As evidence for a causal link between Zika infection and microcephaly and other serious congenital anomalies grew (1), the World Health Organization (WHO) declared the Latin American Zika epidemic a public health emergency of international concern in February 2016 (2). The sheer speed of spread (Fig. 1 and Supplementary Material [SM]) has made formulating an effective public health response challenging. Immediate policy responses have included enhanced vector control (3) and advice to delay pregnancy in a few countries (4), followed by an extended recommendation to all affected countries by WHO in June 2016. These have merits, but are likely to have limited effectiveness (5) and may interact antagonistically. A fuller understanding of the dynamics and drivers of the epidemic is needed to assess longer-term risks posed by Zika and prioritize interventions.
Fig.1.
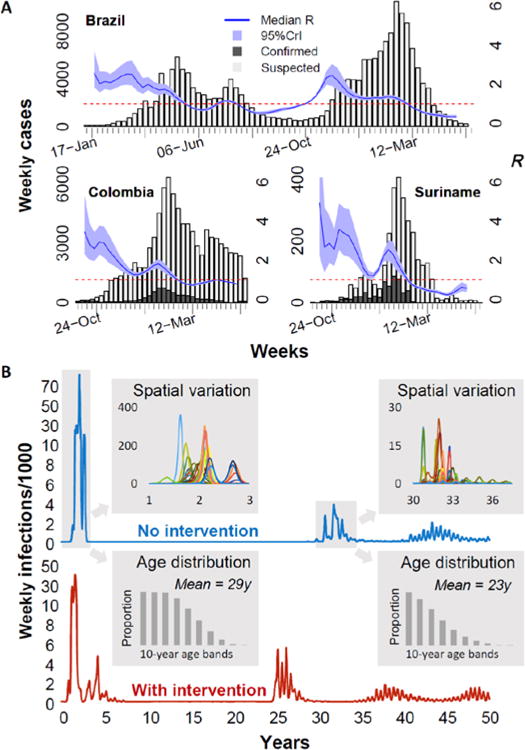
A. Publically available surveillance data on weekly suspected (light grey) and laboratory confirmed (dark grey) Zika cases (left axis and bars) overlaid with estimates of the reproduction number, R (running 5-week average shown, centered on the middle week). The horizontal dashed line marks the R =1 threshold. Results for Brazil Colombia and Suriname are shown; see SM for other countries, sources and estimation methods. B. Typical (see SM for other examples) simulated time series of Zika weekly infection incidence per 1000 people in a population of 600 million for two scenarios: no interventions (blue curve), and assuming interventions which decrease mosquito lifespan by 20% for one year during the initial epidemic (red curve) Incidence is plotted on a non-linear scale (increments of 2 up to 10, then increments of 20) to allow later epidemics to be resolved clearly. Upper and lower shaded insets show, respectively, incidence dynamics (colored curves) in the 20 spatial regions being modelled (axes as main graph) and the age distribution (dark grey bars) of infections, for the first two epidemic periods in the no intervention scenario. Full details provided in SM.
Three key factors determine the scale and speed of spread of an emerging infection in a naïve population and the risk of longer-term endemicity. The first is the transmissibility of the infection, characterised by the reproduction number, R – the average number of secondary infections caused by a typical index case (R<1 stops an epidemic). We provide time-varying estimates of R for affected Latin American countries where surveillance data are available (Fig. 1 and SM). Trends at the country level hide substantial subnational heterogeneity (see SM), likely reflecting geographic variation in vector habitat quality and climate-driven variation in vector density and competence (6).
The generation time (Tg, the time between cycles of infection in an epidemic, see SM) is the second key factor which critically determines the timescale of disease invasions. Taking our estimates of R (Fig. 1a and SM) and the generation time (see SM), we use a stochastic spatial model of Zika transmission (see SM) to illustrate the dynamics of the current epidemic and possible future waves of transmission (Fig. 2a). We expect the current epidemic to be largely over in 3 years, with seasonal oscillations in incidence caused by seasonal variation in mosquito populations and transmissibility. Herd immunity will likely then cause a delay of over a decade until further large epidemics are possible; an epidemic of an immunizing infection peaks when depletion of susceptibles (and consequent growth of herd immunity) drives R down to 1, but transmission then continues as incidence declines – leading to R decreasing to substantially below 1 by the end of the epidemic. Following the epidemic, herd immunity begins to decline (and R to increase) as new births replenish the susceptible population, but sustained transmission is only likely when R exceeds 1 once again.
