Abstract
The proximal aorta acts as a coupling device between heart and brain perfusion, modulating the amount of pressure and flow pulsatility transmitted into the cerebral microcirculation. Stiffening of the proximal aorta is strongly associated with age and hypertension. The detrimental effects of aortic stiffening may result in brain damage as well as heart failure. The resulting cerebral small vessel disease and heart failure may contribute to early cognitive decline and (vascular) dementia. This pathophysiological sequence of events underscores the role of cardiovascular disease as a contributory mechanism in causing cognitive decline and dementia and potentially may provide a starting point for prevention and/or treatment.
Magnetic resonance imaging (MRI) is well suited to assess the function of the proximal aorta and the left ventricle (e.g. aortic arch pulse wave velocity and distensibility) as well as the various early and late manifestations of cerebral small vessel disease (e.g. microbleeds and white matter hyperintensities in strategically important regions of the brain). Specialized MRI techniques are explored for diagnosing preclinical changes in white matter integrity or brain microvascular pulsatility.
This review discusses the potential role of MRI for evaluating the pathophysiology of proximal aortic function with aging and the interaction between aortic function and brain perfusion, as a contributing mechanism to cerebral small vessel disease, resulting in early cognitive decline and vascular dementia.
Introduction
Successful treatment of vascular risk factors and cardiovascular disease may explain the declining incidence of dementia over the last decades.1 Apparently, earlier diagnosis and more effective treatment of stroke and heart disease have contributed to a lower incidence of dementia, including vascular dementia and Alzheimer type dementia.1 Interestingly, dementia shares many risk factors with coronary artery disease, which has shown a similar declining trend over time.2
Multiple vascular risk factors, including apolipoprotein E (APOE) ε4 status, prevalent cardiovascular disease (CVD), transient ischemic attacks, stroke and heart failure, may contribute to the spectrum of cognitive dysfunction, ranging from early memory loss and mild cognitive decline to dementia. In turn, heart failure may lead to decreased cardiac output and consequently to early executive brain dysfunction, whereas treatment of heart failure may reverse cognitive decline as shown in patients who underwent cardiac transplantation, ventricular resynchronization therapy and physical training to improve heart function.3–6 In addition, neurodegenerative disease and dementia may also be associated with activated systemic inflammation in diabetes mellitus, hyperlipidemia, atherosclerosis, hypertension, obesity, metabolic syndrome and infection, presumably by inducing vascular changes and activation of microglia cells in the brain.7
These multiple risk factors may enhance aortic stiffening as an independent and key determinant of brain structure and cognitive function.8 Age and hypertension strongly contribute to vascular stiffening with probably only modest contribution from other CVD risk factors, resulting in subclinical target organ damage, like brain and heart disease.9, 10 Moreover, amyloid-β brain burden as a hallmark of Alzheimer’s disease is related to a number of cardiovascular risk factors, in particular pulse pressure and systolic hypertension as markers of arterial stiffness.11, 12 Hypertension and arterial stiffness are strongly associated with progressive amyloid deposition in the brain.13–15 Accordingly, the term “pulse wave encephalopathy” has been proposed to explain the common vascular pathophysiology that may underlie Alzheimer’s disease and vascular dementia.16
Although diagnostic criteria have been proposed to classify cognitive disorders as probably of vascular in origin, there is a need for well-defined imaging biomarkers for better prediction of cognitive disorders. Magnetic resonance imaging (MRI) is a relatively new tool for comprehensive evaluation of cardiovascular function in conjunction with vascular brain pathology.17 Many important concepts and observations linking CVD and manifestations of vascular brain disease and cognitive dysfunction are based on other imaging modalities, in particular applanation tonometry.18–20 However, MRI is a well established and validated tool to diagnose many manifestations of brain pathology in dementia and other neurological diseases. Furthermore, cardiovascular MRI has been firmly established as a reliable and accurate tool to diagnose many aspects of CVD, such as regional function and structure of the proximal aorta, which may be more difficult to assess and quantify with ultrasound methods. Overall, multi-organ MRI is an expanding and promising approach for comprehensive evaluation of systemic disease processes that may involve different organs.21
Based on the cardiovascular dementia hypothesis, the purpose of this review is to discuss the potential role of MRI for assessing the interaction between the proximal aorta and heart and brain and how this interaction may contribute to cerebral small vessel disease and cognitive decline with aging.
Proximal aortic structure and function: a coupling device between heart and brain perfusion
Pathophysiology
Aortic stiffening is considered one of the earliest manifestations of changes that affect the structure and function of vessel wall integrity with aging. Applanation tonometry is the gold standard for global pulse wave velocity (PWV) assessment owing to its ease of use and superior temporal resolution. Many seminal studies using this imaging method have shown that pulsatile components of blood pressure may be more relevant for prognosis than traditional estimates of mean arterial blood pressure, which is a common target for antihypertensive therapy.18 The Framingham Heart Study has shown that higher forward pressure wave amplitude as a measure of proximal aortic geometry and stiffness, is associated with increased risk for incident CVD, whereas mean arterial pressure and relative wave reflection, as measured by applanation tonometry, were not related to events.22 Of note, these observations may be relevant to understand the potential adverse effect of vasodilatation in antihypertensive therapy, by which increased cardiac output and peak flow may inadvertently increase forward pressure wave amplitude and may also increase penetration of excessive pressure and flow pulsatility into the cerebral microcirculation. Furthermore, recently it was shown that in healthy and relative young adults, increased arterial stiffness assessed by applanation tonometry is superior to blood pressure in predicting cognitive decline in all domains, underscoring the relevance of arterial stiffness as a potential target for therapy.19
The proximal aorta acts as a buffer and cushion for left ventricular systolic load, by which ventricular-aortic coupling between the left ventricle and the elastic proximal aorta and carotid circulation is modulated. Aortic stiffness in combination with stroke volume and ejection velocity is a determinant of arterial pressure wave amplitude, influencing systolic and diastolic blood pressure and pulse pressure.
Left ventricular ejection pattern, aortic wall stiffness and aortic lumen diameter are interrelated and interact resulting in altered hemodynamics and hypertension. For example, systolic blood pressure could be normal in the presence of a stiffened aortic wall if mean arterial pressure is normal or left ventricular peak ejection rate and aortic lumen area are optimized to maintain a low pulse pressure. Conversely, higher mean arterial pressure may increase aortic stiffness in the presence of midlife hypertension due to the effects of distending pressure alone without necessary intrinsic aortic wall abnormalities. 23
The proximal aorta plays a critical role in the transformation of intermittent, highly pulsatile flow from the heart into steady, minimally pulsatile flow throughout the cardiac cycle into the brain microcirculation (Figure 1 A and B, Table 1). The transformation from highly pulsatile to steady flow is achieved by interposing a series of progressively stiffer arteries between heart and microcirculation. In healthy young adults, the aorta is highly compliant whereas the first generation conduit arteries arising from the aorta are relatively stiff (Table 1). The interface between compliant aorta and stiff muscular arteries represents impedance mismatch, which gives rise to wave reflection proportional to the degree of mismatch. Wave reflection in the arterial system is often portrayed as deleterious based on the largely incorrect hypothesis that premature wave reflection is responsible for most of the increase in pulse pressure that occurs with advancing age after midlife.24 However, wave reflection reduces the amplitude of the transmitted flow waveform without affecting mean flow and thereby plays a critical role in the transformation from pulsatile central to steady peripheral flow.8, 25 When the proximal aorta stiffens, the amount of impedance mismatch between aorta and carotid arteries is reduced; the resulting impedance matching reduces wave reflection and thereby increases the amount of pulsatile power that is transmitted into the microcirculation.
Figure 1. A, B and C. The aging cardiovascular system interacting with brain pulsatility and perfusion.
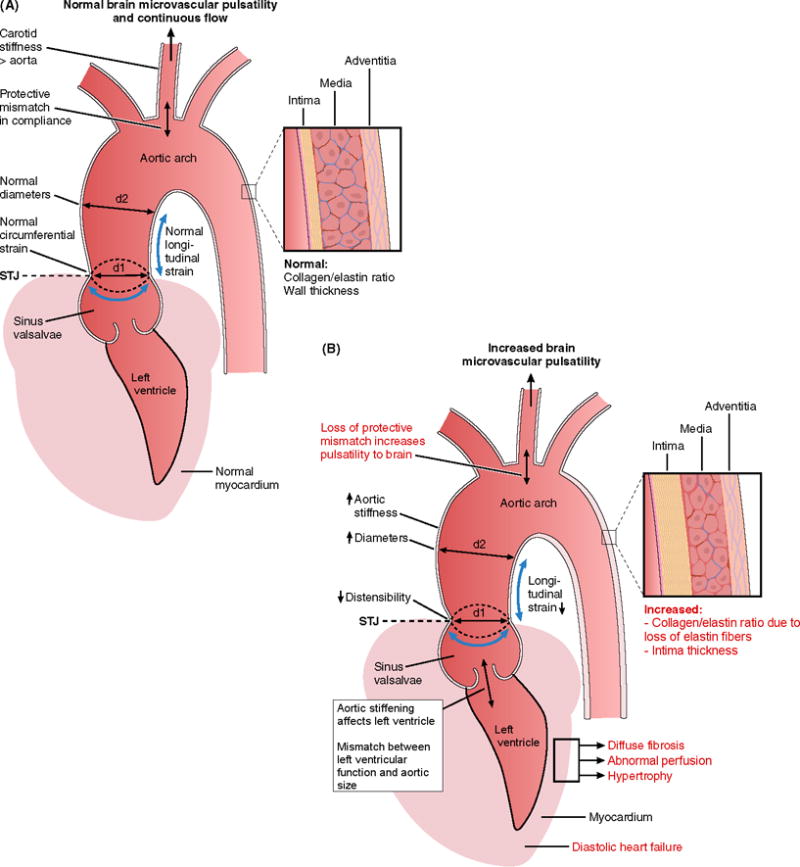
Relationship between proximal aortic stiffness, left ventricular function and brain microvascular pulsatility across the spectrum of age from young (<20 years, Panel A) to older (>70 years, Panel B). The pathophysiological changes with aging contribute to increasing microvascular brain pulsatility that may promote a spectrum of microstructural and macroscopic brain disease processes (Panel C) often associated with cognitive decline and dementia.
