Abstract
Herpes simplex viruses are neurotropic human pathogens that infect and establish latency in peripheral sensory neurons of the host. Herpes Simplex Virus-1 (HSV-1) readily infects the facial mucosa that can result in the establishment of a latent infection in the sensory neurons of the trigeminal ganglia (TG). From latency, HSV-1 can reactivate and cause peripheral pathology following anterograde trafficking from sensory neurons. Under rare circumstances, HSV-1 can migrate into the central nervous system (CNS) and cause Herpes Simplex Encephalitis (HSE), a devastating disease of the CNS. It is unclear whether HSE is the result of viral reactivation within the TG, from direct primary infection of the olfactory mucosa, or from other infected CNS neurons. Areas of the brain that are susceptible to HSV-1 during acute infection are ill-defined. Furthermore, whether the CNS is a true reservoir of viral latency following clearance of virus during acute infection is unknown. In this context, this review will identify sites within the brain that are susceptible to acute infection and harbor latent virus. In addition, we will also address findings of HSV-1 lytic gene expression during latency and the and comment on the pathophysiological consequences HSV-1 infection may have on long-term neurologic performance in animal models and humans.
Keywords: HSV-1 Neural Tropism, Acute Infection, Latent Infection
1. Introduction
The Herpesviridae family consists of a subfamily, the Alphaherpesvirinae, that contains a subset of neurotropic viruses including the common human pathogens, herpes simplex viruses types 1 and 2 (HSV-1, HSV-2) and varicella zoster virus (Smith, 2012). Humans are the natural host of herpes simplex viruses. HSV-1 resides in greater than 60% of the world’s population and can reactivate to cause peripheral disease, such as cold sores, or ocular infection that can lead to recurrent herpes keratitis (Farooq and Shukla, 2012, Looker et al., 2015). HSV-1 is more frequently found in the oral mucosa and ocular areas than HSV- 2 and is one of the leading etiological agents of sporadic encephalitis, known as Herpes Simplex Encephalitis (HSE) (Kollias et al., 2015). In the United States alone, there is an estimated 1,500 cases of HSE per year (Knipe and Cliffe, 2008).
HSV-1 infection is initiated in epithelial cells at mucosal surfaces upon initial binding of viral glycoproteins gB and gC with host cell surface heparan sulfate proteoglycans. This allows attachment of the viral glycoproteins gB, gD, and gL to host cellular receptors such as nectin-1, herpes virus entry mediator, or 3-O-sulfated HS for membrane fusion and viral entry (Agelidis and Shukla, 2015). Following membrane fusion, viral tegument proteins are released in the cytosol such that the viral nucleocapsid is directed to the nucleus along microtubules to release the viral genome (Smith, 2012). In the nucleus, viral DNA circularizes to transcribe immediate-early (IE), early (E) and late (L) viral gene products sequentially (Knipe and Cliffe, 2008). Once viral DNA has replicated, progeny neucleocapsids are assembled, acquire tegument proteins and are enveloped during budding with the inner nuclear membrane (Knipe and Cliffe, 2008). The resulting capsids then bud again with the cytoplasmic membranes of the trans-golgi network for secretion outside of the cell (Johnson and Baines, 2011, Mettenleiter et al., 2009). Once released, virus gains access to the sensory nerve fibers of the peripheral nervous system (PNS) by direct fusion of the axonal membrane and are transported by retrograde microtubule-associated transport to the cell body of the neuron (Cunningham et al., 2006, Mingo et al., 2012). Acute infection is suppressed to a lifelong latent infection that can result in intermittent reactivation.
Herpes simplex viruses are thought to reside in the sensory ganglia of the PNS and share transynaptic dissemination pathways into the central nervous system (CNS). In rodent models, infection with HSV-1 and HSV-2 results in the transynaptic passage through neurons of the PNS to the CNS producing lethal encephalitis. However, it is unclear why the occurrence of HSE is low in humans relative to more common peripheral eye or skin disease. This review aims to describe the neurotropic regions in human case reports and animal models to delineate HSV-1 trafficking in the CNS and the cause(s) of the long term neurological sequelea that is often observed following HSE in human patients.
2. Acute Infection of Herpes Simplex Virus-1 in the Nervous System
2.1 Herpes Simplex Induced Encephalitis in Humans
HSE typically manifests as an acute and focal necrotizing infection most generally found in the frontal and temporal lobes of the brain, yet its direct pathogenesis is largely unknown (Rozenberg et al., 2011). Even with antiviral therapy, survival rates remain at 70% and lifelong neurological deficits are often reported (Aldea et al., 2003, Skelly et al., 2013). Post-HSE sequelae include but are not limited to anterograde amnesia, difficulties with executive function, and aphasia (Skelly, Burger, 2013). The cause of these pathologies has not been identified.
2.2 Evidence of HSV-1 in the Central Nervous System of Human Patients and Correlation with Neurological Disease
Whereas HSV-1 rarely causes encephalitis, it is commonly associated with peripheral manifestations including “cold sores” or ocular keratitis. Nevertheless, HSV-1 DNA is found in a high percentage of PNS and CNS tissue of humans devoid of active clinical signs of disease (Fraser et al., 1981, Mori et al., 2004, Sequiera et al., 1979). Relative to the CNS, upon examination of post-mortem brains of normal and multiple sclerosis patients, 6 of 11 tested positive for HSV-1 DNA (Fraser, Lawrence, 1981). The association of HSV-1 DNA and neurological illness was noted in other small studies including patients who died of chronic psychiatric illness (Sequiera, Jennings, 1979) and familial Alzheimer’s disease (Mori, Yokochi, 2004). While incidental, these reports do raise the possibility that latent/subclinical HSV-1 infection may influence the development of neuronal deficits or neurological disorders themselves.
Clinical investigations have documented the development of HSE following neurosurgery in latently infected patients including an 8-year-old patient suffering from complex partial seizures (Bourgeois et al., 1999) and a 28-year old patient who underwent surgery to excise an oligodendroglioma (Aldea, Joly, 2003). The latter patient did display symptoms consistent with HSE in previous medical history but HSV-1 DNA was never detected by PCR. In fact, precision in defining appropriate conditions that can reproducibly amplify HSV-1 DNA within the cerebral spinal fluid (CSF) is critical prompting concerns that a threshold level is necessary before HSV-1 nucleic acid amplification can be detected with confidence. (Saldanha et al., 1986, Sequiera, Jennings, 1979).
