Abstract
Cystic fibrosis (CF) is a life-limiting, monogenic disorder characterized by chronic sinopulmonary and gastrointestinal involvement. Progressive pulmonary disease leads to death in the majority of patients. Despite its well-defined molecular basis related to defects in the cystic fibrosis transmembrane conductance regulator anion transport channel, there are large gaps in our understanding of the origin of CF lung disease. Disease has been shown to be present in infancy, and there is mounting evidence that abnormalities begin in utero. Heterogeneity of clinical presentations and severity suggest that many factors involved in lung disease have yet to be fully elucidated. Although new advances in therapeutic treatments have shown promise in delaying disease progression, the prevention of pulmonary disease at its origin (primary prevention) should be a key goal of CF care. The objective of this workshop was to (1) review our understanding of the origins of CF lung disease, (2) determine gaps in the knowledge base that are most significant and most likely to enable prevention of CF lung disease, and (3) prioritize new research questions that will promote pulmonary health in both CF and other childhood lung diseases. The goal of this report is to provide recommendations for future research that will improve our understanding of pulmonary development in health and disease, improve outcome measures and biomarkers for early lung disease, and determine therapeutic targets and strategies to prevent the development of lung disease in children with CF.
Background
Although cystic fibrosis (CF) has a well-defined molecular basis, debate continues as to when and how CF lung disease first develops, particularly given the heterogeneous nature of lung disease in this population. The CF subgroup focused on strategies to prevent development of measurable CF lung disease and the prioritization of research strategies to delineate and measure factors leading to disease development.
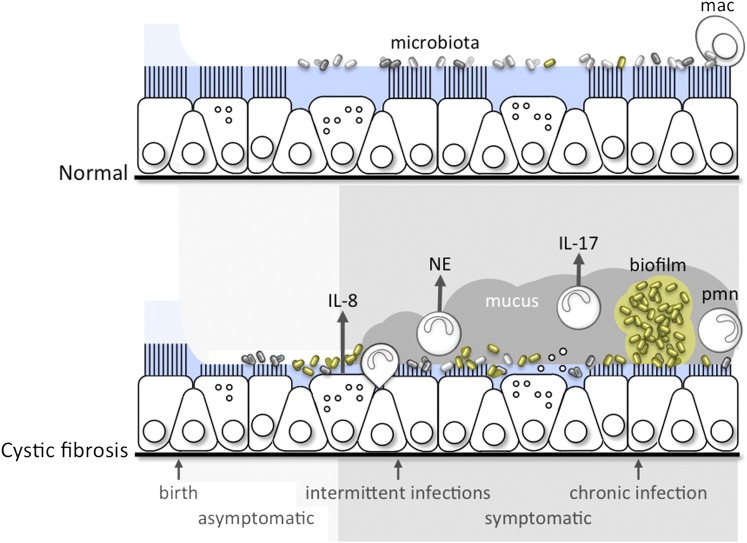
CF is the most common life-shortening autosomal recessive disease of whites. It occurs in approximately 1 in 3,500 live births and is characterized by chronic sinopulmonary and gastrointestinal involvement (1). Although the life expectancy of a child born with CF has gradually improved, the mortality rate remains virtually unchanged, typically due to progressive airway disease. Mutations in the cystic fibrosis transmembrane conductance regulator (CFTR), a gene that encodes an anion transport protein present in the epithelial cell membrane, lead to the manifestations of CF. Defective Cl− and HCO3 transport, coupled with altered regulation of Na+ transport, lead to decreased airway surface liquid and increased mucus viscosity, thereby impeding mucociliary clearance and promoting mucus stasis (2, 3). Mucus stasis, likely coupled with other host defense defects, leads to chronic and progressive inflammation, infection, airways obstruction, bronchiectasis, and deterioration of pulmonary function (Figure 1).
Figure 1.
A conceptual framework for the progression of disruption of mucociliary clearance leading to intermittent, then chronic, infection and inflammation in the lungs of patients with cystic fibrosis. mac = macrophage; NE = neutrophil elastase, pmn = neutrophil.
Variability in the rate of progression of CF lung disease occurs even among individuals carrying identical CFTR mutations (4, 5). Affected twin analysis indicates genetic modifiers independent of CFTR account for approximately half of the variation in lung function, with the remainder attributed to environmental exposures and stochastic effects (6, 7). A number of replicated non-CFTR genetic modifiers of CF lung disease severity have been identified, some of which are associated with inflammation (4, 8). The neutrophilic inflammatory response to chronic infection in CF is both protective and destructive, as inflammation may further worsen mucociliary clearance or itself cause airway damage (Figure 1) (9, 10). Poor nutrition is linked to impaired pulmonary growth, greater morbidity, and increased mortality, but may also impair host defense (11). Viral and bacterial infections are clearly associated with progression of CF lung disease (12–14). New data on the lung microbiota continue to highlight the complex interactions between infectious exposures and the host lung (15).
