Abstract
Post-traumatic stress disorder (PTSD) and alcohol-use disorder (AUD) are highly comorbid in humans. Although we have some understanding of the structural and functional brain changes that define each of these disorders, and how those changes contribute to the behavioral symptoms that define them, little is known about the neurobiology of comorbid PTSD and AUD, which may be due in part to a scarcity of adequate animal models for examining this research question. The goal of this review is to summarize the current state-of-the-science on comorbid PTSD and AUD. We summarize epidemiological data documenting the prevalence of this comorbidity, review what is known about the potential neurobiological basis for the frequent co-occurrence of PTSD and AUD and discuss successes and failures of past and current treatment strategies. We also review animal models that aim to examine comorbid PTSD and AUD, highlighting where the models parallel the human condition, and we discuss the strengths and weaknesses of each model. We conclude by discussing key gaps in our knowledge and strategies for addressing them: in particular, we (1) highlight the need for better animal models of the comorbid condition and better clinical trial design, (2) emphasize the need for examination of subpopulation effects and individual differences and (3) urge cross-talk between basic and clinical researchers that is reflected in collaborative work with forward and reverse translational impact.
Keywords: Alcohol dependence, alcohol use disorder (AUD), alcoholism, post-traumatic stress disorder (PTSD), comorbid PTSD and AUD, amygdala, mesolimbic reward circuit, prefrontal cortex, hippocampus, norepinephrine
Comorbid PTSD and AUD
It has long been recognized that exposure to traumatic events can elicit, in some individuals, the emergence of a spectrum of debilitating and enduring anxiety-related symptoms, but the term post-traumatic stress disorder (PSTD) was not officially recognized as an anxiety disorder until the 3rd edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-III). This term replaced other labels such as ‘shell shock’ and ‘war neurosis’ that had appeared in the literature for hundreds of years (Crocq & Crocq 2000; Trimble 1985). This change was prompted by the acknowledgment that the etiological agent that triggered the symptoms was outside the afflicted individual rather than merely a reflection of some personal weakness or failing, a realization that slowly began to reduce the stigma associated with this disorder. Revisions to the diagnostic criteria of PTSD continued until the latest edition of the DSM (DSM-V) which recognized that, although anxiogenic symptoms are a cornerstone of PTSD, this disease is better described by a new category of mental illnesses, termed trauma- or stressor-related disorders, in which exposure to a traumatic or otherwise adverse environmental event precedes the onset of each disorder. The diagnostic criteria of PTSD include four clusters of symptoms: intrusion, avoidance, negative alterations in cognitions and mood and alterations in arousal and reactivity (Guina et al. 2016). Individuals suffering from these disorders often re-experience a previously traumatic event, make significant efforts to avoid internal or external reminders of the trauma and exhibit hyperarousal in response to relatively neutral stimuli. These revisions to the diagnostic criteria of PTSD are largely evidence-based and have helped to stimulate a much-needed increase in research directed at this debilitating disorder. This is especially critical and timely because the latest estimates using the DSM-V diagnostic criteria indicate that the current past year PTSD prevalence was 4.7% and the lifetime prevalence rate was 8.3 (Kilpatrick 2003).
The diagnostic criteria for alcohol-use disorder (AUD) have also evolved with each iteration of the DSM. In the latest edition (DSM-V), alcohol dependence and abuse have been replaced by a single disease. Alcohol use disorder (AUD), with the diagnosis severity dictated by the expression of a pathological set of behaviors related to the use of alcohol (Hasin et al. 2013). These behaviors fall into four main categories including impaired control (e.g. drinking more than intended), social impairment (e.g. drinking behavior negatively impacts work, family, etc.), risky use (e.g. driving while intoxicated) and altered physiology (e.g. tolerance and withdrawal). As with PTSD, the prevalence of AUD is high. In the United States, data from the 2012–2013 National Epidemiologic Survey on Alcohol and Related Conditions III using the revised DSM-V criteria showed that the 12-month and lifetime prevalence of AUD were 13.9% and 29.1%, respectively (Grant et al. 2015a).
Epidemiology of comorbid PTSD and AUD
Despite the high incidence of AUD in the general population, individuals with PTSD are at even higher risk of developing AUD. Although an anecdotal connection between these disorders had long been appreciated, numerous clinical and epidemiological studies have consistently documented a strong association between PTSD and AUD (Blanco et al. 2013; Debell et al. 2014; Jacobson et al. 2008; Shorter et al. 2015). For example, a large retrospective review of the lifetime co-occurrence of DSM-III-R alcohol dependence and other psychiatric disorders from the US National Comorbidity Study reported that PTSD was associated with a three-fold increase in risk of developing AUD (Kessler et al. 1997). Other studies have reported that the lifetime prevalence of any substance-use disorder (SUD) was twice as high in persons with PTSD vs. those not suffering from this disorder (Shorter et al. 2015) and that alcohol is the most commonly abused drug in PTSD patients (Jacobsen et al. 2001). Indeed, a recent study examined the relationship between the frequency of childhood physical, sexual and emotional abuse and the development of substance use and PTSD in an urban, civilian population. Not only was the lifetime prevalence of substance dependence very high in these individuals (e.g. alcohol: 39%; cocaine: 34%), but also a strong correlation was noted between the levels of childhood abuse and both the prevalence of AUD and current PTSD symptoms (Khoury 2010). Interestingly, more recent data suggest that scoring high on a measure of resilience characteristics such as tenacity, strong self-efficacy and emotional/cognitive control under pressure may reduce the risk of substance misuse and PTSD in this ‘at-risk’ population (Wingo 2014).
Despite the high prevalence of comorbid PTSD/AUD in the civilian population, the situation is even more dire for military personnel. First, the prevalence of PTSD in military and veteran populations may be twice that observed in the civilian population (Gates et al. 2012; Ramchand et al. 2010). Similarly, military personnel are disproportionately impacted by the adverse effects of alcohol. Recent surveys show that rates of alcohol misuse range from 18% to 35% for soldiers returning from Operation Iraqi or Enduring Freedom (Hoge et al. 2004; Wilk et al. 2010). Other data on military personnel that served in Iraq and Afghanistan found that the baseline prevalence of binge drinking among Reserve or National Guard personnel was 53.6% and the incidence of alcohol-related problems was 11.9% (Jacobson et al. 2008). Moreover, individuals who reported combat experience had higher rates of new-onset heavy drinking, binge drinking and alcohol-related problems than those not exposed to combat (Jacobson et al. 2008). Not surprisingly, the rates of comorbid PTSD and AUD are extremely high in military personnel. The Veterans Administration reported that almost 70% of veterans hospitalized for PTSD also suffered from a comorbid SUD, with alcohol being the most commonly abused drug Kulka et al. 1988 and a more recent study found that almost 50% of actively serving or National Guard Infantry personnel deployed to Iraq that screened positive for PTSD also met diagnostic criteria for alcohol misuse (Thomas et al. 2010). Importantly, as noted earlier, the diagnostic criteria for PTSD and AUD have recently been revised. Therefore, new epidemiological studies are needed to obtain estimates of the prevalence of the comorbid condition in the general population and at-risk groups using these revised criteria.
Despite the notion that high rates of comorbidity between PTSD and AUD reflect an attempt by the individual to self-medicate the negative affective state that emerges in trauma and stressor-related disorders, there is also a growing appreciation that all of these disorders may actually share common neural substrates (Enman et al. 2014). Although a number of behavioral and pharmacological treatments are available for both PTSD and AUD, these interventions are frequently ineffective and individuals who suffer from this dual diagnosis are particularly ill-served by current treatment options (Ipser 2015; Najavits 2013; Sofuoglu 2014). As such, there is a great deal of interest in gaining a better understanding of the neurobiological substrates underlying this comorbidity. However, progress has been hampered, to some extent, by a lack of animal models that engender a behavioral phenotype that reflects the human comorbid condition. The overarching goal of this review is to summarize the current state of research on the comorbidity between PTSD and AUD. This article will summarize the epidemiological evidence documenting the prevalence of this comorbidity and review the neurobiological basis for the frequent co-occurrence of these disorders. Current treatment options will also be discussed. This review will also summarize the literature on available animal models, highlighting where the models parallel the human condition, and discuss the strengths and weaknesses of each model. Collectively, this summary may provide guidance for future work directed at improving animal models and developing more effective treatments for comorbid PTSD and AUD. As this article will focus primarily on the literature related to the comorbidity between PTSD and AUD, for manuscripts focused solely on the epidemiology, neurobiology and treatment of each disorder alone, the interested reader is directed to the following PTSD (Admon et al. 2013b; Atwoli et al. 2015; Hoskins et al. 2015; Pitman et al. 2012) and AUD reviews (Batman & Miles 2015; Koob 2013; Samochowiec et al. 2014; Tabakoff & Hoffman 2013; Zindel & Kranzler 2014).
Temporal expression of PTSD and AUD
Although relatively few studies have addressed the order of onset of PTSD and AUD, there is a general consensus that PTSD precedes the onset of AUD (Chilcoat & Breslau 1998; Epstein et al. 1998; Jacobsen et al. 2001; Kessler et al. 1995, although see Cottler et al. 1992). For example, a recent longitudinal study in a cohort of National Guard troops that were screened pre- and post-deployment to Iraq found no effect of pre-deployment alcohol status on the onset of PTSD; however, initial PTSD symptoms significantly increased the risk of screening positive for new-onset alcohol dependence (Kline et al. 2014). These findings lend empirical support to the notion that individuals with PTSD may misuse alcohol in an attempt to alleviate the anxiogenic and hyperarousal symptoms associated with this disorder. Recent work has also highlighted an alarming increase in the prevalence of pediatric PTSD, which may further increase the likelihood of developing AUD following a PTSD diagnosis (Makley & Falcone 2010).
