Abstract
Health-care professionals in India frequently manage injection or infusion therapies in persons with diabetes (PWD). Patients taking insulin should know the importance of proper needle size, correct injection process, complication avoidance, and all other aspects of injection technique from the first visit onward. To assist health-care practitioners in their clinical practice, Forum for Injection Technique and Therapy Expert Recommendations, India, has updated the practical advice and made it more comprehensive evidence-based best practice information. Adherence to these updated recommendations, learning, and translating them into clinical practice should lead to effective therapies, improved outcomes, and lower costs for PWD.
Keywords: Forum for Injection Technique, Forum for Injection Technique and Therapy Expert Recommendations, injection technique, insulin, lipohypertrophy, persons with diabetes
INTRODUCTION
Diabetes and its complications impact the health, well-being, and finances of individual and family. India and China lead the world with the largest number of persons with diabetes (PWD). As per 2015 data, India had 69.2 million people living with diabetes, which is projected to reach 87 million by 2030.[1,2]
Recently, there has been increased emphasis on optimal insulin therapy and blood glucose control in type 2 diabetes mellitus (T2DM). A few PWD, however, realize that correct insulin injection technique is as important in achieving glycemic goal as the type and dose of insulin delivered.[3] Incorrect choice of injection site, delivery devices, and technique may modify insulin absorption parameters, leading to disconnect between maximum glucose load and peak insulin effect. This leads to either glycemic variability or unexplained hypoglycemia and subsequently compromised long-term outcomes.[4,5]
FORUM FOR INJECTION TECHNIQUE INDIA RECOMMENDATIONS
Diabetes management needs lifelong commitment from health-care providers (HCPs) as well as PWD. Even among the literate patient groups, inclination to practice insulin self-administration is low.[5] All injectable agents rely on correct injection technique for optimal delivery and effect.[6] Physician awareness and willingness to convey this information, can help promote correct injection technique among PWD and even other HCPs.[7]
To create recommendations regarding this, the first Indian recommendations for best practice in insulin injection technique were published by the Forum for Injection Technique (FIT), India in 2012. An addendum was published in 2013 covering special populations and insulin pump infusion technique. To make FIT India more comprehensive, another addendum was published in 2014 with focus on injection–mealtime interval, methods for minimizing pain during injections, amyloidosis, and adherence.
Later, the FIT India 2.0 recommendations, 2015 were updated incorporating all additional information and evidence. An addendum to the FIT India 2.0 was published in 2016 with focus on insulin use in hospital indoor settings.
NEED FOR REVISION OF FORUM FOR INJECTION TECHNIQUE INDIA 2.0 RECOMMENDATIONS
The FIT recommendations have addressed the interests of HCPs and PWD and have improved health outcomes by ensuring correct technique and insulin delivery. However, recent advances in device manufacturing, newer research findings, and updated international guidelines demand renewed commitment toward optimizing insulin injection practices.
Numerous reports have described the reuse of insulin syringes, pens, and needles by HCPs as well as patients, potentially exposing them to needlestick injuries (NSIs) and bloodborne diseases. Therefore, it has become imperative to add best practices, specifically addressing the safe use of insulin devices. The sections on adverse safety outcomes of faulty technique and health education to both HCPs and patients on safe disposal of diabetic sharps and wastes need to be strengthened. Measures to enhance awareness of the good injection practices among HCPs, as well as patients, and section on addressing barriers of insulin injection therapy also need to be updated.
MATERIALS AND METHODS
To update these recommendations, literature search was undertaken looking for recent systematic reviews, meta-analysis, and clinical surveys of insulin injection technique used for improving injection practice. Evidence statements were developed for the issues listed, following the process recommended by the FIT scientific advisory board, India. Specific wording of the recommendations and supporting information were collated, and a grading was allocated to the recommendations based on the evidence statements. The subsequent document was circulated to the FIT and Therapy Expert Recommendations (FITTER) board members and expert committee members from India, 11 South Asian experts and 22 members from Afro-Asian Referee group before being finalized for publication.
Grading of the recommendations
The grading method by Frid et al., which includes the activities-specific balance confidence scale for the strength of recommendation and 123 scales for scientific support, has been used to grade the evidence [Figure 1].[8] Certain recommendations, which are supported by manufacturer advice or drug authority guidance, have been ranked 1 in scientific support.
Figure 1.
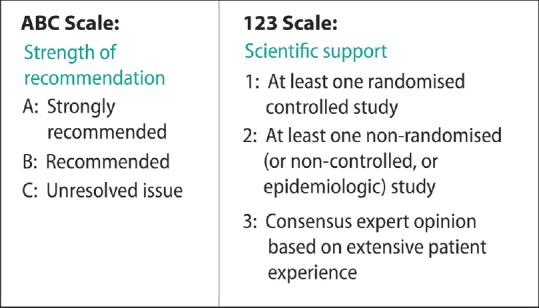
Grading criteria by Frid et al. (2010)
INSULIN INJECTION TECHNIQUE PRACTICES
Global experience
Worldwide Injection Technique Questionnaire (ITQ) Survey, one of the largest surveys in diabetes, was conducted from February 1, 2014, to June 30, 2015. It involved 13,289 insulin-injecting patients from 423 centers in 42 countries. The survey focused on patient characteristics and practical aspects of injection technique. India contributed 1011 participants from 20 centers.[9]
The key observations from this survey were as follows:[9]
Nearly 30% of participants used 4 and 8 mm needle lengths each, while 5 and 6 mm needles each were used by approximately 20%.
Nearly 40% of participants reused needles (pen/syringe) 3–5 times.
Lipohypertrophy (LH) was the most common complication of injecting insulin, self-reported by 29.0%, and found by HCPs in 30.8% of patients.
Patients with LH had higher glycated hemoglobin (HbA1c) (average 0.5%) than in patients without LH. A significantly higher LH was associated with incorrect rotation of sites and with needle reuse.
Patients with LH had high frequency of unexpected hypoglycemia and glucose variability.
Regular injection instructions in the past 6 months led to proper rotation of injection sites, but fewer than 40% of patients received such instructions.
Many used diabetic sharps ended up in public trash and constituted high risk for NSI.
These findings underscored the need for updation of recommendations, their dissemination and implementation.
Indian experience
The Indian results of the recent ITQ survey were recently published in Diabetes Therapy, March 2017.
The key observations from this survey were as follows:[10,11]
Nearly 40% of participants used 4-and 5-mm needle lengths, 8-mm needles were used by approximately 16%.
80% participants reused needles more than 3 times or more.
LH was the most common complication of injecting insulin, self-reported by 26% and found by HCPs in 22 % of patients.
Patients with LH had higher HbA1c than in patient without LH. A significantly higher LH was associated with incorrect rotation of sites and with needle reuse.
Patients with LH had high frequency of unexpected hypoglycemia and glucose variability.
Regular injection instructions in the past 6 months led to proper rotation of injection sites, but limited patients got such instructions.
Most used diabetic sharps ended up in public trash and constituted high risk for NSI.
Evidence from other studies offers similar insight into the current status of injection technique in India. A tertiary care setting reported significant gap between the insulin administration guidelines and current injecting practices. Results showed that appropriate storage of insulin vials, mixing insulin properly before injection, and practice of hand washing were followed by only 75%, 49%, and 70%, respectively. Cleaning of the injection site, injecting with the proper skinfold, injecting insulin at 90° angle were practiced by 76%, 69%, and 55%, respectively. The majority of patients (91%) disposed needle and syringes directly into the garbage and public drainage system. These results highlighted the importance of education and counseling on proper injection technique to all PWD and their caregivers.[4]
INJECTION TECHNIQUE RECOMMENDATIONS
Barriers to insulin injection therapy
Identification of barriers is a critical step toward successful diabetes self-management and takes place through a careful patient assessment. Barriers to initiating and adhering to insulin injection therapy include a wide range of obstacles relating to PWD, providers, and health-care systems.[12] These barriers can be bridged by a systematic process of preinjection assessment and counseling.
Preinjection assessment
Preinjection assessment and counseling should help to choose the correct injection regimen preparation, delivery device, and dose, while encouraging acceptance of, and adherence to therapy. Open-ended, nonjudgmental questions asked by diabetes care providers can help PWD address their concerns and adopt effective solutions.
Clinical assessment
A thorough patient assessment should precede therapy initiation. Optimization of injection technique with respect to the individual patient needs is critical for the success of injectable therapy.[13]
Safe self-administration of insulin also requires assessment of the individual's cognitive and physical abilities to follow instructions and perform the injection technique (B3).
Pharmacological plan
Decide the appropriate insulin regimen and preparation, keeping in mind the potential need for intensification or de-escalation of therapy. Choice of preparation or delivery devices may depend on expected duration of insulin therapy.
Environmental assessment
It is essential to enquire about storage conditions of insulin injection supplies and cold storage facilities (B2).[14] Insulin pens can be used instead of vials in extremes of temperatures (C1).
Sociocultural sensibilities
Sociocultural sensibilities of the community should be respected. It is advised to discuss the site of injection beforehand in Indian women so that their sensibilities are not offended (B3).
Preinjection counseling
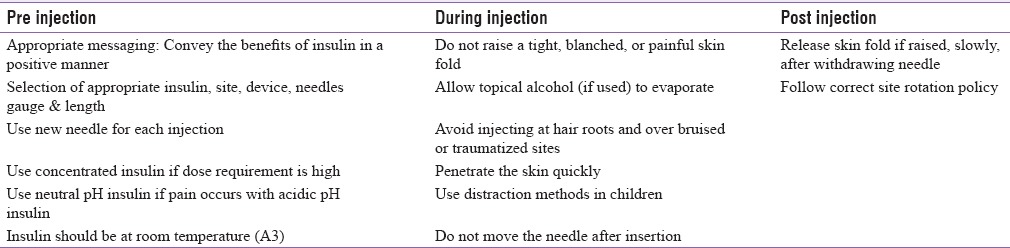
More than 25% of PWD may refuse insulin therapy due to psychological insulin resistance (PIR).[15] The most pronounced reasons associated with PIR are shown in Table 1. In an Indian survey of 198 T2DM patients, the major factors contributing to PIR were found to be pain during injection, fear of injection or hypoglycemia, social stigma, and lack of education. Psychological challenges may vary from person to person.[16] Person-specific communication strategies are required to ensure acceptance of and adherence to therapy. These strategies vary according to age group.
Table 1.
Recommendations to address barriers
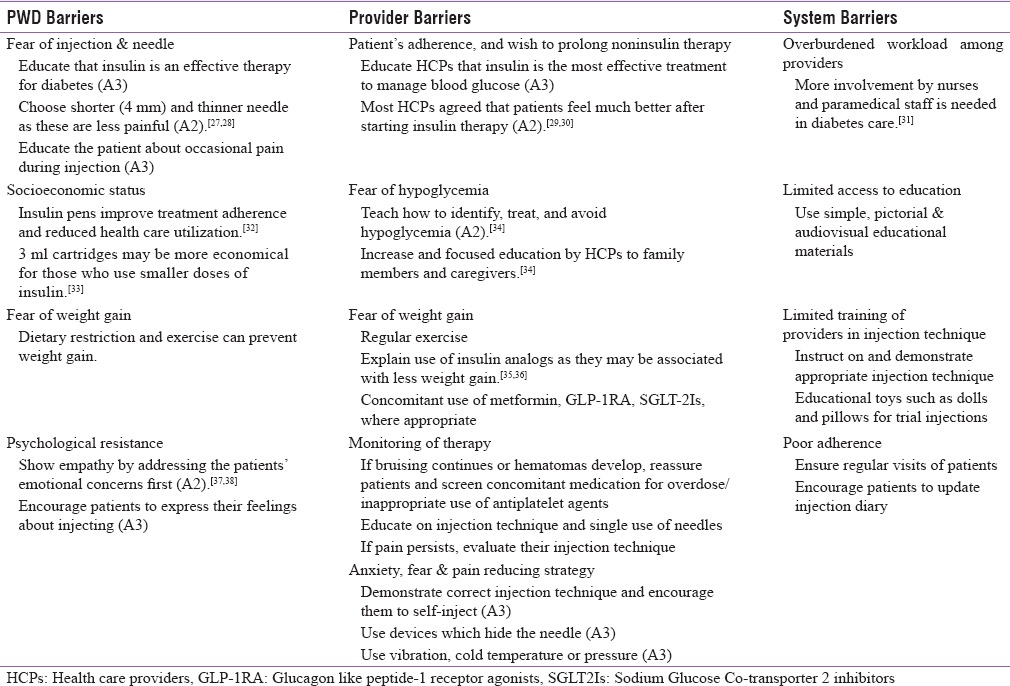
Children
Stress and anxiety developed after diagnosis of diabetes in childhood hinders parent's ability to administer insulin or encourage children to self-administer insulin (A1).[17,18,19] Proper education and demonstration of injection technique on a toy doll by HCPs may help parents, caregivers, and children to overcome anxiety and other issues (A3). Play therapy is a useful method of explaining injection technique.
