Abstract
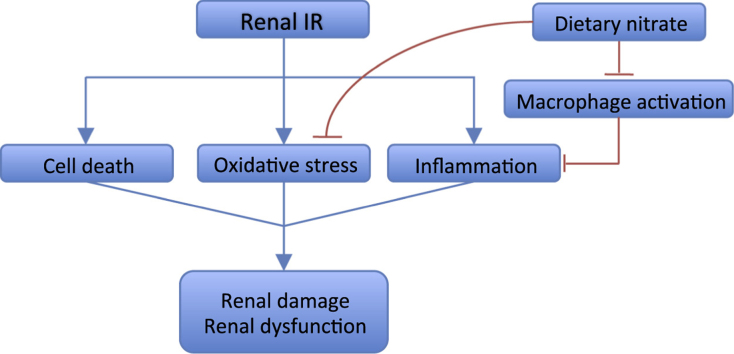
Ischemia-reperfusion (IR) injury involves complex pathological processes in which reduction of nitric oxide (NO) bioavailability is suggested as a key factor. Inorganic nitrate can form NO in vivo via NO synthase-independent pathways and may thus provide beneficial effects during IR. Herein we evaluated the effects of dietary nitrate supplementation in a renal IR model. Male mice (C57BL/6J) were fed nitrate-supplemented chow (1.0 mmol/kg/day) or standard chow for two weeks prior to 30 min ischemia and during the reperfusion period. Unilateral renal IR caused profound tubular and glomerular damage in the ischemic kidney. Renal function, assessed by plasma creatinine levels, glomerular filtration rate and renal plasma flow, was also impaired after IR. All these pathologies were significantly improved by nitrate. Mechanistically, nitrate treatment reduced renal superoxide generation, pro-inflammatory cytokines (IL-1β, IL-6 and IL-12 p70) and macrophage infiltration in the kidney. Moreover, nitrate reduced mRNA expression of pro-inflammatory cytokines and chemo attractors, while increasing anti-inflammatory cytokines in the injured kidney. In another cohort of mice, two weeks of nitrate supplementation lowered superoxide generation and IL-6 expression in bone marrow-derived macrophages. Our study demonstrates protective effect of dietary nitrate in renal IR injury that may be mediated via modulation of oxidative stress and inflammatory responses. These novel findings suggest that nitrate supplementation deserve further exploration as a potential treatment in patients at high risk of renal IR injury.
Abbreviations: BMDMs, bone marrow-derived macrophages; eNOS, endothelial NO synthase; NO3-, nitrate; NO2-, nitrite; NO, nitric oxide; NADPH, nicotinamide adenine dinucleotide phosphate; ROS, reactive oxygen species; O2•−, superoxide anion; IR, ischemia-reperfusion; AKI, acute kidney injury; GFR, glomerular filtration rate; RPF, renal plasma flow
Keywords: Acute kidney injury, Inflammation, Inorganic nitrate, NADPH oxidase, Nitric oxide, Oxidative stress
Graphical abstract
Highlights
-
•
Dietary nitrate ameliorates ischemia reperfusion (IR)-induced renal injuries and dysfunction.
-
•
Nitrate reduces oxidative stress and inflammatory responses in the IR kidney.
-
•
Nitrate lowers O2•− generation and IL-6 expression in bone marrow-derived macrophages.
1. Introduction
Renal ischemia-reperfusion (IR) injury is a common cause of acute kidney injury (AKI) in clinical scenarios such as kidney transplantation, cardiac bypass surgery and shock [1], [2]. A series of complex pathological changes are involved in renal IR injury including the initial sterile hypoxic/ischemic tissue damage, reperfusion-associated oxidative stress and immune cell activation, as well as microvascular dysfunction [3], [4], [5], [6]. Prolonged ischemia can cause acute tubular necrosis and glomerular damage which lead to delayed graft dysfunction and rejection after transplantation, chronic kidney disease, end-stage renal disease and increased mortality [7]. No effective pharmacological therapy is currently available for treating renal IR injury. Local and remote ischemic preconditioning was demonstrated to provide organ protection in subsequent IR injury [8], [9], [10]. However, the dose, type and safety of ischemic preconditioning in the clinic still require further investigation. Temporary local hypothermia was also shown to protect organs against IR injury [11], [12], [13], but the efficacy and clinical applications are limited.
Nitric oxide (NO) has been shown to play pivotal roles during organ IR injuries [14], [15], [16]. However, during ischemia the low tissue oxygen tension markedly reduces oxygen-dependent NO synthesis from endothelial NO synthase (eNOS) [17]. Further, overproduction of reactive oxygen species (ROS), especially superoxide (O2•−), through the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in the early reperfusion phase further consumes endogenous NO [18]. Reduced NO bioavailability further contributes to the endothelial and microvascular dysfunction that leads to the “no-reflow phenomenon” in the ischemic tissue after the initiation of reperfusion [19]. Failure to re-establish the blood flow to the injured kidney will affect the self-repair process of renal tubular cells and the restoration of kidney function [20], [21]. Effective and safe approaches that reduce oxidative stress, resolve inflammation, and maintain NO bioavailability may thus provide a novel strategy to prevent and treat renal IR injury.
The inorganic anions nitrate (NO3-) and nitrite (NO2-), derived either from our daily diet (e.g. green leafy vegetables) or from oxidization of endogenous NO, can be metabolized in vivo to form NO and other bioactive nitrogen oxides [22], [23]. In contrast to the NOSs, the NO3--NO2--NO pathway is not dependent on oxygen and l-arginine, and thus NO formation from this source is not affected during IR injury [24]. In fact, reduction of nitrate and nitrite to NO is greatly enhanced during hypoxia and low pH. The organ protective effects of nitrite in IR injury have been demonstrated in multiple organs such as liver [25], brain [26], lung [27], [28], and particularly in the heart [17], [25], [29]. However, the potential therapeutic value of inorganic nitrate and nitrite in kidney IR injury remains controversial. Tripatara and colleagues used a rat bilateral renal IR model and showed that topical, but not systemic administration of nitrite exerted reno-protective effects [30]. Another study by Basireddy and coworkers demonstrated that administration of nitrite intravenously (i.v) or intraperitoneally (i.p) before or at 22.5 min after induction of ischemia did not provide any beneficial effects in a rat model of unilateral nephrectomy and contralateral ischemia [31]. To date, there is limited knowledge about the effects of inorganic nitrate in renal IR injury. Several studies have demonstrated that dietary supplementation with nitrate has protective effects in experimental and clinical studies of renal and cardiovascular disease [23], [32], via mechanisms that involve restoration of NO signaling and/or reduction of oxidative stress [33], [34], [35], [36]. In addition, recent studies have suggested that anti-inflammatory properties of nitrate and nitrite may contribute substantially to the observed beneficial effects of these anions [37], [38], yet no study has evaluated the efficacy on renal IR.
In the clinical setting, ischemia often occurs in one kidney, however the post-injury total kidney function is not only dependent on the recovery of the ischemic kidney but also on compensatory functional changes of the contralateral kidney. In this study we thus utilized a mouse unilateral renal IR model to investigate the organ protective effect of dietary nitrate supplementation. We hypothesized that chronic intake of nitrate-enriched diet that boosts the NO3--NO2--NO pathway would provide kidney protection against IR injury via modulation of oxidative stress and immune cell activation.
2. Material and methods
2.1. Animals and experimental model
Male C57BL/6 J mice (10 weeks old) were obtained from Charles River (Sulzfeld Germany) and housed under temperature and humidity controlled environment with free access to rodent chow and tap water. All animal procedures were approved by the Stockholm Ethical Committee for Animal Experiments (Protocols: N139/15), and were carried out in accordance with the EU Directive 2010/63/EU for animal experiments. A schematic overview of the experimental protocol is available as Supplementary material.
After 1 week of acclimatization, mice were fed with either regular rodent diet (R34, Lantmannen, Sweden), or diet supplemented with sodium nitrate (NaNO3, 1.0 mmol/kg/day) for 2w before unilateral renal IR was established. During surgery, mice were anesthetized with isoflurane (1.5–2%, Forene; Abbott Scandinavia AB, Solna, Sweden). Buprenorphine (0.05 mg/kg, Schering-Plough, Brussels, Belgium) was injected subcutaneously before abdomen incision. The left kidney was then clamped with a micro serrefine (100 g clamping pressure, Fine Science Tools, Germany) for 30 min. The mice body temperature was controlled at 37 ± 0.5 °C throughout the surgery using a heating lamp and a self-monitored heating pad. After the surgery, mice were fed with the same diet they received prior surgery for 24 h or 2w until termination. Sham operation (exposing kidney without applying serrefine) did not induce changes regarding kidney histology or plasma cytokines.
