Abstract
Purpose
To characterize the current scope and practices of centers performing extracorporeal cardiopulmonary resuscitation (eCPR) on the undifferentiated patient with cardiac arrest in the emergency department.
Methods
We contacted all US centers in January 2016 that had submitted adult eCPR cases to the Extracorporeal Life Support Organization (ELSO) registry and surveyed them, querying for programs that had performed eCPR in the Emergency Department (ED ECMO). Our objective was to characterize the following domains of ED ECMO practice: program characteristics, patient selection, devices and techniques, and personnel.
Results
Among 99 centers queried, 70 responded. Among these, 36 centers performed ED ECMO. Nearly 93% of programs are based at academic/teaching hospitals. 65% of programs are less than 5 years old, and 60% of programs perform ≤ 3 cases per year. Most programs (90%) had inpatient eCPR or salvage ECMO programs prior to starting ED ECMO programs. The majority of programs do not have formal inclusion and exclusion criteria. Most programs preferentially obtain vascular access via the percutaneous route (70%) and many (40%) use mechanical CPR during cannulation. The most commonly used console is the Maquet Rotaflow®. Cannulation is most often performed by cardiothoracic (CT) surgery, and nearly all programs (>85%) involve CT surgeons, perfusionists, and pharmacists.
Conclusions
Over a third of centers that submitted adult eCPR cases to ELSO have performed ED ECMO. These programs are largely based at academic hospitals, new, and have low volumes. They do not have many formal inclusion or exclusion criteria, and devices and techniques are variable.
Keywords: Extracorporeal cardiopulmonary resuscitation—eCPR, Emergency department extracorporeal membrane oxygenation—ED ECMO, Out-of-hospital cardiac arrest—OHCA, In-Hospital Cardiac Arrest—IHCA, Program characteristics, Extracorporeal life support—ECLS
Introduction
The use of extracorporeal life support for adult patients in acute cardiopulmonary arrest is increasing, and was recently added as a consideration for treatment of refractory cardiac arrest by the American Heart Association (AHA).1 Compared to traditional closed chest cardiopulmonary resuscitation (CPR), which results in a low flow perfusion state2,3 with time-limited outcomes,3 extracorporeal CPR provides near-full to full cardiopulmonary support without the physical trauma of chest compressions.4 This may facilitate treatments that are otherwise precluded by standard CPR, such as angiography or other diagnostic imaging; therefore extracorporeal cardiopulmonary resuscitation (eCPR) has been proposed as ideal for patients in refractory cardiac arrest failing traditional CPR.5 For both in- and out-of-hospital cardiac arrest (IHCA and OHCA) treated with eCPR published survival ranges from 13–54%.6–11 While this range is generally equal or superior to the aggregate survivals of 9% and 20% from conventional CPR of OHCA and IHCA,12,13 respectively, the breadth may reflect heterogeneity in arrest etiology, patient selection and management.
The advantages of applying extracorporeal life support (ECLS) utilizing veno-arterial extracorporeal membrane oxygenation (VA-ECMO) during cardiopulmonary arrest include stable and augmented perfusion, allowing providers to cease external chest compressions, which in turn decreases trauma,4 stress,14–16 frequent interruptions, and resultant low flow state of manual external chest compressions;2 disadvantages include increased cost, resources, and care complexity. Unfortunately, the cost/benefit of eCPR compared to conventional CPR is not known, as most studies are retrospective10,17,18 or observational,8,11 and few control for or describe cannulation techniques or post-cannulation management;6 as such, there is a profound knowledge gap around the absolute benefit, use appropriateness and ideal patient management of eCPR, which may lead to inappropriate use and outcome disparities.
In an effort to increase use appropriateness of eCPR, we sought to first characterize the existing practices of centers performing eCPR on the undifferentiated patient in cardiac arrest. We also sought to describe the characteristics of programs in the US that perform Emergency Department ECMO (ED ECMO), including case volume, patient selection criteria, involved personnel, cannulation processes, hardware selection and post-cannulation management.
Methods
Study Design
This was a prospective survey sent to the publically listed program representatives about a hospital program. This project did not meet the definition of human subject research. No previous published surveys of eCPR team composition or management were found to validate; questions were developed by consensus from a convened group of recognized resuscitation experts and from described characteristics of eCPR programs.7–9,11,19–21 Questions were refined and reviewed by the senior researchers of the ERECT Collaborative.
Setting
We contacted all US centers that have submitted adult eCPR cases to the Extracorporeal Life Support Organization (ELSO) international patient registry and surveyed them, identifying programs that had performed eCPR in the Emergency Department (ED) as most exemplary of the fully undifferentiated cardiac arrest patient. While a survey of IHCA eCPR patient selection, application characteristics, and management in the US would increase the pool for analysis, we sought to isolate the use of eCPR in the ED because of the recognition that patients who suffer IHCA often have previous diagnoses, and a higher chance of being peri-operative, which necessarily guides the post arrest management and treatment.
