Abstract
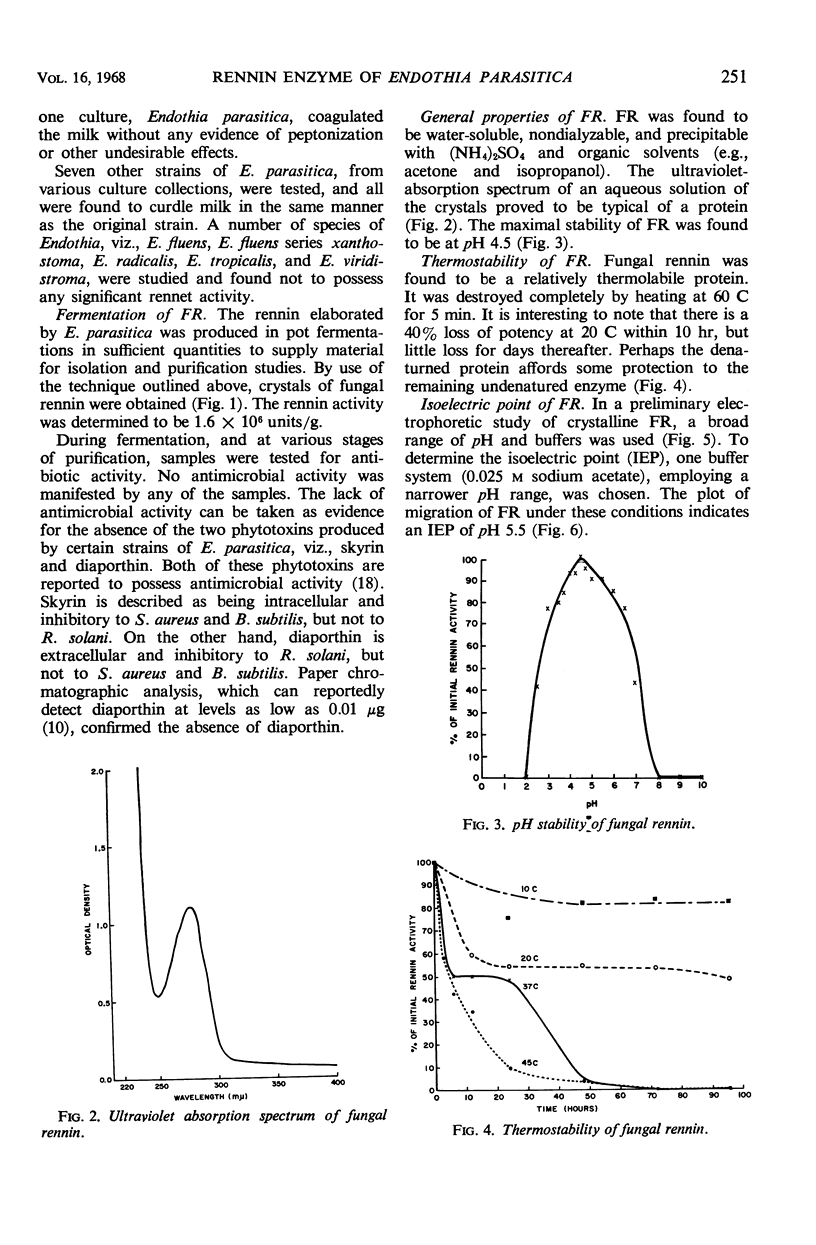
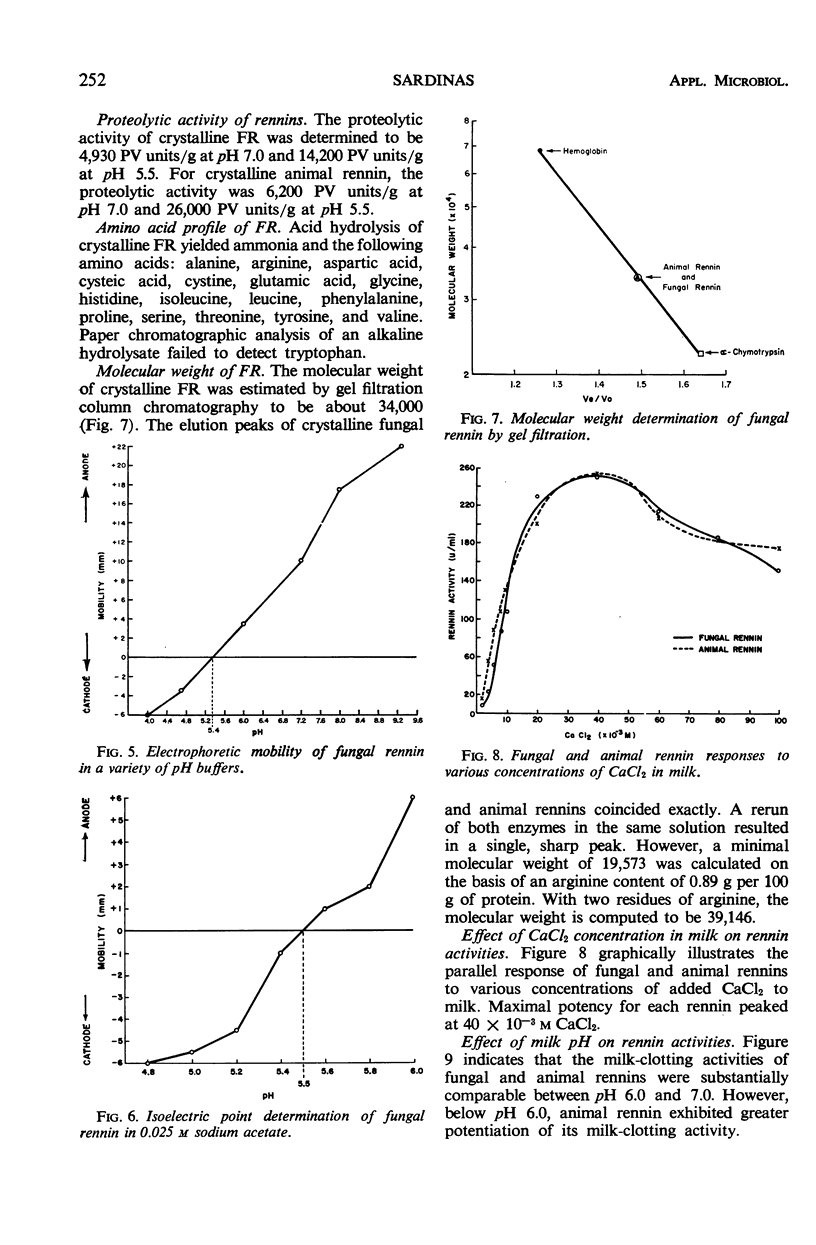
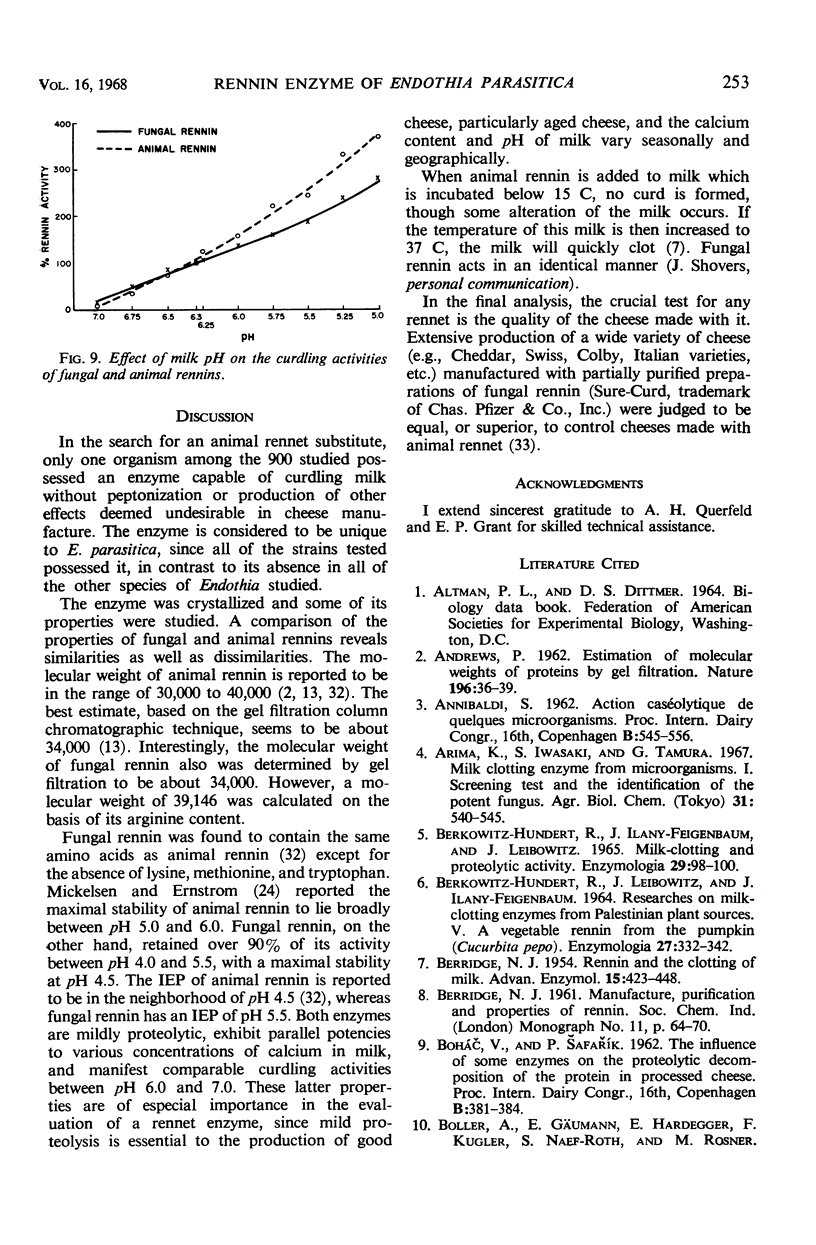
A microbiological screening program was instituted to search for an animal rennet substitute. Among 381 bacteria and 540 fungi tested, only one organism, Endothia parasitica, yielded a suitable enzyme substitute. The fungal rennin enzyme was crystallized and some of its properties were studied. It was found to be water-soluble, nondialyzable, precipitable with (NH4)2SO4 and organic solvents (e.g., acetone and isopropanol), and destroyed by heating for 5 min at 60 C. It was determined to be most stable in water at pH 4.5 and to have an isoelectric point of pH 5.5. On acid hydrolysis, it yielded: alanine, ammonia, arginine, aspartic acid, cysteic acid, cystine, glutamic acid, glycine, histidine, isoleucine, leucine, phenylalanine, proline, serine, threonine, tyrosine, and valine. No tryptophan was detected after alkaline hydrolysis. Its molecular weight was estimated to be in the range of 34,000 to 39,000. The milk-clotting activities of the fungal and animal rennins proved to be essentially identical in milk containing various concentrations of CaCl2. Both rennins manifested comparable clotting activities in milk at pH 6.0 to 7.0.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDREWS P. Estimation of molecular weights of proteins by gel filtration. Nature. 1962 Oct 6;196:36–39. doi: 10.1038/196036a0. [DOI] [PubMed] [Google Scholar]
