Steroid-induced osteonecrosis (ON) is a serious complication of anti-leukemic therapy in children and young adults with acute lymphoblastic leukemia (ALL). Patients with ON experience severe pain and reduced mobility, ultimately leading to surgery with arthroplasty. Survival of ALL has increased to 90%, necessitating improved treatment to reduce the late effects.1,2 However, as the pathogenesis of ON is poorly understood, prevention is challenging. Hyperlipidemia is seemingly a key risk factor for ON, but only few studies have addressed this issue, and the results are inconclusive.3,4 Therefore, to elucidate the role of hyperlipidemia in ON, we studied lipid alterations during ALL therapy. Our findings could lead to future stratified interventions specifically targeting patients at high risk of developing ON.
We included all patients in Denmark, aged 5–45 years at ALL diagnosis, treated with the Nordic Society of Paediatric Haematology and Oncology (NOPHO) ALL2008 protocol (n=182). Registration of symptomatic ON was prospectively reported.5 All patients were followed from diagnosis of ALL until diagnosis of ON, relapse, second malignant neoplasia (SMN) or end of follow up, August 27, 2015, whichever came first. Patients were stratified into standard-risk (SR), intermediate-risk (IR) and high-risk therapy. Therapy lasted 2.5 years and is described in detail elsewhere5–7 (Online Supplementary Material). Retrospectively, we analyzed non-fasting triglycerides (TG), total cholesterol (CH), high-density lipoprotein (HDL), and low-density lipoprotein (LDL) on plasma samples taken at ALL diagnosis (i.e., baseline) and during therapy. Lipid levels were routinely analyzed for clinical purposes throughout the therapy, at two treatment centers (Online Supplementary Material).
Briefly, baseline characteristics were analyzed by simple Cox proportional hazard regression. Baseline lipids were analyzed by multiple Cox regression adjusted for sex and age at diagnosis. For each lipid, we calculated the running mean with standard error (SE) Day 30–250 after ALL diagnosis. If ON or competing events (relapse or SMN) occurred before Day 250, the patient’s subsequent lipid-measurements were excluded. For SR/IR patients, we predefined two treatment intervals: ConsASP Day 50–89, where patients received pegylated asparaginase (PEG-ASP) but not dexamethasone, and DIASP+DEXA Day 90–130, including both PEG-ASP and dexamethasone. In these intervals we used peak values of TG, CH and LDL and trough values of HDL. Subsequently, groups with high/low levels of peak/trough lipids were defined based on cut-off estimated by Youden’s index on the receiver operating characteristic (ROC) curve. Peak/trough levels were analyzed using multiple Cox regression adjusted for sex and age at diagnosis. The cumulative incidences between groups were compared using Gray’s test. Mean lipid-levels were analyzed as time-dependent continuous parameters Day 0–250 in a Cox regression with delayed entry of patients at the first lipid measurement. We recalculated the weighted means every time a patient experienced an event. The weights were the intervals between the two measurements, maximum seven days. Analyses were adjusted for sex and age at ALL diagnosis, and stratified by risk group. In all Cox analyses, competing events were censored. ON-specific hazard ratios (HR) were calculated per mmol/L (Online Supplementary Material).
We included 112 patients diagnosed with ALL from July 2008 to December 2014. Patients were excluded due to lack of consent (n=35), non-protocol treatment (n=11), stem cell transplantation (n=14), death during treatment (n=8), and no available lipid-measurements (n=2) (Online Supplementary Figure S1). Median follow up was 3.2 years. Of the patients, 3 experienced ALL relapse and one developed SMN. Symptomatic ON was diagnosed in 22 patients. Time from ALL diagnosis to ON diagnosis ranged from 153–1362 days (median: 654), using date when ON was first confirmed by radiological imaging. The overall cumulative incidence of ON was 22.9% (95% confidence interval (CI): 13.9–30.8). Age at ALL diagnosis for ON patients ranged 5.2–37 years, which defined the age eligibility criterion by the youngest patient. ON patients were graded according to the Ponte di Legno toxicity working group grade 4 (n=12) and grade 2–3 (n=10)2, and 10 patients underwent surgery. At time of ON diagnosis, 10 patients had multiple joints affected, all with involvement of hip and/or knee. Eleven patients had a single joint affected, including hip (n=5), knee (n=1), ankle (n=2), shoulder (n=2), and elbow (n=1).