2.2. Renal plasma flow (RPF) and glomerular filtration rate (GFR)
2w after reperfusion, RPF and GFR were determined in conscious mice by calculating the clearance of para-amino hippuric acid (PAH) and inulin as described previously [39]. In brief, premixed [14C]-PAH and [3H]-inulin (PerkinElmer, Waltham, MA, USA) were injected via tail vein in conscious mice and blood samples were collected through tail tip using heparinized capillary tubes at 1, 7, 15, 45 and 75 min following injection. Samples were centrifuged at 13,000g for 3 min at room temperature. PAH and inulin concentrations in plasma were determined using liquid scintillation and MicroBeta2 LumiJET 2460 Microplate Counter (PerkinElmer). Clearances were calculated using non-compartmental pharmacokinetic data analysis. RPF was estimated from the PAH clearance using a renal extraction ratio of 0.7.
2.3. Tissue harvest
Mice were anesthetized with isoflurane and blood samples were collected through inferior vena cava. Whole blood with 2 mmol EDTA (Sigma-Aldrich, Stockholm, Sweden) was centrifuged immediately at 4 °C for 7 min (6000g), plasma was collected and stored at −80 °C. Mice were then perfused transcardially with 35 ml ice-cold PBS. The transverse section of kidney including renal hilum (~3 mm) were post-fixed in 4% PBS buffered zinc-formaldehyde (HistoLab Products AB, Gothenburg, Sweden) at 4 °C for 24 h and cut into two pieces for paraffin and Tissue-Tek optimum cutting temperature (OCT) compound embedding respectively. The remaining kidney tissues were snap frozen in dry ice and stored at −80 °C until analysis.
2.4. Histological and stereological examination
Paraffin embedded kidney sections (5 µm thick) were stained with hematoxylin and eosin, as well as periodic acid-Schiff and picrosirius. Kidney injury scores (regarding necrosis, inflammatory cells infiltration, and fibrosis) were calculated essentially as described previously [39] by a pathologist in a blinded manner. The score correlates the severity of pathological change: from 0 represents normal histoarchitecture to 4 represents the most severe changes [34], [39]. The percentage of glomeruli with pathological changes (i.e., mesangial matrix increase, changes in glomerular basement membrane, sclerosis) were evaluated and graded as: 0 (0–10%), 1 (10–25%), 2 (25–40%), 3 (40–55%), and 4 (55–100%).
2.5. Immunohistochemistry staining
After fixation, the kidney tissues were dehydrated in 15% and 30% sucrose, then embedded in OCT compound (Sakura Finetek, USA). Cryostat sections were obtained transversely at 6 µm for immunohistochemistry staining of F4/80. In brief, the sections were washed in 0.1 mol/L PBS and quenched for 30 min with 0.3% hydrogen peroxide in 70% methanol. Sections were then blocked in 3% normal-donkey serum for 1 h at room temperature and incubated in biotin conjugated rat anti-mouse F4/80 (1:100; Isotype: IgG2b; Clone: CI: A3-1; AbD Serotec, USA) for 24 h at 4 °C. The staining was revealed with ABC reagent (standard Vectastain ABC Elite Kit, Vector Labs, Cambridge, UK) and 3,3′-diaminobenzidine (DAB) (Vector Labs). Photomicrographs were acquired with a digital microscope (Axio Scope, Zeiss, Oberkochen, Germany), the F4/80 quantification was calculated by grey scale using ImageJ. For each Section, 3 pictures were obtained randomly from renal cortex and renal medulla area respectively, the average grey scale of each area were used for final quantification.
2.6. Plasma creatinine
The renal function after 24 h reperfusion was evaluated by plasma creatinine using high-performance liquid chromatography tandem mass spectrometry (LC-MS/MS) as previously described [39]. Briefly, 25 µl of plasma were crashed with 225 µl of 0.2% formic acid in isopropanol containing d3-creatinine as internal standards, supernatant was then added into the LC-MS/MS system. Separation was performed with an ACQUITY UPLC System from Waters Corporation (Milford, MA, USA) using an Atlantis HILIC Silica 3 µm (150 × 2.1 mm) column from Waters. Mobile phases consisted of 0.2% formic acid in ACN: MeOH (75:25) and 0.2% formic acid in water. Detection was performed using a Waters Xevo® TQ triple quadrupole equipped with an Electrospray Ion Source working in positive mode. The SRM transition of creatinine (114.0→86.0) and d3-creatinin (117.0→89.0) were used for quantification.
2.7. Cytokines measurement
The inflammatory cytokines (IFN-γ, TNF-α, IL-1β, IL-6, IL-12, KC/GRO and IL-10) in plasma and kidney were detected using mouse pro-inflammatory 7-Plex Ultra-sensitive Kit from MesoScale Discovery (MSD, Rockville, MD, USA) following manufacture instructions [39].
2.8. RNA extraction and cDNA synthesis
Frozen tissue was homogenized in Trisol (Qiagen) using the TissueLyser (Qiagen) at 30 Hz for 2 min or until tissue was completely homogenized. RNA extractions were done using the classical phenol-chloroform method and purified using the RNeasy Lipid Tissue Mini Kit (Qiagen, Hilden, Germany). RNA-quantity and quality was determined by Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Welmington, DE, USA). Total RNA (1.8 μg) was reverse transcribed using the High Capacity RNA-to-CDNA Kit (Applied Biosystems, Foster City, CA, USA), according to the manufacturer's protocol.
2.9. Quantitative reverse transcription PCR (RT-qPCR)
PCR was performed on the 7900HT Fast Real-Time PCR System (Applied Biosystems) in 384-well plates using a final concentration of 1xTaqMan Universal PCR Master Mix (4304437), 1x TaqMan gene expression assay of TNF-α (Mm00443258_m1) and CCL2 (Mm00441242_m1), cDNA (pre-diluted 5x) and RNase free water to a total reaction volume of 10 µl, amplified using the following program: 50 °C for 120 s and 95 °C for 10, then 40 cycles of 95 °C for 15 s and 60 °C for 60 s. Samples were run in duplicates. NTC and no RT controls were included to control for contaminations. B2M (Mm00437762_m1) and HPRT (Mm03024075_m1) were used as reference genes.
2.10. Bone marrow-derived macrophages (BMDMs)
After 2 weeks regular or high nitrate diet feeding, BMDMs were isolated and cultured as previously described [39]. In brief, single-cell suspensions were prepared from femoral and tibia bone marrow, and cultured in Dulbecco's modified Eagle's medium (DMEM) (4.5 g/l glucose, Gibco, Life Technologies, UK) supplemented with 20% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mmol l-glutamine (all reagents from Life Technologies, Stockholm, Sweden) and 20% M-CSF conditioned L929 cell line (ATCC collection) culture medium for 8 days. Cells were then seeded in 12-well plates (1 × 106 cells/well) in DMEM (4.5 g/l glucose, 10% FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mmol l-glutamine) for 24 h and were collected for different analyses.
2.11. NADPH oxidase-mediated O2•− formation
NADPH oxidase-induced O2•− production was determined in both kidney cortex homogenate and BMDMs as previously described [39]. Kidney cortex was homogenized with Bullet Blender™ (Next Advance, Inc., Averill Park, NY, USA) in ice-cold PBS, centrifuged at 4 °C for 20 min (2000g) and the supernatant was collected. BMDMs were incubated in 37 °C DPBS for 20 min and detached by repeated pipetting. NADPH (100 μM, Sigma-Aldrich) and lucigenin (5 μM, Sigma-Aldrich) were added into the reaction tube containing tissue supernatant or BMDMs, O2•− production was determined by measuring lucigenin chemiluminescence every 3 s for 3 min with an AutoLumat LB953 Multi-Tube Luminometer (Berthold Technologies, Bad Wildbad, Germany). Protein was quantified using Bradford protein assay (Bio-Rad Laboratories, Solna, Sweden), and chemiluminescence results were corrected by protein content.
