Significance
Previous studies have demonstrated that Bam protein plays a critical role promoting early germ-line cell differentiation in the Drosophila ovary. Although its regulation and genetic functions have been extensively investigated over the last 20 years, the biochemical nature of Bam has still remained elusive. Here, we show that Bam functions as an ubiquitin-associated protein and regulates the stability of CycA. Our study uncovers a mechanism by which Bam functions as an ubiquitin-associated protein, and cooperates with Otu to deubiquitinate and stabilize CycA, thereby balancing GSC self-renewal and differentiation.
Keywords: germ-line stem cells, bag of marbles, ubiquitin-binding protein, cyclin A deubiquitination
Abstract
Drosophila germ-line stem cells (GSCs) provide an excellent model to study the regulatory mechanisms of stem cells in vivo. Bag of marbles (bam) has been demonstrated to be necessary and sufficient to promote GSC and cystoblast differentiation. Despite extensive investigation of its regulation and genetic functions, the biochemical nature of the Bam protein has been unknown. Here, we report that Bam is an ubiquitin-associated protein and controls the turnover of cyclin A (CycA). Mechanistically, we found that Bam associated with Otu to form a deubiquitinase complex that stabilized CycA by deubiquitination, thus providing a mechanism to explain how ectopic expression of Bam in GSCs promotes differentiation. Collectively, our findings not only identify a biochemical function of Bam, which contributes to GSC fate determination, but also emphasizes the critical role of proper expression of cyclin proteins mediated by both ubiquitination and deubiquitination pathways in balancing stem cell self-renewal and differentiation.
The Drosophila ovary provides an excellent model to study the regulatory mechanisms of how the fate of stem cell self-renewal and differentiation is determined and balanced (1, 2). In adult females, asymmetric division of germ-line stem cells (GSCs) occurs in the anterior region of the germarium to produce two daughter cells. Whereas one daughter cell remains attached to the somatic cap cell for GSC self-renewal, the other becomes a cystoblast (CB). The CB continues to divide four times with incomplete cytokinesis at each division to produce a 16-cell cyst that sustains Drosophila oogenesis (Fig. 1A) (1).
Fig. 1.
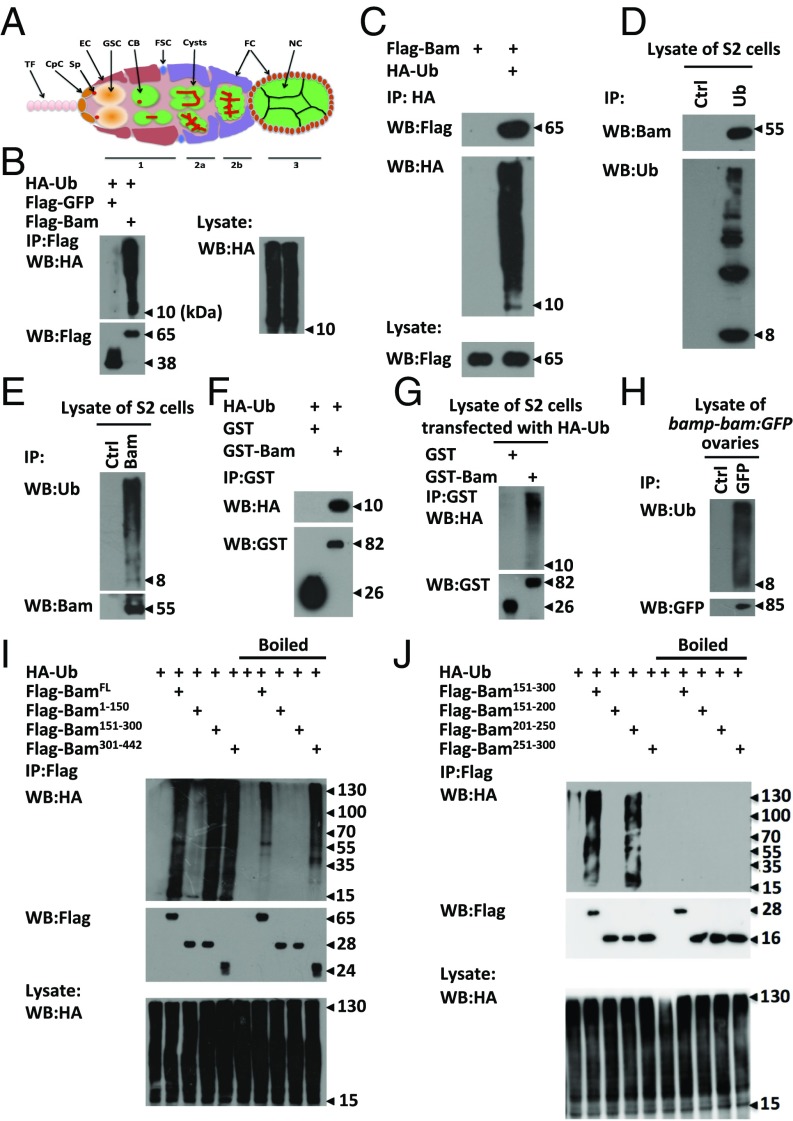
Bam is an ubiquitin-association protein. (A) Schematic diagram displaying the ovarian germarium with various cell types and organelles including the terminal filament (TF), cap cells (CpC), spectrosome (Sp), escort cells (EC), germ-line stem cells (GSC), cystoblasts (CB), follicle stem cells (FSC), cysts, follicle cells (FC), and Nurse cells (NC). (B and C) S2 cells were transfected with indicated plasmids. Cell lysates were subjected to immunoprecipitation assays using Flag beads (B) or anti-HA antibody (C), followed by Western blot assays. (D and E) Lysates of S2 cells were immunoprecipitated with anti-ubiquitin (D) or anti-Bam (E) or mouse IgG (control) antibody, followed by Western blot assays. (F and G) Purified HA-ubiquitin proteins (F) or lysates of S2 cells transfected with HA-Ub plasmids (G) were mixed with GST or GST-Bam, followed by immunoprecipitation and Western blot assays. (H) Ovaries of P{bamp-bam:GFP} were dissected and lysed, followed by immunoprecipitation using anti-GFP or mouse IgG (control) antibody. Western blot assays were performed to detect the levels of indicated proteins. (I and J) S2 cells were transfected with indicated plasmids. Cell lysates were subjected to immunoprecipitation by using Flag beads with or without boiling pretreatment as indicated. Western blot assays were performed to detect the HA signal. All of the biochemical experiments were performed at least three times.
Early genetic studies have demonstrated that the bag of marbles (bam) gene is necessary and sufficient for GSC and CB differentiation, because mutation of bam blocks germ-cell differentiation causing GSC hyperplasty, whereas ectopic expression of bam in GSCs results in their precocious differentiation (3–5). Importantly, previous studies have identified bam as a key gene that responds to niche bone morphogenetic protein (BMP) signaling via the interaction of Smad proteins with a discrete DNA-silencing element in the bam 5′ untranslated region (UTR) (6–8). Thus, transcriptional silencing of bam that directly establishes a link between GSCs and their associated stromal cells (stem cell niche) is essential for GSC fate determination. Blockage of the bam silencing pathway leads to ectopic expression of Bam and loss of GSCs (7, 9–11). However, the molecular mechanism underlying the action of ectopic Bam in GSCs has remained unexplored. In addition to transcriptional control through the niche–stem cell interaction, genetic studies have suggested that the maintenance of GSCs is cell-autonomously regulated by several translational repressor complexes such as Nos–Pum and Ago1/Dcr/Loq–microRNA (miRNA) complexes (12–16). It has been suggested that Bam functions in concert with Bgcn, a DExH box-containing protein, to antagonize the function of Nos/Pum and Ago1/miRNA translational complexes, thus allowing CB differentiation (12, 14). Despite these significant advances in understanding the genetic roles of bam in regulating GSC fate, the biochemical nature of the Bam protein remains elusive.
Cell fate changes (e.g., cell differentiation or regeneration) commonly dictate a change in the cell cycle of daughter cells (17, 18). In Drosophila, it has been proposed that GSC fate determination is tightly regulated by a specific cell cycle program (19, 20). Previous studies have revealed that mutation or dysregulated expression of cyclin proteins, such as cyclin A (CycA), cyclin B (CycB), and cyclin E (CycE), result in defects of GSC maintenance, differentiation, and/or an abnormal cell number of (32- or 8-cell) cysts (19–23). Thus, proper expression of cyclin proteins is critical for GSC fate determination and/or normal germ-line development. We have previously shown that the E2 ubiquitin-conjugating enzyme Effete regulates GSC maintenance by controlling turnover of CycA in GSCs, suggesting that ubiquitin-mediated regulation of CycA plays pivotal roles in determining the fate of GSCs (22). Notably, ectopic expression of the stable form of CycA in germ cells causes loss of GSCs, which is similar to the phenotype resulting from ectopic expression of bam in GSCs. These lines of evidence prompted us to investigate a potential regulatory link between CycA and Bam proteins. In this study, we report that Bam directly associates with the ubiquitin protein and forms a complex with Otu, a putative deubiquitinase, to promote deubiquitination and stabilization of CycA. We further show that this biochemical pathway can explain the precocious GSC differentiation resulting from ectopic expression of Bam in GSCs.