Data Collection
We administered an online survey (SurveyMonkey Inc., Palo Alto, California, USA) through an email sent to the publically listed Program Director and Program Coordinator in January, 2016 for all centers (97) that had submitted adult eCPR cases to ELSO. Additional emails were sent to select centers (2) who had published on or discussed ED ECMO use at national conferences or on social media, as determined by the authors.7,22 Follow up “2nd Notice” emails were sent to all centers who had not responded to the survey after two weeks, based on data suggesting that one followup provides the highest yield for physician surveys.23 This tailored approach resulted in 4 personalized emails going to each program—the suggested near maximum yield for physician surveys24—and avoided overburdening any one person. No other means was used to contact the programs. The survey was closed after 2 months, when there were no on-going responses and a 70% response rate—above the accepted standard for online survey response rates.24,25 The 2 month duration was based upon on our estimate of our population, and by referencing published standards for duration and cost effectiveness of online surveys.24,25 The survey described the purpose of the inquiry, that aggregated results would be analyzed, and results released back to the participants. The survey was sent on behalf of the authors and members of the Extracorporeal REsuscitation ConsorTium (ERECT), a multi-institutional collaborative research group focused on advancing the science and practice of eCPR. The survey consisted of demographics and contact information, followed by 30 multiple-choice questions; most included a space for “Other” with an associated free text response box. Data were not collected on patient survival/success rate, as survey respondents were individually identifiable through the tool, and we felt this fact may dissuade participation and honest responses. A final general free text response box was also included. The questions are entirely reflected in Tables 1–3. No question was required. After demographics were collected, the survey asked programs to continue through the full survey only if they had done eCPR in the ED. Determination of academic / teaching-hospital vs non-teaching hospital was made by review of the public website of each institution or phone call to the program coordinator when this was not clear.
Table 1.
Demographics and Program Characteristics (total n=36)
| Variables | Level | % | N |
|---|---|---|---|
| Academic / teaching vs non-teaching hospital(missing obs.=3) | Non-teaching hospital | 6.1 | 2 |
| Academic | 93.9 | 31 | |
| Formal Program for ED ECMO, or no formal program but ability to place patients in cardiac arrest on ECMO in the Emergency Department | No Formal ED ECMO Program | 66.1 | 22 |
| Formal ED ECMO Program | 38.9 | 14 | |
| Age of ED ECMO Program (missing obs.=4) | <1 | 15.6 | 5 |
| 1–3 years | 31.3 | 10 | |
| 4–5 years | 21.9 | 7 | |
| 6–10 years | 15.6 | 5 | |
| >10 | 15.6 | 5 | |
| Historical rate of eCPR Cannulations per year in the Emergency Department (missing obs.=5) | 0–1 patients / year | 41.9 | 13 |
| 2–3 patients / year | 19.4 | 6 | |
| 4–6 patients / year | 25.8 | 8 | |
| 7–12 patients / year | 12.9 | 4 | |
| Was ICU-based ECMO functioning at your institution prior to ED ECMO program? | Yes | 88.9 | 32 |
| Was salvage ECMO/eCPR available for inpatients before the ED ECMO program start? (missing obs.=4) | Yes | 90.6 | 29 |
| Locations where eCPR has been performed at the institution | Inpatient Hospital Setting | 91.7 | 33 |
| Emergency Department | 100.0 | 36 | |
| Operating Room | 83.3 | 30 | |
| Other | 16.7 | 6 | |
| A mechanical CPR device used during the cannulation process (missing 4) | Yes | 37.5 | 12 |
| Occasionally | 9.4 | 3 | |
| Vascular access is obtained via | Percutaneous | 72.2 | 26 |
| Cut-down | 36.1 | 13 | |
| Modified (Cutdown+Percutaneous) | 25 | 9 | |
| Role of the cannulator (check all that apply) | Emergency Medicine | 11.1 | 4 |
| CT Surgery | 77.8 | 28 | |
| Cardiology | 16.7 | 6 | |
| General Surgery | 8.3 | 3 | |
| Vascular Surgery | 2.8 | 1 | |
| Other | 11.1 | 4 | |
| Does a separate person cannulate for ECMO than the person running the resuscitation? (missing =4) | Yes | 93.8 | 30 |
| Does the ACLS portion of our ECMO resuscitations utilize more protocol than a typical resuscitation (missing =5) | Yes | 35.5 | 11 |
