Abstract
Introduction
Accumulating evidence indicates that arsenic (As), a potent environmental toxicant, may increase cardiovascular disease risk and adversely affect endothelial function at high levels of exposure. Pregnancy is a vulnerable time for both mother and child; however, studies examining the association between prenatal As exposure and plasma biomarkers of inflammation and endothelial function in mothers and newborns are lacking.
Methods
We examined maternal urinary As levels at gestational weeks 24 to 28 and levels of inflammatory biomarkers in plasma from 563 pregnant women and 500 infants’ cord blood. We assessed a multiplexed panel of circulating inflammatory and endothelial function markers, including tumor necrosis factor alpha (TNFα), monocyte chemoattractant protein 1 (MCP1), intercellular adhesion molecule (ICAM1) and vascular cell adhesion molecule (VCAM1).
Results
Compared with the bottom tertile, the highest tertile of maternal urinary As during pregnancy was associated with a 145.2 ng/mL (95% CI 4.1, 286.3; p=0.04) increase in cord blood ICAM1 and 557.3 ng/mL (95% CI −56.4, 1171.1; p=0.09) increase in cord blood VCAM1. Among mothers, the highest tertile of maternal urinary As during pregnancy was related to a 141.8 ng/mL (95% CI 26.1, 257.5; p=0.02) increase maternal plasma VCAM1 levels. Urinary As was unrelated to MCP1 or TNFα in maternal plasma and cord blood. In structural equation models, the association between maternal urinary As and infant VCAM was mediated by maternal levels of VCAM (βmediation: 0.024, 95% CI: 0.002, 0.050).
Conclusion
Our observations indicate that As exposure during pregnancy may affect markers of vascular health and endothelial function in both pregnant women and children, and suggest further investigation of the potential impacts on cardiovascular health in these susceptible populations.
Keywords: Arsenic, pregnancy cohort, New Hampshire, inflammatory markers, endothelial
Introduction
Arsenic (As) exposure continues to be a major public health concern across the globe. Worldwide, the main source of As exposure is contaminated groundwater, with an estimated 200 million individuals exposed to levels exceeding the World Health Organization safety standard and US EPA maximum contaminant level (MCL) of 10μg/L.1–3 In the US, nearly 44.5 million people rely on private wells as their primary water source and an estimated 7% of these systems exceed the As MCL.4–6 Furthermore, a growing number of studies have raised concerns about certain foods, including rice and rice products, as sources of As exposure.7–11
Arsenic’s toxicity at high levels of exposure is well documented. Known primarily for its role as a potent carcinogen of the bladder, skin, kidney, liver and lung, As has also been associated with a multitude of adverse health effects, including respiratory disease, diabetes, neurological impairment, and immune dysfunction.3,4 Epidemiological evidence further supports a relationship between As exposure and cardiovascular effects and pregnant women and children may be especially vulnerable to these effects.12–18 We previously reported that women with higher urinary As levels during pregnancy had greater increases in blood pressure over the course of pregnancy.19 In children, early life As exposure has been associated with early indicators of cardiovascular risk, including increased blood pressure, as well as carotid intima media thickness (cIMT).20–22
Arsenic-related cardiovascular dysfunction may occur by a number of pathways, such as increased inflammation, cytokine induction and production of reactive oxygen species, which can each affect endothelial activation and function.17,23 Cellular damage or toxic insults to the vascular endothelium activate a signaling cascade, triggering expression of pro-inflammatory mediator tumor necrosis factor alpha (TNFα). TNFα induces inflammatory responses by promoting secretion of cytokines and activating endothelial cells by promoting expression of adhesion molecules, such as intercellular adhesion molecule-1 (ICAM1) and vascular adhesion molecule-1 (VCAM1) on their surface.24,25 Adhesion molecules allow mononuclear cells to attach to the endothelium, a key step in atherosclerotic initiation. In experimental studies, As elevates circulating vascular inflammatory marker levels, including ICAM1 and VCAM126–28, increases monocyte adhesion to the vascular endothelium via increased binding to VCAM128 and increases atherosclerotic lesion formation, along with expression of inflammatory mediators, such as monocyte chemoattractant protein 1 (MCP1), at vascular lesion sites.29 Levels of these circulating markers may predict future cardiovascular disease risk30–33 and of these, soluble VCAM1 and ICAM1 have been consistently related to chronic As exposure in studies among adults in Bangladesh.34,35
However, very little is known about whether arsenic may affect these particular markers, which may indicate cardiovascular health, in either children or pregnant women. A recent study from an Iranian birth cohort found positive associations between ambient air pollutants and cord blood ICAM1, VCAM1 and endothelian-1, suggesting that these markers may be impacted by environmental exposures.36 We therefore hypothesized that As exposure may be associated with increases in plasma and cord blood levels of markers of endothelial dysfunction (VCAM1, ICAM1), and mediators of the endothelial inflammatory response (MCP1, TNFα) among mother-infant pairs enrolled in the New Hampshire Birth Cohort Study.
Methods
The New Hampshire Birth Cohort
The New Hampshire Birth Cohort is an ongoing study that began in January 2009, recruiting 18–45 year old pregnant women receiving prenatal care at study clinics, as previously described.9 Women were enrolled at 24–28 weeks gestation if they reported using water from a private well at their residence since their last menstrual period and were not planning to move prior to delivery. Only singleton births were included in the study. All protocols were approved by the Dartmouth College Institutional Review Boards. Participants provided written, informed consent upon enrollment.
Medical Record Review
Participants completed a detailed medical history and lifestyle questionnaire upon enrollment, which ascertained sociodemographic factors (age, race/ethnicity, marital status, education), reproductive history (previous pregnancies, complications, birth outcomes), and health history. Women were asked about habits, including tobacco and alcohol use, along with their home water source and consumption. At two weeks postpartum, mothers were sent a follow-up questionnaire to obtain additional information about pregnancy, delivery and changes in key exposures. Participants also consented to a medical record review, which allowed additional information to be recorded about prenatal infections, medication use, birth outcomes and delivery details, and general health of the women and their infants after birth.
Arsenic Exposure Assessment
Women provided a spot urine sample upon enrollment at 24– 28 weeks gestation, which was collected and stored, as previously described.9 Urine samples were analyzed for levels of arsenite (iAsIII), arsenate (iAsV), monomethylarsonic acid (MMA), dimethylarsinic acid (DMA) and arsenobetaine by high-performance liquid chromatography (HPLC) inductively coupled plasma mass spectrometry (ICP-MS) at the University of Arizona Hazard Identification Core.37–39 Total urinary As was calculated by summing inorganic (iAs = iAsIII+iAsV) and organic (DMA, MMA) metabolites.9 Arsenobetaine, a form of As found in fish and seafood was excluded, as it is thought to pass through the body unmetabolized.40 Urine samples that registered below the As detection limit (ranging from 0.10–0.15 μg/L for individual urine species; 14.0%, 20.8%, and 40.3% of the study population were below the detection limit for DMA, MMA and iAs, respectively) were assigned a value equal to the detection limit divided by the square root of two. Participants were given instructions and prepaid mailing materials upon enrollment to collect samples of their home tap water and return the samples to the study office, which were analyzed by high resolution inductively coupled plasma mass spectrometry (ICP-MS) at the Dartmouth Trace Element Analysis Core, as previously described.9 Water arsenic detection limits ranged from 0.005–0.074 μg/L.