Cases have recorded the development of HSE following neurosurgery in patients without prior evidence of HSE or any indication of previous HSV-1 infection. Two separate cases reported HSE symptoms in patients following removal of a craniopharyngioma which was later (2 weeks) confirmed to be due to HSV-1 (Kwon et al., 2008, Perry et al., 1998). In both instances, it took several months for the patients to improve with blindness reported in one patient due to acute retinal necrosis. These findings are consistent with an older postmortem study that revealed a 65-year old glioma patient displayed signs of herpetic encephalitis (Ochsner, 1981). An additional report recognized HSE symptoms and seizure development following neurosurgectomy of a parasagittal meningioma that resulted in death (Spuler et al., 1999). In a somewhat unique case, HSV-1 antigen was detected in cells of a glioblastoma removed from a 28-year old patient who later died 3 days following the surgery (Sheleg et al., 2001). Whether HSV-1 was acquired or reactivated during the development of the malignancy and may have contributed to the neuropathology associated with the neoplasia is unknown. Collectively, these studies elicit consideration in defining a casual or causal relationship between some forms of CNS disease and HSV-1 acute infection/reactivation.
Patients with HSE present primarily with edema or hemorrhage in the frontal or temporal lobes or orbital cortex (> than 75%) but can also involve other regions including the occipital and parietal lobes (Rozenberg, 2013, Skelly, Burger, 2013). Predilection to traffic and infect these particular brain regions is still unclear but may be related to “hard wiring” between CNS sites. It is thought that the virus directly reaches the temporal lobes via the olfactory neurons that innervate the nasal mucosa (Gilden et al., 2007). Alternatively, HSV-1 can disseminate into the CNS through the brainstem from the TG as trigeminal nuclei are located within all levels of the brainstem (L. Kruger, 1981). Experimentally, it has become evident that the dose and virulence of HSV-1 strains along with the genetic background of the host contributes to greater dissemination throughout the CNS. For example, one study found when mice were infected with a sub-lethal dose of HSV-1 (strain 2) the effects of CNS dissemination were dependent on the mouse strain. In C57BL/6 mice, virus was restricted to the brainstem. However, in more susceptible strains including BALB/c, SJL/J, A/J, and PL/J mice, viral DNA and HSV-1 antigen-positive cells were found throughout the brain at the identical infectious dose (Kastrukoff et al., 2012). Extrapolation of the experimental data to the human condition suggests virus strain, dose of infectious inoculum, and genetic background of the host contribute to the trafficking of virus from the PNS to the CNS and may also influence the severity of encephalitis (Kastrukoff, Lau, 2012, Webb et al., 1989, Yao et al., 2012).
2.3 HSV-1 Dissemination to and from Peripheral Nerves
Upon primary infection, HSV-1 replicates at the portal of entry within epithelial cells at a mucosal surface before fusing with neuronal axon termini of the innervating ganglionic nuclei of the PNS (Enquist et al., 1998, McGavern and Kang, 2011). Virions will then hijack the retrograde transport machinery of the ganglion cell and deliver viral DNA into the nucleus. Once in the neural cell body, viral tegument proteins in combination with cellular components can initiate viral replication resulting in the generation of progeny virus. Infected neurons will either die by cytolysis or virus will infect neighboring cells by non-cytopathic mechanisms (Kollias, Huneke, 2015). During active infection, viral lytic gene replication occurs in an ordered fashion generating products necessary for initiation of the next class of genes. Immediate-early genes are the first class or group of genes to be transcribed and translated followed by the early and late genes (Bloom et al., 2010). Following experimental infection of the cornea or oropharyngeal mucosal epithelial cells, HSV-1 readily infects the innervating TG which receives input from the ophthalmic, mandibular, and maxillary trigeminal branches (Ted L Tewfik, 2015). Alphaherpesviruses infect the CNS by moving from the ganglion cell body to the axon terminal from which virus is released by exocytosis into the synaptic cleft from the presynaptic terminal (Curanovic and Enquist, 2009, Diefenbach et al., 2008).
2.4 Brain Susceptibility During Acute HSV-1 Infection in Mouse Models
Few experimental studies have characterized HSV-1 dissemination pathways in the CNS from the TG following ocular infection. Ocular HSV-1 infection is typically devoted to assessment of virus infection in the TG, establishment of latency, and reactivation of latent virus from neurons in the TG. However, recent work has evaluated the trafficking of virus from the entry point in the CNS, the brainstem, to other regions of the brain. Specifically, it was reported that HSV-1 spread from the brainstem to the ependymal region, which then resulted in infection of brain ependymal cells of the lateral ventricles (Conrady et al., 2013). This infection was found to have physiological consequences that included the loss of ciliated ependymal cells lining the lateral ventricles likely leading to edema of the brain and death of the (immunocompromised) murine host due to insufficient drainage of cerebral spinal fluid (Conrady, Zheng, 2013). A follow-up study aimed to define which areas of the brain were susceptible to infection during the acute phase. Specifically, infectious virus was recovered from all major brain regions examined during acute encephalitis of moribund animals. The greatest amount of infectious virus was recovered in the midbrain, hippocampus, and subventricular zone (Fig. 1) followed by the brainstem, olfactory bulbs, frontal lobes, and cerebellum (Menendez et al., 2016). Taken together, the data suggest that animal mortality was associated with a greater amount of infectious virus reaching distinct brain regions including tissue juxtaposed to the lateral ventricles.
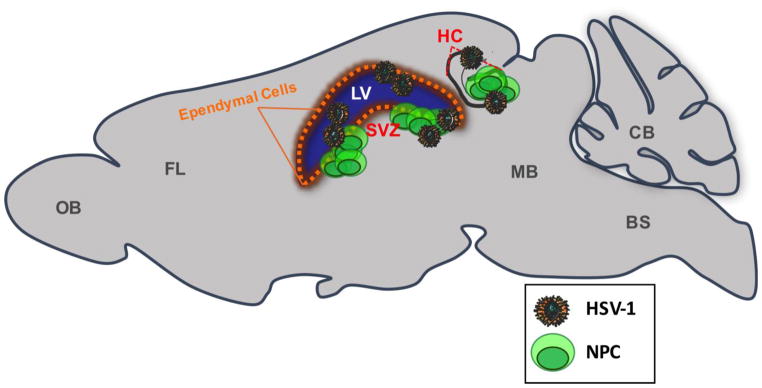
Fig. 1.
Sagittal schematic of brain regions that retain active HSV-1 infection. Infectious HSV-1 is recovered from the olfactory bulbs (OB), the frontal lobe (FL), midbrain (MB), brainstem (BS), and cerebellum (CB) during acute infection of experimental animal models. Most infectious virus is recovered from tissue surrounding the lateral ventricles (LV) and the hippocampus (HC). Several animal model reports have recognized viral tropism towards the HC and ependymal cells (orange) of the LV. Neural progenitor cells (NPC) reside within the subventricular zone (SVZ) of the LV and within the dentate gyrus of the HC in the adult mammalian brain, denoted by red font. NPCs are also prone to HSV-1 infection in vitro and in vivo. Red font also depicts regions of sites of high viral susceptibility.