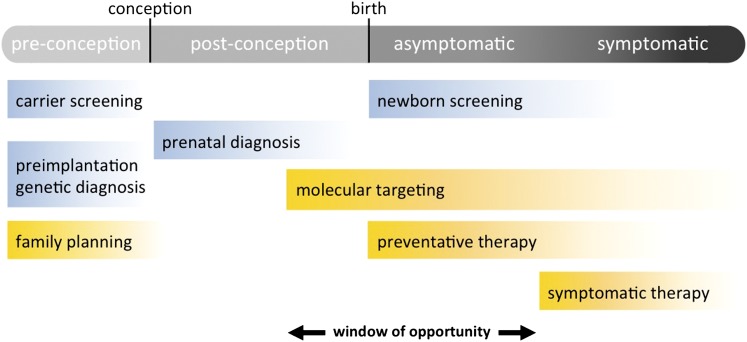
CF lung disease can present in infancy (16) and may be highly heterogeneous within the lung of an individual subject with CF (17). Measurable differences in infant lung function between children with CF and healthy control subjects are evident as early as 6 to 12 months of age, and focal areas of bronchiectasis, mucus plugging, and air trapping may be evident in the first year of life (18, 19). Animal models suggest structural abnormalities in the CF lung may occur in utero, and other developmental defects may also be present. In contrast, some individuals with CF may not have measureable lung disease until much later in life, emphasizing the variable nature of the disease and fueling debate as to when CF lung disease truly begins. One could argue that disease begins at conception, when a sperm and egg combine to create an embryo with two deleterious CFTR mutations. Because of the high penetrance of disease-causing CFTR mutations, we know that the child who develops from this union will manifest features of CF. From this perspective, interventions before conception could be considered as a form of primary prevention of CF lung disease (Figure 2). Even though genetic and molecular pathogenesis is well understood, defining precisely when CF lung disease begins remains challenging. Does lung disease begin shortly after conception? In utero or after birth, when defective ion transport becomes manifest? When the affected child develops functional or structural changes in the lung, or when the child becomes symptomatic? Or does lung disease begin at some other arbitrarily defined point?
Figure 2.
A conceptual framework for early intervention in and primary prevention of cystic fibrosis lung disease. Items highlighted in blue are diagnostic or screening tests; those in yellow are interventions.
Diagnosis of CF in infancy is made by a combination of positive newborn screen and either positive sweat chloride testing or intermediate sweat chloride testing with two disease-causing mutations in CFTR; diagnosis in the nonnewborn screen population relies on sweat chloride testing, genetic testing, and clinical symptoms and/or family history (20). Despite these (typically) straightforward guidelines for diagnosis of CF, definition of the definitive starting point of CF lung disease is not established and may vary from patient to patient. Given that the majority of infants with CF have some abnormalities noted (by imaging, pulmonary function testing, respiratory culture, or inflammatory markers) by 6 to 12 months of age, our group defined a window of intervention as before 12 months of age or before the presence of detectable disease by structural, functional, or biochemical measures.
The clearest mechanism to prevent CF lung disease, excluding in utero measures, is to target intervention at the gene or gene product level (what we will define as primary risk factors) as early in life as possible. This strategy might include interventions directed toward the CFTR gene, genetic modifiers, or protein function. Barring complete recovery or restoration of CFTR function, interventions targeted at secondary risk factors will continue to be important in prevention of CF lung disease. Although many molecular-based therapies are available or currently under investigation, these agents have mostly been studied in older children and adults. Few interventional studies have been performed in the preschool CF population, and there are virtually no data on treatment effects in the first year of life.
Priority Areas for Future Research
Defining the Epidemiology of CF in the Age of Genetic Screening
A discussion of primary prevention of lung disease in single-gene disorders such as CF would be incomplete if we did not consider reproductive options available to carriers. Screening of individuals for CF carrier status has occurred in the United States for 2 decades, and there are a variety of reproductive alternatives available to carrier couples identified in the prepregnancy phase, ranging from egg or sperm donation to preimplantation genetic diagnosis (21) (Figure 2). The vast majority of CF carrier screening is now offered postconception to the pregnant mother. CF carrier screening programs have been reported for some states, but regional and national assessments of program impact are lacking. Another gap in our understanding is whether CF carrier screening has changed the incidence of CF or CFTR allele distribution in the United States, both of which have been reported in smaller studies from individual states in the United States and in European countries (22, 23). National data from the CF Foundation Patient Registry does not reflect a notable decrease in incidence of CF over the past decade, possibly because many couples are opting to continue affected pregnancies to term. Considering that more than 1 million individuals are screened for CF each year, it is remarkable that we do not have a comprehensive assessment of the screening effect.
Potential research questions:
-
•
What is the effectiveness of carrier screening, and has it affected the incidence of CF, distribution of CFTR mutations, or management of newborns known to have CF before birth?