Adolescents
In adolescents, several factors such as peer pressure, lack of seriousness, pain, frustration, and weight gain may also lead to suboptimal compliance. It is important to help adolescents overcome any possible misconceptions related to insulin injection by sharing information and benefits of insulin administration with them (B2).[20,21]
Adults
Proper education about the course of diabetes and the need to start insulin therapy at some time in the future is very important in all newly diagnosed adult PWD (A3). It is important to explore and acknowledge concerns of the patients.
Elderly
Geriatric PWD should be counseled about the course of diabetes and proper injection technique.[8] Kuo et al. 2016, in their study encouraged patients to try a mock self-injection before starting insulin. Group attenders who did a mock self-injection demonstrated greater insulin initiation rates.[22] Limited dexterity, visual impairment, and hearing impairment are some of the common issues to be dealt in geriatric patients.[23]
Needle phobia, trypanophobia, belonephobia, and diabetes stress
Development of trypanophobia or needle phobia may be associated with the lack of confidence that the demands of insulin therapy will be handled, a belief that insulin therapy equates to a personal failure and a perceived loss of control over life and injection-related anxiety.[8] A recent clinical practice guideline recommended exposure-based therapy (vs. no treatment) for managing children 7 years and older and adults with high levels of needle fear.[24] Psychological counseling and acknowledgment of patient's personal obstacles is recommended to overcome needle phobia (A2).[25,26]
Some practical recommendations are listed in Table 1.[27,28,29,30,31,32,33,34,35,36,37,38]
INJECTION STORAGE
Specific storage conditions provided by the manufacturer should be followed. Insulin should be stored in a cool (below 30°C), dark place and must be protected from extremes of temperature such as direct sunlight, kitchen, closed cars, green houses, top of the refrigerator, and television (A3). Insulin pens and vials, which are not in active use, should be refrigerated, but not frozen (A1).[39,40] Pens should never be stored with needles.[13,41] In places where a refrigerator is not available, it is advisable to put the vial in a plastic bag, tie a rubber band, and keep it in a wide mouthed bottle or earthen pitcher filled with water.
Travel: Surface
If the outside temperature is >30°C, insulin should be stored in a flask with ice or in a proper container while travelling.
Travel: Air
While travelling by air, one should ensure that:
DEVICE SELECTION AND USE
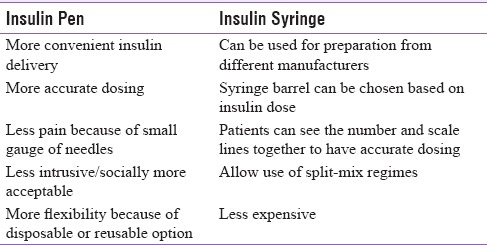
PWD can inject insulin using either syringes or insulin pens. Although the syringe is the primary injection device used in India,[43,44] debate exists over the safest, most effective method of administering insulin. In general, insulin pens are considered to be safer but their inappropriate use may lead to biological contamination of the pen cartridge. Since pens are easier to teach and use, they are frequently the choice for new insulin users. Each has its advantages as shown in Table 2.[8,32,33]
Table 2.
Advantages of insulin pen devices and conventional insulin syringes
A subcutaneous injection aims to deliver medication directly into the subcutaneous tissue without any discomfort or leakage. Ultrasound measurements reveal a mean skin thickness of about 2.2 mm. Multivariate analyses [of age, Body mass index (BMI), ethnicity and gender in adults with diabetes] demonstrate that variation in skin thickness is not clinically significant.[45] Hence, there is no medical reason to recommend needles longer than 4–6 mm to either children or adults. Extremely lean patients should be using a skin fold to inject even with a 4-mm and 5-mm needle.
Syringe and vial
The risk of intramuscular (IM) injection is likely to increase with longer insulin needles and lower BMI.[46] Six millimeter insulin syringes are the shortest needle length available and should be used to minimize accidental IM risk.[27] Shorter needles also help to reduce pain and even simplify the injection technique. Needles longer than 6 mm are not recommended in adolescents or adults.[3]
Syringe and insulin match
In India, insulin is available in the strength of U-40 and U-100 concentrations. U-200 and U-300 insulins are also available but only as pen. To avoid dosing errors, syringes that match the concentration of U-40 and U-100 concentrations must be used (A1).[47,48,49] Insulin syringes of U-100 have an orange cover and black scale markings denoting two units each, whereas U-40 syringes have a red cover and scale marking denoting one unit each.[48] Intravenous (IV) syringes must never be used for insulin administration.[50] Date of opening the vial should be written with a black/marker pen and the same should be used within a month.
Syringe needle length and size
Today needle lengths available for insulin syringes are 6 and 8 mm with 28–31 gauge sizes. Use of syringe needles in very young children (<6 years old) and exceptionally thin adults (BMI < 19) is not recommended, as it increases risk of IM injections.[51]
Pens and pen needles
Insulin pens come in two basic types: durable insulin pens, where cartridge can be reloaded into the pen; and disposable insulin pens are preloaded with insulin and are disposed once emptied. The numbering on the pen dose dial and its magnification, amount of strength and dexterity required to operate the pen should be checked. Before prescription, the anticipated duration of insulin use also determines choice of durable or reusable pen.
Pen needle length and size
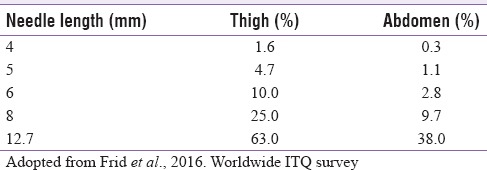
Pen needles are available in 32, 31, and 29 gauges.[52] Choice of needle length (4, 5, and 6 mm) is important. While avoiding intradermal delivery, shorter needles alleviate the risk of IM injections [Table 3].[53] There is no need to raise a skin fold with a 4 mm needle, while injecting in the upper arm.[54]
Table 3.
Estimated IM injection risk by body site
A randomized controlled study in 274 obese (BMI ≥ 30) PWD showed that mean improvement in HbA1c levels with the 4 mm pen needle were 0.08% and 0.10% as compared to 8.0 and 12.7 mm pen needle, respectively, within equivalence margins. However, the 4 mm pen needle was less painful than the larger needles (P < 0.05), with similar leakage rates reported (A2).[28,55,56,57] Although 4 mm pen needle is the needle of choice in all obese patients, a 5 mm needle may be acceptable (A1).[3,28]
One-patient/one-pen policy
Insulin pens are approved for single-patient use only, and Food and Drug Administration (FDA) indicates that even if a new needle is used, the PWD are at risk due to possible biological contamination in the pen cartridges. Therefore, insulin pen cartridges should never be shared between patients (A1).[58,59,60,61]
Needle length recommendations for children, adolescents, and adults
Children, adolescents, and adults should use 4 mm needle with pens and 6 mm needle with syringes (A2).[55,62,63]
Children, extremely lean and elder patients warrants the need of skin fold, especially when using 5 and 6 mm needle, but in children, adolescent and adults, an injection angled at 45° is required while using 6 mm needle (A1).[62,63,64,65]
In adults, injection into limbs and slim abdomen warrants the need for a skin fold with needles longer than the 5 mm (A2).[66,67]
Shorter needle should be given at a 90° angle to the skin surface (A1).[27,62,65,66,68]
No clinical justification is available for recommending needle length more than 4 mm for pen needles and 6 mm for insulin syringes in adults (A3).
PREINJECTION READINESS
Before preparation, insulin should be inspected for temperature, expiry date, possible damage to the bottle, and possible spoilage of insulin. Ideally, insulin should be at room temperature before injection (A3).[69]
Cleansing
A knowledge, attitude, and practice (KAP) survey from India, reported that 72.42% insulin users did not clean the injection site beforehand.[70] Before injection, the site should be thoroughly cleaned either with cotton ball dipped in water or with alcohol swabs (A2).[71,72,73] Cleansing should be started from the center and then move outward in the circular motion. Alcohol if used for cleansing should be evaporated completely, as the dry surface helps to minimize or avoid pain (A3).[74] Do not use soap-based detergent, chloroxylenol, and cetrimide/chlorhexidine to clean before injection (A3). Insulin can be injected provided the site is considered “socially clean.”
Resuspension of cloudy insulin
A study conducted to evaluate how patients mix insulin before injecting showed that only one person out of 180 patients could mix the insulin as per the manufacturers recommendation. In 58 out of 146 pens or cartridges (40%) the opacity of the insulin varied significantly from the expected value.[75]
Neutral protamine hagedorn (NPH) or premixed insulin packaged in vials should not be shaken vigorously, but should be repeatedly inverted for about twenty times, till the suspension is uniformly clouded (A2).[76,77] Failure to resuspend leads to significant variability in action and particularly affects nocturnal plasma insulin concentration. The method of preparation is same for both reusable and prefilled disposable pens. In case of premixed insulin is being used, insulin should be resuspended by rolling the pen and should not be shaken (A2).[77,78,79] Correct resuspension technique has to be regularly evaluated.
Mixing insulins
When short acting (regular) insulin is to be given simultaneously with intermediate acting insulin (NPH), they are usually mixed together in the same syringe. If admixtures which suit the patients insulin requirements are available commercially (premixed insulin), they should be preferred (A1).[80]
Regular insulin should be filled first followed by NPH insulin (A3). Reversal of this order can contaminate the regular insulin vials (A3). Glargine insulin should not be mixed with any other insulin due to unique low pH of its diluents (A2).[43,81,82]
Injection-mealtime gap
Injection-mealtime gap may affect the insulin efficacy. Hence, the timing of injection with respect to meal is critical in controlling glycemic levels (A1).[83,84] Patients should always follow physician's advice in this regard.
Short-acting insulins should be administered 30 min before meal,[83] whereas rapid-acting insulin analogs (RAIAs) can be injected before or immediately after a meal (A2).[85,86]
Intermediate-acting insulins such as NPH and long-acting insulins such as detemir and glargine should not be related to mealtimes and be injected at the same time every day. Ultra-long acting basal insulin degludec can be injected at any time of the day, provided a gap of 8–40 h is maintained between two injections.[85] Co-formulation of degludec and aspart should be injected with the main meal (s) of the day to provide both prandial and basal control.[87] Glucagon-like peptide receptor agonist-like exenatide is injected twice daily, 30 min before meals; whereas liraglutide is injected once daily, without regard to meal timings (A2).[88,89,90] Coformulation of degludec and liraglutide is injected once daily, at the same time each day.[91]
INJECTION SITE AND TECHNIQUE
Choice of site
In ambulatory patients, the most commonly employed route for insulin administration is subcutaneous (SC) tissue. Other routes which are employed only during ketoacidosis or stressful conditions are IV, infusion or IM.[64]
The presence of a fat layer and presence of only a few nerves in these regions makes injections convenient. An Indian cross-sectional, observational, KAP survey found 71.43%, 28.57%, and 5.36% of patients injected insulin in upper arm, abdomen, and thigh, respectively. All the patients rotated injection sites.[70] The most commonly used sites for insulin injection are as follows: abdomen, upper arms, thigh, and buttocks.
Site assessment
The site has to be inspected by the patient for inflammation, wounds or LH before every injection (A2).[81,92,93] and systematically by HCPs at every visit or at least every 6 months (A3). Injection sites should be rotated systematically (A1).[43,81,94]
Anterior abdomen
The site of choice for insulin injections is abdomen (A1).[20,95] The space around a horizontal line drawn 2.5 cm above and below the umbilicus and lateral to vertical lines drawn 5 cm away from the umbilicus may be utilized for SC insulin injections.
Upper arm
Over the arm, the injection site includes the upper lateral mid third of the arm between the shoulder and elbow joint. Needle length more than 6 mm warrants the need for a lifted skin fold, especially in the case of injection into the arm of the patients by a third party.[64,65]
Anterior thigh
Over the thigh, the preferred site is in the anterior and outer aspect of the mid third of the thigh, between the anterior superior iliac spine and knee joint. If there is risk of nocturnal hypoglycemia, evening dose of insulin (NPH) should be injected into the thigh or buttock as these sites have slower absorption (A1).[96,97]
Buttock
The upper outer quadrant of the buttock should be used. The upper outer quadrant may be located by placing index finger on the iliac crest and making a right angle between the index finger and the thumb. This site is not used routinely in adults. It can be used in infants and toddlers.
Injection site rotation
Systematic switching of the injections from one site to another site and within the injection site is important as it helps maintain healthy injection sites, optimizes insulin absorption and reduces the risk of LH (A1).[82] Use same site at the same time each day and rotate injection site to avoid glucose variability and LH (same time same site rule).
Administer insulin injections using a new injection site for each injection, in a systematic manner while ensuring stable insulin absorption. A common and effective scheme is to divide the injection site into quadrants (abdomen) or halves (thighs, buttocks, and arms). One quadrant or half should be used for 1 week and then move either in a clockwise or an anticlockwise fashion to another quadrant or half, next week (A3). New injection site should be at least 1–2 cm apart from the previous site (A3). Do not inject in the area of LH, inflammation, edema, or infections (A2).[98,99] The HCPs should review the site rotation scheme with the patient at least once a year (A1).[100,101,102,103]
Skin fold
Skin folds are considered when the presumptive distance between the skin surface and muscle is less than needle length (A3). Ideally, the thumb and index finger are used to lift a skin-fold properly (possibly with the addition of the middle finger). Use of the whole hand while lifting the skin risks lifting muscle and can lead to IM injections. Correlation between needle length and skin fold has been described in an earlier section of this recommendation.