2.12. Endocytosis and intracellular cytokines in BMDMs
After 2w with regular or high nitrate diet feeding, BMDMs were isolated and cultured as previously described [39]. Endocytic potential of BMDMs was assessed by uptake of fluorescently labeled dextran (ThermoFisher, Sweden). Briefly, BMDMs were cultured in 12-well plate for 24 h then incubated with Alexa Fluor 647-labeled Dextran (1 μg/ml) for 30 min. After washing away the extra dextran with PBS, the cells were detached with 2 mM EDTA. Cells were run in a Gallios flow cytometer (Beckman Coulter, Brea, CA) and analyzed using Kaluza v1.1 software (Beckman Coulter).
The intracellular cytokines of BMDMs was also measured by flow cytometer. Briefly, after 24 h of culture, the cells were first incubated with GolgiPlug (1 µl/ml, BD Biosciences) in complete DMEM for 4 h at 37 °C before incubation with antibodies. Fixation/Permeablization and intracellular staining was conducted using the eBioscience Intracellular Staining Kit and the following antibodies: IL-6 (MP5-20F3, Biolegend), IL-1 beta pro-form (NJTEN3, eBioscience), and TNF alpha (MP6-XT22, Biolegend).
2.13. Statistical analysis
Single comparisons between two groups were tested for significance using the Student's paired or unpaired t-test as appropriate. Multiple comparisons among groups were analyzed by one- or two-way ANOVA followed by recommended post-hoc test.
Scored data from the histology evaluation was analyzed by the nonparametric Kruskal–Wallis test followed by the Dunn's multiple comparisons test. All statistical calculations were made using Graphpad Prism (6.0b, La Jolla, CA, USA). Values are presented as means ± SEM. Statistical significance was defined as p < 0.05.
3. Results
3.1. Dietary nitrate attenuates IR-induced kidney injury
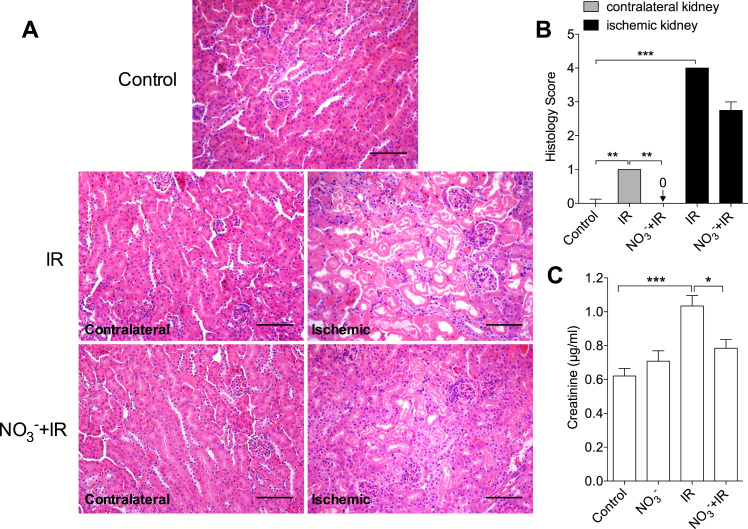
Following ischemia and 24 h reperfusion there were significant morphological changes in the affected kidney, including tubular necrosis and infiltration of inflammatory cells. Nitrate pretreatment tended to attenuate (p = 0.06) this IR-induced kidney damage (Fig. 1 A, B). Interestingly, IR injury was also associated with morphological abnormalities in the contralateral kidney in non-treated IR mice, which was not observed in the nitrate-treated group (Fig. 1 A, B).
Fig. 1.
Dietary nitrate reduces IR induced kidney injury at 24 h after reperfusion. (A) Representative histological pictures of kidney cortex. (B) Dietary nitrate significantly attenuated the IR associated increase of histology score in both ischemic and contralateral kidney at 24 h of reperfusion. (C) IR significantly increased plasma creatinine at 24 h only in the non-treated mice. Data in (B) are shown as median and interquartile range, data in (C) are shown as mean ± SEM. *, *** p < 0.05, 0.001 respectively, n = 6–10/group. Scale bar: 100 µm.
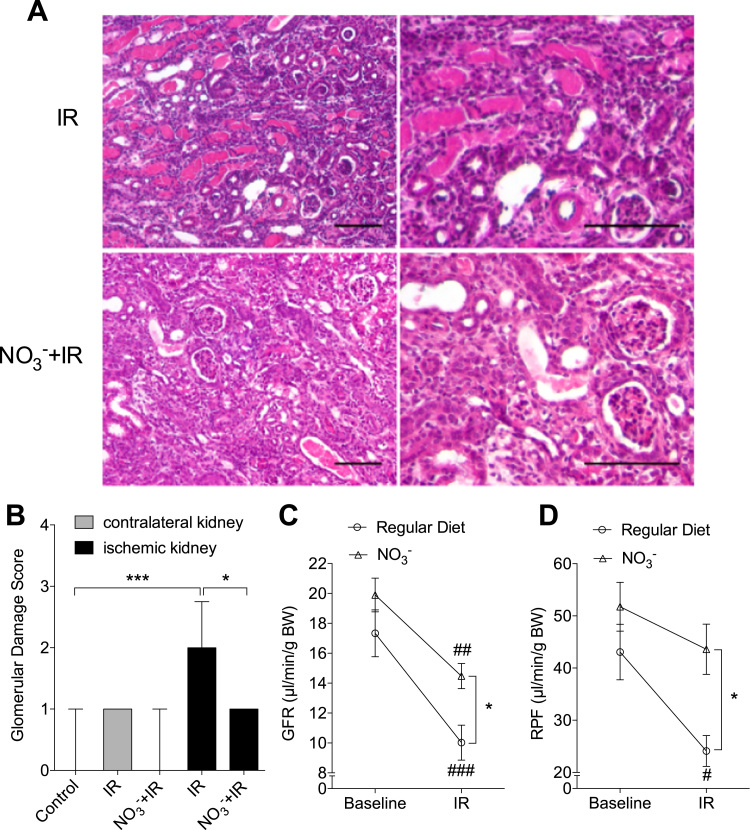
After two weeks (2w) following the ischemic insult the IR group displayed profound glomerular damages together with tubular necrosis and inflammation in the ischemic kidney. These pathological changes were significantly improved by nitrate treatment (Fig. 2 A, B). The histopathological score of the contralateral kidney also tended (p = 0.10) to be higher in the non-treated IR mice compared with the other groups at the same time point.
Fig. 2.
Dietary nitrate improves renal histology and function at 2w of reperfusion. (A) Representative histological pictures of kidney cortex. (B) IR was associated with significantly higher glomerular injury score at two weeks (2w) of reperfusion, which was attenuated by dietary nitrate. In addition, nitrate significantly preserved renal function (GFR and RPF) at 2w of reperfusion (C, D). Data in (B) are shown as median and interquartile range, data in (C, D) are shown as mean ± SEM. * p < 0.05, #, ##, ### p < 0.05, 0.01 and 0.001 vs baseline respectively, n = 8/group. Scale bar: 100 µm.
3.2. Dietary nitrate attenuates IR-induced reduction of glomerular perfusion and filtration
To further validate the IR-induced renal injury we measured renal function at different reperfusion time points. As expected, IR caused a significant increase of plasma creatinine levels at 24 h of reperfusion (1.03 ± 0.06 µg/ml) compared to controls (0.62 ± 0.04 µg/ml, p < 0.001), and this was prevented by nitrate pretreatment (0.79 ± 0.05 µg/ml, p < 0.05 vs IR group, Fig. 1 C). Baseline GFR and RPF were similar between non-treated and nitrate-treated mice.
At 2w of reperfusion IR injury was associated with significant reduction of GFR (17.3 ± 1.5 to 10.0 ± 1.2 µl/min/g BW, p < 0.001) and RPF (from 43.1 ± 5.3 to 24.1 ± 3.0 µl/min/g BW, p < 0.05) in non-treated mice. However, in nitrate-treated mice only GFR was significantly affected by IR injury (from 19.9 ± 1.1 to 14.5 ± 0.8 µl/min/g BW, p < 0.01). Hence, nitrate treatment improved post-IR GFR and RPF compared to that observed in the non-treated group (p<0.05 respectively, Fig. 2 C, D).
