. Some mitotic substrates of PP1, such as Aurora-A, Aurora-B, retinoblastoma, and Cdc25C, possess PP1-docking motifs and are themselves targeting subunits of PP1. By comparison, we observed no direct PP1 and Gwl interaction using purified proteins, suggesting that their association is mediated by an additional protein, presumably a targeting subunit of PP1.
Interestingly, we identified and confirmed two targeting subunits of PP1, PPP1R3B and PPP1R13L, as Gwl-associated proteins. Addition of exogenous PPP1R3B increased PP1 and Gwl association in Xenopus egg extracts. By comparison, PPP1R13L exhibited either non-detectable or weak PP1-binding in extracts; addition of PPP1R13L in extracts did not significantly increase PP1 and Gwl association. These findings suggest PPP1R3B as a targeting subunit that mediates PP1-dependent regulation of Gwl, whereas the nature of Gwl and PPP1R13L association remains to be investigated. We further demonstrated that PPP1R3B promoted Gwl Ser-883 dephosphorylation in both egg extracts and an in vitro phosphatase assay. Thus, in line with its role in Gwl regulation, our study suggests PPP1R3B as a new cell cycle regulator whose up-regulation suppresses mitotic entry and triggers mitotic exit in Xenopus egg extracts. This conclusion, however, will require further validation, as our attempts to immunodeplete PPP1R3B from Xenopus egg extracts were unsuccessful. Moreover, future studies are needed to clarify whether PPP1R3B plays a similar role in mammalian cells and whether additional targeting subunits of PP1 are involved.
Gwl Ser-883 dephosphorylation is an early and essential event of mitotic exit
We report here that Gwl and PPP1R3B dissociate in M phase. This finding is consistent with the cell cycle-dependent association of Gwl and PP1. Presumably, dissociation of PPP1R3B from Gwl upon mitotic entry enables Gwl activation, and their reassociation during mitotic exit silences Gwl kinase. The mechanism underlying the dynamic Gwl and PPP1R3B association is not known but possibly involves CDK1-dependent phosphorylation of Gwl, PP1, or PPP1R3B.
Of perhaps even greater interest is the series of events leading to the silencing Gwl kinase and mitotic exit. By analyzing the fine kinetics of mitotic exit, we showed that dephosphorylation of Gwl Ser-883 occurs prior to that of many other mitotic substrates of PP2A or PP1. This result suggests that various antimitotic phosphatase activities are sequentially activated during mitotic exit and that the portion of PP1 activity targeting Gwl Ser-883 is involved in the initiation of mitotic exit. Notably, several studies provided at least circumstantial evidence that PP1-dependent dephosphorylation of Gwl may serve as one of the initial triggers for PP2A/B55 reactivation and mitotic exit. First, a recent study discovered a PP1-to-PP2A relay controlling mitotic progression and exit (46). Second, modeling suggests that inactivation of Gwl must precede the dephosphorylation of ENSA/ARPP19 by PP2A/B55 to allow the phosphatase to autoreactivate itself as it participates in switch-like regulation of mitotic phosphosubstrates (44, 47). Finally, we showed that Gwl Ser-883 dephosphorylation is an essential event of mitotic exit in Xenopus egg extracts, as replacing endogenous Gwl with phosphomimetic S883E mutant blocked mitotic exit.
The idea that Gwl Ser-883 dephosphorylation by PP1 acts as an upstream event in mitotic exit raises the intriguing question of how this dephosphorylation event is permitted prior to full inactivation of Gwl and CDK. A particular challenge to this model also lies in that Gwl and PPP1R3B/PP1 are dissociated in metaphase-arrested egg extracts or cells, and then they must reassociate rapidly following metaphase-to-anaphase transition to allow Ser-883 dephosphorylation. An interesting although unproven possibility is that the association is regulated through CDK1-dependent phosphorylation of the PP1-PPP1R3B complex, which can then undergo autodephosphorylation upon initial dephosphorylation. Consistently, mathematical modeling illustrated that a small reduction in CDK activity can trigger partial PP1 activation, allowing a rapid response (28). In any event, further delineation of the fine kinetics and mechanisms of Gwl and PPP1R3B association and dissociation will yield new insights into the regulation of mitotic progression.