Alternative routes of HSV-1 inoculation have also been found to infect the brain and lead to morbidity of the host. Specifically, HSV-1 can directly infect the CNS following infection of olfactory receptor neurons of the nasal cavity utilizing the anterograde transport machinery through the glomerulus to reach mitral neurons (Koyuncu et al., 2013). Virus can also spread from the left to the right olfactory bulb via the anterior commissure, as was observed following specific infection of one nostril in rats (Jennische et al., 2015). Consistent with the ocular infection model, viral inoculation via the intranasal cavity has also demonstrated neuroanatomical localization to the hippocampus and entorhinal cortex in Balb/c mice (Webb, Eglin, 1989). The entorhinal cortex lies within the temporal lobe and its axons exert excitatory pathways to the dentate gyrus and entire hippocampus (van Groen et al., 2003). It should be noted that viral tropism manifestations from animal models correlate with trafficking patterns and regions of the brain that are pathologically involved during HSE cases. However, it is still unclear why these particular brain regions are more susceptible to HSV-1 infection.
2.5 Viral Tropism for the Lateral Ventricles and Hippocampus
Predilection of HSV-1 to infect the lateral ventricles and hippocampus also occurs following direct infection of brain tissue without transynaptic passage through neurons from peripheral nerves (Braun et al., 2006, Cohen et al., 2011). Utilization of organotypic brain slice cultures has enabled the study of resident brain tissue apart from the periphery. When either neonate or adult brain tissue slices are incubated with HSV-1 infected media, neurotropism of the virus is largely restricted to the leptomeningeal and cortical cells, cells lining the ventricles, and the hippocampus (Braun, Zimmerman, 2006). Since the lateral ventricles and hippocampus contain sites of cell proliferation in the brain (Seri et al., 2006), it was hypothesized that proliferating cells were more susceptible to HSV-1 infection compared to non-proliferating ones. However, it was later reported that while there was a correlation of HSV-1 infected cells within anatomical areas of high cellular proliferation in brain-slice cultures, only a few infected cells co-localized with proliferation markers (Cohen, Braun, 2011).
In addition to the ventricular system lined by ependymal cells that are susceptible to HSV-1, the lateral ventricles and hippocampus are regions that contain two major neurogenic niches of the adult mammalian brain. Both the subventricular zone of the lateral ventricles and subgranular zone of the hippocampus give rise to neural progenitor cells (NPCs) that proliferate and migrate into existing circuitry and develop into resident neurons and glial cells (Fig. 1) (Kirby et al., 2015). NPCs are a vital part of adult neurogenesis; therefore, it is plausible that HSV-1 along with inflammatory mediators elicited by subtle, persistent infection could skew NPC differentiation. Experimental evidence suggests that HSV-1 readily infects NPCs in vivo following stereotaxic injection of virus into the lateral ventricles and in culture at a very low multiplicity of infection (Chucair-Elliott et al., 2014). This same study also found under conditions that drive NPC differentiation, there is a predilection in blocking neuroblast development without any obvious effect on astrocyte or oligodendrocyte differentiation. This phenotype was recapitulated in vivo where NPC numbers were reduced during the peak of viral encephalitis following both ocular and intranasal infection (Chucair-Elliott, Conrady, 2014, Rotschafer et al., 2013). Since experimental animal models have demonstrated viral tropism towards cortical tissue surrounding the lateral ventricles and hippocampus, these findings correlate with the involvement of similar tissue during human encephalitis. Further research is needed to better illuminate the relationship between infection of the ependymal region of the lateral ventricles and the severity of long-term neurological sequelae that transpires during and following an encephalitic episode.
2.6 HSV-1 Genes Involved in Neurovirulence
The major factors known to specifically cause HSV-1 neurovirulence are largely unknown. What makes HSV-1 a neurotropic virus is its ability to infect, spread throughout the nervous system, and establish a latent infection in neurons. Because of these features, HSV-1 has been idealized as a promising vector for gene therapy of the brain due to its ability to transduce brain regions beyond the initial site of inoculation (Berges et al., 2007). For this purpose, understanding what regions are particularly susceptible to HSV-1 could also benefit therapy’s utilizing herpes simplex vectors. In order for HSV to be exploited as a viral vector for gene therapy, it must be attenuated to prevent HSE. One modification has been to delete the ICP34.5 gene, γ34.5, which has proven attenuation by negating the occurrence of encephalitis (Rampling et al., 2000). Specifically, when cells of neuronal origin, rather than cells of nonneuronal origin, were infected with HSV-1 mutants unable to express the γ34.5 gene, cellular protein synthesis was inhibited thus, reducing infectious progeny virus (Chou and Roizman, 1992). Additionally, HSV-1 γ34.5 indirectly inhibits the type 1 interferon response by reversing translational arrest initiated by eukaryotic translation initiation factor 2, eIF2α, phosphorylation (Gale and Katze, 1998, Wilcox and Longnecker, 2016). HSV-1 γ34.5 also binds TBK1 to prevent IRF3 nuclear translocation thus blocking type 1 IFN expression (Verpooten et al., 2009, Wilcox and Longnecker, 2016). Another feature of ICP34.5 neurovirulence is its ability to directly bind beclin 1 preventing autophagy in neurons (Orvedahl et al., 2007). Multiple other HSV-1 genes have been deleted to create attenuated vectors including the immediate early genes ICP0, ICP4, and ICP27; however, only a few genes, including ICP34.5, have been described to be involved in neurovirulence (Berges, Wolfe, 2007, Lilley et al., 2001). The gene UL49 which encodes the tegument protein, VP16, has demonstrated HSV-1 pathogenicity in cell cultures and reduced the LD50 following intracerebral inoculation (Tanaka et al., 2012). The HSV-1 glycoproteins gB, gD, and GH are also largely responsible for entry in neuronal cells by binding specific receptors such as Nectin-1 (Akhtar and Shukla, 2009, Richart et al., 2003, Spear and Longnecker, 2003). Thus, headway has been made relative to the characterization of neurovirulent genes. However, neurotropic factors that facilitate HSV-1 infection and latency within the CNS remain elusive.
