Abstract
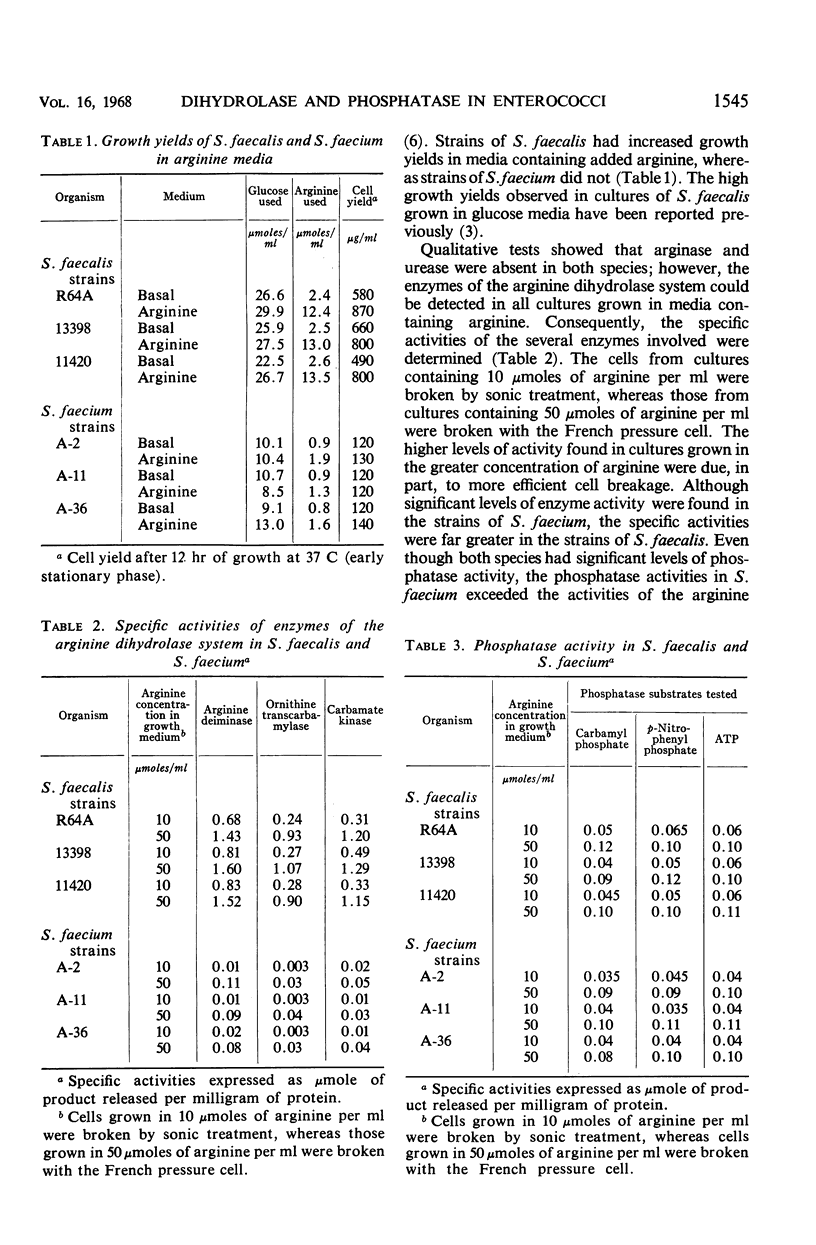
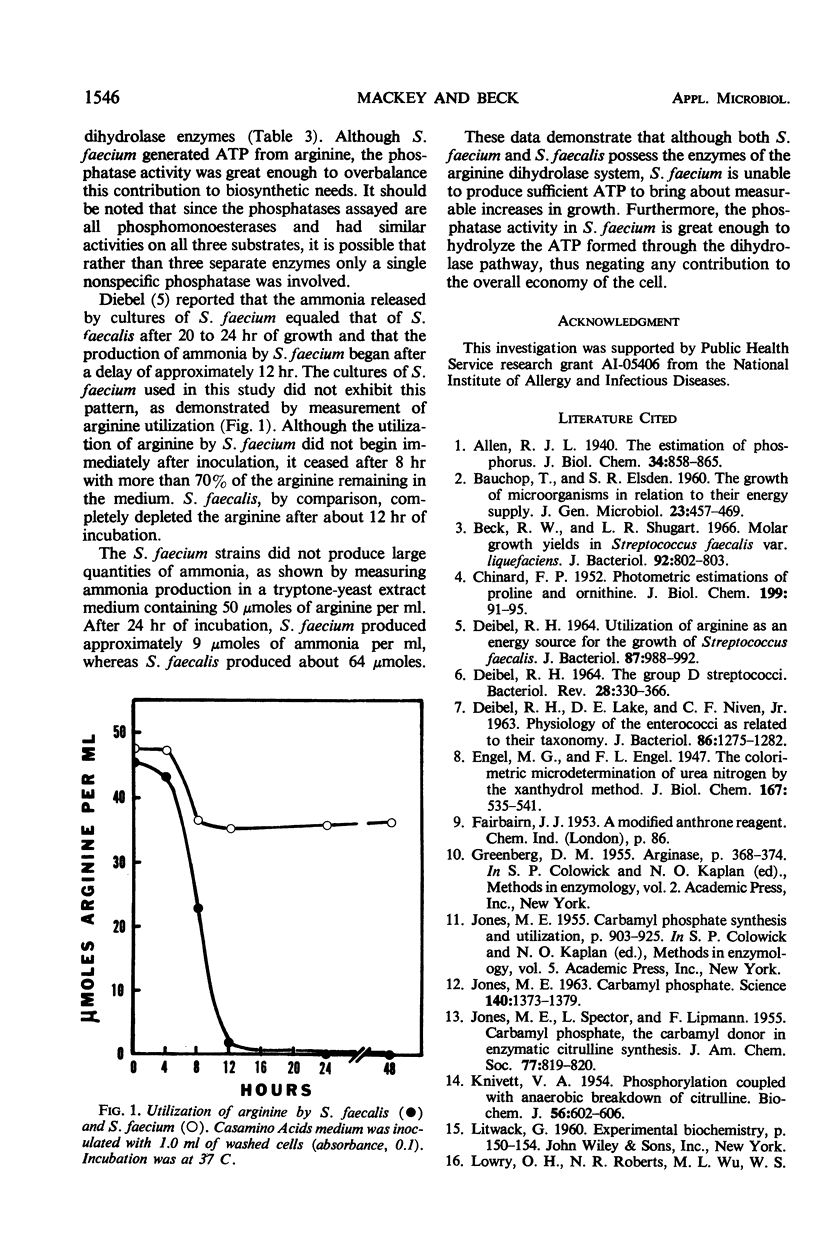
Strains of Streptococcus faecalis and S. faecium are known to produce ammonia from arginine, but only S. faecalis couples the adenosine triphosphate (ATP) produced through the arginine dihydrolase pathway to growth processes. The specific activities of the arginine dihydrolase enzymes were found to be much lower in S. faecium (0.01 to 0.10) than in S. faecalis (0.24 to 1.60). Phosphatase activities in both strains were similar (up to 0.11), but equaled or exceeded the activities of the arginine dihydrolase enzymes in S. faecium. The failure of S. faecium to show increased growth in arginine media is explained on the basis of low activities of the arginine dihydrolase enzymes coupled with sufficient phosphatase activity to negate any benefit from ATP formed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. J. The estimation of phosphorus. Biochem J. 1940 Jun;34(6):858–865. doi: 10.1042/bj0340858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUCHOP T., ELSDEN S. R. The growth of micro-organisms in relation to their energy supply. J Gen Microbiol. 1960 Dec;23:457–469. doi: 10.1099/00221287-23-3-457. [DOI] [PubMed] [Google Scholar]
- Beck R. W., Shugart L. R. Molar growth yields in Streptococcus faecalis var. liquefaciens. J Bacteriol. 1966 Sep;92(3):802–803. doi: 10.1128/jb.92.3.802-803.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHINARD F. P. Photometric estimation of proline and ornithine. J Biol Chem. 1952 Nov;199(1):91–95. [PubMed] [Google Scholar]