Experimental procedures
Antibodies
Commercial antibodies used in this study included rabbit anti-PP1α (A300-904A, lot 2), β (A300-905A, lot 1), and γ (A300-906A, lot 1) and β-Actin (A300-491A, lot 12) antibodies purchased from Bethyl Labs (Montgomery, TX); mouse anti-MBP antibody (E8032S, lot 0091204) from New England Biolabs (Ipswich, MA); rabbit anti-GST antibody (G7781, lot 059K4833) from Sigma; and rabbit anti-phospho-H3 Ser-10 (3377) and phospho-CDK (9477S, lot 2) substrate antibodies from Cell Signaling Technology (Danvers, MA). Gwl antibodies were characterized previously (48) or purchased from Millipore (MABT372, Billerica, MA). A rabbit polyclonal antibody to Gwl Ser-883 was generated using a phosphopeptide derived from the C-terminal sequence of Xenopus Gwl. Xenopus Cdc25C antibody was provided by Drs. Kumagai and Dunphy (Caltech) (49). The antibodies purchased from major commercial resources had been validated in numerous previous studies. In addition, the specificity of antibodies was confirmed by recognition of the immune signals at the expected size with the expected biological behaviors and down-regulation and up-regulation of the targeted proteins. In particular, the Gwl Ser-883 antibody is uniquely characterized in this study. This phospho-specific antibody recognizes mitotic and phosphorylated Gwl in vitro and in extracts; the immune signal using this antibody was diminished with Gwl depletion.
Cell culture
The human cervix carcinoma (HeLa) cell line, authenticated by the ATCC, was maintained in DMEM (Hyclone) with 10% FBS (Hyclone). Cells were synchronized in interphase or M phase with thymidine (2 mm) or nocodazole (2 μm), respectively.
Immunoblotting, immunodepletion, and immunoprecipitation
Immunoblotting, immunodepletion, and immunoprecipitation were performed as described previously (50). For immunoblotting, samples were boiled in 2× Laemmli sample buffer, resolved by SDS-PAGE, and then electrotransferred to PVDF membranes (Millipore). Membranes were blocked with 5% nonfat dry milk in 1× TBST (10 mm Tris HCl (pH 7.5), 150 mm NaCl, and 0.05% Tween 20) and then incubated with specific primary antibodies. Membranes were then washed in 1× TBST and incubated in HRP-conjugated secondary antibodies (Sigma). Immunoreactive signals were detected using the ECL substrate kit (Pierce). For immunodepletion, anti-mouse or anti-rabbit magnetic beads (New England Biolabs) were prewashed in washing buffer (50 mm HEPES (pH 7.5), 150 mm NaCl, 1 mm DTT, and 0.5% Tween 20) and then incubated with antibodies. To reduce nonspecific binding, beads were incubated with washing buffer supplemented with 1% bovine serum albumin. Beads conjugated with antibodies were washed and then mixed with Xenopus egg extracts. After 30 min of incubation, the beads were removed with a magnet, and the remaining extracts were collected. For immunoprecipitation, anti-mouse or anti-rabbit magnetic beads were conjugated to antibodies as described above and then mixed with egg extracts. After 30 min of incubation, the beads were removed with a magnet and washed before elution with 2× Laemmli sample buffer and analysis by immunoblotting.
Protein expression and purification
The Xenopus PP1γ gene was cloned from a Xenopus oocyte cDNA library as described previously (51) using the targeting sequences ATGGCAGATGTTGAC and ATTTATTTCTTTGCTTG. Xenopus PPP1R3B was cloned from the Xenopus oocyte cDNA library using the targeting sequences TCCATGGCGGTAGACATCGCCATG and TTCTTAATAAGGTCCTAGTTTATC. Xenopus PPP1R13L was cloned from the Xenopus oocyte cDNA library using the targeting sequences ATGTCAGGAGAAAAG and TCACTTGGGACTACG. These genes were then inserted into the pGEX4T vector with an N-terminal GST tag or pMAL-parallel plasmid with an N-terminal MBP tag. GST or MBP-tagged Xenopus Gwl was cloned and characterized as in our previous study (52). GST-tagged Pnuts-M was cloned and characterized as in our previous study (50). These proteins were then expressed in BL21 bacterial cells and purified with glutathione or amylose beads.