Risk factors of comorbid PTSD and AUD
As highlighted above, there is compelling evidence that active military personnel and veterans are at increased risk for comorbid PTSD and AUD diagnoses. Are there other risk factors associated with this dual diagnosis? Several studies have noted that exposure to a wide range of early life stressors increases risk for the development of PTSD alone and AUD alone, and that childhood adversity represents an even greater risk factor for the development of comorbid PTSD and AUD. For example, data from the National Survey of Adolescents reported that a history of interpersonal violence was a stronger risk factor for the dual diagnosis of PTSD and AUD than for either non-comorbid diagnoses (Kilpatrick et al. 2013). In a study of alcohol-dependent men with comorbid PTSD, a history of emotional abuse in childhood was found to contribute to impaired quality of life (Evren et al. 2011). A more recent analysis of data from the National Epidemiological Survey on Alcohol and Related Conditions confirmed this relationship, finding that individuals with comorbid PTSD and AUD were more likely to have suffered childhood adversities than those with only one of these conditions (Blanco et al. 2013).
There is also evidence that stressors such as lower socioeconomic status and traumatic life events may place adult victims at greater risk of developing comorbid PTSD and AUD (Riggs et al. 2003; Ullman et al. 2006).
Sex, gender and comorbid PTSD/AUD
Epidemiological studies consistently find that women are more than twice as likely than men to be diagnosed with PTSD over their lifetime (Breslau et al. 1997; Kessler et al. 1995; Olff et al. 2007). In contrast, AUD rates are almost twice as high in men (Erol & Karpyak 2015; Nelson et al. 1998). Given this dichotomy, it may not be surprising that the data on whether men or women are at greater risk for the development of comorbid PTSD and AUD are mixed. Several studies have reported higher prevalence of comorbid PTSD and AUD in men (Conway et al. 2006; Kozaric-Kovacic et al. 2000), whereas others cite slightly higher or comparable rates of comorbidity in women and men (Kessler et al. 1995, 1997; Pietrzak et al. 2012). One complicating factor may be that the type of trauma experienced could influence the likelihood of developing comorbid PTSD and AUD. For example, while sexual assault is a well-established risk factor for PTSD, one study found that the nature of the assault impacts the probability of also developing AUD (Goldstein 2011). While rape victims in this study had a significantly higher incidence of PTSD than non-victims, women who experienced drug/alcohol-facilitated rape (i.e. were incapacitated) with or without forcible rape were even more likely to develop AUD than those who experienced forcible rape alone. Other studies have also begun to identify a variety of correlates that may predict the comorbid condition. For example, among a group of sexual assault survivors who were all diagnosed with PTSD, women who believed that drinking could reduce distress, had less education, or had a history of other traumas were more likely to also suffer from comorbid drinking problems (Ullman 2006). Collectively, these studies suggest that gender likely influences the prevalence of comorbid PTSD and AUD; however, considerable additional research will be needed to better understand the factors that moderate this relationship.
Consequences of comorbid PTSD and AUD
Many studies have examined how the dual diagnosis of PTSD and AUD influences the trajectory and prognosis of these disorders. Here, the literature consistently illustrates that the dual diagnosis of PTSD and AUD exacerbates the symptoms of each disorder. For example, in patients with PTSD, heavy alcohol drinking increases the number (Behar 1987) and severity of PTSD symptoms (Bremner et al. 1996; Jacobsen et al. 2001; Saladin et al. 1995) and prolongs the course of illness (Bremner et al. 1996; Yehuda et al. 1995). One particularly troubling study looked at suicidal ideation and attempts in patients with PTSD and either comorbid major depressive disorder or alcohol dependence. While comorbid depression was not related to suicidal thoughts and actions, PTSD patients with comorbid alcohol dependence showed higher suicidal ideation and suicide attempts (Rojas et al. 2014). Similarly, individuals with comorbid PTSD show an earlier onset of AUD symptoms (Driessen et al. 2008), poorer physical and mental health (Evren et al. 2011) and a much poorer prognosis in recovery (Ipser et al. 2015; Norman et al. 2015; Schafer & Najavits 2007; Shorter et al. 2015).
Modeling comorbid PTSD and AUD in animals
Basic science researchers have long strived to produce animal models that mimic aspects of the clinical diagnoses for PTSD or AUD. As mentioned above, it has also been long acknowledged that PTSD and AUD are highly comorbid in the clinic. Despite that fact, until very recently, few, if any, animal models have existed for testing the neural substrates underlying the symptom clusters associated with PTSD and AUD in the same sets of animals. There are many reasons for this void in the literature, not the least of which is the complexity of combining the models used to study each of these disorders. At the most basic level, it has been notoriously difficult to produce models in which stress reliably increases alcohol drinking for a sustained period of time (Boyce-Rustay et al. 2007, 2008; Croft et al. 2005; Funk et al. 2005; Lopez et al. 2016; McKenzie-Quirk & Miczek 2008; Miczek et al. 2008; Sillaber et al. 2002; van Erp & Miczek 2001). The field has been more successful in producing animal models that mimic the ability of stressors to elicit relapse to seeking of alcohol and other drugs, in which animals are trained to self-administer alcohol or drugs of abuse, this responding is extinguished and stress is used to reinstate responding for an alcohol or drug reinforcer that is not actually available to the animal; these models have been reviewed in detail elsewhere (see Mantsch et al. 2016).
A recent review examined factors that affect whether stress increases alcohol drinking in animals. Among the critical factors are the type and modality of the stress, the species, strain, age and sex of the animal, the acute vs. chronic pattern of stress exposure and whether alcohol drinking is measured using home-cage consumption or operant self-administration models (Noori et al. 2014). Since that time, a variety of rodent models have emerged that seek to reliably produce stress-induced increases in alcohol drinking, in order to study the underlying neural substrates. Typically, these are adaptations of previously existing animal models of stress and alcohol drinking that have been combined and/or modified to yield the desired outcome.
Strategies used to produce stress-induced excessive alcohol drinking in animals can be broadly divided based on the question they are designed to answer. First, if the goal is to understand whether and how traumatic stress produces escalation of alcohol drinking in current drinkers, the animal model should introduce stress in adult animals that have already been trained and had experience drinking low-to-moderate quantities of alcohol over time. Second, if the goal is to understand whether and how traumatic stress history produces escalation of alcohol drinking in future drinkers, the animal model should introduce stress in previously alcohol-naïve animals, perhaps during development depending on the specific question, train animals to drink concurrent with the stress onset or later in life, and then analyze behavior over time. Third, if the goal is to understand whether and how traumatic stress accelerates the development of, exaggerates the magnitude of, or prolongs the effects of alcohol dependence on brain and behavior, the animal model should introduce stress in adult animals that are undergoing forced and/or self-administration of high quantities of alcohol. One difficulty in modeling this scenario is the potential that alcohol dependence produces ceiling effects on alcohol outcomes that mask or dampen additive effects of stress (Anderson et al. 2016). Fourth, if the goal is to understand whether and how traumatic stress elicits relapse in the post-dependent organism, one of several strategies may be useful: either (1) introduce stress in adult animals during alcohol dependence and then impose alcohol abstinence, (2) introduce stress in animals that are already enduring imposed abstinence after being made dependent on alcohol (Sommer et al. 2008; Valdez et al. 2003) or (3) introduce stress to induce reinstatement of previously extinguished responding for alcohol. Footshock and yohimbine reinstate previously extinguished alcohol responding in animals (e.g., Le et al., 2005), but these procedures are not purported to model traumatic stress disorders, and do not produce persistent escalation of alcohol drinking in currently drinking animals (e.g., Meyer et al., 2013).
State-of-the-science: animal models of stress-induced escalation of alcohol drinking
As the human literature suggests that PTSD usually precedes AUD, most animal models have aimed to mimic the human scenario in which traumatic stress precedes, and presumably triggers, the onset of AUD through yet unidentified neurobiological processes. To that end, this nascent research field has developed several animal models aimed at producing stress-induced escalation of alcohol drinking. Here, we introduce the basic structure of these models, but the neurobiological measures will be described in the context of human findings in subsequent sections.
Two laboratories (Edwards et al. 2013; Manjoch et al. 2016) are using predator odor to test the neurobiological correlates and mechanisms of increased alcohol drinking in some, but not all, rats. In these models, adult male rats are either trained to self-administer operant alcohol prior to bobcat urine exposure (Edwards et al. 2013) or trained to drink sweetened alcohol in the home cage prior to soiled cat litter exposure (Manjoch et al. 2016), rats are divided into groups based on several behavioral indices including stress reactivity and then allowed to resume alcohol drinking. In these models, high stress-reactive rats exhibit higher alcohol drinking over 1 week (Manjoch et al. 2016) or 3 weeks (Edwards et al. 2013) post-stress, and also exhibit more compulsive-like responding for alcohol adulterated with escalating concentration of an aversive bitter tastant (Edwards et al. 2013).
To address a slightly different question aimed at understanding additive or synergistic effects of stress and alcohol dependence on alcohol drinking, one research group combined forced swim stress with a mouse model of alcohol dependence that employs chronic intermittent alcohol vapor exposure (Anderson et al. 2016; Lopez et al. 2016). This work shows that daily forced swim stress accelerates the onset of, and perhaps enhances the magnitude of, escalated alcohol drinking in alcohol-dependent adult male C57BL/6J mice (Anderson et al. 2016; Lopez et al. 2016). Highlighting the subtleties of such models, repetition of the forced swim stress may enhance the additive effects of stress and dependence on escalation of alcohol drinking. Furthermore, one of these studies also showed that the same forced swim stress manipulation that enhanced dependence-induced escalation of alcohol drinking did not affect alcohol drinking in binge (i.e. drinking in the dark) or intermittent access procedures, and animals stressed during continuous alcohol access actually decreased their drinking relative to unstressed controls (Anderson et al. 2016).