The large-scale connectivity of human populations is the third key factor. Human mobility determines the chance an infection present in one location will be introduced elsewhere, fundamentally affecting early dynamics when spread is highly stochastic. While the seeding of infection in Brazil was a chance event (7), once a full-blown epidemic was underway, export of infections across the Americas was inevitable and rapid, leading to the widespread epidemics which unfolded from May 2015 (see SM).
Modeling gives insight into how the age distribution of infection will evolve over time – of particular relevance given the risk of congenital Zika syndrome and microcephaly. During the initial epidemic, we would expect all ages to be equally affected unless exposure and/or susceptibility vary substantially with age. The mean age of infection would then fall substantially in future epidemics, given the immunity acquired by older people through past exposure. However, our analysis suggests that this effect is unlikely to be sufficient to prevent an ongoing and substantial risk to pregnant women in future Zika epidemics (Fig. 1b lower inset and SM). This conclusion is supported by analysis of historical Zika seroprevalence data (8).
What should policymakers do?
Advising against pregnancy has been criticized for being infeasible for many women – especially long term (4). Our analysis suggests (Fig. 1b upper inset) that at the provincial scale, the timing of epidemic seeding is unpredictable but that the duration of the first wave of transmission is typically under 6 months. However in some locations the timing of virus introduction can interact with seasonality of transmissibility to extend a local epidemic over two transmission seasons. If recommendations to delay pregnancy were tuned to the local context and adapted in light of local surveillance data, in many areas they could be kept in place for a shorter time – making adherence more feasible while retaining the potential risk-reduction benefits. Local optimization of such control or risk-reduction measures requires timely availability of high-quality geographically stratified surveillance data.
Enhanced vector control is potentially beneficial, but it is critical for policymakers to set realistic expectations. Evidence (8, 9) suggests traditional insecticide-based control is rarely sufficiently effective to stop dengue epidemics. Effectiveness would need to be considerably higher to stop the first epidemic of a new virus in a naive population. But vector control with limited effectiveness could – if sustained – reduce the attack rates seen in the initial epidemic (see SM). Modeling suggests there are downsides, however. First, the epidemic may last longer, which might make it harder for women to adhere to recommendations delaying pregnancy. Second, the epidemic will overshoot the herd-immunity threshold by less than if interventions had not been introduced – leaving a smaller proportion of the population immune and reducing the delay until population susceptibility once again reaches levels which allow sustained endemic transmission (Fig. 1b and SM).
What is the likelihood that the virus will become endemic or that sporadic epidemics will occur with sufficient regularity to pose an equivalent risk? Our analysis suggests that once the current epidemic is over, herd immunity will lead to a delay of at least a decade before large epidemics may recur (SM). However, this prediction has caveats: the delay to resumption of transmission might be substantially reduced by high levels of spatiotemporal heterogeneity in exposure risk (not accounted for in our model) or transient reductions in transmission caused by interventions or population behavior change. In addition, our model makes the conservative assumption that flavivirus transmissibility in Latin America has not been anomalously high in the last 2–3 years (e.g. due to climactic conditions) and so predicts the virus will eventually become endemic. This does not imply predictable annual epidemics in all regions, but rather that sustained transmission would be expected somewhere in the continent every year – akin to what is seen for individual dengue serotypes today. However, if Zika transmissibility is strongly modulated by longer term climatic variation (such as El Niño), the virus may not be able to sustain endemic transmission, resulting in more sporadic but larger scale epidemics when reseeding of infection coincides with favorable conditions for transmission. Last, we have assumed a constant risk of reseeding of the infection into the human population; if a sylvatic reservoir for Zika is established in the Americas (8, 10), background levels of human exposure may increase.