Xenopus egg extracts
Cytostatic factor (CSF) extracts were prepared as described previously (52). Eggs were dejellied with 2% cysteine in 1× XB (1 m KCl, 10 mm MgCl2, 100 mm HEPES (pH 7.7), and 500 mm sucrose) and washed in 1× XB and 1× MEB (1 m KCl, 11 mm MgCl2, 100 mm HEPES (pH 7.7), 500 mm sucrose, and 5 mm EGTA (pH 7.7)). Eggs were packed in centrifuge tubes by low-speed centrifugation and then crushed by centrifugation at 10,000 × g. The cytoplasmic layer was collected and further separated by another centrifugation at 10,000 × g. For cycling extracts, eggs were rinsed with distilled water and then dejellied with 2% cysteine in 1× XB. The eggs were washed five times in 0.2× MMR buffer (100 mm NaCl, 2 mm KCl, 1 mm MgCl2, 2 mm CaCl2, 0.1 mm EDTA, 10 mm HEPES, and KOH to pH 7.8). The Ca2+ ionophore A23187 was added to 10 ng/ml until the animal poles rotated. The eggs were then washed and packed by low-speed centrifugation. The eggs were crushed by centrifugation at 10,000 × g. The cytoplasmic layer was transferred to new tubes, and energy mix (7.5 mm creatine phosphate, 1 mm ATP, and 1 MgCl2) was added. The cytoplasmic layer was further separated by another centrifugation at 10,000 × g.
Author contributions
A. P. conceived and coordinated the study and wrote the paper. D. R. performed most of the biochemical studies in Xenopus egg extracts as related to PP1/Gwl association and PP1-dependent Gwl dephosphorylation. L. A. F. contributed the findings related to PPP1R3B. L. A. F. and J. Z. performed PP1/Gwl association experiments in mammalian cell lysates. L. W. performed experiments related to mitotic exit. B. C. W. and M. L. G. provided assistance for experiments of mitotic exit and manuscript preparation. All authors reviewed the results and approved the final version of the manuscript.
Supplementary Material
Acknowledgments
We thank Drs. Kumagai and Dunphy (Caltech) for providing the Cdc25C antibody.
This work was supported by National Institutes of Health Grants 1R01CA172574 and 5P20GM103489 (to A. P.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains supplemental Figs. S1 and S2.
- CDK
- cyclin-dependent kinase
- Gwl
- Greatwall
- ENSA
- α-endosulfine
- Pnuts
- phosphatase 1 nuclear targeting subunit
- CSF
- cytostatic factor
- IP
- immunoprecipitation
- MBP
- maltose-binding protein.