The aortic wall may thicken and stiffen due to various processes with aging, including intimal thickening, elastin loss in the media, increased collagen deposition, cellular hyperplasia and fiber cross linking. Furthermore, aortic root mobility may decrease with aging and may be characterized by three-dimensional strain as illustrated at the sinotubular junction (STJ) in axial (d1), circumferential and longitudinal components. Note normal flaring out of proximal aorta (d1<d2). At young age the muscular carotid artery has higher stiffness than the aortic arch protecting against transmission of high pulsatility of aortic arch pressure into the brain microcirculation (protective mismatch between aortic and carotid stiffness). With aging aortic stiffness and carotid stiffness equalize transmitting high pulsatility to the brain (loss of protective mismatch). Increased aortic stiffening may also affect myocardial structure and function, leading to diffuse interstitial myocardial fibrosis, replacement scar, decreased myocardial perfusion, hypertrophy and finally heart failure. In turn, heart failure may contribute to decreased brain perfusion and further neurological deterioration. At a young age the stroke volume of the left ventricle matches aortic luminal dimensions. With aging an increase in stroke volume (e.g. in obesity) may cause a mismatch between stroke volume and relative small aortic diameter, thereby increasing pulse pressure and contributing to development of systolic hypertension.
Table 1.
Physiological changes with aging in aortic structure and function affecting microvascular brain pulsatility and heart function.
| YOUNG | OLDER |
|---|---|
|
| |
| - Normal collagen/elastin ratio | - Increasing collagen/elastin ratio |
| - Normal wall thickness | - Wall thickening due to elastin loss, collagen deposition, cell hyperplasia, cross linking |
| - Compliant proximal aorta | - Stiffening of proximal aorta |
| - Normal mobility of aortic root, in 3 dimensions (3D strain) | - Decreased aortic root mobility |
| - Carotid artery stiffness larger than aortic arch stiffness protects the brain against pulsatilty (protective mismatch) | - Aortic arch stiffness equalizes with carotid stiffness. Detrimental for brain, transmitting higher pulsatility. |
| - Normal brain microvascular pulsatility | - Increased microvascular brain pulsatility |
| - Matching LV stroke volume and aortic diameter | - Mismatch between increasing stroke volume and relative small aortic diameter enhancing aortic stiffness, increasing pulse pressure and resulting in systolic hypertension |
| - Normal myocardium | - Myocardial hypertrophy, diffuse fibrosis, decreased perfusion |
| - Normal systolic and diastolic LV function | - Heart failure due to proximal aortic aging and changing physiology |
|
| |
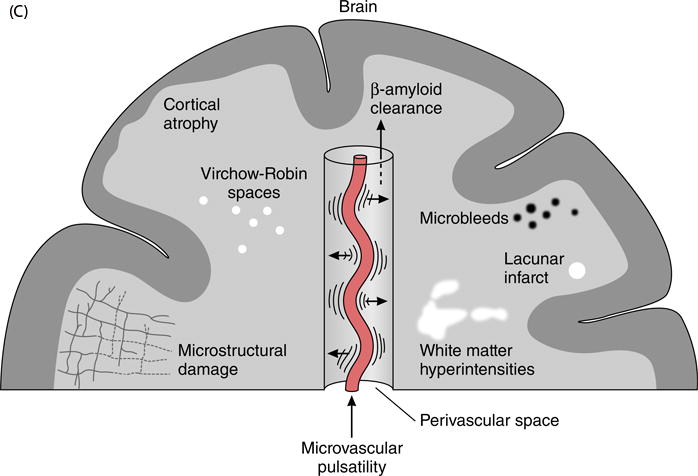
Aortic stiffening is particularly deleterious to high flow organs, such as the brain (Figures 1C and 2) and kidneys, or in the presence of high-flow states, such as diabetes, because high flow is necessarily associated with low microvascular impedance. Impedance mismatch between conduit vessels and microvascular resistance vessels represents the last line of defence against excessive pressure and flow pulsatility. In the presence of obligate high flow (low impedance), as is the case in the brain and kidneys, this last line of defence is compromised, resulting in greater transmission of potentially harmful pulsatility into the microcirculation and veins. Microvascular remodelling in response to excessive aortic stiffness may limit the amount of pulsatility that penetrates into the microcirculation.25 However, remodelled resistance vessels may have impaired reactivity.26 Aortic stiffness is also associated with blood pressure lability, which, in presence of impaired microvascular reactivity, may be associated with frequent episodes of transient brain ischemia that produce tissue damage over time. Thus, excessive aortic stiffness represents a uniquely toxic insult on the brain.
Figure 2. A–D. Common brain MRI manifestations of small vessel disease.
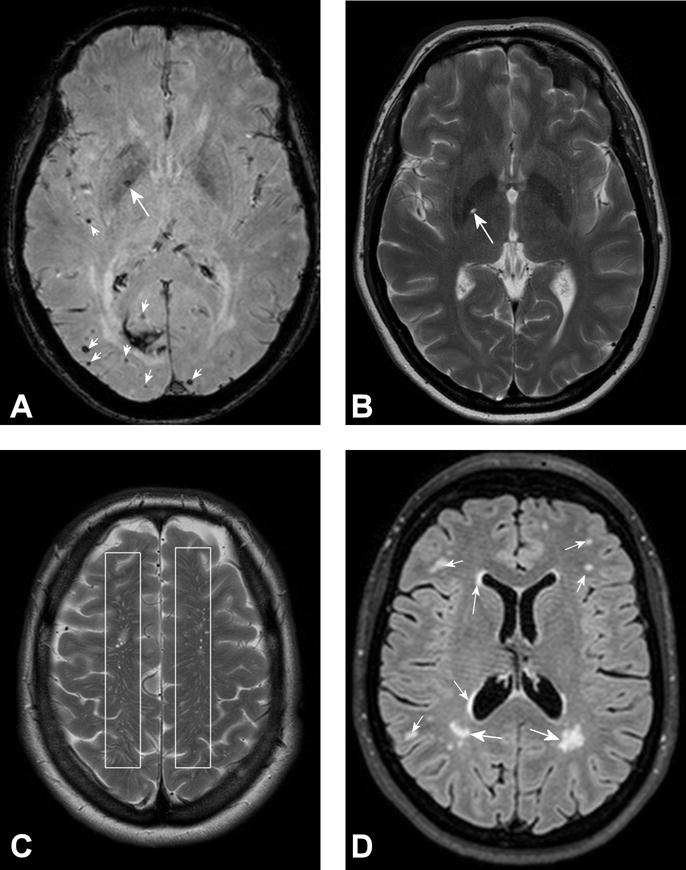
A. Microbleeds visualized as dark foci due to haemorrhage on susceptibility T2* MRI. Microbleed in basal ganglia (large white arrow in A) and number of microbleeds in parenchyma (small white arrows in A). B. Lacunar infarct is seen as bright spot in basal ganglia on T2-weigthed MRI scan (CSF is bright). C. Multiple enlarged Virchow-Robin spaces seen as small bright dots and linear structures (between boxes in C) on T2-weigthed MRI scan (CSF is bright). D. White matter hyperintensities throughout the brain and in periventricular location visualized as irregular shaped bright areas (arrows in D) on FLAIR MRI scan (CSF is dark).
Alterations in stiffness and geometry of the proximal aorta increase load on the left ventricle (LV) through various mechanisms. Stiffening of the aortic wall and mismatch between aortic diameter and flow can increase the amplitude of the forward pressure wave generated by a given LV flow wave.27 Note that mismatch between aortic diameter and flow does not mean that the aorta has gotten smaller in individuals with higher pulse pressure; many studies have shown that aortic lumen diameter increases monotonically across the full lifespan.28 Mismatch represents a failure of aortic lumen diameter remodelling to keep up with increasing flow and wall stiffness. The size of the aorta and amount of elastic fibers in lamellar units, and hence the capacity of the aorta to remodel, are determined genetically and established early in life.29, 30 This fixed pool of elastic fibers in the aorta remodels across the full lifespan to accommodate growth in childhood and increasing adiposity with advancing age in adulthood. Characteristic impedance of the aorta, which represents the relation between pulsatile pressure produced by a given pulsatile flow in the absence of wave reflection, is related directly to aortic wall stiffness and strongly inversely to aortic lumen diameter. If the aorta remodels in response to higher cardiac output, as may occur in the presence of obesity, the fixed volume of elastic fibers in the wall of the aorta will be thinned and will also be under higher tension because of greater lumen diameter. As a result, wall stress and strain will increase, additional collagen will be engaged to bear the load and wall stiffness will increase markedly as collagen is several orders of magnitude stiffer than elastin. If the increase in aortic lumen area is less than the associated increase in peak volumetric flow rate in the proximal aorta, pulse pressure will rise. Importantly, the LV may have a greater capacity than the aorta to remodel in order to accommodate demand for higher flow, resulting in an increase in pulse pressure in those individuals with relatively smaller aortic lumen size.27
The resulting increase in forward pressure wave amplitude increases systolic and pulse pressure and has been show to explain most of the variance in central and peripheral pulse pressure across the full human lifespan.24 Higher forward wave amplitude is associated with increased risk for major CVD events in models that adjust for standard CVD risk factors, including systolic blood pressure and PWV (primarily a measure of aortic wall stiffness). Widening of pulse pressure increases load on the LV and represents a form of abnormal hemodynamic coupling between LV and aorta.
In addition, in the presence of a stiffened proximal aorta, greater force is required to stretch the ascending aorta during systole. Since LV longitudinal shortening supplies this stretching force, proximal aortic stiffening loads the LV, particularly along the longitudinal axis, and represents a form of abnormal mechanical coupling between LV and aorta that contributes to a component of LV hypertrophy that is independent of hemodynamic load.31 If aortic stiffening impairs LV longitudinal shortening, resulting in diminished aortic stretch in systole, there will be a reduction in aortic recoil in early diastole and a commensurate reduction in early diastolic filling and a shift to enhanced late diastolic (atrial systolic).31 The reduction in longitudinal aortic stretch and recoil and related reduction in early diastolic filling may explain inverse relations between higher aortic PWV and lower early diastolic mitral annulus tissue Doppler velocity32 and may contribute to the pathogenesis of heart failure with preserved systolic function.