| The ACLS portion of our ECMO resuscitations are run by (missing =4) | A nurse | 9.4 | 3 |
| A physician | 90.6 | 29 |
Abbreviations: ED--Emergency Department; ECMO--Extracorporeal Membrane Oxygenation; eCPR--Extracorporeal Cardiopulmonary Resuscitation; ICU--Intensive Care Unit; CPR--Cardiopulmonary Resuscitation; ACLS--Advanced Cardiac Life Support
Table 3.
Devices/Equipment/Post ECMO care (total n=36)
| Variables | % | N | |
|---|---|---|---|
| Cannula Selection: For a 70kg male, typical arterial cannula size range (missing obs.=5) | 15–17F | 51.6 | 16 |
| 19–21F | 48.4 | 15 | |
| Cannula Selection: For a70kg male, typical venous cannula size range (missing obs.=5) | 19–21F | 19.4 | 6 |
| 23–25F | 58.1 | 18 | |
| 27F+ | 22.6 | 7 | |
| Routine console for ED ECMO | Maquet Cardiohelp | 27.8 | 10 |
| Maquet Rotaflow | 41.7 | 15 | |
| Thoratec Centrimag | 19.4 | 7 | |
| Sorin Revolution | 19.4 | 7 | |
| Routine use of utilize distal limb perfusion catheters? (missing obs.=4) | Yes | 93.8 | 30 |
| Distal limb perfusion catheter placed by (total n=30) | CT Surgery | 86.7 | 26 |
| Vascular Surgery | 20 | 6 | |
| Cardiology | 30 | 9 | |
| Critical Care | 10 | 3 | |
| Other | 3.3 | 1 | |
| Timing of initial anticoagulation | Upon dilation/cannulation | 38.7 | 12 |
| Upon initiation of ECMO flow | 6.5 | 2 | |
| Vascular access (with catheter/sheath/wire) | 54.8 | 17 | |
| Do you take ED ECMO patients to the cardiac catheterization lab after cannulation? | Always | 12.5 | 4 |
| Yes | 6.3 | 2 | |
| As needed | 62.5 | 20 | |
| No | 18.8 | 6 | |
| Routine anticoagulation for ED ECMO patients with - | |||
| Unfractionated Heparin (UFH) - Use | Exclusively | 60 | 18 |
| Routinely | 36.7 | 11 | |
| Occasionally | 3.3 | 1 | |
| Unfractionated Heparin (UFH) – Monitoring (missing obs.=9) | ACT | 37.0 | 10 |
| ACT & TEG | 3.7 | 1 | |
| ACT & Xa | 3.7 | 1 | |
| PTT | 40.7 | 11 | |
| Xa | 14.8 | 4 | |
| Molecular Weight Heparin (LMWH) – Use (missing obs.=19) | Occasionally | 5.9 | 1 |
| Never | 94.1 | 16 | |
| Low Molecular Weight Heparin (LMWH) – Monitor (missing obs.=19) | PTT | 2.8 | 1 |
| Bivalrudin – Use (missing obs.=17) | Exclusively | 10.5 | 2 |
| Occasionally | 52.6 | 10 | |
| Never | 36.8 | 7 | |
| Bivalrudin – Monitoring (missing obs.=28) | PTT | 87.5 | 7 |
| Xa | 12.5 | 1 | |
| Argatroban - Use(missing obs.=19) | Occasionally | 29.4 | 5 |
| Never | 70.6 | 12 | |
| Argatroban – Monitoring | PTT | 5.6 | 2 |
| Coumadin - Use | Never | 47.2 | 17 |
| Do you use a blender with the sweep gas flow? (missing obs.=4) | Always | 87.5 | 28 |
| Rarely | 3.1 | 1 | |
| Never | 9.4 | 3 | |
| Does your program routinely assess for LV over-distension or unloading? (missing obs.=4) | Yes | 81.3 | 26 |
| Occasionally | 6.3 | 2 | |
| No | 12.5 | 4 | |
| What do you use to decompress the LV? (if applicable) | Atrial Septostomy | 30.6 | 11 |
| LV Impella | 33.3 | 12 | |
| Reduce ECMO flow | 11.1 | 4 | |
| We do not decompress the LV | 5.6 | 2 | |
| Other | 44.4 | 16 |
Abbreviations: kg--kilogram; F--French
Statistical Analysis
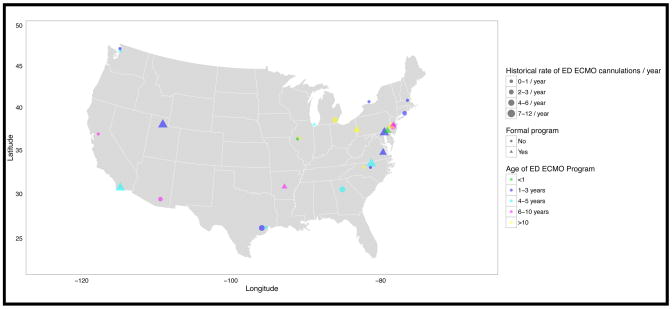
Data were summarized as absolute values and percentages. Geography, services involved throughout the ECMO process, and numerical values of PTT, ACT and anti-factor Xa were displayed graphically. All responses are included, edited for clarity, with “Other” responses summarized in Results. The analysis group was geographically placed (Figure 1) but then de-identified for further analysis. Data are presented in Figures 2–3 and Tables 1–3.
Figure 1.
Adult Emergency Department ECMO Programs in the United States
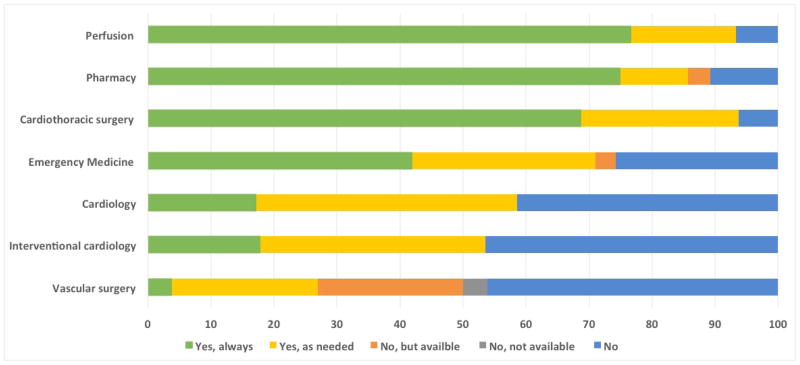
Figure 2.
Services Involved in the Emergency Department ECMO Process
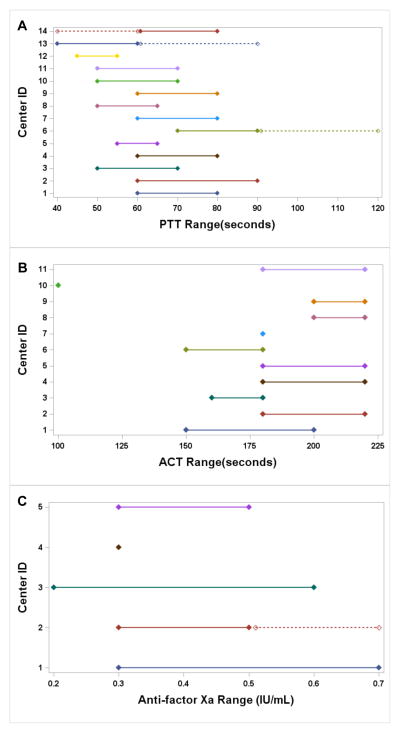
Figure 3.
- Abbreviations: PTT--Activated Partial Thromboplastin Time; ACT-- Activated Clotting Time; IU--international unit, mL--milliliter
-
Notes:3a:#14: Dashed Line: If patient bleeding#13: Dashed Line: If patient tolerates lower range#6: Dashed Line: Two ranges given, not defined
-
3c:#2: Dashed Line: Two ranges given, not defined
Results
Program Characteristics
Among 99 centers contacted, there were 70 unique centers that responded. Among all respondents, 36 centers (analysis group) had performed eCPR in the ED. For the manuscript, this group is referred to as having an ED ECMO program, regardless of program formality.