References
- 1. Nigg E. A. (2001) Mitotic kinases as regulators of cell division and its checkpoints. Nat. Rev. Mol. Cell Biol. 2, 21–32 [DOI] [PubMed] [Google Scholar]
- 2. Ma H. T., and Poon R. Y. (2011) How protein kinases co-ordinate mitosis in animal cells. Biochem. J. 435, 17–31 [DOI] [PubMed] [Google Scholar]
- 3. Lens S. M., Voest E. E., and Medema R. H. (2010) Shared and separate functions of polo-like kinases and aurora kinases in cancer. Nat. Rev. Cancer 10, 825–841 [DOI] [PubMed] [Google Scholar]
- 4. Lorca T., and Castro A. (2013) The Greatwall kinase: a new pathway in the control of the cell cycle. Oncogene 32, 537–543 [DOI] [PubMed] [Google Scholar]
- 5. Hunt T. (2013) On the regulation of protein phosphatase 2A and its role in controlling entry into and exit from mitosis. Adv. Biol. Regul. 53, 173–178 [DOI] [PubMed] [Google Scholar]
- 6. Glover D. M. (2012) The overlooked greatwall: a new perspective on mitotic control. Open Biol. 2, 120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goldberg M. L. (2010) Greatwall kinase protects mitotic phosphosites from barbarian phosphatases. Proc. Natl. Acad. Sci. U.S.A. 107, 12409–12410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mochida S., Maslen S. L., Skehel M., and Hunt T. (2010) Greatwall phosphorylates an inhibitor of protein phosphatase 2A that is essential for mitosis. Science 330, 1670–1673 [DOI] [PubMed] [Google Scholar]
- 9. Gharbi-Ayachi A., Labbé J. C., Burgess A., Vigneron S., Strub J. M., Brioudes E., Van-Dorsselaer A., Castro A., and Lorca T. (2010) The substrate of Greatwall kinase, Arpp19, controls mitosis by inhibiting protein phosphatase 2A. Science 330, 1673–1677 [DOI] [PubMed] [Google Scholar]
- 10. Vigneron S., Brioudes E., Burgess A., Labbé J. C., Lorca T., and Castro A. (2009) Greatwall maintains mitosis through regulation of PP2A. EMBO J. 28, 2786–2793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mochida S., Ikeo S., Gannon J., and Hunt T. (2009) Regulated activity of PP2A-B55 δ is crucial for controlling entry into and exit from mitosis in Xenopus egg extracts. EMBO J. 28, 2777–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Castilho P. V., Williams B. C., Mochida S., Zhao Y., and Goldberg M. L. (2009) The M phase kinase Greatwall (Gwl) promotes inactivation of PP2A/B55 δ, a phosphatase directed against CDK phosphosites. Mol. Biol. Cell 20, 4777–4789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Archambault V., Zhao X., White-Cooper H., Carpenter A. T., and Glover D. M. (2007) Mutations in Drosophila Greatwall/Scant reveal its roles in mitosis and meiosis and interdependence with polo kinase. PLoS Genet. 3, e200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burgess A., Vigneron S., Brioudes E., Labbé J. C., Lorca T., and Castro A. (2010) Loss of human Greatwall results in G2 arrest and multiple mitotic defects due to deregulation of the cyclin B-Cdc2/PP2A balance. Proc. Natl. Acad. Sci. U.S.A. 107, 12564–12569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Voets E., and Wolthuis R. M. (2010) MASTL is the human orthologue of Greatwall kinase that facilitates mitotic entry, anaphase and cytokinesis. Cell Cycle 9, 3591–3601 [DOI] [PubMed] [Google Scholar]
- 16. Álvarez-Fernández M., Sánchez-Martínez R., Sanz-Castillo B., Gan P. P., Sanz-Flores M., Trakala M., Ruiz-Torres M., Lorca T., Castro A., and Malumbres M. (2013) Greatwall is essential to prevent mitotic collapse after nuclear envelope breakdown in mammals. Proc. Natl. Acad. Sci. U.S.A. 110, 17374–17379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang L., Luong V. Q., Giannini P. J., and Peng A. (2014) Mastl kinase, a promising therapeutic target, promotes cancer recurrence. Oncotarget 5, 11479–11489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vera J., Lartigue L., Vigneron S., Gadea G., Gire V., Del Rio M., Soubeyran I., Chibon F., Lorca T., and Castro A. (2015) Greatwall promotes cell transformation by hyperactivating AKT in human malignancies. eLife 