3. Defining HSV-1 Latency
A defining feature of herpesviruses is the ability of the pathogen to establish lifelong infection within sensory neurons. Innate and adaptive immune responses suppress active replication of the genome that results in a quiescent histone-covered viral episome (Skelly, Burger, 2013, Speck and Ganem, 2010). Consequently, latent infection is classically defined as the absence of productive infectious virus in ganglionic tissue that becomes infectious upon co-cultivation of tissue explants with susceptible cells (Knipe and Cliffe, 2008). During latency, the HSV genome is thought to be silent except for one major active region which gives rise to non-coding latency-associated transcripts (LATs) (Stevens et al., 1987). The role of LATs during latency has not been clearly defined. However, evidence suggest that LAT transcripts may silence lytic gene expression in neurons (Knipe and Cliffe, 2008), promote anti-apoptotic and pro-neuronal survival properties, and delay host interferon expression (Peng et al., 2005, Perng et al., 2000).
3.1 HSV-1 Latency in the Peripheral Ganglia
Most experimental studies on HSV-1 latency have focused on peripheral sensory nerves with cell bodies that reside in ganglia, such as the TG, and are viewed as the fundamental reservoir of HSV latency. Periodic reactivation from the TG elicits recurring disease and enables viral shedding to infect neighboring hosts as a result of direct contact with secretions. Specific factors regulating viral latency or reactivation remain to be determined. In addition to LATs, microRNAs (miRNAs) are also transcribed during latency and have been predicted through algorithms to antagonize the expression of immediate early (IE) genes, thereby, maintain latency (Murphy et al., 2008). LAT itself has been found to act as a miRNA precursor capable of encoding distinct miRNAs that can reduce the IE proteins, ICP0, and ICP4 (Umbach et al., 2008). These findings have led to the belief the HSV-1 miRNAs can specifically maintain viral latency by regulating viral gene expression post-transcriptionally.
Post-translational histone modifications are also thought to regulate viral suppression and reactivation. During viral latency, the LAT region of the viral genome is enriched in markers indicative of active transcription (dimethyl H3 K4 and acetyl H3 K9), while the lytic cycle genes contain less of these histone modifications (Bloom, Giordani, 2010). Another defining feature of ganglia harboring latent genomes is the likelihood of expressing the A5 marker (neurons immunoreactive for the calcitonin gene-related peptide and high-affinity nerve growth factor) or the KH10 marker (neurons responsive to glial cell-derived neurotropic factor) (Bertke et al., 2009, Bloom, Giordani, 2010). Apart from these specific markers, the molecular and biological forces driving neural latency in these neurons are not known.
Mouse and rabbit models are most commonly used to study HSV-1 latency. Following inoculation of a peripheral site (flank, eyes, nasal cavity, etc.), the active replication phase of the virus typically lasts up to 21 days within the sensory ganglia in mice (Bloom, Giordani, 2010). By 28 days post infection (DPI), infectious virus is thought to be in its latent form. The inability to detect other viral RNAs other than LAT within ganglia neurons by in situ hybridization has led investigators to believe that LAT positive neurons are negative for other productive cycle viral RNA (Deatly et al., 1987, Feldman et al., 2002, Stevens, Wagner, 1987). However, the notion of complete genome silencing during HSV-1 latency is now appreciated to be a more dynamic process than originally understood.
3.2 Evidence of Sporadic Activation During Latency in the Peripheral Ganglia
Examples of sporadic lytic gene transcription during latency have been demonstrated for over two decades (Kramer et al., 1998, Kramer and Coen, 1995, Maillet et al., 2006). Work by Feldman et al. identified that a frequency of approximately one neuron per 5 mice or 10 TGs expressed high levels of the viral productive lytic gene transcripts, ICP4, TK, and gC at 37–46 days following ocular infection (Feldman, Ellison, 2002). One implication of these findings is that protein (antigen) products encoded by the viral lytic genes are expressed and recognized by immune cells. In fact, early work demonstrated latently infected TG neurons were found to express IFN-γ mRNA and were populated by immune cells (Cantin et al., 1995, Halford et al., 1996, Liu et al., 1996). Chronic exposure of latently infected mice to acyclovir was found to suppress antibody titers to HSV-1 and reduce cytokine mRNA expression in the TG reinforcing the notion that i) immune cells recruited to the TG during acute infection remain active during a latent period and ii) ongoing antigen expression is driving cytokine and antibody levels (Halford et al., 1997). Infiltrating T cells and cytokines residing and expressed locally in the TG were later found to be pivotal in the control of viral reactivation. Reports described cytokines including IL-6 and IFN-γ contributed to or dampened reactivation respectively in vivo (Carr et al., 2009, Decman et al., 2005, Kriesel et al., 1997, Noisakran et al., 1998). HSV-1 specific CD8+ T cells were also found to be critical in blocking reactivation as they remain in direct immunological-synaptic contact with TG neurons and release granzymes that degrade the immediate early lytic transactivator gene, ICP4 (Khanna et al., 2003). An elegant means of demonstrating lytic gene activity in latent tissue was demonstrated using reporter mice and recombinant viruses that “tagged” cells displaying gB and ICP6 lytic gene promoter activity during latency (Russell and Tscharke, 2016). Taken together, these results may explain why gB- and ICP6-specific memory T cells are retained in latently infected human or mouse ganglia (van Velzen et al., 2013, Wallace et al., 1999).
In the HSV-1 skin infection model, a thorough analysis of individual LAT+ neurons found 41% of latently infected dorsal root ganglia neurons expressed all lytic gene classes assayed by qRT-PCR while 36% exhibited partial expression of some classes of lytic genes (Ma et al., 2014). Apart from LAT expression during the latent phase of infection, viral lytic transcript expression could implicate wider biological consequences than originally perceived. Specifically, viral lytic gene expression in latently infected neurons was found to alter expression of anti-viral and survival genes that may simultaneously promote and restrict reactivation as well as cellular genes including Ntrk1+ peptidergic nociceptors that may alter pain perception (Ma, Russell, 2014).
3.3 HSV-1 Latency in the CNS
The inability to successfully reactivate HSV from the CNS of latently infected conventional explant assays may explain the assumption that sensory ganglia are understood to be the primary source of viral latency. However, using a modified ex vivo tissue explant reactivation assay using two different HSV-1 strains, one study found 80% of the brainstem explants effectively demonstrated viral reactivation following latent infection with HSV-1 KOS or McKrae strains (Chen et al., 2006). It was later noted that the more virulent McKrae strain displayed an increase in reactivation kinetics, frequency, and copy number (in vivo) within brainstem tissue in comparison to the TG (Yao, Ling, 2012). Hypothermia-induced in vivo reactivation also enabled virus detection in the brainstem before detection in the TG with an increased reactivation frequency (Yao et al., 2014). When other brain regions were examined, latent viral genomes were also detected in the cerebellum, olfactory bulbs, frontal cortex, and hippocampus. Incredibly, HSV-1 reactivation occurred from all regions except the hippocampus (Chen, Yao, 2006). Whether the success in reactivation was influenced by viral copy number in the tissue is unknown. However, the data is revealing in that it described the occurrence of latent virus within specific anatomical sites of the CNS, and the amount detected per CNS structure was greater than that found in the TG.
