Abstract
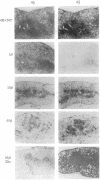
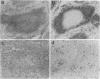
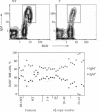
Because allelic polymorphism of the major histocompatibility complex class II antigens affects the immune response at several levels, we wished to characterize the contribution of a particular alpha or beta chain in vivo using transgenic mice. We have established and characterized 12 lines of H-2s/s mice carrying from 1 to 65 copies of an Ak beta transgene. The transgene was coexpressed with the endogenous allele in a tissue-specific manner, and Ak beta mRNA expression correlated well with transgene copy number. High copy number (extreme overexpression) of the transgene was associated with a variety of defects, including a significant reduction in Ia cell-surface expression, a severe decrease in B-cell number, abnormal extramedullary granulopoiesis, and an increased susceptibility to infection. In this paper we describe in detail the phenotype associated with high copy numbers of the Ak beta transgene. The defects we have observed may be relevant to similar phenomena seen in other transgenic mice. In addition, these mice have fortuitously provided a system in which to assess the effect of various levels of class II cell-surface expression in the thymus on selection of the T-cell repertoire.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J., Campbell I. L., Morahan G., Mandel T. E., Harrison L. C., Miller J. F. Diabetes in transgenic mice resulting from over-expression of class I histocompatibility molecules in pancreatic beta cells. Nature. 1988 Jun 9;333(6173):529–533. doi: 10.1038/333529a0. [DOI] [PubMed] [Google Scholar]
- Bjorkman P. J., Saper M. A., Samraoui B., Bennett W. S., Strominger J. L., Wiley D. C. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987 Oct 8;329(6139):506–512. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- Brown J. H., Jardetzky T., Saper M. A., Samraoui B., Bjorkman P. J., Wiley D. C. A hypothetical model of the foreign antigen binding site of class II histocompatibility molecules. Nature. 1988 Apr 28;332(6167):845–850. doi: 10.1038/332845a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. W., Nolan J. A., Conrad P. J., Vasavada H. A., Vasavada H. H., Ploegh H. L., Ganguly S., Janeway C. A., Jr, Weissman S. M. Tissue-specific and cell surface expression of human major histocompatibility complex class I heavy (HLA-B7) and light (beta 2-microglobulin) chain genes in transgenic mice. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7690–7694. doi: 10.1073/pnas.85.20.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R. L., Weissman I. L. B220: a B cell-specific member of th T200 glycoprotein family. Nature. 1981 Feb 19;289(5799):681–683. doi: 10.1038/289681a0. [DOI] [PubMed] [Google Scholar]
- Flavell R. A., Allen H., Burkly L. C., Sherman D. H., Waneck G. L., Widera G. Molecular biology of the H-2 histocompatibility complex. Science. 1986 Jul 25;233(4762):437–443. doi: 10.1126/science.3726537. [DOI] [PubMed] [Google Scholar]
- Folsom V., Gay D., Tonegawa S. The beta 1 domain of the mouse E beta chain is important for restricted antigen presentation to helper T-cell hybridomas. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1678–1682. doi: 10.1073/pnas.82.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Ashwell J. D., Lechler R. I., Margulies D. H., Nickerson K. M., Suzuki G., Tou J. Y. "Exon-shuffling" maps control of antibody- and T-cell-recognition sites to the NH2-terminal domain of the class II major histocompatibility polypeptide A beta. Proc Natl Acad Sci U S A. 1985 May;82(9):2940–2944. doi: 10.1073/pnas.82.9.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain R. N., Bentley D. M., Quill H. Influence of allelic polymorphism on the assembly and surface expression of class II MHC (Ia) molecules. Cell. 1985 Nov;43(1):233–242. doi: 10.1016/0092-8674(85)90028-5. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Norcross M. A., Margulies D. H. Functional expression of a transfected murine class II MHC gene. Nature. 1983 Nov 10;306(5939):190–194. doi: 10.1038/306190a0. [DOI] [PubMed] [Google Scholar]
- Gilfillan S., Aiso S., Michie S. A., McDevitt H. O. The effect of excess beta-chain synthesis on cell-surface expression of allele-mismatched class II heterodimers in vivo. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7314–7318. doi: 10.1073/pnas.87.18.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goding J. W. Conjugation of antibodies with fluorochromes: modifications to the standard methods. J Immunol Methods. 1976;13(3-4):215–226. doi: 10.1016/0022-1759(76)90068-5. [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. Gene transfer into mouse embryos: production of transgenic mice by pronuclear injection. Methods Enzymol. 1983;101:411–433. doi: 10.1016/0076-6879(83)01031-9. [DOI] [PubMed] [Google Scholar]