4, e10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anania M., Gasparri F., Cetti E., Fraietta I., Todoerti K., Miranda C., Mazzoni M., Re C., Colombo R., Ukmar G., Camisasca S., Pagliardini S., Pierotti M., Neri A., Galvani A., and Greco A. (2015) Identification of thyroid tumor cell vulnerabilities through a siRNA-based functional screening. Oncotarget 6, 34629–34648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nagel R., Stigter-van Walsum M., Buijze M., van den Berg J., van der Meulen I. H., Hodzic J., Piersma S. R., Pham T. V., Jiménez C. R., van Beusechem V. W., and Brakenhoff R. H. (2015) Genome-wide siRNA screen identifies the radiosensitizing effect of downregulation of MASTL and FOXM1 in NSCLC. Mol. Cancer Ther. 14, 1434–1444 [DOI] [PubMed] [Google Scholar]
- 21. Manchado E., Guillamot M., de Cárcer G., Eguren M., Trickey M., García-Higuera I., Moreno S., Yamano H., Cañamero M., and Malumbres M. (2010) Targeting mitotic exit leads to tumor regression in vivo: modulation by Cdk1, Mastl, and the PP2A/B55α,δ phosphatase. Cancer Cell 18, 641–654 [DOI] [PubMed] [Google Scholar]
- 22. Blake-Hodek K. A., Williams B. C., Zhao Y., Castilho P. V., Chen W., Mao Y., Yamamoto T. M., and Goldberg M. L. (2012) Determinants for activation of the atypical AGC kinase Greatwall during M phase entry. Mol. Cell Biol. 32, 1337–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vigneron S., Gharbi-Ayachi A., Raymond A. A., Burgess A., Labbé J. C., Labesse G., Monsarrat B., Lorca T., and Castro A. (2011) Characterization of the mechanisms controlling Greatwall activity. Mol. Cell Biol. 31, 2262–2275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yu J., Zhao Y., Li Z., Galas S., and Goldberg M. L. (2006) Greatwall kinase participates in the Cdc2 autoregulatory loop in Xenopus egg extracts. Mol. Cell 22, 83–91 [DOI] [PubMed] [Google Scholar]
- 25. Hégarat N., Vesely C., Vinod P. K., Ocasio C., Peter N., Gannon J., Oliver A. W., Novák B., and Hochegger H. (2014) PP2A/B55 and Fcp1 regulate Greatwall and Ensa dephosphorylation during mitotic exit. PLoS Genet. 10, e1004004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamamoto T. M., Blake-Hodek K., Williams B. C., Lewellyn A. L., Goldberg M. L., and Maller J. L. (2011) Regulation of Greatwall kinase during Xenopus oocyte maturation. Mol. Biol. Cell 22, 2157–2164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ma S., Vigneron S., Robert P., Strub J. M., Cianferani S., Castro A., and Lorca T. (2016) Greatwall dephosphorylation and inactivation upon mitotic exit is triggered by PP1. J. Cell Sci. 129, 1329–1339 [DOI] [PubMed] [Google Scholar]
- 28. Rogers S., Fey D., McCloy R. A., Parker B. L., Mitchell N. J., Payne R. J., Daly R. J., James D. E., Caldon C. E., Watkins D. N., Croucher D. R., and Burgess A. (2016) PP1 initiates the dephosphorylation of MASTL, triggering mitotic exit and bistability in human cells. J. Cell Sci. 129, 1340–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Della Monica R., Visconti R., Cervone N., Serpico A. F., and Grieco D. (2015) Fcp1 phosphatase controls Greatwall kinase to promote PP2A-B55 activation and mitotic progression. eLife 4, e10399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heim A., Konietzny A., and Mayer T. U. (2015) Protein phosphatase 1 is essential for Greatwall inactivation at mitotic exit. EMBO Rep. 16, 1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yanagida M., Kinoshita N., Stone E. M., and Yamano H. (1992) Protein phosphatases and cell division cycle control. CIBA Found. Symp. 170, 130–140; discussion 140–146 [DOI] [PubMed] [Google Scholar]
- 32. Wurzenberger C., and Gerlich D. W. (2011) Phosphatases: providing safe passage through mitotic exit. Nat. Rev. Mol. Cell Biol. 12, 469–482 [DOI] [PubMed] [Google Scholar]
- 33. De Wulf P., Montani F., and Visintin R. (2009) Protein phosphatases take the mitotic stage. Curr. Opin. Cell Biol. 21, 806–815 [DOI] [PubMed] [Google Scholar]