Plasma and cord blood inflammatory markers testing
We selected the first 564 mothers with adequate plasma sample volumes for testing and an available maternal urine sample for As assessment. A paired infant cord blood sample was available for 500 women. A total of 563 mothers and 500 infants were successfully assayed for one or more markers.
Maternal and infant circulating protein marker levels were measured using MILLIPLEX-MAP human magnetic bead multiplexed panels (Millipore, Billerica, MA), according to manufacturers instructions. MCP1 and TNFα were assayed using undiluted plasma samples and had minimum assay sensitivities of 1.9 and 0.7 pg/ml for MCP1 and TNFα, respectively. Plasma samples assayed for soluble ICAM1 and VCAM1 were diluted 1:100 and had minimum assay sensitivities of 0.019 and 0.024 ng/ml for ICAM1 and VCAM1, respectively. Calibration curves of recombinant standards were prepared with three-fold dilution steps in the same matrix as the samples. Standards were measured in triplicate, samples were measured once, and blank values were subtracted from all readings. All assays were carried out directly in a 96-well filtration plate (Millipore) at room temperature and protected from light. Briefly, wells were pre-wet with 100 μl PBS containing 1% BSA. Then, 100 μl volume of beads and either a standard, sample, spikes, or blank were added and incubated at room temperature for 30 min with continuous shaking. Beads were washed with 100 μl PBS containing 1% BSA and 0.05% Tween 20. A cocktail of biotinylated antibodies (50 μl/well) was added to beads for a further 30 min incubation with continuous shaking. Beads were washed, then streptavidin-phycoerythrin was added for 10 min. Beads were again washed and resuspended in 125 μl of PBS containing 1% BSA and 0.05% Tween 20. The fluorescence intensity of the beads was measured using a Bio-Plex Array Reader (Bio-Rad Laboratories, Hercules, CA) and analyzed using Bio-Plex Manager software with five-parametric curve fitting. Markers below the detection limit were assigned a value equal to the detection limit divided by the square root of two.
Maternal and infant samples were randomized across plates. Assays were performed in three separate runs and batch assignment was included in models as a covariate to control for batch-to-batch variability. Approximately 10% of sample wells on each plate were reserved for quality control samples. Replicates of four different non-study plasma samples were included on each plate to account for inter- and intra-plate variability, for which coefficients of variation for these six markers ranged from 9–20% and 3–19%, respectively. All assays were within the range of acceptable variability according to the manufacturer’s standards and generally acceptable in epidemiological biomarker studies.41 In addition to controlling for batch effects within our models, we also evaluated whether there were differences in exposure between plates and batches by examining the mean arsenic exposure by group. We tested this using analysis of variance tests run with SAS PROC GLM and in both cases, we did not find any statistically significant differences between groups by plate (F = 1.11, p=0.35) or by batch (F=2.25, p=0.11).
Statistical Analysis
Characteristics of the study sample and the overall cohort were assessed and frequencies and/or means were compared by chi-squared or one-way ANOVA tests. We computed Spearman’s rank correlations between maternal and infant biomarkers. To assess the shape of the correlations, the median and interquartile ranges of each inflammatory marker on the y-axes were plotted against the median for each tertile of maternal urinary arsenic on the x-axes. Using total urinary As during pregnancy (as a continuous measure and/or categorized by tertiles of exposure) as our measure of exposure for both mothers and infants, we tested for associations with maternal and infant inflammatory markers, using linear regression models, adjusted for potential confounders. These included factors that could potentially influence inflammation or vascular function based on a priori considerations, although they may not be related to As exposure. Final models included age at enrollment, smoking and/or secondhand smoke exposure during pregnancy, education, urinary creatinine and batch assignment. P-values for trends were estimated using urinary As tertiles and statistical significance was determined at p<0.05.
We also considered the possibility of effect modification by pre-pregnancy body mass index (BMI), maternal weight gain, and infant birth size, which were evaluated by inspection of stratum specific estimates and by including a multiplicative interaction term in the multivariable logistic regression models and assessing its statistical significance at p<0.05 using likelihood ratio chi-square tests. In stratified analyses, maternal pre-pregnancy BMI was categorized by World Health Organization standards (i.e. <25 kg/m2: underweight to normal; 25 to 30 kg/m2: overweight; ≥30 kg/m2: obese).42 Maternal weight gain was categorized as normal versus excessive based on IOM standards, based on pre-pregnancy BMI.42 Infant birth size categories were based on gestational age and sex, using methods described by Fenton et al.43
A structural equation model (SEM)44 was applied to simultaneously estimate the direct (maternal urinary As on infant cord blood levels of VCAM and ICAM) and the indirect pathways (with plasma levels of maternal VCAM or ICAM during pregnancy as mediators). This analysis is an extension of multivariable regression analysis that permits the simultaneous modeling of multiple dependent variables to assess potential causal effects among the dependent variables while adjusting for the aforementioned covariates in the regression analysis. The SEM analysis was done in the software Mplus 7.11. Generalized additive models (GAM), adjusted for all aforementioned covariates, with cubic regression splines were used to generate figures in R software.45
Results
A total of 563 mothers and 500 paired infants from the NHBCS were included in this analysis. Nearly 1 in every 7 households (58 of 414 available samples; 14%) tested in this study sample had water As levels above EPA MCL of 10μg/L, with a mean As level of 4.7 μg/L (range: 0.003– 147.7 μg/L) (Table 1). The mean values for water As were similar to the values observed in the overall cohort, but a slightly higher proportion of women in this sample (14.0%) had water levels exceeding the MCL than the overall cohort (11.5%) (p = 0.045). Women in this sample had a mean urinary As concentration of 5.8 μg/L with values ranging from 0.2–288.5 μg/L, which were very similar to the values observed in the overall cohort and not significantly different (Table 1). No other statistically significant differences were observed between the study population and the overall cohort.
Table 1.