-
•
Are there socioeconomic, geographic, or cultural trends to the delivery of CF carrier screening?
-
•
How has carrier screening affected use of preconceptual options by at-risk couples? Would the decision process of carrier couples change if effective molecular-based therapy for CF were available?
Gene/Protein/Molecular Interactions
If lung function is normal in utero, then implementation of therapies targeting CFTR at birth, before the onset of symptoms, provides a critical window for intervention strategies (Figure 1). Even if lung development is altered in utero, restoration of CFTR function as soon as possible would provide the greatest opportunity for prevention of chronic lung disease. Therapies that are of immediate consideration for newborns with CF are those targeted to the basic defect, including nutritional interventions that improve growth and lung development. Given the proven efficacy of ivacaftor in older patients with the p.Gly551Asp (G551D) mutation, it would be a high priority to assess feasibility and safety of administering ivacaftor to newborns with G551D. Correction of mutant CFTR would be the optimal therapy for newly diagnosed patients, but some forms of mutant CFTR are challenging to treat. Thus, resources should be focused on correction of all forms of mutant CFTR. In addition, given recent advances toward in situ genetic correction, such approaches should remain an active area of research. It is also possible that treatments that have limited efficacy in older children and adults might prove to be of greater efficacy in newborns or infants in whom lung disease is in the earliest phases. Examples include agents broadly designed to restore mucus clearance, novel ion transport regulators, and mucolytic agents. Finally, with the advent of technologies that can interrogate the entire human genome, it has been possible to localize genes other than CFTR that influence outcome. Some of these genes are likely to encode therapeutic targets for which drugs are already available.
Treatment of CF at the earliest point requires newborn screening, which allows the opportunity for early intervention. Normal and CF data sets with biomarkers for neonates and children are required for early intervention studies.
Potential research questions:
-
•
What are the efficacy and safety of novel and existing agents targeting molecular defects associated with CF in infants?
-
•
What are the safety and efficacy of novel and existing agents targeting downstream consequences of CFTR dysfunction (e.g., restoration of mucus clearance, ion defects, etc.) used in the first 3 months of life?
-
•
Can individualized care of patients with CF be facilitated by genome sequencing to allow determination of the status of known and newly discovered genetic modifiers?
Defining the Early Infectious Milieu and Implications of Early Infection
Ineffective mucociliary clearance and defective innate defenses allow bacterial infection to become established in the CF airway (24, 25) (Figure 1). Early acquisition of pathogenic bacteria and chronic infection are associated with increased morbidity and mortality in CF (26, 27). Despite aggressive antibiotic treatment for exacerbations, lung disease persists after treatment (28). Management of these early infections has included prophylaxis and eradication protocols; however, bronchiectasis may be present in the absence (or before detection) of these characteristic organisms, suggesting that our understanding of the early microbiota is limited and other infections, such as viruses or anaerobes, could have a significant role in initiating CF lung disease.
The polymicrobial nature of the CF airway has recently been highlighted (15, 29); its diversity appears to be high in the older pediatric population and decrease over time in adults. It is unclear whether current antibiotic regimens produce the narrowing of microbiota diversity. Changes in microbial diversity in the gastrointestinal tract of the CF population have similarly been linked to respiratory disease. More studies are needed to understand the effect of these complex bacterial communities.
Respiratory virus infections play a significant role in pulmonary exacerbations and can lead to morbidity in children with CF. Studies in the CF population have linked infection with established respiratory viruses to clinical deterioration. These viruses injure the airway, induce local inflammatory responses, and may produce airway surface dehydration and mucus stasis. These effects render the lung more susceptible to infection and exaggerate the host inflammatory response by concentrating chemokines and cytokines on the airway surface (30). Viral infections in children with CF tend to be more severe, have a longer duration than in children without CF (26, 31), and have been linked to reduced lung function (32, 33). The precise role of early viral infections in the development of pulmonary disease in CF has yet to be defined.
Potential research questions:
-
•
How do microbiota develop in the normal and CF airway and evolve over time? How does the basic CF defect lead to alterations in the airway microbiota? Does diversity of the microbiota protect against infections and pulmonary deterioration? Does the microbiota modulate the immune system? What is the impact of pathogen-specific interventions on airway microbiota?
-
•
What are the relationships between intestinal and respiratory microbiota? How does infant nutritional status and diet (e.g., breast milk) affect the bacterial flora at both sites? How does infant nutritional status affect pulmonary health?
-
•
What are the importance and impact of viral infections on early CF lung disease? What is the best means of monitoring the airway microbial community and assessing change over time?
Inflammatory Background and Interventions
Normal airways are protected from infection by a complicated host defense system. The respiratory epithelium is the first line of defense of the airways. Mucus, rich with proteolytic and antibacterial enzymes, is constantly produced by the epithelium. It is cleared via the mucociliary escalator, whose efficacy is dependent on the interactions of cilia and the non-Newtonian properties of mucus. Airway macrophages complement epithelial defenses, yet these multiple layers of pulmonary defense are overwhelmed in CF as lung disease develops (34, 35).