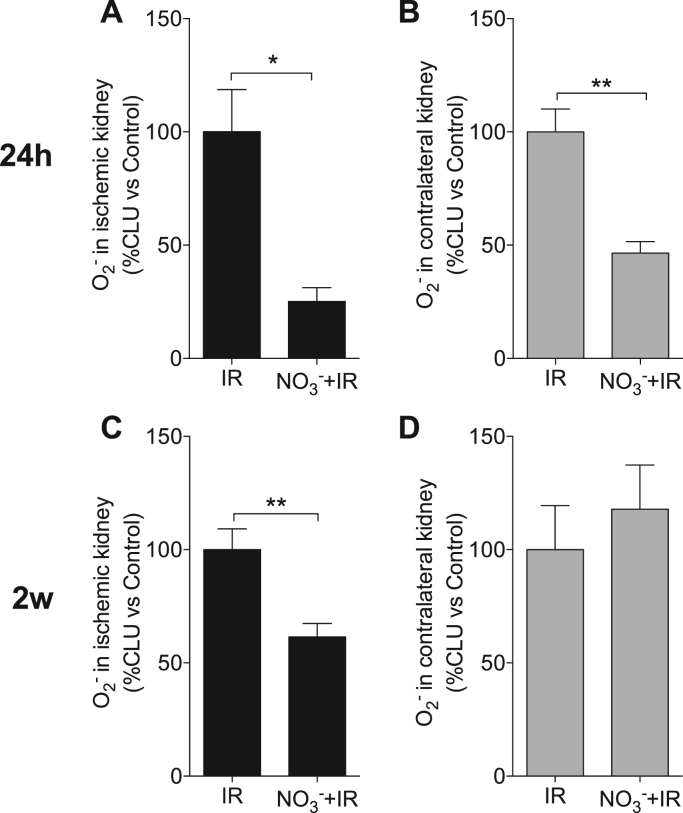
3.3. Dietary nitrate reduces IR-associated O2•− generation in the kidney
Oxidative stress is considered to critically contribute to IR-induced organ injury [40]. We compared the post-IR O2•− levels in both ischemic and contralateral kidneys between the groups. Following ischemia and 24 h reperfusion, nitrate-treated mice had 74.9 ± 6.1% lower O2•− production in the ischemic kidney and 53.5 ± 5.0% lower O2•− production in the contralateral kidney, compared to non-treated IR mice (p < 0.05 and 0.01, respectively Fig. 3 A, B).
Fig. 3.
Dietary nitrate reduces O2•−generation in the kidney. After renal ischemia, dietary nitrate-treated mice showed significantly lower NADPH oxidase-mediated superoxide (O2•−) generation in both the ischemic and contralateral kidney following 24 h of reperfusion (A, B), and in ischemic kidney at two weeks of reperfusion (2w) (C, D). Data are shown as mean ± SEM. *, ** p < 0.05 and 0.01 respectively, n = 6–10/group. CLU: chemiluminescence unit.
After 2w of reperfusion the O2•− level of the ischemic kidney from nitrate-treated mice was 38.5 ± 6.0% lower compared to the non-treated group (p < 0.01, Fig. 3 C), whereas no difference was observed in the contralateral kidneys between the groups (Fig. 3 D).
3.4. Dietary nitrate reduces IR-associated inflammatory responses
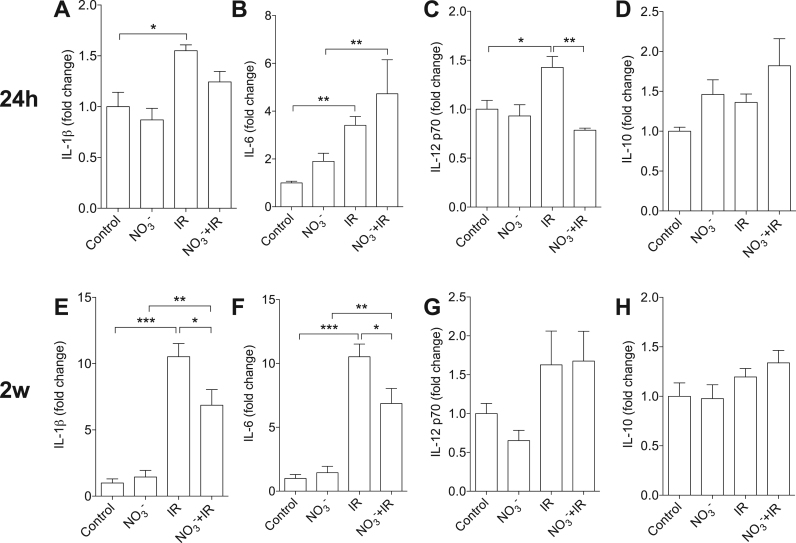
To determine IR injury-associated inflammation, systemic cytokine levels were measured. In non-treated IR mice ischemia followed by 24 h reperfusion was associated with significant elevation of plasma IL-1β (1.5 fold, p < 0.05, Fig. 4 A), IL-6 (3.4 fold, p < 0.01, Fig. 4 B) and IL-12 p70 (1.4 fold, p < 0.05, Fig. 4 C) compared to non-IR control mice. However, in nitrate-treated mice only IL-6 was significantly increased in the plasma at this time point (4.7 fold, p < 0.01, Fig. 4 B). In addition, the plasma IL-12 p70 level at 24 h of reperfusion was significantly lower in nitrate-treated mice compared to non-treated IR mice (p < 0.01, Fig. 4 C).
Fig. 4.
Dietary nitrate modulates circulating cytokines after IR injury. After renal ischemia, the levels of circulating IL-1β (A), IL-6 (B) and IL-12 p70 (C) were significantly higher compared to non-IR controls following 24 h of reperfusion. Dietary nitrate treatment abolished the increase of IL-12 p70 at 24 h (C). No significant changes were observed for IL-10 level at 24 h (D). IR injury was associated with significantly higher plasma IL-1β (E) and IL-6 (F) at two weeks (2w) of reperfusion, and the levels of these cytokines were significantly lower in nitrate-treated mice compared to the non-treated group (E, F). No statistical differences were observed for plasma IL-12 p70 and IL-10 at 2w of reperfusion (G, H). Data are shown as mean ± SEM. *, **, *** p < 0.05, 0.01 and 0.001 respectively, n = 6–8/group.
At 2w of reperfusion plasma IL-1β and IL-6 levels were significantly higher compared to non-IR controls, but the nitrate-treated mice had significantly lower IL-1β and IL-6 compared to the non-treated mice (p < 0.05 respectively, Fig. 4 E, F). Plasma IL-12 p70 levels were similar among the groups at 2w of reperfusion (Fig. 4 G). Plasma IL-10 levels tended to be higher in nitrate-treated mice at both 24 h and 2w of reperfusion, but the differences were not statistically significant (Fig. 4 D, H). No differences were observed in plasma INF-γ, TNF-α, and KC/GRO levels (data not shown).
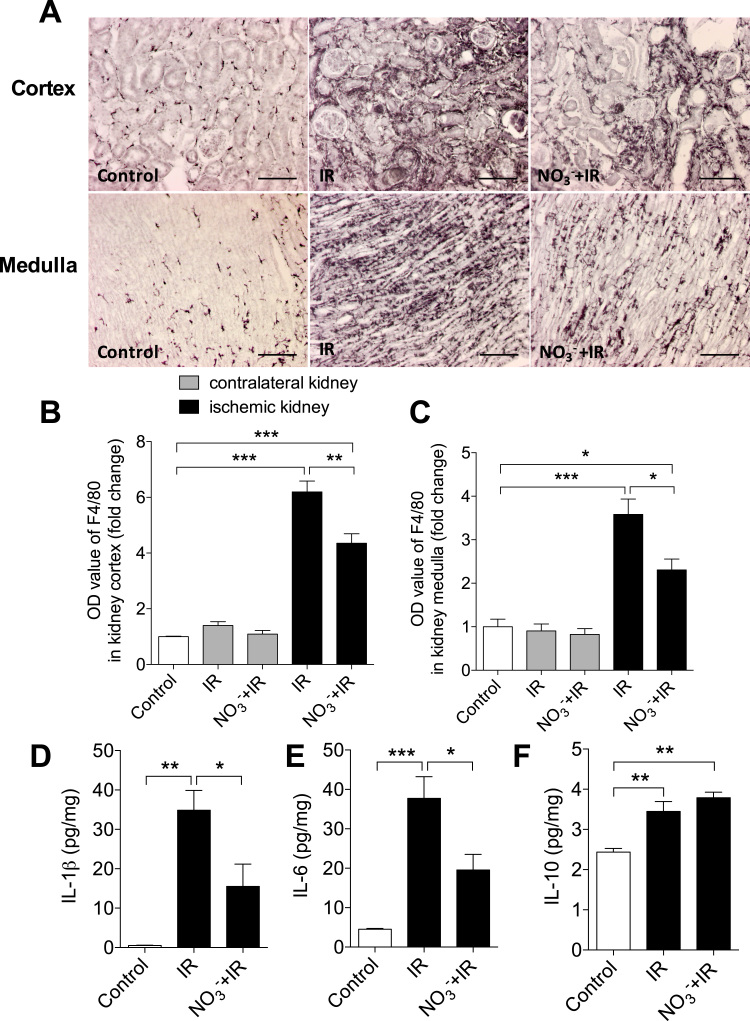
Next we validated the local inflammatory changes in the kidney following IR injury. We analyzed macrophage infiltration using F4/80 immunostaining and measured cytokine levels in ischemic kidneys following 2w of reperfusion. There was substantial infiltration of macrophages in the cortex and medulla of the ischemic kidney (Fig. 5 A). The severity of macrophage infiltration was significantly attenuated in the nitrate-treated mice (Fig. 5 A-C). No obvious macrophage infiltration was observed in the contralateral kidneys 2w after the ischemic insult. Compared to non-IR controls the ischemic kidney from non-treated mice had significantly higher IL-1β (0.5 ± 0.1 vs 34.9 ± 5.1 pg/mg, p < 0.01) and IL-6 (4.5 ± 0.2 vs 37.7 ± 5.5 pg/mg, p < 0.001) levels. In nitrate-treated mice these two pro-inflammatory cytokines were significantly lower (IL-1β: 15.5 ± 5.7 pg/mg, IL-6: 19.5 ± 4.0 pg/mg, p < 0.05 vs IR respectively, Fig. 5 D, E). IR induced similar elevation of IL-10 levels in the ischemic kidney in nitrate-treated (3.7 ± 0.1 pg/mg) and non-treated mice (2.4 ± 0.1 pg/mg, Fig. 5 F).