Selected characteristics for mothers (n=563) and paired infants (n=500) participating in the New Hampshire Birth Cohort Study.
| % or mean (SD) | ||
|---|---|---|
| Maternal Variables | Study population | Overall cohort (n=1033) |
| Maternal age at enrollment | 31.2 (4.8) | 31.06 (4.9) |
| Race, white | 98.1% | 98.5% |
| Educational attainment* | ||
| Less than college | 34.4% | 32.9% |
| College graduate | 39.8% | 39.7% |
| Any post-graduate schooling | 25.8% | 27.5% |
| Relationship status* | ||
| Married | 87.1% | 86.3% |
| Single, separated or divorced | 12.9% | 13.7% |
| Pre-pregnancy BMI* | 26.0 (5.7) | 26.1 (5.9) |
| Weight gain during pregnancy* | 35.6 (14.7) | 34.7 (14.9) |
| Parity* | ||
| Nulliparous | 40.0% | 40.0% |
| 1 to 2 | 51.2% | 51.7% |
| 3+ | 8.8% | 8.3% |
| Ever smoked during pregnancy* | 5.9% | 6.4% |
| Exposed to secondhand smoke during pregnancy* | 12.3% | 13.4% |
| Well water arsenic (μg/L)* | 4.72 (12.22); range 0.003–147.74 | 4.46 (13.37); range 0.002–189.34 |
| Well water arsenic above the 10μg/L MCL | 14.0%# | 11.5% |
| Urinary arsenic (μg/L) | 5.78 (13.34); range 0.21–288.48 | 6.05 (13.19); range 0.21–288.48 |
| Toenail arsenic (μg/g)* | 0.08 (0.08); range 0.001–0.70 | 0.08 (0.08); range 0.001–0.70 |
| Infant Variables | ||
| Sex, male | 48.8% | 48.9% |
| Birth Weight, grams* | 3486.9 (490.7) | 3458.0 (519.6) |
| Gestational age, weeks | 39.6 (1.3) | 39.3 (1.9) |
Missing (n) in study sample: pre-pregnancy BMI (11); pregnancy weight gain (19); relationship status (39); education level (39); parity (2); smoking/secondhand smoke exposure during pregnancy (38); water arsenic (145); toenail arsenic (127); birth weight (6).
Frequencies and means were compared by chi-squared or one-way ANOVA tests, respectively and statistically significant differences (p<0.05) are marked.
We examined correlations between maternal and infant levels of the markers that we investigated. The maternal and infant levels were moderately correlated with the strongest positive correlation observed for maternal ICAM with infant ICAM (ρ=0.76) (Figure 1). Maternal ICAM also was positively correlated with infant VCAM (ρ=0.58) (Figure 1). Maternal VCAM was positively correlated with infant VCAM (ρ=0.56), and infant ICAM (ρ=0.60) (Figure 1). Maternal TNFα was positively correlated with infant VCAM (ρ=0.50) and infant ICAM (ρ=0.43) (Figure 1).
Figure 1.
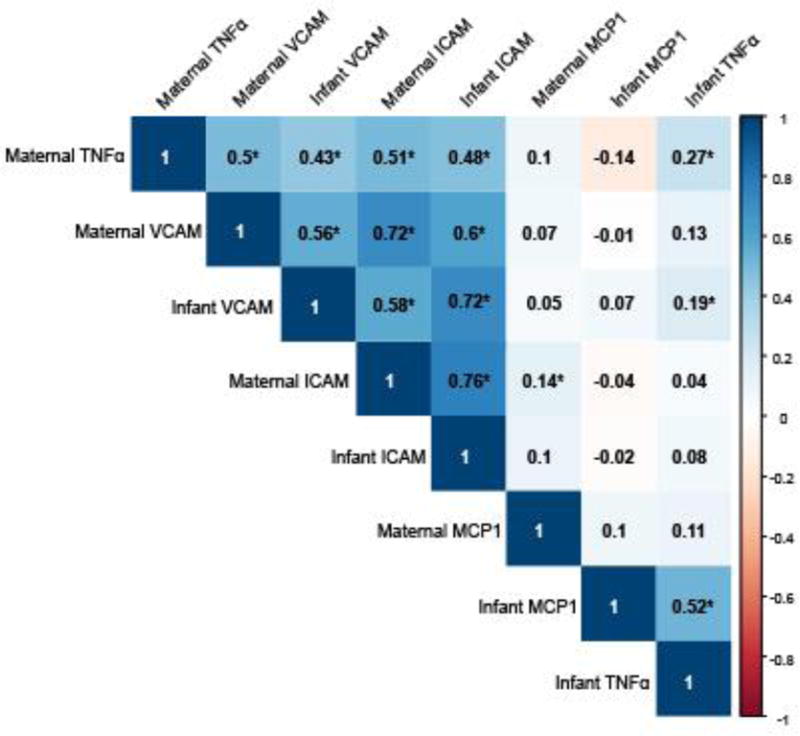
Maternal plasma and infant cord blood marker correlation coefficients. Correlations were examined using Spearman’s rank correlation coefficients. Positive correlations are shown in blue, negative correlations are shown in red, with darker shades indicating stronger correlations. An asterisk (*) indicates statistically significant correlations (p < 0.05).
Using linear regression models, adjusted for covariates, we observed that compared with the lowest As exposure tertile, the highest tertile of maternal urinary As during pregnancy was positively related to cord blood levels of ICAM1 (ng/ml) (βTert3: 145.2, 95% CI: 4.1, 286.3; p-trend= 0.04) (Table 2). We observed a similar positive trend when we examined the dose-response relationship between maternal urinary As and infant ICAM1, using adjusted generalized additive models with cubic regression splines (Figure 2a). We also observed marginally significant increases in cord blood VCAM levels (ng/ml) among infants born to mothers within the higher tertiles of urinary As (βTert2: 532.8, 95% CI: −56.9, 1122.5; βTert3: 557.3, 95% CI: −56.4, 1171.1; p-trend= 0.09), as compared to the level in those born to mothers within the lowest tertile although the trend did not reach statistical significance (Table 2).
Table 2.
Linear regression models of maternal urinary As by tertiles and inflammatory markers in infant cord blood.
| Infant Marker | N | β (95% CI) | p-trend |
|---|---|---|---|
| VCAM1 (ng/ml) | |||
| As Tertile 1 (0.20–2.38 μg/L) | 173 | Ref. | 0.09 |
| As Tertile 2 (2.38–5.30 μg/L) | 160 | 532.8 (−56.9, 1122.5)* | |
| As Tertile 3 (5.30–288.5 μg/L) | 167 | 557.3 (−56.4, 1171.1)* | |
| ICAM1 (ng/ml) | |||
| As Tertile 1 (0.20–2.38 μg/L) | 173 | Ref. | 0.04 |
| As Tertile 2 (2.38–5.30 μg/L) | 160 | 74.8 (−60.8, 210.3) | |
| As Tertile 3 (5.30–288.5 μg/L) | 167 | 145.2 (4.1, 286.3)** | |
| MCP1 (pg/ml) | |||
| As Tertile 1 (0.20–2.38 μg/L) | 173 | Ref. | 0.89 |
| As Tertile 2 (2.38–5.30 μg/L) | 160 | 33.8 (−22.7, 90.3) | |
| As Tertile 3 (5.30–288.5 μg/L) | 167 | 6.5 (−52.3, 65.4) | |
| TNFα (pg/ml) | |||
| As Tertile 1 (0.20–2.38 μg/L) | 173 | Ref. | 0.35 |
| As Tertile 2 (2.38–5.30 μg/L) | 160 | 0.16 (−0.6, 0.9) | |
| As Tertile 3 (5.30–288.5 μg/L) | 167 | 0.36 (−0.4, 1.1) |
0.1>p>0.05;
p<0.05
Adjusted for enrollment age, education, maternal smoking, secondhand smoke exposure, urinary creatinine, and batch assignment.