LV-aorta interaction
Mismatching of ventricular-aortic coupling may result in higher pulse pressure in older people. Regional and global aortic PWV increases monotonically across the human lifespan. In contrast, pulse pressure and aortic characteristic impedance fall modestly with age prior to midlife and then increase dramatically thereafter, particularly in older women. Dissociation between PWV and characteristic impedance is possible because of their differing relations with aortic lumen diameter, which also increases monotonically with age across the full human lifespan. Characteristic impedance has a 5-times greater sensitivity to diameter as compared to PWV, which is only modestly affected by lumen diameter. As a result, variable relations between PWV and characteristic impedance or pulse pressure represent the result of the nonlinear interplay between wall stiffness, which affects both measures similarly, and lumen diameter, which has a much greater effect on characteristic impedance. The concept that higher pulse pressure in older people may be associated with smaller aortic lumen area, greater aortic wall stiffness and thickness and larger ventricular mass and end-diastolic volume was shown in a recent MRI study.27 It has been suggested that the heart may have a greater ability than the aorta to accommodate changes in cardiac output with aging, resulting in dissociation of the heart and aorta to accommodate changing hemodynamics.
Aortic arch stiffness may not only interact with the cerebral circulation, but also with coronary flow and left ventricular function.33 Aortic arch function may act as a coupling device between the heart and the arterial system. Age-related vascular stiffening may be coupled with changes in left ventricular stiffness and diastolic compliance (“aorta-ventricular coupling disease”) (Figure 1A). Aortic stiffness and hypertension will increase the afterload on the left ventricle, leading to left ventricular hypertrophy, diffuse myocardial fibrosis, systolic and diastolic dysfunction. In particular, early diastolic dysfunction of the left ventricle has been recognized as a risk factor for developing cognitive dysfunction. Furthermore, a significant relationship between the size of the left atrium and brain white matter disease as well as cognitive decline in elderly subjects has been recognized.34 Moreover, atrial fibrillation as a frequent complication in heart failure is associated with both vascular and Alzheimer dementia.35 Heart failure may lead to decreased cardiac output and consequently to early executive brain dysfunction and cerebral white matter disease. However, commonly used estimates of left ventricular systolic function like ejection fraction appear to be of limited value to predict vascular brain alterations.36 Direct estimates of stroke volume and hence cardiac output appear to be more reflective of systemic blood flow and associated vascular brain damage.
Reduced cardiac output has been posited as a primary or direct pathway of brain injury independent from shared risk factors, although the human evidence linking systemic blood flow to cerebral blood flow is still limited. In this respect, MRI has a particular advantage by providing direct estimates of stroke volume and cardiac output as derived from velocity-encoded MRI of aortic flow or cine MRI volumetrics of the left ventricle. It has been speculated that intermediate pathways like vascular stiffness or neurohumoral factors may also contribute to both reduced cardiac output and microvascular brain damage. Cardiovascular changes like (subclinical) reduced cardiac output and microemboli may co-occur with Alzheimer’s pathology and may accelerate the progression of disease, thereby fading the distinction between Alzheimer’s and vascular dementia (“mixed dementia”).37
Importance of cerebral small vessel pulsatility
Vascular risk factors may lead through various mechanisms and pathways to brain damage. Aortic stiffness and pressure and flow pulsatility contribute to the development of cerebral small vessel disease, which underlies cognitive decline with aging.38 Putative mechanisms of interaction may occur between the aortic arch and carotid vessels, including loss of a protective mismatch in impedance between aortic arch and carotid arteries as aortic stiffening exceeds carotid stiffening with aging, thereby diminishing wave reflection and enhancing transmission of higher pressure and flow pulsatility into the microcirculation of the brain (Figure 1).8 The higher pulse pressure and higher pulsatility due to aortic stiffening may cause brain damage in specific brain regions like the basal ganglia, which are supplied by short, perforating arteries that arise from the proximal middle cerebral artery (Figure 2A). With increasing age, the brain is exposed to higher pulsatile flow, which penetrates deeper into the low impedance cerebral arterial microvasculature. Cerebral arterial function may also be dependent on the properties of arteries, type of artery, vascular geometry and vascular responsiveness to pulsatile pressure, leading to loss of dampening capacity of cerebral vasculature to increasing pulse pressure.39 Elevated arterial stiffness and pressure pulsatility are associated with longitudinal progression of subclinical vascular brain injury and greater neurocognitive decline in middle-aged and older subjects.40
Basic concepts and MR imaging techniques
Basic concepts pulse wave velocity
The basic concepts of pulse wave velocity analysis have been developed over 100 years ago by physiologists laying the groundwork for today’s applications in medicine.41 Under assumption of a non-viscous fluid and the ratio between vessel wall thickness and luminal diameter being constant, the Moens-Korteweg equation showed the relation between PWV and elasticity modulus: PWV equals the square root of the ratio between the incremental Young’s modulus times vessel wall thickness over the diameter times blood density (Figure 3). The Bramwell-Hill model, described in 1922,42 theoretically links PWV, vessel strain, pulse pressure and blood density together as derived from the Moens-Korteweg equation: under the modeling assumptions of the arterial vessel wall being small compared to the lumen and blood being incompressible and nonviscous, the arterial PWV equals the inverse square root of arterial distensibility times blood density. Nowadays, application of these models enables an estimation of the incremental Young’s modulus of the arterial vessel wall, or of local arterial blood pressure using noninvasive measurement tools such as velocity-encoded MRI (Figures 4 and 5).43, 44
Figure 3. Distensibility and Pulse Wave Velocity (PWV) in proximal aorta and carotid vessels estimated by MRI.
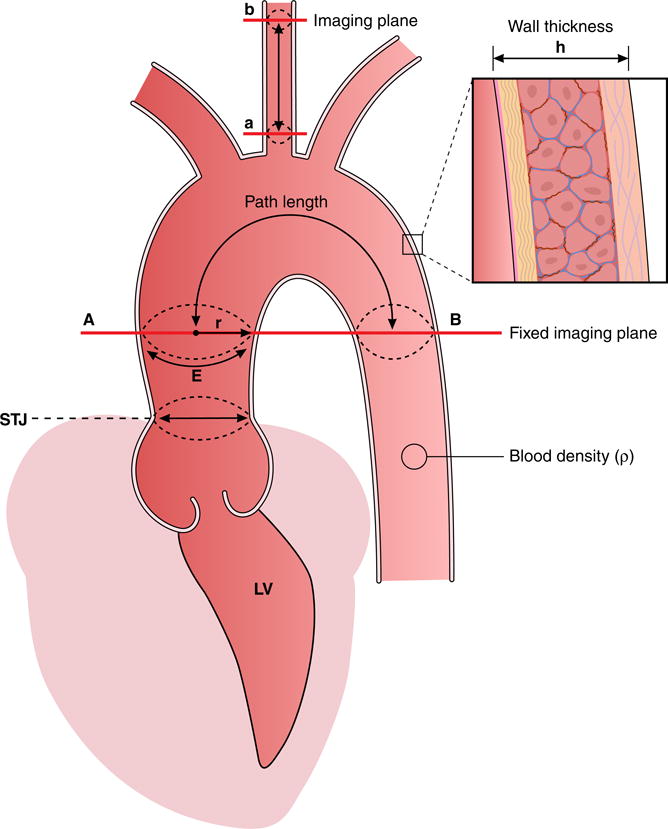
The luminal area change or diameter change of the aorta at the STJ determines local distensibility, according to the formula: Distensibility = (Amax − Amin)/(Amin × PP) (in mmHg−1), where PP is central pulse pressure and Amax and Amin the maximal and minimal cross-sectional area, respectively.
A fixed MRI imaging plane at the level of the ascending aorta (A) and transecting also the proximal descending aorta (B) is used to estimate aortic arch PWV. The path length Δx (in meters) between level A and B is divided by the difference in arrival time Δt (in seconds) between flow curves measured by velocity-encoded MRI at level A and B simultaneously to calculate PWV (expressed in meters/second). Vascular pulse wave velocity is determined by: 1. E (circumferential Young’s modulus, representing material rigidity); 2. h (vessel wall thickness); 3. r (luminal aortic radius); 4. rho ρ (blood density). The relation between these parameters is represented in the Moens-Korteweg equation, according to the formula: PWV = √(E×h / 2r×ρ). PWV and distensibility are inversely related to one another as defined by the Bramwell-Hill equation: PWV = √(1/(ρ×Distensibility)). These factors interact with LV function (e.g. increased cardiac output in obesity) resulting in a stiffer proximal aorta (PWV>) as a consequence of more collagen and less elastin in aortic wall (E>), increased wall thickness (h>), and/or change in aortic radius (> decreases PWV, < increases PWV). When aortic diameter is relatively small as compared to LV function with increased aortic flow and cardiac output, aortic flow and increased aortic stiffness cause increased forward pressure wave amplitude, subsequently resulting in increased pulse pressure and systolic hypertension. Similarly to aortic arch stiffness, carotid artery stiffness can be estimated from 2 imaging levels (a and b) using velocity-encoded MRI to measure the path length between a and b as well as the difference in arrival time between flow curves.
Figure 4. Assessment of pulse wave velocity over the ascending aorta and the aortic arch.
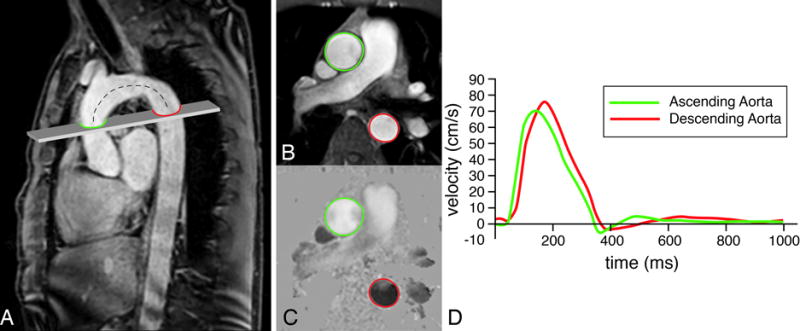
A high-temporal velocity-encoded MRI acquisition plane is positioned perpendicular to the ascending aorta (A), transecting both ascending (in green) and descending aorta (in red). From through-plane velocity encoding, velocity mapping is performed after segmentation in anatomical (B) and velocity images (C). Velocity-time curves are determined (C) and the pulse wave velocity is calculated from the distance Δx between both measurement sites (determined along the centerline of the aorta, dashed line) and the transit time Δt of the onset of the systolic velocity wavefront, measured at each site: PWV = Δx/Δt (in m/s). In this example of a healthy 47 year male volunteer, Δx = 11.5 cm, Δt = 18 ms, resulting in a PWV of 6.4 m/s.
Figure 5. Assessment of pulse wave velocity of the left carotid artery.