Results
Bam Associates with Ubiquitin.
To explore the biochemical function of Bam in the regulation of germ-cell differentiation, we sought to search for Bam-associated partners. According to our described method (11), we expressed Flag epitope-tagged Bam in S2 cells and then performed coimmunoprecipitation experiments followed by mass spectrometric analysis. From this assay, we identified a number of proteins in the Bam immunoprecipitants. In addition to the known Bam-associated partners, Ter94 (24) and eIF4A (25), observed in Bam immunoprecipitants (Fig. S1A), we also found that ubiquitin was highly enriched in Bam complexes in our assays (Fig. S1A). To confirm this finding, we performed independent coimmunoprecipitation experiments in S2 cells, and found that in addition to high molecular weight ubiquitin signal, the free HA-tagged ubiquitin was also present in the Flag-tagged Bam immunoprecipitants (Fig. 1B). Moreover, the unubiquitinated form of Flag-Bam was observed in the ubiquitin immunoprecipitants (Fig. 1C), suggesting that Bam associates with ubiquitin in a noncovalently modified manner. Consistently, we found that endogenous Bam formed a complex with free ubiquitin protein (Fig. 1 D and E). To test whether ubiquitin directly binds Bam, we then performed a GST pull-down assay. As shown in Fig. 1 F and G, the free HA-ubiquitin directly bound to GST-Bam. To examine whether Bam interacts with ubiquitin in germ cells, we performed coimmunoprecipitation experiments in Drosophila ovaries and observed complex formation between Bam and ubiquitin (Fig. 1H).
Fig. S1.
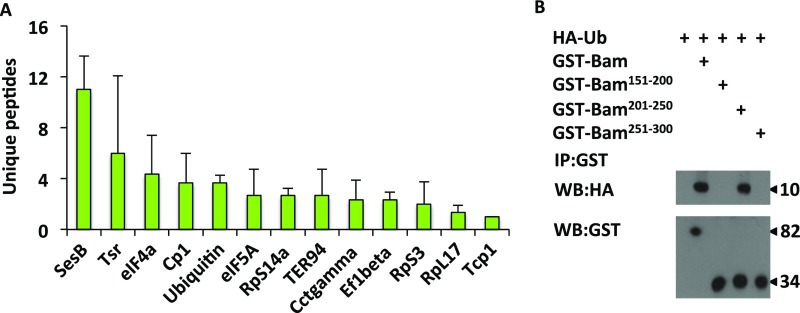
Bam is an ubiquitin binding protein. (A) MS-identified Bam-associating proteins in transfected S2 cells (n = 3). In this assay, S2 cells were transfected with Flag-GFP or Flag-Bam plasmids. After 48 h, cells were harvested and lysed, followed by immunoprecipitation with anti-Flag M2 beads. The immunoprecipitants were eluted and digested by using Trypsin (Promega), and then were subjected to LC-MS/MS assays. Resulting MS/MS data were processed by using Thermo Proteome discovery (version 1.4.1.14), and tandem mass spectra were searched against UniProt-Drosophila database. Unique peptides that were detected only in Flag-Bam immunoprecipitants, or displayed at least 2 folds higher abundance than the Flag-GFP control groups, were selected. From all these peptides, only the ones that emerged in all of the three replicates were considered as Bam associated proteins. (B) HA-Ubiquitin proteins were mixed and incubated with different truncated Bam proteins as indicated. The incubated products were then subjected to immunoprecipitation assays by using GST beads. Western blot assays were performed to detect the indicated proteins.
Bam Interacts with Ubiquitin in a Domain-Specific Manner.
To determine the specific domains of Bam essential for the Bam–ubiquitin interaction, we generated a series of truncated Bam fragments, including the N terminus (amino acids 1–150), center (amino acids 151–300), and C terminus (amino acids 301–442), and then performed coimmunoprecipitation assays. As shown in Fig. 1I, HA-ubiquitin signals were easily detected in immunoprecipitants of Flag-tagged full-length, central, and C-terminal Bam. However, the signals of HA-ubiquitin were dramatically reduced in central Bam when the cell lysates were preboiled before immunoprecipitation assays. Of note, strong HA-ubiquitin signals were still observed in immunoprecipitants of full-length and C-terminal Bam, even with boiling pretreatment (26). The preboiling treatment impaired the physical association of Bam with ubiquitin rather than affecting conjugated binding of ubiquitin with Bam. Therefore, these findings suggest that, whereas the C-terminal region of Bam is modified by ubiquitin, the central region (amino acids 151–300) is necessary for the interaction of Bam with free ubiquitin proteins. To narrow down the region of Bam, which interacts with ubiquitin, we further divided central Bam into smaller fragments including amino acids 151–200, 201–250, and 251–300. Immunoprecipitation assays revealed that a fragment of Bam (amino acids 201–250) is necessary for the Bam–ubiquitin interaction (Fig. 1J). Consistent results were obtained in further GST-pull down assays (Fig. S1B).
Bam Forms a Complex with CycA.
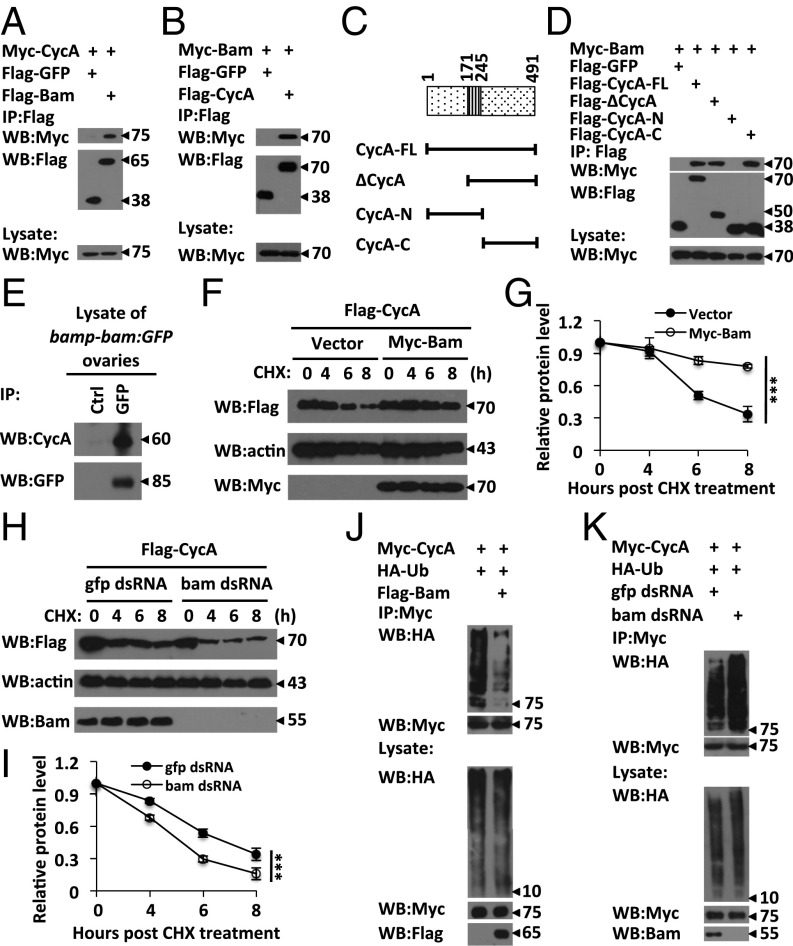
Several lines of evidence promoted us to propose the hypothesis that Bam controls GSC functions by regulating CycA turnover through the ubiquitin-mediated pathway. First, ubiquitin-mediated degradation of CycA is essential for GSC maintenance (22). Second, ectopic expression of either Bam or CycA leads to a GSC-loss phenotype (5, 22). Third, cycA has been shown to genetically interact with bam to control germ cell cyst division (21). To explore the biochemical relationship between Bam and CycA, we first determined whether Bam and CycA form a complex by performing immunoprecipitation assays. As shown in Fig. 2 A and B, Bam and CycA were reciprocally coimmunoprecipitated with each other in transfected S2 cells. CycA is a highly conserved protein and contains multiple domains including d-box and extended d-box domains at its N-terminal region and the cyclin box at its C-terminal region (27). To map the specific region of CycA required for the Bam-CycA association, we generated both N terminus- and C terminus-truncated CycA constructs (Fig. 2C). As shown in a coimmunoprecipitation assay, the C-terminal CycA was sufficient to form a complex with Bam, whereas the N-terminal CycA failed to associate with Bam (Fig. 2D). To test whether endogenous CycA associates with Bam in germ cells, we performed immunoprecipitation experiments in ovaries and obtained similar results (Fig. 2E).
Fig. 2.
