Abstract
Whether non-arctic species persisted in northern Europe during the Last Glacial Maximum (LGM) is highly debated. Until now, the debate has mostly focused on plants, with little consideration for other groups of organisms, e.g. the numerous plant-dependent insect species. Here, we study the late-Quaternary evolution of the European range of a boreo-montane leaf beetle, Gonioctena intermedia, which feeds exclusively on the boreal and temperate trees Prunus padus and Sorbus aucuparia. Using species distribution models, we estimated the congruence between areas of past and present suitable climate for this beetle and its host plants. Then we derived historical hypotheses from the congruent range estimates, and evaluated their compatibility with observed DNA sequence variation at five independent loci. We investigated compatibility using computer simulations of population evolution under various coalescence models. We find strong evidence for range modifications in response to late-Quaternary climate changes, and support for the presence of populations of G. intermedia in northern Europe since the beginning of the last glaciation. The presence of a co-dependent insect in the region through the LGM provides new evidence supporting the glacial survival of cold-tolerant tree species in northern Europe.
Keywords: coalescence simulations, biotic interactions, Nordic glacial refugia, boreo-montane, leaf beetle
1. Background
Where did European species survive during the last glaciation? Traditionally, it has been thought that temperate and boreal species survived the Last Glacial Maximum (21 ky BP, LGM) exclusively in southern Europe [1,2]. This paradigm is increasingly viewed as mistaken, however, contradicted by palaeoecological (e.g. [3–6]) and phylogeographic studies (e.g. [7,8]), as well as from species distribution modelling (SDM) (e.g. [9]). Indeed, these pieces of evidence support the existence of glacial refugia (sensu [10]) in higher latitudes for some temperate and boreal species. Yet, it remains highly controversial whether boreal species survived past glacial maxima further north, in the regions neighbouring the Scandinavian ice sheet. Evidence from pollen, macrofossils and ancient DNA indicate glacial survival of boreal trees (Pinus, Picea) in northern Scandinavia ([11], but see [12,13] for an alternate point of view). Ancient DNA also supports the presence of the cold-tolerant temperate and boreal tree Sorbus aucuparia, in northern Scandinavia since the beginning of the Holocene [11], suggesting either fast postglacial immigration [14] or in situ survival.
Trees are important ecosystem engineers [15] and represent crucial habitats for many species. However, even if trees themselves survived in the high north, they may have been too rare to sustain co-dependent species. Also, whereas studies thoroughly recognize the role of climatic conditions in shaping the geographical distribution of species through time (e.g. [16,17]), few studies also showed the importance of the presence of other species like competitors or required hosts for survival [18,19]. Hence, even if climate is a major determinant for the presence of a species, interactions with other species may also play a significant role, and the nature of their influence (stochastic or deterministic, direct or indirect, positive or negative) needs to be identified. Furthermore, it has been shown that physical barriers and time-lagged migration (e.g. [20]) can prevent species from colonizing areas suitable for their survival after a major climate change. If cryptic northern refuges in the northern hemisphere were more widespread during glacial episodes of Pleistocene than previously thought, their role in re-colonizing northern regions, compared to long-distance migration from southern refuges, need to be re-evaluated.
Here, we investigated the glacial survival and influence of Quaternary climate changes on today's geographical distribution and genetic variation of the boreo-montane leaf-beetle Gonioctena intermedia. Gonioctena intermedia is a univoltine (one generation per year) insect herbivore, characterized by limited dispersal. Its current range spans northern and central-eastern Europe, and its westward distribution limit is the central Alps and the Belgian Ardennes (figure 1; [23]). Both larval and adult stages feed exclusively on the trees Sorbus aucuparia and Prunus padus [22]. These two trees currently occur in the boreal and temperate (nemoral) biomes across northern and central Europe, and are more widespread than G. intermedia. It seems safe to assume that the host plant diet of G. intermedia has remained unchanged during its entire evolutionary history. Indeed, the sister species Gonioctena quinquepunctata also feeds exclusively on the same two host trees, and the genus Gonioctena is believed to have experienced a relatively conserved history of host-plant shifts [24]. It is therefore likely that the host plant diet of G. intermedia and its sister species was inherited from their common ancestor. Here we integrate the relationship between the insect and the two trees through the Quaternary climatic episodes.
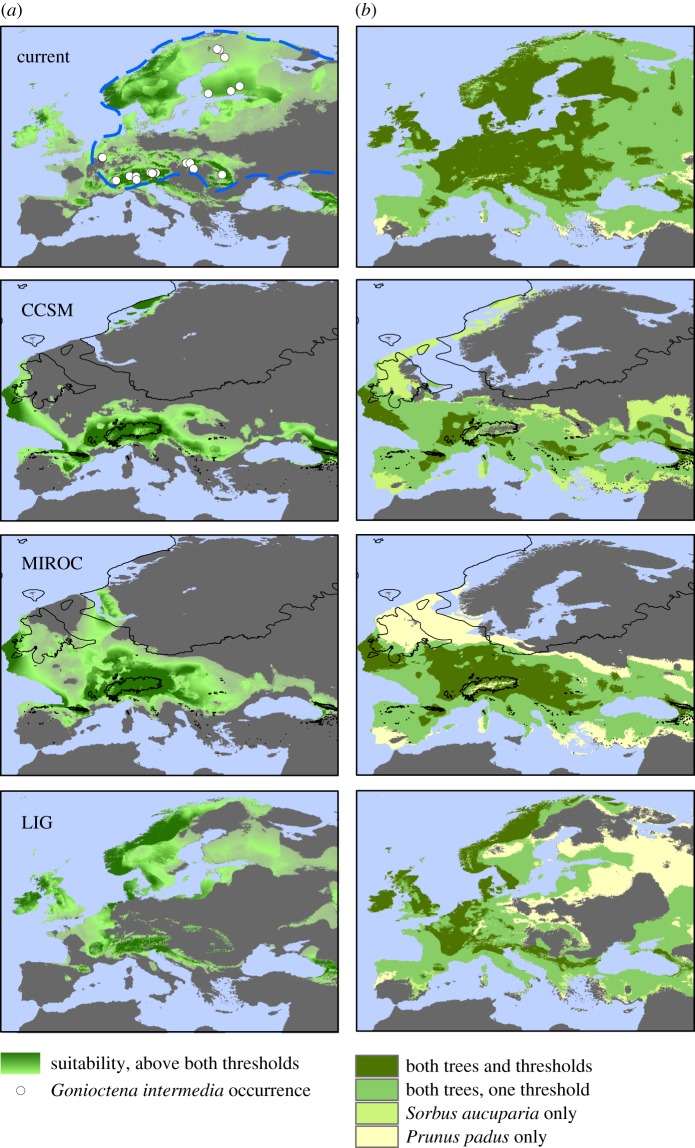
Figure 1.