A more precise assessment of long-term risks requires key data gaps to be filled. We need to measure the extent of (and geographic variation in) herd immunity in populations which have experienced recent Zika epidemics. Studies should not be restricted to Latin America. Currently, we cannot assess whether Asia is also at risk of a major Zika epidemic – or why the scale of transmission in Latin America has been so much greater than anything previously seen. Multiple hypotheses have been proposed (8) but cannot yet be tested: immunological enhancement from prior exposure to dengue, El Niño-driven climate effects, viral evolution and regional genetic differences in the Aedes aegypti populations may all play a role. While data are currently limited (11), cross-reactivity with dengue is a particular concern, as our analysis indicates both cross-protection and enhancement could shorten the time until epidemics can reoccur and increase the chances of long-term endemic transmission (Fig. 2). Age-structured seroprevalence surveys are a priority, using assays which can distinguish exposure to Zika from exposure to other flaviviruses. Such surveys allow estimation of variation in exposure with time and age, of interactions with other flaviviruses, and of overall transmissibility (8). Long-term cohort studies can provide longitudinal data to examine individual variation in exposure and clinical and immunological outcomes.
The traditional model for vaccine and antiviral efficacy trials used for endemic diseases poses major challenges for emerging infections with sporadic and unpredictable epidemics. While phase I safety studies do not require active transmission, efficacy studies do. Our analysis suggests there is limited time to initiate such studies in the current epidemic before incidence may be insufficient to measure impacts. Given the unpredictable timing and intensity of Zika outbreaks, future efficacy trials may need to be pre-approved in a large number of potential trial sites, then rapidly initiated in particular sites once local transmission has been detected. Efficacy studies for vaccines may need to recruit and vaccinate participants now and follow-up for a longer period than is typical. Active case detection in multiple sites over a long time period would be prohibitively expensive, so study protocols need to be adaptive – such as planning to start active surveillance in a trial site only when Zika transmission is detected, even if the outbreak occurs several years after vaccination took place. Evaluating rare endpoints such as microcephaly poses particular difficulties, requiring very large-scale trials if undertaken in advance of an epidemic, or accepting the risks associated with using a novel vaccine in pregnant women if undertaken in the face of an epidemic.
Like Ebola, Zika is another public health crisis where policymakers have had to make decisions in the presence of enormous uncertainty. In such contexts, it is natural to reach for policies which mirror those used previously. However, Zika and Ebola epidemiology and policy options differ fundamentally. The current epidemic is not containable; at best interventions can mitigate its health impacts. More optimistically, the natural dynamics of the epidemic are now likely to give a multi-year window to develop new interventions before further large-scale outbreaks occur.
Supplementary Material
Acknowledgments
The authors acknowledge funding from the UK Medical Research Council, the UK NIHR under the Health Protection Research Unit initiative, NIAID, NIGMS under the MIDAS initiative, the EU Framework 7 PREDEMICS project, the Bill and Melinda Gates Foundation and Imperial College’s Junior Research Fellowship program.
REFERENCES AND NOTES
- 1.Cauchemez S, et al. The Lancet. 2016 pii: S0140-6736. [Google Scholar]
- 2.World Health Organization. WHO Director-General summarizes the outcome of the Emergency Committee regarding clusters of microcephaly and Guillain-Barré syndrome. 2016 http://www.who.int/mediacentre/news/statements/2016/emergency-committee-zika-microcephaly/en/
- 3.World Health Organization. Mosquito (vector) control emergency response and preparedness for Zika virus. 2016 http://www.who.int/neglected_diseases/news/mosquito_vector_control_response/en/
- 4.L D, Schuck-Paim C, Simonsen L, Alonso W. PLoS Currents Outbreaks. 2016 doi: 10.1371/currents.outbreaks.7038a6813f734c1db547240c2a0ba291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tepper NK, et al. MMWR Morb Mortal Wkly Rep. 2016;65:311–314. doi: 10.15585/mmwr.mm6512e1. [DOI] [PubMed] [Google Scholar]
- 6.Johansson MA, Dominici F, Glass GE. PLoS Negl Trop Dis. 2009;3:e382. doi: 10.1371/journal.pntd.0000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faria NR, et al. Science. 2016;352:345–349. doi: 10.1126/science.aaf5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lessler J, et al. Science. 2016 [Google Scholar]
- 9.Achee NL, et al. PLoS Negl Trop Dis. 2015;9:e0003655. doi: 10.1371/journal.pntd.0003655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musso D, Gubler DJ. Clin Microbiol Rev. 2016;29:487–524. doi: 10.1128/CMR.00072-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dejnirattisai W, et al. Nat Immunol. 2016 doi: 10.1038/ni.3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


