Abstract
Molecular phylogenetic analyses of a multigene matrix of partial nuSSU-ITS-LSU rDNA, rpb2 and tef1 sequences were performed to investigate the phylogenetic relationships of Corynespora, Exosporium and Helminthosporium species. Based on phylogenetic analyses and morphology, the genus Exosporium is synonymised with Helminthosporium, and the genus Corynespora is revealed as polyphyletic. Corynespora smithii is confirmed to be closely related to the generic type C. cassiicola and its morphology is described and illustrated. Exosporium tiliae, Corynespora caespitosa, C. endiandrae, C. leucadendri and C. olivacea are recognised in Helminthosporium, and Splanchnonema quercicola and S. kalakadense are combined in Helminthosporium. Based on pure culture studies and DNA sequence data, Massaria heterospora and Massarinula italica are shown to be the sexual morphs of Helminthosporium tiliae and H. microsorum, respectively. European accessions of Splanchnonema quercicola are recognised to differ from the North American type and are described as Helminthosporium quercinum. The sexual morph of H. oligosporum is recorded and described for the first time. The generic type of Helminthosporium, H. velutinum, is epitypified with a recent collection from the type host, Fagus sylvatica. Based on sequence data, Helminthosporium genistae is recognised as a distinct species. Several species for which subperidermal stromata have been reported are shown to be fungicolous on Diaporthales, the “stromata” representing aborted and transformed host stromata or conidiomata: H. caespitosum, H. microsorum, H. quercicola and H. quercinum on Coryneum spp.; H. hispanicum on conidiomata of Juglanconis juglandina; H. juglandinum on conidiomata of Diaporthe sp.; H. oligosporum and H. tiliae on Hercospora tiliae. The newly described H. austriacum is fungicolous on Amphisphaeria cf. millepunctata (Xylariales).
Key words: Ascomycota, Dothideomycetes, Massarinaceae, Phylogenetic analysis, Pleosporales
Taxonomic novelties: New species: Helminthosporium austriacum Voglmayr & Jaklitsch, Helminthosporium hispanicum Voglmayr & Jaklitsch, Helminthosporium juglandinum Voglmayr & Jaklitsch, Helminthosporium quercinum Voglmayr & Jaklitsch
New combinations: Helminthosporium endiandrae (Crous & Summerell) Voglmayr & Jaklitsch, Helminthosporium kalakadense (Subram. & Sekar) Voglmayr & Jaklitsch, Helminthosporium leucadendri (Quaedvl. et al.) Voglmayr & Jaklitsch, Helminthosporium quercicola (M.E. Barr) Voglmayr & Jaklitsch
Epitypifications (basionyms): Coryneum oligosporum Corda, Exosporium caespitosum Ellis & Barthol., Exosporium tiliae Link, Helminthosporium genistae Fr., Helminthosporium microsorum D. Sacc., Helminthosporium velutinum Link, Massaria heterospora G.H. Otth, Massarinula italica D. Sacc., Sporidesmium olivaceum Wallr
Introduction
The genus Helminthosporium produces a conspicuous asexual morph, and its generic type, H. velutinum, is a well-known species of almost world-wide distribution and has been commonly recorded from various hosts. Most Helminthosporium species are considered to be saprobes of chiefly woody hosts (Luttrell, 1964, Alcorn, 1988), but one species, Helminthosporium solani, is an economically important pathogen of potatoes, as it is the causing agent of silver scurf disease of potato tubers (Errampalli et al. 2001).
The taxonomic history of the genus Helminthosporium is complex. About 740 taxa have been placed in Helminthosporium (http://www.indexfungorum.org, Dec. 2016), but most of these are not congeneric with the generic type. After detailed morphological analyses, the genus Helminthosporium was restricted to species having porogenous, distoseptate conidia with conidial scars consisting of simple, flat-ringed pores; conidia are acropleurogenously borne on septate, erect conidiophores which cease growth after the formation of terminal conidia (Ellis, 1961, Luttrell, 1963, Luttrell, 1964). However, Hughes (1958) considered the distinction between pleurogenous vs. acrogenous conidia unsuitable for generic classification and widened the generic circumscription to include also species with acrogenous conidia. The latter were placed in the genera Corynespora and Exosporium by Ellis (1961) and Luttrell (1964), which was subsequently widely accepted.
Applying this restricted circumscription, numerous species pathogenic to hosts from the Poaceae were transferred from Helminthosporium to the genera Bipolaris (= Cochliobolus), Curvularia (= Pseudocochliobolus), Exserohilum (= Setosphaeria), and Pyrenophora (= Drechslera), which are all members of the Pleosporaceae (Sivanesan, 1987, Hyde et al., 2013, Tanaka et al., 2015). Other species like H. asterinum were also shown to be only distantly related (Olivier et al. 2000). In molecular phylogenetic analyses, the generic type, H. velutinum, was revealed to belong to Massarinaceae (Kodsueb et al., 2007, Hyde et al., 2013, Tanaka et al., 2015). However, only few additional Helminthosporium species have been sequenced so far. In the most extensive molecular phylogenetic account available for the genus, Tanaka et al. (2015) included four Helminthosporium species as well as three yet unnamed strains.
Based on extensive morphological investigations, Ellis (1961) synonymised numerous species with H. velutinum, and accepted 10 species in the genus. Subsequently, numerous additional species were described, and Siboe et al. (1999) listed 27 accepted species for Helminthosporium, providing a table summarising their main diagnostic morphological characters. With the recent description of several new species mainly from China and Japan, the number of species currently accepted in Helminthosporium has risen to about 46 (MycoBank, data retrieved December 2016). Unfortunately, for most of these recently described species no sequence data are available.
There are few records of sexual morphs of Helminthosporium, and most are considered dubious as they have not been verified by sequence data. Hughes (1953) reported the production of a Helminthosporium asexual morph in a British ex-ascospore isolate of an unnamed Massaria species from Quercus, but he provided no morphological description of the sexual morph. Also from Quercus, Barr (1993) mentioned Helminthosporium cf. velutinum as presumed asexual morph of her Splanchnonema quercicola, but without a morphological description of the asexual morph, and the connection was not confirmed by pure culture studies. It is tempting to interpret the records of Hughes (1953) and Barr (1993) to represent the same or closely related species, considering that Splanchnonema species have been classified in Massaria until Shoemaker & LeClair (1975) acknowledged the fundamental differences between both genera. However, the lack of a description of the sexual morph by Hughes (1953) and of the asexual morph by Barr (1993) makes this little more than a guess. Subramanian & Sekar (1987) described Splanchnonema kalakadense as the sexual morph of H. velutinum based on pure culture studies. Recently, Tanaka et al. (2015) described a massarina-like sexual morph for H. massarinum based on pure culture and sequence data.
In the course of a survey on corticolous Dothideomycetes, several collections of splanchnonema-like fungi were made on various hosts, which were closely associated with helminthosporium-, corynespora- and exosporium-like asexual morphs. Pure culture as well as DNA sequence data from both sexual and asexual morphs revealed conspecificity of the associated morphs, and phylogenetic analyses revealed that they are all closely related to Helminthosporium velutinum. This prompted us to initiate a detailed morphological and molecular phylogenetic study of several Helminthosporium, Exosporium and Corynespora taxa, which resulted in the taxonomic revision presented here.
Materials and methods
Isolates
The isolates used in this study either originated from ascospores or conidia of fresh specimens or from culture collections. Details of the strains including NCBI GenBank accession numbers of gene sequences used to compute the phylogenetic trees are listed in Table 1. Strain acronyms other than those of official culture collections are used here primarily as strain identifiers throughout the work. Representative isolates have been deposited at the Westerdijk Fungal Biodiversity Centre, Utrecht, The Netherlands (CBS culture collection). Details of the specimens used for morphological investigations are listed in the Taxonomy section under the respective descriptions. The following culture of Pseudosplanchnonema phorcioides was sequenced but is not further treated here: Austria, Wien, Donaustadt, Lobau, Panozzalacke, on dead corticated twigs of Morus alba, 1 Apr. 2006, W. Jaklitsch [WU 38898, culture L16 (ex ascospore) = CBS 122935]. Herbarium acronyms are according to Thiers (2017). Freshly collected specimens have been deposited in the Fungarium of the Department of Botany and Biodiversity Research, University of Vienna (WU).
Table 1.
Isolates and accession numbers used in the phylogenetic analyses. Isolates/sequences in bold were isolated/sequenced in the present study.
| Taxon | Strain | Culture no. | Specimen no.1 | SSU | LSU | ITS | rpb2 | tef1 | Notes5 |
|---|---|---|---|---|---|---|---|---|---|
| Byssothecium circinans | – | CBS 675.92 | – | GU205235 | AY016357 | genome2 | genome2 | genome2 | A |
| Corynespora cassiicola | – | CBS 100822 | CBS H-6061 | GU296144 | GU301808 | – | GU371742 | GU349052 | C |
| C. cassiicola | – | CCP | – | GU296145 | – | KF810854 | genome2 | genome2 | C |
| C. smithii | – | CABI 5649b | – | – | GU323201 | FJ852597 | GU371783 | GU349018 | C |
| L120 | – | WU 38820 | – | KY984297 | KY984297 | KY984361 | KY984435 | C | |
| L130 | – | WU 38821 | KY984419 | KY984298 | KY984298 | KY984362 | KY984436 | C | |
| L133 | CBS 139925 | WU 38822 | – | KY984299 | KY984299 | KY984363 | – | C | |
| L139 | – | WU 38824 | – | KY984300 | KY984300 | KY984364 | – | C | |
| Cyclothyriella rubronotata | TR | CBS 121892 | WU 36862 | – | KX650541 | KX650541 | KX650571 | KX650516 | A |
| C. rubronotata | TR9 | CBS 141486 | WU 36858ET | KX650507 | KX650544 | KX650544 | KX650574 | KX650519 | A |
| Helminthosporium aquaticum | S-096 | MFLUCC 15-0357 | HKAS 89692HT | KU697310 | KU697306 | KU697302 | – | – | C |
| H. austriacum | L132 | CBS 139924 | WU 38826HT | KY984420 | KY984301 | KY984301 | KY984365 | KY984437 | C |
| L137 | – | WU 38825 | – | KY984302 | KY984302 | KY984366 | KY984438 | C | |
| L169 | CBS 142388 | WU 38827 | – | KY984303 | KY984303 | KY984367 | KY984439 | C | |
| L177 | – | WU 38828 | – | KY984304 | KY984304 | – | – | C | |
| H. caespitosum | L141 | – | WU 38831 | – | KY984305 | KY984305 | KY984368 | – | C |
| L151 | – | WU 38829 | – | KY984306 | KY984306 | KY984369 | – | C | |
| L99 | CBS 484.77 | CBS H-713ET | KY984421 | JQ044448 | JQ044429 | KY984370 | KY984440 | C | |
| H. dalbergiae | H 4628 (= TS 36) | MAFF 243853 | HHUF 27971 | AB797231 | AB807521 | LC014555 | – | AB808497 | C |
| H. endiandrae | CPC 22194 | CBS 138902 | CBS H-21984HT | – | KP004478 | KP004450 | – | – | C |
| H. genistae | L125 | – | WU 38836 | – | KY984307 | KY984307 | KY984371 | – | C |
| L128 | CBS 139921 | WU 38835 | KY984422 | KY984308 | KY984308 | KY984372 | – | C | |
| L129 | CBS 139922 | WU 38834 | KY984423 | KY984309 | KY984309 | KY984373 | – | C | |
| L142 | CBS 142597 | WU 38832ET | – | KY984310 | KY984310 | KY984374 | – | C | |
| L143 | CBS 139927 | WU 38837 | – | KY984311 | KY984311 | KY984375 | – | C | |
| L144 | CBS 139928 | WU 38841 | – | KY984312 | KY984312 | KY984376 | – | C | |
| L145 | – | WU 38842 | – | KY984313 | KY984313 | KY984377 | – | C | |
| L147 | – | WU 38838 | – | KY984314 | KY984314 | KY984378 | – | C | |
| L148 | CBS 139929 | WU 38839 | – | KY984315 | KY984315 | KY984379 | – | C | |
| L149 | CBS 139930 | WU 38840 | – | KY984316 | KY984316 | KY984380 | – | C | |
| L173 | – | WU 38833 | – | KY984317 | KY984317 | – | – | C | |
| H. hispanicum | L109 | CBS 136917 | WU 38843HT | KY984424 | KY984318 | KY984318 | KY984381 | KY984441 | C |
| H. juglandinum | L101 | CBS 136912 | WU 38844 | – | KY984319 | KY984319 | KY984382 | KY984442 | C |
| L102 | CBS 136913 | WU 38846 | – | KY984320 | KY984320 | KY984383 | KY984443 | C | |
| L118 | CBS 136922 | WU 38845HT | – | KY984321 | KY984321 | KY984384 | KY984444 | C | |
| L97 | CBS 136911 | WU 38848 | KY984425 | KY984322 | KY984322 | KY984385 | KY984445 | C | |
| H. leucadendri | CPC 19345 | CBS 135133 | CBS H-21323HT | – | KF251654 | KF251150 | KF252159 | KF253110 | C |
| H. magnisporum | H 4627 (= TS 33) | MAFF 239278 | HHUF 27968HT | AB797232 | AB807522 | AB811452 | – | AB808498 | C |
| H. massarinum | KT 1564 | CBS 139690 = JCM 13095 = MAFF 239605 | HHUF 29089HT | AB797234 | AB807524 | AB809629 | – | AB808500 | A |
| KT 838 | JCM 13094 = MAFF 239604 | HHUF 27573PT | AB797233 | AB807523 | AB809628 | – | AB808499 | A | |
| H. microsorum | L108 | CBS 136916 | WU 38863 | – | KY984323 | KY984323 | KY984386 | – | C |
| L123 | – | WU 38861 | – | KY984324 | KY984324 | KY984387 | – | C | |
| L174 | – | WU 38854 | – | KY984325 | KY984325 | – | – | A | |
| L175 | – | WU 38852 | – | KY984326 | KY984326 | – | – | C | |
| L94 | – | WU 38860 | KY984426 | KY984327 | KY984327 | KY984388 | KY984446 | A | |
| L95 | – | WU 38860 | – | KY984328 | KY984328 | KY984389 | KY984447 | C | |
| L96 | CBS 136910 | WU 38850ET | KY984427 | KY984329 | KY984329 | KY984390 | KY984448 | A | |
| H. oligosporum | L106 | – | WU 38869 | – | KY984330 | KY984330 | KY984391 | KY984449 | C |
| L111 | – | WU 38872 | – | KY984331 | KY984331 | KY984392 | – | C | |
| L92 | CBS 136908 | WU 38867 | KY984428 | KY984332 | KY984332 | KY984393 | KY984450 | C | |
| L93 | CBS 136909 | WU 38864ET | – | KY984333 | KY984333 | KY984394 | KY984451 | A | |
| H. quercinum | – | CBS 112393 | – | – | KY984334 | KY984334 | KY984395 | KY984452 | C |
| L105 | – | WU 38877 | – | KY984335 | KY984335 | KY984396 | – | C | |
| L107 | CBS 136915 | WU 38880 | – | KY984336 | KY984336 | KY984397 | – | A | |
| L159 | – | WU 38879 | – | KY984337 | KY984337 | KY984398 | – | A | |
| L170 | – | WU 38878 | – | KY984338 | KY984338 | KY984399 | – | C | |
| L90 | CBS 136921 | WU 38876HT | KY984429 | KY984339 | KY984339 | KY984400 | KY984453 | A | |
| L91 | – | WU 38876HT | – | KY984340 | KY984340 | KY984401 | KY984454 | C | |
| H. solani | – | CBS 365.75 | CBS H-13302 | KY984430 | KY984341 | KY984341 | KY984402 | KY984455 | C |
| – | CBS 640.85 | – | – | KY984342 | KY984342 | KY984403 | – | C | |
| Helminthosporium sp. | yone 38 | MAFF 243857 | HHUF 29740 | AB797237 | AB807527 | NARO3 | – | AB808502 | C |
| H. tiliae | L171 | – | WU 38881 | – | KY984343 | KY984343 | KY984404 | KY984456 | C |
| L87 | CBS 136906 | WU 38884 | – | KY984344 | KY984344 | KY984405 | – | A | |
| L88 | CBS 136907 | WU 38882ET | KY984431 | KY984345 | KY984345 | KY984406 | KY984457 | A | |
| L89 | – | WU 38882ET | – | KY984346 | KY984346 | KY984407 | – | C | |
| H. velutinum | H 4626 (= TS 28) | MAFF 243854 | HHUF 27966 | AB797240 | AB807530 | LC014556 | – | AB808505 | C |
| H 4739 (= TS 58) | MAFF 243855 | HHUF 28243 | AB797235 | AB807525 | LC014557 | – | AB808501 | C | |
| H 4743 (= TS 68) | MAFF 243856 | HHUF 28248 | AB797236 | AB807526 | NARO3 | – | – | C | |
| L115 | CBS 136924 | WU 38891 | – | KY984347 | KY984347 | KY984408 | KY984458 | C | |
| L116 | – | WU 38887 | – | KY984348 | KY984348 | KY984409 | KY984459 | C | |
| L117 | – | WU 38885 | – | KY984349 | KY984349 | KY984410 | KY984460 | C | |
| L126 | – | WU 38894 | – | KY984350 | KY984350 | KY984411 | KY984461 | C | |
| L127 | – | WU 38889 | – | KY984351 | KY984351 | KY984412 | KY984462 | C | |
| L131 | CBS 139923 | WU 38892ET | KY984432 | KY984352 | KY984352 | KY984413 | KY984463 | C | |
| L134 | – | WU 38895 | – | KY984353 | KY984353 | KY984414 | – | C | |
| L135 | – | WU 38896 | – | KY984354 | KY984354 | – | KY984464 | C | |
| L136 | – | WU 38888 | – | KY984355 | KY984355 | – | KY984465 | C | |
| L140 | – | WU 38890 | – | KY984356 | KY984356 | KY984415 | – | C | |
| L163 | – | WU 38893 | – | KY984357 | KY984357 | KY984416 | – | C | |
| L176 | – | WU 38897 | – | KY984358 | KY984358 | – | – | C | |
| L98 | – | WU 38886 | KY984433 | KY984359 | KY984359 | KY984417 | KY984466 | C | |
| S-033 | MFLUCC 15-0423 | HKAS 83990 | KU697308 | KU697304 | KU697300 | – | – | C | |
| S-076 | MFLUCC 15-0243 | HKAS 84000 | KU697309 | KU697305 | KU697301 | – | – | C | |
| S-135 | MFLUCC 15-0428 | HKAS 84015 | KU697307 | KU697303 | KU697299 | – | – | C | |
| yone 63 | MAFF 243858 | HHUF 29741 | AB797238 | AB807528 | NARO3 | – | AB808503 | C | |
| yone 96 | MAFF 243859 | HHUF 30140 | AB797239 | AB807529 | LC014558 | – | AB808504 | C | |
| Massarina cisti | – | CBS 266.62 = JCM 14140 | ZT (Hütter & Loeffler)HT | AB797249 | AB807539 | LC014568 | – | AB808514 | A |
| M. eburnea | – | CBS 473.64 | – | AF164367 | GU301840 | AF383959 | genome2 | genome2 | A |
| H 3953 | CBS 139697 = JCM 14422 | HHUF 26621 | AB521718 | AB521735 | LC014569 | – | AB808517 | A | |
| Periconia byssoides | H 4600 (= TS 29) | MAFF 243872 | HHUF 28238 | AB797280 | AB807570 | LC014581 | – | AB808546 | C |
| P. digitata | – | CBS 510.77 | – | AB797271 | AB807561 | LC014584 | – | AB808537 | C |
| – | CBS 845.96 = JCM 14142 | – | AB797277 | AB807567 | LC014586 | – | AB808543 | C | |
| P. macrospinosa | – | CBS 135663, DSE 20364 | – | KP184080 | KP184038 | KP183999 | genome2,4 | genome2,4 | C |
| P. pseudodigitata | KT 1395 | CBS 139699 = JCM 13166 = MAFF 239676 | HHUF 29370HT | AB797274 | AB807564 | LC014591 | – | AB808540 | A |
| Pseudosplanchnonema phorcioides | L16 | CBS 122935 | WU 38898 | KY984434 | KY984360 | KY984360 | KY984418 | KY984467 | A |
| Stagonospora paludosa | – | CBS 135088 | CBS H-21317NT | – | KF251760 | KF251257 | KF252262 | KF253207 | C |
| S. perfecta | KT 1726A | JCM 13099 = MAFF 239609 | HHUF 29095 | AB797289 | AB807579 | AB809642 | – | AB808555 | A |
| S. pseudoperfecta | KT 889 | CBS 120236 = JCM 13097 = MAFF 239607 | HHUF 29087HT | AB797287 | AB807577 | AB809641 | – | AB808553 | A |
| S. tainanensis | KT 1866 | MAFF 243860 | HHUF 30141 | AB797290 | AB807580 | AB809643 | – | AB808556 | A |
Specimen with ET (epitype), HT (holotype), NT (neotype), and PT (paratype).