Bam associates with CycA and regulates its turnover. (A and B) S2 cells were transfected with indicated plasmids. Cells lysates were subjected to immunoprecipitation assays with Flag beads, followed by Western blot assays. (C) Schematic diagrams of CycA and truncated mutants. (D) S2 cells were transfected with indicated plasmids. Cell lysates were subjected to immunoprecipitation and Western blot assays. (E) Lysates of P{bamp-bam:GFP} ovaries were immunoprecipitated with anti-GFP or mouse IgG (control) antibody, followed by Western blot assays. (F–I) S2 cells were transfected with expression constructs (F) or pretreated with gfp or bam dsRNAs (H). After 48 h, cells were treated with CHX (50 ng⋅mL−1) for various times, followed by immunoblotting to examine CycA levels. Densitometric analyses to quantify CycA expression in F and H are shown in G and I, respectively. Error bars represent SD (n = 3). (J and K) S2 cells were transfected with combinations of plasmids (J) or pretreated with gfp or bam dsRNAs for 48 h (K). Cell lysates were immunoprecipitated with Myc beads, followed by Western blot assays. All of the biochemical experiments were performed at least three times. In G and I, the log rank test was used to analyze statistical variance. ***P < 0.001.
Bam Stabilizes CycA by Negatively Regulating Its Ubiquitination.
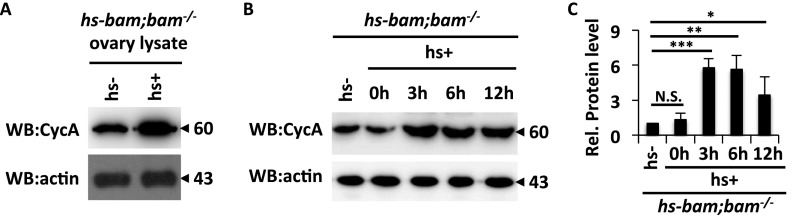
Expressional fluctuation at the protein level is a hallmark of cyclin proteins during the cell cycle (27). Considering that Bam interacts with CycA, we next investigated whether Bam affects the fluctuation of CycA expression. We determined the half-life of Flag-tagged CycA in S2 cells with or without cotransfected Myc-tagged Bam by performing pulse–chase experiments. As shown in Fig. 2 F and G, the half-life of CycA was measured at approximately 6 h in the control experiments, whereas CycA exhibited a much longer half-life when the cells were coexpressed with Myc-Bam, suggesting that Bam is potentially involved in controlling the process of CycA turnover. To confirm this inference, we next tested whether knockdown of bam influences the stability of CycA. As shown in pulse–chase experiments (Fig. 2 H and I), knockdown of bam by dsRNA in S2 cells significantly reduced the half-life of CycA, compared with the control. Collectively, our findings identified a role of Bam in stabilizing CycA in S2 cells. To test whether Bam has the same role in early germ cells, we collected the ovaries from P{hs-bam};bam−/− females at the time point of 6 h after heat-shock treatment to perform Western blot assays. In this assay, P{hs-bam};bam−/− females without heat-shock treatment were used as control. Western blot analysis revealed that overexpression of Bam appeared to increase levels of CycA protein in bam mutant germ cells (Fig. S2 A and C).
Fig. S2.
Elevation of Bam increases CycA protein level in ovarian cells. (A) Ovaries of P{hs-bam};bam−/− with or without heat-shock treatments were collected and lysed. Western blot assays were performed to measure CycA levels. Actin is shown as a loading control. (B) Ovaries of P{hs-bam};bam−/− without heat-shock treatments (hs-) and ovaries at different time points (0, 3, 6, 12 h) after heat-shock treatments (hs+) as indicated were collected and lysed for Western blot assays to measure CycA levels. Actin is shown as a loading control. (C) Densitometric analyses to quantify CycA expression in B are shown in C. Error bars represent SD (n = 3). For C, the Student’s t test was used to analyze the statistical significance, *P < 0.05, **P < 0.01, ***P < 0.001.
We next determined whether Bam controls the ubiquitination status of CycA by performing ubiquitination assays in S2 cells. As shown in Fig. 2J, a strong signal of ubiquitinated CycA was detected when S2 cells were transfected with epitope-tagged CycA and ubiquitin. However, levels of ubiquitinated CycA were significantly reduced when the cells were cotransfected with Bam (Fig. 2J). Consistently, the conjugation of ubiquitin to CycA was evidently increased when S2 cells were treated with dsRNA against bam (Fig. 2K), suggesting that Bam negatively regulates CycA ubiquitination.
CycA Is a Genetic Target of bam in Early Germ Cells.
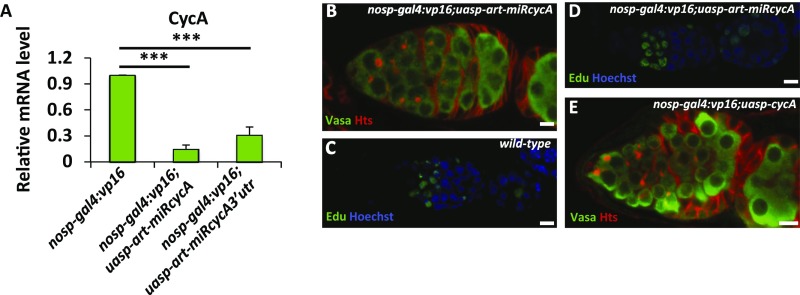
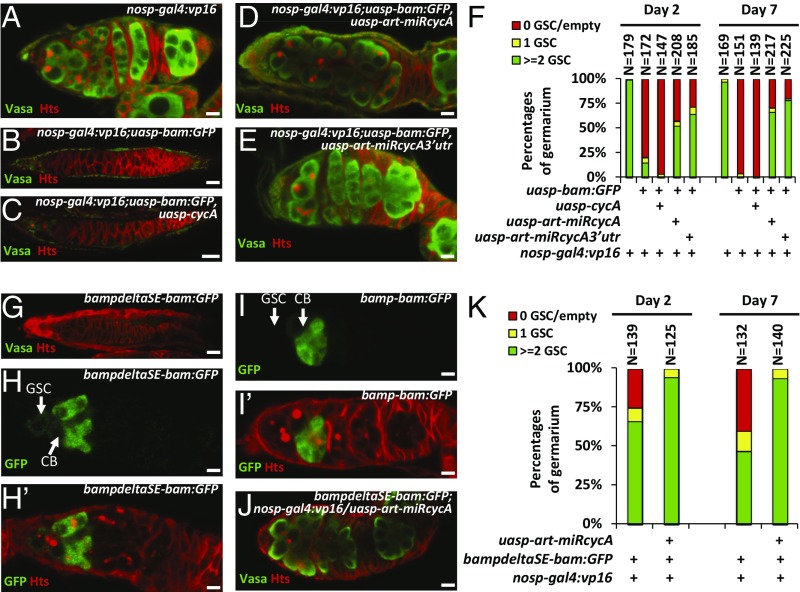
Considering that Bam stabilizes CycA in S2 cells, we hypothesized that ectopic expression of Bam in GSCs would increase the stability of CycA, which may at least in part account for the phenotype of GSC loss induced by Bam. To test this hypothesis, we determined whether down-regulation of CycA antagonized the function of ectopic Bam in GSCs. We first generated two miRNA-based cycA knockdown flies, P{uasp-art-miRcycA} and P{uasp-art-miRcycA3′utr}, according to our described method (28). As shown in Fig. S3A, expression of artificial miRNAs that targeted the coding region or 3′ UTR of cycA with the nanos promoter significantly reduced the levels of cycA transcripts in ovaries. However, knockdown of cycA did not affect the GSC number and allowed normal germ-line development in most ovarioles (Fig. S3B). Additionally, an EdU (5-ethynyl-2'-deoxyuridine)-labeling assay suggested that knockdown of cycA did not obviously affect early germ cells dividing, because wild-type control and cycA knockdown flies showed no significant difference in the number of EdU-positive cells in germaria (Fig. S3 C and D). Previous studies have shown that ectopic expression of Bam in germ cells with the nos promoter by generating P{nosp-gal4:vp16}/P{uasp-bam:GFP} leads to a strong GSC-loss phenotype (6). To establish a robust “read-out” system in our genetic test, we generated a germ cell driver, P{nosp-gal4:vp16}second, which is located on the second chromosome. The transgene combination, P{nosp-gal4:vp16}second;P{uasp-bam:GFP}, resulted in a GSC-loss phenotype in most germaria, but a proportion of germaria (10–20%) containing a normal number of GSCs (Fig. 3 B and F). We next generated P{nosp-gal4:vp16}second;P{uasp-bam:GFP}/P{uasp-cycA} flies, and found that the female flies displayed a stronger GSC-loss phenotype compared with ovaries from P{nosp-gal4:vp16}second;P{uasp-bam:GFP} flies (Fig. 3 A–C and F), suggesting that expression of wild-type CycA enhances Bam function in early germ cells. Of note, P{nosp-gal4:vp16}second;P{uasp-cycA} flies showed no apparent defect in GSCs (Fig. S3E). We next tested whether cycA knockdown antagonizes the function of ectopic Bam in GSCs. As shown in Fig. 3 A, B, and D–F, knockdown of cycA significantly suppressed the GSC-loss phenotype induced by ectopic expression of bam, suggesting that down-regulation of cycA antagonizes the ectopic bam-induced GSC-loss phenotype.
Fig. S3.