3.4 Evidence of Persistent HSV-1 Replication in the CNS During Latency
To further characterize sites of viral latency in the CNS, prominent brain regions that harbored copious amounts of infectious virus in mice that survived the acute phase of infection were studied at 30 and 60 DPI. Consistent with previous studies, LAT mRNA was detected within the ependyma (the lateral ventricles with its surrounding tissue and hippocampus) and brainstem at 30 and 60 DPI (Menendez, Jinkins, 2016). However, the amount of LAT expressed was significantly less than that within the TG. These results were inversely proportional to what was observed during acute infection when significantly more virus was recovered from the ependyma than the TG (Menendez, et al., 2016). In contrast to harboring latent virus as found in the TG, the brain ependyma and brain stem expressed HSV-1 lytic gene transcripts from the immediate early, early, and late replication cycles at 30 DPI. By 60 DPI, lytic gene expression in the ependyma was more pronounced in comparison to the 30 DPI time point, whereas expression had subsided in the brain stem similar to the TG (Menendez, Jinkins, 2016). This study was the first to highlight that the brain ependymal region is unique with an environment conducive for persistent HSV-1 lytic gene expression and associated chronic inflammatory response (Menendez, Jinkins, 2016). Earlier work also reported focal chronic inflammation and evidence of oxidative damage in the CNS of HSV-1 infected animals during latency (30 to 220 DPI) (Valyi-Nagy et al., 2000).
Since susceptible brain regions infected during acute infection including the subventricular zone and hippocampus continue to harbor some type of persistent infection, this brings to question what is unique to this area of the CNS? As indicated earlier, the ependymal region (hippocampus and subventricular zone constitute a portion of the ependyma) harbor NPCs that are susceptible to acute HSV-1 infection (Chucair-Elliott, Conrady, 2014). As such, one study reported when NPCs were sorted from the subventricular zone and hippocampus during latency (30 and 60 days following ocular infection) viral lytic cycle mRNA was detected within the isolated NPCs cells from both regions indicating that the NPCs were infected and potentially harboring infectious virus (Menendez, Jinkins, 2016). By comparison, TGs are not known to maintain NPCs, and lytic gene expression is not found with any consistent frequency during latency in this tissue. Clearly, however, other factors likely contribute to the explanation of the dichotomy between HSV-1 “persistence” in the ependyma in comparison to other nervous system tissue. One likely possibility includes immune surveillance. If HSV antigen is maintained in the ependyma during latency, it is possible that T cell exhaustion would ensue (Wherry and Kurachi, 2015). Consistent with this concept, T cells isolated from the ependyma at 60 DPI were unable to respond to HSV antigen in comparison to T cells simultaneously isolated from the TG (Menendez et al., 2016). Therefore, a plausible combination of factors could explain the continuous lytic gene expression in the ependyma during latency of HSV-1-infected mice. Whether HSV-1 elicits a chronic type of infection within NPCs or the ependyma in the human host following HSE or episodic reactivation from the PNS is currently unknown.
4. Conclusions
The dogmatic understanding that HSV-1 is a characteristic pathogen to model viral latency is currently under scrutiny. Mouse models are more resistant to spontaneous shedding. Therefore, since spontaneous reactivation products are detected within two/thirds of latently infected ganglia (Ma, Russell, 2014) without subjecting the animal to stress, such results portend ongoing persistent infection/replication. Considering the frequency of viral reactivation events, it is plausible that virus spreads to or within the CNS at low levels. Therefore, the establishment and “reactivation of viral latency” in the CNS described by recent publications is proving to be more frequent than originally appreciated. Additionally, evidence of persistent viral activity and inflammation within the ependyma suggests the possibility that HSV-1 establishes a chronic infection. Documentation of pathogenic consequences in the CNS following HSV-1 infection during latency is reported. Chronic inflammation has been associated with oxidative damage (Valyi-Nagy, Olson, 2000) and with focal lesions and atrophy in critical brain regions associated with memory and higher order processing (Armien et al., 2010). In essence, the underlying question that remains from all the experimental findings is whether HSV-1 continues to replicate in the ependymal region or NPCs of human patients following encephalitis or reactivation episodes. This could potentially drive implications for long-term anti-viral treatment in patients with persistent abnormalities associated with HSV-1 reactivation, or in rare neurological disease with unknown etiology.
Highlights.
In this review, we describe recent findings in an experimental animal model of herpes simplex virus encephalitis to persistent lytic infection in a unique and important area of the brain, the ependyma. We describe our results in terms of what is already known experimentally and relate that to the human condition.
Acknowledgments
This work was supported by NIH R01 AI053108 to DJJC. CMM was supported by NIH/NIAID training grant T32 AI007633.