Injection technique (optimal sequence)
A lifted skin fold must be used if necessary (A3).
Push the needle at a 90° angle into the skin.
For lean patients, combined use of an raised skin fold and angled insertion is done.
Avoid indenting the skin during the injection, as the needle may enter the muscle (B3).
Administer insulin slowly and withdraw syringe needle at the same angle (A3).
Hold the needle under the skin for at least 10 s after the plunger has been depressed (A1).[104,105,106]
Release skin fold (if done).
Dispose of used needle safely (A3).
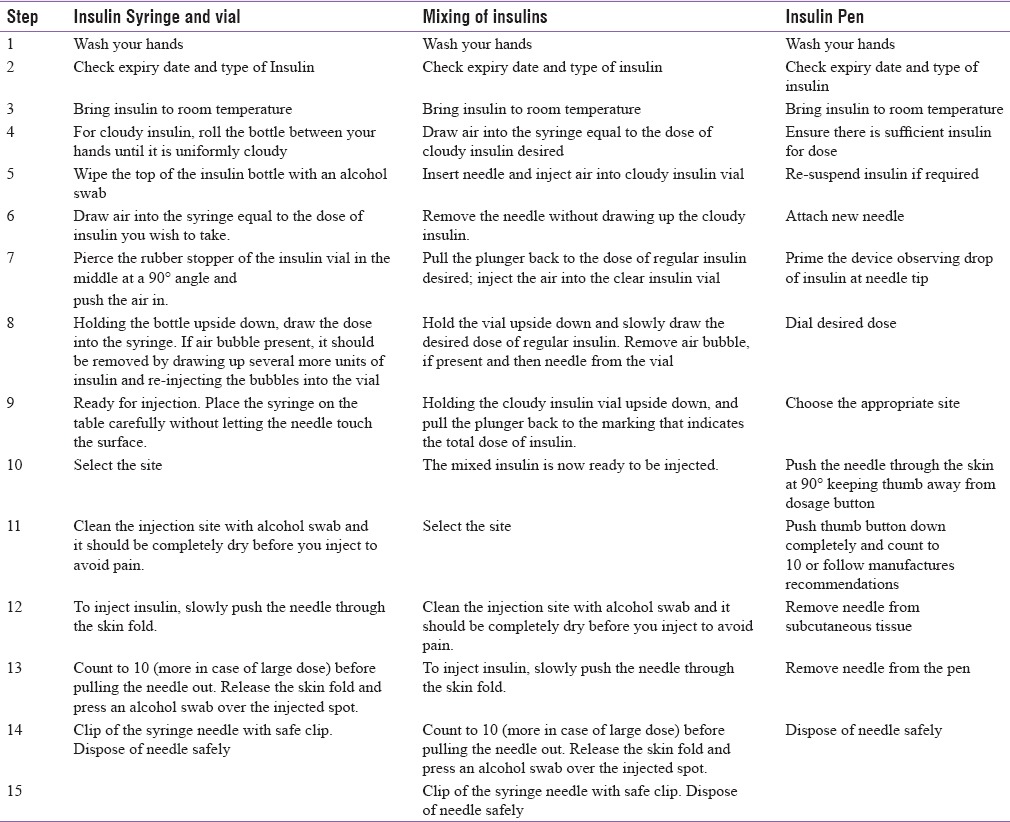
The steps of injection technique are shown in Table 4.[44]
Table 4.
Steps of injection technique
Single use of insulin syringe/pen needle
The United States FDA recommends injection needles for single use only. On the contrary globally and in India, patients often reuse syringes and pen needles for several reasons including cost. Recent ITQ survey showed that 39.7% of insulin pen user reused needles 3–5 times or more and 44% syringe users reused needles an average of 3–5 times.[9,107]
Blanco et al. revealed a clear correlation between the increased incidence of LH and needle reuse.[108] Findings from a recent meta-analysis suggested an association between the presence of LH and reuse of needles. Furthermore, reuse of needles causes more pain at the injection site.[109] Nearly 79% of patient's reported not having received any guidance from HCPs, regarding single-use of needles and syringes.[110]
Disadvantages of reuse of needles
Distorted and bent needle results in more painful injection during reusing.
Needle cleansing with alcohol before use removes the silicone lubricant and results in painful injection with reuse.
Breaking off and lodging of the needles can occur under the skin.
Reuse results in damage to the tissues and levies an increased risk of LH.
Reuse of needles increases the risk of contamination and infection.
Reuse of needles may leads to needle stick injury (NSI) among HCPs in hospital settings.
Impact to patients
Nearly 75% of PWD reported reuse of disposable syringes and needles in a cross-sectional study (n = 28). The prevalence of NSI, LH and hematoma due to syringes and needles reuse was 46%, 7%, and 18% among PWD. Main reasons for syringe and needle changes were pain (54%), guidance of a health professional (14%) and blunt needle (14%).[110] A study by Blanco et al. reported an association between LH and the reuse of needles with significant increase in risk when needles were reused more than five times. Among PWD, who reused needles, 70% reported LH while 61% patients with LH reported needle reuse as a main cause.[108] HCPs should identify and educate PWD who are likely to reuse needles.[9]
Impact to health care providers
A cross-sectional observational study from Gujarat, India conducted in government institutes assessed injection practices and prevalence of NSI among the HCPs. Out of 161 (64.14%) who were practicing unsafe injection methods, 65% had NSI (P < 0.05). Thus, proper training and infection control measures among HCPs can handle this future threat.[111]
A systematic review of economic analyses involving 14 studies related to NSI found significant direct, indirect, potential, and intangible costs borne by the hospital. Efforts directed at preventing NSI may help to lower these costs.[112]
Recommendations
Use a sterile, new needle for each injection (A2).[113]
Reuse increases the risk of LH (A2).[108]
HCPs should provide adequate guidance to PWD, who are likely to reuse syringes and needles (A2).[110]
Patient information leaflet (PIL) with advising statement against the reuse of syringe, pen needle, and lancet boxes should be included in packs (B3).
Photos from microscopy studies should be included in PIL and/or package inserts for patients and HCPs (B3).
HCPs should be aware of reuse practices, in case the PWD makes an informed choice to reuse needles (B3).
Missing injections
All patients should be counseled about the negative effects of missing injections (A2).[114] If a patient is admitted to the hospital and there is uncertainty about his current insulin regimen, rapid or short-acting human insulin should be administered until further information is available. This should always be done under medical supervision.
Factors affecting rate of insulin absorption
The order of the rates of absorption at the sites is abdomen > arm > thigh > buttock.[115] Factors which can speed up the absorption and cause hypoglycemia are hot environment, for example, having a hot bath after the injection, which increases blood flow to the injection area, massage or exercise and IM injection of insulin.
Factors which can slow down absorption and cause a rise in blood glucose levels are large volumes of insulin, injections into damaged, unhealthy tissue, and cold environments (as they reduce blood flow).
“NO” Injection through clothing
This is an uncommon practice among patients, especially when in a hurry, or in a public place. This practice should, however, be firmly discouraged (A3).
SPECIAL POPULATIONS
Pregnancy
Proper education and counseling of antenatal women about the regulation of diabetes and use of insulin therapy (if needed) are very important (A3). Patients should be reassured that abdomen is a safe site for insulin administration during pregnancy (B3).
First trimester: No change in insulin site or technique is needed (B3).
Second trimester: Lateral parts of the abdomen can be used to inject insulin, staying away from the skin overlying the fetus (B3).
Third trimester: Insulin can be injected in the lateral abdomen while ensuring the skin fold is properly raised (B2).[12] Alternative sites for apprehensive patients are thigh, upper arm, or buttock (C3).
Dermatological disease
Injection should not be administered into active or recently healed infections, keloids, or scars. However, stable vitiligo is not a contraindication for insulin injection.
Surgical disease
Different quadrant of the abdomen should be used for insulin injection in patients with open fistulas/ileostomies/colostomies or recent surgical wounds. An alternative approach for apprehensive patients with recent abdominal surgery is thigh, upper arm, or buttocks for injection.
Elderly
Elderly patients should be assisted by a caregiver and the importance of injection therapy, as well as prevention and treatment of hypoglycemia should be emphasized (A2).[12,116] A retrospective study among 3172 insulin-dependent elderly patients with T2DM showed that pen devices improved insulin therapy adherence in a primarily elderly population with T2DM (P < 0.001).[117]
The discreetness, simplicity, convenience of use, dosage accuracy of pen devices and they being less painful to inject allows for widespread acceptability among the elderly (A2).[118,119,120]
Sensory motor impairment: Visual, tactile, and lack of manual dexterity
In visually impaired patients, nonvisual insulin measurement devices, syringe magnifiers, needle gauges, and vial stabilizers help ensure accuracy and aid in insulin delivery. In patients with both visual and dexterity impairment, prefilled syringes may be helpful (A2).[121,122,123]
In patients with impaired hearing and those who use hearing aids, therapy-related discussions should be conducted in a noise-free environment (A2).[124,125,126] In addition, speaking slowly and clearly with normal intonation will also be a benefit. In people with dexterity problems, use of devices with preset doses and easy featuring devices may be beneficial (A2).[124] Pens which require low pressure should be preferred (A3).
Immunocompromised individuals
In some immunocompromised patients such as those with human immune deficiency virus (HIV) and hepatitis, superadded infection is a major concern. Hence, early initiation of insulin therapy should be considered in immunocompromised patients as it improves therapeutic outcomes.[127] Personnel giving injections and those handling sharps are at high risk of exposure and transmission of blood borne pathogens (HIV and hepatitis) through injections and finger sticks administered to affected patients.[128] Therefore, needles, syringes, and lancets should never be reused (A2),[129,130] and should be disposed of carefully.
ADVERSE EVENTS OF FAULTY TECHNIQUE
Pain
Pain is perhaps the most common adverse event associated with insulin use. Good injection practices can minimize or avoid injection-associated pain [Table 5].[20,34,131]
Table 5.
Minimizing pain associated with insulin's
Lipohypertrophy
LH is a thick soft to firm swelling with rubbery consistency which appears on the surface of the skin at the site of insulin infections. It is usually due to repeated reuse of needles at the same injection site. It usually appears as increased swelling, which cannot be pinched together. While large LH sites can be seen on inspections, others may be evident only on palpation.
Blanco et al. found that out of 64.4% of patients who had LH, 98% either did not rotate sites or rotated incorrectly. Furthermore, 39.1% of patients with LH had unexplained hypoglycemia and 49.1% had glycemic variability.[108] The worldwide ITQ survey indicated that the most common complication of insulin injection was LH; self-reported by 29.0% of patients and found by physical examination in 30.8% by HCPs. Further, it was associated with more frequent episodes of diabetic ketoacidosis, incorrect rotation of injection sites, use of smaller injection zones, longer duration of insulin use, and reuse of pen needles (each P < 0.05). Therefore, nonrotation of injection sites and single use of needle should be encouraged.[53] Injection in LH reduces insulin absorption or even causes erratic absorption leading to high glycemic variability.[132,133]
Grassi et al. have shown that targeted individualized training in injection technique, including the switch to a 4 mm needle, is associated with improved glucose control over 3 months, even in patients with LH.[134]
Pain sensation gets reduced at LH sites prompting patients to use this site frequently for injection. This leads to a vicious cycle of significantly unpredictable and delayed absorption causing glycemic variability and unexplained hypoglycemia. Further, unnecessarily larger doses may be used in such cases.[38,135,136] Switching site of injections from LH to normal tissue often requires a decrease in dose of insulin, but it varies from one individual to another and should be monitored by frequent blood glucose measurements (A1).[94,137]
Prevention
Regular inspection and palpation of insulin sites (Self Insulin Site Examination).
Single use of needles.
Do not use the same injection site repeatedly (A2).[109] Follow correct site rotation policy.
Use larger injection surface areas.
Management
Do not inject into LH sites.
Reduce the dose of insulin in habitual LH site injections when shifting to normal SC tissue after consulting physician.
Rule out LH as a cause of poor glycemic control, hypoglycemia, and high glycemic variability.