Inflammation in the CF airway begins early in life and may occur independent of infection. Relatively minor infection may induce an exaggerated and prolonged inflammatory response in the CF lung (Figure 1). Infants with CF and uninfected control subjects have been shown to have similar bronchoalveolar lavage (BAL) profiles (10, 36). At some point, the airway is unable to prevent infection, which becomes persistent, a cardinal feature of CF. The resultant inflammatory response contributes to the progressive, suppurative pulmonary disease in CF. Neutrophils are the prominent inflammatory cell in the lungs of patients with CF, even in mild disease (37). Neutrophils release DNA, oxidants, and proteases into the airway, which contribute to disease pathogenesis. Inflammatory mediators, such as IL-8 and various oxidants, have been found in high concentration in BAL fluid, even in infants with normal lung function and without apparent bacterial colonization (38), and higher BAL fluid neutrophil concentrations are associated with respiratory symptoms. Neutrophil elastase in lavage fluid has been shown to predict early bronchiectasis in children with CF (39).
Dysregulated activity of transcription factors may lead to a hyperinflammatory phenotype of the CF airway epithelium, and ceramide accumulation may induce apoptosis with deposition of DNA in the airway and increase bacterial adhesion in the CF airway (40, 41). Misfolded CFTR, which elicits the unfolded protein response, may contribute to the inflammatory CF phenotype in some patients (42). The function of immune cells may be dysregulated as well, and poor nutrition may further impact the inflammatory milieu (43, 44). Finally, experiments in animal models suggest that mucostasis, in the absence of bacterial infection, can be proinflammatory (45), with increased release of neutrophil chemotactic agents.
Potential research questions:
-
•
Is the CF inflammatory response to infection proportionate or excessive, and, if the latter, is it related to epithelial or immune cell dysfunction?
-
•
What is the role of inflammation in the lung of an infant with CF? How are nutritional health, airway infections, and inflammation related? What is the relationship of “sterile” mucostasis to lung inflammation? Are innate defenses in the CF airway defective early in life?
-
•
What is the effect of antiinflammatory therapies on development of CF lung disease? Do they prevent airways destruction or irreversible disease? Do antiinflammatory therapies change the microbiota or innate defenses of the CF lung? What is the best method for measuring change in inflammation and changes brought about by these agents?
Outcome Measure and Biomarker Development
Pulmonary outcome measures specific to early childhood and sufficiently sensitive to detect early lung disease are lacking, not just in CF, but for the infant population as a whole. Early measures that predict disease development are important as endpoints in young, asymptomatic children and may suggest therapeutic targets. The ideal outcome measure should be simple to perform, feasible across multiple sites, sensitive to early, nonhomogeneous lung disease in CF, and responsive to interventions. Further work is needed to determine the usefulness of existing outcome measures in the infant/preschool population and to develop and validate newer, more sensitive (to early lung disease) or specific (to pulmonary disease) measures. Given the challenges of studying this population, an armamentarium of endpoints will likely be needed (46).
Structural (imaging) and functional measures currently available are hampered by the requirement for sedation, limited reference data, and radiation exposure. Imaging measures that have been used in infants include chest computed tomography (CT), which is sensitive to the presence and heterogeneity of early disease, and chest radiographs, which have limited usefulness in infants with presymptomatic disease (18, 39). Magnetic resonance imaging (MRI) studies are emerging (47). Priorities for further development include chest CT and MRI, improved quantification of chest CTs, hyperpolarized gas MRI (ventilation scans), mucociliary clearance studies, positron emission tomography with fluorodeoxyglucose (PET-FDG), combined MRI/PET, and γ scintigraphy.
Physiologic measures used in recent studies include infant pulmonary function testing, inductance plethysmography, oscillometry, and measurement of resistance and compliance (28, 48). Multiple breath washout (MBW) testing, which assesses ventilation heterogeneity and may be especially sensitive to early peripheral airways disease, is being developed for the preschool population at a few specialized centers (49). Challenges of lung function testing in infants include sedation, expense, limited reference data, and technical expertise. Priorities for development include MBW, with particular emphasis on reference values, testing in infants, and defining longitudinal and clinically significant change; nonsedated physiologic testing; and improvement of reference equations and longitudinal data for all measures.
Currently available biomarkers for infant pulmonary disease are predominantly measured in BAL fluid, necessitating sedation and an invasive procedure, and include inflammatory markers, cell counts and differential, and neutrophil elastase (10). Future development of biomarkers might focus on measures of CFTR function (either airways specific or whole body), serum or urine inflammatory markers, bacterial-specific antibodies, mucin concentrations and mucus biophysical analyses, assessment of microbiome or total lung bacterial burden, macrophage function measurement, and noninvasive measures (exhaled breath condensate, metabolomics).