Figure 2.
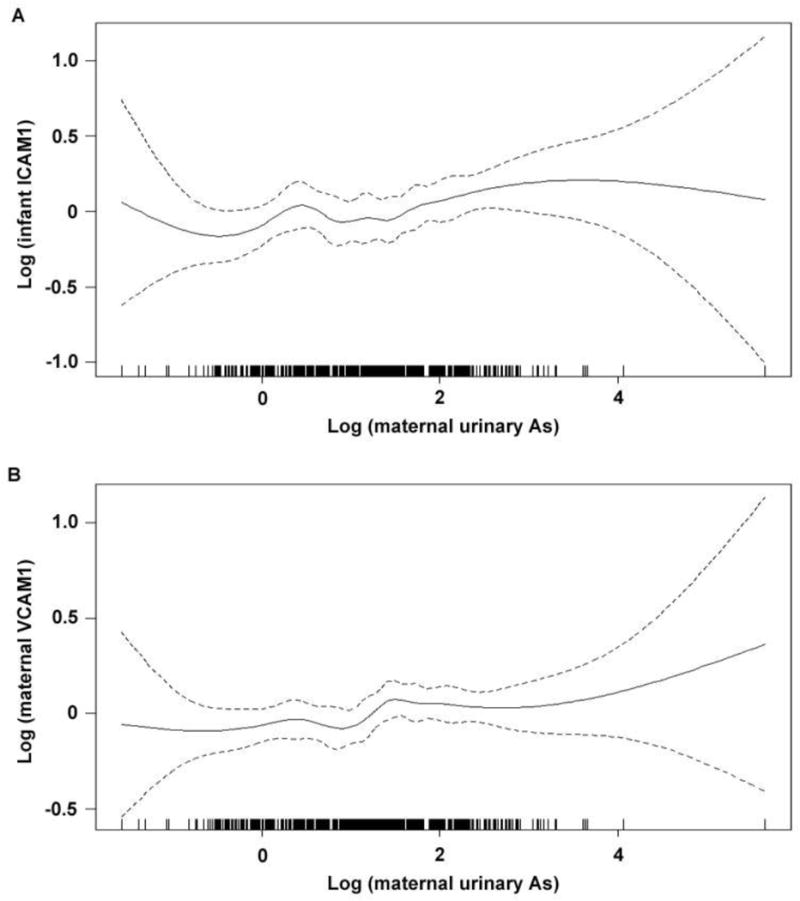
Associations between maternal urinary arsenic and A) infant cord blood ICAM1 and B) maternal plasma VCAM1, as modeled using generalized additive models with cubic regression splines, adjusted for
Among mothers, the higher tertiles of maternal urinary As during pregnancy were positively related to maternal plasma levels of VCAM1 (ng/ml) (βTert2: 97.3, 95% CI: −11.7, 206.3; βTert3: 141.8, 95% CI: 26.1, 257.5; p-trend= 0.02), as compared to the lowest tertile (Table 3). We observed a similar positive trend when we examined the dose-response relationship between maternal urinary As and maternal VCAM1 using adjusted generalized additive models with cubic regression splines (Figure 2b). Further, the highest tertile of maternal urinary As during pregnancy was positively related to maternal plasma levels of MCP1 (pg/ml) (βTert3: 10.4; 95% CI −1.5, 22.3; p-trend= 0.09), as compared to the lowest tertile, although the trend did not reach statistical significance.
Table 3.
Linear regression models of maternal urinary As by tertiles and inflammatory markers in maternal plasma.
| Maternal Marker | N | β (95% CI) | p-trend |
|---|---|---|---|
| VCAM1 (ng/ml) | |||
| As Tertile 1 (0.20–2.38μg/L) | 197 | Ref. | 0.02 |
| As Tertile 2 (2.38–5.30μg/L) | 183 | 97.3 (−11.7, 206.3)* | |
| As Tertile 3 (5.30–288.5 μg/L) | 183 | 141.8 (26.1, 257.5)** | |
| ICAM1 (ng/ml) | |||
| As Tertile 1 (0.20–2.38μg/L) | 197 | Ref. | 0.98 |
| As Tertile 2 (2.38–5.30μg/L) | 183 | 86.9 (−179.6, 353.4) | |
| As Tertile 3 (5.30–288.5 μg/L) | 183 | −3.9 (−286.7, 279.0) | |
| MCP1 (pg/ml) | |||
| As Tertile 1 (0.20–2.38μg/L) | 197 | Ref. | 0.09 |
| As Tertile 2 (2.38–5.30μg/L) | 183 | 8.0 (−3.2, 19.2) | |
| As Tertile 3 (5.30–288.5 μg/L) | 183 | 10.4 (−1.5, 22.3)* | |
| TNFα (pg/ml) | |||
| As Tertile 1 (0.20–2.38μg/L) | 197 | Ref. | 0.12 |
| As Tertile 2 (2.38–5.30μg/L) | 183 | 0.13 (−0.30, 0.56) | |
| As Tertile 3 (5.30–288.5 μg/L) | 183 | 0.39 (−0.07, 0.84)* |
0.1>p>0.05;
p<0.05
Adjusted for enrollment age, education, maternal smoking, secondhand smoke exposure, urinary creatinine, and batch assignment.
We tested for effect modification by infant sex, birth size, maternal BMI and maternal weight gain in stratified models. However, the relation between urinary As and infant cord blood markers did not appreciably vary by infant sex or infant birth size, nor did we observe differences by maternal BMI and maternal weight gain of associations (data not shown).
Given that some studies have suggested that seafood and fish consumption may influence the level of organic As species in urine samples, we performed a sensitivity analysis to explore the impact of seafood consumption on our results. Exclusion of seafood-consumers reduced our sample size to 475 mothers and 426 infants. We found that our results were robust, even when we excluded those individuals who reported consuming fish or seafood within the three days prior to urine collection. Among those mothers who reported that they did not eat seafood within the three days prior to urine collection, maternal urinary arsenic remained positively associated with VCAM1 (Btert2: 113.4, 95% CI: −7.2, 234.0; Btert3: 180.0, 95% CI: 51.1, 309.0; p-trend=0.01). Among infants born to mothers who reported that they did not eat seafood within the three days prior to urine collection, the positive association between maternal urinary arsenic and VCAM1 was marginally improved (Btert2: 674.9, 95% CI: −22.0, 1352.1; Btert3: 814.1, 95% CI: 93.0, 1535.1; p-trend=0.03) and remained similar with ICAM1 (Btert2: 100.6, 95% CI: −55.0, 256.2; Btert3: 174.3, 95% CI: 8.6, 340.0; p-trend=0.04).