Sequence retrieved from genome deposited at JGI-DOE (http://genome.jgi.doe.gov/).
Sequence downloaded from the Microorganism Search System of the Genetic Resources Center (NARO), Tsukuba, Japan (http://www.gene.affrc.go.jp/).
rpb2 and tef1 sequences were retrieved from the genome of strain DSE2036, which has identical ITS and LSU sequences to CBS 135663 (D. Knapp, unpublished data).
Origin of isolates: A, single ascospore; C, single conidium.
Morphology
Microscopic observations were made in tap water except where noted. Morphological investigations of sexual and asexual morphs were consistently done from material on natural substrates. Methods of microscopy included stereomicroscopy using a Nikon SMZ 1500 and Nomarski differential interference contrast (DIC) using a Zeiss Axio Imager.A1 compound microscope equipped with a Zeiss Axiocam 506 colour digital camera. Images and data were gathered using a Nikon DS-U2 digital camera and measured by using the NIS-Elements D v. 3.22.15 or Zeiss ZEN Blue Edition softwares. For certain images of ascomata and conidiomata the stacking software Zerene Stacker v. 1.04 (Zerene Systems LLC, Richland, WA, USA) was used. Measurements are reported as maxima and minima in parentheses and the range representing the mean plus and minus the standard deviation of a number of measurements given in parentheses. Photography of culture plates was performed with a Nikon Coolpix 4500 camera.
Culture preparation, DNA extraction, PCR and sequencing
Single ascospore or conidium isolates were prepared and grown on 2 % malt extract agar (MEA), or on 2 % corn meal agar plus 2 % w/v dextrose (CMD).
Growth of liquid culture and extraction of genomic DNA was performed as reported previously (Voglmayr and Jaklitsch, 2011, Jaklitsch et al., 2012) using the DNeasy Plant Mini Kit (QIAgen GmbH, Hilden, Germany) or the modified CTAB method of Riethmüller et al. (2002).
The following loci were amplified and sequenced: the complete internally transcribed spacer region (ITS1-5.8S-ITS2) and a ca. 900 bp fragment of the large subunit nuclear ribosomal DNA (nuLSU rDNA), amplified and sequenced as a single fragment with primers V9G (de Hoog & Gerrits van den Ende 1998) and LR5 (Vilgalys & Hester 1990); a ca. 1.7–2.2 kb fragment of the small subunit nuclear ribosomal DNA (nuSSU rDNA) with primers SL1 (Landvik et al. 1997) and NS24mod (Voglmayr & Jaklitsch 2011); a ca. 1.2 kb fragment of the RNA polymerase II subunit 2 (rpb2) gene with primers fRPB2-5f and fRPB2-7cr (Liu et al. 1999) or dRPB2-5f and dRPB2-7r (Voglmayr et al. 2016); and a ca. 1.3–1.5 kb fragment of the translation elongation factor 1-alpha (tef1) gene containing introns 4 and 5 and part of the exon with primers EF1-728F (Carbone & Kohn 1999) and TEF1LLErev (Jaklitsch et al. 2005) or EF1-2218R (Rehner & Buckley 2005). PCR products were purified using an enzymatic PCR cleanup (Werle et al. 1994) as described in Voglmayr & Jaklitsch (2008). DNA was cycle-sequenced using the ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit v. 3.1 (Applied Biosystems, Warrington, UK) and the PCR primers; in addition, primers ITS4 (White et al. 1990) and LR3 (Vilgalys & Hester 1990) were used for the ITS-LSU and NSSU1088 (Kauff & Lutzoni 2002) for the SSU rDNA regions. For tef1, the internal primers TEF1_INTF (forward; Jaklitsch 2009) and TEF1_INT2 (reverse; 5′ CCACTTNGTNGTGTCCATCTTRTT 3′) were used for cycle sequencing in certain instances. Sequencing was performed on an automated DNA sequencer (ABI 3730xl Genetic Analyzer, Applied Biosystems).
Data analysis
For phylogenetic analyses, combined matrices of ITS-LSU, SSU, rpb2 and tef1 sequences were produced. GenBank sequences of Massarinaceae and Periconiaceae were selected according to Tanaka et al. (2015) and supplemented with GenBank sequences from additional Corynespora and Helminthosporium species; some ITS sequences of Japanese strains not deposited in GenBank were downloaded via the Microorganism Search System of the Genetic Resources Center (NARO), Tsukuba, Japan (http://www.gene.affrc.go.jp/). For some strains for which the whole genome data are available, sequences were retrieved from JGI-DOE (http://genome.jgi.doe.gov/). Cyclothyriella rubronotata was selected as outgroup (Jaklitsch & Voglmayr 2016). All alignments were produced with the server version of MAFFT (www.ebi.ac.uk/Tools/mafft), checked and refined using BioEdit v. 7.0.9.0 (Hall 1999). Due to alignment problems, 67 nucleotide characters at the 5′ end of the ITS1 were excluded. For phylogenetic analyses, all sequence alignments were combined. For Periconia macrospinosa, ITS, LSU and SSU rDNA GenBank sequences of strain CBS 135663 were combined with the rpb2 and tef1 sequences from the genome of strain DSE 2036, as both strains have identical ITS and LSU sequences (D. Knapp, pers. comm.). Two combined data matrices were produced for subsequent analyses, one including all Helminthosporium accessions for which at least ITS and LSU sequences were available, and a second containing only Helminthosporium accessions for which, in addition to the ITS and LSU, also the rpb2 gene was available. The first combined matrix contained 5 100 nucleotide characters, i.e. 1 462 from the ITS-LSU, 1 024 from the SSU, 1 128 from rpb2 and 1 486 from tef1; the second 5 099 nucleotide characters, with the same number of characters for the various regions except for 1 bp less (1 461) in the ITS-LSU. As for H. leucadendri only comparatively short rpb2 and tef1 sequences are available (313 and 438 bp included characters, respectively), bootstrap analyses were repeated with the second matrix after exclusion of H. leucadendri to evaluate the effect of incomplete rpb2 and tef1 sequences on the topological support. Prior to phylogenetic analyses, the approach of Wiens (1998) was applied to test for significant levels of localised incongruence among the markers used for the combined analyses, using the level of bootstrap support (Sung et al. 2007) as described in Jaklitsch & Voglmayr (2014). For this, the 70 % maximum parsimony (MP) bootstrap consensus trees from 100 bootstrap replicates calculated for each individual partition, with the same parameters given below, were compared. Except for a few nodes within species, no topological conflicts were observed between these bootstrap trees of the various genes, indicating the absence of significant incongruence and combinability of the loci (Wiens 1998).
Maximum parsimony (MP) analyses of the combined matrices were performed using a parsimony ratchet approach. For this, a nexus file was prepared using PRAP v. 2.0b3 (Müller 2004), implementing 1 000 ratchet replicates with 25 % of randomly chosen positions upweighted to 2, which was then run with PAUP v. 4.0a151 (Swofford 2002). The resulting best trees were then loaded in PAUP and subjected to heuristic search with TBR branch swapping (MULTREES option in effect, steepest descent option not in effect). Bootstrap analyses with 1 000 replicates were performed using 5 rounds of replicates of heuristic search with random addition of sequences and subsequent TBR branch swapping (MULTREES option in effect, steepest descent option not in effect) during each bootstrap replicate, with each replicate limited to 1 million rearrangements. In all MP analyses molecular characters were unordered and given equal weight; analyses were performed with gaps treated as missing data; the COLLAPSE command was set to minbrlen.
Maximum likelihood (ML) analyses were performed with RAxML (Stamatakis 2006) as implemented in raxmlGUI v. 1.3 (Silvestro & Michalak 2012), using the ML + rapid bootstrap setting and the GTRGAMMAI substitution model with 1 000 bootstrap replicates. The matrices were partitioned for the individual gene regions, and substitution model parameters were calculated separately for them.
Results
Molecular phylogeny
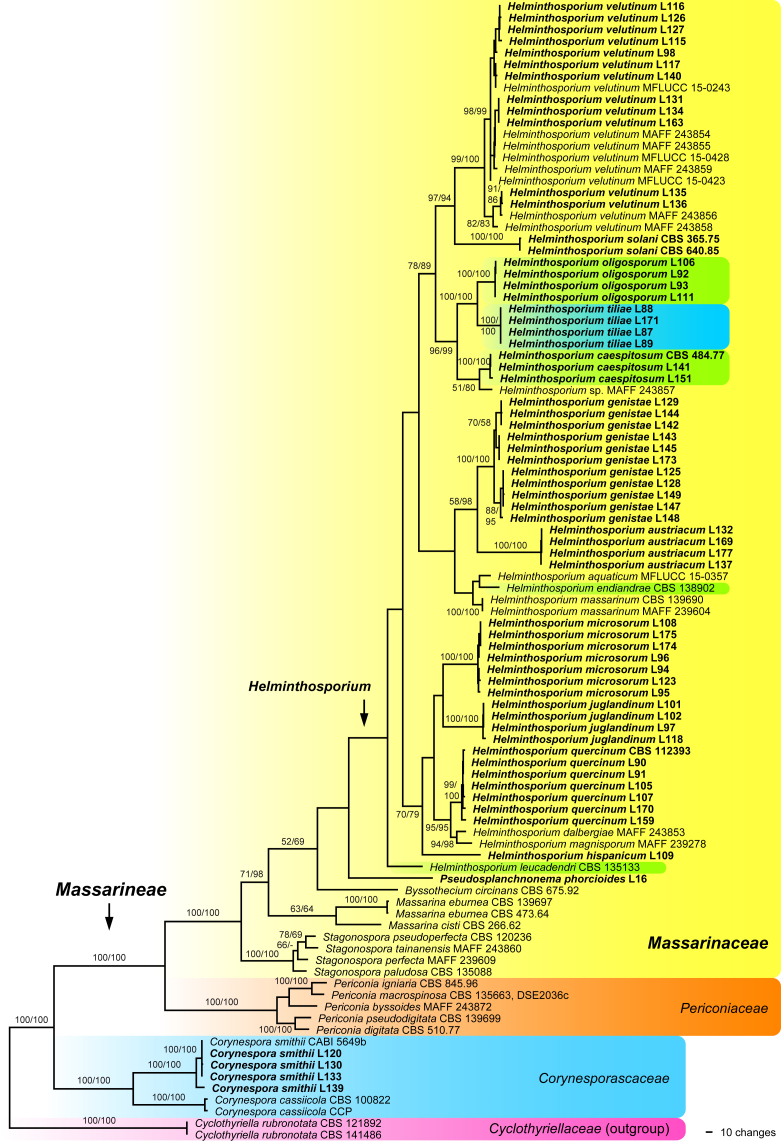
For Helminthosporium genistae, no tef1 sequences could be obtained due to the presence of paralogs. Of the 5 100 and 5 099 nucleotide characters of the two combined matrices used for the phylogenetic analyses, 1 336 and 1 315 are parsimony informative, respectively (408 and 401 of SSU-ITS-LSU, 485 of rpb2, 443 and 429 of tef1). Fig. 1 shows the phylogram of one of 90 761 MP trees of 4 603 steps revealed from the analyses of the combined matrix containing all Helminthosporium accessions for which at least ITS and LSU sequences are available. Tree topologies of all MP trees were identical, except for minor topological differences within species. The backbone of the ML tree revealed by RAxML was similar to the MP strict consensus tree; it differed in a basal position of Periconia byssoides in the Periconia clade, a sister group relationship of Stagonospora perfecta and S. paludosa, an interchanged position of Byssothecium circinans and Pseudosplanchnonma phorcioides and H. aquaticum being placed between them; a sister group relationship of H. endiandrae to H. leucadendri; a slightly different position of H. massarinum; and a sister-group relationship of H. juglandinum to the H. dalbergiae-H. magnisporum-H. quercinum clade (not shown).
Fig. 1.
Phylogram showing one of 90 761 MP trees 4 603 steps revealed by PAUP from an analysis of the combined ITS-LSU-SSU-rpb2-tef1 matrix of Massarinaceae, Periconiaceae and Corynesporascaceae, with Cyclothyriella rubronotata (Cyclothyriellaceae) selected as outgroup. MP and ML bootstrap support above 50 % are given at the first and second position, respectively, above or below the branches; within species bootstrap support is mostly not shown due to lack of space. Strain numbers are given following the taxon names; strains formatted in bold were sequenced in the current study. Helminthosporium taxa formerly classified in Corynespora and Exosporium are marked green and blue, respectively.
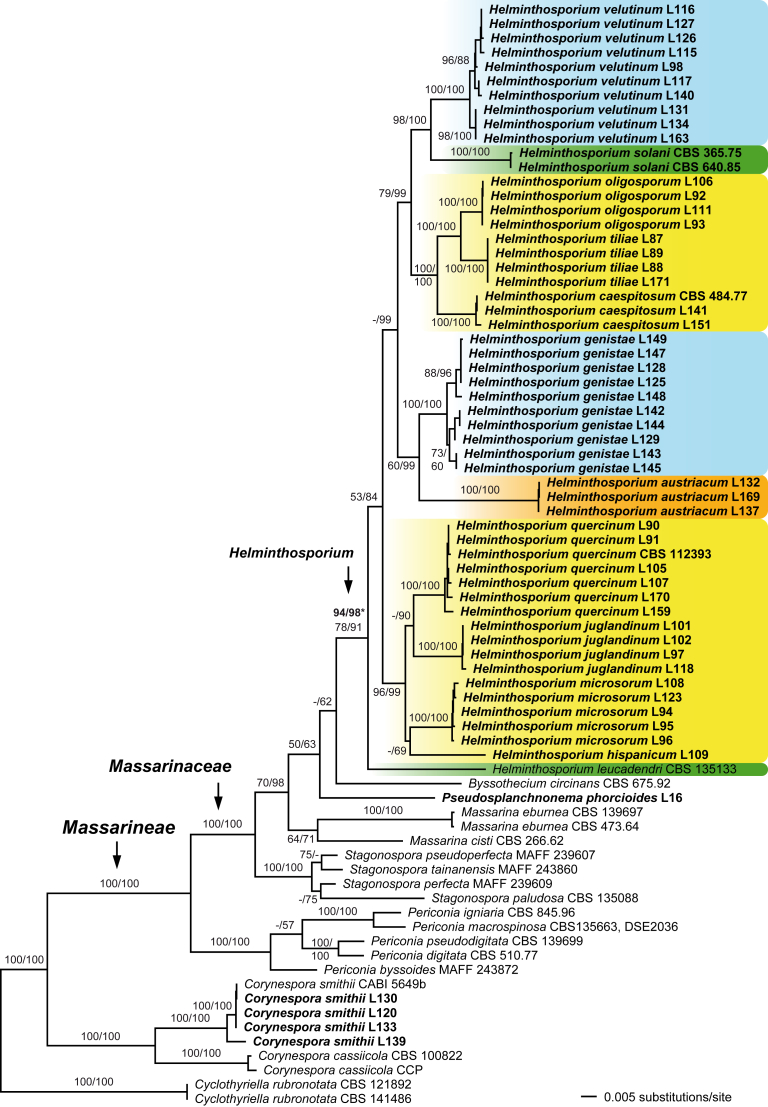
The MP analyses of the combined matrix containing all Helminthosporium accessions, for which at least ITS, LSU and rpb2 sequences are available, revealed 145 MP trees of 4 310 steps (not shown). The best ML tree (lnL = −26619.1191) revealed by RAxML is shown as Fig. 2. The strict consensus tree of all 145 MP trees was fully compatible with Fig. 1, and it was similar to Fig. 2, except for slightly different topologies within Stagonospora.