Nutritional status may also be considered an outcome measure, although nutritional intervention is certainly believed to be an important initial therapy in the newborn with CF. Poor nutritional status (particularly weight or weight-to-height ratio) in infancy has been linked to lower lung function later in life, and it has been shown that infants with CF have lower birth weights, on average, than infants without CF. Thus, there may be a primary prevention intervention that could focus on nutrition as a means of improving pulmonary health and could also use nutritional status as an outcome measure.
Finally, the only currently available patient- and parent-reported outcome in CF, the CFQR (CF Questionnaire, Revised), has recently been extended into preschoolers (50). The CFQR needs further development in infants, as do exacerbation and symptom scores, and perhaps a more sensitive tool specific to symptoms in younger patients with mild symptoms.
Potential research questions:
-
•
What are the best outcome measures for clinical trials in infants with CF?
-
•
How can normative values, applicability, and usefulness of MBW and other minimally invasive techniques be improved and developed?
-
•
How are different measures (e.g., biomarkers and physiologic measures, infant pulmonary function tests, and MBW), associated? Can a better understanding of associations allow better characterization of CF lung disease?
Overall Priorities for Research
The priorities for research outlined below are, in large part, focused on studies that may have a broad impact outside the CF community—refining pulmonary function testing in early childhood; determining the role of microbiota in pulmonary health (including relationships between the enteral microbiome, nutrition, and respiratory health); and linking mucus properties, infections, and inflammation in early childhood disease to structural and functional changes in childhood and beyond (Table 1). Of course, as the science of CF lung disease progresses, these priorities may need to be adjusted to reflect new knowledge and developments.
Table 1.
Primary prevention of cystic fibrosis lung disease: research priorities
| Epidemiology of CF in the age of genetic screening |
| Effectiveness of carrier screening, and effect on distribution of CFTR mutations |
| Socioeconomic, geographic, and cultural trends in the delivery of CF carrier screening |
| Effect of screening on use of preconceptual options by at-risk couples |
| Gene/molecular/protein interactions |
| Efficacy and safety of agents targeting CFTR molecular defects in the infant population |
| Efficacy and safety of agents targeting downstream effects of CFTR dysfunction in infants |
| Feasibility of individualized care via genome sequencing and DNA banking |
| Implications of early infection |
| Development/evolution of microbiota in normal and CF airways, and impact of diversity |
| Relationship between intestinal and respiratory microbiota |
| Importance and impact of viral infections on early CF lung disease |
| Inflammatory background and interventions |
| The CF inflammatory response— appropriate or disproportionate |
| Role of inflammation and sterile mucostasis in the infant lung |
| Effect of antiinflammatory therapies on prevention of CF lung disease |
| Outcome measure and biomarker development |
| Best outcome measures for infant trials—structural, functional, biomarkers, patient-reported outcomes |
| Establish normative values, applicability, and usefulness of noninvasive testing techniques |
| Investigate association of different outcome measures |
Definition of abbreviations: CF = cystic fibrosis; CFTR = cystic fibrosis transmembrane conductance regulator.
Near-term priorities were chosen with the goal of improving efficacy trials in the infant/preschool age group, reflecting our belief that defining more uniform study populations and improving sensitivity and accessibility of outcome measures in heterogeneous lung disease will improve early interventional trials.
-
•
Establish uniform endotypes (genotype and phenotype subpopulations) based on a combination of genetics (CFTR and modifiers), biomarkers, outcome measures (including pulmonary function and nutritional measures), environmental exposures, mucus, inflammatory, and microbiome measures to allow for more targeted interventions based on predicted disease course (e.g., early severe lung disease, early nutritional issues, hyperinflammatory endotypes). Use of endotypes may better demonstrate efficacy of therapeutic interventions by targeting specific populations with CF.
-
•Improve and broaden early and noninvasive outcome measures available for the study of pulmonary disease in young children to strengthen our ability to assess the impact of interventional studies and compare with healthy control subjects. These may include pulmonary function, assessment of structural lung disease, pulmonary-specific inflammatory markers and other markers of lung damage, and investigation of the pulmonary microbiota.
-
○Improvement should include determination of normative values across ages, “normal” longitudinal changes in both healthy subjects and those with disease, and definition of clinically significant change.
-
○
Long-term priorities reflect the goal of eradicating CF lung disease by providing personalized interventions for each patient beginning at birth. These studies would use outcome measures and target subjects based on endotypes described above.
-
•Establish the effect of molecular, genetic, or downstream (therapeutic) interventions initiated in infancy on preventing CF lung disease. Define optimal timing of interventions in infancy and determine the role for prenatal screening in promoting intervention at birth. Special considerations include determination of appropriate duration and interval of treatments (to provide greatest clinical benefits with fewest side effects and lowest cost), identification of CF endotypes most likely to benefit from specific interventions, and detailed monitoring for potential side effects. Outcomes to be considered would include those listed above. Longitudinal study will be necessary, because demonstrable differences in treatment groups may require years of data collection.