Median age at ALL diagnosis was 12.7 years and 10.9 years for ON patients and non-ON patients, respectively. ON was diagnosed in 10 female patients and 12 male patients, and treated according to SR (n=5), IR (n=15) and high-risk (n=2) (Online Supplementary Table S1). We found no association between ON and age at diagnosis (P=0.85), sex (P=0.72), risk group (P=0.40), white blood cell count (P=0.57), steroid during induction (P=0.33) or body mass index at diagnosis (P>0.99).
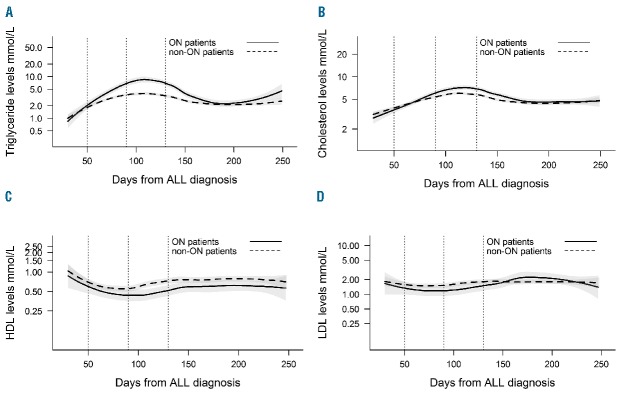
Baseline lipid levels showed no association with ON-specific HR (Table 1). Lipid alterations for treatment Day 30–250 are illustrated for TG, CH, HDL and LDL for all ON patients and non-ON patients (Figure 1A–D). TG and CH increased during therapy, noticeably greater for ON patients (Figure 1A–B). The peak level was observed around Days 100 and 120 for TG and CH, respectively. We observed a decrease of HDL during therapy, marginally greater in ON patients (Figure 1C). The lowest HDL levels were observed around Day 90. LDL alterations were minimal, illustrating no difference between groups (Figure 1D).
Table 1.
Baseline and peak/trough lipid levels for standard-risk and intermediate-risk patients.
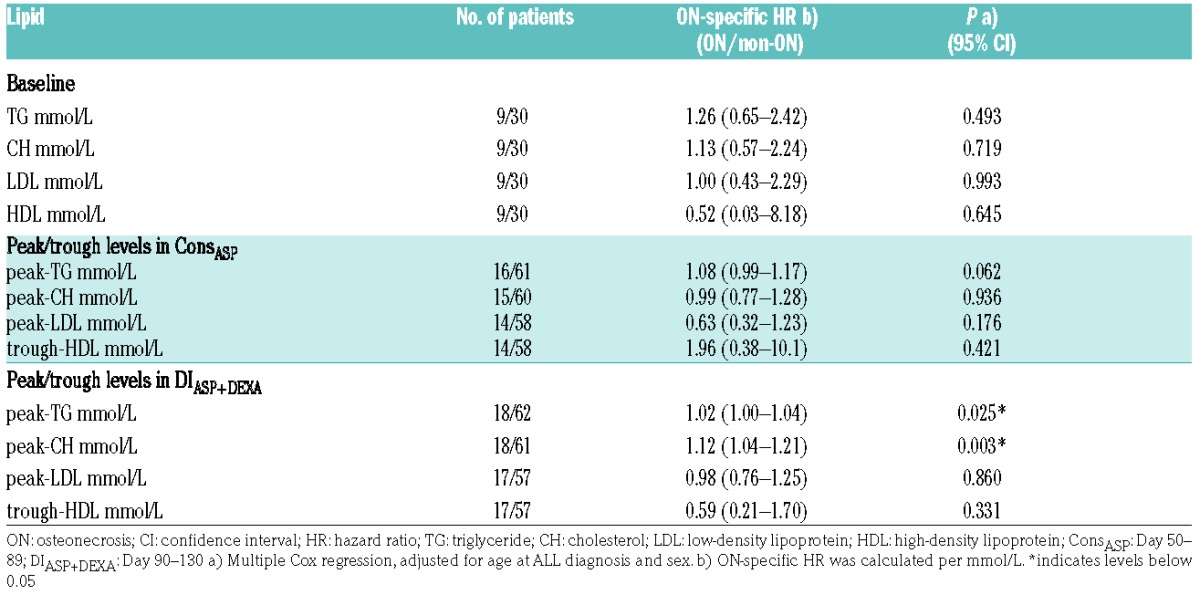
Figure 1.