Abbreviations
- CNS
Central Nervous System
- DPI
Days Post Infection
- FACS
Fluorescence-activated cell sorting
- HSV-1
Herpes Simplex Virus-1
- HSE
Herpes Simplex Encephalitis
- NPCs
Neural Progenitor Cells
- TG
Trigeminal Ganglia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agelidis AM, Shukla D. Cell entry mechanisms of HSV: what we have learned in recent years. Future Virol. 2015;10:1145–54. doi: 10.2217/fvl.15.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar J, Shukla D. Viral entry mechanisms: cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009;276:7228–36. doi: 10.1111/j.1742-4658.2009.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldea S, Joly LM, Roujeau T, Oswald AM, Devaux B. Postoperative herpes simplex virus encephalitis after neurosurgery: case report and review of the literature. Clin Infect Dis. 2003;36:e96–9. doi: 10.1086/368090. [DOI] [PubMed] [Google Scholar]
- Armien AG, Hu S, Little MR, Robinson N, Lokensgard JR, Low WC, et al. Chronic cortical and subcortical pathology with associated neurological deficits ensuing experimental herpes encephalitis. Brain Pathol. 2010;20:738–50. doi: 10.1111/j.1750-3639.2009.00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berges BK, Wolfe JH, Fraser NW. Transduction of brain by herpes simplex virus vectors. Mol Ther. 2007;15:20–9. doi: 10.1038/sj.mt.6300018. [DOI] [PubMed] [Google Scholar]
- Bertke AS, Patel A, Imai Y, Apakupakul K, Margolis TP, Krause PR. Latency-associated transcript (LAT) exon 1 controls herpes simplex virus species-specific phenotypes: reactivation in the guinea pig genital model and neuron subtype-specific latent expression of LAT. J Virol. 2009;83:10007–15. doi: 10.1128/JVI.00559-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom DC, Giordani NV, Kwiatkowski DL. Epigenetic regulation of latent HSV-1 gene expression. Biochim Biophys Acta. 2010;1799:246–56. doi: 10.1016/j.bbagrm.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois M, Vinikoff L, Lellouch-Tubiana A, Sainte-Rose C. Reactivation of herpes virus after surgery for epilepsy in a pediatric patient with mesial temporal sclerosis: case report. Neurosurgery. 1999;44:633–5. doi: 10.1097/00006123-199903000-00106. discussion 5–6. [DOI] [PubMed] [Google Scholar]
- Braun E, Zimmerman T, Hur TB, Reinhartz E, Fellig Y, Panet A, et al. Neurotropism of herpes simplex virus type 1 in brain organ cultures. J Gen Virol. 2006;87:2827–37. doi: 10.1099/vir.0.81850-0. [DOI] [PubMed] [Google Scholar]
- Cantin EM, Hinton DR, Chen J, Openshaw H. Gamma interferon expression during acute and latent nervous system infection by herpes simplex virus type 1. J Virol. 1995;69:4898–905. doi: 10.1128/jvi.69.8.4898-4905.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DJ, Austin BA, Halford WP, Stuart PM. Delivery of Interferon-gamma by an adenovirus vector blocks herpes simplex virus Type 1 reactivation in vitro and in vivo independent of RNase L and double-stranded RNA-dependent protein kinase pathways. J Neuroimmunol. 2009;206:39–43. doi: 10.1016/j.jneuroim.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Yao HW, Huang WY, Hsu KS, Lei HY, Shiau AL, et al. Efficient reactivation of latent herpes simplex virus from mouse central nervous system tissues. J Virol. 2006;80:12387–92. doi: 10.1128/JVI.01232-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou J, Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proc Natl Acad Sci U S A. 1992;89:3266–70. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chucair-Elliott AJ, Conrady C, Zheng M, Kroll CM, Lane TE, Carr DJ. Microglia-induced IL-6 protects against neuronal loss following HSV-1 infection of neural progenitor cells. Glia. 2014;62:1418–34. doi: 10.1002/glia.22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen M, Braun E, Tsalenchuck Y, Panet A, Steiner I. Restrictions that control herpes simplex virus type 1 infection in mouse brain ex vivo. J Gen Virol. 2011;92:2383–93. doi: 10.1099/vir.0.031013-0. [DOI] [PubMed] [Google Scholar]
- Conrady CD, Zheng M, van Rooijen N, Drevets DA, Royer D, Alleman A, et al. Microglia and a functional type I IFN pathway are required to counter HSV-1-driven brain lateral ventricle enlargement and encephalitis. J Immunol. 2013;190:2807–17. doi: 10.4049/jimmunol.1203265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham AL, Diefenbach RJ, Miranda-Saksena M, Bosnjak L, Kim M, Jones C, et al. The cycle of human herpes simplex virus infection: virus transport and immune control. J Infect Dis. 2006;194(Suppl 1):S11–8. doi: 10.1086/505359. [DOI] [PubMed] [Google Scholar]
- Curanovic D, Enquist L. Directional transneuronal spread of alpha-herpesvirus infection. Future Virol. 2009;4:591. doi: 10.2217/fvl.09.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deatly AM, Spivack JG, Lavi E, Fraser NW. RNA from an immediate early region of the type 1 herpes simplex virus genome is present in the trigeminal ganglia of latently infected mice. Proc Natl Acad Sci U S A. 1987;84:3204–8. doi: 10.1073/pnas.84.10.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decman V, Kinchington PR, Harvey SA, Hendricks RL. Gamma interferon can block herpes simplex virus type 1 reactivation from latency, even in the presence of late gene expression. J Virol. 2005;79:10339–47. doi: 10.1128/JVI.79.16.10339-10347.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diefenbach RJ, Miranda-Saksena M, Douglas MW, Cunningham AL. Transport and egress of herpes simplex virus in neurons. Rev Med Virol. 2008;18:35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- Enquist LW, Husak PJ, Banfield BW, Smith GA. Infection and spread of alphaherpesviruses in the nervous system. Adv Virus Res. 1998;51:237–347. doi: 10.1016/s0065-3527(08)60787-3. [DOI] [PubMed] [Google Scholar]
- Farooq AV, Shukla D. Herpes simplex epithelial and stromal keratitis: an epidemiologic update. Surv Ophthalmol. 2012;57:448–62. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci U S A. 2002;99:978–83. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser NW, Lawrence WC, Wroblewska Z, Gilden DH, Koprowski H. Herpes simplex type 1 DNA in human brain tissue. Proc Natl Acad Sci U S A. 1981;78:6461–5. doi: 10.1073/pnas.78.10.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale M, Jr, Katze MG. Molecular mechanisms of interferon resistance mediated by viral-directed inhibition of PKR, the interferon-induced protein kinase. Pharmacol Ther. 1998;78:29–46. doi: 10.1016/s0163-7258(97)00165-4. [DOI] [PubMed] [Google Scholar]
- Gilden DH, Mahalingam R, Cohrs RJ, Tyler KL. Herpesvirus infections of the nervous system. Nat Clin Pract Neurol. 2007;3:82–94. doi: 10.1038/ncpneuro0401. [DOI] [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJ. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–9. [PubMed] [Google Scholar]