Fig. 2.
Phylogram of the best ML tree (lnL = −26619.1191) revealed by RAxML from an analysis of the reduced ITS-LSU-SSU-rpb2-tef1 matrix of Massarinaceae, Periconiaceae and Corynesporascaceae, with Cyclothyriella rubronotata (Cyclothyriellaceae) selected as outgroup. The matrix contains only Helminthosporium accessions for which at least ITS, LSU and rpb2 sequences are available. MP and ML bootstrap support above 50 % are given at the first and second position, respectively, above or below the branches; within species bootstrap support is mostly not shown. Bootstrap support for the Helminthosporium clade in bold marked by an asterisk (*) give results of analyses of the same matrix after the exclusion of H. leucadendri (for detailed explanation see text). Strain numbers are given following the taxon names; strains formatted in bold were sequenced in the current study. Helminthosporium taxa saprobic on woody plant parts are marked blue, those being necrotrophic/parasitic on plant leaves or tubers green, taxa fungicolous on Diaporthales yellow, and taxa fungicolous on Amphisphaeria (Xylariales) orange.
In the MP and ML analyses of both matrices, most basal nodes received high support (Fig. 1, Fig. 2). Our molecular phylogenetic analyses confirm previous investigations (Kodsueb et al., 2007, Hyde et al., 2013, Tanaka et al., 2015) that the genus Helminthosporium belongs to the Massarinaceae. The genus Corynespora is revealed as polyphyletic. While the generic type C. cassiicola and C. smithii are closely related and placed outside the Massarinaceae, the other species included in our analyses (C. caespitosa, C. endiandrae, C. leucadendri and C. olivacea (H. oligosporum); marked green in Fig. 1) are revealed to belong to the genus Helminthosporium. All species here recognised in Helminthosporium are contained in a monophyletic clade, which does not receive support in the analyses of the comprehensive combined matrix (Fig. 1). Remarkably, after removal of the Helminthosporium accessions lacking the rpb2, bootstrap support for the Helminthosporium clade strongly rises to 78 % and 91 % in the MP and ML analyses, respectively, and several additional nodes within the Helminthosporium clade received significantly higher support as well, especially in the ML analyses (Fig. 2). After exclusion of H. leucadendri for which only short rpb2 and tef1 sequences are available, the Helminthosporium clade becomes highly supported even in both analyses (94 % MP and 98 % ML bootstrap support; Fig. 2), whereas support for the other nodes is comparable to the analysis including H. leucadendri (not shown). Within Helminthosporium, neither the species with corynespora-like nor with helminthosporium-like asexual morphs are closely related, but are rather interspersed (Fig. 1, Fig. 2). The corynespora-like Helminthosporium oligosporum and the exosporium-like H. tiliae, both fungicolous on Hercospora tiliae on Tilia spp., are sister species with maximum support, and closely related to the corynespora-like H. caespitosum (Fig. 1, Fig. 2). The fungicolous Helminthosporium species form three clades (Fig. 2). Two clades consist of species growing on old stromata or conidiomata of Diaporthales, whereas H. austriacum, which grows on effete ascomata of Amphisphaeria (Xylariales), is sister species to H. genistae, which is saprobic on Fabaceae (Fig. 2).
Culture characteristics
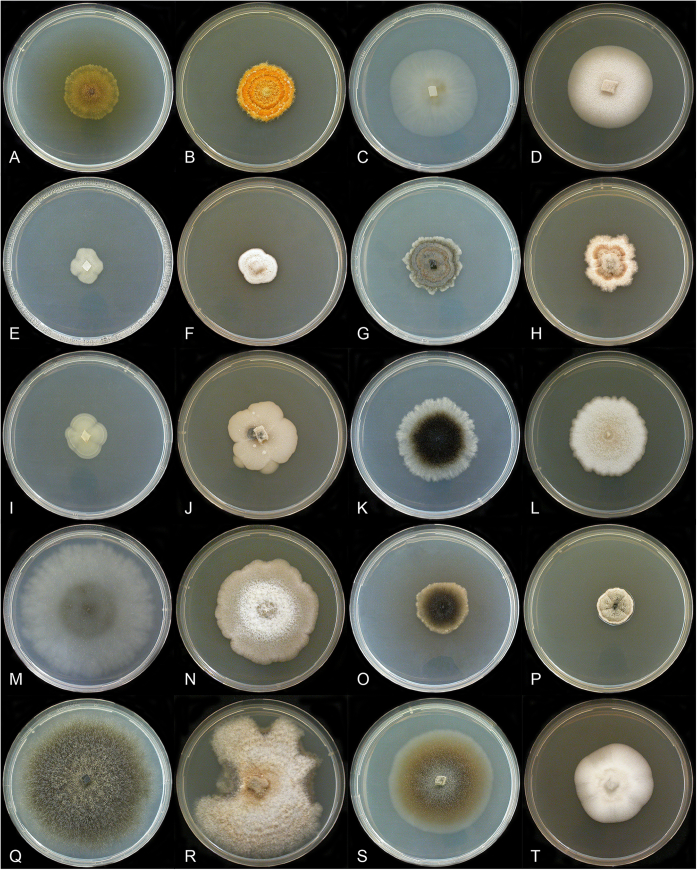
Culture images of nine studied Helminthosporium species grown on MEA and CMD are shown in Fig. 3. Detailed culture descriptions are given under the respective species.
Fig. 3.
Helminthosporium cultures at 22 °C. A, B.H. austriacum (L169). C, D.H. caespitosum (L141). E, F.H. genistae (L142). G, H.H. juglandinum (L118). I, J.H. microsorum (L96). K, L.H. oligosporum (L93). M, N.H. quercinum (L170). O, P.H. tiliae (L171). Q, R.H. velutinum (L115). S, T.H. velutinum (L131). A, C, E, G, I, K, M, O, Q, S. On CMD. B, D, F, H, J, L, N, P, R, T. On MEA. A. After 32 d. B. After 43 d. C, D, G–L, O–T. After 4 wk. E, F. After 3 wk. M, N. After 25 d.
Taxonomy
Corynespora Güssow, Z. PflKrankh. PflSchutz 16: 10. 1906.
Type species: Corynespora mazei Güssow, Consp. Regni Veget. (Leipzig) 16: 13. 1906.
Corynespora smithii (Berk. & Broome) M.B. Ellis, Mycol. Pap. 65: 3. 1957. Fig. 4.
Fig. 4.
Corynespora smithii. A, B. Colony in face view. C, D. Conidiophore bases. E. Stroma cells in section. F, J, K. Conidiophore apices (F. with wide apical pore (arrow), J. with apical conidium, K. proliferating). G–I. Conidiophores (I. with young apical conidium). L–T. Vital conidia (L. young, R, S. proliferating apically). All in water. A, B. WU 38824; C–F. WU 38822; G, K, M–P. WU 38820; H–J, L, Q–T. WU 38821. Scale bars: A = 2 mm; B = 500 μm; C–E, H–T = 10 μm; F = 5 μm, G = 20 μm.
Basionym: Helminthosporium smithii Berk. & Broome [as ‘Helmisporium’], Ann. Mag. nat. Hist., Ser. 2 7: 97. 1851.
Sexual morph unknown. Colony on natural substrate effuse, dark brown or black, velvety or spongy, forming small to widely effused patches up to more than 10 cm long. Mycelium partly superficial, partly immersed in the substrate, composed of branched, septate, subhyaline to brown, smooth-walled, 2–7 μm wide hyphae. Stromata partly superficial, partly immersed, brown, irregular in shape and often extending over large areas, pseudoparenchymatous, composed of cells (5.5–)7.5–11.5(–15.0) μm diam (n = 44). Conidiophores 110–370 μm long, 7–12 μm wide at the base, 8–8.5 μm near the apex, arising singly or more often in dense tufts from superficial hyphae or from cells of the stromata, erect or ascending, simple, straight or flexuous, pale brown to dark brown, septate, with up to four successive cylindrical proliferations. Conidia (140–)170–246(–350) × (9–)11.5–16(–19.5) μm (n = 61), with a 6–7.5 μm wide blackish-brown scar at the base, formed singly or in a short chain through a wide pore at the apex of the conidiophore, often with proliferation through the apical pore and formation of another conidium at the apex of the proliferation, almost cylindrical but usually slightly and gradually tapering towards the rounded apex and more abruptly towards the truncate base, straight or slightly curved, smooth, subhyaline to golden brown, 7–45-distoseptate, with angular lumina; wall up to 5.5 μm thick.
Habitat and host range: Saprobic on dead twigs and trunks of various woody plants.
Distribution: Europe (UK, Austria).
Typification: Lectotype of Helminthosporium smithii, here designated: UK, England, Dorset, Wareham Wood, on dead bark and wood of Ilex aquifolium, 10 Apr. 1850, W. Smith, ex Herb. Berk. (K(M) 233768; MBT376657). Same place, without date, W. Smith, ex herb. C.E. Broome [K(M) 233767, isotype].
Specimens examined: Austria, Niederösterreich, Wöllersdorf, Marchgraben, on Hippocrepis emerus, 9 Oct. 2013, H. Voglmayr [WU 38820, culture L120 (ex conidium)]; Wien, Döbling, Kahlenberg, on Fagus sylvatica, 16 Nov. 2013, W. Jaklitsch [WU 38821, culture L130 (ex conidium)]; Wien, Ottakring, Wilhelminenberg, on Fagus sylvatica, 24 Nov. 2013, H. Voglmayr [WU 38822, culture CBS 139925 = L133 (ex conidium)]; ibid., on Fagus sylvatica, 4 Dec. 2016, H. Voglmayr (WU 38823). UK, England, West Yorkshire, Huddersfield, Gledhold Wood, on Ilex aquifolium, 20 Jan. 2014, C. Yeates [WU 38824, culture L139 (ex conidium)].
Notes: We here provide a description modified from Ellis (1957) for comparison with Helminthosporium, because C. smithii is also found on woody substrates, sometimes in close association with Helminthosporium velutinum. Although the porogenous distoseptate conidia with a dark brown scar and the conidiophores share morphological similarities to some Helminthosporium species as defined here, C. smithii is not closely related to Helminthosporium but forms a separate distant clade together with the generic type, C. cassiicola, which is currently classified as family Corynesporascaceae. Corynespora smithii is characterised by proliferating conidiophores and conidia, a feature which it shares with C. cassiicola. Corynespora smithii has been described from Ilex aquifolium; sequences from a culture from the type host match those obtained from Fagus sylvatica and Hippocrepis emerus, confirming a wide host range of the species given by Ellis (1957).
Helminthosporium Link, Mag. Gesell. naturf. Freunde, Berlin 3(1–2): 10. 1809.
Synonym: Exosporium Link, Mag. Gesell. naturf. Freunde, Berlin 3(1–2): 9. 1809.
Type species: Helminthosporium velutinum Link.
Sexual morph where known massarina- or splanchnonema-like. Pseudostromata formed in the upper bark, usually well-developed, dark (reddish) brown, pseudoparenchymatous, of thick-walled dark brown cells; margin composed of dark brown, verrucose hyphae; less commonly rudimentary and composed of thin-walled, smooth brown hyphae. Ascomata immersed in pseudostromata or upper bark, variably elevating the latter, singly or in small groups, large, ca. 300–1 000 μm diam (including wall of the pseudostroma), globose to depressed globose, often strongly depressed, dark brown to black. Peridium pseudoparenchymatous. Ostioles central, inconspicuous, not protruding above the cortical surface. Hamathecium consisting of numerous filiform, septate, branched, anastomosing, narrow pseudoparaphyses usually embedded in a gel matrix. Asci clavate or fusoid, containing 8 ascospores in irregularly biseriate arrangement, rarely 4 in uniseriate arrangement. Ascospores mostly large, hyaline or first hyaline to pale brown and turning medium to dark brown at full maturity, fusoid, broadly fusoid, subellipsoid, obovoid, less commonly oblong, asymmetric, with 1 eccentric primary septum and often with transverse or oblique distosepta, less commonly ring-like thickenings, in one or both parts, rarely with a longitudinal distoseptum in the larger part, strongly constricted at the primary septum, slightly or not constricted at the secondary distosepta, with subacute to rounded end cells; wall hyaline or brown, smooth or verruculose, sometimes with longitudinal striae; with granular to guttulate contents; each part surrounded by a thick gelatinous sheath.
Habitat and host range: Saprobic, rarely parasitic on plants, or fungicolous.
Distribution: Cosmopolitan, mainly known from Europe and USA.
Colony on natural substrate conspicuous, effuse to punctiform and hairy, or pulvinate, brown to black. Mycelium immersed in the substrate. Stromata usually present. Conidiophores arising solitarily or in fascicles from substrate hyphae or stroma cells, erect, simple, straight or flexuous, brown, single-, few- to many-celled, with a well-defined small pore at the apex, commonly also with lateral pores beneath the upper septa, ceasing growth with the formation of a terminal conidium, usually not proliferating. Conidia formed singly (rarely in short chains), subhyaline to brown, obclavate, obpyriform to lageniform, commonly rostrate, distoseptate, usually with a distinct dark brown to black scar at the base. Cultures on MEA and CMD in most species slow-growing (fast in H. quercinum and H. velutinum), white, shades of brown or grey, rarely orange on MEA (H. austriacum), sometimes (H. austriacum and H. tiliae) with pigment diffusing into agar, odour in most species unpleasant. Culture images of nine studied Helminthosporium species are shown in Fig. 3.
Note: The genera Helminthosporium and Exosporium were described in the same publication (Link 1809). Fries (1832) synonymised Exosporium with Helminthosporium, placing Exosporium tiliae, the generic type, in Helminthosporium, which is therefore to be used as sanctioned name. We provide an emended generic description of Helminthosporium here to include also some species formerly classified in Corynespora and Exosporium, and to appropriately consider the sexual morphs newly linked to several species.
Helminthosporium austriacum Voglmayr & Jaklitsch, sp. nov. MycoBank MB821196. Fig. 5.
Fig. 5.
Helminthosporium austriacum. A. Colony in face view. B. Colony margin with effete ascomata of Amphisphaeria cf. millepunctata. C. Conidiophores with conidia. D, E. Conidiophores. F–H. Conidiophore apices with conidia (F, G) and pores (arrows). I. Conidiophore bases and stroma cells. J–D1. Vital conidia. All in water. A, B, G, W, X. WU 38825; C–F, Y–D1. WU 38826 (holotype); H–V. WU 38827. Scale bars: A = 5 mm; B = 500 μm; C = 200 μm; D, E = 50 μm; F–D1 = 10 μm.
Etymology: Referring to its occurrence in Austria.
Sexual morph unknown. Colony on natural substrate effuse, black, hairy, up to more than 10 cm long. Mycelium mostly immersed, at the surface forming small stroma-like aggregations of dark brown pseudoparenchymatous cells (6.5–)8.7–12.5(–14.0) μm diam (n = 30). Conidiophores 275–700(–920) μm long, 11.5–19 μm wide at the base, tapering to 7–11 μm near the apex, arising solitarily or in fascicles from the stroma cells, erect, simple, straight or flexuous, thick-walled, sub-cylindrical, smooth, brown to dark brown, paler near the apex, with well-defined small pores at the apex and laterally beneath the upper 1–12 septa. Conidia (30–)35–48(–97) × (10.0–)13.7–16.5(–19.8) μm (n = 198), tapering to 4.5–6.0 μm at the distal end, with a blackish-brown 3–6 μm wide scar at the base, obpyriform to lageniform, straight or curved, smooth, pale brown, (4–)5–7(–10)-distoseptate, with angular lumina; wall up to 4.5(–6) μm thick.
Culture characteristics: Culture L169: On CMD colony radius ca. 16 mm after 1 mo at 22 °C. Colony yellow-green, turning dull yellowish brown, centre nearly black, yellowish pigment diffusing into agar (Fig. 3A); odour sweetish or unpleasant (“chemical”). On MEA colony radius 14 mm after 1 mo at 22 °C. Colony thick, dense, zonate, orange, centre whitish to pale greenish, reverse with black and pale orange zones (Fig. 3B).
Habitat and host range: On dead corticated twigs and trunks of Fagus sylvatica and Acer campestre: fungicolous on old ascomata of Amphisphaeria cf. millepunctata.
Distribution: Europe; only known from Austria.
Holotype: Austria, Wien, Döbling, Kahlenberg, on dead corticated twigs of Fagus sylvatica, 16 Nov. 2013, W. Jaklitsch (WU 38826; ex-holotype culture CBS 139924 = L132 (ex conidium); MBT376640).
Other specimens examined (all on corticated dead twigs or trunks): Austria, Kärnten, St. Margareten im Rosental, Zabrde, on Fagus sylvatica, 28 Dec. 2013, W. Jaklitsch [WU 38825, culture L137 (ex conidium)]; Wien, Ottakring, Wilhelminenberg, on Fagus sylvatica, 4 Dec. 2016, H. Voglmayr [WU 38827, culture CBS 142388 = L169 (ex conidium)]; Niederösterreich, Mannersdorf, Naturpark Wüste, on Acer campestre, 11 Feb. 2017, H. Voglmayr & I. Greilhuber [WU 38828, culture L177 (ex conidium)].