A total of 93% of all programs (n=36) are academic or teaching hospitals, yet only 38% describe themselves as having a “formal” ED ECMO program to place eCPR in the ED. Programs are generally new, with >65% of programs ≤5 years old, and 45% ≤3 years old. Furthermore, 60% of programs perform ≤3 cases per year. It should be noted that the vast majority of programs (~90%) had both intensive care unit (ICU) based, and salvage ECMO (ECMO placed when cardiopulmonary collapse is imminent)/eCPR for in-patients and the operating room before starting their ED ECMO program (Table 1).
Patient Selection
The majority of programs do not have many formal inclusion and exclusion criteria (Table 2). Almost all programs included patients with cardiac arrest and an initial shockable rhythm, but around half included non-shockable initial rhythms, respiratory arrests, hypothermia and drug overdoses. Not surprisingly, a thin majority of programs also performed salvage ECMO on ED patients with cardiogenic shock and pulses as part of their ED ECMO program. The majority of programs (>60%) did not have an upper age exclusion limit of >70 years, many did not exclude patients with unwitnessed arrest (44%) or without bystander CPR (>60%). More surprising is how many did not formally exclude patients with >10min of no CPR (~70%), concomitant major trauma (>50%), or known significant medical comorbidities, such as cachexia, obesity or pre-existing renal failure (85–95%). Aggregate examples of exclusion criteria volunteered by respondents included: low exhaled end-tidal carbon dioxide (EtCO2) or poor quality CPR, recent thrombolytics, physician discretion, documented intracranial hemorrhage, or out of hospital arrest.
Table 2.
Inclusion/Exclusion Criteria and Services Involved (total n=36)
| Variables | % | N | |
|---|---|---|---|
| Typically included patient phenotypes | Cardiac arrest with VF/VT initial rhythm | 75 | 27 |
| Cardiac arrest with any initial rhythm | 61.1 | 22 | |
| Respiratory arrest (not otherwise specified) | 50 | 18 | |
| Hypothermia | 55.6 | 20 | |
| Drug Overdose | 47.2 | 17 | |
| Cardiogenic shock with pulses | 55.6 | 20 | |
| Other | 2.8 | 1 | |
| Exclusion criteria (any) | Age >60 | 8.3 | 3 |
| Age >70 | 27.8 | 10 | |
| Obesity | 5.6 | 2 | |
| Cachexia | 13.9 | 5 | |
| Apparent dialysis access/fistula | 11.1 | 4 | |
| Concomitant major trauma | 44.4 | 16 | |
| Suspected/Presumed Drug Overdose | 5.6 | 2 | |
| Unwitnessed | 55.6 | 20 | |
| Non-Shockable Initial Rhythm | 13.9 | 5 | |
| No bystander CPR | 38.9 | 14 | |
| >5min of no bystander CPR | 5.6 | 2 | |
| >10min of no bystander CPR | 25 | 9 | |
| Other | 22.2 | 8 | |
| Services involved throughout entire ECMO Process | |||
| Emergency Medicine (missing obs. =5) | Yes, always | 41.9 | 13 |
| Yes, as needed | 29 | 9 | |
| No, but available | 3.2 | 1 | |
| No | 25.8 | 8 | |
| Cardiothoracic Surgery (missing obs.=4) | Yes, always | 68.8 | 22 |
| Yes, as needed | 25 | 8 | |
| No | 6.3 | 2 | |
| Cardiology (missing obs.=7) | Yes, always | 17.2 | 5 |
| Yes, as needed | 41.4 | 12 | |
| No | 41.4 | 12 | |
| Interventional Cardiology (missing obs. =8) | Yes, always | 17.9 | 5 |
| Yes, as needed | 35.7 | 10 | |
| No | 46.4 | 13 | |
| Vascular Surgery (missing obs. =10) | Yes, always | 3.9 | 1 |
| Yes, as needed | 23.1 | 6 | |
| No, but available | 23.1 | 6 | |
| No, not available | 3.9 | 1 | |
| No | 46.2 | 12 | |
| Perfusion (missing obs. =6) | Yes, always | 76.7 | 23 |
| Yes, as needed | 16.7 | 5 | |
| No | 6.7 | 2 | |
| Neurology (missing obs.=9) | Yes, always | 3.7 | 1 |
| Yes, as needed | 33.3 | 9 | |
| No | 63.0 | 17 | |
| Palliative Care (missing obs.=10) | Yes, always | 19.2 | 5 |
| Yes, as needed | 19.2 | 5 | |
| No | 61.5 | 16 | |
| Pharmacy (missing obs.=8) | Yes, always | 75 | 21 |
| Yes, as needed | 10.7 | 3 | |
| No, but available | 3.6 | 1 | |
| No | 10.7 | 3 |
Abbreviations: VF--Ventricular Fibrillation; VT--Ventricular Tachycardia; CPR--Cardiopulmonary Resuscitation
Devices and Techniques
Nearly 40% of programs use automated compression devices during cannulation, and >70% obtain vascular access percutaneously. Over 30% of programs utilize more protocols for their ED ECMO resuscitations compared to standard resuscitations. Of note, while 36% of programs perform a surgical cut-down to the femoral vessels, nearly 25% perform a modified cut-down, a hybrid of percutaneous and cut down, in which the percutaneous approach enters the vessels within the cut-down site.