Injection sites should be inspected and palpated for LH by both HCPs and PWD (A2).[138]
Bleeding and bruising
Insulin injections can occasionally cause bleeding or bruising, but this is usually not significant. Assessment may be needed in case of frequent or excessive bleeding and/or bruising (A2).[43] Studies have shown less frequent bleeding and bruising incidents with the use of shorter needles. The presence of bleed or bruise appears to have no adverse clinical consequences for the absorption of insulin or for overall diabetes management (A2).[43]
Needlestick injuries
NSI are common among HCPs and warrant training on preventive methods. A cross-sectional study found that prevalence of at least one episode of NSI was about 46%, of which 28% occurred within 1 year before the study and only 24% took prophylaxis for HIV infections. Another study conducted to assess prevalence, causes, and prevention of NSI among nurses found syringe needles and crowded wards as main causes of NSI.[139,140]
Device and circumstances
Pen needle removal and recapping are critical and dangerous steps because the user's fingers come very close to the exposed tip. In one survey, 57% of patients admitted they unscrew pen needles using their own fingers and 29% of NSI injuries occurred during recapping. Safe medical instruments and appropriate training on preventive measures are needed to ensure safe practices.[141]
Severity of injury and risk matrix
As NSI and sharp injuries are common among HCPs, continuing risk assessment, risk elimination, training in the use of devices and awareness of the consequences such as injuries is vital.[141] In 2011, Wittmann developed a standardized risk assessment matrix tool for medical sharps. This tool helps to identify the potential risk associated with the devices or procedures, and the appropriate level of sharps safety required.[142]
Prevalence of blood borne infections (hepatitis B virus, hepatitis C virus, human immunodeficiency virus)
The amount of blood regurgitated into cartridges of insulin pens during injection is sufficient to transmit hepatitis B virus (HBV) infection. Although insulin cartridges contain antimicrobial agents (i.e., phenol or cresol), they are only bactericidal and not active against viruses.[53,58] An epidemiological study conducted in 200 HBcAb+/− anti-HBs T2DM patients compared with well-matched controls (in 1:1 ratio) showed that HBV DNA was detected in 11% of the PWD and 3% of the controls (P < 0.05).[143]
In several studies, the prevalence of hepatitis C infection was found to be higher in PWD than nondiabetics. A cross-sectional study conducted in India to determine the seroprevalence of hepatitis C infection in 192 T2DM, the prevalence rate of hepatitis C virus seropositivity was found to be 5.7%, with male preponderance.[144] Moreover, a recent meta-analysis that included data from 22 studies also confirmed the same fact with an odds ratio of 3.50, at 95% confidence interval = 2.54–4.[145,146]
Prevention and care
Single use of needles
This promotes PWD and HCPs/caregivers to discard needle after single use. Reuse involves recapping needles and increases the risk of NSI.
Education and training
HCPs must receive appropriate education and training in how to minimize risk, by following optimal technique and using available safety devices.[147]
Shorter needles
Through-through NSI risk is higher with the use of longer needle since it may exceed the thickness of folded skin (A2).[148]
Amyloidosis
Amyloidosis is a rare disorder, in which an abnormal protein called amyloid is deposited extracellularly and impairs tissue function.[146] Clinical studies have shown infrequent local amyloid deposition at the site of repeated insulin injection in PWD.[149]
Amyloidosis may be associated with the use of insulin, including human (recombinant) insulin and this may have contributed to the insulin resistance or refractoriness in poorly controlled PWD. The nature of amyloid is considered to be insulin itself or insulin-related substance and has been identified as amyloid insulin type.[150] Marked improvement in blood glucose has been noticed shortly after resection of the mass or change of the injection site.
Hence, to avoid infection, inflammation or mass formation, there is a need to educate PWD about regular check-up of injection site and alternate use of insulin injection sites.
Leakage of insulin
Leakage from pens can occur due to a poor seal between the needle and the cartridge or incorrect positioning of the plunger (A3). Reflux or backflow out of the injection site can happen when the needle is taken out too soon or in obese patient. The needle length does not have a meaningful influence on the amount of leakage.[151,152]
Lipoatrophy
Lipoatrophy (LA) is clinically characterized by visible cutaneous depression and palpable atrophy of SC fat tissue at the injection site.[153] It is an immunological response to insulin aggregates in the presence of high circulating titers of anti-insulin autoantibodies. LA was more prevalent in the prehuman-insulin era due to the use of animal insulin.[154] However, the prevalence of LA has dropped to only 1%–2% with the increasing use of purified insulin.[94] In recent years, case reports on LA have increased in the scientific literature indicating that LA may also develop after treatment with recombinant human insulins or insulin analogs.[155,156]
Scarring
Scar formation is a consequence of the wound healing process that occurs when body tissues are damaged by a physical injury.[157] Repeated injections into the same SC tissue and the increasing use of insulin pump therapy (IPT) carries risks of abscess formation and scarring.[158,159,160]
INJECTION PRACTICES IN INDOOR SETTINGS
Each vial or pen should be labeled with the patient's name and bed number/registration number. To minimize errors, a single strength of insulin should be used in all patients in a hospital as far as possible. Insulin syringes should be stored away from other syringes such as those used for antibiotic sensitivity tests and other purposes.
Recommendations for both critical and noncritical care settings
Ensure compatibility of insulin preparations and delivery devices (A2).[161]
Maintain records of glucose monitoring, insulin dosage, time, and site of insulin (A3).
Site rotations can be followed by marking sites of insulin injections with ink, with consent of the patient (A2).[162]
Ensure adequate training in insulin technique and disposal to patients and/or their caregivers before discharge from hospital (A2).[43]
-
Safe disposal:
- Place sharps containers in every ICU, ward patient room, and at every nursing station and ensure disposal of in accordance with biomedical waste regulations (A2).[164]
-
Audit and appraisal:
- Audit the ICU and ward insulin policy regularly with help of senior nursing and medical staff (A2).[165]
Ensure continuing nursing and medical education (A2).[166]
INSULIN PUMP THERAPY/CONTINUOUS SUBCUTANEOUS INSULIN INFUSION (CSII)
An insulin pump is a pager-sized device, which ensures continuous delivery of rapid acting insulin with the help of an infusion set. One end of this set connects to the insulin filled reservoir and is kept inside the insulin pump, and the other end is connected to the subcutaneously placed needle.[167]
Trials conducted across the world have demonstrated that continuous subcutaneous insulin infusion (CSII) is more beneficial in terms of achieving better metabolic control in T2DM. Unawareness about the multiple benefits of CSII is the main hurdle to its widespread use.[168] In India, CSII is more commonly used in PWD since 2004. Reductions in HbA1c, body weight and total daily dose of insulin are reported in Indian PWD who are on IPT.
A cross-sectional survey conducted among selected Indian PWD, who have been on IPT for more than 3 years reported an improvement in quality of life after being on pump by 92% of patients, the level of satisfaction was rated as “fully satisfied” by 52% of respondents, whereas 26% found being on pump, “satisfactory” 90% thought that the pump met their expectations.[169]
Results of SWEET prospective, multicenter, standardized diabetes patient registry which included 16570 T1DM children reported that relatively good metabolic control was achieved, especially in those treated with insulin pumps and in those at younger ages.[170]
Infusion site
Abdomen is the preferred site for CSII pumps, whereas the alternate sites are upper arm and thighs.
Cannula selection
Short length cannulae (6 mm for 90° sets, 13 mm for 30°–45° angled infusion sets) are most preferred. Steel needle infusion sets are recommended in pregnancy, for patients who have reactions to plastic cannula and who have frequent kinks with plastic cannula.
Angle of insertion
The recommended angle of insertion is 90° and 30°–45° for dexterous lean or muscular patients and pregnant women.
Selection of infusion sets
The most popular infusion set is a 90°, soft cannula infusion set. Variable angle, soft cannula infusion sets are also available for patients who are lean or lead an active lifestyle.
Choice of insulin preparation
The insulins of choice with appropriate stability for use in pumps are RAIAs, as they mimic the endogenous insulin more closely than regular human insulin. Further, the tendency observed for hypoglycemia with RAIAs is significantly less than regular insulin. In comparison to lispro (15.7%) and glulisine (40.9%), Insulin aspart (9.2%) led to greatest chemical and physical stability in the insulin pump with the lowest rates of overall occlusion and is thus considered the most compatible of the three RAIAs for pump use.[171] The use of insulin mixture in pump is not recommended as their stability needs evaluation and therefore is not recommended.[172] When injected subcutaneously, insulin aspart has a rapid onset and shorter duration of action than soluble insulin.[173]
Troubleshooting
Adhesive tape allergy
In India, this is rare and use of oral antihistamines for a few days usually suffices.
Infusion site infection prophylaxis
The infusion site must be clean and dry before insertion of the cannula. Changing the infusion set once in every 2–3 days is recommended. In India, most of the pump users retain the same infusion set for 5–7 days due to the high cost of consumables.[167] Customized advice and recommendations are to be made based on affordability, work pattern, and level of education.
Lipohypertrophy
LH has been described in the earlier part of the recommendations.
Loss of insulin potency
The potency of insulin may be compromised if it is used for longer than 3 days or if it is frozen.
Pump occlusion
Cannula and infusion set should be changed if occlusion occurs.[174,175]
Unexplained hyperglycemia
Emerging data indicate that insulin pump infusion sets are sometimes responsible for unexplained hyperglycemia. This under-reported, and under-discussed etiology, often leads to a significant psychological burden and discontinuation of pump therapy and/or diabetic ketoacidosis.[176] Check if hyperglycemia responds to bolus dose and replace infusion set immediately.
Recommendations
INJECTION DEVICE DISPOSAL
Responsibility of environmental-friendly and safe disposal of insulin sharps has to be shared by all stake–holders including prescriber, consumer to waste disposer and recycler. HCPs should shoulder responsibility of awareness and sensitization regarding safe disposal.[180] A study from New Delhi has showed that 84.1% PWD discarded the sharps directly into their household waste bins.[181] Recent worldwide ITQ survey including 7.6% of patients from India found that a very large number of used diabetes sharps still end up in the general community trash. 8.6% of the total population agreed to sharps injuries in community because of wrong disposal practices.[9]
Lack of knowledge about proper method of disposal, lack of counseling by HCPs and fear of revelation of diabetes status are major factors that compromise safe disposal of insulin delivery sharps in India.[182]
Learning from other country practices and guidelines
A review of 12 community-based program from the United States, Canada, and Australia for syringe disposal among PWD showed that drop boxes, puncture proof containers disposed in the trash and sharps container disposal at designated sites were the preferred practices.[183] The harm reduction strategy propagated by NACO has issued guidelines for disposal of waste needles and syringes. Green diabetology campaign has propagated practices regarding proper waste disposal. This helps to prevent the adverse impact of diabetes waste on the environment.
Recommendations
Collect the used needles or syringes in puncture proof box/safety box/strong cardboard/glass container
Label the box as biohazard and hand it over to nearby health-care facility
Proper education and training about the safe disposal of the sharps to PWD (A3)
Adopt simple strategies depending on sociodemographic, economic, and cultural practices inherent to the area.[182,183,184,185]
DISASTER MANAGEMENT
Avoiding or missing insulin therapy in persons with T1DM can be life threatening. Hence, patient's education about the disaster management is important (A2).[13] A portable, insulated, and waterproof diabetes disaster kit should be kept handy (A3).
The kit should have a supply of insulin syringes for at least 30 days and insulin vials or pens and needles along with cold packs (A2).[13] In addition, it should also contain blood testing supplies including lancets, test strips, and a glucose meter (preferably two) with extra batteries. A separate sharps container for the disposal of lancets and needles should also be included.
At least 3 days supply of nonperishable food and bottled water is also recommended.[44]
HEALTH EDUCATION INTERVENTIONS
Initiatives to enhance diabetes education in patients and caregivers can improve self-care behaviors, knowledge, and attitude domains profoundly.
Linetzky et al. 2016 in a long-term multicenter education trial showed that combined education of patients and physicians provided the significant and sustained clinical and metabolic improvement in the intervention group than in the control group.[166]
Assessing outcome
When educating in a group setting, there is evidence that better adherence and lower subsequent HbA1c values are achieved if the HCPs has formal training as an educator.[186]
Patient education on insulin technique can:
Reduce the risk of complications significantly
Support well-being and satisfaction of the patient
Support target Hb1Ac while minimizing need for additional insulin.
Periodic clinical audits
A periodic audit of injection practices in PWD by their clinicians is highly recommended (A3). Mutual audits can be performed in pairs by members of diabetes clubs or patient organizations.
Measures to improve adherence to insulin injection therapy
Encouraging patients to ask questions and clarify doubts is important. Arranging periodic refresher sessions with patients is helpful in addressing any new issues that arise during therapy.[187] The message should be personalized, and information relevant to the patient's perspective should be provided.[188] The use of pens makes insulin injections more convenient and promote better adherence to schedule and increases patient compliance.[189]
The WATER approach explained below has been suggested to fulfill the purpose. The patient must be welcomed warmly in the clinic, from the outpatient counter onward. The clinicians should ask and assess carefully making use of various cues and sequencing the questions appropriately. They should tell truthfully making use of metaphors analogies, keeping in mind both verbal, as well as nonverbal cues from the patient. They should explain with empathy, making use of experience sharing, practical demonstration, and imparting coping skills training. Finally, the clinicians must reassure the patient and tell him/her to return for any clarifications.[190]
SEVEN GOLDEN RULES
The golden rules developed for proper injection technique are shown in Table 6.[191,192,193,194,195,196,197]
Table 6.