Fig. 5.
Dietary reduces inflammatory responses in the kidney. (A) Representative immunohistochemistry (F4/80) pictures of macrophage infiltration of the renal cortex and and the medulla. Dietary nitrate significantly reduced IR-induced macrophage infiltration in both kidney cortex and medulla at two weeks (2w) (B, C). In addition, IR was associated with significantly increased levels of IL-1β (D) and IL-6 (E) in the kidney at 2w of reperfusion, compared to non-IR controls, which were both attenuated by dietary nitrate treatment. However, dietary nitrate did not influence the IR injury-associated increase of IL-10 in the kidney (F). Data are shown as mean ± SEM. *, **, *** p < 0.05, 0.01 and 0.001 respectively, n = 6–8/group.
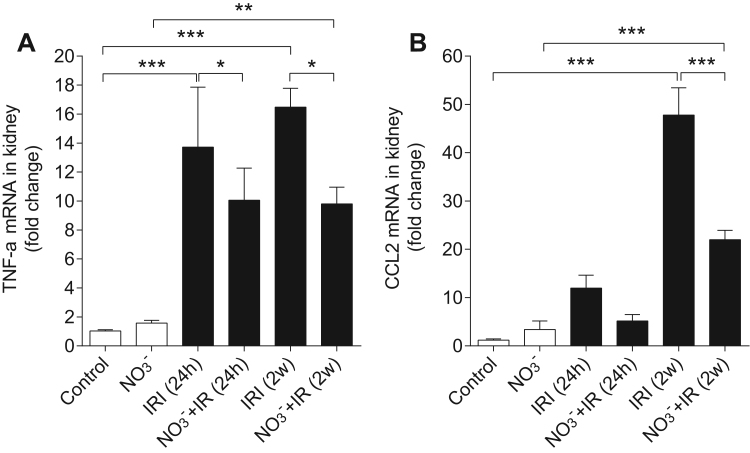
We then compared mRNA expression levels of TNF-α and CCL2 in the kidney at both 24 h and 2w after reperfusion. IR injury was associated with increased TNF-α at both time points (p < 0.001 vs control, respectively), which were significantly attenuated by nitrate treatment (p < 0.05 vs IR, respectively, Fig. 6 A). Similarly, nitrate significantly reduced IR-induced increase of CCL2 expression at 2w of reperfusion (p < 0.001, Fig. 6 B).
Fig. 6.
Dietary nitrate reduces TNF-α and CLL2 mRNA expression in kidney. IR induce significant increase of TNF-α mRNA expression at both 24 h and 2w of reperfusion. These increases were attenuated by dietary nitrate treatment (A). The CCL2 mRNA expression was significant increased at 2w after IR, which was also attenuated by dietary nitrate treatment (B). Data are shown as mean ± SEM. *, **, *** p < 0.05, 0.01 and 0.001 respectively, n = 6–8/group.
3.5. Nitrate diet alters the phenotype of BMDMs
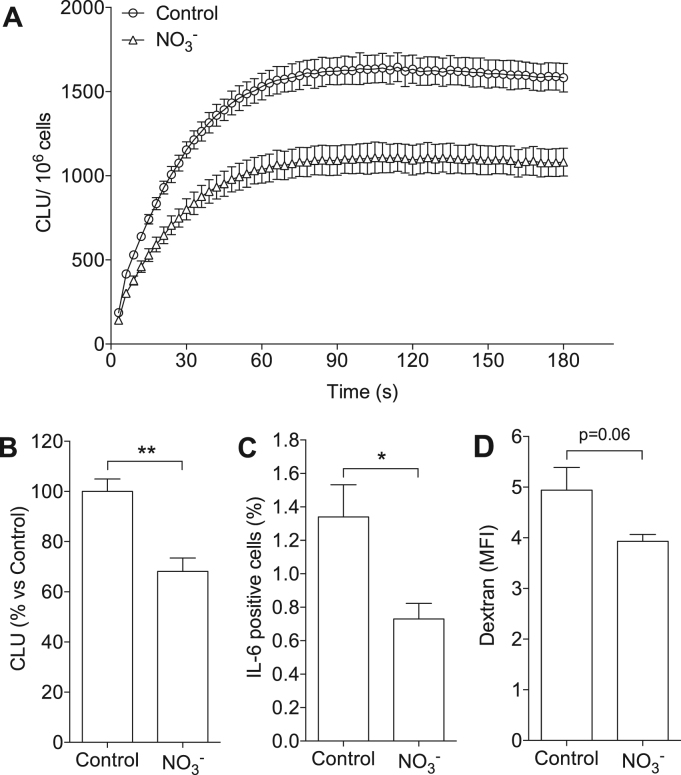
Next we investigated whether dietary treatment with nitrate influenced macrophage phenotype. Bone marrow cells from mice fed with nitrate-supplemented or regular chow were differentiated into macrophages in vitro, and O2•− generation, levels of intracellular cytokines and endocytic function were measured. BMDMs isolated from nitrate-treated mice had 32 ± 5% lower O2•− generation compared to control (i.e. BMDMs from mice fed with regular diet) (p < 0.01, Fig. 7 A, B). The proportion of IL-6+ cells, as determined by flow cytometry, was also significantly lower in the BMDMs isolated from nitrate-treated mice compared to controls (1.3 ± 0.2 vs 0.7 ± 0.09%, p < 0.05, Fig. 7 C). The expression of intracellular IL-1β and TNF-α were similar between groups (data not shown). There was also a trend of reduced dextran endocytosis in the BMDMs from nitrate-treated mice compared with controls (Fig. 7 D).
Fig. 7.
Dietary nitrate affects the phenotype of primary macrophages. BMDMs isolated from mice fed with high nitrate diet showed significant lower NADPH oxidase-derived superoxide (O2•−) generation (A, B) and a lower proportion of IL-6+ cells (C). The endocytosis function, indicated by the uptake of fluorescent-labeled dextran, also trended to be reduced in BMDMs from nitrate-treated mice compared with controls (D). Data are shown as mean ± SEM. *, **, p < 0.05 and 0.01 respectively, n = 4/group in panel A, B, n = 6/group in panel C, D. CLU: chemiluminescence unit.
4. Discussion
In this study we demonstrate that dietary nitrate supplementation significantly reduces the tubular and glomerular damages as well as the loss of renal function following IR injury. These salutary effects are coupled to an altered inflammatory response with reduced generation of ROS and changes in macrophage function. This is the first description of the beneficial effects of nitrate-enriched diet in protecting the kidney against AKI and associated renal complications after an ischemia-reperfusion insult.