Lastly, we built a structural equation model to investigate how maternal VCAM and ICAM plasma levels may contribute to the effect of maternal urinary As on infant cord blood levels of VCAM and ICAM. We modeled VCAM and ICAM independently, adjusted for covariates. According to the model estimates, maternal urinary As was associated with increased maternal VCAM levels (β= 0.024, 95% CI: 0.002, 0.05) which in turn were related to increased infant VCAM levels (β= 0.52, 95% CI: 0.41, 0.63) (Supplementary Figure 1). For VCAM, the overall mediational effect was statistically significant (β= 0.024, 95% CI: 0.002, 0.05). We did not observe a statistically significant mediational effect in our model of maternal urinary As with maternal and infant ICAM, but we observed a similar trend, indicating that increases in maternal ICAM may mediate the effect on infant ICAM levels (β= 0.16, 95% CI: 0.09, 0.24) (Supplementary Figure 1).
Discussion
In this cohort study of US women and their newborns, we evaluated the associations between prenatal As exposure and circulating levels of maternal plasma and infant cord blood levels of markers of inflammation. We observed a positive association between maternal pregnancy urinary As and infant cord blood levels of ICAM and a similar, albeit weaker positive association with cord blood levels of VCAM. We also found a relationship between maternal urinary As and maternal plasma levels of VCAM, as well as an association with levels of MCP1 of borderline statistical significance. Subsequent investigation of maternal VCAM and ICAM as mediators of As-related effects on infant VCAM and ICAM suggested that As may impact markers of infant endothelial function via modulation of maternal markers. Although the overall levels of arsenic exposure in our study population are relatively lower than other areas of the world (<50μg/L in well water), our results are consistent with previous epidemiological studies in Bangladesh among adults with much higher levels of As exposure that have found that As increases circulating plasma levels of soluble VCAM1 and ICAM1.34,35 These results indicate that maternal As exposure at relatively low levels during pregnancy may have an effect on inflammatory pathways and/or endothelial function in both the mother and her child.
To our knowledge, no other epidemiological studies have examined the association between As exposure during pregnancy and levels of endothelial function markers in both pregnant women and their infants. A number of studies have observed relationships between maternal As exposure and alterations in expression of markers of immune function, including inflammatory cytokines46, and glucocorticoid and TNF signaling pathways.47,48 However, existing studies of endothelial function markers in this vulnerable population have been generally limited to investigations of these biomarkers in relation to pregnancy complications.49–51 During pregnancy, women may experience improved endothelial function, a cardiovascular adaption to support the growing fetus52,53. This pregnancy-associated improvement in endothelial response could potentially blunt the effects of environmental insults on the endothelium. However, in our study we observed a positive association between urinary As and maternal plasma marker VCAM and a possible positive association with MCP1, which have been related to inflammation and endothelial dysfunction.17,29 This could signal greater cardiovascular risks for more highly As exposed women later in life, as these markers have been associated with cardiovascular risk in adults.30–33 For example, one study found that the highest quartile of baseline ICAM1 (>260 ng/mL) was associated with an increased risk of myocardial infarction (RR: 1.6, 95% Cl 1.1–2.4), while another group observed a 2.1 fold (95% Cl: 1.1, 4.0) greater risk of death among coronary artery disease patients within the top quartile of baseline VCAM1 concentrations compared with lower quartiles.30,33 Recent work has indicated that women who experience cardiovascular complications or hypertensive disorders in pregnancy are at sharply increased risks of cardiomyopathy, acute myocardial infarction, stroke, or new-onset heart failure beginning within just a few years of giving birth.54–56 Altered vascular function also may increase the risk of adverse pregnancy outcomes and placental insufficiency. Increased maternal plasma levels of endothelial markers during pregnancy, including VCAM1 and ICAM1, have been implicated in the development of pre-eclampsia, fetal endothelial function and increased risk of preterm delivery and lower birth weight.49–51 Some have speculated that As’s vascular impacts could affect the placenta by leading to placental insufficiency and intrauterine growth retardation, thus potentially underlying As-related effects on fetal growth and birth weight.57–61 While it is unclear whether As-related changes in a mother’s vascular health may affect her child’s health, our structural equation models suggest that As’s effects on biomarkers of maternal endothelial function may in turn mediate similar changes in these same biomarkers in infants. Nonetheless, the long-term impacts of these changes have yet to be explored.
Our work indicates that prenatal As exposure may be related to elevated levels of inflammatory markers in infants. Our findings are similar to a recent study from a birth cohort in Iran, which found associations between prenatal exposure to ambient air pollution and elevated VCAM1, ICAM1 and endothelian-1 levels in cord blood.36 Additional studies are needed to determine whether these higher marker levels in early life may be related to any long-term health impacts. Although clinical symptoms of cardiovascular dysfunction occur much later in life, there is clear evidence that atherogenesis is a lifelong process and vascular changes can begin very early in childhood.62,63 While speculative, these inflammatory markers are hypothesized to play a role in the early phases of disease based on their role in CVD in adults and several studies have demonstrated that children at higher cardiovascular risk due to obesity, dyslipidemia, or hypertension have higher observed levels of VCAM1, ICAM1, and MCP1 as compared to healthy children.64–68 Nevertheless, growing evidence supports a role for As in the promotion of adult cardiovascular disease and studies prospectively examining the effects of early life As exposure on cardiovascular function in children have found associations between in utero and early life As exposure to increased blood pressure, carotid intima media thickness (cIMT) and biomarkers of oxidative stress.20,21 While these inflammatory markers may be related to other chronic disease pathways and have yet to be fully explored in early life, our results are in line with previous studies that have suggested that early life As exposure in children may alter early biomarkers and preclinical indicators of cardiovascular function.
The strengths of our study include a relatively large sample of mother and infant pairs, with a range of As exposure levels, and rich covariate information collected from medical records and questionnaires. Our results are consistent with previous studies in adults that have observed similar associations between As exposure and endothelial function exposed to higher concentrations of water As.34,35 Unlike previous studies that have observed relationships between As exposure and pro-inflammatory cytokines46,47 we did not see an association between As exposure and TNFα. This could suggest that the lower levels of As exposure in our population have more targeted effects on endothelial function and may not have broader effects on systemic inflammation. It is likely that other key pathways may be impacted by As exposure that we have not investigated in this study, thus a wider range of markers of inflammation and endothelial function should be explored in future studies to more fully elucidate the cardiovascular effects of As. A potential limitation of this study is that, to our knowledge, these markers have not been validated in cord blood using this multiplexed platform. However, another study has recently assessed these markers in the context of air pollution using enzyme-linked immune-sorbent assays (ELISA) and reported concentrations of VCAM1 and ICAM1 in cord blood within a similar range as our study.36 Lastly, our study population of women is relatively well educated and primarily white, which may underrepresent different racial or socioeconomic groups, limiting the generalizability of our results.