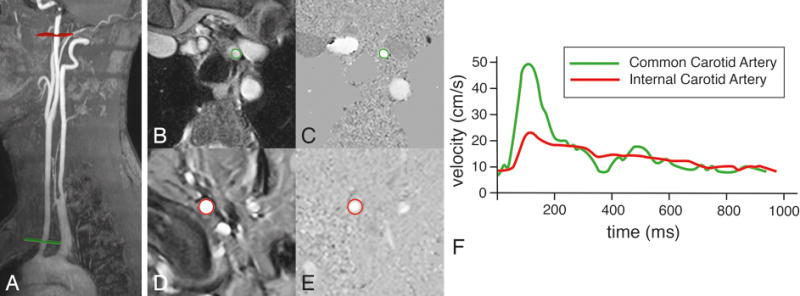
Two acquisition planes are positioned, one perpendicular to the common carotid artery just above the aortic arch (in green) and one perpendicular to the internal carotid artery just proximal to the petrous portion of the artery (in red). Velocity mapping is performed after segmentation in common carotid artery (B and C) and the internal carotid artery (D and E). Velocity-time curves are determined (F) and the pulse wave velocity is calculated from the distance Δx between both measurement sites (determined along the centerline of the vasculature) and the transit time Δt of the onset of the systolic velocity wavefront, measured at each site: PWV = Δx/Δt (in m/s). In this example of a healthy 47 year male volunteer, Δx = 19.6 cm, Δt = 25 ms, resulting in a PWV of 7.8 m/s.
MRI measurement of aortic function
MRI may provide an alternative method to estimate vascular function regionally and in different vascular beds simultaneously, thereby allowing evaluation of organ interaction and vascular physiology in a comprehensive way. However, MRI is expensive, not widely available, and technically demanding in terms of image acquisition and image analysis tools. On the other hand, the potential utility appears quite unique in terms of direct unlimited vascular access, unsurpassed reproducibility and dimensional accuracy, and the available wide array of MRI methods for visualizing brain and CVD processes unseen with other imaging modalities. However, despite these potential advantages large, prospective studies will be required to demonstrate the additional and incremental utility of MRI for assessing cardiovascular function and cerebral vascular disease manifestations in a comprehensive imaging session.
MRI allows an exact assessment of the aortic anatomy, independent of acoustic windows and is therefore an optimal tool to detect wall thickening, dilatation and aneurysm formation.45 Accurate MRI measurements of the mechanical and functional properties of the proximal aorta are a prerequisite for elucidating pathophysiological changes (Figures 3 and 6). Simple diameter or area measurements of aortic size are commonly performed perpendicular to the flow direction in double angulated views to optimize measurement precision. During follow-up studies of aortic dimensions, it is mandatory to carefully ascertain that similar levels of the aorta are being measured to optimize precision and to detect changes in size over time. The aneurysmal aorta grows slowly, approximately 1 mm per year on average, requiring high precision of measurements to detect significant changes.46 A number of confounders has to be controlled for in order to obtain consistent estimates of aortic dimensions and function, such as definition of diameter measurements (e.g. inner wall to inner wall or outer wall to outer wall measures), to account for diameter changes over the cardiac cycle (systolic versus diastolic diameter), and to correct for through-plane motion of the aortic root over the cardiac cycle (longitudinal displacement). It has been recognized that the elastic proximal aorta shows displacement over the cardiac cycle varying with systolic contraction and diastolic relaxation in both longitudinal and circumferential directions. Aortic circumferential strain is estimated by determination of lumen area changes over the cardiac cycle. The sinotubular junction (serving as a marker in the proximal aorta) shows marked displacement over the cardiac cycle, along the long axis of the aorta as shown by MRI due to longitudinal strain (Figure 6). In turn, variation in longitudinal strain may affect the accuracy of estimating circumferential strain, which results in erroneous estimates of proximal aortic stiffness if uncorrected, particularly in women who have generally greater longitudinal strain than men.47 Combining measurements of circumferential aortic strain with central pulse pressure provides an estimate of local aortic distensibility. Several other indices for arterial wall stiffness have been proposed, such as compliance and the stiffness index, which are based on the pressure-diameter relationship of arterial distention.48 Arterial compliance is defined by the absolute change in lumen area over the pulse pressure that is required for distension, whereas distensibility is defined as the relative change in vessel diameter or lumen area over local pulse pressure.
Figure 6. Aortic root motion and expansion during the cardiac cycle.
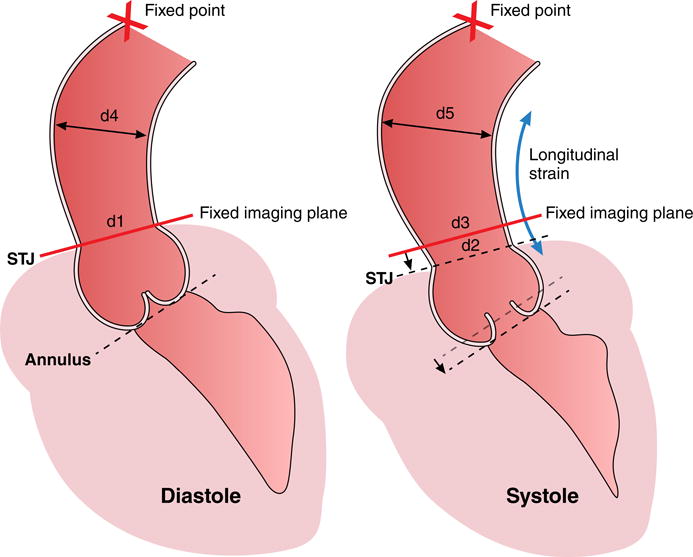
The sinotubular junction (STJ) and annulus show longitudinal strain from diastole to systole as compared to a relative stationary point at the level of the aortic arch (X=fixed point). A fixed imaging plane (as used in MRI) at the level of the STJ in diastole will intersect the ascending aorta more cranially to the STJ in systole due to longitudinal strain of the aortic root. The section of aorta that lies within the diastolic and systolic image planes is flaring out (d4>d1 in diastole and d5>d2 and d3>d2, respectively in systole), leading to overestimation of strain if through-plane motion is ignored. The fixed imaging plane would compute strain as (d3 - d1)/d1. The correct calculation should take (d2 - d1)/d1 and then adjust circumferential strain for longitudinal strain.
A correction for longitudinal strain is not really relevant to PWV but is highly relevant to cross-sectional compliance. Most studies measure the aortic arch length in late diastole, when the aorta is relative static. The arch will get a bit longer in systole, but assuming there is 10% stretch of the ascending segment only (and possibly even a bit of compression of the descending segment since the root is being pulled downward), the error in transit distance will be small (less than half of 10%), so maybe a 5% error in resulting PWV. Furthermore, the foot of the aortic flow wave (used in the transit time method) propagates effectively at diastolic pressure, therefore the diastolic length of the arch is used to compute PWV. On the other hand, if raw circumferential strain is 5% and there is 10% longitudinal strain in the ascending aorta, the true effective circumferential strain would be 5% + (10%/2) = 10%, meaning this results in 100% error.
Estimates of aortic PWV generally are based on measurements that span the length of the aorta and hence represent global stiffness of the full aorta. Aortic PWV is defined as the velocity of the systolic pulse wave front propagating through the arterial system and is assessed by registering flow waves at multiple positions along the arterial path length. The incremental Young’s modulus, however, represents the local mechanical elastic properties of tissue defined by the non-linear relationship between stress (i.e. force per unit area) and strain (i.e. proportional deformation). This local measure can depict more subtle, regional changes in the relative proportions of the constituents of the arterial wall.49 Beware that Young’s modulus in older people can be misleading if the wall thickens because of expansion of non-load bearing elements (e.g. in the intima), Young’s modulus will be underestimated because the wall thickness that implicitly appears in the denominator will be inappropriately large.
MRI with velocity encoding provides an alternative method for estimating vascular PWV locally in various segments of the aorta as well as in other vascular territories, allowing direct measurements of the path length, even in tortuous and elongated vessels, without geometric assumptions (Figures 3, 4 and 5). Temporal resolution of commonly-used high-temporal velocity-encoded MRI sequences is on the order of 5 ms, allowing reliable PWV measurements locally in the aortic arch (~10 cm length) of up to 10 m/s according to the Nyquist–Shannon sampling theorem. Temporal resolution may be increased when using one-dimensional scanning and Fourier velocity-encoding50; however, this approach is not available on all MRI platforms. Several algorithms have been evaluated with respect to accuracy and consistency of aortic PWV assessment51, 52, but the transit-time foot-to-foot definition is currently the mostly used and validated method.53
Aortic arch PWV as measured by MRI was the best method to predict aging across the full age range, not confounded by other factors as is the case for applanation tonometry. Furthermore, it may be important to recognize regional heterogeneity of aortic stiffness with aging for different aortic locations (e.g. aortic arch versus abdominal aorta). It has been shown by MRI that the greatest difference in systolic diameter and length between old and young subjects occurs in the aortic arch, which may be a compensatory mechanism to increase capacitance when the wall stiffens.54 Also more pronounced age-related increase of wall stiffening in the proximal aorta has been demonstrated by MRI in patients with Marfan syndrome, suggesting accelerated media degeneration in the proximal aorta.55 MRI with in-plane velocity-encoding proved to be the optimal approach for evaluating Bramwell-Hill associations between local aortic arch PWV and aortic distensibility.44
MRI of brain small vessel disease and aortic function
MRI is the primary imaging modality to assess the various imaging manifestations of cerebral small vessel disease related to increased small vessel pulsatility, such as microstructural changes in the white and gray matter, enlargement of perivascular spaces, small areas of hemorrhage adjacent to small arteries (so-called microbleeds), lacunes in sensitive deep brain structures, white matter hyperintensities (WMHs), small subcortical brain infarcts and generalized brain atrophy (Figures 1B and 2, Table 2).40 Standards for image interpretation, MRI image acquisition and reporting have been proposed using consistent terminology and definition of the various manifestations of cerebral small vessel disease.56 Elevated arterial pulsations may cause a water hammer mediated repetitive injury to the brain, resulting in leukoaraiosis (i.e. periventricular and subcortical WMHs), “état criblé” (i.e. dilatations of Virchow-Robin spaces), gliosis, demyelination, and failure to clear perivascular spaces from beta amyloid (in particular implicated in Alzheimer’s disease progression).57 The glymphatic pathway is a key modulator to the clearance of soluble beta amyloid from the brain interstitium and it has been proposed that failure of this clearance may be a driver of Alzheimer’s disease progression.58–60 Conceivably a cascade of increasing brain damage related to small vessel pulsatility and decreased brain perfusion starts at an early age and progresses throughout life depending on risk factors that modulate aortic stiffness and may result in early subclinical brain deficits, finally leading to cognitive decline and dementia (Table 2). In older persons, aortic stiffness is associated with remodelled cerebral microcirculation, microvascular parenchymal brain damage and lower performance on memory tests.61 White matter tracts and hippocampus may be important in maintenance of memory and share a susceptibility to pulsatile microvascular damage due to perfusion by short branches that arise from the circle of Willis.