-
○Determine the impact of CFTR correctors and potentiators on the prevention of CF lung disease when treatment is initiated early, shortly after diagnosis.
-
○Determine the impact of agents designed to improve mucociliary clearance (hypertonic saline, dornase-alfa, N-acetylcysteine, mannitol) on the prevention of detectable lung disease in infants with CF.
-
○Determine the impact of antiinflammatory agents (azithromycin, ibuprofen) on the prevention of lung disease in infants with CF.
-
○
-
•
Investigate the normal development of the microbiota of the lung and the impact of alterations in respiratory flora on lung health in children with CF.
Summary
Great progress has been made in the past quarter century in the understanding and treatment of CF, with the potential to slow progression of structural damage and functional impairment beginning in the first few months of life. This is reflected in a steadily increasing median life expectancy in patients with CF. Neonatal diagnosis, early interventions, and newer mutation-based treatment options have led some to consider what was unthinkable only a few years ago—primary prevention of CF disease. To shift the treatment paradigm for CF, however, we need to fully understand the pathophysiological origins of CF lung disease, identify relevant biomarkers, and better define outcomes. Primary prevention would profoundly change the trajectory of CF lung disease and improve the morbidity and mortality of future children.
Footnotes
Report from the NHLBI Workshop September 19–20, 2013
Author Contributions: All authors contributed to the drafting and preparation of all portions of this manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Davies JC, Alton EW, Bush A. Cystic fibrosis. BMJ. 2007;335:1255–1259. doi: 10.1136/bmj.39391.713229.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis PB. Pulmonary disease in cystic fibrosis. Kendig’s disorders of the respiratory tract in children. In: Chernick V, Boat TF, Wilmott RW, Bush A, editors. Philadelphia: Saunders Elsevier; 2006. pp. 873–886. [Google Scholar]
- 3.Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, Boucher RC, Rubinstein M. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drumm ML, Ziady AG, Davis PB. Genetic variation and clinical heterogeneity in cystic fibrosis. Annu Rev Pathol. 2012;7:267–282. doi: 10.1146/annurev-pathol-011811-120900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfenden LL, Schechter MS. Genetic and non-genetic determinants of outcomes in cystic fibrosis. Paediatr Respir Rev. 2009;10:32–36. doi: 10.1016/j.prrv.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 6.Vanscoy LL, Blackman SM, Collaco JM, Bowers A, Lai T, Naughton K, Algire M, McWilliams R, Beck S, Hoover-Fong J, et al. Heritability of lung disease severity in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:1036–1043. doi: 10.1164/rccm.200608-1164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collaco JM, Blackman SM, McGready J, Naughton KM, Cutting GR.Quantification of the relative contribution of environmental and genetic factors to variation in cystic fibrosis lung function J Pediatr 2010157802–807.e1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cutting GR. Modifier genes in Mendelian disorders: the example of cystic fibrosis. Ann N Y Acad Sci. 2010;1214:57–69. doi: 10.1111/j.1749-6632.2010.05879.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belessis Y, Dixon B, Hawkins G, Pereira J, Peat J, MacDonald R, Field P, Numa A, Morton J, Lui K, et al. Early cystic fibrosis lung disease detected by bronchoalveolar lavage and lung clearance index. Am J Respir Crit Care Med. 2012;185:862–873. doi: 10.1164/rccm.201109-1631OC. [DOI] [PubMed] [Google Scholar]
- 10.Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, Robertson CF, Grimwood K. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol. 2005;40:500–510. doi: 10.1002/ppul.20294. [DOI] [PubMed] [Google Scholar]
- 11.Stephenson AL, Mannik LA, Walsh S, Brotherwood M, Robert R, Darling PB, Nisenbaum R, Moerman J, Stanojevic S. Longitudinal trends in nutritional status and the relation between lung function and BMI in cystic fibrosis: a population-based cohort study. Am J Clin Nutr. 2013;97:872–877. doi: 10.3945/ajcn.112.051409. [DOI] [PubMed] [Google Scholar]