In conclusion, we found that As exposure during pregnancy was associated with increases in markers of endothelial function in a US population of mothers. Our results further suggest that As’s effects on markers of infant endothelial function may be mediated by impacts on maternal levels of these markers. Whether these effects are associated with long-term adverse cardiovascular health consequences will need to be assessed in future studies.
Supplementary Material
Highlights.
Arsenic (As) exposure has been associated with elevated cardiovascular disease risk Plasma inflammatory and endothelial function markers may indicate future CVD risks Studies of As exposure and maternal-infant inflammatory markers are lacking Increased As was associated with greater maternal VCAM and infant ICAM Prenatal As exposure may increase endothelial dysfunction in mothers and infants
Acknowledgments
Funding sources and ethical considerations
S.F. Farzan is supported by a NIEHS K99/R00 (R00ES024144). Funding for this study was provided by the NIEHS Superfund Research Program (P42ES007373) and the Children’s Center for Environmental Health and Disease Prevention Research (NIEHS and USEPA grants ES022832 and RD83459901). The funding agencies that supported this work had no role in the planning, design, or execution of this study, nor any role in data analysis or manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no competing personal or financial interests. All participants provided written, informed consent upon enrollment. All protocols were approved by the Dartmouth College Institutional Review Board (Dartmouth Committee for the Protection of Human Subjects Approval Reference #20844).
References
- 1.World Health Organization (WHO) Guidelines for drinking-water quality. 4. 2011. [Google Scholar]
- 2.International Agency for Research on Cancer (IARC) IARC monographs on the evaluation of carcinogenic risks to humans: Some drinking-water disinfectants and contaminants, including arsenic. Lyon, France: 2004. [PMC free article] [PubMed] [Google Scholar]
- 3.Naujokas MF, Anderson B, Ahsan H, Aposhian HV, Graziano JH, Thompson C, Suk WA. The broad scope of health effects from chronic arsenic exposure: Update on a worldwide public health problem. Environ Health Perspect. 2013;121(3):295–302. doi: 10.1289/ehp.1205875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Research Council. Critical aspects of EPA’s IRIS assessment of inorganic arsenic: Interim report. Washington, DC: National Research Council; 2014. [Google Scholar]
- 5.Ayotte J, Gronberg J, Apodaca L. Trace elements and radon in groundwater across the United States, 1992–2003: US Geological survey scientific investigations report. 2011. [Google Scholar]
- 6.Maupin MA, Kenny JF, Hutson SS, Lovelace JK, Barber NL, Linsey KS, et al. Estimated use of water in the United States in 2010. United States Geological Survey; 2014. [Google Scholar]
- 7.Cottingham KL, Karimi R, Gruber JF, Zens MS, Sayarath V, Folt CL, Punshon T, Morris JS, Karagas MR. Diet and toenail arsenic concentrations in a New Hampshire population with arsenic-containing water. Nutr J. 2013;12(1):149. doi: 10.1186/1475-2891-12-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis MA, Mackenzie TA, Cottingham KL, Gilbert-Diamond D, Punshon T, Karagas MR. Rice consumption and urinary arsenic concentrations in US children. Environ Health Perspect. 2012;120(10):1418–24. doi: 10.1289/ehp.1205014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert-Diamond D, Cottingham KL, Gruber JF, Punshon T, Sayarath V, Gandolfi AJ, Baker ER, Jackson BP, Folt CL, Karagas MR. Rice consumption contributes to arsenic exposure in US women. Proc Natl Acad Sci U S A. 2011;108(51):20656–60. doi: 10.1073/pnas.1109127108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nachman KE, Baron PA, Raber G, Francesconi KA, Navas-Acien A, Love DC. Roxarsone, inorganic arsenic, and other arsenic species in chicken: A US-based market basket sample. Environ Health Perspect. 2013;121(7):818–24. doi: 10.1289/ehp.1206245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wong WW, Chung SW, Chan BT, Ho YY, Xiao Y. Dietary exposure to inorganic arsenic of the Hong Kong population: Results of the first Hong Kong total diet study. Food Chem Toxicol. 2013;51:379–85. doi: 10.1016/j.fct.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Abhyankar LN, Jones MR, Guallar E, Navas-Acien A. Arsenic exposure and hypertension: A systematic review. Environ Health Perspect. 2012;120(4):494–500. doi: 10.1289/ehp.1103988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Graziano JH, Parvez F, Liu M, Slavkovich V, Kalra T, Argos M, Islam T, Ahmed A, Rakibuz-Zaman M, Hasan R, Sarwar G, Levy D, Van Geen A, Ahsan H. Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ. 2011;342:d2431. doi: 10.1136/bmj.d2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Wu F, Graziano JH, Parvez F, Liu M, Paul RR, et al. Arsenic exposure from drinking water, arsenic methylation capacity, and carotid intima-media thickness in Bangladesh. Am J Epidemiol. 2013;178(3):372–81. doi: 10.1093/aje/kwt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farzan SF, Chen Y, Rees JR, Zens MS, Karagas MR. Risk of death from cardiovascular disease associated with low-level arsenic exposure among long-term smokers in a US population-based study. Toxicol Appl Pharmacol. 2015 doi: 10.1016/j.taap.2015.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, et al. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: a prospective cohort study. Ann Intern Med. 2013;159(10):649–59. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu F, Molinaro P, Chen Y. Arsenic exposure and subclinical endpoints of cardiovascular diseases. Curr Environ Health Rep. 2014;1(2):148–62. doi: 10.1007/s40572-014-0011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Farzan SF, Karagas MR, Chen Y. In utero and early life arsenic exposure in relation to long-term health and disease. Toxicol Appl Pharmacol. 2013;272(2):384–90. doi: 10.1016/j.taap.2013.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farzan SF, Chen Y, Wu F, Jiang J, Liu M, Baker E, et al. Blood pressure changes in relation to arsenic exposure in a US pregnancy cohort. Environ Health Perspect. 