Table 2.
Spectrum of brain damage related to small vessel disease.
| Type of brain damage | Prefered location(s) | Pathophysiology | MRI application |
|---|---|---|---|
| Virchow-Robin spaces | Putamen, globus pallidus, centrum semiovale | Increased vessel pulsatility | 3d-T1-w |
| Microbleeds | Basal ganglia | Increased vessel pulsatility | Susceptibility T2* MRI |
| Lacunes | Periventricular, deep white matter, basal ganglia | Low flow | T2 MRI/FLAIR |
| Microinfarcts | Gray/white matter | Low flow | T2 MRI/FLAIR |
| White matter Hyperintensities | Periventricular white matter Deep white matter | Low flow | T2 MRI/FLAIR |
| (Sub) Cortical atrophy | Hippocampus Cortex | Low flow | 3d-T1-w |
| White matter integrity | Periventricular white matter, deep white matter, corpus callosum, corona radiata | Low flow | DTI, MTR, Low b value DWI |
| Gray matter integrity | Cerebral cortex, thalamus | Low flow | Resting state fMRI, ASL |
3d-T1-w= three-dimensional T1 weigthed MRI technique; T2 MRI= T2-weigthed MRI technique; FLAIR= Fluid Attenuated Inversion Recovery; DTI=diffusion tensor imaging; MTR= magnetization transfer imaging; DWI= diffusion weighted imaging; fMRI= functional MRI; ASL= arterial spin labeling.
Data from the AGES-Reykjavik Study, in which the potential mechanisms that may underlie the association of aortic stiffness and cognitive function were studied, clearly showed that higher carotid-femoral PWV was associated with lower memory scores, higher WMH volume and increased cerebral vascular resistance, and higher odds for the presence of subcortical infarcts.8 Generally, the association between increased arterial PWV and higher WMH burden is well established, even in relatively healthy populations.62–65 In contrast to accumulation of microbleeds in the basal ganglia, which seems only associated with vascular stiffness in older populations or with cardiovascular comorbidity (e.g. hypertension, diabetes), the association between WMHs and increased arterial PWV is already present in younger populations or older populations with a relatively lower PWV. The pathophysiology behind the origin of WMHs is not fully clarified. In addition to the concept that WMHs reflect low flow areas in the brain, preceding the occurrence of low flow infarctions or lacunar infarctions with confluent WMHs, it has recently been proposed that endothelial dysfunction may play a key role in the development of small vessel disease, which may result in stiffness of the small blood vessels.66 This endothelial dysfunction affects the cerebrovascular reactivity which is the capability to adapt vessel diameter to increase brain blood flow when needed, thereby impacting the supply of oxygen and nutrients to the brain leading to lacunar infarctions or infarctions in low flow regions, especially in watershed territories.
Overt changes in the deep white matter are commonly visualized in high risk patients by using T2-weighted MRI techniques, whereas the gray matter seems to be relatively spared, although few studies report a relation between increased arterial stiffness and gray matter atrophy. It has been postulated that especially those regions in the brain that are located at the far end of the deep penetrating arteries are highly susceptible for low flow infarctions that are secondary to decreased global CBF rather than changes in pulsatility.67, 68
Several studies have reported independent associations between the presence of deep subcortical and periventricular WMHs, number of microbleeds or presence of lacunar infarcts with impaired cognitive function. It is currently widely accepted that microbleeds in the pallidum and putamen are strongly associated with hypertension.69 Cerebral microbleeds are small perivascular haemorrhages and consist of iron deposits, mainly haemosiderin, and are expected to occur due to hypertensive arteriopathy that causes small vascular leaks. Although it has been shown that microbleeds in the pallidum and putamen are strongly associated with hypertension, the association with aortic stiffening is less obvious. Recent larger studies assessing the association between aortic stiffening and the presence of microbleeds demonstrate a slight association with increased vascular stiffening, 70, 71 although not all studies support this finding.62, 63
In this respect it is important to distinguish lobar microbleeds from deep or infratentorial microbleeds. Lobar microbleeds are uniquely attributed to the presence of cerebral amyloid angiopathy and not directly associated with hypertension.72
The presence of dilated Virchow–Robin spaces may be an interesting marker for the presence of vascular damage.73 Dilated Virchow-Robin spaces are associated with endothelial dysfunction, and are commonly visualized as punctate or linear hyperintensities on T2-weighted MRI pulse sequences, frequently observed in the basal ganglia or centrum semiovale (Figure 2C). Marked dilatation of Virchow-Robin spaces has been independently associated with age and hypertension, and considered as another MRI marker of cerebral small vessel disease in the elderly. It has been suggested that increased arterial pulsations produce increased perivascular shear stresses and could induce large pulsations in the perivascular space leading to dilation of these spaces. Therefore, in particular in the pallidum and putamen, dilated Virchow-Robin spaces may be a marker for vascular damage directly caused by high pressure variability and flow pulsatility, as was demonstrated in the AGES-Reykjavik Study.74 Although the mechanisms by which dilated Virchow–Robin spaces are formed are not completely elucidated, they particularly occur in the pallidum and putamen, in its worst form known as “état criblé” describing diffusely widened perivascular spaces. Widened perivascular spaces are also a common finding in deep white matter in older and/or hypertensive persons.73
MRI of microstructural brain disease
Whereas in the proximal part of the arterial tree, damage may be mainly driven by high pressure fluctuations and flow pulsatility, more distally, additional effects caused by impaired microvascular reactivity in combination with decreased cerebral blood flow will occur. It is conceivable that a cascade of vascular changes ranging from subtle “MRI-invisible” (i.e. normal-appearing brain tissue on conventional imaging techniques) microstructural changes up to clearly identifiable lacunar infarcts may occur over time (Figure 1B). These early vascular changes may become visible and quantifiable by specialized MRI techniques, such as by microstructural MRI (diffusion tensor imaging) or by functional brain network connectivity techniques (resting-state functional MRI), by techniques that probe the integrity of the myelin sheaths (Magnetization Transfer Imaging) and by techniques that may assess microvascular pulsatility (Intravoxel Incoherent Motion MRI).
Diffusion tensor imaging defines microstructural brain tissue integrity in the normal-appearing brain tissue, unseen with structural and conventional MRI methods. Water diffusion in the brain is anisotropic due to restriction of lipid bilayers and cellular components. Diffusion tensor imaging estimates the direction and magnitude of water diffusion by providing metrics like fractional anisotropy, axial diffusivity and radial diffusivity. A recent MRI study showed that increased aortic arch PWV in hypertensive patients is associated with decreased fractional anisotropy in brain white matter, increased axial diffusivity in brain gray matter and increased radial diffusivity in both white and gray matter, independent of age, sex, body mass index, smoking and presence of WMHs. It was concluded that aortic arch stiffness relates to incipient brain injury before overt brain abnormalities become apparent in hypertensive patients.75, 76 Also in diabetic patients an independent association between MRI-based estimates of aortic pulse wave velocity and diffusion tensor imaging of brain white matter integrity has been shown. Furthermore, the Framingham Heart Study revealed that higher aortic stiffness is associated with reduced white matter and gray matter integrity as measured by diffusion tensor imaging in brain regions implicated in cognitive decline and Alzheimer’s disease, already evident at a young age.77 Specific regions of the brain appear to be vulnerable to increased aortic stiffness, such as the corpus callosum, corona radiata and internal capsule for white matter and the thalamus for gray matter, respectively.
Magnetization transfer imaging is another sensitive MRI method to detect early microstructural alterations in normal-appearing brain tissue. This imaging method probes the protons bound to large molecules like the myelin lipids and proteins, reflecting myelinisation of white matter fibers. Magnetization transfer imaging of the brain has revealed that incipient brain injury occurs in middle-aged to elderly overweight persons with increased cardiovascular risk or metabolic syndrome, before overt small vessel brain disease becomes manifest.78, 79 The utility of serial measurements of magnetization transfer imaging markers was recently shown for predicting cognitive decline. Magnetization transfer imaging markers appeared to be related to cognitive test performance at baseline and 3.3-year follow-up in older subjects with increased cardiovascular risk.80
Resting-state functional MRI is another sensitive tool to detect early changes (i.e. before structural disease becomes evident) in brain activity in subjects with subjective cognitive decline, presumably prior to onset of mild cognitive decline and Alzheimer disease.81, 82 This technique is able to detect altered functional brain activity related to spontaneous fluctuations in blood oxygenation level-dependent signals reflecting regional glucose metabolism in specific brain regions that are linked with mild cognitive decline and Alzheimer disease. Direct changes in regional cerebral blood flow (rCBF) can be studied by arterial spin labelling (ASL) techniques in which rCBF can be established non-invasively.83
Intravoxel incoherent motion MRI at very low-b values provides perfusion and pulsatility data from the microcirculation in the capillary networks of the brain without the use of contrast agents.84 In view of the primary role of cerebral small vessel pulsatility in developing cognitive decline, non-invasive assessment of microvascular pulsatility by using intravoxel incoherent motion MRI may open new avenues for exploring the importance of vascular pulsatility for brain function and disease.85
Clinical relevance of aortic arch PWV
Combined MRI and ultrasound studies have shown that aortic distensibility may be the most sensitive marker of aortic aging in individuals below 50 years of age.86 In addition, the predictive value of MRI-estimated proximal aortic distensibility for all-cause mortality and incident cardiovascular events has been well documented, in particular in low to intermediate risk individuals.87 Of note, this predictive value of proximal aortic function is independent from classic cardiovascular risk factors.