- BERKOWITZ-HUNDERT R., LEIBOWITZ J., ILANY-FEIGENBAUM J. RESEARCHES ON MILK-CLOTTING ENZYMES FROM PALESTINIAN PLANT SOURCES. V. A VEGETAL RENNIN FROM THE PUMPKIN (CUCURBITA PEPO). Enzymologia. 1964 Oct 15;27:332–342. [PubMed] [Google Scholar]
- BERRIDGE N. J. Rennin and the clotting of milk. Adv Enzymol Relat Subj Biochem. 1954;15:423–448. doi: 10.1002/9780470122600.ch10. [DOI] [PubMed] [Google Scholar]
- DJURTOFT R., FOLTMANN B., JOHANSEN A. STUDIES ON RENNIN. 8. ON THE MOLECULAR WEIGHT OF PRORENNIN AND RENNIN. C R Trav Lab Carlsberg. 1964;34:287–298. [PubMed] [Google Scholar]
- GANGULI N. C., BHALERAO V. R. DIFFERENTIAL ACTION OF ANIMAL, VEGETABLE, AND MICROBIAL RENNETS ON CASEINS AS REVEALED BY CASEIN AGAR PLATE ASSAY METHOD. J Dairy Sci. 1965 Apr;48:438–443. doi: 10.3168/jds.s0022-0302(65)88249-2. [DOI] [PubMed] [Google Scholar]
- Knight S. G. Production of a rennin-like enzyme by molds. Can J Microbiol. 1966 Apr;12(2):420–422. doi: 10.1139/m66-059. [DOI] [PubMed] [Google Scholar]
- Loo Y. H., Skell P. S., Thornberry H. H., Ehrlich J., McGuire J. M., Savage G. M., Sylvester J. C. Assay of Streptomycin by the Paper-Disc Plate Method. J Bacteriol. 1945 Dec;50(6):701–709. [PMC free article] [PubMed] [Google Scholar]