2015 doi: 10.1289/ehp.1408472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hawkesworth S, Wagatsuma Y, Kippler M, Fulford AJ, Arifeen SE, Persson LA, et al. Early exposure to toxic metals has a limited effect on blood pressure or kidney function in later childhood, rural Bangladesh. Int J Epidemiol. 2012 doi: 10.1093/ije/dys215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osorio-Yanez C, Ayllon-Vergara JC, Aguilar-Madrid G, Arreola-Mendoza L, Hernandez-Castellanos E, Barrera-Hernandez A, et al. Carotid intima-media thickness and plasma asymmetric dimethylarginine in Mexican children exposed to inorganic arsenic. Environ Health Perspect. 2013 doi: 10.1289/ehp.1205994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Osorio-Yanez C, Ayllon-Vergara JC, Arreola-Mendoza L, Aguilar-Madrid G, Hernandez-Castellanos E, Sanchez-Pena LC, et al. Blood pressure, left ventricular geometry, and systolic function in children exposed to inorganic arsenic. Environ Health Perspect. 2015;123(6):629–35. doi: 10.1289/ehp.1307327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.States JC, Srivastava S, Chen Y, Barchowsky A. Arsenic and cardiovascular disease. Toxicol Sci. 2009;107(2):312–23. doi: 10.1093/toxsci/kfn236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Modur V, Zimmerman GA, Prescott SM, Mcintyre TM. Endothelial cell inflammatory responses to tumor necrosis factor alpha. Ceramide-dependent and -independent mitogen-activated protein kinase cascades. J Biol Chem. 1996;271(22):13094–102. doi: 10.1074/jbc.271.22.13094. [DOI] [PubMed] [Google Scholar]
- 25.Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA, Jr, Luster AD, Luscinskas FW, Rosenzweig A. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature. 1999;398(6729):718–23. doi: 10.1038/19546. [DOI] [PubMed] [Google Scholar]
- 26.Hou YC, Hsu CS, Yeh CL, Chiu WC, Pai MH, Yeh SL. Effects of glutamine on adhesion molecule expression and leukocyte transmigration in endothelial cells exposed to arsenic. J Nutr Biochem. 2005;16(11):700–4. doi: 10.1016/j.jnutbio.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Soucy NV, Mayka D, Klei LR, Nemec AA, Bauer JA, Barchowsky A. Neovascularization and angiogenic gene expression following chronic arsenic exposure in mice. Cardiovasc Toxicol. 2005;5(1):29–41. doi: 10.1385/ct:5:1:029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lemaire M, Negro Silva LF, Lemarie CA, Bolt AM, Flores Molina M, Krohn RM, et al. Arsenic exposure increases monocyte adhesion to the vascular endothelium, a pro-atherogenic mechanism. PloS One. 2015;10(9):e0136592. doi: 10.1371/journal.pone.0136592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Srivastava S, Vladykovskaya EN, Haberzettl P, Sithu SD, D’souza SE, States JC. Arsenic exacerbates atherosclerotic lesion formation and inflammation in APOE−/− mice. Toxicol Appl Pharmacol. 2009;241(1):90–100. doi: 10.1016/j.taap.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, et al. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104(12):1336–42. doi: 10.1161/hc3701.095949. [DOI] [PubMed] [Google Scholar]
- 31.Haim M, Tanne D, Boyko V, Reshef T, Goldbourt U, Leor J, et al. Soluble intercellular adhesion molecule-1 and long-term risk of acute coronary events in patients with chronic coronary heart disease. Data from the bezafibrate infarction prevention (BIP) study. J Am Coll Cardiol. 2002;39(7):1133–8. doi: 10.1016/s0735-1097(02)01728-x. [DOI] [PubMed] [Google Scholar]
- 32.Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Jr, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: The atherosclerosis risk in communities (ARIC) study. Circulation. 1997;96(12):4219–25. doi: 10.1161/01.cir.96.12.4219. [DOI] [PubMed] [Google Scholar]
- 33.Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet. 1998;351(9096):88–92. doi: 10.1016/S0140-6736(97)09032-6. [DOI] [PubMed] [Google Scholar]
- 34.Wu F, Jasmine F, Kibriya MG, Liu M, Wojcik O, Parvez F, et al. Association between arsenic exposure from drinking water and plasma levels of cardiovascular markers. Am J Epidemiol. 2012;175(12):1252–61. doi: 10.1093/aje/kwr464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Santella RM, Kibriya MG, Wang Q, Kappil M, Verret WJ, et al. Association between arsenic exposure from drinking water and plasma levels of soluble cell adhesion molecules. Environ Health Perspect. 2007;115(10):1415–20. doi: 10.1289/ehp.10277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Poursafa P, Baradaran-Mahdavi S, Moradi B, Haghjooy Javanmard S, Tajadini M, Mehrabian F, et al. The relationship of exposure to air pollutants in pregnancy with surrogate markers of endothelial dysfunction in umbilical cord. Environ Res. 2016;146:154–60. doi: 10.1016/j.envres.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 37.Le XC, Lu XF, Ma MS, Cullen WR, Aposhian HV, Zheng BS. Speciation of key arsenic metabolic intermediates in human urine. Anal Chem. 2000;72(21):5172–7. doi: 10.1021/ac000527u. [DOI] [PubMed] [Google Scholar]
- 38.Wei HY, Brockhoff-Schwegel CA, Creed JT. A comparison of urinary arsenic speciation via direct nebulization and on-line photo-oxidation-hydride generation with IC separation and icp-ms detection. J Anal At Spectrom. 2001;16(1):12–9. [Google Scholar]
- 39.Larsen EH, Pritzl G, Hansen SH. Speciation of 8 arsenic compounds in human urine by high-performance liquid-chromatography with inductively-coupled plasma-mass spectrometric detection using antimonate for internal chromatographic standardization. J Anal At Spectrom. 1993;8(4):557–63. [Google Scholar]
- 40.Tseng CH. A review on environmental factors regulating arsenic methylation in humans. Toxicol Appl Pharmacol. 2009;235(3):338–50. doi: 10.1016/j.taap.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 41.Tworoger S, Hankinson S. Use of biomarkers in epidemiologic studies: Minimizing the influence of measurement error in the study design and analysis. Cancer Causes Control. 2006;17(7):889–99. doi: 10.1007/s10552-006-0035-5. [DOI] [PubMed] [Google Scholar]
- 42.World Health Organization (WHO) Global database on body mass index 2016. 2016 Mar 08; Available from: http://apps.who.int/bmi/index.jsp?introPage=intro_3.html.