Running means of lipid levels. The running means of A. levels of triglyceride, B. cholesterol, C. high-density lipoprotein (HDL) and D. low-density lipoprotein (LDL) in mmol/L, Day 30–250 after diagnosis of acute lymphoblastic leukemia (ALL) for osteonecrosis patients (filled lines) and non-osteonecrosis patients (dashed lines). The shaded areas show the standard error for each group. Lipid levels are shown in absolute values on a logarithmic scale. The vertical dotted lines indicate treatment intervals in consolidation Day 50–89 (ConsASP) and delayed intensification Day 90–130 (DIASP+DEXA).
Peak/trough lipid levels were analyzed in the predefined treatment interval, i.e., ConsASP and DIASP+DEXA (Table 1). In DIASP+DEXA, we found significantly increased ON-specific HR with higher peak-TG (HR=1.02, P=0.025) and higher peak-CH (HR=1.12, P=0.003). In ConsASP, we similarly found increased ON-specific HR with higher peak-TG, though non-significant (HR=1.08, P=0.062), but no significant association for peak-CH (HR=0.99, P=0.936). For peak-LDL and trough-HDL, we found no significant association with ON in either interval.
Subsequently, we estimated the cut-off at 18.1 mmol/L for peak-TG in DIASP+DEXA (ROC: area under the curve (AUC)=0.653, sensitivity=0.50, specificity=0.85). Patients were grouped into high/low peak-TG based on the cutoff (Online Supplementary Figure S2). The 3-year cumulative ON incidence for patients with high peak-TG was significantly higher (54.0%) than for patients with low peak-TG (16.8%, P<0.001) (Figure 2A). The cut-off estimation for peak-CH in DIASP+DEXA was 7.7 mmol/L (ROC: AUC=0.696, sensitivity=0.72, specificity=0.69). Using this cut-off, we found a significantly higher 3-year cumulative ON incidence for patients with high peak-CH (45.3%) compared with patients with low peak-CH (12.2%, P=0.0014) (Figure 2B). Analyses were made for all peak/trough lipids in both intervals, but for the rest ROC showed AUC<0.60 and sensitivity or specificity below 0.5, indicating poor estimation of the cut-off value (results not shown). In DIASP+DEXA, 13/18 (72%) ON patients had either peak-TG or peak-CH above the cutoff and 9/18 (50%) had simultaneous elevation.
Figure 2.
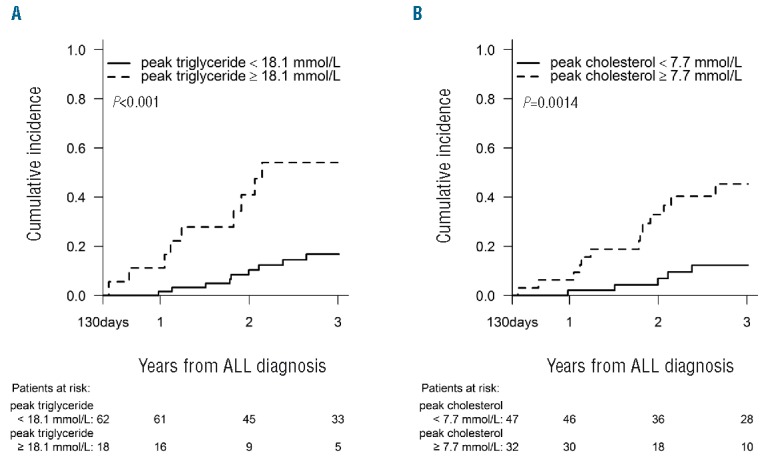
Cumulative incidence of osteonecrosis. The cumulative incidence of osteonecrosis grouping patients according to a cut-off level during DIASP+DEXA of (A) 18.1 mmol/L for peak-triglyceride, and (B) 7.7 mmol/L for peak-cholesterol. The follow up starts at the end of the interval DIASP+DEXA (Day 130 after diagnosis of acute lymphoblastic leukemia). P-values are from Gray’s test comparing the cumulative incidences.