Notes: Helminthosporium austriacum is well characterised by its small, distinctly lageniform conidia in combination with Amphisphaeria cf. millepunctata, mostly on Fagus sylvatica. These hosts are shared with the polyphagous H. velutinum, with which it can co-occur. Helminthosporium austriacum is apparently fungicolous as all four collections were associated with old ascomata of Amphisphaeria cf. millepunctata. Also the orange colony colour seems to be characteristic, at least among the studied species. Helminthosporium austriacum has conidia of similar length as H. mauritanicum and H. acaciae (Ellis 1961); however, in the latter they are of different shape and significantly narrower (8–13 μm, mean 11.1 μm, and 10–14 μm, mean 12 μm, in H. mauritanicum and H. acaciae, respectively, vs. (10.0–)13.7–16.5(–19.8) μm, mean 15.1 μm, in H. austriacum). In addition, H. mauritanicum and H. acaciae occur on different hosts in (sub)tropical areas.
Helminthosporium caespitosum (Ellis & Barthol.) S. Hughes [as ‘Helmisporium cespitosum’], Canad. J. Bot. 36: 775. 1958. Fig. 6.
Fig. 6.
Helminthosporium caespitosum. A–E. Conidiomata in face view. F. Conidiomata in side view. G. Old stroma of Coryneum lanciforme in section below Helminthosporium conidioma. H–M. Conidiogenous cells with apical pore (L). N–F1. Conidia (young in N–R and mature in T–F1); N–X. vital, Y–F1. dead; F1. showing coarse verrucae on conidial wall. H–L, C1–F1. in 3 % KOH, M–B1. in water. A, C, D, F, M–X. WU 38829; B. WU 38831; E, G, H–L, Y–F1. CBS-H 713 (epitype). Scale bars: A = 2 mm; B = 1 mm; C, D, F = 500 μm; E, G = 200 μm; H–F1 = 20 μm.
Basionym: Exosporium caespitosum Ellis & Barthol. [as ‘cespitosum’], J. Mycol. 8(4): 178. 1902.
Synonyms: Corynespora caespitosa (Ellis & Barthol.) M.B. Ellis [as ‘cespitosa’], Mycol. Pap. 87: 39. 1963.
Corynespora bramleyi M.B. Ellis, Mycol. Pap. 76: 34. 1960.
Sexual morph unknown. Colonies on natural substrate forming conspicuous dark red brown, scattered or crowded conidiomata. Mycelium immersed, growing in aborted stromata or conidiomata of Coryneum below the periderm. Conidiomata 0.3–1.7(–3.6) mm wide (n = 63), 250–650 μm high (n = 30), superficial, stromatic, erumpent through the periderm, pulvinate to discoid, sometimes confluent, circular to ellipsoid, often irregularly lobed, internally composed of loose branched hyphae tending to be more compacted towards the surface. Conidiophores densely crowded, arising from the outer cell layer of the conidiomata, erect, simple, straight or curved, obpyriform, 0–2 septate, medium to dark reddish brown, (21–)27–37(–44) μm long, (11.2–)12.2–14.5(–16.5) μm wide (n = 80), with a swollen apex and a single conspicuous apical pore bearing the single conidium. Conidia (67–)82–109(–119) × (22.0–)27.3–35.5(–40.5) μm (n = 173), tapering to 3.5–9 μm at the distal end, with a 2.5–6 μm wide, dark brown to black scar at the base, broadly ellipsoid to obclavate, sometimes rostrate, straight or slightly curved, with coarse scale-like flat verrucae, medium to dark reddish brown, paler toward the apex, (3–)6–10-distoseptate with angular lumina; wall up to 8 μm thick.
Culture characteristics: Culture L141: On CMD colony radius ca. 22 mm after 4 wk at 22 °C. Colony white turning dull brownish from the centre, dense, thin, aerial hyphae inconspicuous or lacking (Fig. 3C); odour strong, unpleasant. On MEA colony radius 20 mm after 4 wk at 22 °C. Colony roundish, surface velvety, covered by a white dense flat mat of aerial hyphae, reverse yellowish (Fig. 3D); odour strong, unpleasant.
Habitat and host range: On dead corticated twigs of Betula spp.: fungicolous on old stromata of Coryneum lanciforme.
Distribution: North America, Northern Europe; widespread but uncommon.
Typification: USA, Michigan, Mackinac Island, on dead birch limbs, 10 Jul. 1899, E.T. Harper 452 (NY 00928681, holotype). Epitype, here designated: Canada, Québec, Gatineau Park, Pinks Lake, on dead corticated branches of Betula sp., without date, S.J. Hughes & W. Gams (CBS-H 000713; ex-epitype culture CBS 484.77; MBT376641).
Other specimens examined (all on dead corticated twigs of Betula spp.): Norway, Prov. Aust-Agder, Froland kommune, Ytre Lauvrak, on Betula pendula, 3 Oct. 2014, H. Voglmayr [WU 38829, culture L151 (ex conidium)]. Poland, Ruciane-Nida, Niedźwiedzi Róg, on Betula pubescens, 19 Jul. 2015, H. Voglmayr & I. Greilhuber (WU 38830). UK, England, West Yorkshire, Brighouse, on Betula pubescens, 18 Apr. 2014, C.S.V. Yeates [WU 38831, culture L141 (ex conidium)].
Notes: In the original description, the incorrect spelling “cespitosum” was used, which is here corrected to “caespitosum”, in accordance with MycoBank. Helminthosporium caespitosum is well characterised by its host (Betula spp.) and its large, dark red-brown conidiomata superficially resembling immature stromata of Hypoxylon. Conspecificity of North American and European accessions was confirmed by sequence data. A Canadian collection housed at Westerdijk Institute is chosen as epitype; this collection was misidentified as Exosporium tiliae, probably due to misidentification of the host, which is given as Tilia americana on the label. The sequences of this strain have been published as Corynespora olivacea (Crous et al. 2011). However, bark anatomy undoubtedly reveal the host as a Betula sp., and morphology as well as sequence data fully agree with the European collections.
Helminthosporium endiandrae (Crous & Summerell) Voglmayr & Jaklitsch, comb. nov. MycoBank MB821197.
Basionym: Corynespora endiandrae Crous & Summerell, in Crous et al., Persoonia 33: 229. 2014.
Holotype: Australia, New South Wales, Nightcap National Park, S28.33.918 E153.20.228, on leaves of Endiandra introrsa (Lauraceae), 9 Mar. 2013, B.A. Summerell (CBS H-21984; ex-holotype culture CPC 22194 = CBS 138902).
Notes: Corynespora endiandrae is not closely related to the generic type of Corynespora, C. cassiicola, but embedded within the Helminthosporium clade (Fig. 1). Also morphologically it fits the genus Helminthosporium as re-defined here in its non-proliferating conidiophores. In contrast to most other Helminthosporium species, H. endiandrae grows on leaves. For detailed descriptions and illustrations see Crous et al. (2014).
Helminthosporium genistae Fr. [as ‘Helmisporium’], Syst. mycol. (Lundae) 3(2): 360. 1832. Fig. 7.
Fig. 7.
Helminthosporium genistae. A. Colony in face view. B–D. Conidiophores with apical and lateral conidia in side view. E–H. Conidiophores. I. Conidiophore base and stroma cells. J, K. Conidiophore apices with apical (J, K) and lateral (K) conidia and pores (arrows). L–J1. Conidia (vital in L–F1, dead in G1–J1). E–G, L–F1. in water; H–K, G1–J1. in 3 % KOH. A–D, H–K, G1–J1. WU 38832 (epitype); E, G, Q–U. WU 38841; F, L–P, E1, F1. WU 38839; V–D1. WU 38834. Scale bars: A = 5 mm; B–D = 100 μm; E, G, H = 50 μm; F = 25 μm; I–J1 = 10 μm.
Sexual morph unknown. Colony on natural substrate effuse, black, hairy. Mycelium immersed, at the substrate surface forming stroma-like aggregations of subhyaline to dark brown pseudoparenchymatous cells (4.5–)6.0–11.8(–22.8) μm diam (n = 71). Conidiophores (155–)280–460(–560) μm long (n = 112), 15–23 μm wide at the base, tapering to 10.5–15 μm near the apex, arising usually in fascicles from stroma cells, simple, straight or flexuous, thick-walled, sub-cylindrical, smooth, brown to dark brown, with well-defined small pores at the apex and laterally beneath the upper 1–7 septa. Conidia (41–)51–73(–93) × (10.5–)12.7–15.8(–17.5) μm (n = 98), gradually tapering to 3–6.5(–8) μm at the distal end, with a 2–5 μm wide, blackish-brown to black scar at the base, straight or flexuous, obclavate to rostrate, smooth-walled, pale golden brown to brown, 5–12-distoseptate, with angular lumina; wall up to 6.5 μm thick.
Culture characteristics: Culture L142: On CMD colony radius ca. 10 mm after 4 wk at 22 °C. Colony white, dense, thick, zonate after exposure to light; aerial hyphae inconspicuous or lacking (Fig. 3E); odour unpleasant (cabbage-like). On MEA colony radius 9 mm after 4 wk at 22 °C. Colony with a slightly uneven margin, thick, with a white dense mat of aerial hyphae containing large drops; reverse yellow, brown in the centre (Fig. 3F); odour strong, unpleasant.
Habitat and host range: Saprobic on dead twigs of various fabaceous shrubs from the tribe Genisteae.
Distribution: Europe (France, Italy, Spain); apparently common in the mediterranean to submediterranean region.
Typification: France, on dead twigs of Cytisus scoparius, J.B. Mougeot, ex Herb. E. Fries (UPS: BOT: F-783304, holotype). Epitype, here designated: France, Côte-d'Or (21), Vieux-Château, on dead corticated twigs of Cytisus scoparius, 15 Apr. 2014, A. Gardiennet, A.G. 14089 [WU 38832; ex-epitype culture CBS 142597 = L142 (ex conidium); MBT376642].
Other specimens examined (all on dead corticated twigs): Greece, Crete, Chania, SW Lakki, on Chamaecytisus creticus, 5 Jun. 2015, H. Voglmayr & W. Jaklitsch [WU 38833, culture L173 (ex conidium)]. Italy, Lazio, Viterbo, Bomarzo, Monte Casoli, on Cytisus scoparius, 17 Oct. 2013, H. Voglmayr & W. Jaklitsch [WU 38834, culture CBS 139922 = L129 (ex conidium)]; Viterbo, Gradoli, Il Purgarorio, on Cytisus scoparius, 13 Oct. 2013, H. Voglmayr & W. Jaklitsch [WU 38835, culture CBS 139921 = L128 (ex conidium)]; Viterbo, Norchia, on Cytisus scoparius, 14 Oct. 2013, H. Voglmayr & W. Jaklitsch [WU 38836, culture L125 (ex conidium)]. Portugal, Sintra, Castelo dos Mouros, on Cytisus cf. striatus, 16 Feb. 2017, H. Voglmayr & W. Jaklitsch (WU 38899). Spain, Andalucia, Cádiz, Alcalá de los Gazules, El Picacho, on Cytisus baeticus, 1 Apr. 2014, W. Jaklitsch [WU 38837, culture L143 (ex conidium) = CBS 139927]; Huelva, Castaño de Robledo, on Ulex parviflorus, 8 Apr. 2014, W. Jaklitsch [WU 38838, culture L147 (ex conidium)]; Huelva, Castaño de Robledo, on Cytisus striatus, 8 Apr. 2014, W. Jaklitsch [WU 38839, culture CBS 139929 = L148 (ex conidium)]; Jaén, Otiñar, La Castañeda, on Cytisus fontanesii, 12 May 2014, W. Jaklitsch [WU 38840, culture CBS 139930 = L149 (ex conidium)]; Jimena, Montes de Jimena, Puerto Galis, on Calicotome villosa, 4 Apr. 2014, W. Jaklitsch [WU 38841, culture CBS 139928 = L144 (ex conidium)]; Málaga, Cortes de la Frontera, La Sauceda, on Cytisus baeticus, 4 Apr. 2014, W. Jaklitsch [WU 38842, culture L145 (ex conidium)]; Canarias, La Gomera, Alto de Garajonay, on Chamaecytisus proliferus, 21 Mar. 2016, H. Voglmayr (WU 35976).
Notes: Helminthosporium genistae is morphologically similar to the polyphagous H. velutinum, which is also found on the type host, Cytisus scoparius, and it has been synonymised with the latter by Ellis (1961). However, sequence data reveal H. genistae as a distinct taxon. Culture morphology and growth rates also differ substantially between the species. The type collection of H. genistae preserved in the Fries herbarium at UPS has been collected by Mougeot, presumably in eastern France, and sequence data from a recent French collection confirm that the species occurs in this area. Due to the morphological similarities with H. velutinum (in absence of cultures), which also occurs on the type host, and due to the depauperate type collection which is not sent out for study, we here epitypify H. genistae with a recent collection for which sequence data and a culture are available.
Helminthosporium hispanicum Voglmayr & Jaklitsch, sp. nov. MycoBank MB821198. Fig. 8.
Fig. 8.
Helminthosporium hispanicum (WU 38843, holotype). A. Two conidiomata in face view. B, C. Conidiophores with apical conidia in side view. D. Conidiophores. E, F, H. Conidiophore apices with apical pore (E, arrow) and apical young (F) and mature conidia (H, arrow). G. Conidiophore base and stroma cells. I–O. Conidia (vital in I–N, dead in O). All in water; except G, O in 3 % KOH. Scale bars: A = 200 μm; B, C = 100 μm; D = 20 μm; E–O = 10 μm.
Etymology: Referring to Spain, where the type has been collected.
Sexual morph unknown. Colony on natural substrate punctiform, black, hairy, 140–700 μm diam. Mycelium mostly immersed, towards the surface forming stroma-like aggregations of light to dark brown pseudoparenchymatous cells (6.3–)9.5–15.0(–20) μm diam (n = 80). Conidiophores 130–540 μm long, 13–22.5 μm wide at the base, tapering to 8–15 μm near the apex, arising solitarily or in small groups from the stroma cells, erect, simple, straight or flexuous, thick-walled, sub-cylindrical, smooth, dark to blackish brown, paler near the apex, with well-defined small pores at the apex and rarely laterally beneath the upper 1–2 septa. Conidia 69–99(–130) × (17–)18–21(–24) μm (n = 20), tapering to 5.5–8 μm at the distal end, with a blackish-brown 4–6 μm wide scar at the base, obclavate, straight or flexuous, thin-walled, smooth, pale brown, (4–)6–11(–14)-distoseptate, with angular lumina; wall up to 7 μm thick.
Habitat and host range: On dead corticated twigs of Juglans regia: fungicolous on old conidiomata of Juglanconis juglandina.
Distribution: Only known from the type collection in Asturias (Spain).
Holotype: Spain, Asturias, Selviella, on dead corticated twigs of Juglans regia, 1 Jun. 2013, W. Jaklitsch & H. Voglmayr [WU 38843; ex-holotype culture CBS 136917 = L109 (ex conidium); MBT376644].
Notes: Helminthosporium hispanicum grows on Juglans regia, a host which is also colonised by H. juglandinum, H. juglandis and the polyphagous H. velutinum; for comparison see notes under H. juglandinum below. Both H. hispanicum and H. juglandinum are fungicolous but colonise different hosts: H. hispanicum grows on old conidiomata of Juglanconis juglandina and H. juglandinum on conidiomata of a Diaporthe sp.
Helminthosporium juglandinum Voglmayr & Jaklitsch, sp. nov. MycoBank MB821199. Fig. 9.
Fig. 9.
Helminthosporium juglandinum. A. Colony in face view. B. Punctiform conidiomata in face view. C. Conidiomata in side view with column-like subcortical stromata representing transformed conidiomata of Diaporthe. D, E. Conidiophores, in E with young apical conidium. F–I. Conidiophore apices with apical conidia in H, I. J. Thick-walled stroma cells in section. K. Conidiophore base (arrow) and stroma cells. L. Conidiophores on stroma in section. M–G1. Vital conidia. All in water. A–C, G, P, Q, S, T, V–Y, B1–D1, F1, G1. WU 38845 (holotype); D, Z, E1. WU 38844; E, F, J–L. WU 35975; H, I, M–O, R. WU 38847; U, A1. WU 38848. Scale bars: A = 5 mm; B = 500 μm; C = 100 μm; D, E = 20 μm; F–K, M–G1 = 10 μm; L = 50 μm.
Etymology: Referring to its growth on Juglans spp.
Sexual morph unknown. Colonies on natural substrate discrete, punctiform, 0.3–1 mm wide, sometimes confluent, usually in large groups, blackish brown. Mycelium immersed, growing in aborted conidiomata of Diaporthe sp., the latter becoming transformed into distinct column-like, 0.3–1.1 mm wide and 200–450 μm high stromata below the periderm. Conidiophores (175–)215–325(–455) μm long (n = 120), 11–23 μm wide at the base, 8.5–14 μm wide near the slightly inflated apex, fasciculate, arising from the upper cells of the stromata, erect, simple, straight or flexuous, thick-walled, sub-cylindrical, smooth, brown to dark brown, darker to black at the apex, the latter with a well-defined apical pore. Conidia (69–)89–145(–205) × (15.0–)16.5–20.0(–25.0) μm (n = 83), tapering to 4.5–10 μm at the distal end, with a 3.5–7 μm wide blackish-brown scar at the base, rostrate, straight or flexuous, thin-walled, smooth, pale brown, (5–)9–17(–20)-distoseptate, with angular lumina; wall up to 12 μm thick.
Culture characteristics: Culture L118: On CMD colony radius 15 mm after 4 wk at 22 °C. Colony dense, thin, with brown and bluish zones eventually turning black, irregular whitish margin with bluish shimmer; surface resinous due to condensed excretions of shimmery organic compounds (Fig. 3G); odour unpleasant (“chemical”). On MEA colony radius 14 mm after 4 wk at 22 °C. Colony irregularly lobate, reddish brown, with a white to rosy mat of aerial hyphae at the margin, reverse rosy-brown (Fig. 3H); odour weak, fruity.