Species geographical models of G. intermedia (a) and its two host trees (b) inferred for three periods of time: Present, Last Glacial Maximum (LGM) and Last Interglacial (LIG). CCSM and MIROC were the two climate models we used for the LGM (see text). The black line represents a model of the maximum ice-sheet extent during the LGM [21]. Gonioctena intermedia: white dots represent sampling localities. The presumed current range of this species in Europe (excluding the Urals mountain range, outside the figure) is outlined using a dashed purple line [22]. Note however that this range is highly fragmented, so that the insect is not found in all areas inside this limit. Regions in dark green or grey suggest a high probability of presence or absence, respectively, while light green indicates ambiguous estimates or low probability of presence. Host trees: for clarity of the map overlap for the two species, two different thresholds were used to infer presence/absence of the species in a given area (see text). Regions in dark green or grey suggest a high probability of presence or absence for both trees, respectively, while light colours suggest presence of one host tree or under one threshold only. See also the electronic supplementary material, figures S2 and S3.
Overall, we aim to provide an understanding of the geographical dynamic of trophic interactions through the last glacial-interglacial cycle, with special emphasis on in situ survival in northern Europe. In the absence of fossils, insights on the evolution of species ranges can be obtained through modelling the current and past geographical distributions based on environmental variables and current genetic diversity patterns. The combination of SDM techniques with coalescence models of DNA sequence variation appears particularly promising (e.g. [25–27]). In this study, we maximized the synergy of the two approaches, by directly integrating the SDM outputs into alternative scenarios of population history. These scenarios can be compared through coalescence simulations of DNA sequences and then comparing simulated sequences to observed DNA sequences sampled across the beetle species range.
More specifically, we first estimate the potential LGM survival of the two host tree species within northern Europe using species-distribution-based hindcasting [25]. We then estimate potential northern Europe LGM-survival of the beetle species taking both climate and host tree distributions into account. Based on this, we develop alternative historical demographic scenarios for the beetle and test these against current genetic diversity patterns (studied with five sequenced loci) to assess whether they are consistent with in situ LGM survival in northern Europe.
2. Material and methods
(a). DNA sequences
Sequences from five genetic markers, one mitochondrial (cytochrome oxydase subunit I, COI) and four nuclear loci (Actin, elongation factor 1-alpha or ELFAC, ribosomal protein P0 or RpP0, and Wingless), were obtained from 23 sampled populations (1–11 individuals per population, including at least 10 individuals per considered region; electronic supplementary material, table S1) located over most of the assumed range of G. intermedia ([22]; figure 1, and electronic supplementary material, table S1 and figure S1). Each of the four nuclear and the mitochondrial markers displayed allelic richness ranging from 3 to 4.13 per geographical region, and nucleotide diversity ranging from 0.001 to 0.003 per geographical region (electronic supplementary material, table S1), therefore providing sufficient variability for the current investigation.
(b). Species distribution models
Areas of suitable climatic conditions were computed for G. intermedia and its host tree species with MaxEnt v. 3.3.3a [28] for the present, the LGM (c. 21 kya) and Last Interglacial (LIG) (c. 120 kya). The models were calibrated with all 23 records of sampling localities in Europe for G. intermedia (figure 1) and projected on layers of past climate without clamping. Given that G. intermedia is difficult to differentiate from its sister taxon G. quinquepunctata based on morphology alone, that both species feed on the same two host plants, and that they have largely overlapping geographical distributions, the potential for misidentification in occurrence databases (e.g. Global Biodiversity Information Facility (GBIF)) is strong. We therefore only considered the 23 occurrence data points for which genetic information was available to confirm correct species identification. The distribution of S. aucuparia and P. padus were digitalized on a 50 × 50 km grid based on the maps used in Montoya et al. [29] to use occurrence data in the study area (electronic supplementary material, table S2). Current and past climate data were obtained from the WorldClim database ([30]; http://www.worldclim.org) at a 30 arc-seconds spatial resolution (c. 1 × 1 km). For the LGM, we used two different climate simulations: the Community Climate System Model (CCSM; [31]) and the Model for Interdisciplinary Research on Climate (MIROC; v. 3.2; [32]). We used three different variable selection procedures to select a small set of ecologically relevant bioclimatic variables for each species: (i) six non-correlated variables that we assumed important for a species inhabiting cold regions and in the case of G. intermedia, for example, suggested sensitive to heat (absent in southern Europe mountains and valleys in central Europe) like temperature during the hottest months. Additionally, among the six we further selected: (ii) those contributing most to the model after successive reduction steps (i.e. taking out one variable at a time), or (iii) those performing best according to the Akaike information criterion scores (computed in ENMtools; [33]) (see the electronic supplementary material, Materials and Methods S1). We hindcasted SDMs for the LGM and the LIG in order to investigate past climatic transitions from the glacial to interglacial period, and the inverse, because G. intermedia is thought to have diverged from its sister species, G. quinquepunctata, before the LIG [22].
For the purpose of comparing different SDMs, the predicted suitability maps were transformed to presence–absence maps based on two different thresholds: the maximum training sensitivity plus specificity threshold as recommended by Jiménez-Valverde & Lobo [34] and the minimum presence threshold. These thresholds were selected to represent a strict and a less restrictive approach, respectively, and thus to overcome potential biases in the models. The congruence between the potential distribution of the leaf beetle species and that of its hosts was studied (figure 1 and electronic supplementary material, figure S3) and used to build potential scenarios that were tested with the genetic data.
(c). Coalescence simulations and hypotheses testing
The scenarios were subsequently confronted to genetic variation data. For this purpose, coalescence models were built based on the continuous probability of presence of the species, i.e. SDM models without constraining them to presence/absence by the two thresholds mentioned above.