- PIEZ K. A., MORRIS L. A modified procedure for the automatic analysis of amino acids. Anal Biochem. 1960 Nov;1:187–201. doi: 10.1016/0003-2697(60)90045-2. [DOI] [PubMed] [Google Scholar]
- Partridge S. M. Filter-paper partition chromatography of sugars: 1. General description and application to the qualitative analysis of sugars in apple juice, egg white and foetal blood of sheep. with a note by R. G. Westall. Biochem J. 1948;42(2):238–250. doi: 10.1042/bj0420238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., TUPPY H. The amino-acid sequence in the phenylalanyl chain of insulin. I. The identification of lower peptides from partial hydrolysates. Biochem J. 1951 Sep;49(4):463–481. doi: 10.1042/bj0490463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRINIVASAN R. A., IYENGAR M. K., BABBAR I. J., CHAKRAVORTY S. C., DUDANI A. T., IYA K. K. MILK-CLOTTING ENZYMES FROM MICROORGANISMS. Appl Microbiol. 1964 Nov;12:475–478. doi: 10.1128/am.12.6.475-478.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TENDLER M. D., BURKHOLDER P. R. Studies on the thermophilic actinomycetes. I. Methods of cultivation. Appl Microbiol. 1961 Sep;9:394–399. doi: 10.1128/am.9.5.394-399.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD D. N., ARNOTT M. S. GEL FILTRATION OF PROTEINS, WITH PARTICULAR REFERENCE TO THE GLYCOPROTEIN, LUTEINIZING HORMONE. Anal Biochem. 1965 Aug;12:296–302. doi: 10.1016/0003-2697(65)90094-1. [DOI] [PubMed] [Google Scholar]