Habitat and host range: On dead corticated twigs of Juglans regia: fungicolous on conidiomata of Diaporthe sp.
Distribution: Europe (Austria, Italy).
Holotype: Austria, Niederösterreich, Gießhübl, on dead corticated twigs of Juglans regia, 1 Sep. 2013, H. Voglmayr (WU 38845; ex-holotype culture CBS 136922 = L118 (ex conidium); MBT376645).
Other specimens examined (all on dead corticated twigs of Juglans regia except where noted): Austria, Kärnten, St. Margareten im Rosental, Wograda, 30 Dec. 2012, W. Jaklitsch [WU 38844, culture L101 (ex conidium) = CBS 136912]; Niederösterreich, Orth/Donau, on Juglans nigra, 26 Jan. 2013, H. Voglmayr & I. Greilhuber [WU 38846, culture L102 (ex conidium) = CBS 136913]; Orth/Donau, on Juglans nigra, 19 May 2013, H. Voglmayr & W. Jaklitsch (WU 38847); Mühlleiten, on Juglans nigra, 4 Dec. 2016, H. Voglmayr & I. Greilhuber (WU 35975). Italy, Toscana, Grosseto, Pitigliano, 23 Oct. 2012, W. Jaklitsch & H. Voglmayr [WU 38848, culture L97 (ex conidium) = CBS 136911]; Grosseto, Sovana, 23 Oct. 2012, W. Jaklitsch & H. Voglmayr (WU 38849).
Notes: Helminthosporium juglandinum appears to be the most common of the three species known on Juglans in Europe and is apparently confined to that host. Another species, Helminthosporium juglandis, has been described from Juglans in China (Zhao & Zhao 2012), but it clearly differs by much narrower conidia (10–12.7 μm). Helminthosporium hispanicum is morphologically highly similar to Helminthosporium juglandinum but differs by growth on old conidiomata of Juglanconis juglandina and by sequence data.
The description and illustrations of Exosporium stylobatum Curzi & Barbaini (1927), described in Italy from Juglans regia, closely resemble H. juglandinum. However, no original material could be obtained for investigation, and ITS (JQ044428) and LSU (JQ044447) sequences from the ex-type culture (CBS 160.30) are almost identical to those of Massarina corticola, which is not a member of Massarinaceae but of the distantly related Amorosiaceae (Thambugala et al. 2015). Interestingly, we isolated Massarina corticola from Juglans regia close to a colony of H. juglandinum, but the connection with Exosporium stylobatum remains obscure. In the light of these discrepancies, and due to the fact that three similar Helminthosporium species are known from Juglans in Europe (H. hispanicum, H. juglandinum and H. velutinum), Exosporium stylobatum remains a mystery, and it should be considered a nomen dubium.
Helminthosporium kalakadense (Subram. & Sekar) Voglmayr & Jaklitsch, comb. nov. MycoBank MB821200.
Basionym: Splanchnonema kalakadense Subram. & Sekar, Kavaka 15(1–2): 89. 1989 [1987].
Holotype: India, Tamil Nadu, Tirunelveli, Kalakad, Sengaltheri Forest, on dead unidentified twig, 24 Aug. 1980, G. Sekar (IMI 324680).
Notes: Ex-ascospore isolates of S. kalakadense produced a helminthosporium-like asexual morph closely resembling Helminthosporium velutinum (Subramanian & Sekar 1987). The morphological features of its sexual morph match the splanchnonema-like sexual morphs recorded for Helminthosporium in the present study. Although no sequence data are available, there is no doubt that the species belongs to Helminthosporium. However, we do not consider it to be conspecific with H. velutinum, for which no sexual morph is known and which differs by wider conidia [13–15 vs. (11–)14–18.5(–25) μm in H. velutinum]. Therefore we combine S. kalakadense in Helminthosporium here.
Helminthosporium leucadendri (Quaedvl. et al.) Voglmayr & Jaklitsch, comb. nov. MycoBank MB821201.
Basionym: Corynespora leucadendri Quaedvl. et al., Stud. Mycol. 75: 382. 2013.
Holotype: South Africa, Western Cape Province, Helderberg Nature Reserve, on leaves of Leucadendron sp. (Proteaceae), 14 Aug. 2000, S. Lee (CBS H-21323; ex-holotype culture CBS 135133 = CPC 19345).
Notes: Corynespora leucadendri is not closely related to the generic type of Corynespora, C. cassiicola, but was revealed as a member of the Helminthosporium clade (Tanaka et al. 2015, Fig. 1, Fig. 2). Also morphologically it fits the genus Helminthosporium as re-defined here in its non-proliferating conidiophores. In contrast to most other Helminthosporium species, H. leucadendri grows on leaves. For detailed descriptions and illustrations see Quaedvlieg et al. (2013).
Helminthosporium microsorum D. Sacc. [as ‘Helmisporium’], Malpighia 12: 219. 1898. Fig. 10, Fig. 11.
Fig. 10.
Helminthosporium microsorum, sexual morph. A–D. Ascomata in horizontal (A, B) and vertical (C, D) section, surrounded by well-developed pseudostromata. E. Three ostioles in face view. F, G. Peridium and pseudostroma in section. H. Pseudostroma with coarsely verrucose marginal hyphae in section. I. Coarsely verrucose marginal hyphae. J. Ascus with vital ascospores. K. Pseudoparaphyses. L. Immature ascospore. M–H1. Mature vital (sub)hyaline ascospores surrounded by gel sheath, eventually brown in age (X, Y, F1–H1). All in water, except F–I. in 3 % KOH. A, C–R, W–Y. WU 38860; B, S–V. WU 38850 (epitype); Z. WU 38852; A1–H1. WU 38854. Scale bars: A–E = 200 μm; F, H, J = 20 μm; G, I, K–H1 = 10 μm.
Fig. 11.
Helminthosporium microsorum, asexual morph. A. Colony in face view. B. Two punctiform conidiomata in face view. C. Conidioma in side view. D. Old stroma of Coryneum in section below Helminthosporium conidioma. E. Conidiophores. F, I. Conidiophore apices with apical and lateral pores (arrows). G. Conidiophore base and stroma cells in section. H. Stroma cells in section. J–U. Conidia (vital in J–T, dead in U). All in water, except I. in 3 % KOH. A–C, E–H. WU 38861; D, N, O, Q–S. WU 38860; I. WU 38858; J–M, P, T, U. WU 38850 (epitype). Scale bars: A = 2 mm; B, D = 200 μm; C = 100 μm; E = 50 μm; F–U = 10 μm.
Synonym: Massarinula italica D. Sacc., Malpighia 12: 207. 1898.
Sexual morph. Pseudostromata formed in the upper bark, well-developed, dark reddish brown, pseudoparenchymatous, of thick-walled dark brown cells (5.2–)9.0–14.5(–17.5) × (3.8–)5.5–9.2(–13.3) μm (n = 90); margin composed of dark brown, coarsely verrucose hyphae (16–)23–37(–41) × (5.2–)5.5–8.0(–9.0) μm (n = 19). Ascomata immersed in pseudostromata, distinctly elevating the bark, singly or sometimes in small groups, 425–713 μm diam (n = 20), 136–320 μm high (n = 10) (including pseudostromatal margin), strongly depressed, entirely filled by pure white hymenium, peridium (10.6–)13.8–24.5(–30.2) μm thick (n = 27), pseudoparenchymatous, of pale to medium brown cells (3.4–)4.1–6.3(–8.4) μm wide (n = 34). Ostioles central, (86–)123–234(–262) μm long, (86–)95–159(–178) μm wide (n = 7). Hamathecium of filiform, septate, branched, anastomosing, 1.8–3.5 μm wide pseudoparaphyses, extending and filling the ostiole. Asci (154–)169–206(–218) × (36–)39–47(–49) μm (n = 15), clavate, containing 8 irregularly biseriate ascospores. Ascospores (35–)43–61(–86) × (16–)18–21.5(–25.5) μm, l/w = (1.9–)2.2–3.1(–4) (n = 140), hyaline to subhyaline, turning light to medium brown at full maturity, dark brown after ejection, subellipsoid to obovoid, rarely fusoid, asymmetric, 1-septate, with few to numerous ring-like thickenings of the inner wall giving the inner wall an irregularly wavy outline, at full maturity sometimes developing into thin transverse distosepta, strongly constricted at the primary septum, with usually rounded, rarely subacute end cells; length of larger hemisphere/total length of ascospore = (0.52–)0.57–0.62(–0.66), mean = 0.59 (n = 110); wall smooth, hyaline, at maturity light brown; the contents granular, sometimes with a large and several smaller guttules per cell; each hemisphere surrounded by a thick gelatinous sheath. Asexual morph. Colonies on natural substrate punctiform, black, hairy, usually in patches. Mycelium immersed, growing in aborted stromata or conidiomata of Coryneum below the periderm. Conidiophores (96–)167–383(–564) μm long (n = 55), (11.5–)12.5–15.8(–17.2) wide at the base (n = 28), (8.8–)10.2–12.0(–13.5) μm wide near the apex (n = 27), fasciculate, arising from upper cells of the stromata, simple, flexuous, cylindrical, dark brown, smooth-walled, septate, with a pore at the apex and often 1–2 lateral pores beneath the upper 1–2 septa. Conidia (85–)93–121(–141) × (16–)17–20(–22) μm (n = 25), tapering to 5–9 μm at the distal end, with a 5–7.5 μm wide blackish-brown to black scar at the base, arising terminally and sometimes laterally through pores or thin areas in the conidiophore wall, obclavate, pale to golden-brown, smooth-walled, 7–11(–17)-distoseptate, with angular lumina; wall up to 5.7 μm thick.
Culture characteristics: Culture L96: On CMD colony radius ca. 10 mm after 4 wk at 22 °C. Colony whitish to pale yellowish, dense, thick, aerial hyphae inconspicuous or lacking (Fig. 3I); odour strong, chemical to fruity or rancid. On MEA colony radius up to 21 mm after 4 wk at 22 °C. Colony margin irregular, dense, whitish, with long white aerial hyphae, reverse pale yellowish, centre nearly black (Fig. 3J); odour strong, unpleasant (rancid-fruity).
Habitat and host range: On dead corticated twigs of Quercus spp. (confirmed for Q. brachyphylla, Q. cerris, Q. coccifera, Q. ilex, Q. macrolepis, Q. suber): fungicolous on old stromata or conidiomata of Coryneum sp.
Distribution: With certainty only known from Europe (Croatia, England, Greece, Italy, Portugal, Spain).
Typification: Lectotype of Helminthosporium microsorum, here designated: Italy, Padova, Orto Botanico, on branches of Quercus ilex, Jun. 1897, D. Saccardo, Mycotheca italica 194 [K(M) 233086!; MBT376646]. Lectotype of Massarinula italica, here designated: D. Saccardo, Contribuzione alla micologia veneta e modenese, Malpighia 12, 1898, tav. VII, Fig. 3a–d (iconotype); MBT376647. Epitype of Helminthosporium microsorum and of Massarinula italica, here designated: Italy, Toscana, Grosseto, Pitigliano, on dead corticated twigs of Quercus ilex, 23 Oct. 2012, H. Voglmayr & W. Jaklitsch [WU 38850; ex-epitype culture CBS 136910 = L96 (ex ascospore); MBT376648, MBT376649].
Other specimens examined (all on dead corticated twigs of Quercus ilex except where noted): Croatia, Istria, Rovinj, 14 May 2015, H. Voglmayr (WU 38851). Greece, Crete, between Lakki and Omalos, on Quercus coccifera, 5 Jun. 2015, H. Voglmayr & W. Jaklitsch [WU 38852, culture L175 (ex conidium)]; N Omalos, on Quercus coccifera, 5 Jun. 2015, H. Voglmayr & W. Jaklitsch (WU 38853); NE Askifou, on Quercus coccifera, 6 Jun. 2015, H. Voglmayr & W. Jaklitsch [WU 38854, culture L174 (ex ascospore)]; Pananiana, 4 Jun. 2015, H. Voglmayr & W. Jaklitsch (WU 38855); Rethymno, Kaloniktis, on Quercus macrolepis, 7 Jun. 2015, H. Voglmayr & W. Jaklitsch (WU 38856); Rethymno, Palelimnos, on Quercus coccifera, 7 Jun. 2015, H. Voglmayr & W. Jaklitsch (WU 38857); Crete, Chania, Zounaki, on Quercus brachyphylla, 4 Jun. 2015, H. Voglmayr & W. Jaklitsch (WU 35974). Italy, Padova, without date, P.A. Saccardo, in Briosi & Cavara, Fungi parassitici 332 [K(M) 233087!, PAD!]. Padova, Orto Botanico, 6 Apr. 2016, H. Voglmayr & W. Jaklitsch (WU 38858); Toscana, Pisa, Tirrenia, 30 Oct. 2015, W. Jaklitsch (WU 38859); Lazio, Viterbo, Bomarzo, La Pyramide, on Quercus cerris, 22 Oct. 2012, H. Voglmayr & W. Jaklitsch [WU 38860, culture L94 (ex ascospore), L95 (ex conidium)]; Viterbo, Vulci, 15 Oct. 2013, H. Voglmayr & W. Jaklitsch [WU 38861, culture L123 (ex conidium)]. Portugal, Sintra, Castelo dos Mouros, 16 Feb. 2017, H. Voglmayr & W. Jaklitsch (WU 35971); Sintra, Monserrate, on Quercus suber, 18 Feb. 2017, H. Voglmayr & W. Jaklitsch (WU 35972). Spain, Andalucia, Granada, SW Montefrio, 11 May 2014, W. Jaklitsch (WU 38862); Asturias, Pola de Somiedo, 2 Jun. 2013, H. Voglmayr [WU 38863, culture CBS 136916 = L108 (ex conidium)].
Notes: Helminthosporium microsorum and its sexual morph, Massarinula italica, were described and illustrated in the same publication (Saccardo 1898), but no connection was made between them. DNA sequence data from cultures obtained from sexual and asexual morphs revealed conspecificity of both morphs. At PAD, only a duplicate from Briosi & Cavara's Fungi parassitici 332 is extant, which was collected at the type locality by P.A. Saccardo; no date is given but it has likely been collected after the description of the species. We therefore lectotypify H. microsorum with specimen K(M) 233086 distributed as part of D. Saccardo's Mycotheca italica 194, which is cited in the protologue. As no collection of Massarinula italica appears to be extant in PAD, we select the illustrations in Saccardo (1898) as lectotype. For nomenclatural stability, we epitypify both names with the same recent collection containing the holomorph, for which a culture and DNA sequence data are available. Helminthosporium microsorum is a common species particularly on Quercus ilex in the Mediterranean. It grows on senescent stromata or conidiomata of Coryneum sp., which are commonly entirely filled and transformed by its hyphae; they have been mistaken for immersed stromata by Ellis (1961).
The ascospores from the Cretan collections from Quercus coccifera occasionally developed additional thin distosepta (see Fig. 10E1–H1) and were longer than those from the other collections [(49–)52–68(–86) vs. (35–)42–50.5(–54) μm], but as their ITS-LSU sequences were (almost) identical, this is considered to be within the range of the species.
Helminthosporium oligosporum (Corda) Hughes [as ‘Helmisporium’], Canad. J. Bot. 36: 775. 1958. Fig. 12.
Fig. 12.
Helminthosporium oligosporum, sexual (A–V) and asexual (W–X1) morph. A. Three ostioles in face view. B–D. Ascomata in horizontal (B, C) and vertical (D) section, surrounded by a well-developed pseudostroma (B, D. showing fresh hydrated ascomata). E. Peridium and pseudostroma in section. F. Ascus with vital ascospores. G. Pseudoparaphyses. H–V. Mature vital ascospores surrounded by gel sheath; in M. showing germinating ascospore, in N. verruculose ascospore wall. W–Y. Conidiomata in face view. Z. Conidioma on old Hercospora tiliae stroma in vertical section. A1. Old stroma of Hercospora tiliae in section below conidioma. B1–D1. Conidiogenous cells with apical pore and young conidium (D1). E1–X1. Vital conidia (young in E1–H1, mature in I1–X1). All in water. A, B, D, G–R, Y. WU 38866; C, E, F, S–V, P1. WU 38864 (epitype); W, X, B1–E1, I1–M1, O1, Q1–W1. WU 38867; Z, A1, F1–H1. WU 38870; N1, X1. WU 38872. Scale bars: A, X, Y = 500 μm; B–D, A1 = 200 μm; E, F = 20 μm; G–V, B1–X1 = 10 μm; W = 1 mm; Z = 300 μm.
Basionym: Coryneum oligosporum Corda, Icon. Fung. 5: 81. 1842.
Synonyms: Sporidesmium olivaceum Wallr., Fl. crypt. Germ. (Norimbergae) 2: 228. 1833, non Helminthosporium olivaceum Berk. & Ravenel, in Berkeley, Grevillea 3(no. 27): 102. 1875.
Clasterosporium olivaceum (Wallr.) Sacc., Syll. fung. (Abellini) 4: 390. 1886.
Corynespora olivacea (Wallr.) M.B. Ellis, Mycol. Pap. 76: 32. 1960.
For additional synonyms, see Hughes (1958).