To determine which evolutionary scenarios fit the genetic data best, we translated them into models of population evolution for the purpose of computer simulations. Two kinds of models of coalescence were generated: (i) spatially explicit models, that directly integrate a two-dimensional representation of the hypothesized range and take into account the restricted movement of individuals; and (ii) classic models that translate a fragmented species range into a series of panmictic populations among which migration rates are specified. PhyloGeoSim 1.0 [35] was used to simulate sequences under the first type of models. In this case, a grid is directly superimposed onto a geographical map, and grid cells in which the species is present are specified, for different periods of time. Coalescence simulations are then conducted on this grid. This spatially explicit approach offers, among others, the ability to implement isolation by distance, which is assumed for populations of G. intermedia given its low capacity to disperse and its fragmented distribution. The second type of models were investigated within an Approximate Bayesian Computation (ABC) framework [36] with the program ABCtoolbox [37]. ABC is a popular approach for comparing historical hypotheses in a rigorous statistical framework [38,39], which allows a more thorough exploration of parameter values, whereas it is restricted to simpler (less geographically explicit) models of coalescence, to avoid unrealistically large computing resources. We thus investigated a wider range of demographic parameter values, mainly for: (i) times of range modification, and (ii) migration rates among populations, with the ABC approach.
The continuous projections of the current, LGM (represented either by the CCSM or MIROC simulation) and LIG distributions were used as a basis to define the tested coalescence models (electronic supplementary material, figures S2 and S3). Evolutionary scenarios tested with ABCtoolbox are described in figure 3 and the electronic supplementary material, figure S5. To develop less complex models for the ABC approach, only the most important parts of the species ranges were included in the models, corresponding to the major portions of the fragmented ranges: (i) the Alps, (ii) the Carpathians, and (iii) Fennoscandia (Scandinavia + Finland). We then evaluated the persistence of a Nordic population at the LGM by estimating the divergence time separating Fennoscandia from the remaining of the distribution. This was done via the inference of the posterior probability distribution of this model parameter. We also investigated the current and past connectivity among the three main currently inhabited regions from the beginning of the LIG.
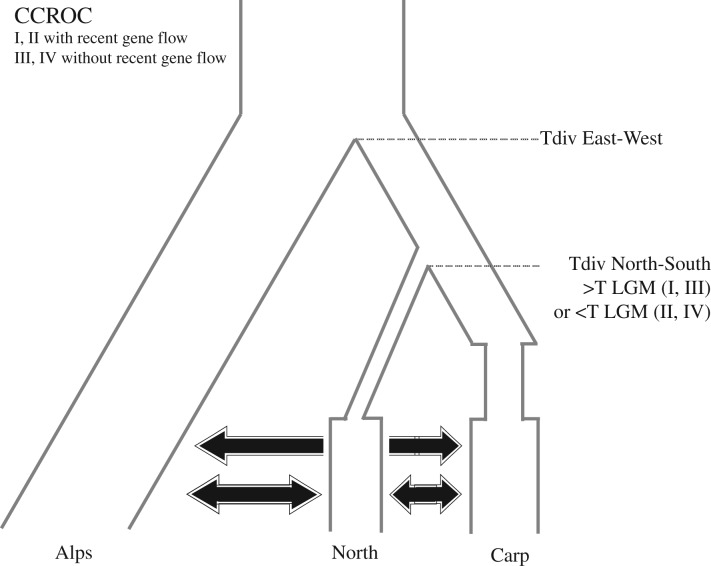
Figure 3.
Graphical representation of some of the various historical scenarios evaluated with the ABC approach for G. intermedia. Each scenario includes two splitting events among three main regions, the Alps (Alps), the Carpathians (Carp), and Scandinavia (North) represented by Tdiv East-West and Tdiv North-South; the former indicates the divergence time between the eastern and the western regions, i.e. the ancestral lineage of current populations in the Alps and the Carpathians, and the latter indicates the divergence between northern and southern Europe regions, i.e. the ancestral lineages of current populations in the Alps and Scandinavia. Depending on the scenarios, migration among populations was implemented (CCROC I and II) or not (CCROC III and IV). Graphical representations of the other tested scenarios are given in the electronic supplementary material, figure S5.
See the electronic supplementary material, Materials and Methods S1, for a complete description of all analytic procedures (SDMs and hypothesis testing) and all tested coalescence models.
3. Results
(a). Species potential geographical distribution through time
We first modelled the geographical extent of suitable environmental conditions for G. intermedia and its two host trees. For all three species suitable environmental conditions appeared to have been more restricted during the LIG than it is today (figure 1; electronic supplementary material, figures S2 and S3). In addition, for G. intermedia, areas of suitable climate were less fragmented at the LGM than during the LIG and at present, but not necessarily more widespread. However, the question of connectivity among the three main regions of known occurrence (the Alps, the Carpathians and Scandinavia) remains unresolved with the results of the SDM analysis alone, because different suitability thresholds resulted in various connection patterns, especially for the LGM. A strict threshold, when applied in the host distribution models, indicated that the habitat of G. intermedia was limited by the absence of its two host trees during the LGM and LIG (electronic supplementary material, figure S3 A2–4) despite suitable climatic conditions for the beetle in southwest Norway according to the MIROC model (electronic supplementary material, figure S3 A3), or in northwestern Norway under the CCSM model (electronic supplementary material, figure S3 A4). By contrast, less restrictive threshold (electronic supplementary material, figure S3 B3–4) suggested the presence of both the insect and its host trees in these regions.
Given these SDM estimates, we investigated the potential presence of northern refugia in areas influenced by oceanic climate in southwest (MIROC) or northwest Norway (CCSM) at the LGM through the use of geographically explicit coalescent simulations of genetic data. In addition, according to our SDM analyses, suitable environmental conditions are currently available for G. intermedia in western Europe, while the species is in fact absent from most of that region (e.g. France, including the Pyrenees and the Massif Central). This suggests that factors other than climatic conditions or the presence of the host plant are constraining the geographical range in the west. We therefore derived 12 historical scenarios (P1–P12) directly from the SDM results, six from the CCSM and six from the MIROC glacial climate estimates (electronic supplementary material, figure S4).