We analyzed prolonged exposure to hyperlipidemia Day 0–250. We found significantly higher ON-specific HR per mmol/L for increased TG levels (HR=1.08, P=0.038), likewise for CH levels (HR=1.26, P=0.039). We also found a tendency of an inverse association between higher HDL and ON (HR=0.22, P=0.053). Prolonged exposure of LDL was not associated with ON (HR=1.23, P=0.495).
In this population-based study, hyperlipidemia increased the risk of developing ON. Adolescents are at increased risk of both hyperlipidemia8 and ON.1 We found cut-off levels for both peak-TG and peak-CH, identifying groups of patients with exceptionally high incidence of ON. This suggests that, apart from age, peak-TG and peak-CH are important predictors. Additionally, we found that prolonged exposure to hypertriglyceridemia or hypercholesterolemia was associated with increased risk of ON, demonstrating that hyperlipidemia during longer periods could induce ON. To our knowledge, no other human studies have investigated similar associations; we suggest validation in other cohorts.
The pathogenesis behind hyperlipidemia-induced ON is poorly understood. A possible mechanism is increased blood viscosity leading to reduced blood flow.9 Furthermore, Powell et al. proposed that “compartment syndrome” in the bone could induce ON10. Increased intraosseous pressure caused by hyperlipidemia, through deposition of fat in the intramedullary tissue11 and enlargements of fat cells in the bone marrow cavity,12 might induce this “compartment syndrome state”.
Kawedia et al. reported a link between ON and hypercholesterolemia.3 Conversely, no human studies have found that hypertriglyceridemia induces ON.3,4 Compared with similar phases in previous studies, the NOPHO ALL2008 protocol uses intense dexamethasone therapy for SR/IR patients, possibly influencing the severity of lipid alterations.3,4 Additional differences between studies could occur due to i) predefined/estimated cut-off levels,4 ii) capturing of the actual peak levels, iii) ethnicity and host-specific genetics,13 and iv) fasting/non-fasting lipid measurements.
ON is primarily induced by glucocorticosteroids.14 However, due to pharmacokinetic interaction, lipid alterations are the most pronounced during simultaneous PEG-ASP and dexamethasone therapy.8,15 Therefore, timing and severity of hyperlipidemia seemingly depend on scheduling of both dexamethasone and PEG-ASP. We speculate that PEG-ASP could play a larger role in the pathogenesis of ON than previously assumed. The NOPHO ALL2008 protocol includes a randomized trial investigating continuous vs. non-continuous PEG-ASP treatment. Asparaginase-related toxicities will be analyzed, including the effect on risk of ON. Moreover, we propose interventional studies, using antihyperlipidemic drugs, physical activity and diet, targeted toward reducing TG and CH in hyperlipidemic children and young adults.
In conclusion, we find hypertriglyceridemia and hypercholesterolemia to be important risk factors for developing ON. Interventional studies are needed to elucidate whether lowering TG and CH during combined PEG-ASP and dexamethasone therapy can reduce the risk of ON.
Supplementary Material
Acknowledgments
The authors would like to thank laboratory technicians Lene Ravn, Jane Hagelskjær Knudsen and Emel Korkmaz for their assistance with all lipid analyses for the study. We also thank Steen Rosthøj for collecting patient consent and samples from the patients from Aalborg Hospital. A special thanks to all the patients and their families for participating in the study.