Velocity-encoded MRI provides direct local estimates of flow in both aortic arch and carotid artery to estimate the protective stiffness mismatch at young age or increased match with aging in compliance between aortic arch and carotid arteries.88 Using MRI it has been shown that increased leveling or matching of PWV between the proximal aorta and carotid artery circulation with aging is associated with a reduction in blood flow volume towards the brain as a mechanism causing brain alterations.88 Increased aortic arch PWV measured by MRI is related to lacunar brain infarcts in hypertensive patients, independent of age, sex, and hypertension duration.89 In a large sample (n=1270) drawn from the Dallas Heart Study, the relationship between MRI-based estimates of aortic arch PWV and cerebral microvascular disease was evaluated, revealing that aortic arch PWV is a highly significant independent predictor of subsequent white matter disease, with a higher standardized effect than any other cardiovascular risk factor except for age.90 In type 1 diabetic patients a two-directional relationship between MRI-based estimates of aortic stiffness and systolic cardiac function and cerebral small vessel disease has been demonstrated. Aortic stiffness was inversely associated with systolic left ventricular function and cerebral white matter hyperintensities, independently of age, gender, mean arterial pressure, heart rate, body mass index, smoking, diabetes duration and hypertension, indicating the prognostic implications of aortic PWV in its own right.91
A subsequent study from the population Dallas Heart Study provided evidence that smaller volumes of specific brain regions (e.g. hippocampus, precuneus, posterior cingulate gyrus), considered to be early imaging markers of dementia, are associated with specific cardiovascular risk factors and cognitive deficits in predominantly midlife subjects, suggesting that these imaging biomarkers for brain insult may be useful for early diagnosis of dementia.92
Summary and perspective.
Aortic stiffness is a key novel indicator of cardiovascular health and global cardiovascular disease risk. Multiple cardiovascular disease risk factors, including genetic determinants, result in accelerated vascular aging beyond biological age. Regional aortic function has various effects on specific end-organs related to aortic function and its change with age and various exposures. For example, the aging of the proximal aorta is directly related to end-organ damage to the brain and heart. In this respect, the proximal aorta serves as a coupling device between cardiovascular and brain function. Aging and accelerated stiffening of the proximal aorta cause increased microvascular brain pulsatility, which accelerates the development of cerebral small vessel disease. MRI is well suited to assess the function of the proximal aorta and to define several stages of brain damage, which may contribute to early memory loss, mild cognitive decline and eventually dementia. Therefore, proximal aortic stiffening may act both as a biomarker of vascular health and as a risk factor for cerebrovascular damage. Recognition of accelerated aging of the proximal aorta may help to identify high-risk individuals and may be a starting point for preventive measures to reduce cardiovascular disease and related brain damage. Hopefully treating cardiovascular risk factors and other modifiers of vascular aging will help to improve brain health, thereby reducing the various manifestations of declining brain function, such as cognitive decline and dementia. Further studies are required to validate and substantiate the concept that cognitive decline and dementia are causally related to cardiovascular disease.
Acknowledgments
The authors like to thank Soraya Arthur-Kovacs for excellent support in manuscript preparation.
Funding: Dr. Mitchell is funded by research grants from Novartis and the National Institutes of Health. An abbreviated funding statement that pertains just to work on the review: Dr. Mitchell was supported by a research grant (HL126136) from the National Institutes of Health.). Other authors no funding.
Footnotes
Disclosures:
Dr. Mitchell is owner of Cardiovascular Engineering, Inc., a company that develops and manufactures devices to measure vascular stiffness, serves as a consultant to and receives honoraria from Novartis, Merck, Servier and Philips.
Other authors no disclosures.
References
- 1.Satizabal CL, Beiser AS, Chouraki V, Chene G, Dufouil C, Seshadri S. Incidence of Dementia over Three Decades in the Framingham Heart Study. The New England journal of medicine. 2016;374:523–532. doi: 10.1056/NEJMoa1504327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones DS, Greene JA. Is Dementia in Decline? Historical Trends and Future Trajectories. The New England journal of medicine. 2016;374:507–509. doi: 10.1056/NEJMp1514434. [DOI] [PubMed] [Google Scholar]
- 3.Angermann CE, Frey A, Ertl G. Cognition matters in cardiovascular disease and heart failure. European heart journal. 2012;33:1721–1723. doi: 10.1093/eurheartj/ehs128. [DOI] [PubMed] [Google Scholar]
- 4.Flynn KE, Pina IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, Fine LJ, Howlett JG, Keteyian SJ, Kitzman DW, Kraus WE, Miller NH, Schulman KA, Spertus JA, O’Connor CM, Weinfurt KP, Investigators H-A Effects of exercise training on health status in patients with chronic heart failure: HF-ACTION randomized controlled trial. Jama. 2009;301:1451–1459. doi: 10.1001/jama.2009.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deshields TL, McDonough EM, Mannen RK, Miller LW. Psychological and cognitive status before and after heart transplantation. General hospital psychiatry. 1996;18:62S–69S. doi: 10.1016/s0163-8343(96)00078-3. [DOI] [PubMed] [Google Scholar]
- 6.Dixit NK, Vazquez LD, Cross NJ, Kuhl EA, Serber ER, Kovacs A, Dede DE, Conti JB, Sears SF. Cardiac resynchronization therapy: a pilot study examining cognitive change in patients before and after treatment. Clinical cardiology. 2010;33:84–88. doi: 10.1002/clc.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lenart N, Brough D, Denes A. Inflammasomes link vascular disease with neuroinflammation and brain disorders. J Cereb Blood Flow Metab. 2016;36:1668–1685. doi: 10.1177/0271678X16662043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain: a journal of neurology. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coutinho T, Turner ST, Kullo IJ. Aortic pulse wave velocity is associated with measures of subclinical target organ damage. JACC Cardiovascular imaging. 2011;4:754–761. doi: 10.1016/j.jcmg.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cecelja M, Chowienczyk P. Dissociation of aortic pulse wave velocity with risk factors for cardiovascular disease other than hypertension: a systematic review. Hypertension. 2009;54:1328–1336. doi: 10.1161/HYPERTENSIONAHA.109.137653. [DOI] [PubMed] [Google Scholar]
- 11.Toledo JB, Toledo E, Weiner MW, Jack CR, Jr, Jagust W, Lee VM, Shaw LM, Trojanowski JQ. Cardiovascular risk factors, cortisol, and amyloid-beta deposition in Alzheimer’s Disease Neuroimaging Initiative. Alzheimer’s & dementia: the journal of the Alzheimer’s Association. 2012;8:483–489. doi: 10.1016/j.jalz.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langbaum JB, Chen K, Launer LJ, Fleisher AS, Lee W, Liu X, Protas HD, Reeder SA, Bandy D, Yu M, Caselli RJ, Reiman EM. Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiology of aging. 2012;33:827.e11–19. doi: 10.1016/j.neurobiolaging.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodrigue KM, Rieck JR, Kennedy KM, Devous MD, Sr, Diaz-Arrastia R, Park DC. Risk factors for beta-amyloid deposition in healthy aging: vascular and genetic effects. JAMA neurology. 2013;70:600–606. doi: 10.1001/jamaneurol.2013.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes TM, Kuller LH, Barinas-Mitchell EJ, Mackey RH, McDade EM, Klunk WE, Aizenstein HJ, Cohen AD, Snitz BE, Mathis CA, Dekosky ST, Lopez OL. Pulse wave velocity is associated with beta-amyloid deposition in the brains of very elderly adults. Neurology. 2013;81:1711–1718. doi: 10.1212/01.wnl.0000435301.64776.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes TM, Kuller LH, Barinas-Mitchell EJ, McDade EM, Klunk WE, Cohen AD, Mathis CA, Dekosky ST, Price JC, Lopez OL. Arterial stiffness and beta-amyloid progression in nondemented elderly adults. JAMA neurology. 2014;71:562–568. doi: 10.1001/jamaneurol.2014.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bateman GA. Pulse wave encephalopathy: a spectrum hypothesis incorporating Alzheimer’s disease, vascular dementia and normal pressure hydrocephalus. Medical hypotheses. 2004;62:182–187. doi: 10.1016/S0306-9877(03)00330-X. [DOI] [PubMed] [Google Scholar]
- 17.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke; a journal of cerebral circulation. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell GF. Arterial stiffness: insights from Framingham and Iceland. Current opinion in nephrology and hypertension. 2015;24:1–7. doi: 10.1097/MNH.0000000000000092. [DOI] [PubMed] [Google Scholar]
- 19.Hajjar I, Goldstein FC, Martin GS, Quyyumi AA. Roles of Arterial Stiffness and Blood Pressure in Hypertension-Associated Cognitive Decline in Healthy Adults. Hypertension. 2016;67:171–175. doi: 10.1161/HYPERTENSIONAHA.115.06277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pase MP, Himali JJ, Mitchell GF, Beiser A, Maillard P, Tsao C, Larson MG, DeCarli C, Vasan RS, Seshadri S. Association of Aortic Stiffness With Cognition and Brain Aging in Young and Middle-Aged Adults: The Framingham Third Generation Cohort Study. Hypertension. 2016;67:513–519. doi: 10.1161/HYPERTENSIONAHA.115.06610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Meer RW, Lamb HJ, Smit JW, de Roos A. MR imaging evaluation of cardiovascular risk in metabolic syndrome. Radiology. 2012;264:21–37. doi: 10.1148/radiol.12110772. [DOI] [PubMed] [Google Scholar]