(b). Spatially explicit coalescence simulations
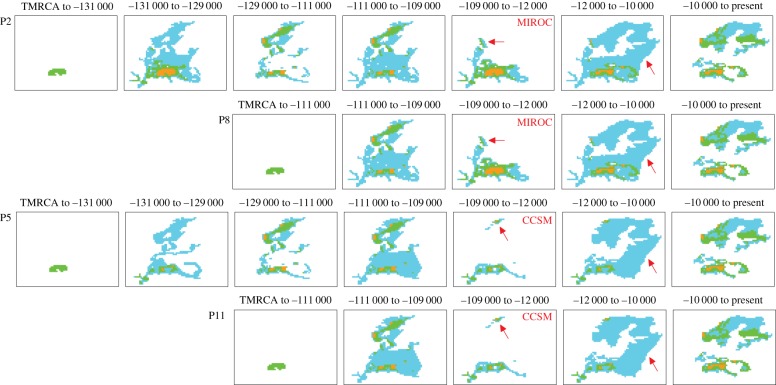
Scenarios P2 (p-value = 0.271) and P8 (p-value = 0.251) were identified as the most likely among tested spatially explicit hypotheses (the third most likely scenario is P5 with a p-value of only 0.04; electronic supplementary material, table S3). Both supported scenarios featured a relatively continuous distribution during the LGM and the presence of isolated glacial refugia in southern Norway (figure 2). They mainly differed in the timing of occurrence of the most ancestral range, when the distribution of G. intermedia is restricted to the Alps (figure 2): in contrast to scenario P8, P2 suggested an additional expansion/contraction cycle that occurred before the LIG (before approx. −130 000 yr BP).
Figure 2.
Selected historical scenarios for G. intermedia with the program PhyloGeoSim. Each line corresponds to one scenario. Each scenario is made of several chronological layers, each layer displaying a different matrix of maximum effective population sizes per grid cell, and includes the matrices based on SDM outputs for three different time periods. Time periods of each scenario layer is given in numbers of years. Blue cells have a maximum effective population size of 0.5*Ne, green cells a maximum effective population size of 1*Ne and orange cells a maximum effective population size of 2*Ne. These colours correspond to SDMs predictions of high, intermediate and low potential presence, respectively. Layers are dated in forward generation time (one generation per year in Goniomena species). Red arrows or circles indicate notable differences between concurrent layers from the 12 different scenarios—all four likely scenarios do not differ for the recent connection between northern and central Europe. See text for details on different evolutionary scenarios and also the electronic supplementary material, figure S4.
Because the pattern of variation at the mitochondrial locus differed somewhat from nuclear loci, we ran the same comparison of scenarios, but based only on nuclear loci. In this case, the p-value of scenario P11 approached those of P2 and P8 and the p-value of scenario P5 was slightly higher than the standard significant threshold of 0.05 (maximum p-values are 0.736 for P2, 0.768 for P8, 0.06 for P5, and 0.349 for P11; electronic supplementary material, table S3). All four supported scenarios involved an early Holocene connection between Scandinavia and eastern Europe (figure 2). All scenarios either: (i) excluding a northern refuge or (ii) including one but without a Holocene connection with central Europe (electronic supplementary material, figure S4 and table S3), were rejected (p-value < 0.001). These results therefore suggest that a combination of northern refuge and early Holocene connection explain the current distribution.
(c). Coalescence simulations in Approximate Bayesian Computation framework
To complement our analyses, we also conducted simulations following simpler (less geographically explicit) models of coalescence, under another statistical framework, using ABC. Through the use of ABC, we can more intensively explore the space of parameter values like divergence time. The best historical scenario (scenario CCROC I; figure 3 and electronic supplementary material, table S4; marginal density of 2.5844 × 10−4 along with an observed p-value of 0.1813 that indicated a relatively good model fit to the observed data) involves recent migration (Late Pleistocene - early Holocene) among the three regions, bottleneck events for the Carpathian and northern regions after their separation, and both divergence times largely predating the LGM (HDI95 for the most recent divergence, between the Carpathian and northern regions: 83 030–194 590 years ago). This scenario has the highest degree of complexity compared to all other tested models and was only slightly favoured (Bayes factor of 1.273) over the best simpler scenario of complete isolation, ISOL II (electronic supplementary material, figure S5; mean posterior probability of 2.03 × 10−4 along with an observed p-value of 0.103, revealing a relatively good model fit to the observed data; electronic supplementary material, table S4). Compared to CCROC I, this scenario involves no gene flow among regions after their separation, and no bottleneck event (although population sizes of the Carpathian and northern regions are smaller than that of the Alps), but also includes old divergence times among regions notably between southern and northern Europe (more than 160 generations/years ago). Therefore, ABC analyses also favoured a long-term existence (i.e. older than the LGM) of G. intermedia in northern Europe.
4. Discussion
For cold-hardy species with current widespread geographical ranges including Fennoscandia and other cold European regions, two main scenarios are likely to explain the existence of contemporary Nordic populations: in situ survival in northern Europe or recent colonization of northern regions from southern, western and/or eastern refugia (constantly inhabitable areas; e.g. [40,41]; see [42]). The potential for in situ survival in northern Europe during the LGM remains controversial for many species, even cold-adapted ones but especially for plants, is receiving growing support (e.g. [11,43–46]). Our study evaluated this hypothesis by investigating the evolutionary history of a boreo-montane herbivorous insect, G. intermedia, also considering its specialized trophic interaction with boreal and temperate tree species. Our results shed light on the range evolution of the insect that must reflect its host plant, since it is required for the survival of the insect. Their survival together in northern Europe may result from a combination of in situ survival and more recent colonization. We therefore encourage further exploration of the in situ survival hypothesis for other organisms.
SDM is often used to estimate the palaeo-distribution of species in the absence of fossil data [25]. In this study, we built SDMs using climatic variables corresponding to current and past suitable regions in Europe for the leaf beetle G. intermedia and its two host trees, P. padus and S. aucuparia. Models portray a fragmented geographical range during the LIG, as previously suggested for several plant taxa [47], followed by a less fragmented range at the LGM in central Europe, but not obviously associated with a range expansion. Gonioctena intermedia diverged from its sister species G. quinquepunctata at least 240 000 yr BP [22]. This implies it survived several climatic cycles, with a more continuous range during colder episodes and confinement to smaller isolated regions (generalized range fragmentation) during warmer episodes. According to our SDMs, G. intermedia appears currently more widespread than during LIG, although strong discontinuities in the range are still observed today between the north and the rest of Europe, and among the main inhabited mountain ranges of central and southern Europe (figure 1). Notably, LGM models suggest the existence of a habitable region located in the most western coastal region of Norway in northern Europe. Western coastal Norway is an area the continental ice sheet may not have reached, contrary to what is inferred by some models ([48]: but see [49]), and in which the discovery of plant macrofossils also suggest the glacial survival of certain tree species [11].