Footnotes
Funding: the study was funded by the Danish Childhood Cancer Foundation, the Swedish Childhood Cancer Foundation, the Dagmar Marshalls Foundation, the Foundation for Danish Cancer Research, the Otto Christensen Foundation and the Axel Muusfeldts Foundation.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Kunstreich M, Kummer S, Laws H-J, et al. Osteonecrosis in children with acute lymphoblastic leukemia. Haematologica. 2016;28(56):90–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmiegelow K, Attarbaschi A, Barzilai S, et al. Consensus definitions of 14 severe acute toxic effects for childhood lymphoblastic leukaemia treatment: a Delphi consensus. Lancet Oncol. 2016; 17(6):e231–e239. [DOI] [PubMed] [Google Scholar]
- 3.Kawedia JD, Kaste SC, Pei D, et al. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117(8):2340–2347; quiz 2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhojwani D, Darbandi R, Pei D, et al. Severe hypertriglyceridaemia during therapy for childhood acute lymphoblastic leukaemia. Eur J Cancer. 2014;50(15):2685–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frandsen TL, Heyman M, Abrahamsson J, et al. Complying with the European Clinical Trials directive while surviving the administrative pressure - An alternative approach to toxicity registration in a cancer trial. Eur J Cancer. 2014;50(2):251–259. [DOI] [PubMed] [Google Scholar]
- 6.Toft N, Birgens H, Abrahamsson J, et al. Risk group assignment differs for children and adults 1–45 yr with acute lymphoblastic leukemia treated by the NOPHO ALL-2008 protocol. Eur J Haematol. 2013;90(5):404–412. [DOI] [PubMed] [Google Scholar]
- 7.Raja RA, Schmiegelow K, Albertsen BK, et al. Asparaginase-associated pancreatitis in children with acute lymphoblastic leukaemia in the NOPHO ALL2008 protocol. Br J Haematol. 2014;165(1):126–133. [DOI] [PubMed] [Google Scholar]
- 8.Tong WH, Pieters R, de Groot-Kruseman HA, et al. The toxicity of very prolonged courses of PEGasparaginase or Erwinia asparaginase in relation to asparaginase activity, with a special focus on dyslipidemia. Haematologica. 2014;99(11):1716–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rosenson RS, Shott S, Tangney CC. Hypertriglyceridemia is associated with an elevated blood viscosity Rosenson: Triglycerides and blood viscosity. Atherosclerosis. 2002;161(2):433–439. [DOI] [PubMed] [Google Scholar]
- 10.Powell C, Chang C, Gershwin ME. Current concepts on the pathogenesis and natural history of steroid-induced osteonecrosis. Clin Rev Allergy Immunol. 2011;41(1):102–113. [DOI] [PubMed] [Google Scholar]
- 11.Jaffe WL, Epstein M, Heyman N, et al. The effect of cortisone on femoral and humeral heads in rabbits. An experimental study. Clin Orthop Relat Res. 1972;82:221–228. [PubMed] [Google Scholar]
- 12.Miyanishi K, Yamamoto T, Irisa T, et al. Bone marrow fat cell enlargement and a rise in intraosseous pressure in steroid-treated rabbits with osteonecrosis. Bone. 2002;30(1):185–190. [DOI] [PubMed] [Google Scholar]
- 13.Kahn J, Barrera S, Davila R, et al. Higher Incidence of Treatment-Related Toxicities in Non-Hispanic Patients Undergoing Therapy for Newly Diagnosed Pediatric Acute Lymphoblastic Leukemia on Dana-Farber Cancer Institute ALL Consortium Protocol 05-001. Blood. 2015;126(23):248. [Google Scholar]
- 14.Mattano LA, Devidas M, Nachman JB, et al. Effect of alternate-week versus continuous dexamethasone scheduling on the risk of osteonecrosis in paediatric patients with acute lymphoblastic leukaemia: results from the CCG-1961 randomised cohort trial. Lancet Oncol. 2012;13(9):906–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang L, Panetta JC, Cai X, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol. 2008;26(12):1932–1939. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.