Bam genetically interacts with cycA. (A) Ovaries of P{nosp-gal4:vp16}, P{nosp-gal4:vp16};P{uasp-art-miRcycA}, and P{nosp-gal4:vp16};P{uasp-art-miRcycA3′utr} flies were lysed for total RNA extraction, followed by cDNA synthesis and qRT-PCR to measure the mRNA levels of cycA (n = 3). (B) Ovaries of P{nosp-gal4:vp16};P{uasp-art-miRcycA} were stained with anti-Vasa (green) and anti-Hts (red) antibodies as indicated. (C and D) Distribution of mitotic cells in the ovaries from w1118 (wild-type, C), and P{nosp-gal4:vp16};P{uasp-art-miRcycA} (D) adults. (E) Immunostaining same as in B, except that flies used were P{nosp-gal4:vp16};P{uasp-cycA} instead of {nosp-gal4:vp16};P{uasp-art-miRcycA}. (Scale bars: 10 μm.) For A, the Student’s t test was used to analyze the statistical significance, ***P < 0.001.
Fig. 3.
CycA is a downstream target of Bam. (A–E) Ovaries from P{nosp-gal4:vp16} (A, wild-type control), P{nosp-gal4:vp16}; P{uasp-bam:GFP} (B), P{nosp-gal4:vp16};P{uasp-bam:GFP}/P{uasp-cycA} (C), P{nosp-gal4:vp16};P{uasp-bam:GFP}/P{uasp-art-miRcycA} (D), and P{nosp-gal4:vp16};P{uasp-bam:GFP}/P{uasp-art-miRcycA3′utr} (E) were stained with anti-Vasa (green) and anti-Hts (red) antibodies. Vasa was used to visualize germ cells. Hts was used to outline the germarium and morphology of fusomes. (F) Quantification of the germarium phenotypes of ovaries in A–E. (G and J) Ovaries of P{bampdeltaSE-bam:GFP} (G) and P{nosp-gal4:vp16}; P{bampdeltaSE-bam:GFP}/P{uasp-art-miRcycA} (J) were stained with anti-Vasa (green) and anti-Hts (red) antibodies. (H–I′) Ovaries of P{bampdeltaSE-bam:GFP} (H and H′) and P{bamp-bam:GFP} (I and I′) were stained with anti-GFP (green) and anti-Hts (red) antibodies. (Scale bars: 10 μm.) (K) Statistical quantification of the germarium phenotypes of ovaries in G and J.
We have previously shown that removal of bam silencer element (SE) leads to ectopic expression of bam gene at native levels in GSCs and GSC-loss phenotype (6). To test whether GSC loss caused by derepressed expression of bam in GSCs can be rescued by loss of CycA, we used the transgene, P{bampdeltaSE-bam:GFP}, in which the Bam:GFP conding sequences are under the control of the bam promoter without bam SE. As shown in Fig. 3 G and K, absence of bam silence element (SE) resulted in GSC-loss phenotype induced by ectopic Bam:GFP in GSCs, compared with control flies carrying P{bamp-bam:GFP} (Fig. 3 I and I′). Notably, low levels of ectopic Bam:GFP expression were detected in GSCs (Fig. 3 H and H′). To validate our argument, we performed further genetic experiments. As shown in Fig. 3 G, J, and K, knockdown of cycA significantly suppressed the GSC-loss phenotype induced by removal of bam SE. Taken together, our genetic findings suggest that cycA is one of the downstream targets of bam in GSCs.
Identification of Otu as a Bam Cofactor in the Deubiquitination Complex.
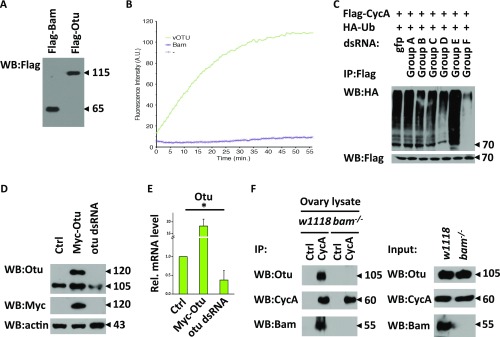
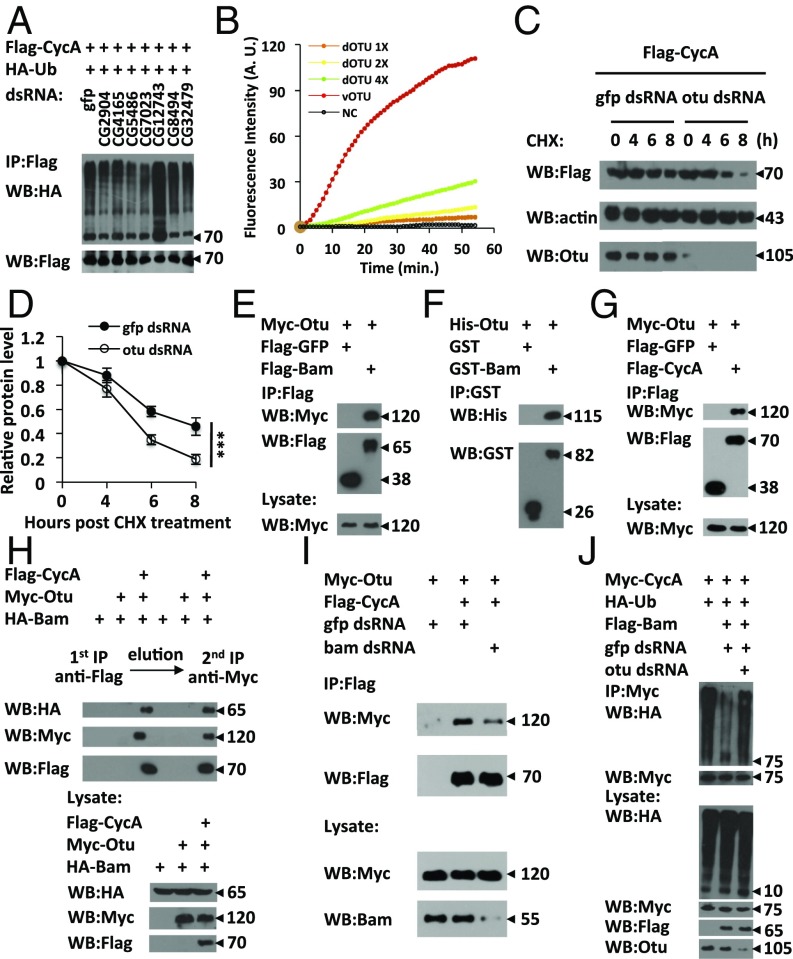
Considering that Bam is an ubiquitin-interacting protein and its role in negatively regulating CycA ubiquitination, we examined whether Bam has an intrinsic deubiquitinating enzymatic activity. To this end, we carried out deubiquitination assays by using eukaryotic-expressed Flag-tagged Bam and found that the purified Flag-Bam had no deubiquitinase activity (Fig. S4 A and B). Based on these observations, we reasoned that Bam might form a complex with other cofactors, likely deubiquitinating enzymes, to negatively regulate CycA ubiquitination. To test this possibility, we used a dsRNA library that targets 41 deubiquitinating enzymes in Drosophila and performed screening to search for a deubiquitinating enzyme involved in regulating CycA deubiquitination (Table S1). In this screening, we identified a pair of dsRNA that target Drosophila Otu. As shown in Fig. 4A and Fig. S4C, knockdown of otu significantly increased the abundance of ubiquitin conjugating to CycA. A recent study reported that Drosophila Otu has no detectable deubiquitinating enzyme (Dub) activity (29). However, given the fact that Drosophila Otu is required for inhibiting the CycA ubiquitination in S2 cells, we sought to determine the issue of whether Drosophila Otu is a Dub, and used Ub-Rhodamine110 as a substrate to perform quantitative deubiquitinating enzyme assays (30). As shown in Fig. 4B and Fig. S4A, although it exhibited much lower Dub activity compared with the positive control vOtu, Drosophila Otu was able to cleave the amide bond between the C-terminal glycine of ubiquitin and rhodamine in a dose-dependent manner, suggesting that Drosophila Otu possesses a Dub enzyme activity. We next asked whether Drosophila Otu regulates CycA protein turnover by designing another pair of otu dsRNA (Fig. S4 D and E) and found that knockdown of otu led to destabilization of the CycA protein (Fig. 4 C and D). These findings suggest that Drosophila Otu negatively regulates CycA ubiquitination and, thus, stabilizes CycA.
Fig. S4.
Otu is involved in CycA deubiquitination. (A) Western blots showing the Flag-Bam and Flag-Otu proteins that were purified from human 293T cells. (B) In vitro Dub assays were performed to measure the enzymatic activity of Drosophila Bam. In this assay, viral Otu and the BSA protein were used as positive and negative control, respectively. In the reaction, the Ub-Rhodamine110 was used as Dub enzyme substrate. The rhodamine fluorescence signal could increase, if cleavage of the amide bond between the C-terminal glycine of ubiquitin and rhodamine catalyzed by a positive deubiquitinase occurs. Dynamic fluorescence signals could be recorded with excitation and emission wavelengths set at 485/20 and 535/20 nm, respectively, at various time points. (C) S2 cells were treated with indicated groups of dsRNAs for 48 h, followed by plasmids transfection. Cells were then collected and subjected to ubiquitination assay to measure the ubiquitination pattern of CycA. (D and E) S2 cells were treated with otu dsRNA or gfp dsRNA (control) for 48 h. Cells were then transfected with Myc-Otu plasmids as indicated. Western blot and qRT-PCR assays were performed to measure the levels of Otu protein (D) or mRNA (E, n = 3). (F) Ovaries from w1118 or bam−/− adults were collected and lysed, followed by immunoprecipitation with indicated antibodies. Western blot assays were performed to detect indicated protein levels. For E, the Student’s t test was used to analyze the statistical significance, *P < 0.05.