Several limitations of SDM are recognized (e.g. [50–52]). One limitation is that probabilities of occurrence are inferred from a subset of environmental parameters. In addition, the presence of a species depends on other important parameters that are rarely taken into account in SDM, e.g. the species capacity to disperse, or its interactions with other species [19] such as trophically linked taxa (e.g. the host of a specialized insect; [15]). Therefore, SDMs provide an estimate of the potential presence of a species in a given area, rather than actual presence [53]. For example, one factor that could explain the current distribution of G. intermedia, which we know is narrower than its SDM estimate suggests, is the apparent exclusion by its sister species G. quinquepunctata, that feeds on the same host plants, but never occurs in the same area. The sister species is widely distributed in western Europe, including areas identified by SDM estimates as suitable for G. intermedia, but from which G. intermedia is absent. On the other hand, the absence of G. intermedia in the western portion of its potential distribution (as estimated by SDM) could simply be owing to low dispersal capacity.
To improve our initial SDM estimates of the leaf beetle ranges, we identified portions of the potential ranges that did not overlap (i.e. where co-occurrence is rejected) with the potential presence of at least one of its host plants. Climate change can indeed generate a mismatch among the geographical distributions of interacting species [54], thereby interrupting trophic networks. The corrected estimated range remained compatible with a Scandinavian refugium for G. intermedia (in association with S. aucuparia), and continued to support the occurrence of multiple transitions between a continuous and a fragmented leaf beetle distribution, following shifts between glacial and interglacial episodes (electronic supplementary material, figure S3). However, given the noted limitations associated with SDM, and the variation among distribution estimates derived from different climatic models and modelling parameters, independent data should be sought to test these historical hypotheses further. In this context, genetic data were collected to challenge the historical hypotheses of G. intermedia suggested by SDM. Our simulations of sequence data in coalescence models clearly favoured the presence of a northern refuge separate from the Alps, Carpathians, or Ardennes regions, during the last glacial period (figure 2).
The results from ABC analyses confirmed some inferences from the spatially explicit analyses. Notably, it confirmed the presence of a northern population of G. intermedia during the LGM. Indeed, it favoured models featuring an older split between northern and central Europe, 80 000–200 000 yr ago over models featuring such split at, or after, the LGM. These models suggest that the current distribution of genetic diversity of G. intermedia was shaped by a combination of in situ survival in northern Europe with post-glacial colonization of the remaining current range from central Europe refuges (Alps and possibly Carpathians) after the retreat of the continental ice sheet. Our ABC analyses suggest the following times of major events: a first separation between the Alps (west) and the Carpathians (east) most likely preceding the LIG (approx. −145 000 yr BP), followed by a north/south split of the eastern portion of the range that occurred after the LIG (approx. −92 000 yr BP) (electronic supplementary material, table S4).
SDMs suggested a more continuous range during the cold periods and more fragmentation during warm episodes of the Late Pleistocene. However, contrary to what has been suggested for other cold-adapted species (e.g. [55]), the range of G. intermedia was probably not larger during glacial episodes. In fact, SDM analyses suggested an overall reduced range for this species at the LGM, with northern Europe and the Carpathians populations experiencing contraction. Furthermore, ABC analyses suggest changes in population size for the Carpathian and northern regions: size reduction of the Carpathian population (±30 000 yr BP) followed by size expansion of northern and Carpathian populations (ABC results, electronic supplementary material, table S4; HDI95 between −15 100 and 19 700 yr BP), which probably corresponds to the end of the coldest phase of the last glaciation episode (approx. −19 000 yr BP). These demographic events could reflect, at least partially, the impact of the climatic oscillations at the end of the Pleistocene and associated dynamics of the ice sheet. In addition, the environment in Europe seems to have experienced significant changes during the sub-glacial/sub-interglacial episodes that lasted 1000–1500 years or even 4000–5000 years for the most important stadials and interstadials (e.g. [48]) at the Holocene, which may explain patterns of recent gene flow occurring among currently isolated regions of the distribution.
Our coalescence simulations clearly rejected the hypothesis of a recent colonization of Fennoscandia from central Europe at the end of the last glaciation (late-glacial/early Holocene; e.g. [56]). The persistence of a northern population isolated from central Europe at the LGM could be owing to the presence of a refugium situated west of the Scandinavian continental ice sheet, but perhaps elsewhere, e.g. in the Russian plains and/or in the Ural mountains (e.g. [5,44,57]), where patches of vegetation, even forests, persisted through the last glacial episode (e.g. [41,58]), although SDM analyses showed no evidence of suitable habitats in northeast Europe at the LGM.
DNA sequence variation strongly suggests the existence of a northern refuge for G. intermedia, isolated from the Alpine and Carpathian regions for a long period of time (more than 100 000 years), although it might have been briefly in contact at the end of the Pleistocene. Combining genetic data with SDM results suggests that this northern refuge might have been located in an extreme western coastal region of Norway, although we cannot exclude other locations in northern Europe. Future sampling should target the presumed northern refugium to test this hypothesis further. The plausibility of a Scandinavian refuge is further supported by the inferred potential range estimates for S. aucuparia, one of the beetle's host trees. Sorbus aucuparia is a cold-hardy tree with a current geographical distribution in Europe from Iceland and northern Finland to mountain ranges of central Spain and southern Italy [59]. Thus S. aucuparia populations could have survived locally in Scandinavia at the LGM, offering suitable habitats for its insect in that region. A recent debate has emerged in the literature, from available evidences for the presence of other boreal plant species in western Norway during the last glaciation (e.g. [60–62]). Evidence for the presence of S. aucuparia in northern Europe is available at least for the late glacial period (e.g. in Denmark) and early Holocene [11]. Although the controversy mainly focuses on plant taxa, the literature also discusses fossils that reveal larger Scandinavian ranges than expected from climatic and ice sheet models for several animal species (e.g. [63]; but see [4]).