Golden rules for injection technique

CONCLUSION
FITTER, India 2017 recommendations provide new, evidence-based, practical, and comprehensive set of recommendations for patients and professionals. The tools, approaches, and practices will help the health system to adopt correct injection technique and safe use of anti-diabetic injectable therapies.
DUALITY OF INTEREST
The authors are members of FIT India advisory board, who have helped develop the Indian Insulin Injection Technique Recommendations 2017. FIT India is supported by Becton Dickinson India Private Limited (BD), a manufacturer of injecting devices. Members of the FIT advisory board have not received any honorarium from BD for their contribution to the recommendations. FIT India is constituted to provide evidence-based information on best practices on injection technique, to all those using injectable therapies for diabetes care, to achieve best possible health outcomes, ensuring that the right dose is delivered at the right injection site, using the right technique, each time.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
The authors would like to acknowledge the significant contribution made by members of the South Asian experts, Afro-Asian Referee Group and the Indian Review Panel. Authors also acknowledge WorkSure India for assistance in medical writing.
REFERENCES
- 1.World Health Organization. Global Report on Diabetes 2016. (NLM Classification: WK 810) 2016. [Last accessed on 2017 Jan 03]. Available from: http://www.apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf .
- 2.Akhtar SN, Dhillon P. Prevalence of diagnosed diabetes and associated risk factors: Evidence from the large-scale surveys in India. J Soc Health Diabetes. 2017;5:28. [Google Scholar]
- 3.Davidson JA. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(Suppl 2):S2. doi: 10.1016/S1262-3636(10)70001-X. [DOI] [PubMed] [Google Scholar]
- 4.Patil M, Sahoo J, Kamalanathan S, Selviambigapathy J, Balachandran K, Kumar R, et al. Assessment of insulin injection techniques among diabetes patients in a tertiary care centre. Diabetes Metab Syndr. 2016:pii: S1871-402130211-9. doi: 10.1016/j.dsx.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor U, Ramasamy G, Selvaraj K, Sahoo JP, Kar SS. Does one-to-one demonstration with insulin pads by health-care providers improves the insulin administration techniques among diabetic patients of a Tertiary Care Teaching Hospital in South India? Indian J Endocrinol Metab. 2016;20:767–71. doi: 10.4103/2230-8210.192904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davel H, Berg G, Allie R, Van der Merwe L. Injection technique guidelines for diabetes: Sharp and to the point. J Endocrinol Metab Diabetes S Afr. 2014;19:8–13. [Google Scholar]
- 7.Tandon N, Kalra S, Balhara YP, Baruah MP, Chadha M, Chandalia HB, et al. Forum for Injection Technique (FIT), India: The Indian recommendations 2.0, for best practice in Insulin Injection Technique, 2015. Indian J Endocrinol Metab. 2015;19:317–31. doi: 10.4103/2230-8210.152762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frid A, Hirsch L, Gaspar R, Hicks D, Kreugel G, Liersch J, et al. New injection recommendations for patients with diabetes. Diabetes Metab. 2010;36(Suppl 2):S3–18. doi: 10.1016/S1262-3636(10)70002-1. [DOI] [PubMed] [Google Scholar]
- 9.Frid AH, Hirsch LJ, Menchior AR, Morel DR, Strauss KW. Worldwide Injection Technique Questionnaire study: Population parameters and injection practices. Mayo Clin Proc. 2016;91:1212–23. doi: 10.1016/j.mayocp.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 10.Kalra S, Mithal A, Sahay R, John M, Unnikrishnan AG, Saboo B, et al. Indian Injection Technique Study: Population Characteristics and Injection Practices. Diabetes Ther. 2017;8:1–21. doi: 10.1007/s13300-017-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalra S, Mithal A, Sahay R, John M, Unnikrishnan AG, Saboo B, et al. Indian Injection Technique Study: Injecting Complications, Education, and the Health Care Professional. Diabetes Ther. 2017;8:1–4. doi: 10.1007/s13300-017-0244-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siminerio L, Kulkarni K, Meece J, Williams A, Cypress M, Haas L, et al. Strategies for insulin injection therapy in diabetes self-management. Diabetes Educ. 2011;37:1–10. [Google Scholar]
- 13.Dolinar R. The importance of good insulin injection practices in diabetes management. US Endocrinol. 2009;5:49–52. [Google Scholar]
- 14.Vimalavathini R, Gitanjali B. Effect of temperature on the potency and pharmacological action of insulin. Indian J Med Res. 2009;130:166–9. [PubMed] [Google Scholar]
- 15.Okazaki K, Goto M, Yamamoto T, Tsujii S, Ishii H. Barriers and facilitators in relation to starting insulin therapy in type 2 diabetes. Diabetes. 1999;48:SA319–SA319. [Google Scholar]
- 16.Jha S, Panda M, Kumar S, Gupta R, Neemani A, Jacob J, et al. Psychological insulin resistance in patients with type 2 diabetes. J Assoc Physicians India. 2015;63:33–9. [PubMed] [Google Scholar]
- 17.Cahill SM, Polo KM, Egan BE, Marasti N. Interventions to promote diabetes self-management in children and youth: A scoping review. Am J Occup Ther. 2016;70:7005180020p1–8. doi: 10.5014/ajot.2016.021618. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson S. How young people can learn about newly diagnosed type 1 diabetes. Nurs Child Young People. 2016;28:22–6. doi: 10.7748/ncyp.28.3.22.s21. [DOI] [PubMed] [Google Scholar]
- 19.Hanas R, Ludvigsson J. Experience of pain from insulin injections and needle-phobia in young patients with IDDM. Pract Diabetes Int. 1997;14:95–9. [Google Scholar]
- 20.Meece J. Dispelling myths and removing barriers about insulin in type 2 diabetes. Diabetes Educ. 2006;32(1 Suppl):9S–18S. doi: 10.1177/0145721705285638. [DOI] [PubMed] [Google Scholar]
- 21.Cocoman A, Barron C. Administering subcutaneous injections to children: What does the evidence say. J Child Young Peoples Nurs. 2008;2:84–9. [Google Scholar]
- 22.Kuo CR, Quan J, Kim S, Tang AH, Heuerman DP, Murphy EJ, et al. Group visits to encourage insulin initiation: Targeting patient barriers? J Clin Nurs. 2016:DOI: 10.1111/jocn.13577. doi: 10.1111/jocn.13577. [DOI] [PubMed] [Google Scholar]
- 23.Hendra TJ. Starting insulin therapy in elderly patients. J R Soc Med. 2002;95:453–5. doi: 10.1258/jrsm.95.9.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMurtry CM, Taddio A, Noel M, Antony MM, Chambers CT, Asmundson GJ, et al. Exposure-based interventions for the management of individuals with high levels of needle fear across the lifespan: A clinical practice guideline and call for further research. Cogn Behav Ther. 2016;45:217–35. doi: 10.1080/16506073.2016.1157204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mollema ED, Snoek FJ, Adèr HJ, Heine RJ, van der Ploeg HM. Insulin-treated diabetes patients with fear of self-injecting or fear of self-testing: Psychological comorbidity and general well-being. J Psychosom Res. 2001;51:665–72. doi: 10.1016/s0022-3999(01)00229-x. [DOI] [PubMed] [Google Scholar]
- 26.DiMatteo MR, DiNicola DD. Pergamon General Psychology Series. New York, NY: Pergamon Press; 1982. Achieving Patient Compliance: The Psychology of the Medical Practitioner's Role; p. 335. [Google Scholar]
- 27.Gibney MA, Arce CH, Byron KJ, Hirsch LJ. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: Implications for needle length recommendations. Curr Med Res Opin. 2010;26:1519–30. doi: 10.1185/03007995.2010.481203. [DOI] [PubMed] [Google Scholar]
- 28.Bergenstal RM, Strock ES, Peremislov D, Gibney MA, Parvu V, Hirsch LJ. Safety and efficacy of insulin therapy delivered via a 4 mm pen needle in obese patients with diabetes. Mayo Clin Proc. 2015;90:329–38. doi: 10.1016/j.mayocp.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Hayes RP, Fitzgerald JT, Jacober SJ. Primary care physician beliefs about insulin initiation in patients with type 2 diabetes. Int J Clin Pract. 2008;62:860–8. doi: 10.1111/j.1742-1241.2008.01742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sorli C, Heile MK. Identifying and meeting the challenges of insulin therapy in type 2 diabetes. J Multidiscip Healthc. 2014;7:267–82. doi: 10.2147/JMDH.S64084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siminerio LM, Funnell MM, Peyrot M, Rubin RR. US nurses' perceptions of their role in diabetes care: Results of the cross-national Diabetes Attitudes Wishes and Needs (DAWN) study. Diabetes Educ. 2007;33:152–62. doi: 10.1177/0145721706298194. [DOI] [PubMed] [Google Scholar]
- 32.Pearson TL. Practical aspects of insulin pen devices. J Diabetes Sci Technol. 2010;4:522–31. doi: 10.1177/193229681000400304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandran A, Bonafede MK, Nigam S, Saltiel-Berzin R, Hirsch LJ, Lahue BJ. Adherence to insulin pen therapy is associated with reduction in healthcare costs among patients with type 2 diabetes mellitus. Am Health Drug Benefits. 2015;8:148–58. [PMC free article] [PubMed] [Google Scholar]
- 34.Nefs G, Pouwer F, Holt RI, Skovlund S, Hermanns N, Nicolucci A, et al. Correlates and outcomes of worries about hypoglycemia in family members of adults with diabetes: The second Diabetes Attitudes, Wishes and Needs (DAWN2) study. J Psychosom Res. 2016;89:69–77. doi: 10.1016/j.jpsychores.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 35.Boehm BO, Vaz JA, Brøndsted L, Home PD. Long-term efficacy and safety of biphasic insulin aspart in patients with type 2 diabetes. Eur J Intern Med. 2004;15:496–502. doi: 10.1016/j.ejim.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Riddle MC, Rosenstock J, Gerich J. Insulin Glargine Study Investigators. The treat-to-target trial: Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 37.Zambanini A, Newson RB, Maisey M, Feher MD. Injection related anxiety in insulin-treated diabetes. Diabetes Res Clin Pract. 1999;46:239–46. doi: 10.1016/s0168-8227(99)00099-6. [DOI] [PubMed] [Google Scholar]
- 38.Polonsky WH, Jackson RA. What's so tough about taking insulin? Addressing the problem of psychological insulin resistance in type 2 diabetes. Clin Diabetes. 2004;22:147–50. [Google Scholar]
- 39.Kansra U, Sircar S. Insulin therapy: Practical points. J Indian Acad Clin Med. 2000;1:285–93. [Google Scholar]
- 40.American Diabetes Association. Insulin administration. Diabetes Care. 2004;27(Suppl 1):S106–9. doi: 10.2337/diacare.27.2007.s106. [DOI] [PubMed] [Google Scholar]
- 41.Shafaat K, Hussain A, Kumar B, ul Hasan R, Prabhat P, Kumar Yadav V. An overview: Storage of pharmaceutical products. World J Pharm Pharm Sci. 2013;2:2499–515. [Google Scholar]
- 42.Aye MM, Atkin SL. Patient safety and minimizing risk with insulin administration – Role of insulin degludec. Drug Healthc Patient Saf. 2014;6:55–67. doi: 10.2147/DHPS.S59566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bajwa SS, Bajwa S, Baruah M, Kalra S. Insulin injection guidelines for peri-operative and critically ill patients. J Sci Soc. 2013;40:68. [Google Scholar]
- 44.Kalra S, Balhara YP, Baruah MP, Chadha M, Chandalia HB, Chowdhury S, et al. Forum for injection techniques, India: The first Indian recommendations for best practice in insulin injection technique. Indian J Endocrinol Metab. 2012;16:876–85. doi: 10.4103/2230-8210.102929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strauss K, Gols HD, Hannet I, Partanen TM, Frid A. A pan-European epidemiologic study of insulin injection technique in patients with diabetes. Pract Diabetes Int. 2002;19:71–6. [Google Scholar]
- 46.Hirsch L, Byron K, Gibney M. Intramuscular risk at insulin injection sites – measurement of the distance from skin to muscle and rationale for shorter-length needles for subcutaneous insulin therapy. Diabetes Technol Ther. 2014;16:867–73. doi: 10.1089/dia.2014.0111. [DOI] [PubMed] [Google Scholar]
- 47.Caffrey RM. Are all syringes created equal? Am J Nurs. 2003;103:46–9. doi: 10.1097/00000446-200306000-00022. [DOI] [PubMed] [Google Scholar]
- 48.Gitanjali B. A tale of too many strengths: Can we minimize prescribing errors and dispensing errors with so many formulations in the market? J Pharmacol Pharmacother. 2011;2:147–9. doi: 10.4103/0976-500X.83277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Insulin Syringes. [Last accessed on 2017 Jan 23]. Available from: http://www.bd.com/us/diabetes/page.aspx?cat=7001 and id=7251 .