- 22.Cooper LL, Rong J, Benjamin EJ, Larson MG, Levy D, Vita JA, Hamburg NM, Vasan RS, Mitchell GF. Components of hemodynamic load and cardiovascular events: the Framingham Heart Study. Circulation. 2015;131:354–361. doi: 10.1161/CIRCULATIONAHA.114.011357. discussion 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smulyan H, Mookherjee S, Safar ME. The two faces of hypertension: role of aortic stiffness. Journal of the American Society of Hypertension: JASH. 2016;10:175–183. doi: 10.1016/j.jash.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell GF, Wang N, Palmisano JN, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS. Hemodynamic correlates of blood pressure across the adult age spectrum: noninvasive evaluation in the Framingham Heart Study. Circulation. 2010;122:1379–1386. doi: 10.1161/CIRCULATIONAHA.109.914507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. Journal of applied physiology (Bethesda, Md: 1985) 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D, Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- 27.Torjesen AA, Sigurethsson S, Westenberg JJ, Gotal JD, Bell V, Aspelund T, Launer LJ, de Roos A, Gudnason V, Harris TB, Mitchell GF. Pulse pressure relation to aortic and left ventricular structure in the Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. Hypertension. 2014;64:756–761. doi: 10.1161/HYPERTENSIONAHA.114.03870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lam CS, Xanthakis V, Sullivan LM, Lieb W, Aragam J, Redfield MM, Mitchell GF, Benjamin EJ, Vasan RS. Aortic root remodeling over the adult life course: longitudinal data from the Framingham Heart Study. Circulation. 2010;122:884–890. doi: 10.1161/CIRCULATIONAHA.110.937839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circulation research. 1967;20:99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- 30.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiological reviews. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell V, Sigurdsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, Harris TB, Gudnason V, de Roos A, Mitchell GF. Relations between aortic stiffness and left ventricular structure and function in older participants in the Age, Gene/Environment Susceptibility–Reykjavik Study. Circulation Cardiovascular imaging. 2015;8:e003039. doi: 10.1161/CIRCIMAGING.114.003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Cheng S, Aragam J, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Relations of Central Hemodynamics and Aortic Stiffness with Left Ventricular Structure and Function: The Framingham Heart Study. Journal of the American Heart Association. 2016;4:e002693. doi: 10.1161/JAHA.115.002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antonini-Canterin F, Carerj S, Di Bello V, Di Salvo G, La Carrubba S, Vriz O, Pavan D, Balbarini A, Nicolosi GL. Arterial stiffness and ventricular stiffness: a couple of diseases or a coupling disease? A review from the cardiologist’s point of view. European journal of echocardiography: the journal of the Working Group on Echocardiography of the European Society of Cardiology. 2009;10:36–43. doi: 10.1093/ejechocard/jen236. [DOI] [PubMed] [Google Scholar]
- 34.Oh JE, Shin JW, Sohn EH, Jung JO, Jeong SH, Song HJ, Kim JM, Lee AY. Effect of cardiac function on cognition and brain structural changes in dementia. Journal of clinical neurology (Seoul, Korea) 2012;8:123–129. doi: 10.3988/jcn.2012.8.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaita F, Corsinovi L, Anselmino M, Raimondo C, Pianelli M, Toso E, Bergamasco L, Boffano C, Valentini MC, Cesarani F, Scaglione M. Prevalence of silent cerebral ischemia in paroxysmal and persistent atrial fibrillation and correlation with cognitive function. Journal of the American College of Cardiology. 2013;62:1990–1997. doi: 10.1016/j.jacc.2013.05.074. [DOI] [PubMed] [Google Scholar]
- 36.Jefferson AL. Cardiac output as a potential risk factor for abnormal brain aging. Journal of Alzheimer’s disease: JAD. 2010;20:813–821. doi: 10.3233/JAD-2010-100081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jefferson AL, Tate DF, Poppas A, Brickman AM, Paul RH, Gunstad J, Cohen RA. Lower cardiac output is associated with greater white matter hyperintensities in older adults with cardiovascular disease. Journal of the American Geriatrics Society. 2007;55:1044–1048. doi: 10.1111/j.1532-5415.2007.01226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singer J, Trollor JN, Baune BT, Sachdev PS, Smith E. Arterial stiffness, the brain and cognition: a systematic review. Ageing research reviews. 2014;15:16–27. doi: 10.1016/j.arr.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Zarrinkoob L, Ambarki K, Wahlin A, Birgander R, Carlberg B, Eklund A, Malm J. Aging alters the dampening of pulsatile blood flow in cerebral arteries. J Cereb Blood Flow Metab. 2016;36:1519–1527. doi: 10.1177/0271678X16629486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsao CW, Himali JJ, Beiser AS, Larson MG, DeCarli C, Vasan RS, Mitchell GF, Seshadri S. Association of arterial stiffness with progression of subclinical brain and cognitive disease. Neurology. 2016;86:619–626. doi: 10.1212/WNL.0000000000002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Middeke M. The pioneer in hemodynamics and pulse-wave analysis, Otto Frank. Journal of the American Society of Hypertension: JASH. 2016;10:290–296. doi: 10.1016/j.jash.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Bramwell J, Hill A. The velocity of the pulse wave in man. Proc R Soc Lond B. 1922:298–306. [Google Scholar]
- 43.Dogui A, Kachenoura N, Frouin F, Lefort M, De Cesare A, Mousseaux E, Herment A. Consistency of aortic distensibility and pulse wave velocity estimates with respect to the Bramwell-Hill theoretical model: a cardiovascular magnetic resonance study. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic Resonance. 2011;13:11. doi: 10.1186/1532-429X-13-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westenberg JJ, van Poelgeest EP, Steendijk P, Grotenhuis HB, Jukema JW, de Roos A. Bramwell-Hill modeling for local aortic pulse wave velocity estimation: a validation study with velocity-encoded cardiovascular magnetic resonance and invasive pressure assessment. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic Resonance. 2012;14:2. doi: 10.1186/1532-429X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roes SD, Westenberg JJ, Doornbos J, van der Geest RJ, Angelie E, de Roos A, Stuber M. Aortic vessel wall magnetic resonance imaging at 3.0 Tesla: a reproducibility study of respiratory navigator gated free-breathing 3D black blood magnetic resonance imaging. Magnetic resonance in medicine. 2009;61:35–44. doi: 10.1002/mrm.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elefteriades JA, Farkas EA. Thoracic aortic aneurysm clinically pertinent controversies and uncertainties. Journal of the American College of Cardiology. 2010;55:841–857. doi: 10.1016/j.jacc.2009.08.084. [DOI] [PubMed] [Google Scholar]
- 47.Bell V, Mitchell WA, Sigurethsson S, Westenberg JJ, Gotal JD, Torjesen AA, Aspelund T, Launer LJ, de Roos A, Gudnason V, Harris TB, Mitchell GF. Longitudinal and circumferential strain of the proximal aorta. Journal of the American Heart Association. 2014;3:e001536. doi: 10.1161/JAHA.114.001536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen F, Bergqvist D, Mangell P, Ryden A, Sonesson B, Lanne T. Non-invasive measurement of pulsatile vessel diameter change and elastic properties in human arteries: a methodological study. Clinical physiology (Oxford, England) 1993;13:631–643. doi: 10.1111/j.1475-097x.1993.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 49.Claridge MW, Bate GR, Hoskins PR, Adam DJ, Bradbury AW, Wilmink AB. Measurement of arterial stiffness in subjects with vascular disease: Are vessel wall changes more sensitive than increase in intima-media thickness? Atherosclerosis. 2009;205:477–480. doi: 10.1016/j.atherosclerosis.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 50.Taviani V, Patterson AJ, Graves MJ, Hardy CJ, Worters P, Sutcliffe MP, Gillard JH. Accuracy and repeatability of fourier velocity encoded M-mode and two-dimensional cine phase contrast for pulse wave velocity measurement in the descending aorta. Journal of magnetic resonance imaging: JMRI. 2010;31:1185–1194. doi: 10.1002/jmri.22143. [DOI] [PubMed] [Google Scholar]
- 51.Ibrahim el SH, Johnson KR, Miller AB, Shaffer JM, White RD. Measuring aortic pulse wave velocity using high-field cardiovascular magnetic resonance: comparison of techniques. Journal of cardiovascular magnetic resonance: official journal of the Society for Cardiovascular Magnetic Resonance. 2010;12:26. doi: 10.1186/1532-429X-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dogui A, Redheuil A, Lefort M, DeCesare A, Kachenoura N, Herment A, Mousseaux E. Measurement of aortic arch pulse wave velocity in cardiovascular MR: comparison of transit time estimators and description of a new approach. Journal of magnetic resonance imaging: JMRI. 2011;33:1321–1329. doi: 10.1002/jmri.22570. [DOI] [PubMed] [Google Scholar]
- 53.Grotenhuis HB, Westenberg JJ, Steendijk P, van der Geest RJ, Ottenkamp J, Bax JJ, Jukema JW, de Roos A. Validation and reproducibility of aortic pulse wave velocity as assessed with velocity-encoded MRI. Journal of magnetic resonance imaging: JMRI. 2009;30:521–526. doi: 10.1002/jmri.21886. [DOI] [PubMed] [Google Scholar]
- 54.Hickson SS, Butlin M, Graves M, Taviani V, Avolio AP, McEniery CM, Wilkinson IB. The relationship of age with regional aortic stiffness and diameter. JACC Cardiovascular imaging. 2010;3:1247–1255. doi: 10.1016/j.jcmg.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 55.Westenberg JJ, Scholte AJ, Vaskova Z, van der Geest RJ, Groenink M, Labadie G, van den Boogaard PJ, Radonic T, Hilhorst-Hofstee Y, Mulder BJ, Kroft LJ, Reiber JH, de Roos A. Age-related and regional changes of aortic stiffness in the Marfan syndrome: assessment with velocity-encoded MRI. Journal of magnetic resonance imaging: JMRI. 2011;34:526–531. doi: 10.1002/jmri.22646. [DOI] [PubMed] [Google Scholar]