5. Conclusion
The study sheds light on the history of a trophic interaction between a specialized insect and its host through major Pleistocene climatic changes. Results suggest that the leaf beetle G. intermedia survived the harsh environmental conditions of northern Europe at the LGM, although the exact location of survival remains open. This, in turn, implies the glacial survival of at least one of its host trees, most likely S. aucuparia, providing further evidence for the survival of cold-adapted trees in northern Europe at the LGM. This study should stimulate search for other sources of evidence in favour or against glacial survival of boreal species in Fennoscandia. In addition, the study illustrates how we can improve estimates of past and present species ranges from species distribution modelling by combining the estimates from interacting species, and by analysing DNA variation data using spatially explicit models of coalescence and ABC.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
The authors thank three anonymous reviewers for making constructive suggestions for the improvement of this paper. The authors are grateful to J. Powell for his careful reading of an earlier version of our manuscript.
Data accessibility
The multilocus dataset includes sequences recorded in GenBank: KJ785947 to KJ785959, KJ785974 to KJ786010, KJ786063 to KJ786085, KJ786135 to KJ786144, and KJ786170 to KJ786186 [22].
Authors' contributions
M.C.Q., S.N. and P.M. designed the project. M.C.Q. and P.M. collected the samples. M.C.Q performed the laboratory work and most computer analyses. S.N. and J.-C.S. provided data for some analyses. S.N. and S.D. performed some computer analyses. M.C.Q. drafted the manuscript with assistance from all authors. All authors read and approved the final manuscript.
Competing interests
We declare we have no competing interests.
Funding
M.C.Q. and S.D. were supported by a FRIA scholarship from the Belgian Fund for Scientific Research (FRS-FNRS) and Van buuren Funds. M.C.Q. is a post-doctoral research fellow of the Belgian American Educational foundation with a grant funded by the Francqui Foundation. S.D. is a post-doctoral research fellow funded by the Fonds Wetenschappelijk Onderzoek (FWO, Belgium). This research project was funded by a grant from the FRS-FNRS (FRFC – convention no. 2.4554.09), awarded to P.M. Part of this work was supported by the Danish Council for Independent Research | Natural Sciences (grant no. 10-085056 to S.N.) and the Aarhus University Research Foundation (grant no. AUFF F2010-2-34 to S.N. and J.-C.S. and the AU IDEAS Center for Informatics Research on Complexity in Ecology, CIRCE, to J.-C.S.).
References
- 1.Bennett K, Tzedakis P, Willis K. 1991. Quaternary refugia of north European trees. J. Biogeogr. 18, 103–115. ( 10.2307/2845248) [DOI] [Google Scholar]
- 2.Hewitt G. 1999. Post-glacial re-colonization of European biota. Biol. J. Linn. Soc. 68, 87–112. ( 10.1111/j.1095-8312.1999.tb01160.x) [DOI] [Google Scholar]
- 3.Binney HA, et al. 2009. The distribution of late-Quaternary woody taxa in northern Eurasia: evidence from a new macrofossil database. Quat. Sci. Rev. 28, 2445–2464. ( 10.1016/j.quascirev.2009.04.016) [DOI] [Google Scholar]
- 4.Ukkonen P, et al. 2011. Woolly mammoth (Mammuthus primigenius Blum.) and its environment in northern Europe during the last glaciation. Quat. Sci. Rev. 30, 693–712. ( 10.1016/j.quascirev.2010.12.017) [DOI] [Google Scholar]
- 5.Väliranta M, Kaakinen A, Kuhry P, Kultti S, Salonen JS, Seppä H. 2011. Scattered late-glacial and early Holocene tree populations as dispersal nuclei for forest development in north-eastern European Russia. J. Biogeogr. 38, 922–932. ( 10.1111/j.1365-2699.2010.02448.x) [DOI] [Google Scholar]
- 6.De Lafontaine G, Amasifuen Guerra CA, Ducousso A, Petit RJ. 2014. Cryptic no more: soil macrofossils uncover Pleistocene forest microrefugia within a periglacial desert. New Phytol. 204, 715–772. ( 10.1111/nph.12833) [DOI] [PubMed] [Google Scholar]
- 7.Magri D, et al. 2006. A new scenario for the quaternary history of European beech populations: palaeobotanical evidence and genetic consequences. New Phytol. 171, 199–221. ( 10.1111/j.1469-8137.2006.01740.x) [DOI] [PubMed] [Google Scholar]
- 8.Edwards CJ, et al. 2012. Temporal genetic variation of the red fox, Vulpes vulpes, across western Europe and the British Isles. Quat. Sci. Rev. 57, 95–104. ( 10.1016/j.quascirev.2012.10.010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svenning J, Normand S, Skov F. 2008. Postglacial dispersal limitation of widespread forest plant species in nemoral Europe. Ecography 31, 316–326. ( 10.1111/j.0906-7590.2008.05206.x) [DOI] [Google Scholar]
- 10.Holderegger R, Thiel-Egenter C. 2009. A discussion of different types of glacial refugia used in mountain biogeography and phylogeography. J. Biogeogr. 36, 476–480. ( 10.1111/j.1365-2699.2008.02027.x) [DOI] [Google Scholar]
- 11.Parducci L, et al. 2012. Glacial survival of boreal trees in northern Scandinavia. Science 335, 1083–1086. ( 10.1126/science.1216043) [DOI] [PubMed] [Google Scholar]
- 12.Birks HH, et al. 2012. Comment on ‘Glacial survival of boreal trees in northern Scandinavia’. Science 338, 742 ( 10.1126/science.1225345) [DOI] [PubMed] [Google Scholar]
- 13.Vorren TO, et al. 2013. Palaeoenvironment in northern Norway between 22.2 and 14.5 cal. ka BP. Boreas 42, 876–895. ( 10.1111/bor.12013) [DOI] [Google Scholar]