A plurality of programs utilizes the Maquet Rotaflow® console (41%, Table 3), a small centrifugal pump console, which may be due to the comparative cost of the Rotaflow®.26 Almost all programs utilize distal limb perfusion catheters, which are equally likewise placed by cardiothoracic (CT) surgery. The majority of programs systematically assess for left ventricular over-distension, and use of a left ventricular impellar-type decompression pump or an atrial septostomy are common ways to decompress. Among the “Other” category, 8 programs volunteered that they place a left ventricular or left atrial vent in a “Y” configuration with the venous cannula. While multiple approaches and methods have been described,27 for percutaneous VA ECMO this is generally a femoral venous approach percutaneous cannula (8–15 Fr) passed across the atrial septum into the left atrium and connected to the pre-pump side of the ECMO circuit in order to continuously aspirate blood.
All programs used anticoagulation (not shown) and most programs begin anticoagulation around the time of vascular access or dilation (Table 3). The choice of anticoagulant varied highly, as did the target ranges for laboratory monitoring (Figure 3). First line anticoagulant medications included unfractionated heparin (60%) and bivalrudin (10%), whereas bivalrudin and argatroban were often second line (>50% and 29%, respectively), whereas low molecular weight heparin (LMWH) and coumadin were almost never used. Programs used a variety of laboratory tests for monitoring anticoagulation levels for each anticoagulant that was used.
Personnel
Nearly all ED ECMO programs (>85%) involve CT surgery, perfusion, and pharmacy and most (70%) involve emergency medicine (Table 2). Unsurprisingly, as ECMO in adults has historically been managed by cardiothoracic surgery, in the vast majority of programs surveyed, the cannula is placed by CT surgery, with only 4 programs having emergency medicine physicians cannulate (Table 1). In up to 45% of ED ECMO programs, cardiology/interventional cardiology is not defined as “always” involved. Over half of programs (60%) neither involve neurology nor palliative care. Very few programs (2, 6%) operate without regular perfusion involvement.
Discussion
In an effort to further the study of eCPR and therefore use appropriateness, we sought to characterize eCPR practice patterns and current use for the undifferentiated cardiac arrest in the US. Attempts have been made to characterize practices surrounding eCPR by systematically reviewing the literature,28 but unfortunately, to date, the eCPR literature is mostly single center with limited descriptions of team composition or management and we found no eCPR practice surveys. To our knowledge, our study is the first to describe in detail the practice and program characteristics of centers actively performing eCPR on undifferentiated patients in cardiac arrest in the emergency department. We identified 36 centers that are actively engaged in providing ED eCPR in the United States. Nearly all centers are academic or teaching, and involve the core services of CT surgery, perfusion and pharmacy, with most cannulations performed by CT surgery and only rarely by emergency physicians. Few programs are formally multidisciplinary, but a majority have similar peri-cannulation and core management similarities, including exclusion criteria, cannula size, separation of the cannulation procedure from the resuscitation, and the use of anticoagulation, though differences exist in post cannulation management, including drug selection, routine cardiac catheterization and left ventricular decompression.
Patient Selection
Patient selection practices are highly varied. The significance of the general lack of strict selection criteria to identify arrest etiologies or patient types may signify that most ED ECMO programs in the US are not systematically targeting a particular patient type or post-ECMO therapy. It is not clear from our study whether a lack of inclusion and exclusion criteria imply a lack of post eCPR etiology targeted therapies, such as coronary revascularization, pulmonary thrombectomy or toxin hemodialysis, though we did observe that coronary angiography is only used “always” in 4 (12%) of programs. Though in light of the broad inclusion criteria of our study group and resultant heterogeneity of arrest etiologies, the necessity of requiring angiography after every eCPR can be debated. Publications of eCPR include heterogenous etiologies ranging from hypothermia to refractory arrhythmias,6,9 with some of the best outcomes in those series with narrow inclusion criteria and multidisciplinary coordinated therapies post eCPR.6,9 Without strict inclusion and exclusion criteria, it is unlikely that a homogenous population will be captured in any eCPR program. Juxtaposed to this is the observation that higher volume ECMO centers have been associated with improved outcomes.29
Devices and Techniques
The majority of cannulations in our study occurred via the percutaneous peripheral route. Traditionally, ECMO cannulations were performed centrally during cardiopulmonary bypass surgery, or peripherally via surgical cutdown. Since the 1990s, use of the peripheral percutaneous approach for patients not undergoing cardiopulmonary bypass has increased in usage.30 For peripheral VA-ECMO in adults, access is mostly commonly femoral,31,32 with other peripheral locations (axillary33) and methods (triple wire34) described. For emergent VA cannulations, the peripheral femoral approach predominates in adults.7–10,21,31,35,36 Given the known risk of vascular injury, ischemia and edema associated with femoral cannulations,32,35,37 it is not surprising that vascular surgery is involved in >25% of US programs.
Personnel
In our study, cardiothoracic surgeons perform cannulation at most centers, though several report cannulation by emergency physicians. We also found that most programs limit mandated personnel to the specialties of CT surgery, perfusion and pharmacy, with emergency physicians and cardiologists “always” involved in <50% and <20% of the programs, respectively.