- 12.Callaghan M, McClean S. Bacterial host interactions in cystic fibrosis. Curr Opin Microbiol. 2012;15:71–77. doi: 10.1016/j.mib.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Rosenfeld M, Emerson J, McNamara S, Joubran K, Retsch-Bogart G, Graff GR, Gutierrez HH, Kanga JF, Lahiri T, Noyes B, et al. EPIC Study Group Participating Clinical Sites. Baseline characteristics and factors associated with nutritional and pulmonary status at enrollment in the cystic fibrosis EPIC observational cohort. Pediatr Pulmonol. 2010;45:934–944. doi: 10.1002/ppul.21279. [DOI] [PubMed] [Google Scholar]
- 14.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, Stokes DC, Wohl ME, Wagener JS, Regelmann WE, Johnson CA.Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis J Pediatr 2007151134–139.139.e1 [DOI] [PubMed] [Google Scholar]
- 15.Rabin HR, Surette MG. The cystic fibrosis airway microbiome. Curr Opin Pulm Med. 2012;18:622–627. doi: 10.1097/MCP.0b013e328358d49a. [DOI] [PubMed] [Google Scholar]
- 16.Ranganathan SC, Dezateux C, Bush A, Carr SB, Castle RA, Madge S, Price J, Stroobant J, Wade A, Wallis C, et al. London Collaborative Cystic Fibrosis Group. Airway function in infants newly diagnosed with cystic fibrosis. Lancet. 2001;358:1964–1965. doi: 10.1016/s0140-6736(01)06970-7. [DOI] [PubMed] [Google Scholar]
- 17.Davis SD, Fordham LA, Brody AS, Noah TL, Retsch-Bogart GZ, Qaqish BF, Yankaskas BC, Johnson RC, Leigh MW. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med. 2007;175:943–950. doi: 10.1164/rccm.200603-343OC. [DOI] [PubMed] [Google Scholar]
- 18.Hall GL, Logie KM, Parsons F, Schulzke SM, Nolan G, Murray C, Ranganathan S, Robinson P, Sly PD, Stick SM, et al. AREST CF. Air trapping on chest CT is associated with worse ventilation distribution in infants with cystic fibrosis diagnosed following newborn screening. PLoS ONE. 2011;6:e23932. doi: 10.1371/journal.pone.0023932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ranganathan S, Linnane B, Nolan G, Gangell C, Hall G. Early detection of lung disease in children with cystic fibrosis using lung function. Paediatr Respir Rev. 2008;9:160–167. doi: 10.1016/j.prrv.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, Durie PR, Legrys VA, Massie J, Parad RB, et al. Cystic Fibrosis Foundation. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goetzinger KR, Cahill AG. An update on cystic fibrosis screening. Clin Lab Med. 2010;30:533–543. doi: 10.1016/j.cll.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 22.Hale JE, Parad RB, Comeau AM. Newborn screening showing decreasing incidence of cystic fibrosis. N Engl J Med. 2008;358:973–974. doi: 10.1056/NEJMc0707530. [DOI] [PubMed] [Google Scholar]
- 23.Castellani C, Picci L, Tamanini A, Girardi P, Rizzotti P, Assael BM. Association between carrier screening and incidence of cystic fibrosis. JAMA. 2009;302:2573–2579. doi: 10.1001/jama.2009.1758. [DOI] [PubMed] [Google Scholar]
- 24.Matsui H, Grubb BR, Tarran R, Randell SH, Gatzy JT, Davis CW, Boucher RC. Evidence for periciliary liquid layer depletion, not abnormal ion composition, in the pathogenesis of cystic fibrosis airways disease. Cell. 1998;95:1005–1015. doi: 10.1016/s0092-8674(00)81724-9. [DOI] [PubMed] [Google Scholar]
- 25.Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–577. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lipuma JJ. The changing microbial epidemiology in cystic fibrosis. Clin Microbiol Rev. 2010;23:299–323. doi: 10.1128/CMR.00068-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA. 2010;303:2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 28.Pittman JE, Johnson RC, Davis SD. Improvement in pulmonary function following antibiotics in infants with cystic fibrosis. Pediatr Pulmonol. 2012;47:441–446. doi: 10.1002/ppul.21575. [DOI] [PubMed] [Google Scholar]
- 29.Willner D, Haynes MR, Furlan M, Schmieder R, Lim YW, Rainey PB, Rohwer F, Conrad D. Spatial distribution of microbial communities in the cystic fibrosis lung. ISME J. 2012;6:471–474. doi: 10.1038/ismej.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Farberman MM, Ibricevic A, Joseph TD, Akers KT, Garcia-Medina R, Crosby S, Clarke LL, Brody SL, Ferkol TW. Effect of polarized release of CXC-chemokines from wild-type and cystic fibrosis murine airway epithelial cells. Am J Respir Cell Mol Biol. 2011;45:221–228. doi: 10.1165/rcmb.2009-0249OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Ewijk BE, van der Zalm MM, Wolfs TF, Fleer A, Kimpen JL, Wilbrink B, van der Ent CK. Prevalence and impact of respiratory viral infections in young children with cystic fibrosis: prospective cohort study. Pediatrics. 2008;122:1171–1176. doi: 10.1542/peds.2007-3139. [DOI] [PubMed] [Google Scholar]