Sexual morph. Pseudostromata formed in the upper bark, well-developed, dark brown. Ascomata surrounded by pseudostroma, not to slightly elevating the bark and scarcely noticeable from outside, single, (580–)645–890(–1 045) μm diam (n = 32) (including pseudostromatal margin), globose to depressed globose, dark brown, peridium (including pseudostromatal margin) (38–)55–85(–93) μm thick (n = 36), pseudoparenchymatous, of medium to dark brown cells (4.2–)8.0–16.2(–22) μm (n = 52). Ostioles central, scarcely visible in surface view, not protruding above the cortical surface. Hamathecium of densely packed filiform, septate, branched, anastomosing, 2–4 μm wide pseudoparaphyses embedded in a tough gel matrix. Asci (202–)230–318(–376) × (34–)39.5–51.5(–54.5) μm (n = 21), clavate, containing 8 irregularly biseriate ascospores. Ascospores (44–)49–62(–70) × (11.5–)13.0–15.5(–18.5) μm, l/w = (2.6–)3.3–4.5(–5.9) (n = 129), light to medium brown, fusoid to elongate, strongly asymmetric, first 1-septate, developing (2–)3(–5) additional transverse, occasionally oblique distosepta in the larger and 1(–2) in the smaller (lower) hemisphere, sometimes with a thin longitudinal septum in the inner cell of the larger hemisphere, strongly constricted at the primary septum, slightly or not constricted at the secondary distosepta, with subacute to rounded end cells; length of larger hemisphere/total length of ascospore = (0.56–)0.60–0.67(–0.72), mean = 0.63 (n = 111); wall finely verruculose, brown; the contents granular; each hemisphere surrounded by a thick gelatinous sheath. Asexual morph. Colonies on natural substrate of conspicuous scattered or crowded dark brown to black conidiomata. Mycelium immersed, growing in aborted stromata or conidiomata of Hercospora tiliae below the periderm. Conidiomata 0.1–2.8 mm wide, 70–960 μm high (n = 42), superficial, stromatic, erumpent through the periderm, hemispherical to pulvinate, sometimes confluent, circular, sometimes irregularly lobed, inside composed of loosely compacted, branched, anastomosing and very thick-walled (up to 6 μm) hyphae, outside forming a brown to dark brown continuous layer of pseudoparenchymatous cells. Conidiophores (17–)22–35(–46) μm long, (8.0–)8.5–10.5(–11.5) μm wide (n = 23), densely crowded, arising from the outer conidiomatal cells, erect, simple, straight, cylindrical to slightly swollen at the apex, brown to dark brown, darker at the apex, 0–2 septate, smooth, with a single conspicuous apical pore bearing the single conidium. Conidia (37–)59–80(–124) × (14.8–)15.8–18.0(–20.0) μm (n = 111), tapering to 4–10.5 μm at the distal end, with a 4–8 μm wide dark brown to black scar at the base, obclavate, sometimes rostrate, straight or curved, smooth but occasionally wrinkled with age, pale brown to brown, paler toward the apex, 6–12(–16)-distoseptate, with angular lumina; wall up to 6 μm thick.
Culture characteristics: Culture L93: On CMD colony radius ca. 24 mm after 4 wk at 22 °C. Colony black, margin white (Fig. 3K); odour unpleasant. On MEA colony radius 22 mm after 4 wk at 22 °C. Colony whitish floccose by aerial hyphae, reverse yellowish (Fig. 3L); odour unpleasant.
Habitat and host range: On dead corticated twigs of Tilia spp.: fungicolous on aborted conidiomata and stromata of Hercospora tiliae.
Distribution: Widespread in Europe and North America (Hughes 1983).
Typification: Holotype of Sporidesmium olivaceum: Germany, on rotten branches of Tilia, Herb. Wallroth (Wallroth genus no. 192, Wallroth species no. 1700) (STR 91001). Lectotype of Coryneum oligosporum, here designated: Czech Republic, S Praha, Zbraslav (Königsaal), on rotten branches of Corylus (re-identified as Tilia, based on bark anatomy), without date, Corda (PRM 155452; MBT376650). Same data, ex herb Berkeley [K(M) 233686, IMI 74988, isotypes]. Epitype of Sporidesmium olivaceum and of Coryneum oligosporum, here designated: Austria, Niederösterreich, Heiligenkreuz, Kreuzweg, on dead corticated twigs of Tilia cordata, 14 Oct. 2012, H. Voglmayr & I. Greilhuber [WU 38864; ex-epitype culture CBS 136909 = L93 (ex ascospore); MBT376651, MBT376652].
Other specimens examined (all on dead corticated twigs): Austria, Niederösterreich, Heiligenkreuz, Kreuzweg, on Tilia cordata, 13 Jan. 2013, H. Voglmayr & I. Greilhuber (WU 38865); ibid., 8 Dec. 2016, H. Voglmayr & I. Greilhuber (WU 38866); St. Corona/Schöpfl, on Tilia platyphyllos, 14 Oct. 2012, H. Voglmayr & I. Greilhuber [WU 38867, culture CBS 136908 = L92 (ex conidium)]; Steiermark, Graz, Botanical Garden of the University, on Tilia platyphyllos, 15 Oct. 2012, C. Scheuer (WU 38868); Wien, Floridsdorf, Marchfeldkanalweg, on Tilia cordata, 14 Oct. 2012, W. Jaklitsch [WU 38869, culture L106 (ex conidium)]; Währing, Türkenschanzpark, on Tilia platyphyllos, 16 May 2013, H. Voglmayr (WU 38870). France, Côte-d'Or (21), Véronnes, on Tilia sp., 20 Nov. 2013, A. Gardiennet, A.G. 13220 (WU 38871). Spain, Asturias, Pola de Somiedo, on Tilia platyphyllos, 3 Jun. 2013, H. Voglmayr & W. Jaklitsch [WU 38872, culture L111 (ex conidium)]; Pola de Somiedo, Saliencia, on Tilia platyphyllos, 2 Jun. 2013, H. Voglmayr & W. Jaklitsch (WU 35977).
Notes: The description of the asexual morph has been modified from Hughes (1983). Helminthosporium oligosporum has been commonly known as Corynespora olivacea. Classification in Corynespora goes back to Ellis (1960), who found that the conidiophore “sometimes proliferates through the apical pore and forms another conidium at the apex of the proliferation”. However, neither Luttrell (1963) nor Hughes (1983), who investigated about 70 collections from North America and Europe, observed a regular proliferation of conidiophores, which concurs with our observations. According to the results of molecular phylogeny, Hughes (1958) was correct in placing the species in Helminthosporium. As in Helminthosporium the epithet olivaceum is pre-occupied by a different species (Helminthosporium olivaceum Berk. & Ravenel), the next available name, Coryneum oligosporum Corda, was combined in Helminthosporium by Hughes (1958). The host for the latter was given as Corylus, but bark and wood anatomy of the type specimen (PRM 155452) reveals it as Tilia. To stabilise the connection between both names, we here epitypify Sporidesmium olivaceum and Coryneum oligosporum with a recent collection for which cultures and DNA data are available from the sexual and asexual morphs.
According to our knowledge, the sexual morph is here described for the first time. Sister group relationship to H. tiliae, which also occurs on Tilia spp. and which is the generic type of Exosporium (see below), received maximum support.
Like its close relative H. tiliae, H. oligosporum consistently grows on aborted conidiomata or stromata of Hercospora tiliae. On the same twigs we observed apparently healthy stromata close to infected ones bearing conidiomata of H. oligosporum, in which aborted host perithecia were still visible (see Fig. 12A1). However, most host stromata are completely filled and transformed by hyphae of H. oligosporum, but usually the diagnostic black line delimiting the stromata of Hercospora tiliae is still visible. The true nature of these structures has not been correctly recognised before; they have been interpreted as subperidermal stromata by Ellis (1960) and Hughes (1983).
Helminthosporium quercicola (M.E. Barr) Voglmayr & Jaklitsch, comb. nov. MycoBank MB821202. Fig. 13.
Fig. 13.
Helminthosporium quercicola (NY 00914609, holotype), sexual (A–U) and asexual (V–C1) morph. A. Ostioles in face view. B. Ascoma in face view. C. Ascoma in horizontal section; the dark zone surrounding the ascoma representing blackened host tissue, no pseudostroma apparent. D. Two ascomata in vertical section. E. Peridium in section. F. Smooth hyphae at the peridial margin. G. Pseudoparaphyses. H–U. Mature ascospores, in Q, R. showing transversely sectioned spores, in S–U. densely disposed striations in the ascospore wall. V. Conidiomata in face view. W, X. Conidiophores with apical conidia (arrows) in side view. Y. Conidiophore base and stroma cells in section. Z. Conidiophore apex with apical pore (arrow). A1. Conidiophore fragment showing a lateral pore (arrow). B1, C1. Young (B1) and mature (C1) conidium. G, P, U, Y, B1, C1. in water; Z, A1. in 3 % KOH; all others from permanent slides. Scale bars: A–C, V = 200 μm; D, W, X = 100 μm; E–R, Y–C1 = 10 μm; S–U = 5 μm.
Basionym: Splanchnonema quercicola M.E. Barr, Mycotaxon 49: 140. 1993.
Sexual morph. Ascomata immersed in the upper bark, strongly elevating the bark, towards the apex sometimes covered by a weakly developed, rudimentary pseudostroma of thin-walled, light brown, smooth hyphae, singly or in groups of two, 300–600 μm diam, 200–300 μm high, depressed subglobose, dark reddish brown, peridium 24–45 μm thick (n = 15), pseudoparenchymatous, of reddish brown cells (4.0–)4.5–11.5(–19.2) × (2.0–)2.5–4.5(–6.0) μm (n = 51). Ostioles central, 100–160 μm wide. Hamathecium of filiform, septate, branched, anastomosing, 1–3 μm wide pseudoparaphyses. Asci 150–175 × 30–40 μm, clavate, containing 8 irregularly biseriate ascospores. Ascospores (46–)49–57(–64) × (18.5–)19.3–21.5(–22.8) μm, l/w = (2.2–)2.4–2.8(–3.1) (n = 29), brown, subellipsoid to obovoid, distinctly asymmetric, first 1-septate, developing 0–3 additional transverse distosepta in the larger and 0–3 in the smaller hemisphere, strongly constricted at the primary septum, slightly or not constricted at the secondary distosepta, with subacute to rounded end cells; length of larger hemisphere/total length of ascospore = (0.55–)0.58–0.62(–0.66), mean = 0.60 (n = 34); wall smooth, pale to medium brown, with densely disposed longitudinal striae, apparently within the wall; guttulate when young; each hemisphere surrounded by a gelatinous sheath.
Asexual morph. Colony on natural substrate effuse, black, hairy. Mycelium immersed, apparently growing in aborted conidiomata of Coryneum below the periderm. Conidiophores (115–)133–226(–300) μm long (n = 47), 14–20 μm wide at the base, tapering to 10–15 μm near the apex, arising solitarily or in fascicles from the cells of a reduced stroma, simple, straight or flexuous, cylindrical, thick-walled, smooth, brown to dark brown, with well-defined small pores at the apex and laterally beneath the upper septa. Conidia ca. 60–100 × 15–22 μm, gradually tapering to 8–9 μm at the distal end, with a ca. 7 μm wide blackish-brown to black scar at the base, straight or flexuous, obclavate, smooth-walled, brown, 8–10-distoseptate, with angular lumina; wall up to 5.5 μm thick.
Habitat and host range: On dead corticated twigs of Quercus cf. reticulata: apparently fungicolous on old stromata or conidiomata of Coryneum sp.
Distribution: North America (USA).
Holotype: USA, Arizona, Pima Co., Santa Catalina Mts., Creek, 1 219 m (4 000 ft.), on dead corticated branches of Quercus cf. reticulata, 2 Aug. 1980, M.E. Barr Bigelow 6797 (NY 00914609!).
Notes: Helminthosporium quercicola is so far only known from the type collection. As the material is very sparse, we based our observations and illustrations primarily on the permanent slides included in the type, which had already partly dried out. The description given above is modified from that of Barr (1993) and the comprehensive unpublished notes of R.A. Shoemaker attached to the type (as NY 00914610), and was supplemented with our own observations. As also the asexual morph is sparse and only few conidia were seen, conidial measurements likely do not represent the full range.
Helminthosporium quercicola was described as Splanchnonema quercicola by Barr (1993) from Quercus cf. reticulata collected in Arizona (USA), and Helminthosporium cf. velutinum was mentioned as its presumed asexual morph, but not described in detail. In an extensive unpublished note attached to the type specimen (NY 00914610), R.A. Shoemaker compared S. quercicola with European material (IMI 19472) for which Hughes (1953) demonstrated by pure culture studies the connection of a massaria-like sexual morph with an unidentified Helminthosporium species. Shoemaker concluded that the European material was morphologically distinct by larger ascomata with thicker walls as well as different ascospore ornamentation and therefore not conspecific with S. quercicola. He also doubted that a helminthosporium-like asexual morph was present on the type specimen of S. quercicola, and interpreted the conidiophores as setae. As he found conidia of Coryneum in microscope mounts of conidiophores from the type specimen, but no Helminthosporium conidia, he concluded that Barr (1993) mistook ascomatal setae mixed with Coryneum conidia as Helminthosporium cf. velutinum.
Unfortunately, no material from North America was available for DNA sequencing. Re-investigation of the type collection confirmed morphological differences of the sexual morph of Helminthosporium quercicola from European collections, which are described as H. quercinum below (for details, see notes below). However, we do not agree with the conclusion of Shoemaker that setae were misidentified as conidiophores by Barr (1993). Although the type collection contains only a sparse sexual as well as asexual morph, and most conidiophores are in a very young stage before conidiation, a few conidiophores bearing young conidia were found during a thorough search under the stereomicroscope (see Fig. 13W, X). In addition, a conidiophore fragment showing a typical lateral pore as well as a few typical helminthosporium-like conidia were found in a microscope mount of old conidiophores (see Fig. 13Z–C1). Like in the European H. microsorum and H. quercinum, H. quercicola apparently grows on old conidiomata of Coryneum, which explains the presence of Coryneum conidia in the microscope mounts mentioned by Shoemaker.
Helminthosporium quercinum Voglmayr & Jaklitsch, sp. nov. MycoBank MB821203. Fig. 14, Fig. 15.
Fig. 14.
Helminthosporium quercinum, sexual morph. A, B. Ostioles. C–H. Ascomata in horizontal (C–F) and vertical (G, H) section, surrounded by a well-developed pseudostroma and subiculum (E–H). I, J. Peridium and pseudostroma in section. K. Peridium of ascoma basis in section. L. Pseudostroma with verrucose marginal cells. M–O. Verrucose subicular hyphae. P. Ascus with vital ascospores. Q. Pseudoparaphyses. R–M1. Mature ascospores surrounded by gel sheath (R–J1. vital, K1–M1. dead; in K1–M1 showing widely disposed striae in the ascospore wall). All in water, except I–K, N, O, L1 in 3 % KOH. A–E, G–N, P, X–C1, K1, M1. WU 38876 (holotype); F, O, L1. IMI 219012; Q. WU 38874; R, S. WU 38879; T–W. WU 38878; D1–J1. WU 38880. Scale bars: A = 500 μm; B–E, G, H = 200 μm; F, I = 100 μm; J–O, Q–M1 = 10 μm; P = 20 μm.
Fig. 15.
Helminthosporium quercinum, asexual morph. A. Colony in face view; bark cracks containing old stromata of Coryneum. B. Conidiomata in face view; densely aggregated conidiophores on left side on old conidiomata of Coryneum. C, D. Conidiophores with conidia in side view. E. Old stroma of Coryneum in section below conidioma. F, H. Conidiophores. G. Conidiophore base and stroma cells in section. I–L. Conidiophore apices with young conidia and lateral pores (arrows in I). M–Z. Vital (M–V) and dead (W–Z) conidia. All in water. A–I, K, M–Z. WU 38876 (holotype); J, L. WU 38877. Scale bars: A = 1 mm; B = 500 μm; C, D = 100 μm; E = 200 μm; F = 50 μm; G, I–Z = 10 μm; H = 20 μm.
Etymology: Referring to its growth on Quercus spp.
Sexual morph. Pseudostromata formed in the upper bark, well-developed, dark reddish brown, pseudostroma wall 65–180 μm wide (n = 14), pseudoparenchymatous, of thick-walled dark brown cells (4.0–)6.7–10.5(–12.3) μm wide (n = 30), at the margin and on top surrounded by dark brown, distinctly verrucose, (4.5–)4.8–6.5(–7.0) μm wide subicular hyphae. Ascomata surrounded by pseudostroma, distinctly elevating the bark, single or in small groups, 570–910 μm diam (n = 24), 365–540 μm high (n = 10) (including pseudostromatal margin), strongly depressed, often entirely filled by pure white hymenium, peridium 18–60 μm thick at the margin (n = 12), 22–40 μm at the base (n = 11), pseudoparenchymatous, of pale to medium brown cells (4.0–)5.5–10.0(–12.3) × (2.0–)3.0–5.5(–7.5) μm at the margin (n = 64) and (8.0–)9.5–15.2(–18.8) × (2.5–)3.7–6.3(–7.8) μm at the base (n = 41). Ostioles central, 90–260 μm long, (100–)104–210 μm wide. Hamathecium of filiform, septate, branched, anastomosing, 1.2–4 μm wide pseudoparaphyses. Asci (160–)183–256(–320) × (32–)36–43(–46) μm (n = 19), clavate or fusoid, containing 8 bi- to triseriate ascospores. Ascospores (46–)50–59(–72) × (12–)15–19(–21) μm, l/w = (2.5–)3.0–3.7(–5.2) (n = 154), brown, obovoid to fusoid, distinctly asymmetric, first 1-septate, developing (1–)3 additional transverse, rarely oblique distosepta in the larger and 1(–2) in the smaller hemisphere, strongly constricted at the primary septum, slightly or not constricted at the secondary distosepta, with subacute to rounded end cells; length of larger hemisphere/total length of ascospore = (0.52–)0.57–0.63(–0.66), mean = 0.60 (n = 94); wall smooth, pale to medium brown, with distinct longitudinal, distantly disposed striae apparently within the wall; the contents granular, sometimes with a large and several smaller guttules per cell; each hemisphere surrounded by a thick gelatinous sheath. Asexual morph. Colony on natural substrate effuse, black, hairy. Mycelium mostly immersed, at the substrate surface forming stroma-like aggregations of light to dark brown pseudoparenchymatous cells (5.0–)7.3–12.0(–15.0) μm diam (n = 70). Conidiophores (40–)74–199(–332) μm long (n = 86), 11–18 μm wide at the base, tapering to 8.5–13.5 μm near the apex, arising solitarily or more commonly in fascicles from the stroma cells, simple, straight or flexuous, cylindrical, thick-walled, smooth, brown to dark brown, with well-defined small pores at the apex and laterally beneath the upper 1–5 septa. Conidia (47–)78–130(–201) × (13.2–)15.3–18.0(–20.5) μm (n = 122), gradually tapering to 4–10 μm at the distal end, with a 4–6.7 μm wide blackish-brown to black scar at the base, straight or flexuous, rostrate, smooth-walled, brown, 8–13(–20)-distoseptate, with angular lumina; wall up to 7 μm thick.