We could not find clear published documentation of ECMO team composition for eCPR. First described in 1966 by a surgeon,38 the first eCPR/salvage ECMO case series was published by a thoracic surgeon in the 1970s.39 Subsequent series from thoracic surgeons and cardiologists were published in the 1990s,40,41 followed by the first publication by an emergency physician in 1997.42 While there were notable subsequent larger series of eCPR43 and a prospective observational study in 2008,8 the next publication of emergency physician-initiated eCPR for OHCA was not published until 2012.7,19 Since that time, team composition for emergency department eCPR has not well characterized. This is the first study to describe in detail emergency department eCPR team composition and roles. Given the potential complexity of care coordination for post-eCPR interventions (ex: angiography, LV decompression) and management, we agree that a rehearsed and multidisciplinary team approach to the entire ED ECMO process may be best for patient care.5
In summary, we present the first national survey of ED ECMO practices in the US. We have demonstrated that this practice is in its infancy in the United States, and optimal patient selection, techniques, and personnel are still being defined. Most importantly, because ED ECMO provides cardiopulmonary support, but may not reverse the cause of the arrest, we submit that that the range of survival observed through published eCPR cases could be improved through defined matching of eCPR to therapies directed at identification and reversal of the arrest. We agree with previous suggestions that this is most easily achieved through studies focusing on narrow selection criteria to isolate arrest etiologies.5 This may be best demonstrated in the CHEER trial, where eCPR was followed by angiography, revascularization and hypothermia, and in 51% of patients who underwent coronary angiography, a culprit lesion was identified and opened.21
Lastly, our study demonstrated that even among the low numbers of ED ECMO at many centers, nearly all centers had experience with ICU based and inpatient eCPR/salvage ECMO before expanding to the ED. We advocate that given the complexity of eCPR and the association of higher volume ECMO with improved outcomes,29 that use appropriateness of eCPR and ED ECMO be studied in experienced, high volume centers. We do not yet know if eCPR is superior to traditional CPR for cardiac arrest, or only superior in those with refractory cardiac arrest. We call for structured randomized clinical trials among experienced centers with strict inclusion and exclusion criteria, structured cannulation processes and post arrest management derived from those programs with the best published eCPR outcomes.
Limitations
The survey was generated based on published characteristics of eCPR series and studies in the literature, but not previously validated. We would encourage further studies to use our survey and validate it, which we will provide. As may be noted from the Discussion, we did not define ED ECMO as only applying to OHCA patients. Of note, only two centers listed OHCA as exclusion for eCPR. One short coming was that the definitions of some terminology were not specified; for example, the term “involvement” varied, such that while 70% of respondents reported that patients would “always” or “as needed” go to the cardiac catheterization laboratory after ED ECMO, 45% of programs also reported that cardiology/interventional cardiology was not involved throughout the entire ED ECMO process. It seems that either other services are performing the cardiac catheterization, or many programs operate with auxiliary support services that are not considered to be involved throughout the entire eCPR/ED ECMO program.
Conclusions
Among all centers submitting eCPR cases to the ELSO registry, approximately one-third have performed eCPR in the ED. Among these, the majority expanded from in-patient salvage ECMO, also perform in-patient eCPR, and do not have many formal inclusion or exclusion criteria. We hope that describing the national landscape brings awareness to this growing rescue technique, and enables emerging programs to refine their approaches.
Acknowledgments
This study had no external funding source and was conducted on behalf of ERECT (www.erectcollaborative.org), a non-profit collaborative of centers and researchers dedicated to furthering research on extracorporeal resuscitation and emergency department ECMO. This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-02 (formerly 8UL1TR000105 and UL1RR025764).
Footnotes
Conflicts of Interest Statement
None of the authors declares any conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Joseph E. Tonna, Assistant Professor, Associate Director, ECMO Services, Division of Cardiothoracic Surgery, Critical Care, Division of Emergency Medicine, Department of Surgery, University of Utah School of Medicine, 30 North 1900 East, 3C127, Department of Surgery, University of Utah, Salt Lake City, UT 84132, @JoeTonnaMD.
Nicholas J Johnson, Division of Pulmonary and Critical Care Medicine, Department of Medicine, University of Washington, Seattle, WA 98195-6522.
John Greenwood, Assistant Professor, Department of Emergency Medicine, Department of Anesthesiology & Critical Care, Perelman School of Medicine at the University of Pennsylvania, 3400 Spruce Street, Ground Ravdin, Philadelphia, PA 19104.
David F Gaieski, Associate Professor, Sidney Kimmel Medical College at Thomas Jefferson University, Department of Emergency Medicine, Vice Chair for Resuscitation Services, Director, Emergency Critical Care, 1025 Walnut Street, 300 College Building, Philadelphia, PA 19107.
Zachary Shinar, Department of Emergency Medicine, Sharpe Memorial Hospital, 7901 Frost Street, San Diego, CA 92123.
Joseph M. Bellezo, Chairman, Department of Emergency Medicine, Director, Emergency Department ECMO Services, Department of Emergency Medicine, Sharpe Memorial Hospital, 7901 Frost Street, San Diego, CA 92123.
Lance Becker, Professor and Chair, Hofstra Northwell School of Medicine, Chairman of Emergency Medicine at Long Island Jewish Medical Center &, North Shore University Hospital, 270-05 76th Ave., New Hyde Park, NY 11040.
Atman P. Shah, Clinical Director, Section of Cardiology, Co-Director, Adult Cardiac Catheterization Laboratory, Associate Professor of Medicine, The University of Chicago, 5841 S. Maryland Avenue, MC 6080, Chicago, IL 60637.
Scott T. Youngquist, Medical Director, Salt Lake City Fire Department, Associate Professor of Surgery, Research Director, Division of Emergency Medicine, Department of Surgery, University of Utah School of Medicine, 30 North 1900 East, 1C26 SOM, Salt Lake City, Utah 84132, Cell (801) 540-5594, Fax (801) 585-0603, Twitter: @ScottYoungquist.
Michael P Mallin, Assistant Professor of Surgery, Ultrasound Director, Education Director, Division of Emergency Medicine, Department of Surgery, University of Utah, 30N 1900E Rm 1c026, Salt Lake City, UT 84132.
James Franklin Fair, III, Visiting Instructor, Division of Emergency Medicine, Department of Surgery, University of Utah School of Medicine, 30N 1900E Rm 1c026, Salt Lake City, UT 84132.
Kyle J. Gunnerson, Associate Professor, Departments of Emergency Medicine, Anesthesiology, and Internal Medicine, Michigan Center for Integrative Research In Critical Care (MCIRCC), University of Michigan, 1500 E Medical Center Dr., Ann Arbor, MI 48109-5303.