Another set of animal models aimed at understanding the effect of traumatic stress on future alcohol drinking has adapted an early life stress model to show that social isolation during adolescence, but not during adulthood, increases alcohol drinking. Rats and, to a lesser extent mice, are highly social creatures, and in the wild live in large social groups of hundreds of animals (McEwen et al. 2015). Many studies have shown that depriving rats of social interaction during adolescence leads to enduring alterations in a wide range of emotional behaviors in adulthood (Marsden et al. 2011; Ratajczak et al. 2013; Toth & Neumann 2013), many of which are considered risk factors of PTSD or AUD, including increases in measures of unconditioned anxiety-like behavior and impulsivity. Although several early studies noted that adolescent social isolation generally promotes an increase in alcohol drinking (Deatherage 1972; Hall et al. 1998; Schenk et al. 1990; Wolffgramm 1990), only recently have researchers leveraged this model to study neural correlates of PTSD/AUD comorbidity.
One group employs male Long Evans rats and socially isolates them for 6 weeks in standard single rodent cages, starting at postnatal day (PD) 28. Group-housed control subjects are housed in cohorts of four animals in larger cages in an otherwise identical environment. Thus, the primary experimental variable is that socially isolated rats are deprived of any physical interaction with their peers. Recent studies show that this adolescent social isolation procedure produces a wide range of behaviors that have all been linked with heightened vulnerability to AUD and/or PTSD including hyperactivity in a novel environment (Chappell et al. 2013), increased anxiety-like behavior on the elevated-plus maze (Chappell et al. 2013; McCool & Chappell 2009; Yorgason et al. 2013), deficits in sensory gating (McCool & Chappell 2009) and impaired extinction of fear learning (Skelly et al. 2015). Notably, many of the behavioral alterations engendered by this model persist for months into adulthood, even when comparisons are made with adolescent group-housed rats that are then also singly housed in adulthood. Thus, the behavioral changes associated with social isolation only manifest when this stressor is experienced early in life.
In addition to the behavioral risk factors of PTSD and AUD noted above, this model also promotes long-lasting increases in ethanol self-administration using a variety of drinking procedures. For example, McCool and Chappell (2009) reported greater ethanol, but not sucrose, consumption in a limited-access, operant procedure and, using the home-cage intermittent two-bottle choice paradigm, increases in ethanol intake and preference lasting up to 8 weeks were observed (Skelly et al. 2015). Another research group employed a similar social isolation model in male Sprague-Dawley rats and found that isolation during a shorter window, from PD 21 to 42, promoted increased conditioned place preference for ethanol (Whitaker et al. 2013). One caveat with this model, at least in Long Evans rats, is that adolescent social isolation may not engender long-lasting increases in anxiety-like behaviors or ethanol drinking-related behaviors in females (Butler et al. 2014).
As noted above, this model is most commonly employed in rats. However, several groups have reported similar behavioral changes in mice that have been socially isolated in adolescence. For example, adolescent social isolation in mice has been shown to lead to increases in anxiety-like behavior on the elevated-plus maze (Koike et al. 2009; Kumari et al. 2016; Wei et al. 2007, although see Voikar et al. 2005; Zhang et al. 2014), sensory gating deficits (Gan et al. 2014) as well as increased contextual fear and impaired fear extinction (Liu et al. 2015; Pibiri et al. 2008). Other studies have reported increases in home-cage ethanol intake and preference (Advani et al. 2007; Lopez et al. 2011; Talani et al. 2014), with one of these studies showing that this effect endured for at least 1 month into adulthood (Advani et al. 2007). Again, as observed in rats, the effect of adolescent social isolation on ethanol drinking was primarily observed in male mice (Advani et al. 2007; Lopez et al. 2011).
Social defeat stress has been examined in mice and rats for its effects on alcohol drinking over time. One group reported that 10 episodes of moderate (30 bites) but not mild (15 bites) social defeat stress increases subsequent acquisition of continuous two-bottle choice home-cage alcohol drinking in outbred mice (Norman 2012). Another group similarly reported that repeated (three episodes) social defeat stress increases alcohol drinking in mice on a mixed background that were previously trained to drink alcohol in a two-bottle choice home-cage situation (Molander et al. 2012). A more recent study reported that social defeat stress increases alcohol drinking in C57BL/6J mice with intermittent (every other day) home-cage alcohol access (Hwa 2016). Less work has been carried out on rats, with one group reporting that a history of social defeat stress increases alcohol preference (but not intake levels) during subsequent acquisition of operant alcohol self-administration, and that after extinction of alcohol responding, low drinkers (but not high drinkers) with a history of social defeat stress exhibit higher intakes relative to unstressed low drinking controls (Logrip & Zorrilla 2012). This social defeat stress effect on acquisition of drinking in rats is similar to what is observed in adult male rats exposed to a series of high-intensity footshocks: one group used a model called stress-enhanced fear learning, developed originally to measure the effect of traumatic stress history on subsequent fear learning (Rau & Fanselow 2009), to show that repeated footshock facilitates acquisition of two-bottle choice home-cage alcohol drinking in previously alcohol-naïve rats, but does not alter alcohol drinking in rats with prior alcohol drinking history (Meyer et al. 2013).
Individual differences in stress-induced escalation of alcohol drinking
One final comment on animal models that seek to produce stress-induced escalation of alcohol drinking in rodent models is the consideration of subpopulation effects or individual differences between animals. Historically, animal models have examined mean group effects of stress on alcohol drinking, which may mask stress effects in subpopulations of animals, especially because it is well accepted that traumatic stress disorders develop only in a portion of individuals exposed to the stress event. It is important to understand that in humans, and therefore in animal models, subgroups of individuals may respond to traumatic stress with different coping strategies, and changes in brain function that are of different magnitudes, opposite directions or qualitatively different. Individual differences in brain function may be the cause or consequence (or both) of effective vs. ineffective coping strategies, and may be responsible for eventual emergence of lasting psychiatric disturbances, or protection from those effects. Some of the models described above do attempt to index animals on their stress reactivity or baseline alcohol drinking, and examine the effects of stress or stress history on alcohol drinking over time in subgroups of rats. For example, the two predator odor stress models described above index high vs. low stress-reactive rats from a genetically heterogeneous stock, and show that some but not all animals exhibit sustained increases in alcohol drinking over time post-stress (Edwards et al. 2013; Manjoch et al. 2016). Understanding why some but not all animals escalate their alcohol drinking after stress may be particularly important because we know that the activity of brain reward circuits differs in genetically heterogeneous animals that consume high vs. low alcohol quantities (Juarez & Han 2016). This divergence of neurobiology across animals has been exploited in various selective breeding programs designed to produce animals that consume high vs. low quantities of alcohol (McBride et al. 2014; Murphy et al. 2002), or that diverge on novelty seeking (Flagel et al. 2014) or motivational response to reward cues (Robinson et al. 2014), all of which are thought to have a role in addiction susceptibility.
Anatomy and circuitry of comorbid PTSD and AUD
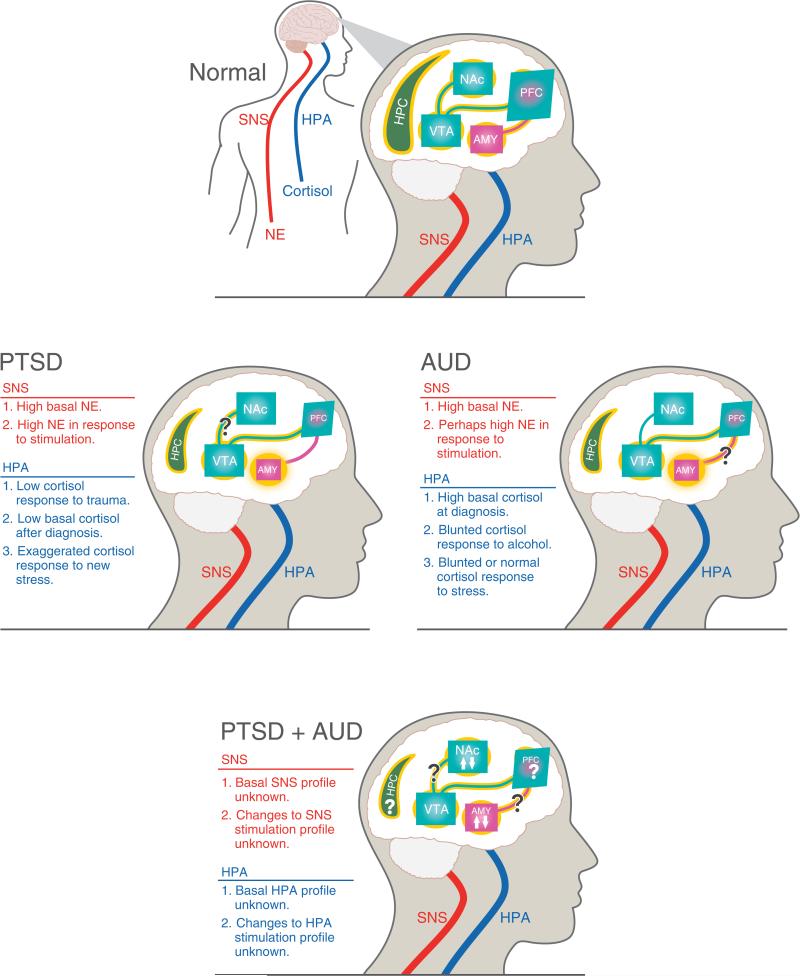
Humans diagnosed with AUD exhibit specific structural and functional brain changes (Buhler & Mann 2011). Similarly, individuals that have endured traumatic stress exhibit structural and functional brain changes that are associated with specific symptom clusters in the PTSD diagnosis (Weiss 2007). Although PTSD likely shares some, but certainly not all, of its neurobiology with innate anxiety and fear learning (Calhoon & Tye 2015), we do not discuss the neurobiology of innate anxiety, fear learning or threat processing in healthy humans (for excellent reviews on these topics and their relevance for understanding PTSD, see Delgado et al. 2006; Etkin & Wager 2007; Jovanovic & Ressler 2010; Mahan & Ressler 2012; Milad et al. 2014) nor do we discuss the neurobiology of alcohol dependence (for review, see Gilpin & Koob 2008). Instead, we focus below on discussing the neurobiology and neurocircuitry of comorbid PTSD and AUD (see Fig. 1) in three specific ways: points of overlap in the hallmark findings from humans with PTSD and humans with AUD, discussion of findings from humans with comorbid PTSD/AUD and findings from animal models that aim to model the comorbid condition (not one condition or the other alone).