- 50.Safer Administration of Insulin. London: NHS; 2010. [Last accessed on 2017 Feb 09]. Rapid Response Report Reference: National Patient Safety Agency. Available from: http://www.bit.ly/2bmZKu7 . [Google Scholar]
- 51.The UK Injection and Infusion Technique Recommendations 4th edition. [Published on 2016 Oct]. Available from: http://www.fit4diabetes.com .
- 52.Syringe and Pen Needle Sizes. [Last accessed on 2016 Nov 23]. Available from: http://www.bd.com/us/diabetes/page.aspx?cat=7001 and id=7253 .
- 53.Frid AH, Hirsch LJ, Menchior AR, Morel DR, Strauss KW. Worldwide Injection Technique Questionnaire study: Injecting complications and the role of the professional. Mayo Clin Proc. 2016;91:1224–30. doi: 10.1016/j.mayocp.2016.06.012. [DOI] [PubMed] [Google Scholar]
- 54.Skin Thickness and Needle Size. [Last accessed on 2016 Nov 23]. Available from: http://www.bd.com/us/diabetes/page.aspx?cat=7001 and id=32371 .
- 55.Miwa T, Itoh R, Kobayashi T, Tanabe T, Shikuma J, Takahashi T, et al. Comparison of the effects of a new 32-gauge×4-mm pen needle and a 32-gauge×6-mm pen needle on glycemic control, safety, and patient ratings in Japanese adults with diabetes. Diabetes Technol Ther. 2012;14:1084–90. doi: 10.1089/dia.2012.0170. [DOI] [PubMed] [Google Scholar]
- 56.Nagai Y, Ohshige T, Arai K, Kobayashi H, Sada Y, Ohmori S, et al. Comparison between shorter straight and thinner microtapered insulin injection needles. Diabetes Technol Ther. 2013;15:550–5. doi: 10.1089/dia.2012.0334. [DOI] [PubMed] [Google Scholar]
- 57.Chantelau E, Lee DM, Hemmann DM, Zipfel U, Echterhoff S. What makes insulin injections painful? BMJ. 1991;303:26–7. doi: 10.1136/bmj.303.6793.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sonoki K, Yoshinari M, Iwase M, Tashiro K, Iino K, Wakisaka M, et al. Regurgitation of blood into insulin cartridges in the pen-like injectors. Diabetes Care. 2001;24:603–4. doi: 10.2337/diacare.24.3.603. [DOI] [PubMed] [Google Scholar]
- 59.Herdman ML, Larck C, Schliesser SH, Jelic TM. Biological contamination of insulin pens in a hospital setting. Am J Health Syst Pharm. 2013;70:1244–8. doi: 10.2146/ajhp120728. [DOI] [PubMed] [Google Scholar]
- 60.Best practices in ensuring the safe use of insulin pens in the hospital. Am J Health Syst Pharm. 2016;73(19 Suppl):S45–7. doi: 10.1093/ajhp/73.19_Supplement_5.S45. [DOI] [PubMed] [Google Scholar]
- 61.Brown KE, Hertig JB. Determining current insulin pen use practices and errors in the inpatient setting. Jt Comm J Qual Patient Saf. 2016;42:568–82. doi: 10.1016/S1553-7250(16)30109-X. [DOI] [PubMed] [Google Scholar]
- 62.Hofman PL, Lawton SA, Peart JM, Holt JA, Jefferies CA, Robinson E, et al. An angled insertion technique using 6-mm needles markedly reduces the risk of intramuscular injections in children and adolescents. Diabet Med. 2007;24:1400–5. doi: 10.1111/j.1464-5491.2007.02272.x. [DOI] [PubMed] [Google Scholar]
- 63.Birkebaek NH, Solvig J, Hansen B, Jorgensen C, Smedegaard J, Christiansen JS. A 4-mm needle reduces the risk of intramuscular injections without increasing backflow to skin surface in lean diabetic children and adults. Diabetes Care. 2008;31:e65. doi: 10.2337/dc08-0977. [DOI] [PubMed] [Google Scholar]
- 64.Lo Presti D, Ingegnosi C, Strauss K. Skin and subcutaneous thickness at injecting sites in children with diabetes: Ultrasound findings and recommendations for giving injection. Pediatr Diabetes. 2012;13:525–33. doi: 10.1111/j.1399-5448.2012.00865.x. [DOI] [PubMed] [Google Scholar]
- 65.Hildebrandt P. Skinfold thickness, local subcutaneous blood flow and insulin absorption in diabetic patients. Acta Physiol Scand Suppl. 1991;603:41–5. [PubMed] [Google Scholar]
- 66.Sim KH, Hwang MS, Kim SY, Lee HM, Chang JY, Lee MK. The appropriateness of the length of insulin needles based on determination of skin and subcutaneous fat thickness in the abdomen and upper arm in patients with type 2 diabetes. Diabetes Metab J. 2014;38:120–33. doi: 10.4093/dmj.2014.38.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Neal KS, Johnson J, Swar S. Nontraditional considerations with insulin needle length selection. Diabetes Spectr. 2015;28:264–7. doi: 10.2337/diaspect.28.4.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirsch LJ, Gibney MA, Albanese J, Qu S, Kassler-Taub K, Klaff LJ, et al. Comparative glycemic control, safety and patient ratings for a new 4 mm×32G insulin pen needle in adults with diabetes. Curr Med Res Opin. 2010;26:1531–41. doi: 10.1185/03007995.2010.482499. [DOI] [PubMed] [Google Scholar]
- 69.Perriello G, Torlone E, Di Santo S, Fanelli C, De Feo P, Santeusanio F, et al. Effect of storage temperature of insulin on pharmacokinetics and pharmacodynamics of insulin mixtures injected subcutaneously in subjects with type 1 (insulin-dependent) diabetes mellitus. Diabetologia. 1988;31:811–5. doi: 10.1007/BF00277482. [DOI] [PubMed] [Google Scholar]
- 70.Gawand KS, Gawali UP, Kesari HV. A study to assess knowledge, attitude and practice concerning insulin use in adults patients with diabetes mellitus in tertiary care centre. Indian J Med Res Pharm Sci. 2016;3:52–6. [Google Scholar]
- 71.McCarthy JA, Covarrubias B, Sink P. Is the traditional alcohol wipe necessary before an insulin injection? Dogma disputed. Diabetes Care. 1993;16:402. doi: 10.2337/diacare.16.1.402a. [DOI] [PubMed] [Google Scholar]
- 72.Sexson K, Lindauer A, Harvath TA. Administration of subcutaneous injections. Am J Nurs. 2016;116:49–52. doi: 10.1097/01.NAJ.0000508671.49210.ba. [DOI] [PubMed] [Google Scholar]
- 73.Khawaja RA, Sikandar R, Qureshi R, Jareno RJ. Routine skin preparation with 70% isopropyl alcohol swab: Is it necessary before an injection? Quasi study. J Liaquat Univ Med Health Sci. 2013;12:109. [Google Scholar]
- 74.Gorman CK. Good hygiene versus alcohol swabs before insulin injections. Diabetes Care. 1993;16:960–1. doi: 10.2337/diacare.16.6.960. [DOI] [PubMed] [Google Scholar]
- 75.Brown A, Steel JM, Duncan C, Duncan A, McBain AM. An assessment of the adequacy of suspension of insulin in pen injectors. Diabet Med. 2004;21:604–8. doi: 10.1111/j.1464-5491.2004.01206.x. [DOI] [PubMed] [Google Scholar]
- 76.Jehle PM, Micheler C, Jehle DR, Breitig D, Boehm BO. Inadequate suspension of neutral protamine Hagendorn (NPH) insulin in pens. Lancet. 1999;354:1604–7. doi: 10.1016/S0140-6736(98)12459-5. [DOI] [PubMed] [Google Scholar]
- 77.Karch AM, Karch FE. Troubleshooting insulin self-administration. Am J Nurs. 2000;100:24. [PubMed] [Google Scholar]
- 78.Nath C. Mixing insulin: shake, rattle, or roll? Nursing. 2002;32:10. doi: 10.1097/00152193-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 79.Kaiser P, Maxeiner S, Weise A, Nolden F, Borck A, Forst T, et al. Assessment of the mixing efficiency of neutral protamine Hagedorn cartridges. J Diabetes Sci Technol. 2010;4:652–7. doi: 10.1177/193229681000400320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Davidson MB. Self-mixed/split insulin regimen: a serious omission in the ADA/EASD position statement. Diabetes Care. 2014;37:3–4. doi: 10.2337/dc13-1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gentile S, Grassi G, Armentano V. AMD-OSDI consensus on injection techniques for people with diabetes mellitus. Med Clin Rev. 2016;2:3. [Google Scholar]
- 82.Yuan J, Chen Y, Xuan Y, Cao L, Zhu J, Wang F, et al. Can the upper inner side of the thigh become a new option for insulin injection? Curr Med Res Opin. 2016;32:1319–24. doi: 10.1185/03007995.2016.1174107. [DOI] [PubMed] [Google Scholar]
- 83.Czupryniak L, Drzewoski J. Insulin injection-meal time interval – It is not its length that matters. Pract Diabetes Int. 2001;18:338. [Google Scholar]
- 84.International Diabetes Federation Guideline Development Group. Guideline for management of postmeal glucose in diabetes. Diabetes Res Clin Pract. 2014;103:256–68. doi: 10.1016/j.diabres.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 85.Fowler MJ. Diabetes treatment, part 3: Insulin and incretins. Clin Diabetes. 2008;26:35–9. [Google Scholar]
- 86.Rizvi AA. Treatment of type 2 diabetes with biphasic insulin analogues. Eur Med J Diabetes. 2016;4:74–83. [PMC free article] [PubMed] [Google Scholar]
- 87.Unnikrishnan AG, Singh AK, Modi KD, Saboo B, Garcha SC, Rao PV. Review of clinical profile of IDegAsp. J Assoc Physicians India. 2015;63(5 Suppl):15–20. [PubMed] [Google Scholar]
- 88.Unger JR, Parkin CG. Glucagon-like peptide-1 (GLP-1) receptor agonists: Differentiating the new medications. Diabetes Ther. 2011;2:29–39. doi: 10.1007/s13300-010-0013-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Buse JB, Rosenstock J, Sesti G, Schmidt WE, Montanya E, Brett JH, et al. Liraglutide once a day versus exenatide twice a day for type 2 diabetes: A 26-week randomised, parallel-group, multinational, open-label trial (LEAD-6) Lancet. 2009;374:39–47. doi: 10.1016/S0140-6736(09)60659-0. [DOI] [PubMed] [Google Scholar]
- 90.Alshali KZ, Karawagh AM. A review of glycemic efficacy of liraglutide once daily in achieving glycated hemoglobin targets compared with exenatide twice daily, or sitagliptin once daily in the treatment of type 2 diabetes. Saudi Med J. 2016;37:834–42. doi: 10.15537/smj.2016.8.15800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hughes E. IDegLira: Redefining insulin optimisation using a single injection in patients with type 2 diabetes. Prim Care Diabetes. 2016;10:202–9. doi: 10.1016/j.pcd.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 92.Seyoum B, Abdulkadir J. Systematic inspection of insulin injection sites for local complications related to incorrect injection technique. Trop Doct. 1996;26:159–61. doi: 10.1177/004947559602600406. [DOI] [PubMed] [Google Scholar]
- 93.Annersten M, Willman A. Performing subcutaneous injections: A literature review. Worldviews Evid Based Nurs. 2005;2:122–30. doi: 10.1111/j.1741-6787.2005.00030.x. [DOI] [PubMed] [Google Scholar]
- 94.Gentile S, Strollo F, Ceriello A. AMD-OSDI Injection Technique Study Group. Lipodystrophy in insulin-treated subjects and other injection-site skin reactions: Are we sure everything is clear? Diabetes Ther. 2016;7:401–9. doi: 10.1007/s13300-016-0187-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zehrer C, Hansen R, Bantle J. Reducing blood glucose variability by use of abdominal insulin injection sites. Diabetes Educ. 1990;16:474–7. doi: 10.1177/014572179001600609. [DOI] [PubMed] [Google Scholar]
- 96.Karges B, Boehm BO, Karges W. Early hypoglycaemia after accidental intramuscular injection of insulin glargine. Diabet Med. 2005;22:1444–5. doi: 10.1111/j.1464-5491.2005.01654.x. [DOI] [PubMed] [Google Scholar]
- 97.Bantle JP, Neal L, Frankamp LM. Effects of the anatomical region used for insulin injections on glycemia in type I diabetes subjects. Diabetes Care. 1993;16:1592–7. doi: 10.2337/diacare.16.12.1592. [DOI] [PubMed] [Google Scholar]
- 98.Ariza-Andraca CR, Altamirano-Bustamante E, Frati-Munari AC, Altamirano-Bustamante P, Graef-Sánchez A. Delayed insulin absorption due to subcutaneous edema. Arch Invest Med (Mex) 1991;22:229–33. [PubMed] [Google Scholar]
- 99.Gentile S, Agrusta M, Guarino G, Carbone L, Cavallaro V, Carucci I, et al. Metabolic consequences of incorrect insulin administration techniques in aging subjects with diabetes. Acta Diabetol. 2011;48:121–5. doi: 10.1007/s00592-009-0172-x. [DOI] [PubMed] [Google Scholar]
- 100.Thatcher G. Insulin injections. The case against random rotation. Am J Nurs. 1985;85:690–2. [PubMed] [Google Scholar]