In mammals, inorganic nitrate from dietary sources can be reduced to nitrite by commensal bacteria in the oral cavity, and then nitrite is subsequently swallowed, absorbed in the gut and further metabolized to NO and other bioactive nitrogen oxides in blood and tissues through multiple pathways [23], [41]. The NO3--NO2--NO pathway is greatly enhanced in conditions with ischemia and hypoxia when NOS-dependent NO generation is compromised [22], [23]. In an ex vivo setting we have previously reported that the renal microvasculature is exquisitely responsive to nitrite-mediated vasodilatation [35] and that the effects of nitrite are significantly enhanced at lower pH and oxygen tensions mimicking the in vivo ischemic environment [42]. Dietary sources of nitrate can thus serve as an ideal alternative NO progenitor pool during tissue IR injury. Our study indicates that chronic intake of nitrate-supplemented diet attenuates IR-induced tubular necrosis at 24 h. By 2w of reperfusion the mice fed with nitrate diet showed significantly improved tubular repair and less glomerular damage compared to non-treated mice.
Previous studies have demonstrated that AKI can occur following injuries in remote organs [43], [44], [45]. Similarly, we found that IR injury in one kidney also caused mild morphological abnormalities in the contralateral kidney of non-treated mice, whereas this was not observed in nitrate-treated animals. These changes in the contralateral kidneys occurred at an early post reperfusion time point (24 h), persisted for at least 2w and are likely mediated through circulating toxic factors as well as inflammatory cytokines and oxidative stress [46]. Along with the effects of reducing renal structural damage the nitrate-enriched diet preserved kidney function after IR injury. At 24 h, plasma creatinine levels in non-treated mice were significantly increased compared to non-IR controls and nitrate-IR mice. Because plasma creatinine usually returns to normal levels one week after reperfusion [47], [48], we measured GFR and RPF at 2w to evaluate renal function at this delayed reperfusion time point. Our data indicate that both the GFR and RPF were significantly improved in nitrate-treated mice compared to non-treated mice.
The overall improved post-IR kidney function in nitrate-treated mice may be due to attenuation of renal pathological changes in both the ischemic and contralateral kidneys. Although two previous studies have shown that systemic administration of nitrite did not protect against renal IR injury in rats [30], [31] the conditions of our present study are quite different. We used chronic dietary nitrate treatment instead of a single dose of nitrite and demonstrated that daily intake of nitrate can provide protection against subsequent renal IR injury. This is a clinically relevant scenario and suggests that patients with high risk of renal IR injury may benefit from a high nitrate diet. We cannot exclude that a shorter period of nitrate intake before and/or after the insult could have had similar effects, although this is unlikely considering the alteration in the bone marrow-derived immune cells which requires some time. When comparing the current results to earlier studies one should also note that the pharmacokinetics of nitrite and nitrate are vastly different, with nitrate having a substantially longer half-life, resulting in a prolonged low grade formation of nitrite, NO and other possibly bioactive nitrogen oxides. Conversely, nitrite peaks rapidly and declines at an equally high pace. In addition, earlier studies using nitrite in other models of IR injury have shown that the dose-response curve for this anion is U-shaped with loss of protection at higher doses [25], [26].
Our previous studies in models of hypertension have demonstrated that dietary nitrate exerts renal and cardiovascular protective effects through increased NO bioavailability and reduction of oxidative stress [34], [35]. During IR the oxygen burst during the early reperfusion phase leads to profound ROS generation, which is a key contributor to the reperfusion-associated tissue injury [19]. In this study we found that dietary nitrate significantly reduced NADPH-dependent O2•− generation in the ischemic kidney up to 2w. In the contralateral kidney, O2•− levels were also significant lower in the nitrate-treated group compared to non-treated animals up to 24 h post-reperfusion. These data further support the anti-oxidative properties of dietary nitrate and suggest that the protective effects of nitrate may at least in part be mediated through reduction of NADPH oxidase activity and O2•− generation.
Apart from oxidative stress the inflammatory response following sterile cell death is another pivotal biological process during IR injury [19]. The immune responses with inflammatory cells infiltrating in the ischemic tissue are critical mechanisms for cellular debris clearance and adequate tissue repair. However, prolonged and unresolved inflammation can further exacerbate tissue injury [5], [6], [19], [49]. In order to understand if nitrate can alter inflammatory responses during IR injury we measured several circulating cytokines. We determined that at 24 h only IL-12 p70 was significantly reduced in the nitrate-treated mice compared to the non-treated group. However, by 2w nitrate-treated mice showed lower plasma IL-1β and IL-6 levels. IL-12 p70 is produced by multiple immune cells, in particular dendritic cells (DCs) [50] in response to antigen stimulation and it is involved in initiating T cell activation [51]. Kidney-resident DCs can excrete IL-12 following renal IR injury and trigger downstream innate and adaptive immune responses [52]. IL-1β and IL-6 at delayed reperfusion time points are dominantly released by activated macrophage in the ischemic tissue and related to the severity of structural damage [53], [54]. To further validate the inflammation in the kidney we detected macrophage infiltration using F4/80 staining as well as measuring local cytokine production in the ischemic kidney at 2w of reperfusion. Macrophage infiltration was significantly reduced in nitrate-treated animals. This is in agreement with a recently published article by Ahluwalia and colleagues who found reduced macrophage accumulation in atherosclerotic plaques after dietary nitrate in apolipoprotein (Apo) E knockout (KO) mice [38].
In accordance with the circulating cytokine levels in our study, IR injury was associated with increased IL-1β and IL-6 production in the ischemic kidney, as well as TNF-α and CCL2 mRNA expression. CCL2 is considered to play an important role in recruiting monocytes, T cells and DCs to the inflammatory sites [55]. We also observed significant infiltration of macrophages in the ischemic kidney following 2w of reperfusion. These elevations in local pro-inflammatory cytokines and macrophage infiltration were significantly attenuated by dietary nitrate. Taken together, our data demonstrate an anti-inflammatory role of dietary nitrate in protecting the kidney against IR injury. This is in agreement with our previous findings that dietary nitrate decrease leukocyte recruitment during intestinal injury [56]. IL-10 is important for limiting the IR injury and facilitating tissue repair [57], [58]. However, nitrate did not affect the levels of this cytokine in the ischemic kidney at 2w.
Our previous work showed that nitrite can modulate macrophage phenotype and function in response to lipopolysaccharide stimulation in vitro [37]. In the current study we demonstrate that dietary supplementation with nitrate can also cause significant reduction of O2•− generation in primary BMDMs. Interestingly, this was an intrinsic effect of nitrate that occurred in the absence of IR injury. In addition, the intracellular IL-6 expression in BMDMs from nitrate-treated mice was decreased. Although BMDMs from nitrate-treated mice also tended to have reduced endocytic function (p = 0.06 vs control), our previous work demonstrated that inorganic nitrate and nitrite do not influence the in vivo bacterial clearance function [56]. However, the exact in vivo endocytic function of macrophages following dietary nitrate treatment requires further investigation. This direct impact of dietary nitrate on BMDMs phenotypes cannot be simply attributed to the generation of NO via the NO3--NO2--NO pathway due to the short physiological half-life of NO [59]. The underlying mechanisms of how dietary nitrate modulates the immune system is a critical question that warrants further study. Addressing this question may add novel mechanisms to the organ- protective effects of dietary nitrate and nitrite.
In conclusion, oral intake of inorganic nitrate can effectively protect the kidney against IR injury. This protection by nitrate is coupled to phenotype changes in bone marrow-derived immune cells and anti-oxidative as well as anti-inflammatory properties of nitrate. Future clinical trials shall elucidate if nitrate-supplementation can provide beneficial effects in patients with high risk of acquiring renal IR injury.
Sources of funding
The work was supported by Swedish Heart and Lung Foundation (Dnr: 20140448), the Swedish Research Council (Dnr: 2016-01381), and by KID-funding from the Karolinska Institutet (Dnr 2415/2012-225 and Dnr 2–3707/2013).
Author contributions
TY, MC designed the research studies, conducted experiments, analyzed the data, drafted and edited the manuscript, and approved the final version. XMY, LT, MP, ZZ, NT conceived or designed aspects of the research work, acquired data, and played important role in interpreting the results. RAH, PSO, EL, AEG contributed to the design of the research studies, offered insight in data analysis, and edited the manuscript. JOL, EW contributed to the design of the research studies, offered insight in data analysis, and drafted and edited the manuscript. MC and TY are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgements
We thank Carina Nihlén, Annika Olsson and Margareta Stensdotter (Karolinska Institutet, Stockholm) for their technical assistance.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2017.06.002.