- 14.Kullman L. 1998. The occurrence of thermophilous trees in the Scandes Mountains during the early Holocene: evidence for a diverse tree flora from macroscopic remains. J. Ecol. 86, 421–428. ( 10.1046/j.1365-2745.1998.00266.x) [DOI] [Google Scholar]
- 15.Linder HP, et al. 2012. Biotic modifiers, environmental modulation and species distribution models. J. Biogeogr. 39, 2179–2190. ( 10.1111/j.1365-2699.2012.02705.x) [DOI] [Google Scholar]
- 16.Root TL, Price JT, Hall KR, Schneider SH. 2003. Fingerprints of global warming on wild animals and plants. Nature 421, 57–60. ( 10.1038/nature01333) [DOI] [PubMed] [Google Scholar]
- 17.Field R, et al. 2009. Spatial species-richness gradients across scales: a meta-analysis. J. Biogeogr. 36, 132–147. ( 10.1111/j.1365-2699.2008.01963.x) [DOI] [Google Scholar]
- 18.Araújo MB, Luoto M. 2007. The importance of biotic interactions for modelling species distributions under climate change. Glob. Ecol. Biogeogr. 16, 743–753. ( 10.1111/j.1466-8238.2007.00359.x) [DOI] [Google Scholar]
- 19.Wisz MS, et al. 2013. The role of biotic interactions in shaping distributions and realised assemblages of species: implications for species distribution modelling. Biol. Rev. Camb. Philos. Soc. 88, 15–30. ( 10.1111/j.1469-185X.2012.00235.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Svenning J-C, Skov F. 2004. Limited filling of the potential range in European tree species. Ecol. Lett. 7, 565–573. ( 10.1111/j.1461-0248.2004.00614.x) [DOI] [Google Scholar]
- 21.Ehlers J, Gibbard PL. 2004. Quaternary glaciations—extent and chronology. Part I: Europe. Developments in Quaternary Sciences. [Google Scholar]
- 22.Quinzin MC, Mardulyn P. 2014. Multi-locus DNA sequence variation in a complex of four leaf beetle species with parapatric distributions: mitochondrial and nuclear introgressions reveal recent hybridization. Mol. Phylogenet. Evol. 78C, 14–24. ( 10.1016/j.ympev.2014.05.003) [DOI] [PubMed] [Google Scholar]
- 23.Palmen E. 1948. Zur Systematik finnischer Chrysomeliden. 4. Phytodecta (Goniomena) quinquepunctatus F. und P. intermedius Hellies. Annales Entomologici Fennici, 14, 1–10. [Google Scholar]
- 24.Mardulyn P, Milinkovitch MC, Pasteels JM. 1997. Phylogenetic analyses of DNA and allozyme data suggest that Gonioctena leaf beetles (Coleoptera; Chrysomelidae) experienced convergent evolution in their history of host-plant family shifts. Syst. Biol. 46, 722–747. ( 10.1093/sysbio/46.4.722) [DOI] [PubMed] [Google Scholar]
- 25.Svenning J-C, Fløjgaard C, Marske KA, Nógues-Bravo D, Normand S. 2011. Applications of species distribution modeling to paleobiology. Quat. Sci. Rev. 30, 2930–2947. ( 10.1016/j.quascirev.2011.06.012) [DOI] [Google Scholar]
- 26.Kidd DM, Ritchie MG. 2006. Phylogeographic information systems : putting the geography into phylogeography. J. Biogeogr. 33, 1851–1865. ( 10.1111/j.1365-2699.2006.01574.x) [DOI] [Google Scholar]
- 27.Carstens BC, Richards CL. 2007. Integrating coalescent and ecological niche modeling in comparative phylogeography. Evolution 61, 1439–1454. ( 10.1111/j.1558-5646.2007.00117.x) [DOI] [PubMed] [Google Scholar]
- 28.Phillips SJ, Anderson RP, Schapire RE. 2006. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190, 231–259. ( 10.1016/j.ecolmodel.2005.03.026) [DOI] [Google Scholar]
- 29.Montoya D, Rodriguez MA, Zavala MA, Hawkins BA. 2007. Contemporary richness of Holarctic trees and the historical pattern of glacial retreat. Ecography 30, 173–182. ( 10.1111/j.0906-7590.2007.04873.x) [DOI] [Google Scholar]
- 30.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978. ( 10.1002/joc.1276) [DOI] [Google Scholar]
- 31.Collins WD, et al. 2006. The Community Climate System Model version 3 (CCSM3). J. Clim. 19, 2122–2143. ( 10.1175/JCLI3761.1) [DOI] [Google Scholar]
- 32.Hasumi H, Emori S. 2004. K-1 coupled GCM (MIROC) description. Tokyo, Japan: Center for Climate System Research, University of Tokyo. [Google Scholar]
- 33.Warren DL, Glor RE, Turelli M. 2010. ENMTools: a toolbox for comparative studies of environmental niche models. Ecography 33, 607–611. ( 10.1111/j.1600-0587.2009.06041.x) [DOI] [Google Scholar]
- 34.Jiménez-Valverde A, Lobo JM. 2007. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecol. 31, 361–369. ( 10.1016/j.actao.2007.02.001) [DOI] [Google Scholar]
- 35.Dellicour S, Kastally C, Hardy OJ, Mardulyn P. 2014. Comparing phylogeographic hypotheses by simulating DNA sequences under a spatially explicit model of coalescence. Mol. Biol. Evol. 31, 3359–3372. ( 10.1093/molbev/msu277) [DOI] [PubMed] [Google Scholar]
- 36.Beaumont MA, Zhang W, Balding DJ. 2002. Approximate Bayesian computation in population genetics. Genetics 162.4, 2025–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wegmann D, Leuenberger C, Neuenschwander S, Excoffier L. 2010. ABCtoolbox: a versatile toolkit for approximate Bayesian computations. BMC Bioinformatics 11, 116 ( 10.1186/1471-2105-11-116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beaumont MA, et al. 2010. In defence of model-based inference in phylogeography. Mol. Ecol. 19, 436–446. ( 10.1111/j.1365-294X.2009.04515.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertorelle G, Benazzo A, Mona S. 2010. ABC as a flexible framework to estimate demography over space and time: some cons, many pros. Mol. Ecol. 19, 2609–2625. ( 10.1111/j.1365-294X.2010.04690.x) [DOI] [PubMed] [Google Scholar]
- 40.Taberlet P, Fumagalli L, Wust-Saucy AG, Cosson JF. 1998. Comparative phylogeography and postglacial colonization routes in Europe. Mol. Ecol. 7, 453–464. ( 10.1046/j.1365-294x.1998.00289.x) [DOI] [PubMed] [Google Scholar]