Figure 1. Neurobiology of comorbid PTSD and AUD.
Illustration of hallmark central and peripheral nervous system changes associated with PTSD (left), AUD (right), PTSD + AUD (bottom) and relative to healthy controls (top). All denoted biological changes reflect the human literature on PTSD, AUD or comorbid PTSD + AUD, although it should be noted that very few human studies have focused specifically on the neurobiology and neurophysiology of comorbid PTSD + AUD. Smaller size of a structure reflects literature showing volumetric reductions in that brain structure. Yellow glow of structures and connections reflects normal function in healthy controls (top). Absence or exaggeration of yellow glow in other panels indicates functional deficits or hyperactivity in those structures and/or pathways. Upward and downward arrows indicate evidence in support of effects in both directions in a particular brain region. Question marks indicate ambiguity of findings or lack of information, as summarized in the text.
Hypoactive vmPFC
Although rats and humans both have medial prefrontal cortex (mPFC), it can be difficult to determine analogous regions of rodent and human brain. That said, efforts are made in preclinical neuroscience research to determine rodent analogs of human brain regions based on anatomy, connectivity (inputs and outputs) and function. In the case of the rat mPFC, this brain region is often divided into its dorsal and ventral areas, referred to as dorsomedial PFC (dmPFC) and ventromedial PFC (vmPFC). A finer separation of regions divides the mPFC into anterior cingulate cortex (ACC), prelimbic cortex (PL) and infralimbic cortex (IL), with the ACC and top half of PL composing the dmPFC and the IL and bottom half of PL composing the vmPFC. It is generally thought that the rodent PL is analogous to Brodmann area 32 in human and monkey brain, whereas the rodent IL is analogous to Brodmann area 25 in human and monkey brain (Myers-Schulz & Koenigs 2012). In reality, the picture is likely to be more ambiguous because, for example, human functional imaging data show that fear extinction recall correlates not with activity in Brodmann area 25 as would be predicted based on this model, but just anterior to this region in Brodmann area 10 (Milad et al. 2007; Phelps et al. 2004). These findings and others have led to a further separation of human vmPFC into posterior and perigenual subregions that may serve negative affective and positive affective roles, respectively (for excellent review, see Myers-Schulz & Koenigs 2012). As higher resolution imaging techniques become available in humans, the evolving answer to this comparative neuroscience question is likely to become clearer.
Humans with PTSD exhibit vmPFC structural deficits. For example, youth diagnosed with vmPFC have lower right vmPFC volume relative to both maltreated youth without PTSD and healthy controls (Morey et al. 2016). A meta-analysis of nine voxel-based morphometry studies showed that PTSD adults exhibit gray matter loss in vmPFC relative to individuals exposed to trauma that did not develop PTSD (Kuhn & Gallinat 2013). Youth with PTSD also exhibit lower gray matter volume in vmPFC that is inversely correlated with PTSD duration (i.e. lower volume with longer duration), but not with symptom severity (Keding & Herringa 2015).
In general, PTSD is associated with lower vmPFC function during a negative emotional condition (for meta-analysis, see Etkin & Wager 2007), although opposite results have been reported (St Jacques et al. 2011). Combat veterans with PTSD exhibit lower vmPFC activity when exposed to learned danger signals, relative to combat controls (Garfinkel et al. 2014). A small-scale study also showed that PTSD patients undergoing treatment showed improvements on the Clinician-Administered PTSD Scale (CAPS) that correlated with increases in right ACC activity from pretreatment to posttreatment imaging time points (Felmingham et al. 2007), suggesting that vmPFC abnormalities in PTSD patients can be rescued. It is becoming increasingly recognized that altered activity in specific brain regions of PTSD patients (and in general) during functional imaging tasks may reflect changes in the activity of a larger cortical network (Spielberg et al. 2015). It should be noted that vmPFC damage reduces the likelihood of developing PTSD (reviewed in Koenigs & Grafman 2009), and that this pattern of results is not consistent with the notion that low vmPFC activity contributes to PTSD. There is also some disagreement among imaging studies regarding vmPFC activity patterns in mood, anxiety and stress disorders, and some of this ambiguity may be attributable to the poor delineation of vmPFC subregions described above (Myers-Schulz & Koenigs 2012).
Humans with alcohol dependence and/or AUD also exhibit structural and functional changes in the vmPFC. Comprehensive reviews of the literature showing PFC changes in human addicts exist elsewhere (Goldstein & Volkow 2016), and can be seen at rest, during emotional reactivity tasks, and also during inhibitory control tasks. Several findings warrant mention here as they relate to vmPFC and alcohol. First, alcohol dependence is associated with loss of gray matter volume in dorsolateral PFC (dlPFC) that is lasting and is associated with executive dysfunction (Chanraud et al. 2007; Fein et al. 2002; Wobrock et al. 2009). Metabolism in cingulate cortex (Brodmann area 32) is associated with alcohol craving and D2-like receptor availability in the striatum of alcoholics, but not healthy controls (Heinz et al. 2004; Volkow et al. 2007). Similarly, opioid receptor binding in ACC is associated with alcohol craving in alcoholics (Williams et al. 2009). Interestingly, low dynamic activity in the vmPFC during a stress task predicts maladaptive coping behaviors and binge alcohol drinking in otherwise healthy humans (Sinha 2016). Similarly, low vmPFC reactivity to alcohol and stress cues in the laboratory predicts higher risk of relapse to alcohol drinking in abstinent patients recovering from alcohol dependence (Blaine et al. 2015). This vmPFC hyporeactivity was predicted by high circulating cortisol:adrenocorticotropic hormone (ACTH) ratios, although it should be noted that these results were correlational and there was no control group without a history of AUD (Blaine et al. 2015). A recent surge in the field has been to explore whether the cortico-amygdalar circuits involved in fear learning and extinction, which is impaired in PTSD, may also be important for learning and extinguishing alcohol and drug cues (Gass & Chandler 2013; Peters et al. 2009).
There is a dearth of literature examining structural and functional changes in the vmPFC of humans diagnosed with both PTSD and AUD, especially relative to groups with one diagnosis or the other. For example, one study reported that PTSD patients with comorbid alcohol and cocaine abuse exhibit lower frontal cortex blood flow, when compared with healthy controls (Semple et al. 2000). There is also very little preclinical data exploring changes in vmPFC of animals after exposure to both traumatic stress and chronic alcohol. One study showed that rats that exhibit high avoidance of a predator odor-paired chamber (i.e. Avoider rats) consume more alcohol than Non-Avoiders and unstressed Controls, and that these rats exhibit increases in extracellular signal-regulated kinases phosphorylation in vmPFC after re-exposure to the stress-paired context relative to Non-Avoiders (Edwards et al. 2013). Nine days after stress, these Avoider rats have more corticotropin-releasing factor (CRF)-positive cells in vmPFC (but not in dmPFC) than Non-Avoider rats and unstressed controls, the distribution of cell counts suggest that this effect is driven by the stress event, and antagonism of CRF-1 receptors in vmPFC reduces avoidance but not escalated alcohol drinking in Avoider rats (Schreiber et al. 2016).
Hyperactive amygdala
The gross anatomy, connectivity and cellular composition of amygdaloid nuclei is generally considered to be well-enough conserved across species to allow for the formation of translational hypotheses in humans based on rodent work, although there are some important cross-species differences in terms of spatial organization and relative size of various subnuclei (Fox et al. 2015). Structural and functional neuroimaging in humans broadly supports the amygdaloid connectivity described in rodent tract tracing studies, e.g. the reciprocal connections between central amygdala (CeA) and bed nucleus of the stria terminals via the ventral amygdalofugal pathway (Avery et al. 2014, 2016; Oler et al. 2012; Roy et al. 2009). Many of the human studies mentioned below describe findings in whole amygdala, as those studies were not able to use an imaging resolution sufficient to distinguish amygdaloid subnuclei.
Humans with PTSD exhibit structural and functional changes in the amygdala, although functional data are more abundant. Children who have endured early life stress (physical abuse, low socioeconomic status and neglect) have lower amygdala volumes that are quantitatively associated with the amount of cumulative stress exposure and the extent to which the individual exhibits behavioral problems (Hanson et al. 2015). PTSD children have lower left amygdala volume relative to both maltreated children without PTSD and healthy controls, and lower amygdala volumes inversely correlate with greater PTSD symptomatology (Morey et al. 2016). A similar pattern of structural changes is observed in adults, e.g. cancer survivors with intrusive recollections that resemble PTSD symptoms have lower amygdala volumes (Matsuoka et al. 2003).
Perhaps, the most universally accepted biomarker of PTSD in humans is a hyperactive and/or hyperreactive amygdala (see Etkin & Wager 2007). For example, combat veterans with PTSD exhibit exaggerated amygdala responses to fearful faces when compared with combat veterans without PTSD (Rauch et al. 2000). Combat veterans with PTSD show higher amygdala activity that correlates with impaired extinction recall in a safe context, as well as lower amygdala activity that correlates with impaired fear renewal in a dangerous context, which suggests deficits in discrimination and generalization of signals related to safety and danger (Garfinkel et al. 2014). In one example of civilian PTSD, after partner violence, women with PTSD exhibit higher amygdala activity during exposure to fearful faces (Fonzo et al. 2010). Individuals exposed to early life stress appear to undergo ‘re-mapping’ of amygdala connectivity, both between subnuclei and with other brain regions, as it pertains to the threat response in adulthood (Grant et al. 2015b). Trauma survivors exhibit increased attentional bias to threat that is associated with lower peripheral anandamide levels as well as increased cannabinoid type 1 receptor availability in amygdala (Pietrzak et al. 2014a). Because most of these studies are either retrospective (i.e. post-trauma) or they test the response to laboratory stress rather than life trauma, the question remains whether preexisting differences in amygdala activity are predictive of future trauma responses and related diagnoses in some or all individuals. One elegant study tested this possibility and reported no predictive value of amygdala reactivity for future observation of internalizing symptoms associated with PTSD, panic disorder, generalized anxiety disorder and major depression, but did see this predictive association in individuals that experienced high life stress, suggesting that amygdala reactivity may be a promising biomarker in this subpopulation (Swartz et al. 2015).