Table S1.
Identification of the Dub that regulates CycA deubiquitination by a cell-based screening
| Group | CG numbers of potential Dub genes |
| A | CG1490, CG3251, CG4265, CG5794, CG7857, CG8877, CG14884 |
| B | CG1945, CG3416, CG4751, CG5798, CG8232, CG9448, CG15817 |
| C | CG1950, CG3431, CG4968, CG6091, CG8334, CG9769, CG18174 |
| D | CG2224, CG3781, CG5384, CG6932, CG8445, CG12082, CG30421 |
| E | CG2904, CG4165, CG5486, CG7023, CG8494, CG12743, CG32479 |
| F | CG3016, CG4166, CG5505, CG7288, CG8830, CG14619 |
To identify the specific Dub(s) that regulates CycA deubiquitination, we used indicated dsRNAs in Table S1 to treat S2 cells and performed cell-based deubiquitination assays. In this experiment, 41 candidate genes encoding potential Dubs were divided into 6 groups and indicated dsRNAs of each group were mixed to treat S2 cells for 48 h, the treated cells were then transfected with HA-Ub and Flag-CycA plasmids. Cell lysates were used to perform in vivo deubiquitination assays.
Fig. 4.
Bam targets CycA in an Otu-dependent manner. (A) S2 cells were treated with dsRNAs targeting deubiquitination genes for 48 h, followed by plasmid transfections. Cells were lysed for immunoprecipitation and Western blot assays. (B) In vitro Dub assays showed the enzymatic activity of Drosophila Otu (the detailed information was shown in SI Text). (C and D) S2 cells were pretreated with gfp or otu dsRNAs for 48 h and then transfected with indicated plasmids (C). Cells were treated with CHX (50 ng⋅mL−1) for various time points as indicated, followed by immunoblotting to measure expression levels of CycA. Densitometric analysis for quantifying CycA expression in C is shown in D. Error bars represent SD (n = 3). (E) S2 cells were transfected with indicated plasmids. Cell lysates were prepared and immunoprecipitated, followed by Western blot assays. (F) His-tagged Otu protein purified from Escherichia coli was coimmunoprecipitated with GST (control) or GST-Bam, followed by Western blot assays. (G) Transfection and coimmunoprecipitation experiments were performed as in E, except that the Flag-CycA plasmid was used instead of Flag-Bam. (H) S2 cells were transfected with indicated plasmids. Cell lysates were prepared and subjected to a two-step immunoprecipitation method by using Flag and Myc beads successively, followed by Western blot assays. (I) S2 cells were treated with gfp or bam dsRNAs for 48 h, followed by transfection with indicated plasmids. Cells lysates were subjected to immunoprecipitation and Western blot assays. (J) S2 cells were treated with dsRNA targeting gfp or otu for 48 h and then transfected with indicated expression vectors. Cells were lysed and subjected to immunoprecipitation assays, followed by Western blot assays. All of the biochemical experiments were performed at least three times. In D, the log rank test was used to analyze statistical variance. ***P < 0.001.
Bam Acts in Concert with Otu To Promote CycA Deubiquitination.
We next sought to investigate whether Otu form a complex to regulate CycA deubiquitination. As shown by immunoprecipitation assays (Fig. 4E), a Bam-Otu protein association was detected in S2 cells cotransfected with both Flag-Bam and Myc-Otu. GST-pull down experiments confirmed that Bam and Otu proteins directly bound to each other (Fig. 4F). Because CycA associated with Otu in coimmunoprecipitation assays (Fig. 4G), and both Otu and Bam caused CycA deubiquitination, we next asked whether CycA, Bam, and Otu formed a trimeric complex by performing two-step immunoprecipitation assays in S2 cells. As shown in Fig. 4H, after two-step immunoprecipitations, both HA-Bam and Flag-CycA were present in the Myc–Otu complex, suggesting that CycA, Bam, and Otu form a trimeric complex. Of note, knockdown or mutation of bam significantly impaired the association between Otu and CycA, suggesting that Bam is required for Otu to target CycA both in S2 cells and in germ cells (Fig. 4I and Fig. S4F). Next, we evaluated whether Otu participates in Bam-mediated CycA deubiquitination in S2 cells. As shown in Fig. 4J, overexpression of Bam reduced the conjugation of ubiquitin to CycA compared with the control. However, the deubiquitination of CycA induced by overexpression of Bam was significantly suppressed when otu was knocked down in S2 cells, suggesting that Bam stabilizes CycA by promoting its deubiquitination in a manner that depends on the activity of Otu.
Otu and Bam Cooperatively Control Early Germ Cell Development.
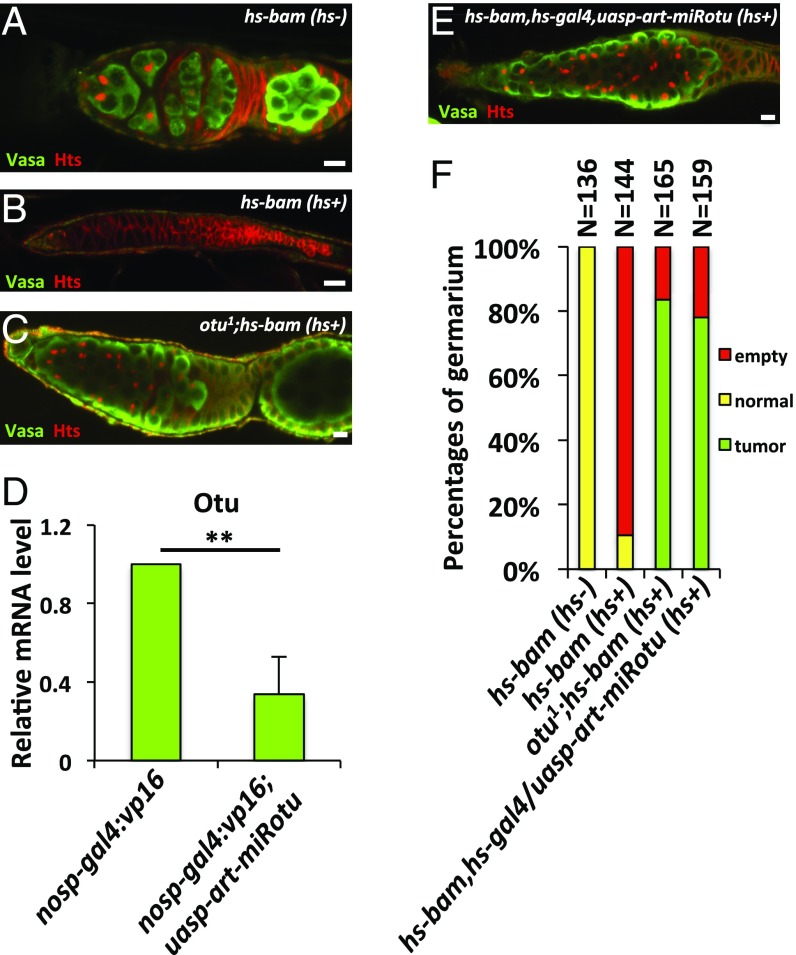
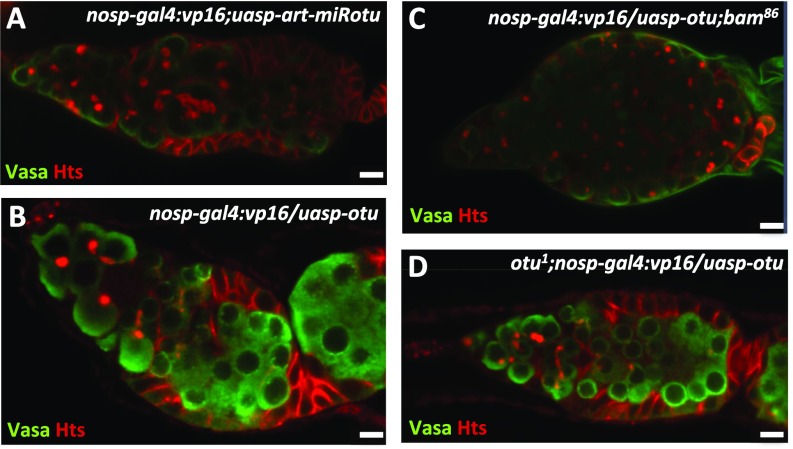
Previous studies have shown that the loss of otu results in severe germ cell hyperplasty (31), similar to the tumorous phenotype caused by mutation of the bam gene. To elucidate the genetic relationships between bam and otu in regulating ovarian germ cell development, we generated otu1;P{hs-bam} flies and examined whether Otu is required for the Bam function in promoting precocious differentiation of GSCs. As shown in Fig. 5 A–C and F, ectopic expression of Bam in wild-type background resulted in a strong GSC-loss phenotype; by contrast, ectopic Bam in the otu mutant had no effect on GSC loss. Immunostaining showed that otu1;P{hs-bam} ovaries still displayed tumorous germarium phenotypes that were similar to those in females carrying the otu mutant alone (Fig. 5 C and F). To confirm these observations, we generated the transgenic line P{uasp-art-miRotu} that carried artificial miRNAs targeting the otu gene (28). As shown in Fig. 5D, knockdown of otu by the nos promoter effectively reduced the level of otu, and accordingly caused the tumorous germarium phenotype (Fig. S5A). Importantly, further genetic experiments revealed that knockdown of otu efficiently suppressed the GSC-loss phenotype induced by overexpression of Bam (Fig. 5 E and F). Additionally, we did not observe any detectable phenotypes in ovaries from P{nosp-gal4:vp16}/P{uasp-otu} or P{nosp-gal4:vp16}/P{uasp-otu};bam86 flies (Fig. S5 B and C). One explanation is that the levels of endogenous Otu could be saturated in germ cells. Of note, the otu transgene could fully rescue otu mutant phenotype in germ cells (Fig. S5D). Collectively, these findings suggest that Otu is critical for the function of ectopic Bam in GSCs.