- 43.Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013;13:59. doi: 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mackinnon DP. Introduction to statistical mediation analysis. New York: Lawrence Erlbaum Associates; 2008. p. 477. x. [Google Scholar]
- 45.Wood SN. Generalized additive models: An Introduction with R. Boca Raton, FL: Chapman and Hall/CRC; 2006. [Google Scholar]
- 46.Ahmed S, Mahabbat-E Khoda S, Rekha RS, Gardner RM, Ameer SS, Moore S, et al. Arsenic-associated oxidative stress, inflammation, and immune disruption in human placenta and cord blood. Environ Health Perspect. 2011;119(2):258–64. doi: 10.1289/ehp.1002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey KA, Laine J, Rager JE, Sebastian E, Olshan A, Smeester L, Drobna Z, Styblo M, Rubio-Andrade M, Garcia-Vargas G, Fry RC. Prenatal arsenic exposure and shifts in the newborn proteome: Interindividual differences in tumor necrosis factor (TNF)-responsive signaling. Toxicol Sci. 2014;139(2):328–37. doi: 10.1093/toxsci/kfu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rager JE, Bailey KA, Smeester L, Miller SK, Parker JS, Laine JE, Drobna Z, Currier J, Douillet C, Olshan AF, Rubio-Andrade M, Styblo M, Garcia-Vargas G, Fry RC. Prenatal arsenic exposure and the epigenome: Altered microRNAs associated with innate and adaptive immune signaling in newborn cord blood. Environ Mol Mutagen. 2014;55(3):196–208. doi: 10.1002/em.21842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paradisi G, Mattoli MV, Tomei C, Zuppi C, Lulli P, Quagliozzi L, et al. Cardiovascular risk factors in healthy women with previous small for gestational age infants. J Obstet Gynaecol Res. 2011;37(10):1397–404. doi: 10.1111/j.1447-0756.2011.01547.x. [DOI] [PubMed] [Google Scholar]
- 50.Redman CW, Sacks GP, Sargent IL. Preeclampsia: An excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 Pt 1):499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 51.Veas CJ, Aguilera VC, Munoz IJ, Gallardo VI, Miguel PL, Gonzalez MA, Lamperti LI, Escudero CA, Aguayo CR. Fetal endothelium dysfunction is associated with circulating maternal levels of sE-selectin, sVCAM1, and sFLT-1 during pre-eclampsia. J Matern Fetal Neona. 2011;24(11):1371–7. doi: 10.3109/14767058.2011.556204. [DOI] [PubMed] [Google Scholar]
- 52.Anumba DO, Ford GA, Boys RJ, Robson SC. Stimulated nitric oxide release and nitric oxide sensitivity in forearm arterial vasculature during normotensive and preeclamptic pregnancy. Am J Obstet Gynecol. 1999;181(6):1479–84. doi: 10.1016/s0002-9378(99)70394-7. [DOI] [PubMed] [Google Scholar]
- 53.Dorup I, Skajaa K, Sorensen KE. Normal pregnancy is associated with enhanced endothelium-dependent flow-mediated vasodilation. Am J Physiol. 1999;276(Pt 2):H821–5. doi: 10.1152/ajpheart.1999.276.3.H821. [DOI] [PubMed] [Google Scholar]
- 54.Behrens I, Basit S, Lykke JA, Ranthe MF, Wohlfahrt J, Bundgaard H, et al. Association between hypertensive disorders of pregnancy and later risk of cardiomyopathy. J Am Med Assoc. 2016;315(10):1026–33. doi: 10.1001/jama.2016.1869. [DOI] [PubMed] [Google Scholar]
- 55.Yeh JS, Cheng HM, Hsu PF, Sung SH, Liu WL, Fang HL, et al. Synergistic effect of gestational hypertension and postpartum incident hypertension on cardiovascular health: A nationwide population study. J Am Heart Assoc. 2014;3(6) doi: 10.1161/JAHA.114.001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arnaout R. Cardiovascular complications in pregnancy quickly boost future risk Presentation at American Heart Association Annual Conference. New Orleans, LA.: Ob Gyn News; 2017. [Google Scholar]
- 57.Davis MA, Higgins J, Li Z, Gilbert-Diamond D, Baker ER, Das A, et al. Preliminary analysis of in utero low-level arsenic exposure and fetal growth using biometric measurements extracted from fetal ultrasound reports. Environmental Health. 2015;14:12. doi: 10.1186/1476-069X-14-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilbert-Diamond D, Emond JA, Baker ER, Korrick SA, Karagas MR. Relation between in utero arsenic exposure and birth outcomes in a cohort of mothers and their newborns from New Hampshire. Environ Health Perspect. 2016;124(8):1299–307. doi: 10.1289/ehp.1510065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hopenhayn C, Ferreccio C, Browning SR, Huang B, Peralta C, Gibb H, et al. Arsenic exposure from drinking water and birth weight. Epidemiology. 2003;14(5):593–602. doi: 10.1097/01.ede.0000072104.65240.69. [DOI] [PubMed] [Google Scholar]
- 60.Huyck KL, Kile ML, Mahiuddin G, Quamruzzaman Q, Rahman M, Breton CV, Dobson CB, Frelich J, Hoffman E, Yousuf J, Afroz S, Islam S, Christiani DC. Maternal arsenic exposure associated with low birth weight in Bangladesh. J Occup Env Med. 2007;49(10):1097–104. doi: 10.1097/JOM.0b013e3181566ba0. [DOI] [PubMed] [Google Scholar]
- 61.Rahman A, Vahter M, Smith AH, Nermell B, Yunus M, El Arifeen S, et al. Arsenic exposure during pregnancy and size at birth: A prospective cohort study in Bangladesh. Am J Epidemiol. 2009;169(3):304–12. doi: 10.1093/aje/kwn332. [DOI] [PubMed] [Google Scholar]
- 62.Berenson GS, Srinivasan SR, Hunter SM, Nicklas TA, Freedman DS, Shear CL, et al. Risk factors in early life as predictors of adult heart disease: The Bogalusa heart study. Am J Med Sci. 1989;298(3):141–51. doi: 10.1097/00000441-198909000-00001. [DOI] [PubMed] [Google Scholar]
- 63.Louisana State University Medical Center, Mayo Clinic, University of Iowa Hospitals, National Heart, Lung and Blood Institute. Cardiovascular profile of 15,000 children of school age in three communities, 1971–1975. U.S. Department of Health, Education, and Welfare, Public Health Service, National Institutes of Health, National Heart, Lung, and Blood Institute; 1978. [Google Scholar]
- 64.Desideri G, De Simone M, Iughetti L, Rosato T, Iezzi ML, Marinucci MC, et al. Early activation of vascular endothelial cells and platelets in obese children. J Clin Endocr Metab. 2005;90(6):3145–52. doi: 10.1210/jc.2004-1741. [DOI] [PubMed] [Google Scholar]
- 65.Glowinska-Olszewska B, Tolwinska J, Urban M. Relationship between endothelial dysfunction, carotid artery intima media thickness and circulating markers of vascular inflammation in obese hypertensive children and adolescents. J Pediatr Endocr Met. 2007;20(10):1125–36. [PubMed] [Google Scholar]
- 66.Jimenez MV, Estepa RM, Camacho RMM, Estrada RC, Luna FG, Guitarte FB. Endothelial dysfunction is related to insulin resistance and inflammatory biomarker levels in obese prepubertal children. Eur J Endocrinol. 2007;156(4):497–502. doi: 10.1530/EJE-06-0662. [DOI] [PubMed] [Google Scholar]
- 67.Kavazarakis E, Moustaki M, Gourgiotis D, Zeis PM, Bossios A, Mavri A, et al. The impact of serum lipid levels on circulating soluble adhesion molecules in childhood. Pediatr Res. 2002;52(3):454–8. doi: 10.1203/00006450-200209000-00025. [DOI] [PubMed] [Google Scholar]
- 68.Martos R, Valle M, Morales RM, Canete R, Gascon F, Urbano MM. Changes in body mass index are associated with changes in inflammatory and endothelial dysfunction biomarkers in obese prepubertal children after 9 months of body mass index SD score loss. Metabolism. 2009;58(8):1153–60. doi: 10.1016/j.metabol.2009.03.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