Amygdala activity has also been related to treatment response in humans with PTSD. For example, low amygdala activity during fear processing in the laboratory predicts positive outcomes after exposure therapy in humans diagnosed with PTSD (Felmingham et al. 2007). In a related case study, deep brain stimulation via electrodes implanted bilaterally in the basolateral amygdala (BLA) promoted extinction of a fear reminder during re-exposure, reduced the frequency of nightmares, and improved scores on the CAPS (Langevin et al. 2016), which agrees with data from macaques showing a role for the BLA in fear generalization (Resnik & Paz 2015).
Similar to humans diagnosed with PTSD, humans diagnosed with an AUD or alcohol dependence (in DSM-IV) exhibit structural and functional changes in the amygdala. Alcohol-dependent humans have lower amygdala volumes, and these lower amygdala volumes are predictive of higher alcohol craving and higher alcohol drinking up to 6 months after imaging (Wrase et al. 2008). It appears that alcohol-dependent humans may transfer genetically encoded amygdala abnormalities to their offspring, because one study shows that young adults with at least one biological parent diagnosed with AUD, but that do not themselves have a diagnosable SUD, exhibit blunted (or absent) amygdala response to fearful faces (Glahn et al. 2007).
Very few studies have performed neuroimaging in humans with PTSD and AUD. One such study showed that PTSD patients with comorbid AUD (and cocaine-use disorder) exhibit higher amygdala blood flow compared with healthy controls (Semple et al. 2000), but this study lacked PTSD-only and AUD-only comparison groups. A separate study examined neural activation patterns as they are related to stress-induced alcohol drinking in humans. This study elegantly showed that drinking problems are highest in the context of stress and in participants that exhibit either (1) high threat-related reactivity of the amygdala combined with low reward-related activity of the ventral striatum (VS) or (2) low threat-related reactivity of the amygdala combined with high reward-related activity of the VS (Nikolova et al. 2016). These data suggest that the relationship between neural activity and stress-related drinking problem may not be simply related to activity in one brain region, but rather may depend on convergent or divergent (re)activity in multiple brain regions.
Few animal studies have examined amygdala changes as they relate to comorbid stress pathology and excessive alcohol drinking, although our laboratories have published preliminary work on this question. In one study, we showed that rats exhibiting persistent avoidance of a predator odor-paired chamber (i.e. Avoiders) go on to drink more alcohol (Edwards et al. 2013) and have higher levels of CRF in CeA (Itoga et al. 2016), but whether these higher CeA CRF levels are responsible for stress-induced escalation of alcohol drinking in Avoiders remain to be determined. Similarly, rats socially isolated during adolescence go on to drink more alcohol in adulthood (Chappell et al. 2013; Skelly et al. 2015), and BLA pyramidal neurons of these rats exhibit higher intrinsic excitability as well as lower expression and activity of small-conductance calcium-activated potassium (SK) channels (Rau et al. 2015). Pharmacological treatment with an SK channel modulator 1-Ethyl-2-benzimidazolinone reduces anxiety-like behavior and rescues BLA physiology changes in these animals (Rau et al. 2015), but it remains to be determined whether these BLA changes mediate the escalated alcohol drinking seen in these animals during adulthood.
PFC-to-amygdala
The dorsal and ventral areas of the mPFC each send highly organized projections to specific amygdaloid subnuclei. Our understanding of the organization of those projections is evolving, but advancing rapidly, and comes mainly from the rodent fear conditioning and fear extinction literature (see review by Marek et al. 2013). It has been proposed that mPFC subregion projections to extended amygdala nuclei control both fear and drug-seeking behaviors, and that dysregulation of these top-down PFC projections may lead to abnormal fear conditioning and compulsive drug-seeking behavior (Peters et al. 2009). The PFC projections to amygdala have been attributed a role not only in drug seeking and extinction learning, but also in the extinction of drug-related memories themselves (Gass & Chandler 2013). This is a nascent field, and it is understandably complicated to study the relationship between two brain regions in individuals diagnosed with two comorbid psychiatric disorders; the following is a brief summary of recent findings on PFC-to-amygdala function in each of those disorders in humans, as well as animal findings supporting the notion that PFC projections to amygdala may contribute to the high rates of comorbidity between traumatic stress and AUDs.
It is generally thought that amygdala hyperactivity is permitted, at least in part, by hypoactive PFC function. For example, PTSD patients show weaker positive connectivities between mPFC and amygdala (and other subcortical regions), and this weaker connectivity is predictive of higher CAPS scores in those patients (Jin et al. 2014). A recent study showed that amygdalo-frontal hypo-connectivity in patients with PTSD (and major depressive disorder) was associated with depression-related symptomatology, suggesting this neuropathology may associate with overlapping symptoms from different clinical groups (Satterthwaite et al. 2016). Youth (aged 8–18) diagnosed with PTSD also exhibit decreased mPFC–amygdala connectivity that is negatively correlated with PTSD severity, and this connectivity is weaker in older youth, suggesting the possibility that it worsens with time, in contrast to positively correlated age and mPFC-amygdala connectivity in healthy youth (Wolf & Herringa 2016). A follow-up study from that group showed that youth with PTSD exhibit reduced connectivity between dmPFC and amygdala, and also between ventrolateral PFC and amygdala, in response to angry faces, but exhibit increased connectivity between these regions in response to happy faces (Keding & Herringa 2016).
Non-diagnosed trauma-exposed children and adolescents fail to engage inhibitory circuits between the pre-genual cingulate cortex and amygdala during regulation of emotional conflict (Marusak et al. 2015). In agreement with this observation, high PFC–amygdala connectivity is associated with lower PTSD symptom severity in disaster survivors diagnosed with PTSD (Yoon et al. 2016). Another study showed that certain risk allele carriers diagnosed with PTSD exhibit white matter deficits in the uncinate fasciculus, which serves as a primary connection between ventral PFC and amygdala, suggesting gene × trauma interaction effects on long-term brain structure and function (Almli et al. 2014).
It is important to note that amygdalo-frontal hypo-connectivity is not a universal finding, because studies have also shown no change (Gilboa et al. 2004) or increases (St Jacques et al. 2011) in vmPFC–amygdala coupling in humans diagnosed with PTSD. Trauma-exposed veterans with focal damage to either vmPFC or anterior temporal area containing amygdala exhibit lower incidence of PTSD, arguing against the contribution of inversely related activity in these two regions to a PTSD diagnosis (Koenigs et al. 2008). The same research group showed recently that humans who have sustained focal bilateral vmPFC damage (but have not been diagnosed with PTSD) exhibit potentiated amygdala responses to aversive images and elevated resting-state amygdala functional connectivity (Motzkin et al. 2015). Another study using functional magnetic resonance imaging (fMRI) in young monkeys and children with anxiety disorders showed that reduced functional connectivity between the dlPFC and CeA was associated with increased anxiety outside the scanner, and that elevated CeA metabolism statistically mediated this association between prefrontal–amygdalar connectivity and elevated anxiety (Birn et al. 2014). Finally, work on rodents has shown that chronic intermittent exposure to alcohol doses sufficient to produce dependence impairs fear extinction encoding by vmPFC neurons, suggesting that alcohol may increase risk for development of fear-conditioning deficits customarily observed in traumatic stress disorders (Holmes et al. 2012).
Hypofunctional reward pathway
Virtually all conceptual frameworks of addiction include an integral role for the canonical ‘brain reward’ circuitry, which is highly conserved across species (Berridge & Kringelbach 2008; Kelley & Berridge 2002). Although many brain regions contribute to reward, the most studied element involves the dopamine (DA)-ergic projection from the ventral tegmental area (VTA) to the nucleus accumbens (NAc), a pathway that is particularly well conserved in all mammals (Scaplen & Kaun 2016). Early animal studies, using electrical stimulation, showed that activation of this pathway was sufficient to maintain motivated behavior and countless subsequent studies have shown that acute administration of all drugs of abuse, including alcohol, stimulates mesolimbic DA release in the NAc (for reviews, see Bressan & Crippa 2005; Olds & Fobes 1981; Volkow et al. 2010). Initial conceptual frameworks for the neurobiology of alcohol addiction focused on the competing ideas that repeated alcohol exposure increased the sensitivity of the brain reward system (Robinson & Berridge 2008) or that preexisting deficits in this system led to a negative affective state that could be relieved by excessive alcohol intake (Koob et al. 2014). A more integrated hypothesis suggests that both processes play an integral role in the etiology of AUD. In this theory, advanced by Koob and others, alcohol drinking is initially motivated by positive reinforcement, driven in large part by acute alcohol stimulation of mesolimbic DA release. However, as the addiction process takes hold, there is an allostatic shift in the addict's affective set point, driven in part by hypoactivity and hyporesponsivity of the mesolimbic DA system. In this latter stage of the addiction process, alcohol drinking is motivated largely by negative reinforcement. Regardless of which theory is correct, the central place of the reward circuit in all theories of addiction has generated a great deal of human and animal research into the role of the VTA–NAc pathway in the pathophysiology of addiction (Koob 2013; Volkow et al. 2016). Importantly, there is also compelling evidence that exposure to a wide range of stressors, ranging from childhood adversity (Andersen 2009) to traumatic events in adulthood (Felmingham 2007), can also disrupt mesolimbic DA signaling. In fact, as reviewed below, the brain reward circuitry is also compromised in PTSD patients and contributes to the symptoms of this disease.