- 56.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, Lindley RI, O’Brien JT, Barkhof F, Benavente OR, Black SE, Brayne C, Breteler M, Chabriat H, Decarli C, de Leeuw FE, Doubal F, Duering M, Fox NC, Greenberg S, Hachinski V, Kilimann I, Mok V, Oostenbrugge R, Pantoni L, Speck O, Stephan BC, Teipel S, Viswanathan A, Werring D, Chen C, Smith C, van Buchem M, Norrving B, Gorelick PB, Dichgans M. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. The Lancet Neurology. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell GF. Cerebral small vessel disease: role of aortic stiffness and pulsatile hemodynamics. Journal of hypertension. 2015;33:2025–2028. doi: 10.1097/HJH.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 58.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. The Journal of clinical investigation. 2013;123:1299–1309. doi: 10.1172/JCI67677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, Weller RO. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathology and applied neurobiology. 2008;34:131–144. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- 60.Schreiber S, Drukarch B, Garz C, Niklass S, Stanaszek L, Kropf S, Bueche C, Held F, Vielhaber S, Attems J, Reymann KG, Heinze HJ, Carare RO, Wilhelmus MM. Interplay between age, cerebral small vessel disease, parenchymal amyloid-beta, and tau pathology: longitudinal studies in hypertensive stroke-prone rats. Journal of Alzheimer’s disease: JAD. 2014;42(Suppl 3):S205–S215. doi: 10.3233/JAD-132618. [DOI] [PubMed] [Google Scholar]
- 61.Cooper LL, Woodard T, Sigurdsson S, van Buchem MA, Torjesen AA, Inker LA, Aspelund T, Eiriksdottir G, Harris TB, Gudnason V, Launer LJ, Mitchell GF. Cerebrovascular Damage Mediates Relations Between Aortic Stiffness and Memory. Hypertension. 2016;67:176–182. doi: 10.1161/HYPERTENSIONAHA.115.06398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henskens LH, Kroon AA, van Oostenbrugge RJ, Gronenschild EH, Fuss-Lejeune MM, Hofman PA, Lodder J, de Leeuw PW. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension. 2008;52:1120–1126. doi: 10.1161/HYPERTENSIONAHA.108.119024. [DOI] [PubMed] [Google Scholar]
- 63.Poels MM, Zaccai K, Verwoert GC, Vernooij MW, Hofman A, van der Lugt A, Witteman JC, Breteler MM, Mattace-Raso FU, Ikram MA. Arterial stiffness and cerebral small vessel disease: the Rotterdam Scan Study. Stroke; a journal of cerebral circulation. 2012;43:2637–2642. doi: 10.1161/STROKEAHA.111.642264. [DOI] [PubMed] [Google Scholar]
- 64.Webb AJ, Simoni M, Mazzucco S, Kuker W, Schulz U, Rothwell PM. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke; a journal of cerebral circulation. 2012;43:2631–2636. doi: 10.1161/STROKEAHA.112.655837. [DOI] [PubMed] [Google Scholar]
- 65.Saji N, Ogama N, Toba K, Sakurai T. White matter hyperintensities and geriatric syndrome: An important role of arterial stiffness. Geriatrics & gerontology international. 2015;15(Suppl 1):17–25. doi: 10.1111/ggi.12673. [DOI] [PubMed] [Google Scholar]
- 66.Markus HS, Hunt B, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian Stroke Prevention Study. Stroke; a journal of cerebral circulation. 2005;36:1410–1414. doi: 10.1161/01.STR.0000169924.60783.d4. [DOI] [PubMed] [Google Scholar]
- 67.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR American journal of neuroradiology. 1990;11:431–439. [PMC free article] [PubMed] [Google Scholar]
- 68.Bogousslavsky J, Regli F. Unilateral watershed cerebral infarcts. Neurology. 1986;36:373–377. doi: 10.1212/wnl.36.3.373. [DOI] [PubMed] [Google Scholar]
- 69.Romero JR, Preis SR, Beiser A, DeCarli C, D’Agostino RB, Wolf PA, Vasan RS, Polak JF, Seshadri S. Carotid Atherosclerosis and Cerebral Microbleeds: The Framingham Heart Study. Journal of the American Heart Association. 2016;4:e002377. doi: 10.1161/JAHA.115.002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song TJ, Kim J, Kim YD, Nam HS, Lee HS, Nam CM, Heo JH. The distribution of cerebral microbleeds determines their association with arterial stiffness in non-cardioembolic acute stroke patients. European journal of neurology. 2014;21:463–469. doi: 10.1111/ene.12332. [DOI] [PubMed] [Google Scholar]
- 71.Seo WK, Lee JM, Park MH, Park KW, Lee DH. Cerebral microbleeds are independently associated with arterial stiffness in stroke patients. Cerebrovascular diseases (Basel, Switzerland) 2008;26:618–623. doi: 10.1159/000166837. [DOI] [PubMed] [Google Scholar]
- 72.Wu Y, Chen T. An Up-to-Date Review on Cerebral Microbleeds. J Stroke Cerebrovasc Dis. 2016;24:1301–1306. doi: 10.1016/j.jstrokecerebrovasdis.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 73.Potter GM, Doubal FN, Jackson CA, Chappell FM, Sudlow CL, Dennis MS, Wardlaw JM. Enlarged perivascular spaces and cerebral small vessel disease. International journal of stroke: official journal of the International Stroke Society. 2015;10:376–381. doi: 10.1111/ijs.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van Sloten TT, Sigurdsson S, van Buchem MA, Phillips CL, Jonsson PV, Ding J, Schram MT, Harris TB, Gudnason V, Launer LJ. Cerebral Small Vessel Disease and Association With Higher Incidence of Depressive Symptoms in a General Elderly Population: The AGES-Reykjavik Study. The American journal of psychiatry. 2015;172:570–578. doi: 10.1176/appi.ajp.2014.14050578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sala M, van den Berg-Huysmans A, van der Grond J, Huisman M, Brandts A, Westenberg JJ, de Roos A. Aortic Arch Stiffness Is Associated With Incipient Brain Injury in Patients With Hypertension. Am J Hypertens. 2016;29:705–712. doi: 10.1093/ajh/hpv161. [DOI] [PubMed] [Google Scholar]
- 76.Tjeerdema N, Van Schinkel LD, Westenberg JJ, Van Elderen SG, Van Buchem MA, Smit JW, Van der Grond J, De Roos A. Aortic stiffness is associated with white matter integrity in patients with type 1 diabetes. Eur Radiol. 2014;24:2031–2037. doi: 10.1007/s00330-014-3179-9. [DOI] [PubMed] [Google Scholar]
- 77.Maillard P, Mitchell GF, Himali JJ, Beiser A, Tsao CW, Pase MP, Satizabal CL, Vasan RS, Seshadri S, DeCarli C. Effects of Arterial Stiffness on Brain Integrity in Young Adults From the Framingham Heart Study. Stroke. 2016;47:1030–1036. doi: 10.1161/STROKEAHA.116.012949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sala M, de Roos A, van den Berg A, Altmann-Schneider I, Slagboom PE, Westendorp RG, van Buchem MA, de Craen AJ, van der Grond J. Microstructural brain tissue damage in metabolic syndrome. Diabetes care. 2014;37:493–500. doi: 10.2337/dc13-1160. [DOI] [PubMed] [Google Scholar]
- 79.Sala M, van der Grond J, de Mutsert R, van Heemst D, Slagboom PE, Kroft LJ, de Roos A. Liver Fat Assessed With CT Relates to MRI Markers of Incipient Brain Injury in Middle-Aged to Elderly Overweight Persons. AJR Am J Roentgenol. 2016;206:1087–1092. doi: 10.2214/AJR.15.15251. [DOI] [PubMed] [Google Scholar]
- 80.Sala M, de Roos A, Blauw GJ, Middelkoop HA, Jukema JW, Mooijaart SP, van Buchem MA, de Craen AJ, van der Grond J. Association between changes in brain microstructure and cognition in older subjects at increased risk for vascular disease. BMC neurology. 2015;15:133. doi: 10.1186/s12883-015-0396-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun Y, Dai Z, Li Y, Sheng C, Li H, Wang X, Chen X, He Y, Han Y. Subjective Cognitive Decline: Mapping Functional and Structural Brain Changes-A Combined Resting-State Functional and Structural MR Imaging Study. Radiology. 2016:151771. doi: 10.1148/radiol.2016151771. [DOI] [PubMed] [Google Scholar]
- 82.Hafkemeijer A, van der Grond J, Rombouts SA. Imaging the default mode network in aging and dementia. Biochimica et biophysica acta. 2012;1822:431–441. doi: 10.1016/j.bbadis.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 83.Detre JA, Alsop DC. Perfusion magnetic resonance imaging with continuous arterial spin labeling: methods and clinical applications in the central nervous system. Eur J Radiol. 1999;30:115–124. doi: 10.1016/s0720-048x(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 84.Iima M, Le Bihan D. Clinical Intravoxel Incoherent Motion and Diffusion MR Imaging: Past, Present, and Future. Radiology. 2016;278:13–32. doi: 10.1148/radiol.2015150244. [DOI] [PubMed] [Google Scholar]
- 85.Federau C, Hagmann P, Maeder P, Muller M, Meuli R, Stuber M, O’Brien K. Dependence of brain intravoxel incoherent motion perfusion parameters on the cardiac cycle. PloS one. 2013;8:e72856. doi: 10.1371/journal.pone.0072856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Redheuil A, Yu WC, Wu CO, Mousseaux E, de Cesare A, Yan R, Kachenoura N, Bluemke D, Lima JA. Reduced ascending aortic strain and distensibility: earliest manifestations of vascular aging in humans. Hypertension. 2010;55:319–326. doi: 10.1161/HYPERTENSIONAHA.109.141275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Redheuil A, Wu CO, Kachenoura N, Ohyama Y, Yan RT, Bertoni AG, Hundley GW, Duprez DA, Jacobs DR, Jr, Daniels LB, Darwin C, Sibley C, Bluemke DA, Lima JA. Proximal aortic distensibility is an independent predictor of all-cause mortality and incident CV events: the MESA study. J Am Coll Cardiol. 2014;64:2619–2629. doi: 10.1016/j.jacc.2014.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kroner ES, Lamb HJ, Siebelink HM, Cannegieter SC, van den Boogaard PJ, van der Wall EE, de Roos A, Westenberg JJ. Pulse wave velocity and flow in the carotid artery versus the aortic arch: effects of aging. J Magn Reson Imaging. 2014;40:287–293. doi: 10.1002/jmri.24470. [DOI] [PubMed] [Google Scholar]
- 89.Brandts A, van Elderen SG, Westenberg JJ, van der Grond J, van Buchem MA, Huisman MV, Kroft LJ, Tamsma JT, de Roos A. Association of aortic arch pulse wave velocity with left ventricular mass and lacunar brain infarcts in hypertensive patients: assessment with MR imaging. Radiology. 2009;253:681–688. doi: 10.1148/radiol.2533082264. [DOI] [PubMed] [Google Scholar]
- 90.King KS, Chen KX, Hulsey KM, McColl RW, Weiner MF, Nakonezny PA, Peshock RM. White matter hyperintensities: use of aortic arch pulse wave velocity to predict volume independent of other cardiovascular risk factors. Radiology. 2013;267:709–717. doi: 10.1148/radiol.13121598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.van Elderen SG, Brandts A, Westenberg JJ, van der Grond J, Tamsma JT, van Buchem MA, Romijn JA, Kroft LJ, Smit JW, de Roos A. Aortic stiffness is associated with cardiac function and cerebral small vessel disease in patients with type 1 diabetes mellitus: assessment by magnetic resonance imaging. Eur Radiol. 2010;20:1132–1138. doi: 10.1007/s00330-009-1655-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Srinivasa RN, Rossetti HC, Gupta MK, Rosenberg RN, Weiner MF, Peshock RM, McColl RW, Hynan LS, Lucarelli RT, King KS. Cardiovascular Risk Factors Associated with Smaller Brain Volumes in Regions Identified as Early Predictors of Cognitive Decline. Radiology. 2016;278:198–204. doi: 10.1148/radiol.2015142488. [DOI] [PMC free article] [PubMed] [Google Scholar]