Culture characteristics: Culture L170: On CMD colony radius ca. 40 mm after 25 d at 22 °C. Colony whitish with a dull greyish to brownish centre (Fig. 3M); odour slightly unpleasant. On MEA colony radius ca. 31 mm after 25 d at 22 °C. Colony roundish with slightly uneven margin, thick and dense, light brown, covered by a thick white mat of aerial hyphae from the centre (Fig. 3N).
Habitat and host range: On dead corticated twigs of Quercus spp. (confirmed for Quercus brachyphylla, Q. cerris, Q. faginea, Q. petraea, Q. pubescens, Q. robur): fungicolous on old stromata or conidiomata of Coryneum sp.
Distribution: Europe (Austria, England, France, Greece, Netherlands, Spain).
Holotype: Austria, Niederösterreich, Spitzerberg, on dead corticated twigs of Quercus petraea, 16 Sep. 2012, H. Voglmayr & W. Jaklitsch [WU 38876; ex-holotype cultures CBS 136921 = L90 (ex ascospore), L91 (ex conidium); MBT376653].
Other specimens examined (all on dead corticated twigs of Quercus): Austria, Burgenland, Purbach, Purbacher Heide, on Quercus pubescens, 4 Feb. 2017, H. Voglmayr & I. Greilhuber (WU 388739); Niederösterreich, Bisamberg S Hagenbrunn, on Quercus cerris, 5 Feb. 2017, H. Voglmayr & W. Jaklitsch (WU 38874); Mödling, Kalenderberg, on Quercus petraea, 11 Feb. 2013, H. Voglmayr & I. Greilhuber (WU 38875); Unterzögersdorf, on Quercus robur, 25 Apr. 2013, H. Voglmayr & I. Greilhuber [WU 38877, culture L105 (ex conidium)]. Greece, Crete, Rethymno, Kaloniktis, on Quercus brachyphylla, 7 Jun. 2015, H. Voglmayr & W. Jaklitsch [WU 38878, culture L170 (ex conidium)]. Portugal, Sintra, Monserrate, on Quercus faginea, 17 Feb. 2017, H. Voglmayr & W. Jaklitsch (WU 35973). Spain, Andalucia, Guadalajara, Auñón, on Quercus faginea, 8 Apr. 2015, W. Jaklitsch [WU 38879, culture L159 (ex ascospore)]; Asturias, Viescas, on Quercus petraea, 4 Jun. 2013, H. Voglmayr & W. Jaklitsch [WU 38880, culture CBS 136915 = L107 (ex ascospore)]. U.K., England, Surrey (VC: 17), Esher, West End Common, map grid TQ1263, on dead, attached twigs of Quercus robur, 25 Jan. 2004, B.M. Spooner [K(M)121279!]; Devon, Steps Bridge, on dead, corticated twigs of Quercus sp., 15 Sep. 1947, ex herb. C.O.C. Chesters No. 948, 949 (IMI 19472; recently re-numbered IMI 219012!).
Notes: Helminthosporium quercinum is morphologically similar to H. quercicola from the USA, under which name European collections have been identified and recorded (Tello Mora, 2015, Osieck and Koopmans, 2016). However, H. quercinum differs from H. quercicola in distinctly larger ascomata embedded in a well-developed stroma surrounded by a subiculum of verrucose hyphae and in more distinct but shorter and less densely disposed striations of the ascospore wall. Therefore, we consider the European material to represent a distinct species, which is described here.
Hughes (1953) experimentally proved the connection of a massaria-like sexual morph with an unnamed Helminthosporium species by pure culture studies of a British collection from Quercus, but it was never formally described. Based on a detailed morphological comparison, R.A. Shoemaker concluded that the collection studied by Hughes was not conspecific with the North American H. quercicola (unpubl. notes in the type collection of H. quercicola; see notes above). A re-investigation of the material investigated by Hughes (IMI 19472; recently re-numbered IMI 219012) by us showed that it fully matches H. quercinum, except for somewhat shorter conidiophores (40–150 μm vs. 80–330 μm in the other collections studied). As the ascoma, ascospore and conidium characters fully agree with our sequenced collections, we identify this material as H. quercinum. Under the name Splanchnonema quercicola, Osieck & Koopmans (2016) recorded and illustrated the sexual morph from the Netherlands; the holomorph of a Spanish collection is illustrated by Tello Mora (2015).
The asexual morph of H. quercinum is very similar to that of H. microsorum but differs by conidiophore aggregations, which are distinctly punctiform in H. microsorum and more effuse in H. quercinum. In addition, the conidiophores of H. quercinum are shorter. Both species usually grow tightly associated with old Coryneum stromata or conidiomata on oaks, but usually on different oak (and Coryneum) species.
Culture CBS 112393, which was isolated as endophytic mycelium from Fagus sylvatica in Italy and deposited as Corynespora proliferata, was revealed to represent H. quercinum in our phylogenetic analyses (Fig. 1). Culture CBS 112393 was evidently misidentified as Corynespora proliferata, which was described from dead wood of Fagus sylvatica but differs substantially from H. quercinum according to the original description (Loerakker 1975).
Helminthosporium tiliae (Link) Fr. [as ‘Helmisporium’], Syst. mycol. (Lundae) 3(2): 360. 1832. Fig. 16.
Fig. 16.
Helminthosporium tiliae, sexual (A–U) and asexual (V–K1) morph. A. Three ostioles in face view. B–D. Ascomata in horizontal (B, C) and vertical (D) section. E. Peridium in section. F. Ascus with dead ascospores. G. Pseudoparaphyses. H–U. Mature vital (H–O) and dead (P–U) ascospores surrounded by gel sheath (N, Q–U). V. Conidiomata in face view. W. Conidioma on old Hercospora tiliae stroma in vertical section. X. Old stroma of Hercospora tiliae in section below conidioma. Y–A1. Conidiophores and stroma cells (Y, Z) with apical pores (arrows). B1–K1. Vital (B1–I1) and dead (J1, K1) conidia. All in water, except R–U, A1, J1, K1. in 3 % KOH. A–Q, V–Y. WU 38882 (epitype); R–U, J1. B 700014746 (holotype of Massaria heterospora); Z, B1–I1. WU 38881; A1, K1. B 700016453 (holotype of Exosporium tiliae). Scale bars: A, B = 200 μm; C, D, W, X = 100 μm; E, F = 20 μm; G–U, Y–K1 = 10 μm; V = 500 μm.
Basionym: Exosporium tiliae Link, Mag. Gesell. naturf. Freunde, Berlin 3(1–2): 10. 1809.
Synonym: Massaria heterospora G.H. Otth, Mitt. naturf. Ges. Bern: 49. 1868.
Sexual morph. Pseudostromata weakly developed, rudimentary. Ascomata (345–)422–572(–722) μm diam (n = 29), (257–)270–370(–398) μm high (n = 9), depressed subglobose, immersed in the upper bark, slightly elevating the bark, single, dark brown, peridium (28–)33–56(–76) μm thick (n = 36), pseudoparenchymatous, cells (6–)9–16(–21) μm long (n = 53), dark brown, black in KOH. Ostioles central, not protruding above the bark surface. Hamathecium of densely packed filiform, septate, branched, anastomosing, 1.5–3.5 μm wide pseudoparaphyses embedded in a tough gel matrix. Asci (185–)206–245(–257) × (38–)40–48(–52) μm (n = 30), clavate or fusoid, usually containing 8 irregularly biseriate, rarely 4 uniseriate ascospores. Ascospores (39–)47–58(–73) × (15.0–)17.7–21.2(–23.5) μm, l/w = (2.0–)2.4–3.1(–3.8) (n = 146), first light brown, becoming dark brown at full maturity, obovoid to fusoid, distinctly asymmetric, first 1-septate, developing 2–3 transverse, sometimes oblique distosepta in the larger and 1(–2) in the smaller hemisphere, rarely with a longitudinal septum in the larger hemisphere, strongly constricted at the primary septum, slightly or not constricted at the secondary distosepta, with subacute to rounded end cells; length of larger hemisphere/total length of ascospore = (0.50–)0.57–0.63(–0.66), mean = 0.60 (n = 134); wall finely verrucose, brown; the contents granular; each hemisphere surrounded by a thick gelatinous sheath. Asexual morph. Colonies on natural substrate discrete, punctiform, blackish brown. Mycelium immersed, growing in aborted stromata or conidiomata of Hercospora tiliae below the periderm, at the substrate surface forming stroma-like aggregations of light to dark brown pseudoparenchymatous cells. Conidiophores (68–)79–133(–150) μm long (n = 35), 9–15 μm wide at the base, 8–12 μm wide near the slightly inflated apex, fasciculate, arising from the upper cells of the stromata, simple, straight or flexuous, cylindrical, finely verrucose, brown to dark brown, very dark brown to black at the apex, 3–6 septate, forming one to several conidia. Conidia (57–)74–111(–122) × (13.5–)13.7–19.0(–24.5) μm (n = 20), tapering to 6–9.5 μm at the distal end, with a 5–7 μm wide, blackish-brown to black scar at the base, straight or curved, obclavate to rostrate, smooth-walled, pale to golden brown, 7–18(–25)-distoseptate, with angular lumina; wall up to 6.5 μm thick.
Culture characteristics: Culture L171: On CMD colony radius ca. 16 mm after 4 wk at 22 °C. Colony dull grey-brown, turning black from the centre, with whitish margin and a pale rosy halo around the colony, with long white aerial hyphae (Fig. 3O); odour indistinct. On MEA colony radius 10 mm after 4 wk at 22 °C. Colony thick, dense, silvery-grey with small black dots and white margin, reverse brownish (Fig. 3P); odour indistinct to pleasant.
Habitat and host range: On dead corticated twigs of Tilia spp.: fungicolous on aborted conidiomata and stromata of Hercospora tiliae.
Distribution: Widespread in Europe.
Typification: Holotype of Exosporium tiliae: Germany, without place, date and collector, on dead twigs of Tilia sp. (B 700016453!). Holotype of Massaria heterospora: Switzerland, Bern, on dead twigs of Tilia cordata, without date, G. Otth 16 (B 700014746!). Epitype of Exosporium tiliae and Massaria heterospora, here designated: Austria, Oberösterreich, Raab, Wetzlbach, on dead corticated branches of Tilia platyphyllos, 8 Sep. 2012, H. Voglmayr [WU 38882; ex-epitype cultures CBS 136907 = L88 (ex ascospore), L89 (ex conidium); MBT376654 and MBT376655, respectively].
Other specimens examined (all on dead corticated twigs of Tilia): Austria, Kärnten, St. Margareten im Rosental, Wograda, on Tilia cordata, 22 Aug. 2012, W. Jaklitsch [WU 38884, culture CBS 136906 = L87 (ex ascospore)]; Niederösterreich, Mayerling, on Tilia sp., 8 Dec. 2016, H. Voglmayr & I. Greilhuber [WU 38881, culture L171 (ex conidium)]; Oberösterreich, Raab, Wetzlbach, on Tilia platyphyllos, 11 May 2013, H. Voglmayr (WU 38883).
Notes: Helminthosporium tiliae is the type of the genus Exosporium and has been commonly known as Exosporium tiliae. No sexual morph was known for H. tiliae, and Massaria heterospora is here proven to be its sexual morph. A recent holomorphic collection, for which cultures and sequence data are available, is here designated as epitype to firmly establish the connection between the sexual and asexual morphs. Sister group relationship to H. oligosporum, also growing on Tilia spp. and formerly classified within Corynespora (see above), received maximum support.
Like its close relative H. oligosporum, H. tiliae consistently grows on aborted conidiomata or stromata of Hercospora tiliae. Usually the host stromata and conidiomata are fully transformed and filled with brown hyphae of H. tiliae, but occasionally host perithecia are still recognisable. However, the diagnostic black line delimiting the stromata of Hercospora tiliae is usually well seen. Growth on Hercospora is here reported for the first time; the infected transformed host stromata and conidiomata have been erroneously interpreted as immersed stromata by Ellis (1961).
Helminthosporium velutinum Link [as ‘Helmisporium’], Mag. Gesell. naturf. Freunde, Berlin 3(1–2): 10, tab. 1:9. 1809. Fig. 17.
Fig. 17.
Helminthosporium velutinum. A. Colony in face view. B–E. Conidiophores with apical and lateral conidia in side view. F. Conidiophores. G, H. Young (G) and old (H) conidiophore apices with lateral pores (arrows). I. Conidiophore base and stroma cells. J–G1. Conidia (J–N. dead, O–G1. vital). All in water. A–C, V–E1. WU 38892 (epitype); D, G, Q–U. WU 38891; E. WU 38889; F. WU 38885; H, O, P, F1, G1. WU 38887; I–N. B 700016457 (holotype). Scale bars: A = 1 cm; B–E = 100 μm; F = 50 μm; G–G1 = 10 μm.
Sexual morph unknown. Colony on natural substrate effuse, black, hairy. Mycelium immersed, on the substrate surface forming stroma-like aggregations of light to dark brown pseudoparenchymatous cells (5.0–)6.5–11.0(–19.8) μm diam (n = 58). Conidiophores (163–)340–698(–960) μm long (n = 247), 14–26 μm wide at the base, tapering to 8–12 μm near the apex, arising solitarily or in fascicles from the stroma cells, simple, straight or flexuous, cylindrical, thick-walled, smooth, brown to dark brown, with well-defined small pores at the apex and laterally beneath the upper 1–12 septa. Conidia (42–)56–89(–142) × (11–)14.3–18.5(–24.7) μm (n = 351), gradually tapering to (3.5–)5–8 μm at the distal end, with a 1.4–3.7 μm wide, blackish-brown to black scar at the base, straight or flexuous, obclavate to rostrate, smooth-walled, pale golden brown to brown, 6–18-distoseptate, with angular lumina; wall up to 4.5 μm thick.
Culture characteristics: Cultures L115 and L131: On CMD colony radius (20–)28–45 mm after 4 wk, i.e. sometimes mycelium (culture L115) filling a centrally inoculated 90 mm diam Petri dish entirely at 22 °C. Colony more or less circular, dense, brown with yellow tint or olivaceous fading to colourless or whitish at the margin, with short or long and dense white aerial hyphae spreading from the centre (Fig. 3Q, S); odour indistinct to slightly unpleasant (cabbage-like). On MEA growth irregular, depending on the condition of the mycelium; colony radius e.g. 42 mm, i.e. reaching the plate margin at one side after 4 wk at 22 °C or ca. 20 mm after 4 wk. Colony roundish or irregular, dense, thick, with shades of grey plus white patches of dense tufts of white aerial hyphae with yellow to black drops of clear fluid or a dense flat white mat or a thick zonate white and brownish mat of aerial hyphae, sometimes with partial black marginal patches (Fig. 3R, T); odour indistinct or slightly unpleasant (cabbage-like).
Habitat and host range: Saprobic on various plants; usually on dead twigs of various trees and shrubs, sometimes on herbaceous stems.
Distribution: Widespread and common in temperate Eurasia and America, probably almost cosmopolitan.
Typification: Holotype of Helminthosporium velutinum: Germany, without place and date, on dead twigs of Fagus sylvatica (B 700016457!). Epitype, here designated: Austria, Wien, Döbling, Kahlenberg, on dead corticated twigs of Fagus sylvatica, 16 Nov. 2013, W. Jaklitsch [WU 38892; ex-epitype culture L131 (ex conidium) = CBS 139923; MBT376656].
Other specimens examined (all on dead corticated twigs): Austria, Kärnten, St. Margareten im Rosental, Dullach, Drau-Auen, on Euonymus europaeus, 10 Aug. 2013, W. Jaklitsch & H. Voglmayr [WU 38885, culture L117 (ex conidium)]; St. Margareten im Rosental, Ferm-Wograda, on Juglans regia, 16 Nov. 2012, W. Jaklitsch [WU 38886, culture L98 (ex conidium)]; ibid., on Juglans regia, 10 Aug. 2013, H. Voglmayr & W. Jaklitsch [WU 38887, culture L116 (ex conidium)]; St. Margareten im Rosental, Gupf, on Corylus avellana, 8 Nov. 2013, W. Jaklitsch [WU 38888, culture L136 (ex conidium)]; Niederösterreich, Krems, Egelsee, on Genista tinctoria, 27 Oct. 2013, W. Jaklitsch & H. Voglmayr [WU 38889, culture L127 (ex conidium)]; Krems, Senftenberg, on Cytisus scoparius, 15 Feb. 2014, H. Voglmayr & W. Jaklitsch [WU 38890, culture L140 (ex conidium)]; Oberösterreich, St. Willibald, Aichet, on Sambucus nigra, 16 Aug. 2013, H. Voglmayr [WU 38891, culture CBS 136924 = L115 (ex conidium)]. Germany, Hessen, Rheingau, Lorch, on Cytisus scoparius, 3 Apr. 2015, W. Jaklitsch [WU 38893, culture L163 (ex conidium)]. Italy, Lazio, Viterbo, Norchia, on Acer campestre, 14 Oct. 2013, H. Voglmayr & W. Jaklitsch [WU 38894, culture L126 (ex conidium)]. Spain, Canarias, Teneriffe, Los Batanes, on Prunus lusitanica, 17 Dec. 2013, W. Jaklitsch [WU 38895, culture L134 (ex conidium)]; La Gomera, El Cedro, on Gesnouinia arborea, 24 Mar. 2016, H. Voglmayr [WU 38897, culture L176 (ex conidium)]. Sweden, Skåne, NE Helsingborg, Kropp parish, Vasatorp, 3.8 km SSW of the church, 56°03′13″ N, 12°46′47″ E, on Ribes rubrum, 27 Oct. 2013, S.-A. Hanson [WU 38896, culture L135 (ex conidium)].