- 34. Bollen M., Gerlich D. W., and Lesage B. (2009) Mitotic phosphatases: from entry guards to exit guides. Trends Cell Biol. 19, 531–541 [DOI] [PubMed] [Google Scholar]
- 35. Rebelo S., Santos M., Martins F., da Cruz e Silva E. F., and da Cruz e Silva O. A. (2015) Protein phosphatase 1 is a key player in nuclear events. Cell. Signal. 27, 2589–2598 [DOI] [PubMed] [Google Scholar]
- 36. Lesage B., Beullens M., Nuytten M., Van Eynde A., Keppens S., Himpens B., and Bollen M. (2004) Interactor-mediated nuclear translocation and retention of protein phosphatase-1. J. Biol. Chem. 279, 55978–55984 [DOI] [PubMed] [Google Scholar]
- 37. da Cruz e Silva E. F., Fox C. A., Ouimet C. C., Gustafson E., Watson S. J., and Greengard P. (1995) Differential expression of protein phosphatase 1 isoforms in mammalian brain. J. Neurosci. 15, 3375–3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Virshup D. M., and Shenolikar S. (2009) From promiscuity to precision: protein phosphatases get a makeover. Mol. Cell 33, 537–545 [DOI] [PubMed] [Google Scholar]
- 39. Peng A., and Maller J. L. (2010) Serine/threonine phosphatases in the DNA damage response and cancer. Oncogene 29, 5977–5988 [DOI] [PubMed] [Google Scholar]
- 40. Moorhead G. B., Trinkle-Mulcahy L., and Ulke-Lemée A. (2007) Emerging roles of nuclear protein phosphatases. Nat. Rev. Mol. Cell Biol. 8, 234–244 [DOI] [PubMed] [Google Scholar]
- 41. Wang P., Galan J. A., Normandin K., Bonneil É., Hickson G. R., Roux P. P., Thibault P., and Archambault V. (2013) Cell cycle regulation of Greatwall kinase nuclear localization facilitates mitotic progression. J. Cell Biol. 202, 277–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Doherty M. J., Moorhead G., Morrice N., Cohen P., and Cohen P. T. (1995) Amino acid sequence and expression of the hepatic glycogen-binding (GL)-subunit of protein phosphatase-1. FEBS Lett. 375, 294–298 [DOI] [PubMed] [Google Scholar]
- 43. Li Y., Ahmad A., and Sarkar F. H. (2015) ASPP and iASPP: implication in cancer development and progression. Cell Mol. Biol. 61, 2–8 [PubMed] [Google Scholar]
- 44. Williams B. C., Filter J. J., Blake-Hodek K. A., Wadzinski B. E., Fuda N. J., Shalloway D., and Goldberg M. L. (2014) Greatwall-phosphorylated endosulfine is both an inhibitor and a substrate of PP2A-B55 heterotrimers. eLife 3, e01695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wang P., Larouche M., Normandin K., Kachaner D., Mehsen H., Emery G., and Archambault V. (2016) Spatial regulation of greatwall by Cdk1 and PP2A-Tws in the cell cycle. Cell Cycle 15, 528–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Grallert A., Boke E., Hagting A., Hodgson B., Connolly Y., Griffiths J. R., Smith D. L., Pines J., and Hagan I. M. (2015) A PP1-PP2A phosphatase relay controls mitotic progression. Nature 517, 94–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mochida S., Rata S., Hino H., Nagai T., and Novák B. (2016) Two bistable switches govern M phase entry. Curr. Biol. 26, 3361–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang L., Fisher L. A., Wahl J. K. 3rd, Peng A. (2011) Monoclonal antibodies against Xenopus Greatwall kinase. Hybridoma 30, 469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kumagai A., and Dunphy W. G. (1992) Regulation of the cdc25 protein during the cell cycle in Xenopus extracts. Cell 70, 139–151 [DOI] [PubMed] [Google Scholar]
- 50. Fisher L. A., Wang L., Wu L., and Peng A. (2014) Phosphatase 1 nuclear targeting subunit is an essential regulator of M-phase entry, maintenance, and exit. J. Biol. Chem. 289, 23745–23752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peng A., Lewellyn A. L., Schiemann W. P., and Maller J. L. (2010) Repo-Man controls a protein phosphatase 1-dependent threshold for DNA damage checkpoint activation. Curr. Biol. 20, 387–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Peng A., Wang L., and Fisher L. A. (2011) Greatwall and Polo-like kinase 1 coordinate to promote checkpoint recovery. J. Biol. Chem. 286, 28996–29004 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.