- Halford WP, Gebhardt BM, Carr DJ. Acyclovir blocks cytokine gene expression in trigeminal ganglia latently infected with herpes simplex virus type 1. Virology. 1997;238:53–63. doi: 10.1006/viro.1997.8806. [DOI] [PubMed] [Google Scholar]
- Jennische E, Eriksson CE, Lange S, Trybala E, Bergstrom T. The anterior commissure is a pathway for contralateral spread of herpes simplex virus type 1 after olfactory tract infection. J Neurovirol. 2015;21:129–47. doi: 10.1007/s13365-014-0312-0. [DOI] [PubMed] [Google Scholar]
- Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nat Rev Microbiol. 2011;9:382–94. doi: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- Kastrukoff LF, Lau AS, Thomas EE. The effect of mouse strain on herpes simplex virus type 1 (HSV-1) infection of the central nervous system (CNS) Herpesviridae. 2012;3:4. doi: 10.1186/2042-4280-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ED, Kuwahara AA, Messer RL, Wyss-Coray T. Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF. Proc Natl Acad Sci U S A. 2015;112:4128–33. doi: 10.1073/pnas.1422448112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol. 2008;6:211–21. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- Kollias CM, Huneke RB, Wigdahl B, Jennings SR. Animal models of herpes simplex virus immunity and pathogenesis. J Neurovirol. 2015;21:8–23. doi: 10.1007/s13365-014-0302-2. [DOI] [PubMed] [Google Scholar]
- Koyuncu OO, Hogue IB, Enquist LW. Virus infections in the nervous system. Cell Host Microbe. 2013;13:379–93. doi: 10.1016/j.chom.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MF, Chen SH, Knipe DM, Coen DM. Accumulation of viral transcripts and DNA during establishment of latency by herpes simplex virus. J Virol. 1998;72:1177–85. doi: 10.1128/jvi.72.2.1177-1185.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MF, Coen DM. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J Virol. 1995;69:1389–99. doi: 10.1128/jvi.69.3.1389-1399.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriesel JD, Gebhardt BM, Hill JM, Maulden SA, Hwang IP, Clinch TE, et al. Anti-interleukin-6 antibodies inhibit herpes simplex virus reactivation. J Infect Dis. 1997;175:821–7. doi: 10.1086/513977. [DOI] [PubMed] [Google Scholar]
- Kwon JW, Cho BK, Kim EC, Wang KC, Kim SK. Herpes simplex encephalitis after craniopharyngioma surgery. J Neurosurg Pediatr. 2008;2:355–8. doi: 10.3171/PED.2008.2.11.355. [DOI] [PubMed] [Google Scholar]
- Kruger LRFY. Specialized Features of the Trigeminal Nerve and Its Central Connections. In: DmMS, PJJMD, editors. The Cranial Nerves. Springer; Berlin Heidelberg: 1981. pp. 273–301. [Google Scholar]
- Lilley CE, Groutsi F, Han Z, Palmer JA, Anderson PN, Latchman DS, et al. Multiple immediate-early gene-deficient herpes simplex virus vectors allowing efficient gene delivery to neurons in culture and widespread gene delivery to the central nervous system in vivo. J Virol. 2001;75:4343–56. doi: 10.1128/JVI.75.9.4343-4356.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Tang Q, Hendricks RL. Inflammatory infiltration of the trigeminal ganglion after herpes simplex virus type 1 corneal infection. J Virol. 1996;70:264–71. doi: 10.1128/jvi.70.1.264-271.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looker KJ, Magaret AS, May MT, Turner KM, Vickerman P, Gottlieb SL, et al. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS One. 2015;10:e0140765. doi: 10.1371/journal.pone.0140765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JZ, Russell TA, Spelman T, Carbone FR, Tscharke DC. Lytic gene expression is frequent in HSV-1 latent infection and correlates with the engagement of a cell-intrinsic transcriptional response. PLoS Pathog. 2014;10:e1004237. doi: 10.1371/journal.ppat.1004237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maillet S, Naas T, Crepin S, Roque-Afonso AM, Lafay F, Efstathiou S, et al. Herpes simplex virus type 1 latently infected neurons differentially express latency-associated and ICP0 transcripts. J Virol. 2006;80:9310–21. doi: 10.1128/JVI.02615-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGavern DB, Kang SS. Illuminating viral infections in the nervous system. Nat Rev Immunol. 2011;11:318–29. doi: 10.1038/nri2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menendez CM, Jinkins JK, Carr DJ. Resident T Cells Are Unable To Control Herpes Simplex Virus-1 Activity in the Brain Ependymal Region during Latency. J Immunol. 2016;197:1262–75. doi: 10.4049/jimmunol.1600207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter TC, Klupp BG, Granzow H. Herpesvirus assembly: an update. Virus Res. 2009;143:222–34. doi: 10.1016/j.virusres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- Mingo RM, Han J, Newcomb WW, Brown JC. Replication of herpes simplex virus: egress of progeny virus at specialized cell membrane sites. J Virol. 2012;86:7084–97. doi: 10.1128/JVI.00463-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori I, Yokochi T, Koide N, Sugiyama T, Yoshida T, Kimura Y, et al. PCR search for the herpes simplex virus type 1 genome in brain sections of patients with familial Alzheimer’s disease. J Clin Microbiol. 2004;42:936–7. doi: 10.1128/JCM.42.2.936-937.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy E, Vanicek J, Robins H, Shenk T, Levine AJ. Suppression of immediate-early viral gene expression by herpesvirus-coded microRNAs: implications for latency. Proc Natl Acad Sci U S A. 2008;105:5453–8. doi: 10.1073/pnas.0711910105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noisakran S, Halford WP, Veress L, Carr DJ. Role of the hypothalamic pituitary adrenal axis and IL-6 in stress-induced reactivation of latent herpes simplex virus type 1. J Immunol. 1998;160:5441–7. [PubMed] [Google Scholar]
- Ochsner F. Contamination of a glioma by the herpes virus. Schweiz Arch Neurol Neurochir Psychiatr. 1981;129:19–30. [PubMed] [Google Scholar]
- Orvedahl A, Alexander D, Talloczy Z, Sun Q, Wei Y, Zhang W, et al. HSV-1 ICP34.5 confers neurovirulence by targeting the Beclin 1 autophagy protein. Cell Host Microbe. 2007;1:23–35. doi: 10.1016/j.chom.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Peng W, Henderson G, Inman M, BenMohamed L, Perng GC, Wechsler SL, et al. The locus encompassing the latency-associated transcript of herpes simplex virus type 1 interferes with and delays interferon expression in productively infected neuroblastoma cells and trigeminal Ganglia of acutely infected mice. J Virol. 2005;79:6162–71. doi: 10.1128/JVI.79.10.6162-6171.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perng GC, Jones C, Ciacci-Zanella J, Stone M, Henderson G, Yukht A, et al. Virus-induced neuronal apoptosis blocked by the herpes simplex virus latency-associated transcript. Science. 2000;287:1500–3. doi: 10.1126/science.287.5457.1500. [DOI] [PubMed] [Google Scholar]
- Perry JD, Girkin CA, Miller NR, Kerr DA. Herpes simplex encephalitis and bilateral acute retinal necrosis syndrome after craniotomy. Am J Ophthalmol. 1998;126:456–60. doi: 10.1016/s0002-9394(98)00108-1. [DOI] [PubMed] [Google Scholar]