- Greaves D. R., Wilson F. D., Lang G., Kioussis D. Human CD2 3'-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989 Mar 24;56(6):979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- Grosschedl R., Weaver D., Baltimore D., Costantini F. Introduction of a mu immunoglobulin gene into the mouse germ line: specific expression in lymphoid cells and synthesis of functional antibody. Cell. 1984 Oct;38(3):647–658. doi: 10.1016/0092-8674(84)90259-9. [DOI] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Hayakawa K., Hardy R. R., Parks D. R., Herzenberg L. A. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983 Jan 1;157(1):202–218. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzenberg L. A., Stall A. M., Braun J., Weaver D., Baltimore D., Herzenberg L. A., Grosschedl R. Depletion of the predominant B-cell population in immunoglobulin mu heavy-chain transgenic mice. Nature. 1987 Sep 3;329(6134):71–73. doi: 10.1038/329071a0. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney J. F., Cooper M. D., Klein J., Abney E. R., Parkhouse R. M., Lawton A. R. Ontogeny of Ia and IgD on IgM-bearing B lymphocytes in mice. J Exp Med. 1977 Jul 1;146(1):297–301. doi: 10.1084/jem.146.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Sun L., Watanabe T. Monoclonal rat antibodies to murine IgM determinants. J Immunol Methods. 1981;42(1):17–26. doi: 10.1016/0022-1759(81)90220-9. [DOI] [PubMed] [Google Scholar]
- Lang G., Wotton D., Owen M. J., Sewell W. A., Brown M. H., Mason D. Y., Crumpton M. J., Kioussis D. The structure of the human CD2 gene and its expression in transgenic mice. EMBO J. 1988 Jun;7(6):1675–1682. doi: 10.1002/j.1460-2075.1988.tb02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur M., Waltzinger C., Gerlinger P., Benoist C., Mathis D. Restricted assembly of MHC class II molecules in transgenic mice. J Immunol. 1989 Jan 1;142(1):323–327. [PubMed] [Google Scholar]
- Lechler R. I., Norcross M. A., Germain R. N. Qualitative and quantitative studies of antigen-presenting cell function by using I-A-expressing L cells. J Immunol. 1985 Nov;135(5):2914–2922. [PubMed] [Google Scholar]
- Ledbetter J. A., Herzenberg L. A. Xenogeneic monoclonal antibodies to mouse lymphoid differentiation antigens. Immunol Rev. 1979;47:63–90. doi: 10.1111/j.1600-065x.1979.tb00289.x. [DOI] [PubMed] [Google Scholar]
- Lo D., Burkly L. C., Widera G., Cowing C., Flavell R. A., Palmiter R. D., Brinster R. L. Diabetes and tolerance in transgenic mice expressing class II MHC molecules in pancreatic beta cells. Cell. 1988 Apr 8;53(1):159–168. doi: 10.1016/0092-8674(88)90497-7. [DOI] [PubMed] [Google Scholar]
- Matis L. A., Glimcher L. H., Paul W. E., Schwartz R. H. Magnitude of response of histocompatibility-restricted T-cell clones is a function of the product of the concentrations of antigen and Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6019–6023. doi: 10.1073/pnas.80.19.6019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matis L. A., Jones P. P., Murphy D. B., Hedrick S. M., Lerner E. A., Janeway C. A., Jr, McNicholas J. M., Schwartz R. H. Immune response gene function correlates with the expression of an Ia antigen. II. A quantitative deficiency in Ae:E alpha complex expression causes a corresponding defect in antigen-presenting cell function. J Exp Med. 1982 Feb 1;155(2):508–523. doi: 10.1084/jem.155.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNicholas J. M., Murphy D. B., Matis L. A., Schwartz R. H., Lerner E. A., Janeway C. A., Jr, Jones P. P. Immune response gene function correlates with the expression of an Ia antigen. I. Preferential association of certain Ae and E alpha chains results in a quantitative deficiency in expression of an Ae:E alpha complex. J Exp Med. 1982 Feb 1;155(2):490–507. doi: 10.1084/jem.155.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mond J. J., Kessler S., Finkelman F. D., Paul W. E., Scher I. Heterogeneity of Ia expression on normal B cells, neonatal B cells, and on cells from B cell-defective CBA/N mice. J Immunol. 1980 Apr;124(4):1675–1682. [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Parham P. Intolerable secretion in tolerant transgenic mice. Nature. 1988 Jun 9;333(6173):500–503. doi: 10.1038/333500a0. [DOI] [PubMed] [Google Scholar]
- Pierres M., Devaux C., Dosseto M., Marchetto S. Clonal analysis of B- and T-cell responses to Ia antigens. I. Topology of epitope regions on I-Ak and I-Ek molecules analyzed with 35 monoclonal alloantibodies. Immunogenetics. 1981 Dec;14(6):481–495. doi: 10.1007/BF00350120. [DOI] [PubMed] [Google Scholar]
- Sarvetnick N., Liggitt D., Pitts S. L., Hansen S. E., Stewart T. A. Insulin-dependent diabetes mellitus induced in transgenic mice by ectopic expression of class II MHC and interferon-gamma. Cell. 1988 Mar 11;52(5):773–782. doi: 10.1016/0092-8674(88)90414-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. Immune response (Ir) genes of the murine major histocompatibility complex. Adv Immunol. 1986;38:31–201. doi: 10.1016/s0065-2776(08)60006-1. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Stall A. M., Loken M. R. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol. 1984 Feb;132(2):787–795. [PubMed] [Google Scholar]