Notes: Helminthosporium velutinum is by far the most commonly recorded and best-known species of the genus. Remarkably, it has been recorded world-wide from a wide range of woody and herbaceous substrates; more than 100 hosts are listed in Farr & Rossman (2016). Numerous species have been synonymised with H. velutinum by Ellis (1961) based on morphology, but it is uncertain whether all of them are conspecific. Based on sequence data, one of these putative synonyms, H. genistae, is here shown to represent a distinct species, which is apparently confined to fabaceous hosts (see above). Although some of the literature records may represent misidentifications, polyphagous nature of H. velutinum has been confirmed by sequence data in the present study, and GenBank sequences confirm presence of this species in Europe, Asia and the Americas.
The holotype specimen in B is in good condition; however, considering the presence of cryptic species within Helminthosporium, we designate an epitype of H. velutinum from the original host, Fagus sylvatica, for which a culture and sequence data are available, to stabilise the application of the name.
Discussion
In the molecular phylogenetic analyses (Fig. 1, Fig. 2), several species previously classified in Corynespora and Exosporium are revealed as closely related to Helminthosporium, and are thus here recognised as members of the genus Helminthosporium. Although revealed as monophyletic, the genus Helminthosporium receives no significant bootstrap support in the extended combined matrix, and also the relationships within Helminthosporium remain partly unsupported (Fig. 1). This significantly changes after removal of the accessions for which no rpb2 sequences are available, and the Helminthosporium clade becomes moderately to highly supported (78 % MP and 91 % ML bootstrap support for the Helminthosporium clade; Fig. 2), and the same is also observed in several additional nodes within Helminthosporium. After exclusion of H. leucadendri, for which only comparatively short rpb2 and tef1 sequences are available, the Helminthosporium clade becomes highly supported in both analyses (94 % MP and 98 % ML bootstrap support; Fig. 2). This once again demonstrates that the ribosomal genes commonly provide insufficient phylogenetic resolution on the generic to familial level, challenging the common practice of generic and familial reclassification solely based on phylogenies of ribosomal genes. The results of our phylogenetic analyses also show that inclusion of accessions in multi-gene analyses, for which only ITS and/or LSU sequences are available, can be problematic, as the low phylogenetic signal can result in significantly decreasing overall phylogenetic resolution. At least the rpb2 should be sequenced and included in phylogenetic analyses of ascomycetes in general and dothideomycetes in particular, as this marker usually significantly increases the phylogenetic resolution. Also the tef1 locus, which has been included in the multi-gene analyses of Massarineae by Tanaka et al. (2015), and which has been shown to resolve well in many ascomycete lineages, is a marker of good resolution at the generic level and below, if the introns are included; however, as it contains paralogs in Helminthosporium genistae it could not be obtained for all species.
The results of the molecular phylogenetic analyses necessitate a critical re-evaluation of the morphological characters traditionally considered to be diagnostic for Corynespora and Helminthosporium. The main character used for distinction between Corynespora and Helminthosporium by Ellis (1961) and Luttrell, 1963, Luttrell, 1964, acrogenous vs. acropleurogenous conidiation, is shown to be insignificant in a phylogenetic context. Already Luttrell (1964) pointed out that another character used by Ellis (1961), i.e. percurrent (Corynespora, Exosporium) vs. non-percurrent (Helminthosporium) conidiophore proliferation, does not separate the genera. On the one hand, percurrent proliferation has been observed in H. velutinum by Luttrell (1964), whereas on the other hand Luttrell (1963) and Hughes (1983) could not confirm regular percurrent proliferation in Helminthosporium oligosporum, which was classified in Corynespora by Ellis (1961). Considering that numerous species described in Corynespora have not been critically studied, it is likely that several of them belong to Helminthosporium.
The present investigations significantly extend the knowledge of sexual morphs of Helminthosporium. Based on pure culture and sequence data, Tanaka et al. (2015) described a massarina-like sexual morph for Helminthosporium massarinum, but challenged the few previous reports of massaria- and splachnonema-like sexual morphs for Helminthosporium. Based on pure culture and sequence data as well as herbarium studies, we here experimentally confirm sexual morphs for four Helminthosporium species, three of which are splanchnonema-like. In addition, our morphological re-investigation of the type specimen of Splanchnonema quercicola revealed that Barr (1993) was correct reporting the associated asexual morph to be helminthosporium-like. Although the North American S. quercicola is morphologically very close to the massaria-like sexual morph reported by Hughes (1953) for an unnamed Helminthosporium species from Europe, our extensive investigations of the material of Hughes as well as of freshly collected material revealed several morphological differences. Therefore, we consider the European records attributed to S. quercicola to represent a distinct species, which is here described as H. quercinum. Massaria heterospora, a little-known and recorded splanchnonema-like sexual morph is here proven to be connected with Helminthosporium tiliae, and an apparently undescribed splanchnonema-like sexual morph is reported for H. oligosporum for the first time. In addition, we show that Massarinula italica is the sexual morph of H. microsorum. In light of this experimental evidence, we consider it fully justified to combine Splanchnonema quercicola and S. kalakadense, for which helminthosporium-like asexual morphs have been reported (Subramanian and Sekar, 1987, Barr, 1993) but for which no sequence data are available, in Helminthosporium. In this context, it may also be worth mentioning that Pseudosplanchnonema phorcioides, another splanchnonema-like species lacking a helminthosporium-like asexual morph, is closely related to Helminthosporium (Fig. 1, Fig. 2).
With the exception of H. solani, a plant parasite on Solanum, most species of Helminthosporium have been reported as saprobes of plants, usually from woody substrates. With the transfer of several Corynespora species parasitic on leaves, the genus now contains additional plant pathogens. In addition, the current detailed investigations revealed that some well-known Helminthosporium species are fungicolous, mostly on Diaporthales, and the prominent subperidermal stromata of e.g. H. caespitosum, H. oligosporum and H. tiliae actually represent transformed host stromata or conidiomata. However, it is unknown whether these Helminthosporium species are parasitic or saprobic on their fungal hosts.
Acknowledgements
We thank the fungarium curators of B, CBS, K, NY and Walter Till at WU for sending and managing collections; Rossella Marcucci for support in locating and investigating specimens at PAD; Alain Gardiennet, Chris Yeates, Sven-Åke Hanson and Christian Scheuer for providing fresh material and literature; Irmgard Greilhuber and her family for organising and participating in numerous collecting trips together with HV; Daniel Knapp for providing information about unpublished ITS and LSU sequences of Periconia macrospinosa strain DSE 2036; Walter Gams for his hospitality and organisation of collecting trips around Bomarzo, Italy; Begoña Aguirre-Hudson (K) for invaluable information about several type collections held at K; and Michel Hoff (STR), Åsa Kruys (UPS), and Jan Holec and Markéta Šandová (PRM) for providing details about the type collections of Sporidesmium olivaceum, Helmisporium genistae and Coryneum oligosporum, respectively. The financial support by the Austrian Science Fund (FWF; project P25870-B16) to WJ is gratefully acknowledged.
Footnotes
Peer review under responsibility of Westerdijk Fungal Biodiversity Institute.
References
- Alcorn J.L. The taxonomy of “Helminthosporium” species. Annual Review of Phytopathology. 1988;26:37–56. [Google Scholar]
- Barr M.E. Notes on the Pleomassariaceae. Mycotaxon. 1993;49:129–142. [Google Scholar]
- Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. [Google Scholar]
- Crous P.W., Summerell B.A., Shivas R.G. Fungal Planet description sheets: 92–106. Persoonia. 2011;27:130–162. doi: 10.3767/003158511X617561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crous P.W., Wingfield M.J., Schumacher R.K. Fungal Planet description sheets: 281–319. Persoonia. 2014;33:212–289. doi: 10.3767/003158514X685680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzi M., Barbaini M. Fungi aternenses ad fungorum italicorum cognitionem aliquo incremento augendam digesti ac descripti. Atti dell' Istituto botanico dell' Università di Pavia, Ser. 3. 1927;33:147–208. [Google Scholar]
- de Hoog G.S., Gerrits van den Ende A.H.G. Molecular diagnostics of clinical strains of filamentous basidiomycetes. Mycoses. 1998;41:183–189. doi: 10.1111/j.1439-0507.1998.tb00321.x. [DOI] [PubMed] [Google Scholar]
- Ellis M.B. Some species of Corynespora. Mycological Papers. 1957;65:1–15. [Google Scholar]
- Ellis M.B. Dematiaceous Hyphomycetes. I. Mycological Papers. 1960;76:1–36. [Google Scholar]
- Ellis M.B. Dematiaceous Hyphomycetes. III. Mycological Papers. 1961;82:1–55. [Google Scholar]
- Errampalli D., Saunders J.M., Holley J.D. Emergence of silver scurf (Helminthosporium solani) as an economically important disease of potato. Plant Pathology. 2001;50:141–153. [Google Scholar]
- Farr D.F., Rossman A.Y. Systematic Mycology and Microbiology Laboratory, ARS, USDA; 2016. Fungal databases – fungus-host distributions.http://nt.ars-grin.gov/fungaldatabases/ (retrieved November 16, 2016) [Google Scholar]
- Fries EM (1832). Systema Mycologicum3(2): i–ii, 261–524. Sumptibus Ernesti Mauritii, Greifswald.
- Hall T.A. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series. 1999;41:95–98. [Google Scholar]
- Hughes S.J. Conidiophores, conidia, and classification. Canadian Journal of Botany. 1953;31:577–659. [Google Scholar]
- Hughes S.J. Revisiones hyphomycetum aliquot cum appendice de nominibus rejiciendis. Canadian Journal of Botany. 1958;36:727–836. [Google Scholar]
- Hughes S.J. Helminthosporium oligosporum. Fungi Canadenses. 1983;245 [Google Scholar]
- Hyde K.D., Jones E.B.G., Liu J.K. Families of Dothideomycetes. Fungal Diversity. 2013;63:1–313. [Google Scholar]
- Jaklitsch W.M. European species of Hypocrea – part I. Studies in Mycology. 2009;63:1–91. doi: 10.3114/sim.2009.63.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Komon M., Kubicek C.P. Hypocrea voglmayrii sp. nov. from the Austrian Alps represents a new phylogenetic clade in Hypocrea/Trichoderma. Mycologia. 2005;97:1365–1378. doi: 10.3852/mycologia.97.6.1365. [DOI] [PubMed] [Google Scholar]
- Jaklitsch W.M., Stadler M., Voglmayr H. Blue pigment in Hypocrea caerulescens sp. nov. and two additional new species in sect. Trichoderma. Mycologia. 2012;104:925–941. doi: 10.3852/11-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Voglmayr H. Persistent hamathecial threads in the Nectriaceae, Hypocreales: Thyronectria revisited and re-instated. Persoonia. 2014;33:182–211. doi: 10.3767/003158514X685211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaklitsch W.M., Voglmayr H. Hidden diversity in Thyridaria and a new circumscription of the Thyridariaceae. Studies in Mycology. 2016;85:35–64. doi: 10.1016/j.simyco.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauff F., Lutzoni F. Phylogeny of Gyalectales and Ostropales (Ascomycota, Fungi): among and within order relationships based on nuclear ribosomal RNA small and large subunits. Molecular Phylogenetics and Evolution. 2002;25:138–156. doi: 10.1016/s1055-7903(02)00214-2. [DOI] [PubMed] [Google Scholar]
- Kodsueb R., Lumyong S., Ho W.H. Morphological and molecular characterization of Aquaticheirospora and phylogenetics of Massarinaceae (Pleosporales) Botanical Journal of the Linnean Society. 2007;155:283–296. [Google Scholar]
- Landvik S., Egger K., Schumacher T. Towards a subordinal classification of the Pezizales (Ascomycota): phylogenetic analyses of SSU rDNA sequences. Nordic Journal of Botany. 1997;17:403–418. [Google Scholar]
- Link J.H.F. Observationes in ordines plantarum naturales. Dissertatio 1ma. Magazin der Gesellschaft Naturforschender Freunde zu Berlin. 1809;3:3–42. [Google Scholar]
- Liu Y.L., Whelen S., Hall B.D. Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- Loerakker W.M. A new species of Corynespora. Persoonia. 1975;8:220–222. [Google Scholar]
- Luttrell E.S. Taxonomic criteria in Helminthosporium. Mycologia. 1963;55:643–674. [Google Scholar]
- Luttrell E.S. Systematics of Helminthosporium and related genera. Mycologia. 1964;56:119–132. [Google Scholar]
- Müller K. PRAP – calculation of Bremer support for large data sets. Molecular Phylogenetics and Evolution. 2004;31:780–782. doi: 10.1016/j.ympev.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Olivier C., Berbee M.L., Shoemaker R.A. Molecular phylogenetic support from ribosomal DNA sequences for origin of Helminthosporium from Leptosphaeria-like loculoascomycete ancestors. Mycologia. 2000;92:736–746. [Google Scholar]
- Osieck E., Koopmans D. Two species of Pleomassariaceae new for The Netherlands: Pleomassaria carpini and Splanchnonema quercicola. Coolia. 2016;59:33–37. [Google Scholar]
- Quaedvlieg W., Verkley G.J.M., Shin H.D. Sizing up Septoria. Studies in Mycology. 2013;75:307–390. doi: 10.3114/sim0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- Riethmüller A., Voglmayr H., Göker M. Phylogenetic relationships of the downy mildews (Peronosporales) and related groups based on nuclear large subunit ribosomal DNA sequences. Mycologia. 2002;94:834–849. doi: 10.1080/15572536.2003.11833177. [DOI] [PubMed] [Google Scholar]
- Saccardo D. Contribuzione alla micologia veneta e modenese. Malpighia. 1898;12:201–228. Tav. VII–VIII. [Google Scholar]
- Shoemaker R.A., LeClair P.M. Type studies of Massaria from the Wehmeyer collection. Canadian Journal of Botany. 1975;53:1568–1598. [Google Scholar]
- Siboe G.M., Kirk P.M., Cannon P.F. New dematiaceous hyphomycetes from Kenyan rare plants. Mycotaxon. 1999;73:283–302. [Google Scholar]
- Silvestro D., Michalak I. raxmlGUI: a graphical front-end for RAxML. Organisms Diversity & Evolution. 2012;12:335–337. [Google Scholar]
- Sivanesan A. Graminicolous species of Bipolaris, Curvularia, Exserohilum and their teleomorphs. Mycological Papers. 1987;158:1–261. [Google Scholar]
- Stamatakis E. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- Subramanian C.V., Sekar G. Three bitunicate ascomycetes and their tretic anamorphs. Kavaka. 1987;15:87–98. [Google Scholar]
- Sung G.H., Sung J.M., Hywel-Jones N.L. A multigene phylogeny of Clavicipitaceae (Ascomycota, Fungi): identification of localised incongruence using a combinational bootstrap approach. Molecular Phylogenetics and Evolution. 2007;44:1204–1223. doi: 10.1016/j.ympev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Swofford D.L. Sinauer Associates; Sunderland, Massachusetts: 2002. PAUP* 4.0b10: phylogenetic analysis using parsimony (*and other methods) [Google Scholar]
- Tanaka K., Hirayama K., Yonezawa H. Revision of the Massarineae (Pleosporales, Dothideomycetes) Studies in Mycology. 2015;82:75–136. doi: 10.1016/j.simyco.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tello Mora S. Splanchnonema quercicola en España. Micobotánica-Jaén. 2015;10 http://www.micobotanicajaen.com/Revista/Inicio.html [Google Scholar]
- Thambugala K.M., Hyde K.D., Tanaka K. Towards a natural classification and backbone tree for Lophiostomataceae, Floricolaceae, and Amorosiaceae fam. nov. Fungal Diversity. 2015;74:199–266. [Google Scholar]
- Thiers B. New York Botanical Garden's Virtual Herbarium; 2017. Index Herbariorum: a global directory of public herbaria and associated staff.http://sweetgum.nybg.org/ih/ [Google Scholar]
- Vilgalys R., Hester M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology. 1990;172:4238–4246. doi: 10.1128/jb.172.8.4238-4246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H., Akulov O.Y., Jaklitsch W.M. Reassessment of Allantonectria, phylogenetic position of Thyronectroidea, and Thyronectria caraganae sp. nov. Mycological Progress. 2016;15:921. doi: 10.1007/s11557-016-1218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voglmayr H., Jaklitsch W.M. Prosthecium species with Stegonsporium anamorphs on Acer. Mycological Research. 2008;112:885–905. doi: 10.1016/j.mycres.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Voglmayr H., Jaklitsch W.M. Molecular data reveal high host specificity in the phylogenetically isolated genus Massaria (Ascomycota, Massariaceae) Fungal Diversity. 2011;46:133–170. [Google Scholar]
- Werle E., Schneider C., Renner M. Convenient single-step, one tube purification of PCR products for direct sequencing. Nucleic Acids Research. 1994;22:4354–4355. doi: 10.1093/nar/22.20.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White T.J., Bruns T., Lee S. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis M.A., Gelfand D.H., Sninsky J.J., White T.J., editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego: 1990. pp. 315–322. [Google Scholar]
- Wiens J.J. Combining datasets with different phylogenetic histories. Systematic Biology. 1998;47:568–581. doi: 10.1080/106351598260581. [DOI] [PubMed] [Google Scholar]
- Zhao G.C., Zhao R.L. Yunnan Science and Technology Press; Kunming: 2012. The higher microfungi from forests of Yunnan province. [Google Scholar]