- Rampling R, Cruickshank G, Papanastassiou V, Nicoll J, Hadley D, Brennan D, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Ther. 2000;7:859–66. doi: 10.1038/sj.gt.3301184. [DOI] [PubMed] [Google Scholar]
- Richart SM, Simpson SA, Krummenacher C, Whitbeck JC, Pizer LI, Cohen GH, et al. Entry of herpes simplex virus type 1 into primary sensory neurons in vitro is mediated by Nectin-1/HveC. J Virol. 2003;77:3307–11. doi: 10.1128/JVI.77.5.3307-3311.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotschafer JH, Hu S, Little M, Erickson M, Low WC, Cheeran MC. Modulation of neural stem/progenitor cell proliferation during experimental Herpes Simplex encephalitis is mediated by differential FGF-2 expression in the adult brain. Neurobiol Dis. 2013;58:144–55. doi: 10.1016/j.nbd.2013.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozenberg F. Acute viral encephalitis. Handb Clin Neurol. 2013;112:1171–81. doi: 10.1016/B978-0-444-52910-7.00038-6. [DOI] [PubMed] [Google Scholar]
- Rozenberg F, Deback C, Agut H. Herpes simplex encephalitis : from virus to therapy. Infect Disord Drug Targets. 2011;11:235–50. doi: 10.2174/187152611795768088. [DOI] [PubMed] [Google Scholar]
- Russell TA, Tscharke DC. Lytic Promoters Express Protein during Herpes Simplex Virus Latency. PLoS Pathog. 2016;12:e1005729. doi: 10.1371/journal.ppat.1005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha J, Sutton RN, Gannicliffe A, Farragher B, Itzhaki RF. Detection of HSV1 DNA by in situ hybridisation in human brain after immunosuppression. J Neurol Neurosurg Psychiatry. 1986;49:613–9. doi: 10.1136/jnnp.49.6.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequiera LW, Jennings LC, Carrasco LH, Lord MA, Curry A, Sutton RN. Detection of herpes-simplex viral genome in brain tissue. Lancet. 1979;2:609–12. doi: 10.1016/s0140-6736(79)91667-2. [DOI] [PubMed] [Google Scholar]
- Seri B, Herrera DG, Gritti A, Ferron S, Collado L, Vescovi A, et al. Composition and organization of the SCZ: a large germinal layer containing neural stem cells in the adult mammalian brain. Cereb Cortex. 2006;16(Suppl 1):i103–11. doi: 10.1093/cercor/bhk027. [DOI] [PubMed] [Google Scholar]
- Sheleg SV, Nedzved MK, Nedzved AM, Kulichkovskaya IV. Contamination of glioblastoma multiforme with type 1 herpes simplex virus. Case illustration. J Neurosurg. 2001;95:721. doi: 10.3171/jns.2001.95.4.0721. [DOI] [PubMed] [Google Scholar]
- Skelly MJ, Burger AA, Adekola O. Herpes simplex virus-1 encephalitis: a review of current disease management with three case reports. Antivir Chem Chemother. 2013;23:13–8. doi: 10.3851/IMP2129. [DOI] [PubMed] [Google Scholar]
- Smith G. Herpesvirus transport to the nervous system and back again. Annu Rev Microbiol. 2012;66:153–76. doi: 10.1146/annurev-micro-092611-150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear PG, Longnecker R. Herpesvirus entry: an update. J Virol. 2003;77:10179–85. doi: 10.1128/JVI.77.19.10179-10185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck SH, Ganem D. Viral latency and its regulation: lessons from the gamma-herpesviruses. Cell Host Microbe. 2010;8:100–15. doi: 10.1016/j.chom.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spuler A, Blaszyk H, Parisi JE, Davis DH. Herpes simplex encephalitis after brain surgery: case report and review of the literature. J Neurol Neurosurg Psychiatry. 1999;67:239–42. doi: 10.1136/jnnp.67.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens JG, Wagner EK, Devi-Rao GB, Cook ML, Feldman LT. RNA complementary to a herpesvirus alpha gene mRNA is prominent in latently infected neurons. Science. 1987;235:1056–9. doi: 10.1126/science.2434993. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kato A, Satoh Y, Ide T, Sagou K, Kimura K, et al. Herpes simplex virus 1 VP22 regulates translocation of multiple viral and cellular proteins and promotes neurovirulence. J Virol. 2012;86:5264–77. doi: 10.1128/JVI.06913-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ted L, Tewfik M, Arlen D, Meyers . In: Trigeminal Nerve Anatomy, Gross Anatomy. Arlen D, Meyers M MBA, editors. Medscape; Online 2015. [Google Scholar]
- Umbach JL, Kramer MF, Jurak I, Karnowski HW, Coen DM, Cullen BR. MicroRNAs expressed by herpes simplex virus 1 during latent infection regulate viral mRNAs. Nature. 2008;454:780–3. doi: 10.1038/nature07103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valyi-Nagy T, Olson SJ, Valyi-Nagy K, Montine TJ, Dermody TS. Herpes simplex virus type 1 latency in the murine nervous system is associated with oxidative damage to neurons. Virology. 2000;278:309–21. doi: 10.1006/viro.2000.0678. [DOI] [PubMed] [Google Scholar]
- van Groen T, Miettinen P, Kadish I. The entorhinal cortex of the mouse: organization of the projection to the hippocampal formation. Hippocampus. 2003;13:133–49. doi: 10.1002/hipo.10037. [DOI] [PubMed] [Google Scholar]
- van Velzen M, Jing L, Osterhaus AD, Sette A, Koelle DM, Verjans GM. Local CD4 and CD8 T-cell reactivity to HSV-1 antigens documents broad viral protein expression and immune competence in latently infected human trigeminal ganglia. PLoS Pathog. 2013;9:e1003547. doi: 10.1371/journal.ppat.1003547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpooten D, Ma Y, Hou S, Yan Z, He B. Control of TANK-binding kinase 1-mediated signaling by the gamma(1)34.5 protein of herpes simplex virus 1. J Biol Chem. 2009;284:1097–105. doi: 10.1074/jbc.M805905200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace ME, Keating R, Heath WR, Carbone FR. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J Virol. 1999;73:7619–26. doi: 10.1128/jvi.73.9.7619-7626.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SJ, Eglin RP, Reading M, Esiri MM. Experimental murine herpes simplex encephalitis: immunohistochemical detection of virus antigens. Neuropathol Appl Neurobiol. 1989;15:165–74. doi: 10.1111/j.1365-2990.1989.tb01218.x. [DOI] [PubMed] [Google Scholar]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nat Rev Immunol. 2015;15:486–99. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox DR, Longnecker R. The Herpes Simplex Virus Neurovirulence Factor gamma34.5: Revealing Virus-Host Interactions. PLoS Pathog. 2016;12:e1005449. doi: 10.1371/journal.ppat.1005449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao HW, Ling P, Chen SH, Tung YY, Chen SH. Factors affecting herpes simplex virus reactivation from the explanted mouse brain. Virology. 2012;433:116–23. doi: 10.1016/j.virol.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Yao HW, Ling P, Tung YY, Hsu SM, Chen SH. In vivo reactivation of latent herpes simplex virus 1 in mice can occur in the brain before occurring in the trigeminal ganglion. J Virol. 2014;88:11264–70. doi: 10.1128/JVI.01616-14. [DOI] [PMC free article] [PubMed] [Google Scholar]