- DEIBEL R. H., LAKE D. E., NIVEN C. F., Jr PHYSIOLOGY OF THE ENTEROCOCCI AS RELATED TO THEIR TAXONOMY. J Bacteriol. 1963 Dec;86:1275–1282. doi: 10.1128/jb.86.6.1275-1282.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEIBEL R. H. THE GROUP D STREPTOCOCCI. Bacteriol Rev. 1964 Sep;28:330–366. doi: 10.1128/br.28.3.330-366.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deibel R. H. Utilization of arginine as an energy source for the growth of Streptococcus faecalis. J Bacteriol. 1964 May;87(5):988–992. doi: 10.1128/jb.87.5.988-992.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNIVETT V. A. Phosphorylation coupled with anaerobic breakdown of citrulline. Biochem J. 1954 Apr;56(4):602–606. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., WU M. L., HIXON W. S., CRAWFORD E. J. The quantitative histochemistry of brain. II. Enzyme measurements. J Biol Chem. 1954 Mar;207(1):19–37. [PubMed] [Google Scholar]
- SHUGART L. R., BECK R. W. PURIFICATION AND ACTIVITY OF PROTEINASE OF STREPTOCOCCUS FAECALIS VAR. LIQUEFACIENS. J Bacteriol. 1964 Sep;88:586–590. doi: 10.1128/jb.88.3.586-590.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLADE H. D., SLAMP W. C. The formation of arginine dihydrolase by streptococci and some properties of the enzyme system. J Bacteriol. 1952 Oct;64(4):455–466. doi: 10.1128/jb.64.4.455-466.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TERNBERG J. L., HERSHEY F. B. Colorimetric determination of blood ammonia. J Lab Clin Med. 1960 Nov;56:766–776. [PubMed] [Google Scholar]
- TOMBS M. P., SOUTER F., MACLAGAN N. F. The spectrophotometric determination of protein at 210 millimicrons. Biochem J. 1959 Sep;73:167–171. doi: 10.1042/bj0730167. [DOI] [PMC free article] [PubMed] [Google Scholar]