Contributor Information
Ting Yang, Email: ting.yang2@duke.edu.
Mattias Carlstrom, Email: mattias.carlstrom@ki.se.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Thadhani R., Pascual M., Bonventre J.V. Acute renal failure. N. Engl. J. Med. 1996;334:1448–1460. doi: 10.1056/NEJM199605303342207. [DOI] [PubMed] [Google Scholar]
- 2.Bellomo R., Kellum J.A., Ronco C. Acute kidney injury. Lancet. 2012;380:756–766. doi: 10.1016/S0140-6736(11)61454-2. [DOI] [PubMed] [Google Scholar]
- 3.Bonventre J.V., Yang L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011;121:4210–4221. doi: 10.1172/JCI45161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edelstein C.L., Ling H., Schrier R.W. The nature of renal cell injury. Kidney Int. 1997;51:1341–1351. doi: 10.1038/ki.1997.183. [DOI] [PubMed] [Google Scholar]
- 5.Bonventre J.V. Mechanisms of ischemic acute renal failure. Kidney Int. 1993;43:1160–1178. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- 6.Bonventre J.V., Zuk A. Ischemic acute renal failure: an inflammatory disease? Kidney Int. 2004;66:480–485. doi: 10.1111/j.1523-1755.2004.761_2.x. [DOI] [PubMed] [Google Scholar]
- 7.Liano F., Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int. 1996;50:811–818. doi: 10.1038/ki.1996.380. [DOI] [PubMed] [Google Scholar]
- 8.Joo J.D., Kim M., D'Agati V.D., Lee H.T. Ischemic preconditioning provides both acute and delayed protection against renal ischemia and reperfusion injury in mice. J. Am. Soc. Nephrol. 2006;17:3115–3123. doi: 10.1681/ASN.2006050424. [DOI] [PubMed] [Google Scholar]
- 9.Hausenloy D.J., Yellon D.M. Ischaemic conditioning and reperfusion injury. Nat. Rev. Cardiol. 2016;13:193–209. doi: 10.1038/nrcardio.2016.5. [DOI] [PubMed] [Google Scholar]
- 10.Zimmerman R.F., Ezeanuna P.U., Kane J.C., Cleland C.D., Kempananjappa T.J., Lucas F.L., Kramer R.S. Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney Int. 2011;80:861–867. doi: 10.1038/ki.2011.156. [DOI] [PubMed] [Google Scholar]
- 11.Zager R.A., Gmur D.J., Bredl C.R., Eng M.J. Degree and time sequence of hypothermic protection against experimental ischemic acute renal failure. Circ. Res. 1989;65:1263–1269. doi: 10.1161/01.res.65.5.1263. [DOI] [PubMed] [Google Scholar]
- 12.De Rosa S., Antonelli M., Ronco C. Hypothermia and kidney: a focus on ischaemia-reperfusion injury. Nephrol. Dial. Transplant. 2016 doi: 10.1093/ndt/gfw038. [DOI] [PubMed] [Google Scholar]
- 13.Kanemoto S., Matsubara M., Noma M., Leshnower B.G., Parish L.M., Jackson B.M., Hinmon R., Hamamoto H., Gorman J.H., 3rd, Gorman R.C. Mild hypothermia to limit myocardial ischemia-reperfusion injury: importance of timing. Ann. Thorac. Surg. 2009;87:157–163. doi: 10.1016/j.athoracsur.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schulz R., Kelm M., Heusch G. Nitric oxide in myocardial ischemia/reperfusion injury. Cardiovasc Res. 2004;61:402–413. doi: 10.1016/j.cardiores.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 15.Meade M.O., Granton J.T., Matte-Martyn A., McRae K., Weaver B., Cripps P., Keshavjee S.H., Toronto Lung Transplant P. A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am. J. Respir. Crit. Care Med. 2003;167:1483–1489. doi: 10.1164/rccm.2203034. [DOI] [PubMed] [Google Scholar]
- 16.Peralta C., Jimenez-Castro M.B., Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J. Hepatol. 2013;59:1094–1106. doi: 10.1016/j.jhep.2013.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Webb A., Bond R., McLean P., Uppal R., Benjamin N., Ahluwalia A. Reduction of nitrite to nitric oxide during ischemia protects against myocardial ischemia-reperfusion damage. Proc. Natl. Acad. Sci. USA. 2004;101:13683–13688. doi: 10.1073/pnas.0402927101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weitzberg E., Hezel M., Lundberg J.O. Nitrate-nitrite-nitric oxide pathway: implications for anesthesiology and intensive care. Anesthesiology. 2010;113:1460–1475. doi: 10.1097/ALN.0b013e3181fcf3cc. [DOI] [PubMed] [Google Scholar]
- 19.Eltzschig H.K., Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffield J.S., Bonventre J.V. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005;68:1956–1961. doi: 10.1111/j.1523-1755.2005.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffield J.S., Park K.M., Hsiao L.L., Kelley V.R., Scadden D.T., Ichimura T., Bonventre J.V. Restoration of tubular epithelial cells during repair of the postischemic kidney occurs independently of bone marrow-derived stem cells. J. Clin. Investig. 2005;115:1743–1755. doi: 10.1172/JCI22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundberg J.O., Gladwin M.T., Ahluwalia A., Benjamin N., Bryan N.S., Butler A., Cabrales P., Fago A., Feelisch M., Ford P.C., Freeman B.A., Frenneaux M., Friedman J., Kelm M., Kevil C.G., Kim-Shapiro D.B., Kozlov A.V., Lancaster J.R., Jr, Lefer D.J., McColl K., McCurry K., Patel R.P., Petersson J., Rassaf T., Reutov V.P., Richter-Addo G.B., Schechter A., Shiva S., Tsuchiya K., van Faassen E.E., Webb A.J., Zuckerbraun B.S., Zweier J.L., Weitzberg E. Nitrate and nitrite in biology, nutrition and therapeutics. Nat. Chem. Biol. 2009;5:865–869. doi: 10.1038/nchembio.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lundberg J.O., Weitzberg E., Gladwin M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 24.Zweier J.L., Wang P., Samouilov A., Kuppusamy P. Enzyme-independent formation of nitric oxide in biological tissues. Nat. Med. 1995;1:804–809. doi: 10.1038/nm0895-804. [DOI] [PubMed] [Google Scholar]
- 25.Duranski M.R., Greer J.J., Dejam A., Jaganmohan S., Hogg N., Langston W., Patel R.P., Yet S.F., Wang X., Kevil C.G., Gladwin M.T., Lefer D.J. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J. Clin. Investig. 2005;115:1232–1240. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung K.H., Chu K., Ko S.Y., Lee S.T., Sinn D.I., Park D.K., Kim J.M., Song E.C., Kim M., Roh J.K. Early intravenous infusion of sodium nitrite protects brain against in vivo ischemia-reperfusion injury. Stroke. 2006;37:2744–2750. doi: 10.1161/01.STR.0000245116.40163.1c. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto T., Tang X., Janocha A., Farver C.F., Gladwin M.T., McCurry K.R. Nebulized nitrite protects rat lung grafts from ischemia reperfusion injury. J. Thorac. Cardiovasc. Surg. 2013;145:1108–1116. doi: 10.1016/j.jtcvs.2012.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Sugimoto R., Okamoto T., Nakao A., Zhan J., Wang Y., Kohmoto J., Tokita D., Farver C.F., Tarpey M.M., Billiar T.R., Gladwin M.T., McCurry K.R. Nitrite reduces acute lung injury and improves survival in a rat lung transplantation model. Am. J. Transplant. 2012;12:2938–2948. doi: 10.1111/j.1600-6143.2012.04169.x. [DOI] [PubMed] [Google Scholar]
- 29.Omar S.A., Webb A.J., Lundberg J.O., Weitzberg E. Therapeutic effects of inorganic nitrate and nitrite in cardiovascular and metabolic diseases. J. Intern. Med. 2016;279:315–336. doi: 10.1111/joim.12441. [DOI] [PubMed] [Google Scholar]
- 30.Tripatara P., Patel N.S., Webb A., Rathod K., Lecomte F.M., Mazzon E., Cuzzocrea S., Yaqoob M.M., Ahluwalia A., Thiemermann C. Nitrite-derived nitric oxide protects the rat kidney against ischemia/reperfusion injury in vivo: role for xanthine oxidoreductase. J. Am. Soc. Nephrol. 2007;18:570–580. doi: 10.1681/ASN.2006050450. [DOI] [PubMed] [Google Scholar]