- 32.Wat D, Gelder C, Hibbitts S, Cafferty F, Bowler I, Pierrepoint M, Evans R, Doull I. The role of respiratory viruses in cystic fibrosis. J Cyst Fibros. 2008;7:320–328. doi: 10.1016/j.jcf.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Olesen HV, Nielsen LP, Schiotz PO. Viral and atypical bacterial infections in the outpatient pediatric cystic fibrosis clinic. Pediatr Pulmonol. 2006;41:1197–1204. doi: 10.1002/ppul.20517. [DOI] [PubMed] [Google Scholar]
- 34.Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, Karp PH, Wohlford-Lenane CL, Haagsman HP, van Eijk M, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–113. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moskwa P, Lorentzen D, Excoffon KJ, Zabner J, McCray PB, Jr, Nauseef WM, Dupuy C, Bánfi B. A novel host defense system of airways is defective in cystic fibrosis. Am J Respir Crit Care Med. 2007;175:174–183. doi: 10.1164/rccm.200607-1029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- 37.Konstan MW, Hilliard KA, Norvell TM, Berger M. Bronchoalveolar lavage findings in cystic fibrosis patients with stable, clinically mild lung disease suggest ongoing infection and inflammation. Am J Respir Crit Care Med. 1994;150:448–454. doi: 10.1164/ajrccm.150.2.8049828. [DOI] [PubMed] [Google Scholar]
- 38.Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med. 2009;180:146–152. doi: 10.1164/rccm.200901-0069OC. [DOI] [PubMed] [Google Scholar]
- 39.Sly PD, Gangell CL, Chen L, Ware RS, Ranganathan S, Mott LS, Murray CP, Stick SM, Investigators AC AREST CF Investigators. Risk factors for bronchiectasis in children with cystic fibrosis. N Engl J Med. 2013;368:1963–1970. doi: 10.1056/NEJMoa1301725. [DOI] [PubMed] [Google Scholar]
- 40.Venkatakrishnan A, Stecenko AA, King G, Blackwell TR, Brigham KL, Christman JW, Blackwell TS. Exaggerated activation of nuclear factor-kappaB and altered IkappaB-beta processing in cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol. 2000;23:396–403. doi: 10.1165/ajrcmb.23.3.3949. [DOI] [PubMed] [Google Scholar]
- 41.Brodlie M, McKean MC, Johnson GE, Gray J, Fisher AJ, Corris PA, Lordan JL, Ward C. Ceramide is increased in the lower airway epithelium of people with advanced cystic fibrosis lung disease. Am J Respir Crit Care Med. 2010;182:369–375. doi: 10.1164/rccm.200905-0799OC. [DOI] [PubMed] [Google Scholar]
- 42.DiMango E, Ratner AJ, Bryan R, Tabibi S, Prince A. Activation of NF-kappaB by adherent Pseudomonas aeruginosa in normal and cystic fibrosis respiratory epithelial cells. J Clin Invest. 1998;101:2598–2605. doi: 10.1172/JCI2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Petit-Bertron AF, Tabary O, Corvol H, Jacquot J, Clément A, Cavaillon JM, Adib-Conquy M. Circulating and airway neutrophils in cystic fibrosis display different TLR expression and responsiveness to interleukin-10. Cytokine. 2008;41:54–60. doi: 10.1016/j.cyto.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Tan HL, Regamey N, Brown S, Bush A, Lloyd CM, Davies JC. The Th17 pathway in cystic fibrosis lung disease. Am J Respir Crit Care Med. 2011;184:252–258. doi: 10.1164/rccm.201102-0236OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, Randell SH, O’Neal WK. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol. 2012;5:397–408. doi: 10.1038/mi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davis SD, Brody AS, Emond MJ, Brumback LC, Rosenfeld M. Endpoints for clinical trials in young children with cystic fibrosis. Proc Am Thorac Soc. 2007;4:418–430. doi: 10.1513/pats.200703-041BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puderbach M, Eichinger M, Haeselbarth J, Ley S, Kopp-Schneider A, Tuengerthal S, Schmaehl A, Fink C, Plathow C, Wiebel M, et al. Assessment of morphological MRI for pulmonary changes in cystic fibrosis (CF) patients: comparison to thin-section CT and chest x-ray. Invest Radiol. 2007;42:715–725. doi: 10.1097/RLI.0b013e318074fd81. [DOI] [PubMed] [Google Scholar]
- 48.Rosenfeld M, Ratjen F, Brumback L, Daniel S, Rowbotham R, McNamara S, Johnson R, Kronmal R, Davis SD, Group IS ISIS Study Group. Inhaled hypertonic saline in infants and children younger than 6 years with cystic fibrosis: the ISIS randomized controlled trial. JAMA. 2012;307:2269–2277. doi: 10.1001/jama.2012.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robinson PD, Latzin P, Verbanck S, Hall GL, Horsley A, Gappa M, Thamrin C, Arets HG, Aurora P, Fuchs SI, et al. Consensus statement for inert gas washout measurement using multiple- and single- breath tests. Eur Respir J. 2013;41:507–522. doi: 10.1183/09031936.00069712. [DOI] [PubMed] [Google Scholar]
- 50.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128:2347–2354. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]