- 101.Ampudia-Blasco FJ, Girbes J, Carmena R. A case of lipoatrophy with insulin glargine: long-acting insulin analogs are not exempt from this complication. Diabetes Care. 2005;28:2983. doi: 10.2337/diacare.28.12.2983. [DOI] [PubMed] [Google Scholar]
- 102.Lumber T. Tips for site rotation. When it comes to insulin, where you inject is just as important as how much and when. Diabetes Forecast. 2004;57:68–70. [PubMed] [Google Scholar]
- 103.Teft G. Lipohypertrophy: Patient awareness and implications for practice.(Clinical Audit) J Diabetes Nurs. 2002;6:20–4. [Google Scholar]
- 104.Broadway CA. Prevention of insulin leakage after subcutaneous injection. Diabetes Educ. 1991;17:90. doi: 10.1177/014572179101700203. [DOI] [PubMed] [Google Scholar]
- 105.Rissler J, Jørgensen C, Rye Hansen M, Hansen NA. Evaluation of the injection force dynamics of a modified prefilled insulin pen. Expert Opin Pharmacother. 2008;9:2217–22. doi: 10.1517/14656566.9.13.2217. [DOI] [PubMed] [Google Scholar]
- 106.Ginsberg BH, Parkes JL, Sparacino C. The kinetics of insulin administration by insulin pens. Horm Metab Res. 1994;26:584–7. doi: 10.1055/s-2007-1001764. [DOI] [PubMed] [Google Scholar]
- 107.De Coninck C, Frid A, Gaspar R, Hicks D, Hirsch L, Kreugel G, et al. Results and analysis of the 2008-2009 Insulin Injection Technique Questionnaire Survey. J Diabetes. 2010;2:168–79. doi: 10.1111/j.1753-0407.2010.00077.x. [DOI] [PubMed] [Google Scholar]
- 108.Blanco M, Hernández MT, Strauss KW, Amaya M. Prevalence and risk factors of lipohypertrophy in insulin-injecting patients with diabetes. Diabetes Metab. 2013;39:445–53. doi: 10.1016/j.diabet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 109.Zabaleta-Del-Olmo E, Vlacho B, Jodar-Fernández L, Urpí-Fernández AM, Lumillo-Gutiérrez I, Agudo-Ugena J, et al. Safety of the reuse of needles for subcutaneous insulin injection: A systematic review and meta-analysis. Int J Nurs Stud. 2016;60:121–32. doi: 10.1016/j.ijnurstu.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 110.Guterres C, Rollin G, Ribeiro R, Bastos G, Lima K, Barrionuevo F, et al. Reuse of disposable syringes and needles in patients with type 2 diabetes. Diabetol Metab Syndr. 2015;7(Suppl 1):A189. [Google Scholar]
- 111.Shah H, Mangal A, Solanki H, Parmar D. Unsafe injection practices: An occupational hazard for health care providers and a potential threat for community: A detailed study on injection practices of health care providers. Int J Health Allied Sci. 2014;3:28–32. [Google Scholar]
- 112.Mannocci A, De Carli G, Di Bari V, Saulle R, Unim B, Nicolotti N, et al. How much do needlestick injuries cost? A systematic review of the economic evaluations of needlestick and sharps injuries among healthcare personnel. Infect Control Hosp Epidemiol. 2016;37:635–46. doi: 10.1017/ice.2016.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Thompson D, Bowdey L, Brett M, Cheek J. Using medical student observers of infection prevention, hand hygiene, and injection safety in outpatient settings: A cross-sectional survey. Am J Infect Control. 2016;44:374–80. doi: 10.1016/j.ajic.2015.11.029. [DOI] [PubMed] [Google Scholar]
- 114.Harashima SI, Nishimura A, Inagaki N. Attitudes of patients and physicians to insulin therapy in Japan: An analysis of the Global Attitude of Patients and Physicians in Insulin Therapy study. Expert Opin Pharmacother. 2017;18:5–11. doi: 10.1080/14656566.2016.1260547. [DOI] [PubMed] [Google Scholar]
- 115.Yadav S, Parakh A. Insulin therapy. Indian Pediatr. 2006;43:863–72. [PubMed] [Google Scholar]
- 116.Langa KM, Vijan S, Hayward RA, Chernew ME, Blaum CS, Kabeto MU, et al. Informal caregiving for diabetes and diabetic complications among elderly americans. J Gerontol B Psychol Sci Soc Sci. 2002;57:S177–86. doi: 10.1093/geronb/57.3.s177. [DOI] [PubMed] [Google Scholar]
- 117.Slabaugh SL, Bouchard JR, Li Y, Baltz JC, Meah YA, Moretz DC. Characteristics relating to adherence and persistence to basal insulin regimens among elderly insulin-naïve patients with type 2 diabetes: Pre-filled pens versus vials/syringes. Adv Ther. 2015;32:1206–21. doi: 10.1007/s12325-015-0266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Korytkowski M, Bell D, Jacobsen C, Suwannasari R. FlexPen Study Team. A multicenter, randomized, open-label, comparative, two-period crossover trial of preference, efficacy, and safety profiles of a prefilled, disposable pen and conventional vial/syringe for insulin injection in patients with type 1 or 2 diabetes mellitus. Clin Ther. 2003;25:2836–48. doi: 10.1016/s0149-2918(03)80337-5. [DOI] [PubMed] [Google Scholar]
- 119.Bohannon NJ, Jack DB. Type II diabetes: Tips for managing your older patients. Geriatrics. 1996;51:28–35. [PubMed] [Google Scholar]
- 120.Baruah MP, Kalra S, Unnikrishnan AG, Raza SA, Somasundaram N, John M, et al. Management of hyperglycemia in geriatric patients with diabetes mellitus: South Asian consensus guidelines. Indian J Endocrinol Metab. 2011;15:75–90. doi: 10.4103/2230-8210.81935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fischer JS, Edelman SV, Schwartz SL. United States patient preference and usability for the new disposable insulin device Solostar versus other disposable pens. J Diabetes Sci Technol. 2008;2:1157–60. doi: 10.1177/193229680800200626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.de Luis DA, Aller R, Cuellar L, Terroba MC, Ovalle HF, Izaola O, et al. Effect on quality of life with a new insulin injection device in elderly patients with diabetes mellitus type 2. J Diabetes Complications. 2004;18:216–9. doi: 10.1016/S1056-8727(03)00089-8. [DOI] [PubMed] [Google Scholar]
- 123.Heinemann L, Drossel D, Freckmann G, Kulzer B. Usability of medical devices for patients with diabetes who are visually impaired or blind. J Diabetes Sci Technol. 2016;10:1382–7. doi: 10.1177/1932296816666536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Livshitz L, Ghanayim R, Kraus C, Farah R, Even-Tov E, Avraham Y, et al. Application-based hearing screening in the elderly population. Ann Otol Rhinol Laryngol. 2017;126:36–41. doi: 10.1177/0003489416672873. [DOI] [PubMed] [Google Scholar]
- 125.Kim J, Nam KW, Yook S, Hong SH, Jang DP, Kim IY. Effect of the degree of sensorineural hearing impairment on the results of subjective evaluations of a noise-reduction algorithm. Speech Commun. 2015;68:1–10. [Google Scholar]
- 126.Contrera KJ, Wallhagen MI, Mamo SK, Oh ES, Lin FR. Hearing loss health care for older adults. J Am Board Fam Med. 2016;29:394–403. doi: 10.3122/jabfm.2016.03.150235. [DOI] [PubMed] [Google Scholar]
- 127.Palios J, Kadoglou NP, Lampropoulos S. The pathophysiology of HIV-/HAART-related metabolic syndrome leading to cardiovascular disorders: The emerging role of adipokines. Exp Diabetes Res. 2012;2012:103063. doi: 10.1155/2012/103063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kalra S, Unnikrishnan AG, Raza SA, Bantwal G, Baruah MP, Latt TS, et al. South Asian Consensus Guidelines for the rational management of diabetes in human immunodeficiency virus/acquired immunodeficiency syndrome. Indian J Endocrinol Metab. 2011;15:242–50. doi: 10.4103/2230-8210.85573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Dzekashu LG, Akoachere JF, Mbacham WF. Medical waste management and disposal practices of health facilities in Kumbo East and Kumbo West health districts. Int J Med Med Sci. 2017;9:1–11. [Google Scholar]
- 130.Treloar C, Mao L, Wilson H. Beyond equipment distribution in Needle and Syringe Programmes: An exploratory analysis of blood-borne virus risk and other measures of client need. Harm Reduct J. 2016;13:18. doi: 10.1186/s12954-016-0107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lantus Product Monograph. [Last accessed on 2016 Nov 23]. Available from: http://www.products.sanofi.ca/en/lantus.pdf .
- 132.Yuan T, Zhao W, Wang L, Dong Y, Li N. Continuous subcutaneous insulin infusion as an effective method of desensitization therapy for diabetic patients with insulin allergy: A 4-year single-center experience. Clin Ther. 2016;38:2489–94.e1. doi: 10.1016/j.clinthera.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 133.Kalra S, Jawad F. Lipohypertrophy. J Pak Med Assoc. 2016;66:779–80. [PubMed] [Google Scholar]
- 134.Grassi G, Scuntero P, Trepiccioni R, Marubbi F, Strauss K. Optimizing insulin injection technique and its effect on blood glucose control. J Clin Transl Endocrinol. 2014;1:145–50. doi: 10.1016/j.jcte.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Asche CV, Shane-McWhorter L, Raparla S. Health economics and compliance of vials/syringes versus pen devices: A review of the evidence. Diabetes Technol Ther. 2010;12(Suppl 1):S101–8. doi: 10.1089/dia.2009.0180. [DOI] [PubMed] [Google Scholar]
- 136.McCall AL. Insulin therapy and hypoglycemia. Endocrinol Metab Clin North Am. 2012;41:57–87. doi: 10.1016/j.ecl.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Spollett G, Edelman SV, Mehner P, Walter C, Penfornis A. Improvement of insulin injection technique: Examination of current issues and recommendations. Diabetes Educ. 2016;42:379–94. doi: 10.1177/0145721716648017. [DOI] [PubMed] [Google Scholar]
- 138.Ji L, Sun Z, Li Q, Qin G, Wei Z, Liu J, et al. Lipohypertrophy in China: Prevalence, risk factors, insulin consumption, and clinical impact. Diabetes Technol Ther. 2017;19:61–7. doi: 10.1089/dia.2016.0334. [DOI] [PubMed] [Google Scholar]
- 139.Balouchi A, Shahdadi H, Ahmadidarrehsima S, Rafiemanesh H. The frequency, causes and prevention of needlestick injuries in nurses of Kerman: A cross-sectional study. J Clin Diagn Res. 2015;9:DC13–5. doi: 10.7860/JCDR/2015/16729.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kaweti G, Abegaz T. Prevalence of percutaneous injuries and associated factors among health care workers in Hawassa referral and adare district hospitals, Hawassa, Ethiopia, January 2014. BMC Public Health. 2016;16:8. doi: 10.1186/s12889-015-2642-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.FIT4Safety. Injection Safety in UK and Ireland; Safety of Sharps in Diabetes Recommendations. 1st ed. UK: FIT4Safety; 2012. [Last accessed on 2017 Feb 09]. Available from: http://www.fit4diabetes.com/files/1413/4727/6994/BD4224_FIT_Safety_STG07_AW2_PP.pdf . [Google Scholar]
- 142.Adams D, Hicks D, Down S. Needlestick and sharps injuries in diabetes: RU FIT4Safety? J Diabetes Nurs. 2012;16:391. [Google Scholar]
- 143.Demir M, Serin E, Göktürk S, Ozturk NA, Kulaksizoglu S, Ylmaz U. The prevalence of occult hepatitis B virus infection in type 2 diabetes mellitus patients. Eur J Gastroenterol Hepatol. 2008;20:668–73. doi: 10.1097/MEG.0b013e3282f55e1e. [DOI] [PubMed] [Google Scholar]
- 144.Laloo D, Walke P, Bhimo T, Prasad L, Ranabir S. Seroprevalence of hepatitis C infection in type 2 diabetes mellitus. Indian J Endocrinol Metab. 2015;19:296–9. doi: 10.4103/2230-8210.149325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Guo X, Jin M, Yang M, Liu K, Li JW. Type 2 diabetes mellitus and the risk of hepatitis C virus infection: A systematic review. Sci Rep. 2013;3:2981. doi: 10.1038/srep02981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kalra S, Balhara YS, Baruah M, Chadha M, Chandalia H, Chowdhury S, et al. Addendum 2: Forum for injection technique, India. Indian J Endocrinol Metab. 2014;18:800. [Google Scholar]
- 147.Strauss K. Synopsis of the WISE meeting. Diabetes Metab. 2012;38(Suppl 1):S15–26. doi: 10.1016/S1262-3636(12)70977-1. [DOI] [PubMed] [Google Scholar]
- 148.Strauss K. WISE Consensus Group. WISE recommendations to ensure the safety of injections in diabetes. Diabetes Metab. 2012;38(Suppl 1):S2–8. doi: 10.1016/S1262-3636(12)70975-8. [DOI] [PubMed] [Google Scholar]