Fig. 5.
Otu genetically interacts with bam. (A–C) Ovaries from P{hs-bam} (hs-) (A, wild-type control), P{hs-bam} (hs+) (B), and otu1;P{hs-bam} (hs+) (C) flies were stained with anti-Vasa (green) and anti-Hts (red) antibodies. (D) Ovaries from P{nosp-gal4:vp16} (wild-type control) and P{nosp-gal4:vp16};P{uasp-art-miRotu} were dissected and subjected to quantitative RT-PCR (qRT-PCR) assays. Error bars represent SD (n = 3). (E) The staining was performed as in C, except that flies used were P{uasp-art-miRotu}/P{hs-bam,hs-gal4} (hs+). (Scale bars: 10 μm.) (F) Quantification of the germarium phenotypes of ovaries in A–C and E. In D, the Student’s t test was used to analyze statistical variance. **P < 0.01.
Fig. S5.
Bam/Otu cooperatively control germ cell development through regulating CycA. Ovaries from P{nosp-gal4:vp16};P{uasp-art-miRotu} (A), P{nosp-gal4:vp16}/P{uasp-otu} (B), P{nosp-gal4:vp16}/P{uasp-otu};bam86 (C), and otu1;P{nosp-gal4:vp16}/P{uasp-otu} (D) were stained with anti-Vasa (green) and anti-Hts (red) antibodies. (Scale bars: 10 μm.)
Discussion
Previous studies have shown that Bam plays an essential role in promoting early germ cell differentiation in Drosophila ovaries. To protect GSCs from this powerful differentiation inducer, the expression of bam is inhibited in GSCs by the BMP/Dpp signaling pathway. Disrupting the BMP/Dpp pathway, or ectopically inducing bam expression in GSCs, causes them to differentiate, resulting in a loss of the germ line (5, 7–9). However, little is known about how ectopic expression of Bam drives GSC differentiation. In this study, we showed that knockdown of CycA significantly suppressed the GSC-loss phenotype induced by ectopic Bam in GSCs. Importantly, we found that Bam acts in concert with Otu to negatively regulate CycA ubiquitination. Thus, our findings suggest ectopically expressed Bam acts as a component of an Otu-containing Dub complex that promotes accumulation of deubiquitinated CycA in GSCs, and CycA-dependent differentiation.
A hallmark of early germ cell development is that each round of cyst division is accompanied by a morphological change of a germ cell-specific organelle called the fusome (32). While it is observed as a spherical dot in GSCs/CBs, the fusome progressively grows upon cell division to become elongated and finally a branched structure in matured cysts. Because a significant proportion of CycA is associated with the fusome, regulation of CycA at the fusome likely plays important roles in controlling asymmetric division of GSCs and germ cell-specific mitosis (21). Interestingly, previous and current studies have shown that Bam is partially associated with the fusome and required to promote proper incomplete cytokinesis (detailed information shown in SI Text and Fig. S6). In particular, the BamF protein is detectable in the fusome at a low level in GSCs (4). Of note, loss of function of the otu gene encoding a Bam cofactor leads to fragmented fusomes in tumorous egg chambers (31). Based on previous and current findings, we propose that the turnover of CycA mediated by Bam/Otu-dependent deubiquitination is likely processed in a fusome-dependent manner. Indeed, considering that Cul1 and 19S-S1 proteins are also present in the fusome, previous studies suggest that the ubiquitin-based proteolysis machinery is localized at the fusome and regulates the turnover of cyclins (e.g., CycA and CycE) (20). Thus, our findings emphasize that proper balancing of CycA turnover either by ubiquitination or deubiquitination processes is critical for GSC asymmetric division.
Fig. S6.
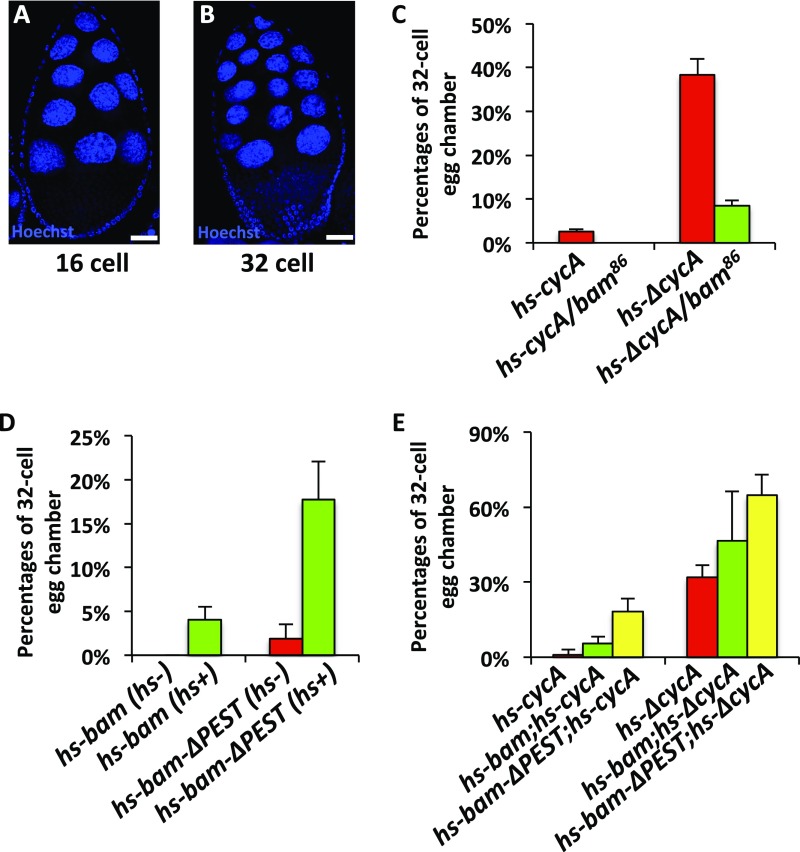
The Bam-CycA regulatory axis controls the division of cyst cells. (A and B) Examples of 16-cell (A) and 32-cell (B) egg chambers. (Scale bars: 10 μm.) (C–E) Statistical quantification showing the percentages of 32-cell egg chambers in females with the indicated genotypes. Error bars represent SD (n = 3).
Bam has been shown to form a complex with its obligate cofactor Bgcn to regulate CB differentiation (33). Previous studies show that the Bam/Bgcn complex antagonizes Nos expression likely via the nos 3′ UTR, thus derepressing CB-promoting factors (12). However, the molecular basis of how Bam regulates translational repression of nos has remained poorly understood. Nevertheless, the current findings suggest that Bam plays multiple roles in regulating early germ cell differentiation through both translational repression and posttranslational control. Recent studies have suggested that ubiquitin, a regulator of protein degradation, also contributes to regulation of mRNA turnover through the action of a family of RNA-binding proteins that also have an E3 ubiquitin ligase activity, thus establishing a link between translational repression and posttranslational control (34, 35). Considering that Bam is an ubiquitin-associated protein, it would be interesting to explore the detailed function of Bam in the future.
SI Text
Drosophila Otu Possesses a Dub Activity.
A recent study reported that Drosophila Otu has no enzymatic activity for substrate deubiquitination (Dub), because Drosophila Otu lacks a conserved cysteine residue critical for Dub activity (29). However, a previous work studying on the structure of Ubc13, an ubiquitin-conjugating enzyme (E2), described that mutation of the active site cysteine in Ubc13 to serine doesn’t completely abolish its E2 activity, albeit with reduced catalytic efficiency (36). Given the fact that Drosophila Otu negatively regulates ubiquitination of the CycA in S2 cells, we reasoned that Drosophila Otu itself might have a low Dub activity, and its in vivo activity likely would be regulated by other factors. To determine the issue of whether Drosophila Otu is a deubiquitinating enzyme, we used Ub-Rhodamine110 as a substrate to perform quantitative deubiquitinating enzyme assays (30). Ub-Rhodamine110 is an exquisitely sensitive deubiquitinating enzyme substrate for detecting ubiquitin C-terminal hydrolytic activity, because cleavage of the amide bond between the C-terminal glycine of ubiquitin and rhodamine by deubiquitinase leads to an increase in rhodamine fluorescence, and dynamic fluorescence could be quantitatively monitored with excitation and emission wavelengths set at 485/20 and 535/20 nm, respectively. To measure the potential Dub activity of Drosophila Otu, we used a commonly used Dub, viral Otu (vOtu) as a positive control, and the BSA protein as a negative control. As shown in Fig. 4B and Fig. S4A, although it exhibited much lower Dub activity compared with vOtu, Drosophila Otu was able to cleave the amide bond between the C-terminal glycine of ubiquitin and rhodamine in a dose-dependent manner, suggesting that Drosophila Otu itself possesses Dub enzyme activity.