Although PTSD is most frequently associated with increased anxiety and stress reactivity, anhedonia is also common. Almost two-thirds of PTSD patients report decreased interest in pleasurable activities and reduced positive affect, even in the absence of comorbid depression (Carmassi et al. 2014; Franklin & Zimmerman 2001). Behavioral studies have shown that PTSD patients show reduced approach behavior or motivation for positive reinforcers, lower expectancy of, and satisfaction with, monetary rewards (Hopper et al. 2008; Nawijn et al. 2015) and expend less effort to obtain reward (Elman et al. 2005). Structural imaging studies of striatum in humans with PTSD are scarce, but functional imaging studies have reported dysregulation of the mesolimbic brain reward pathway in patients with PTSD. For example, several fMRI studies in individuals with PTSD have observed blunted striatal activation in response to positive stimuli (Elman et al. 2009; Felmingham et al. 2014; Sailer et al. 2008). Moreover, decreased accumbal reactivity to reward was correlated with increased post-deployment PTSD symptom severity in a small cohort of soldiers (Admon et al. 2013a).
One recent study used Single-photon emission computed tomography imaging to show increased striatal levels of the DA transporter (DAT) in PTSD patients (Hoexter et al. 2012). Another study used a selective kappa opioid receptor (KOR) radioligand and high-resolution positron emission tomography (PET) imaging to show a negative correlation between dysphoric symptoms and KOR bioavailability in ventral striatal circuits of individuals with trauma-related psychopathology (Pietrzak et al. 2014b). Because DAT and KORs play an integral role in regulating ambient DA levels, these striatal changes may contribute to a hypo-dopaminergic state that is often associated with decreased reward sensitivity and depressive-like symptoms.
As noted earlier, the mesolimbic reward circuit features prominently in most theories of addiction, and this has stimulated many human imaging studies that have characterized the acute and chronic alcohol effects on VTA–NAc DA signaling. Several studies have employed PET imaging to explore the effects of alcohol consumption on ventral striatal DA levels in healthy subjects, and most (Aalto et al. 2015; Boileau et al. 2003; Oberlin et al. 2015; Urban et al. 2010), but not all (Salonen et al. 1997), reported significant increases in DA levels in this brain region. Several recent studies used a dynamic intravenous alcohol clamp procedure to control for chemo- and somatosensory alcohol cues that may affect mesolimbic DA signaling. Interestingly, initial studies using these methods failed to detect a pharmacological effect of alcohol on ventral striatal DA release in healthy, social drinkers (Yoder et al. 2005, 2007). However, a subsequent study by these investigators that examined the effect of alcohol-associated flavor cues, found that the flavor of a preferred beer, in the absence of any alcohol exposure, increased ventral striatal DA release, with the strongest effect observed in subjects with a first-degree alcoholic relative (Oberlin et al. 2013). An even more recent paper by these investigators explored the distinct effects of taste cues and alcohol, in heavy drinking male subjects, and reported that alcohol alone increased DA, but only in the left NAc region, whereas the combination of beer flavor and alcohol caused a bilateral increase in NAc DA (Oberlin et al. 2015).
A series of seminal PET studies by Volkow et al. reported lower D2 receptor availability in the striatum of drug abusers (Volkow et al. 1990; Wang et al. 1997) and alcoholics (Volkow et al. 1996). These findings, along with the other data from this group showing a blunting of methamphetamine-stimulated striatal DA release in these individuals, relative to non-addicted control subjects (Volkow et al. 1997, 2004, 2007), provide some support for the reward deficiency hypothesis. However, it should be noted that these studies were not able to resolve whether these deficits in the striatal DA system were present before the addiction or were a consequence of excessive drug or alcohol exposure (Hommer et al. 2011).
A few studies have also used fMRI to characterize the mesolimbic DA system in alcoholics. These studies used the monetary incentive delay task, which activates the VS and provides a measure of an individual's sensitivity to anticipation of working for reward, as well as sensitivity to notification that a reward has been won. Two studies using this kind of task reported decreased blood-oxygen-level dependent (BOLD) activation of the VS of alcoholics vs. non-alcoholics during anticipation of reward (Beck et al. 2009; Wrase et al. 2007). However, another study found no significant difference in this measure, but reported that alcoholics had greater ventral striatal BOLD response to notification of reward delivery, and greater reactivity to both positive and negative reward outcomes (Bjork et al. 2008). These seemingly discrepant findings may be explained by a recent report that both low and high ventral striatal reactivity in a reward task is associated with greater risks for drinking problems in young adults, depending on the balance between this response and threat-related amygdala reactivity (Nikolova et al. 2016).
Although no human imaging studies have examined the VTA–NAc reward circuit in patients with comorbid PTSD and AUD, recent animal studies provide further evidence that dysregulation of mesolimbic dopaminergic transmission may contribute to a PTSD–AUD dual diagnosis. For example, rodent studies suggest that adolescent social isolation, a putative model of heightened vulnerability to PTSD and AUD, leads to an increase in the firing rate and burst-like activity of VTA DA neurons (Fabricius et al. 2010), as well as an increase in long-term potentiation (LTP) of N-methyl-d-aspartate receptor-mediated synaptic transmission in these cells (Whitaker et al. 2013). Other studies, using ex vivo fast-scan cyclic voltammetry, suggest that adolescent social isolation leads to a long-lasting increase in electrically stimulated DA release and uptake in the NAc (Yorgason et al. 2013), with the latter effect possibly mediated by an increase in DAT expression (Yorgason et al. 2016), as observed in human patients with PTSD. Using in vivo microdialysis in freely moving rats, Karkhanis et al. (2014) found that an acute ethanol injection resulted in greater ethanol-stimulated accumbal DA release in adolescent socially isolated rats vs. group-housed controls. Although these findings seem to suggest that vulnerability to comorbid PTSD and AUD are actually associated with a sensitization of the mesolimbic DA system, a more recent study reported lower baseline DA levels in the NAc of socially isolated rats that can be rescued by blockade of KORs (Karkhanis et al. 2016). As noted earlier, lower baseline levels of DA are hypothesized to contribute to anhedonia in humans and animals. Together, these findings suggest that low baseline accumbal DA and heightened responsivity of the VTA–NAc DA circuit may increase the risk of developing PTSD and AUD (or both), and may contribute to the expression of these highly comorbid disorders.
HPA axis function
It is beyond the scope of this review to discuss all findings of hypothalamic–pituitary–adrenal (HPA) axis dysfunction in humans diagnosed with AUD or PTSD. The literature is rife with reviews focusing specifically on the role of HPA axis function and dysfunction in acute alcohol effects (Rivier 2014), development of AUD (Clarke et al. 2008), fear conditioning and extinction (Stockhorst & Antov 2015), acute and chronic stress effects (Herman et al. 2002; McEwen et al. 2015; Wamsteeker & Bains 2010) and PTSD pathology and diagnosis (Daskalakis et al. 2013). Here, we describe several of the most pertinent findings as they relate to AUD and PTSD trajectory in humans, as well as animal data aimed at understanding the role of the HPA axis in comorbid high traumatic stress reactivity and escalated alcohol drinking.
One of the hallmark findings related to the neuroendocrine stress axis in PTSD is that low cortisol levels immediately post-trauma are predictive of a subsequent PTSD diagnosis (Delahanty et al. 2000, 2005), and this finding appears to hold across a range of human samples and traumatic events (e.g. child trauma and motor vehicle accident). Although perhaps a bit less clean cut, other studies have reported that these low cortisol levels persist in humans diagnosed with PTSD well after the trauma event (i.e. post-diagnosis; Wahbeh & Oken 2013; Yehuda et al. 1990). More specifically, one study showed that low cortisol levels in PTSD patients are associated with higher disengagement coping strategies associated with the avoidance-numbing symptom subscore defined in the DSM-III-R (Mason et al. 2001); avoidance has been separated out into its own symptom cluster in the DSM-5. It is worth reiterating here that different individuals respond to traumatic stress with different coping strategies that may be the cause or consequence of neural changes that are of different magnitudes, opposite directions or qualitatively different across individuals, dictating the likelihood of a PTSD diagnosis. Work by our group and others have used predator odor stress to show that low corticosterone levels immediately post-trauma predicts higher avoidance of a traumatic stress-paired context and higher anxiety-like behavior in rats (Cohen et al. 2006; Whitaker & Gilpin 2015). Corticosterone treatment prior to stress exposure reduces the incidence of avoidance and anxiety-like behaviors (Cohen et al. 2006; Whitaker et al. 2016), which is particularly interesting because rats exposed to predator odor in these models go on to consume more alcohol over weeks post-stress (Edwards et al. 2013; Manjoch et al. 2016). The ‘centralization’ of HPA feedback effects on brain regions outside the hypothalamus is not well understood, but one example of this type of work used fMRI in humans to show that cortisol signaling through mineralocorticoid receptors mediates stress-induced enhancement of CeA connectivity with striatum (Vogel et al. 2015). Furthermore, traumatic stress produces brain region-specific changes in glucocorticoid receptor (GR) chaperone protein expression (Whitaker et al. 2016).