- 31.Basireddy M., Isbell T.S., Teng X., Patel R.P., Agarwal A. Effects of sodium nitrite on ischemia-reperfusion injury in the rat kidney. Am. J. Physiol. Ren. Physiol. 2006;290:F779–F786. doi: 10.1152/ajprenal.00334.2005. [DOI] [PubMed] [Google Scholar]
- 32.Lundberg J.O., Carlstrom M., Larsen F.J., Weitzberg E. Roles of dietary inorganic nitrate in cardiovascular health and disease. Cardiovasc. Res. 2011;89:525–532. doi: 10.1093/cvr/cvq325. [DOI] [PubMed] [Google Scholar]
- 33.Kapil V., Khambata R.S., Robertson A., Caulfield M.J., Ahluwalia A. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320–327. doi: 10.1161/HYPERTENSIONAHA.114.04675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ridderstrale W., Saluveer O., Carlstrom M., Jern S., Hrafnkelsdottir T.J. The impaired fibrinolytic capacity in hypertension is unaffected by acute blood pressure lowering. J. Thromb. Thrombolysis. 2011;32:399–404. doi: 10.1007/s11239-011-0595-4. [DOI] [PubMed] [Google Scholar]
- 35.Gao X., Yang T., Liu M., Peleli M., Zollbrecht C., Weitzberg E., Lundberg J.O., Persson A.E., Carlstrom M. NADPH oxidase in the renal microvasculature is a primary target for blood pressure-lowering effects by inorganic nitrate and nitrite. Hypertension. 2015;65:161–170. doi: 10.1161/HYPERTENSIONAHA.114.04222. [DOI] [PubMed] [Google Scholar]
- 36.Hezel M., Peleli M., Liu M., Zollbrecht C., Jensen B.L., Checa A., Giulietti A., Wheelock C.E., Lundberg J.O., Weitzberg E., Carlstrom M. Dietary nitrate improves age-related hypertension and metabolic abnormalities in rats via modulation of angiotensin II receptor signaling and inhibition of superoxide generation. Free Radic. Biol. Med. 2016;99:87–98. doi: 10.1016/j.freeradbiomed.2016.07.025. [DOI] [PubMed] [Google Scholar]
- 37.Yang T., Peleli M., Zollbrecht C., Giulietti A., Terrando N., Lundberg J.O., Weitzberg E., Carlstrom M. Inorganic nitrite attenuates NADPH oxidase-derived superoxide generation in activated macrophages via a nitric oxide-dependent mechanism. Free Radic. Biol. Med. 2015;83:159–166. doi: 10.1016/j.freeradbiomed.2015.02.016. [DOI] [PubMed] [Google Scholar]
- 38.Khambata R.S., Ghosh S.M., Rathod K.S., Thevathasan T., Filomena F., Xiao Q., Ahluwalia A. Antiinflammatory actions of inorganic nitrate stabilize the atherosclerotic plaque. Proc. Natl. Acad. Sci. USA. 2017;114:E550–E559. doi: 10.1073/pnas.1613063114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang T., Zollbrecht C., Winerdal M.E., Zhuge Z., Zhang X.M., Terrando N., Checa A., Sallstrom J., Wheelock C.E., Winqvist O., Harris R.A., Larsson E., Persson A.E., Fredholm B.B., Carlstrom M. Genetic abrogation of adenosine A3 receptor prevents uninephrectomy and high salt-induced hypertension. J. Am. Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zweier J.L., Talukder M.A. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 41.Duncan C., Dougall H., Johnston P., Green S., Brogan R., Leifert C., Smith L., Golden M., Benjamin N. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat. Med. 1995;1:546–551. doi: 10.1038/nm0695-546. [DOI] [PubMed] [Google Scholar]
- 42.Liu M., Zollbrecht C., Peleli M., Lundberg J.O., Weitzberg E., Carlstrom M. Nitrite-mediated renal vasodilatation is increased during ischemic conditions via cGMP-independent signaling. Free Radic. Biol. Med. 2015;84:154–160. doi: 10.1016/j.freeradbiomed.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 43.Lee H.T., Park S.W., Kim M., D'Agati V.D. Acute kidney injury after hepatic ischemia and reperfusion injury in mice. Lab Investig. 2009;89:196–208. doi: 10.1038/labinvest.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palomba H., de Castro I., Neto A.L., Lage S., Yu L. Acute kidney injury prediction following elective cardiac surgery: AKICS score. Kidney Int. 2007;72:624–631. doi: 10.1038/sj.ki.5002419. [DOI] [PubMed] [Google Scholar]
- 45.Lewington A.J., Cerda J., Mehta R.L. Raising awareness of acute kidney injury: a global perspective of a silent killer. Kidney Int. 2013;84:457–467. doi: 10.1038/ki.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Basile D.P., Leonard E.C., Tonade D., Friedrich J.L., Goenka S. Distinct effects on long-term function of injured and contralateral kidneys following unilateral renal ischemia-reperfusion. Am. J. Physiol. Ren. Physiol. 2012;302:F625–F635. doi: 10.1152/ajprenal.00562.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basile D.P., Fredrich K., Alausa M., Vio C.P., Liang M., Rieder M.R., Greene A.S., Cowley A.W., Jr. Identification of persistently altered gene expression in the kidney after functional recovery from ischemic acute renal failure. Am. J. Physiol. Ren. Physiol. 2005;288:F953–F963. doi: 10.1152/ajprenal.00329.2004. [DOI] [PubMed] [Google Scholar]
- 48.Leemans J.C., Stokman G., Claessen N., Rouschop K.M., Teske G.J., Kirschning C.J., Akira S., van der Poll T., Weening J.J., Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J. Clin. Investig. 2005;115:2894–2903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buckley C.D., Gilroy D.W., Serhan C.N., Stockinger B., Tak P.P. The resolution of inflammation. Nat. Rev. Immunol. 2013;13:59–66. doi: 10.1038/nri3362. [DOI] [PubMed] [Google Scholar]
- 50.Kalinski P., Hilkens C.M., Snijders A., Snijdewint F.G., Kapsenberg M.L. IL-12-deficient dendritic cells, generated in the presence of prostaglandin E2, promote type 2 cytokine production in maturing human naive T helper cells. J. Immunol. 1997;159:28–35. [PubMed] [Google Scholar]
- 51.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 52.Li L., Huang L., Vergis A.L., Ye H., Bajwa A., Narayan V., Strieter R.M., Rosin D.L., Okusa M.D. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Investig. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haq M., Norman J., Saba S.R., Ramirez G., Rabb H. Role of IL-1 in renal ischemic reperfusion injury. J. Am. Soc. Nephrol. 1998;9:614–619. doi: 10.1681/ASN.V94614. [DOI] [PubMed] [Google Scholar]
- 54.Cao Q., Harris D.C., Wang Y. Macrophages in kidney injury, inflammation, and fibrosis. Physiology. 2015;30:183–194. doi: 10.1152/physiol.00046.2014. [DOI] [PubMed] [Google Scholar]
- 55.Carr M.W., Roth S.J., Luther E., Rose S.S., Springer T.A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. USA. 1994;91:3652–3656. doi: 10.1073/pnas.91.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jadert C., Petersson J., Massena S., Ahl D., Grapensparr L., Holm L., Lundberg J.O., Phillipson M. Decreased leukocyte recruitment by inorganic nitrate and nitrite in microvascular inflammation and NSAID-induced intestinal injury. Free Radic. Biol. Med. 2012;52:683–692. doi: 10.1016/j.freeradbiomed.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 57.Deng J., Kohda Y., Chiao H., Wang Y., Hu X., Hewitt S.M., Miyaji T., McLeroy P., Nibhanupudy B., Li S., Star R.A. Interleukin-10 inhibits ischemic and cisplatin-induced acute renal injury. Kidney Int. 2001;60:2118–2128. doi: 10.1046/j.1523-1755.2001.00043.x. [DOI] [PubMed] [Google Scholar]
- 58.Godet C., Goujon J.M., Petit I., Lecron J.C., Hauet T., Mauco G., Carretier M., Robert R. Endotoxin tolerance enhances interleukin-10 renal expression and decreases ischemia-reperfusion renal injury in rats. Shock. 2006;25:384–388. doi: 10.1097/01.shk.0000209528.35743.54. [DOI] [PubMed] [Google Scholar]
- 59.Beckman J.S., Koppenol W.H. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am. J. Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material