The Bam-CycA Regulatory Axis Controls the Division of Cyst Cells.
It has been shown that overexpression of CycA or ΔCycA forces cysts into an extra round of division, resulting in 32-cell cysts (21). Notably, the percentages of egg chambers containing 32-cell cysts could be significantly reduced by introduction of one copy of mutant bam (21) (Fig. S6 A–C). Consistent with these observations, we found that overexpression of bam alone by using P{hs-bam} flies could induce the 32-cell–cyst phenotypes (Fig. S6D). Moreover, overexpression of bam significantly enhanced the 32-cell–cyst phenotypes induced by overexpression of CycA or ΔCycA, when P{hs-bam};P{hs-cycA} or P{hs-bam};P{hs-ΔcycA} females were used in our assays (Fig. S6E), suggesting that elevation of Bam expression enhances the CycA function in promoting the extra round of cyst division. To substantiate our argument, we have also used another transgene line, P{hs-bam-ΔPEST}, in which a sequence encoding Bam without its “PEST” domain was under the control of the heat-shock promoter. Of note, overexpression of Bam-ΔPEST led to stronger 32-cell cysts phenotypes than the wild-type form of Bam did, and the phenotypes became much more severe when the Bam-ΔPEST was cooverexpressed with CycA or ΔCycA, compared with individual overexpression of Bam-ΔPEST, CycA, or ΔCycA (Fig. S6 D and E). Thus, our findings support a notion that the Bam-CycA regulatory axis plays an important role in regulating the proper division of cyst cells in Drosophila ovaries.
Materials and Methods
All fly strains were maintained under standard culture conditions. The w1118 strain was used as the host for all P-element–mediated transformation. Fly strains, including P{hs-bam}, P{uasp-bam:GFP}, P{uasp-cycA}, P{nosp-gal4:vp16}, and P{bamp-bam:GFP} have been described (5, 6, 21, 22). Knockdown transgenic flies, including P{uasp-art-miRcycA}, P{uasp-art-miRcycA3′utr}, and P{uasp-art-miRotu}, were generated according to methods that we have described (28). For the P{bampdeltaSE-bam:GFP} transgene, the coding sequences of Bam and GFP were successively inserted after the bam promoter without silence element (SE). The otu1 was obtained from the Bloomington stock center. Additional materials and methods are available in SI Materials and Methods.
SI Materials and Methods
Co-IP and LC-MS/MS Analysis.
S2 cells were transfected with Flag-GFP or Flag-Bam plasmids in three biological replicates, followed by lysates preparation and immunoprecipitation. Proteins were eluted and digested by using Trypsin (Promega), and then were subjected to LC-MS/MS assays. Resulting MS/MS data were processed by using Thermo Proteome discovery (version 1.4.1.14), and tandem mass spectra were searched against UniProt-Drosophila database. Unique peptides that were detected only in Flag-Bam immunoprecipitants, or displayed at least 2 folds higher abundance than the Flag-GFP control groups were selected. From all these peptides, only the ones that emerged in all of the three replicates were considered as Bam-associated proteins.
Immunohistochemistry of Fly Ovaries.
Dissected ovaries were fixed in fixation buffer (4% formaldehyde and 0.25% Tween-20 in PBS) for 15 min and blocked in PBTA (0.3% Tween-20 and 1.5% BSA in PBS) as described (4). Primary antibodies were added to PBTA and incubated with ovaries overnight, followed by washing for three times (10 min per time) in PBTA. Secondary antibodies, goat anti-mouse Alexa 568, and goat anti-rabbit Alexa 488 (1:2000, Molecular Probes) were applied for 3–4 h at room temperature, followed by washing for 30 min.
For 32-cell cysts phenotype assays, indicated flies were subjected to a 5-d-long heat-shock treatment. For each daily heat-shock treatment, food vials containing flies were placed in a 37 °C incubator for 1 h, and 6 h later for another heat-shock treatment (three times in total for 1 d). Between heat shocks, flies were placed in the 25 °C incubator. After the final heat shock, flies were cultured for 1 d in the 25 °C incubator. Dissected ovaries were then fixed and stained by using Hoechst (1:5000). Images were acquired under a Zeiss LSM 710 Meta confocal microscope.
EdU Staining.
For EdU staining, we used a Click-iT EdU Alexa Fluor 488 Imaging Kit. Briefly, ovaries were treated with 100 µM EdU for 1 h, followed by fixing in 4% formaldehyde for 15 min, permeabilizing in 1% Triton X-100/PBS for 30 min, and blocking in PBTA for 30 min. Samples were then incubated with Alexa Fluor 488 for 30 min and washed in PBTA for 20 min. Images were acquired under the Zeiss LSM 710 Meta confocal microscope.
Cell Culture, Immunoprecipitation, and Western Blotting.
S2 cells were cultured in Drosophila medium (Sigma) at 27 °C. Plasmids were transfected by using standard Lipo2000 transfection reagents (Invitrogen). Cells were harvested and lysed (50 mM Tris, pH 7.5, 150 mM NaCl, 1% Triton X-100, 10% glycerol) for 30 min. Immunoprecipitation and Western blot assays were performed by using standard protocols. The following materials and antibodies were used for immunoprecipitation: Flag-beads (Sigma), Myc-beads (MBL), protein A/G (Sigma), GST-beads (Sigma), HA-Ub (Recombinant human HA-Ubiquitin protein, U-110, R&D Systems), mouse anti-ubiquitin (Santa Cruz Biotechnology), mouse anti-GFP (Santa Cruz Biotechnology) antibodies. Antibodies used for Western blotting include the following: rabbit anti-Myc (MBL, 1:5000), rabbit anti-Flag (Sigma, 1:5000), rabbit anti-HA (MBL, 1:5000), rabbit anti-GFP (Santa Cruz Biotechnology, 1:3000), mouse anti-Bam (1:10000), mouse anti-CycA (1:5000), mouse anti-Otu (1:10000). Antibodies against Bam and CycA have been described (22). The antibody against Otu was generated by immunizing mice with a recombinant protein His6-Otu (amino acids 253–352) that was produced in E. coli.
DsRNA Synthesis.
Purified DNAs with T7 elements were subjected to in vitro transcription reaction by using dsRNA production system (RiboMAX, Promega). Primers for dsRNA synthesis are shown as follows.
bam-s: 5′-ACTATAGGGAGATCGGCCGCCCTTAGCTCGGCA-3′,
bam-as: 5′-ACTATAGGGAGACGCCGATTTGGGCTTCTCGCG-3′,
otu-s: 5′-ACTATAGGGAGAGATACTTCTACA-3′,
otu-as: 5′-ACTATAGGGAGACTGTCCTTGAGC-3′,
gfp-s: 5′-ACTATAGGGAGAAGCAAGGGCGAGGAGCTGTT-3′,
gfp-as: 5′-ACTATAGGGAGAGGTAGTGGTTGTCGGGCAGC-3′.
In Vivo Ubiquitination Assay.
In vivo ubiquitination experiments were performed by using standard protocols as described (11, 22). In brief, plasmids were transfected into S2 cells for 48 h followed by MG132 (50 μM) treatment for 6 h. Ub lysis buffer (50 mM Tris, pH 7.5, 500 mM NaCl, 0.5% Nonidet P-40, and 10% glycerol) containing 1% (wt/vol) SDS was added to cell pellets, followed by heating at 98 °C for 5 min. Total lysates were combined with binding buffer (50 mM Tris, pH 7.5, 500 mM NaCl, 0.5% Nonidet P-40, and 10% glycerol) to adjust the SDS to a final concentration of 0.1%, followed by sonication and immunoprecipitation using the indicated beads. Immunoprecipitated beads were then washed and subjected to Western blot assays to detect the ubiquitination patterns of target proteins.
In Vitro Deubiquitination Assay.
Purified proteins including Drosophila Bam, Drosophila Otu, viral Otu (vOtu), or BSA was mixed with Ub-Rhodamine110 (Boston Biochem, final concentration at 1 μM) in a total volume of 30 μL in the reaction buffer (20 mM Tris⋅HCl, pH 7.5, 200 mM NaCl, 5 mM MgCl2, 2 mM DTT). Ub-Rhodamine110 is an exquisitely sensitive Dub enzyme substrate for detecting ubiquitin C-terminal hydrolytic activity, because cleavage of the amide bond between the C-terminal glycine of ubiquitin and rhodamine by deubiquitinase leads to an increase in rhodamine fluorescence. The mixture was added into a black 384-well low volume plate (Corning) and incubated at 37 °C by using MD SpectraMax M5 Microplate Reader. Dynamic fluorescence was monitored with excitation and emission wavelengths set at 485/20 and 535/20 nm, respectively. Fluorescence intensity for each condition was averaged from duplicates and plotted as a function of time.
qRT-PCR.