Acute exposure to intoxicating doses of alcohol is generally believed to increase neuroendocrine stress axis activity, as indicated by a rise in circulating ACTH and cortisol levels, in both humans and rats without a chronic alcohol history (Jenkins & Connolly 1968; Rivier 2014; but see Inder et al. 1995). In some but not all individuals with AUD, chronic alcohol can produce a pseudo-Cushing's syndrome, defined by higher circulating cortisol levels and insufficient suppression after low-dose dexamethasone, and the opposite effects during withdrawal (Besemer et al. 2011), that is difficult to distinguish clinically from organic Cushing's syndrome, except that symptoms dissipate upon cessation of alcohol use (Groote Veldman & Meinders 1996). Animals with a chronic alcohol history exhibit blunted ACTH/corticosterone rise in response to acute alcohol that is administered by an experimenter (Lee & Rivier 1997) or voluntarily consumed (Richardson et al. 2008). In a human laboratory study, psychosocial stress increased circulating cortisol levels and drinking in non-treatment-seeking alcoholics (Thomas et al. 2011), although that study did not include non-AUD controls. Another study reported blunted cortisol response to personalized stressful imagery in alcohol-dependent individuals (Sinha et al. 2009), although this effect may or may not hold in alcohol-dependent individuals co-diagnosed with PTSD (McRae et al. 2006). A separate study showed that prior alcohol use is positively associated with cortisol levels in women after rape, and that this effect is dampened in women with a prior assault history (Resnick et al. 1997), suggesting complex interactions between trauma, trauma history and alcohol-use history. These studies in humans, along with decades of work in animals (Mantsch et al. 2016), make the case that acute stress is sufficient to trigger relapse to alcohol-seeking and -drinking behaviors. Indeed, for some time, physical, psychological and pharmacological stressors have been used in human laboratory studies to examine the relationship between stress reactivity and alcohol abuse (Thomas et al. 2012). More specifically, humans diagnosed with comorbid PTSD and AUD exhibit increases in circulating ACTH and cortisol levels and concurrent increases in alcohol craving in response to acute psychosocial stress (Kwako et al. 2015b). Furthermore, stress-induced increases in circulating ACTH and cortisol may be useful biomarkers for relapse risk in those recovering from AUD (Sinha 2012). Centralization of HPA feedback appears to play a role in alcohol abuse but the details of this process are not yet well understood; for example, alcohol-dependent rats exhibit brain region-specific changes in GR mRNA during withdrawal (Vendruscolo et al. 2012). Showing the translational utility of this work, systemic treatment with the GR antagonist mifepristone reduces escalated alcohol drinking by alcohol-dependent rats and also reduces cue-induced alcohol craving in non-treatment-seeking alcohol-dependent humans (Vendruscolo et al. 2015).
High NE and hyperarousal
A well-documented feature of both PTSD and AUD is a state of hyperarousal and heightened responsivity to stress. Indeed, hyperarousal is a central diagnostic criterion for PTSD (Bremner 2006; Nutt 2000), and many studies have shown that individuals with AUD exhibit increased responsivity to a wide range of stressors (e.g. alcohol cues, psychological stressors, etc.; for reviews, see Seo & Sinha 2014; Sinha 2012). Although other neural systems are likely to be involved, consistent evidence suggests that hyperactive central and peripheral norepinephrine (NE) signaling contribute to these behaviors (for detailed reviews, see Krystal & Neumeister 2009; O'Donnell et al. 2004; Strawn & Geracioti 2008).
The primary stress-related central NE system originates in the locus coeruleus (LC), includes projections to the amygdala, hippocampus and PFC, and plays an integral role in normal fear and stress responses (Itoi & Sugimoto 2010; Shin et al. 2006). There is now overwhelming evidence that hyperactivity of brain NE systems, under basal and stimulated conditions, contributes to the pathophysiology of PTSD. Early studies showed that pharmacological challenges with yohimbine, a presynaptic α2-adrenoceptor antagonist, induced larger increases in heart rate and systolic blood pressure in PTSD patients relative to healthy controls, and exacerbated core PTSD symptoms (e.g. anxiety and flashbacks) in affected individuals, without any untoward effects in control subjects (Bremner et al. 1997; Southwick 1993). Other evidence suggests that tonic CSF NE levels are significantly higher in men with PTSD than in healthy controls and that CSF NE levels positively correlate with PTSD symptom severity (Geracioti et al. 2001).
Many studies have examined peripheral markers of NE and, although this literature is more mixed, the preponderance of the evidence is consistent with hyperactivity of peripheral NE signaling in PTSD. Physiological measures of sympathetic tone that are thought to involve peripheral NE release are elevated in PTSD patients, including increased blood pressure, heart rate, skin conductance (Blanchard et al. 1996; Orr et al. 1997a,1997b; Pitman & Orr 1990; Pitman et al. 1990) and an exaggerated startle reflex (Orr et al. 2002). Several studies have observed higher urinary (De Bellis et al. 1997; Kosten et al. 1987) and plasma (Blanchard et al. 1991; McFall et al. 1990; Yehuda et al. 1998) levels of epinephrine and NE in PTSD patients compared with healthy controls or even individuals with other psychiatric illnesses. However, other studies have not replicated these findings (Mellman et al. 1995; Pitman & Orr 1990), including one of the studies cited above which documented higher CSF NE in PTSD patients relative to controls but no difference in plasma NE levels (Geracioti et al. 2001).
There is very little imaging research directed at central NE system changes in PTSD, possibly because of the lack of effective radioligands. One recent study used the radioligand [11C]methylreboxetine to examine in vivo availability of the NE transporter (NET) in the LC of PTSD patients, trauma-exposed subjects who did not develop PTSD, and healthy controls (Pietrzak et al. 2013). Prior preclinical work suggested that repeated stress decreases NET expression in this brain region and other limbic areas (Zafar et al. 1997). Indeed, the imaging study found a significant reduction in NET bioavailability in PTSD patients relative to healthy controls, and a negative correlation between NET bioavailability and the severity of anxious arousal or hypervigilance symptoms in these patients. These findings are consistent with the idea that PTSD is associated with lower NET levels in the LC, which could lead to excessive NE signaling in downstream projections such as the amygdala and PFC. However, it should be noted that no differences in NET availability were noted between trauma-exposed individuals who developed PTSD and those who did not. Another set of interesting fMRI studies reported LC hyperactivity in PTSD patients relative to healthy controls (Steuwe et al. 2014), as well as a strengthening of functional connectivity between the LC and a distributed network that includes the thalamus, caudate putamen, insula, cingulate gyrus and amygdala (Steuwe et al. 2015).
Although fewer studies have focused on NE and AUD, increased sympathetic drive has been well documented in alcoholics, particularly during acute withdrawal (for reviews, see Fitzgerald 2013; Muzyk et al. 2011). For example, alcoholics in early withdrawal exhibit tachycardia, increases in blood pressure and pulse rate (Mayo-Smith 1997; Myrick & Anton 1998), as well as elevated CSF levels of NE (Bayard et al. 2004). Many of these acute symptoms can be mitigated by the α2-adrenoceptor agonist clonidine, which decreases NE release (Muzyk et al. 2011). The literature on NE signaling in protracted abstinence is more mixed. Alcoholics abstinent for at least 2 weeks were found to have increased plasma NE levels relative to healthy controls (Eisenhofer et al. 1985); however, other studies have reported that CSF NE levels may be decreased in abstinent alcoholics (Geracioti et al. 1994). One study showed that abstinent alcoholics exhibit greater cortisol and prolactin release in response to an acute yohimbine challenge (Krystal et al. 1996), but reported effects of this challenge on measures of craving in detoxified patients with AUD have been mixed (Krystal et al. 1994; Umhau et al. 2011). One interesting study examined the effect of sleep deprivation, which activates the sympathetic nervous system on alcohol-dependent men abstinent for 2–3 weeks and healthy control subjects (Irwin & Ziegler 2005). This study reported no baseline differences in heart rate, blood pressure or peripheral NE or epinephrine levels, but a 3-h disruption of the latter stages of a normal sleep cycle resulted in a greater increase in heart rate and circulating NE and epinephrine levels in patients with AUD.
Given the evidence that hyperactivity of central NE signaling likely contributes to the pathophysiology of PTSD and AUD, a number of studies have examined the efficacy of pharmacological modulators of NE neurotransmission in the treatment of these disorders. Although no selective NE modulators are currently approved for the treatment of PTSD, a wide range of drugs that target the NE system have been shown to improve PTSD symptoms, including the α1-adrenoceptor antagonist prazosin (Peskind et al. 2003; Raskind et al. 2003, 2007; Taylor et al. 2006), the α2-adrenoceptor agonists clonidine (Harmon & Riggs 1996; Porter & Bell 1999) and guanfacine (Horrigan 1996; Horrigan & Barnhill 1996) (for pediatric PTSD), and the β blocker propranolol (Famularo et al. 1988; Pitman et al. 2002; Vaiva et al. 2003). Interestingly, several small studies have suggested that the serotonin/NE reuptake inhibitor duloxetine, which has higher affinity for the NET, may reduce PTSD symptoms (Aikins et al. 2011; Villarreal et al. 2010) even in patients refractory to the two Food and Drug Administration (FDA)-approved treatments for this disorder (Walderhaug et al. 2010), sertraline and paroxetine (which preferentially inhibit serotonin transporters). There is also emerging evidence that targeting the NE system may be beneficial in the treatment of AUD, with prazosin showing the most early promise (Fox et al. 2012; Simpson et al. 2009). The potential utility of prazosin in the treatment of comorbid PTSD and AUD is discussed in a later section.
There is also extensive evidence from preclinical studies that NE transmission is dysregulated in animal models of PTSD and AUD. A detailed discussion of these findings is beyond the scope of this article; however, these topics have been comprehensively reviewed (PTSD and NE: Berridge et al. 2012; Morilak et al. 2005; Otis et al. 2015; AUD and NE: Becker 2012; Weinshenker & Schroeder 2007). Very little work has focused on pathophysiological changes in NE transmission in animal models of comorbid PTSD and AUD. We recently reported increased responsivity of brain NE neurotransmission in the rodent adolescent social isolation model that engenders increases in many behavioral risk factors of PTSD and AUD (Karkhanis et al. 2014, 2015). Using microdialysis in awake, freely moving rats, no differences in baseline NE levels were observed in either the NAc or basolateral amygdala between rats that were socially isolated or group-housed during adolescence. However, marked differences were noted in both brain regions in response to an acute ethanol injection. In adolescent group-housed animals, ethanol exposure had no effect on NE levels in either the NAc or the basolateral amygdala. However, in the socially isolated cohort, the same ethanol treatment significantly increased NE levels in these brain areas. Given that the NAc receives its primary noradrenergic input from the nucleus of the solitary tract (Delfs et al. 1998), whereas the basolateral amygdala receives dense innervation from the LC (Jones & Yang 1985; Moore & Bloom 1979), these initial findings suggest that a sensitization of multiple networks of noradrenergic cells may contribute to the behavioral phenotypes associated with this model. Another study found that systemic administration of prazosin, propranolol and duloxetine, three medications that dampen NE neurotransmission via distinct mechanisms, significantly inhibited ethanol intake in socially isolated rats at doses that had no effect in group-housed controls (Skelly et al. 2015). These findings are similar to those showing greater efficacy of these same drugs in reducing alcohol drinking by alcohol-dependent rats relative to non-dependent controls (Gilpin & Koob 2010; Ji et al. 2008; Walker et al. 2008). Collectively, these data are consistent with the idea that the escalation in ethanol intake that manifests following early life stress or repeated episodes of intermittent ethanol exposure may be mediated, at least in part, by increased central noradrenergic signaling.