Cindy Weng, Department of Pediatrics, University of Utah, 295 Chipeta Way, Salt Lake City, Utah 84108.
Stephen McKellar, Assistant Professor of Surgery, Director of ECMO Services, Division of Cardiothoracic Surgery, Department of Surgery, University of Utah School of Medicine, 30 N 1900 E, SOM 3C 127, Salt Lake City, UT 84132.
References
- 1.Brooks SC, Anderson ML, Bruder E, et al. Part 6: Alternative Techniques and Ancillary Devices for Cardiopulmonary Resuscitation. Circulation. 2015;132(18 suppl 2):S436–S443. doi: 10.1161/CIR.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 2.Berg RA, Sanders AB, Kern KB, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104(20):2465–2470. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 3.Goto Y, Funada A, Goto Y. Relationship Between the Duration of Cardiopulmonary Resuscitation and Favorable Neurological Outcomes After Out-of-Hospital Cardiac Arrest: A Prospective, Nationwide, Population-Based Cohort Study. Journal of the American Heart Association. 2016;5(3):e002819. doi: 10.1161/JAHA.115.002819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ihnát Rudinská L, Hejna P, Ihnát P, Tomášková H, Smatanová M, Dvořáček I. Intra-thoracic injuries associated with cardiopulmonary resuscitation - Frequent and serious. Resuscitation. 2016;103:66–70. doi: 10.1016/j.resuscitation.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Müller T, Lubnow M. The future of E-CPR: A joint venture. Resuscitation. 2013;84(11):1463–1464. doi: 10.1016/j.resuscitation.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial) Resuscitation. 2015;86:88–94. doi: 10.1016/j.resuscitation.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Bellezzo JM, Shinar Z, Davis DP, et al. Emergency physician-initiated extracorporeal cardiopulmonary resuscitation. Resuscitation. 2012;83(8):966–970. doi: 10.1016/j.resuscitation.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y-S, Lin J-W, Yu H-Y, et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: an observational study and propensity analysis. The Lancet. 2008;372(9638):554–561. doi: 10.1016/S0140-6736(08)60958-7. [DOI] [PubMed] [Google Scholar]
- 9.Fagnoul D, Taccone FS, Belhaj A, et al. Extracorporeal life support associated with hypothermia and normoxemia in refractory cardiac arrest. Resuscitation. 2013;84(11):1519–1524. doi: 10.1016/j.resuscitation.2013.06.016. [DOI] [PubMed] [Google Scholar]
- 10.Johnson NJ, Acker M, Hsu CH, et al. Extracorporeal life support as rescue strategy for out-of-hospital and emergency department cardiac arrest. Resuscitation. 2014;85(11):1527–1532. doi: 10.1016/j.resuscitation.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 11.Sakamoto T, Morimura N, Nagao K, et al. Extracorporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation. 2014;85(6):762–768. doi: 10.1016/j.resuscitation.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 12.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics-2015 update: a report from the american heart association. Circulation. 2015;131(4):e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 13.Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS. Trends in Survival after In-Hospital Cardiac Arrest. The New England journal of medicine. 2012;367(20):1912–1920. doi: 10.1056/NEJMoa1109148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hunziker S, Semmer NK, Tschan F, Schuetz P, Mueller B, Marsch S. Dynamics and association of different acute stress markers with performance during a simulated resuscitation. Resuscitation. 2012;83(5):572–578. doi: 10.1016/j.resuscitation.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 15.Hunziker S, Pagani S, Fasler K, Tschan F, Semmer NK, Marsch S. Impact of a stress coping strategy on perceived stress levels and performance during a simulated cardiopulmonary resuscitation: a randomized controlled trial. BMC Emergency Medicine. 2013;13(1):8. doi: 10.1186/1471-227X-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunziker S, Laschinger L, Portmann-Schwarz S, Semmer NK, Tschan F, Marsch S. Perceived stress and team performance during a simulated resuscitation. Intensive care medicine. 2011;37(9):1473–1479. doi: 10.1007/s00134-011-2277-2. [DOI] [PubMed] [Google Scholar]
- 17.Choi DS, Kim T, Ro YS, et al. Extracorporeal life support and survival after out-of-hospital cardiac arrest in a nationwide registry: A propensity score-matched analysis. Resuscitation. 2016;99:26–32. doi: 10.1016/j.resuscitation.2015.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Shin TG, Jo IJ, Sim MS, et al. Two-year survival and neurological outcome of inhospital cardiac arrest patients rescued by extracorporeal cardiopulmonary resuscitation. International journal of cardiology. 2013;168(4):3424–3430. doi: 10.1016/j.ijcard.2013.04.183. [DOI] [PubMed] [Google Scholar]
- 19.Shinar Z, Bellezzo J, Paradis N, et al. Emergency department initiation of cardiopulmonary bypass: a case report and review of the literature. JEM. 2012;43(1):83–86. doi: 10.1016/j.jemermed.2011.06.134. [DOI] [PubMed] [Google Scholar]
- 20.Paden ML, Conrad SA, Rycus PT, Thiagarajan RR. Extracorporeal Life Support Organization Registry Report 2012. ASAIO Journal. 2013;59(3):202–210. doi: 10.1097/MAT.0b013e3182904a52. [DOI] [PubMed] [Google Scholar]