- 149.Swift B. Examination of insulin injection sites: An unexpected finding of localized amyloidosis. Diabet Med. 2002;19:881–2. doi: 10.1046/j.1464-5491.2002.07581.x. [DOI] [PubMed] [Google Scholar]
- 150.Dische FE, Wernstedt C, Westermark GT, Westermark P, Pepys MB, Rennie JA, et al. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158–61. doi: 10.1007/BF00276849. [DOI] [PubMed] [Google Scholar]
- 151.Wittmann A, Köver J, Kralj N, Gasthaus K, Lerch H, Rommel M, et al. Insulin leakage value in relation to pen needle length and administered dose after subcutaneous injection. Diabetes Technol Ther. 2010;12:587–90. doi: 10.1089/dia.2010.0050. [DOI] [PubMed] [Google Scholar]
- 152.Heise T, Nosek L, Dellweg S, Zijlstra E, Præstmark KA, Kildegaard J, et al. Impact of injection speed and volume on perceived pain during subcutaneous injections into the abdomen and thigh: A single-centre, randomized controlled trial. Diabetes Obes Metab. 2014;16:971–6. doi: 10.1111/dom.12304. [DOI] [PubMed] [Google Scholar]
- 153.Kordonouri O, Biester T, Schnell K, Hartmann R, Tsioli C, Fath M, et al. Lipoatrophy in children with type 1 diabetes: An increasing incidence? J Diabetes Sci Technol. 2015;9:206–8. doi: 10.1177/1932296814558348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chakraborty PP, Biswas SN. Distant site lipoatrophy: A rare complication of subcutaneous insulin therapy. Postgrad Med J. 2016;92:57–8. doi: 10.1136/postgradmedj-2015-133639. [DOI] [PubMed] [Google Scholar]
- 155.Theofanidis D. In-hospital administration of insulin by nurses in Northern Greece: An observational study. Diabetes Spectr. 2017;25:ds160001. doi: 10.2337/ds16-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kohara K, Kaneto H, Kamei S, Shimoda M, Hamamoto S, Tawaramoto K, et al. Ice cube tray-shaped insulin lipoatrophy throughout the abdomen in a subject with type 2 diabetes. Diabetes Care. 2016;39:e4–5. doi: 10.2337/dc15-1778. [DOI] [PubMed] [Google Scholar]
- 157.Rosique RG, Rosique MJ, Farina Junior JA. Curbing inflammation in skin wound healing: A review. Int J Inflam. 2015;2015:316235. doi: 10.1155/2015/316235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Richardson T, Kerr D. Skin-related complications of insulin therapy: Epidemiology and emerging management strategies. Am J Clin Dermatol. 2003;4:661–7. doi: 10.2165/00128071-200304100-00001. [DOI] [PubMed] [Google Scholar]
- 159.Conwell LS, Pope E, Artiles AM, Mohanta A, Daneman A, Daneman D. Dermatological complications of continuous subcutaneous insulin infusion in children and adolescents. J Pediatr. 2008;152:622–8. doi: 10.1016/j.jpeds.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 160.Binder E, Lange O, Edlinger M, Meraner D, Abt D, Moser C, et al. Frequency of dermatological side effects of continuous subcutaneous insulin infusion in children and adolescents with type 1 diabetes. Exp Clin Endocrinol Diabetes. 2015;123:260–4. doi: 10.1055/s-0034-1394381. [DOI] [PubMed] [Google Scholar]
- 161.Pisano M. Overview of insulin and non-insulin delivery devices in the treatment of diabetes. P T. 2014;39:866–76. [PMC free article] [PubMed] [Google Scholar]
- 162.Frid AH, Kreugel G, Grassi G, Halimi S, Hicks D, Hirsch LJ, et al. New insulin delivery recommendations. Mayo Clin Proc. 2016;91:1231–55. doi: 10.1016/j.mayocp.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 163.Hutin Y, Hauri A, Chiarello L, Catlin M, Stilwell B, Ghebrehiwet T, et al. Best infection control practices for intradermal, subcutaneous, and intramuscular needle injections. Bull World Health Organ. 2003;81:491–500. [PMC free article] [PubMed] [Google Scholar]
- 164.Kalra S, Girdhar R, Sahay R. Green diabetology. Indian J Endocrinol Metab. 2014;19:698–700. doi: 10.4103/2230-8210.164030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rachabattula H, Simmons E, Ghatahora S, Grant P. The variable rate intravenous insulin infusion in clinical practice 2015: An audit against the new JBDS guidance. Br J Diabetes. 2016;16:25–9. [Google Scholar]
- 166.Linetzky B, Curtis B, Frechtel G, Montenegro R, Jr, Escalante Pulido M, Stempa O, et al. Challenges associated with insulin therapy progression among patients with type 2 diabetes: Latin American MOSAIc study baseline data. Diabetol Metab Syndr. 2016;8:41. doi: 10.1186/s13098-016-0157-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kalra S, Chadha M, Chandalia H, Chowdhury S, Kesavadev J, Kumar KM, et al. Addendum:First injection technique recommendations for patients with diabetes, forum for injection techniques India. Indian J Endocrinol Metab. 2013;17:1005. [Google Scholar]
- 168.Kesavadev J, Das AK, Unnikrishnan RS, Joshi SR, Ramachandran A, Shamsudeen J, et al. Use of insulin pumps in India: Suggested guidelines based on experience and cultural differences. Diabetes Technol Ther. 2010;12:823–31. doi: 10.1089/dia.2010.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kesavadev J, Shankar A, Sadasrian Pillai PB, Saboo B, Joshi S, Krishnan G, et al. CSII as an alternative therapeutic strategy for managing type 2 diabetes: Adding the Indian experience to a global perspective. Curr Diabetes Rev. 2016;12:312–4. doi: 10.2174/1573399812666151208112607. [DOI] [PubMed] [Google Scholar]
- 170.Szypowska A, Schwandt A, Svensson J, Shalitin S, Cardona-Hernandez R, Forsander G, et al. Insulin pump therapy in children with type 1 diabetes: Analysis of data from the SWEET registry. Pediatr Diabetes. 2016;17(Suppl 23):38–45. doi: 10.1111/pedi.12416. [DOI] [PubMed] [Google Scholar]
- 171.Bode BW. Comparison of pharmacokinetic properties, physicochemical stability, and pump compatibility of 3 rapid-acting insulin analogues-aspart, lispro, and glulisine. Endocr Pract. 2011;17:271–80. doi: 10.4158/EP10260.RA. [DOI] [PubMed] [Google Scholar]
- 172.American Diabetes Association. Continuous subcutaneous insulin infusion. Diabetes Care. 2004;27(Suppl 1):S110. doi: 10.2337/diacare.27.2007.s110. [DOI] [PubMed] [Google Scholar]
- 173.Mooradian AD, Bernbaum M, Albert SG. Narrative review: A rational approach to starting insulin therapy. Ann Intern Med. 2006;145:125–34. doi: 10.7326/0003-4819-145-2-200607180-00010. [DOI] [PubMed] [Google Scholar]
- 174.Keay S, Callander C. The safe use of infusion devices. Contin Educ Anaesth Crit Care Pain. 2004;4:81–5. [Google Scholar]
- 175.Ponder SW, Skyler JS, Kruger DF, Matheson D, Brown BW. Unexplained hyperglycemia in continuous subcutaneous insulin infusion: Evaluation and treatment. Diabetes Educ. 2008;34:327–33. doi: 10.1177/0145721708315682. [DOI] [PubMed] [Google Scholar]
- 176.Deiss D, Adolfsson P, Alkemade-van Zomeren M, Bolli GB, Charpentier G, Cobelli C, et al. Insulin infusion set use: European perspectives and recommendations. Diabetes Technol Ther. 2016;18:517–24. doi: 10.1089/dia.2016.07281.sf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schmid V, Hohberg C, Borchert M, Forst T, Pfützner A. Pilot study for assessment of optimal frequency for changing catheters in insulin pump therapy-trouble starts on day 3. J Diabetes Sci Technol. 2010;4:976–82. doi: 10.1177/193229681000400429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.George MM, Ruiz-Elizalde AR, Beck JK. A continuous subcutaneous insulin infusion needle break. Clin Diabetes. 2015;33:195–7. doi: 10.2337/diaclin.33.4.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Saboo BD, Talaviya PA. Continuous subcutaneous insulin infusion: Practical issues. Indian J Endocrinol Metab. 2012;16(Suppl 2):S259–62. doi: 10.4103/2230-8210.104055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kalra S, Girdhar R, Sahay R. Green diabetology. Indian J Endocrinol Metab. 2015;19:698–700. doi: 10.4103/2230-8210.164030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Singh AP, Chapman RS. Knowledge, attitude and practices (KAP) on disposal of sharp waste, used for home management of type-2 diabetes mellitus, in New Delhi, India. J Health Res. 2011;25:135–40. [Google Scholar]
- 182.Majumdar A, Sahoo J, Roy G, Kamalanathan S. Improper sharp disposal practices among diabetes patients in home care settings: Need for concern? Indian J Endocrinol Metab. 2015;19:420–5. doi: 10.4103/2230-8210.152792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Macalino GE, Springer KW, Rahman ZS, Vlahov D, Jones TS. Community-based programs for safe disposal of used needles and syringes. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;18(Suppl 1):S111–9. doi: 10.1097/00042560-199802001-00019. [DOI] [PubMed] [Google Scholar]
- 184.Recommendations on Safe Disposal of Used Needles and Syringes in the Context of Targeted Intervention for Injecting Drug Users. [Last accessed on 2016 Dec 23]. Available from: http://www.nacoonline.org/upload/NGO%20 and %20Targeted/waste%20disposal%20recommendations%20for%20IDU%20TI.pd .
- 185.Memon MS, Arain ZI, Naz F, Zaki M, Kumar S, Burney AA. Prevalence of type 2 diabetes mellitus in hepatitis C virus infected population: A Southeast Asian study. J Diabetes Res. 2013;2013:539361. doi: 10.1155/2013/539361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zareban I, Karimy M, Niknami S, Haidarnia A, Rakhshani F. The effect of self-care education program on reducing HbA1c levels in patients with type 2 diabetes. J Educ Health Promot. 2014;3:123. doi: 10.4103/2277-9531.145935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Beran D. Improving access to insulin: What can be done? Diabetes Manag. 2011;1:67–76. [Google Scholar]
- 188.Kalra S, Kalra B, Batra P. Patient motivation for insulin/injectable therapy: The karnal model. Int J Clin Cases Investig. 2010;1:11–5. [Google Scholar]
- 189.Diadiun TV. Study of key commodity aspects of injection pens for insulin administration and needles for them. Asian J Pharm. 2016;10:S717–20. [Google Scholar]
- 190.Kalra S, Kalra B. A good diabetes counselor 'Cares': Soft skills in diabetes counseling. Internet J Health. 2010;11:1–3. [Google Scholar]
- 191.Association for Diabetes Care Professionals (EADV) Guideline: The Administration of Insulin with the Insulin Pen. Utrecht: Association for Diabetes Care Professionals; 2008. [Google Scholar]
- 192.Hansen B, Kirketerp G, Ehlers G, Nordentoft E, Hansen G. Evidence based clinical guidelines for injection of insulin for adults with diabetes mellitus. Denmark: Danish Nurses Organization; 2006. [Google Scholar]
- 193.Koivisto VA, Felig P. Alterations in insulin absorption and in blood glucose control associated with varying insulin injection sites in diabetic patients. Ann Intern Med. 1980;92:59–61. doi: 10.7326/0003-4819-92-1-59. [DOI] [PubMed] [Google Scholar]
- 194.Vidal M, Colungo C, Jansà M. Update on insulin administration techniques and devices (I) Av Diabetol. 2008;24:255–69. [Google Scholar]
- 195.Calles DL, Collier MG, Khudyakov Y, Mixson-Hayden T, VanderBusch L, Weninger S, et al. Hepatitis C virus transmission in a skilled nursing facility, North Dakota, 2013. Am J Infect Control. 2017;45:126–32. doi: 10.1016/j.ajic.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 196.Healthcare Inspection Inappropriate Use of Insulin Pens VA Western New York Healthcare System Buffalo, New York. [Last accessed on 2017 Feb 09]. Available from: https://www.va.gov/oig/pubs/VAOIG-13-01320-200.pdf .
- 197.Pugliese G, Germanson TP, Bartley J, Luca J, Lamerato L, Cox J, et al. Evaluating sharps safety devices: Meeting OSHA's intent. Occupational Safety and Health Administration. Infect Control Hosp Epidemiol. 2001;22:456–8. doi: 10.1086/501934. [DOI] [PubMed] [Google Scholar]