Total RNA was extracted by using TRIZOL reagent (Invitrogen) according to the manufacturer’s instructions. cDNA was synthesized by using a first-strand cDNA synthesis kit (Transgen). qRT-PCR was performed in triplicate by using SYBR Green Master Mix (Kangwei) on a Light Cycler 480. Relative mRNA levels were normalized to rp49 in each sample. Primers for qRT-PCR were as follows.
rp49-s: 5′-CACGATAGCATACAGGCCCAAGATCGG-3′,
rp49-as: 5′-GCCATTTGTGCGACAGCTTAG-3′,
cycA-s: 5′-GACATGCCTGAGAAGCTGAA-3′,
cycA-as: 5′-CGACTGAAGCGGATGACATAA-3′,
otu-s: 5′-CTTCCTTCTCCACCGCTAAAT-3′,
otu-as: 5′-CGCCCATGTTGTAGAAGTATCT-3′.
Statistical Methods.
Data from qRT-PCR assays were analyzed from three biological replicates and significant differences were determined by two-tailed Student’s t test. Data of protein stability assays were collected from three biological replicates and significant differences were analyzed by the log-rank test using PASW Statistics 18 software. All data are shown as means ± SD. For all tests, a P < 0.05 was considered to be statistically significant. All of the Western blots were performed at least three times, from which the typical results were shown in figures.
Acknowledgments
This work was supported by the National Basic Research Program of China Grant 2013CB945002; Natural Science Foundation of China Grants 3159830021, 91640204, and 31130036; and Strategic Priority Research Program of the Chinese Academy of Sciences Grant XDB19000000.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 6154.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1619188114/-/DCSupplemental.
References
- 1.Spradling A, Fuller MT, Braun RE, Yoshida S. Germline stem cells. Csh Perspect Biol. 2011;3:a002642. doi: 10.1101/cshperspect.a002642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losick VP, Morris LX, Fox DT, Spradling A. Drosophila stem cell niches: A decade of discovery suggests a unified view of stem cell regulation. Dev Cell. 2011;21:159–171. doi: 10.1016/j.devcel.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKearin DM, Spradling AC. bag-of-marbles: A Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- 4.McKearin D, Ohlstein B. A role for the Drosophila bag-of-marbles protein in the differentiation of cystoblasts from germline stem cells. Development. 1995;121:2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- 5.Ohlstein B, McKearin D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development. 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- 6.Chen D, McKearin DM. A discrete transcriptional silencer in the bam gene determines asymmetric division of the Drosophila germline stem cell. Development. 2003;130:1159–1170. doi: 10.1242/dev.00325. [DOI] [PubMed] [Google Scholar]
- 7.Chen D, McKearin D. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol. 2003;13:1786–1791. doi: 10.1016/j.cub.2003.09.033. [DOI] [PubMed] [Google Scholar]
- 8.Song X, et al. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development. 2004;131:1353–1364. doi: 10.1242/dev.01026. [DOI] [PubMed] [Google Scholar]
- 9.Xie T, Spradling AC. Decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- 10.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 11.Xia L, et al. The Fused/Smurf complex controls the fate of Drosophila germline stem cells by generating a gradient BMP response. Cell. 2010;143:978–990. doi: 10.1016/j.cell.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Minor NT, Park JK, McKearin DM, Maines JZ. Bam and Bgcn antagonize Nanos-dependent germ-line stem cell maintenance. Proc Natl Acad Sci USA. 2009;106:9304–9309. doi: 10.1073/pnas.0901452106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen D, McKearin D. Gene circuitry controlling a stem cell niche. Curr Biol. 2005;15:179–184. doi: 10.1016/j.cub.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Yang L, et al. Argonaute 1 regulates the fate of germline stem cells in Drosophila. Development. 2007;134:4265–4272. doi: 10.1242/dev.009159. [DOI] [PubMed] [Google Scholar]
- 15.Jin Z, Xie T. Dcr-1 maintains Drosophila ovarian stem cells. Curr Biol. 2007;17:539–544. doi: 10.1016/j.cub.2007.01.050. [DOI] [PubMed] [Google Scholar]
- 16.Park JK, Liu X, Strauss TJ, McKearin DM, Liu Q. The miRNA pathway intrinsically controls self-renewal of Drosophila germline stem cells. Curr Biol. 2007;17:533–538. doi: 10.1016/j.cub.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 17.Kalaszczynska I, et al. Cyclin A is redundant in fibroblasts but essential in hematopoietic and embryonic stem cells. Cell. 2009;138:352–365. doi: 10.1016/j.cell.2009.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pauklin S, Vallier L. The cell-cycle state of stem cells determines cell fate propensity. Cell. 2013;155:135–147. doi: 10.1016/j.cell.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Lin H. The division of Drosophila germline stem cells and their precursors requires a specific cyclin. Curr Biol. 2005;15:328–333. doi: 10.1016/j.cub.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Ohlmeyer JT, Schüpbach T. Encore facilitates SCF-Ubiquitin-proteasome-dependent proteolysis during Drosophila oogenesis. Development. 2003;130:6339–6349. doi: 10.1242/dev.00855. [DOI] [PubMed] [Google Scholar]
- 21.Lilly MA, de Cuevas M, Spradling AC. Cyclin A associates with the fusome during germline cyst formation in the Drosophila ovary. Dev Biol. 2000;218:53–63. doi: 10.1006/dbio.1999.9570. [DOI] [PubMed] [Google Scholar]
- 22.Chen D, et al. Effete-mediated degradation of Cyclin A is essential for the maintenance of germline stem cells in Drosophila. Development. 2009;136:4133–4142. doi: 10.1242/dev.039032. [DOI] [PubMed] [Google Scholar]
- 23.Ables ET, Drummond-Barbosa D. Cyclin E controls Drosophila female germline stem cell maintenance independently of its role in proliferation by modulating responsiveness to niche signals. Development. 2013;140:530–540. doi: 10.1242/dev.088583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.León A, McKearin D. Identification of TER94, an AAA ATPase protein, as a Bam-dependent component of the Drosophila fusome. Mol Biol Cell. 1999;10:3825–3834. doi: 10.1091/mbc.10.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shen R, Weng C, Yu J, Xie T. eIF4A controls germline stem cell self-renewal by directly inhibiting BAM function in the Drosophila ovary. Proc Natl Acad Sci USA. 2009;106:11623–11628. doi: 10.1073/pnas.0903325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandrasekharan MB, Huang F, Sun ZW. Decoding the trans-histone crosstalk: Methods to analyze H2B ubiquitination, H3 methylation and their regulatory factors. Methods. 2011;54:304–314. doi: 10.1016/j.ymeth.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaspar M, Dienemann A, Schulze C, Sprenger F. Mitotic degradation of cyclin A is mediated by multiple and novel destruction signals. Curr Biol. 2001;11:685–690. doi: 10.1016/s0960-9822(01)00205-6. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Mu Y, Chen D. Effective gene silencing in Drosophila ovarian germline by artificial microRNAs. Cell Res. 2011;21:700–703. doi: 10.1038/cr.2011.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Louis M, Hofmann K, Broemer M. Evolutionary loss of activity in de-ubiquitylating enzymes of the OTU family. PLoS One. 2015;10:e0143227. doi: 10.1371/journal.pone.0143227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hassiepen U, et al. A sensitive fluorescence intensity assay for deubiquitinating proteases using ubiquitin-rhodamine110-glycine as substrate. Anal Biochem. 2007;371:201–207. doi: 10.1016/j.ab.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 31.Storto PD, King RC. Multiplicity of functions for the otu gene products during Drosophila oogenesis. Dev Genet. 1988;9:91–120. doi: 10.1002/dvg.1020090203. [DOI] [PubMed] [Google Scholar]
- 32.McKearin D. The Drosophila fusome, organelle biogenesis and germ cell differentiation: If you build it... BioEssays. 1997;19:147–152. doi: 10.1002/bies.950190209. [DOI] [PubMed] [Google Scholar]
- 33.Ohlstein B, Lavoie CA, Vef O, Gateff E, McKearin DM. The Drosophila cystoblast differentiation factor, benign gonial cell neoplasm, is related to DExH-box proteins and interacts genetically with bag-of-marbles. Genetics. 2000;155:1809–1819. doi: 10.1093/genetics/155.4.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cano F, Miranda-Saavedra D, Lehner PJ. RNA-binding E3 ubiquitin ligases: Novel players in nucleic acid regulation. Biochem Soc Trans. 2010;38:1621–1626. doi: 10.1042/BST0381621. [DOI] [PubMed] [Google Scholar]
- 35.Cano F, Rapiteanu R, Sebastiaan Winkler G, Lehner PJ. A non-proteolytic role for ubiquitin in deadenylation of MHC-I mRNA by the RNA-binding E3-ligase MEX-3C. Nat Commun. 2015;6:8670. doi: 10.1038/ncomms9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eddins MJ, Carlile CM, Gomez KM, Pickart CM, Wolberger C. Mms2-Ubc13 covalently bound to ubiquitin reveals the structural basis of linkage-specific polyubiquitin chain formation. Nat Struct Mol Biol. 2006;13:915–920. doi: 10.1038/nsmb1148. [DOI] [PubMed] [Google Scholar]