- 21.Stub D, Bernard S, Pellegrino V, et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial) Resuscitation. 2014:1–7. doi: 10.1016/j.resuscitation.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Shinar Z, Bellezzo JM, Weingart SD. ED ECMO. [Accessed January 31, 2016];ED ECMO. Audio-Podcast http://edecmo.org/category/podcasts/
- 23.Willis GB, Smith T, Lee HJ. Do Additional Recontacts to Increase Response Rate Improve Physician Survey Data Quality? Medical Care. 2013;51(10):945–948. doi: 10.1097/MLR.0b013e3182a5023d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dillman DA, Smyth Jolene D, Christian Leah M. Internet, Mail, and Mixed Mode Surveys: The Tailored Design Method. 3. New York: John Wiley & Sons; 2009. [Google Scholar]
- 25.Klabunde CN, Willis GB, McLeod CC, et al. Improving the Quality of Surveys of Physicians and Medical Groups: A Research Agenda. Evaluation & the Health Professions. 2012;35(4):477–506. doi: 10.1177/0163278712458283. [DOI] [PubMed] [Google Scholar]
- 26.Palanzo DA, Baer LD, El-Banayosy A, Wang S, Undar A, Pae WE. Choosing a pump for extracorporeal membrane oxygenation in the USA. Artif Organs. 2014;38(1):1–4. doi: 10.1111/aor.12215. [DOI] [PubMed] [Google Scholar]
- 27.Aiyagari RM, Rocchini AP, Remenapp RT, Graziano JN. Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Crit Care Med. 2006;34(10):2603–2606. doi: 10.1097/01.CCM.0000239113.02836.F1. [DOI] [PubMed] [Google Scholar]
- 28.Ortega-Deballon I, Hornby L, Shemie SD, Bhanji F, Guadagno E. Extracorporeal resuscitation for refractory out-of-hospital cardiac arrest in adults: A systematic review of international practices and outcomes. Resuscitation. 2016;101:1–9. doi: 10.1016/j.resuscitation.2016.01.018. [DOI] [PubMed] [Google Scholar]
- 29.Barbaro RP, Odetola FO, Kidwell KM, et al. Association of Hospital-Level Volume of Extracorporeal Membrane Oxygenation Cases and Mortality. Analysis of the Extracorporeal Life Support Organization Registry. American Journal of Respiratory and Critical Care Medicine. 2015;191(8):894–901. doi: 10.1164/rccm.201409-1634OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kolla S, Awad SS, Rich PB, Schreiner RJ, Hirschl RB, Bartlett RH. Extracorporeal life support for 100 adult patients with severe respiratory failure. Annals of Surgery. 1997;226(4):544–564. doi: 10.1097/00000658-199710000-00015. discussion 565–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Napp LC, Kühn C, Hoeper MM, et al. Cannulation strategies for percutaneous extracorporeal membrane oxygenation in adults. Clinical Research in Cardiology. 2015;105(4):283–296. doi: 10.1007/s00392-015-0941-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reeb J, Olland A, Renaud S, et al. Vascular access for extracorporeal life support: tips and tricks. Journal of thoracic disease. 2016;8(S4):S353–S363. doi: 10.21037/jtd.2016.04.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Biscotti M, Bacchetta M. The “sport model”: extracorporeal membrane oxygenation using the subclavian artery. The Annals of Thoracic Surgery. 2014;98(4):1487–1489. doi: 10.1016/j.athoracsur.2014.02.069. [DOI] [PubMed] [Google Scholar]
- 34.Grasselli G, Pesenti A, Marcolin R, et al. Percutaneous vascular cannulation for extracorporeal life support (ECLS): a modified technique. Int J Artif Organs. 2010;33(8):553–557. doi: 10.1177/039139881003300806. [DOI] [PubMed] [Google Scholar]
- 35.Rupprecht L, Lunz D, Philipp A, Lubnow M, Schmid C. Pitfalls in percutaneous ECMO cannulation. Heart, lung and vessels. 2015;7(4):320–326. [PMC free article] [PubMed] [Google Scholar]
- 36.Ventetuolo CE, Muratore CS. Extracorporeal Life Support in Critically Ill Adults. American Journal of Respiratory and Critical Care Medicine. 2014;190(5):497–508. doi: 10.1164/rccm.201404-0736CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Foley PJ, Morris RJ, Woo EY, et al. Limb ischemia during femoral cannulation for cardiopulmonary support. Journal of vascular surgery. 2010;52(4):850–853. doi: 10.1016/j.jvs.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy JH. The role of assisted circulation in cardiac resuscitation. JAMA. 1966;197(8):615–618. [PubMed] [Google Scholar]
- 39.Mattox KL, Beall AC. Resuscitation of the moribund patient using portable cardiopulmonary bypass. The Annals of thoracic surgery. 1976;22(5):436–442. doi: 10.1016/s0003-4975(10)64452-9. [DOI] [PubMed] [Google Scholar]
- 40.Hill JG, Bruhn PS, Cohen SE, et al. Emergent applications of cardiopulmonary support: A multiinstitutional experience. The Annals of thoracic surgery. 1992;54(4):699–704. doi: 10.1016/0003-4975(92)91014-z. [DOI] [PubMed] [Google Scholar]
- 41.Mooney M, Arom K, Joyce L, et al. Emergency cardiopulmonary bypass support in patients with cardiac arrest. The Journal of Thoracic and Cardiovascular Surgery. 1991;101(3):450–454. [PubMed] [Google Scholar]
- 42.Younger JG, Schreiner RJ, Swaniker F, Hirschl RB, Chapman RA, Bartlett RH. Extracorporeal Resuscitation of Cardiac Arrest. Academic emergency medicine : official journal of the Society for Academic Emergency Medicine. 1999;6(7):700–707. doi: 10.1111/j.1553-2712.1999.tb00438.x. [DOI] [PubMed] [Google Scholar]
- 43.Chen Y-S, Chao A, Yu H-Y, et al. Analysis and results of prolonged resuscitation in cardiac arrest patients rescued by extracorporeal membrane oxygenation. JAC. 2003;41(2):197–203. doi: 10.1016/s0735-1097(02)02716-x. [DOI] [PubMed] [Google Scholar]