- 41.Markova AK, Simakova AN, Puzachenko AY. 2009. Ecosystems of Eastern Europe at the time of maximum cooling of the Valdai glaciation (24-18 kyr BP) inferred from data on plant communities and mammal assemblages. Quat. Int. 201, 53–59. ( 10.1016/j.quaint.2008.05.020) [DOI] [Google Scholar]
- 42.Provan J, Bennett KD. 2008. Phylogeographic insights into cryptic glacial refugia. Trends Ecol. Evol. 23, 564–571. ( 10.1016/j.tree.2008.06.010) [DOI] [PubMed] [Google Scholar]
- 43.Stewart JR, Lister AM, Barnes I, Dalen L. 2010. Refugia revisited: individualistic responses of species in space and time. Proc. R. Soc. B 277, 661–671. ( 10.1098/rspb.2009.1272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Birks HH. 1994. Plant macrofossils and the nunatak theory of per-glacial survival. Diss. Bot. 234, 129–143. [Google Scholar]
- 45.Brochmann C, Gabrielsen TM, Nordal I, Landvik J, Elven R. 2003. Glacial survival or tabula rasa? The history of North Atlantic biota revisited. Taxon 52, 417–450. ( 10.2307/3647444) [DOI] [Google Scholar]
- 46.Westergaard KB, Alsos IG, Popp M, Engelskjøn T, Flatberg KI, Brochmann C. 2011. Glacial survival may matter after all: nunatak signatures in the rare European populations of two west-arctic species. Mol. Ecol. 20, 376–393. ( 10.1111/j.1365-294X.2010.04928.x) [DOI] [PubMed] [Google Scholar]
- 47.Velichko AA, Novenko EY, Pisareva VV, Zelikson EM, Boettger T, Junge FW. 2005. Vegetation and climate changes during the Eemian interglacial in Central and Eastern Europe: comparative analysis of pollen data. Boreas 34, 207–209. ( 10.1111/j.1502-3885.2005.tb01016.x) [DOI] [Google Scholar]
- 48.Svendsen J. 2004. Late Quaternary ice sheet history of northern Eurasia. Quat. Sci. Rev. 23, 1229–1271. ( 10.1016/j.quascirev.2003.12.008) [DOI] [Google Scholar]
- 49.Arnold N, van Andel TH, Valen V. 2002. Extent and dynamics of the Scandinavian ice sheet during oxygen isotope Stage 3 (65 000–25 000 yr B.P.). Quat. Res. 57, 38–48. ( 10.1006/qres.2001.2298) [DOI] [Google Scholar]
- 50.Wiens JJ, Graham CH. 2005. Niche conservatism: integrating evolution, ecology, and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36, 519–539. ( 10.1146/annurev.ecolsys.36.102803.095431) [DOI] [Google Scholar]
- 51.Araújo MB, Guisan A. 2006. Five (or so) challenges for species distribution modelling. J. Biogeogr. 33, 1677–1688. ( 10.1111/j.1365-2699.2006.01584.x) [DOI] [Google Scholar]
- 52.Gavin DG, et al. 2014. Climate refugia: joint inference from fossil records, species distribution models and phylogeography. New Phytol. 204, 37–54. ( 10.1111/nph.12929) [DOI] [PubMed] [Google Scholar]
- 53.Guisan A, Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009. ( 10.1111/j.1461-0248.2005.00792.x) [DOI] [PubMed] [Google Scholar]
- 54.Schweiger O, Settele J, Kudrna O, Klotz S, Kühn I. 2008. Climatic change can cause spatial mismatch of trophically interacting species. Ecology 89, 3472–3479. ( 10.1890/07-1748.1) [DOI] [PubMed] [Google Scholar]
- 55.Dalen L, Fuglei E, Hersteinsson P, Kapel CMO, Roth JD, Samelius G, Tannerfeldt M, Angerbjörn A. 2005. Population history and genetic structure of a circumpolar species: the arctic fox. Biol. J. Linn. Soc. 84, 79–89. ( 10.1111/j.1095-8312.2005.00415.x) [DOI] [Google Scholar]
- 56.Theissinger K, Bálint M, Feldheim KA, Haase P, Johannesen J, Laube I, Pauls SU, Comes H-P. 2013. Glacial survival and post-glacial recolonization of an arctic-alpine freshwater insect (Arcynopteryx dichroa, Plecoptera, Perlodidae) in Europe. J. Biogeogr. 40, 236–248. ( 10.1111/j.1365-2699.2012.02793.x) [DOI] [Google Scholar]
- 57.Theissinger K, Bálint M, Haase P, Johannesen J, Laube I, Pauls SU. 2011. Molecular data and species distribution models reveal the Pleistocene history of the mayfly Ameletus inopinatus (Ephemeroptera: Siphlonuridae)1. Freshw. Biol. 56, 2554–2566. ( 10.1111/j.1365-2427.2011.02681.x) [DOI] [Google Scholar]
- 58.Birks HJB, Willis KJ. 2008. Alpines, trees, and refugia in Europe. Plant Ecol. Divers. 1, 147–160. ( 10.1080/17550870802349146) [DOI] [Google Scholar]
- 59.Hultén E, Fries M (eds). 1986. Atlas of North European vascular plants. North of the Tropic of Cancer, Vol. II Königstein, Germany: Koeltz Scientic Books. [Google Scholar]
- 60.Kullman L. 2002. Boreal tree taxa in the central Scandes during the Late-Glacial: implications for Late-Quaternary forest history. J. Biogeogr. 29, 1117–1124. ( 10.1046/j.1365-2699.2002.00743.x) [DOI] [Google Scholar]
- 61.Kullman L. 2005. On the presence of late-glacial trees in the Scandes. J. Biogeogr. 32, 1499–1500. ( 10.1111/j.1365-2699.2005.01332.x) [DOI] [Google Scholar]
- 62.Birks HH, Larsen E, Birks HJB. 2005. Did tree-Betula, Pinus and Picea survive the Last glaciation along the west coast of Norway? A review of the evidence in light of Kullman 2002. J. Biogeogr. 32, 1461–1471. ( 10.1111/j.1365-2699.2005.01287.x) [DOI] [Google Scholar]
- 63.Ukkonen P, Lunkka JP, Jungner H, Donner J. 1999. New radiocarbon dates on Finnish mammoths indicate large ice-free area in Fennoscandia during the Middle Weichselian. J. Quat. Sci. 14, 711–714. ( 10.1002/(SICI)1099-1417(199912)14:7%3C711::AID-JQS506%3E3.0.CO;2-E) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The multilocus dataset includes sequences recorded in GenBank: KJ785947 to KJ785959, KJ785974 to KJ786010, KJ786063 to KJ786085, KJ786135 to KJ786144, and KJ786170 to KJ786186 [22].