Hippocampal deficits
Given the integral role that the hippocampus plays in learning, memory and regulation of the HPA axis, and the many findings showing that both PTSD and AUD are associated with profound alterations in memory function and stress hormone signaling (Anderson et al. 2014; Phillips et al. 2006), many human and animal studies have characterized the role of structural and functional changes in the hippocampus in the pathophysiology of these two disorders.
Motivated by initial observations that patients with PTSD exhibit significant deficits in short-term memory, Bremner et al. (1995, 1997) were the first to report reduced hippocampal volume in patients with PTSD. Since those initial observations, a number of studies have sought to replicate these findings. Indeed, several recent meta-analyses have confirmed a significant relationship between smaller hippocampal volume and PTSD when comparisons are made with healthy subjects (Karl et al. 2006; Kitayama et al. 2005; O'Doherty et al. 2015; Smith 2005) or even trauma-exposed subjects that did not develop PTSD (Karl et al. 2006; O'Doherty et al. 2015). Despite these findings, a surprisingly large number of other reports have not observed a significant association between smaller hippocampal volume and PTSD (for review, see O'Doherty et al. 2015). Complicating the story further, a study in identical twins showed no difference in hippocampal volume between siblings who developed PTSD and those who did not, although this study did report a significant negative correlation between hippocampal volume and the probability of developing PTSD after exposure to a traumatic event (Gilbertson et al. 2002). These findings raise the possibility that small hippocampal volume may represent a biological risk factor that increases susceptibility to PTSD.
There is also a large body of evidence suggesting that AUD may be associated with a smaller hippocampal volume (Hoefer et al. 2014). For example, an early MRI study reported decreased hippocampal size in alcoholic men and women, independent of comorbid psychiatric illnesses, including PTSD (Agartz et al. 1999). In fact, even a short history of alcohol abuse has been linked with decreased hippocampal volume in adolescent men and women (De Bellis et al. 2000; Medina et al. 2007; Nagel et al. 2005). However, whether hippocampal volume is directly related to measures of alcohol consumption is less clear. One study reported that hippocampal size was positively correlated with age of onset of alcohol dependence and negatively correlated with the duration of alcohol use (De Bellis et al. 2000), but several other studies have not observed these relationships (Agartz et al. 1999; Gazdzinski et al. 2008; Nagel et al. 2005). As with some PTSD studies, these findings may suggest that a smaller hippocampal volume represents a biological risk factor for the development of AUD or may be a consequence of other environmental factors, such as cigarette smoking.
Given some of the disparate findings noted above, particularly in relation to PTSD and hippocampal volume, a few studies have specifically addressed whether comorbid AUD contributed to associations between hippocampal size and PTSD. However, this literature is also somewhat mixed. For example, a small pilot study in four patients with combat-related PTSD and no history of drug or alcohol abuse reported significantly smaller hippocampal volume in these subjects relative to healthy controls (Hedges et al. 2003). Similarly, larger studies that also controlled for severe major depression and substance-related disorders reported smaller hippocampal volume in subjects diagnosed with PTSD relative to healthy (Bossini et al. 2008) or trauma-exposed controls (Lindauer et al. 2004). In contrast, several other studies that excluded subjects with comorbid AUD found no relationship between PTSD and hippocampal volume (Carrion et al. 2001; De Bellis et al. 1999, 2002; Fennema-Notestine et al. 2002; Schuff et al. 2001; Woodward et al. 2006), although a recent study reported that the diagnosis of comorbid AUD significantly strengthened the negative correlation between hippocampal size and PTSD (Starcevic et al. 2015).
Despite a large literature that has established an important role for the hippocampus in behavioral phenotypes associated with PTSD and AUD (for reviews, see Jin & Maren 2015; Matthews & Morrow 2000; Mulholland 2012; Reznikov et al. 2016), relatively few studies have examined hippocampal function in models of comorbid PTSD and AUD. A series of studies using a mouse adolescent social isolation model that alters behaviors associated with both PTSD and AUD found that, relative to mice group-housed during adolescence, socially isolated mice exhibit altered expression and function of hippocampal gamma-aminobutyric acid (GABA)A receptors along with a decrease in hippocampal levels of allopregnanolone, a neurosteroid that potently enhances GABAergic inhibition (Pibiri et al. 2008; Sanna et al. 2011; Serra et al. 2000, 2006; Talani et al. 2016). These studies also reported decreased hippocampal synaptic excitability and LTP in socially isolated rats. More recent work by our group also reported a significant reduction in LTP in the CA1 region of the hippocampus in recordings from socially isolated rats (Almonte et al. 2016). However, this reduction in synaptic plasticity was associated with marked increases in measures of synaptic excitability in this brain region. Moreover, these social isolation-associated changes were restricted to the ventral hippocampus, which is known to play an integral role in regulating anxiety-like behaviors and contextual fear extinction (McDonald & Mott 2016; Strange et al. 2014).
Treatment of comorbid PTSD and AUD
From the preceding review of the epidemiology and neurobiology of PTSD and AUD, it is clear that these disorders are frequently comorbid and that this dual diagnosis exacerbates the severity of both disorders and worsens the prognosis for recovery. The considerable overlap in the neural substrates underlying PTSD and AUD would seem to offer many putative targets for the development of pharmacotherapies that would effectively treat the core symptoms of these diseases. It is therefore somewhat surprising that, until fairly recently, relatively little clinical attention has been directed specifically at this comorbid patient population. Reasons for this include the fact that PTSD was characterized relatively recently, only recognized in the DSM as a distinct disorder in 1980 (Crocq & Crocq 2000) and only in the last 20 years has sufficient epidemiological evidence accumulated documenting (1) the high prevalence of the comorbidity of these disorders and (2) the challenges associated with treating patients suffering from both diseases. There was previously a belief amongst many clinicians that addressing the symptoms of AUD could potentially hinder the treatment of PTSD (Hien et al. 2004; McGovern et al. 2009; Mills 2013; Najavits & Hien 2013; Solomon et al. 1992). Fortunately, recent evidence has begun to challenge this ill-founded notion (Foa et al. 2013; Najavits & Hien 2013) and the last decade has seen a sharp increase in clinical trials that specifically target individuals with comorbid PTSD and AUD (see Table 1). The treatment of PTSD (Kirkpatrick & Heller 2014; Koller & Stein 2013) and AUD (Muller et al. 2014; Seneviratne & Johnson 2015; Zindel & Kranzler 2014) has been extensively reviewed; therefore, the following section focuses on studies that have targeted patients diagnosed with both disorders.
Table 1.
Characteristics and results of clinical trials for the treatment of comorbid PTSD and AUD
| Medication | Patient population | Treatment setting | Study type | Results | Reference |
|---|---|---|---|---|---|
| Sertraline | 9 Non-veterans (3 M/6 F) | Outpatients | Open label | Sertraline reduced PTSD symptoms, decreased average number of drinks and increased abstinence days | Brady et al. (1995) |
| Sertraline | 94 Non-veterans (51 M/43 F) | Outpatients | Double-blind, RCT | Sertraline modestly improved PTSD symptoms and improved AUD symptoms only in patients with early-onset PTSD and less severe AUD | Brady et al. (2005) |
| Paroxetine vs. desipramine (in combination with naltrexone or placebo) | 88 (81 veterans) (80 M/8 F) | Outpatients | Double-blind, RCT | Paroxetine and desipramine equally reduced PTSD symptoms. Desipramine improved retention and alcohol-use outcomes. Naltrexone had no effect on any drinking measures | Petrakis et al. (2012) |
| Quetiapine | 50 veterans (50 M) | Outpatients | Retrospective | Quetiapine treatment was associated with increased number of abstinent days and lower number of hospitalizations. PTSD symptoms were not reported | Monnelly et al. (2004) |
| Naltrexone vs. disulfiram | 254 veterans (247 M/7 F) | Outpatients | Secondary analysis of a double-blind, RCT | Naltrexone and disulfiram, alone or in combination, decreased alcohol-use measures; however, there was no benefit of combining these medications. Only complete abstainers showed an improvement of PTSD symptoms and, within this group, disulfiram was the most effective medication | Petrakis et al. (2006) |
| Topiramate | 30 veterans (29 M/1 F) | Outpatients | Double-blind, RCT | Topiramate improved PTSD symptoms and decreased alcohol use and craving. Treatment was associated with mild cognitive impairment that recovered by the end of the 12-week treatment period | Batki et al. (2014) |
| Prazosin | 30 (9 veterans) (19 M/11 F) | Outpatients | Double-blind, RCT | Prazosin had no effect on PTSD symptoms but significantly decreased the percentage of drinking days/week and the percentage of heavy drinking days/week | Simpson et al. (2015) |
| Prazosin | 96 veterans (89 M/6 F) | Outpatients | Double-blind, RCT | Prazosin had no significant effect on either PTSD symptoms or alcohol consumption | Petrakis et al. (2016) |
| Aprepitant (NK1R antagonist) | 53 Non-veterans (29 M/24 F) | Outpatients | Double-blind, RCT | Aprepitant had no significant effect on PTSD symptoms or behavioral/neuroendocrine response to stress or alcohol cues | |
| Naltrexone vs. prolonged exposure therapy vs. supportive counseling | 165 Veterans and non-veterans (108 M/57 F) | Outpatients | Double-blind, RCT | PTSD symptoms were reduced in all treatment groups, although the main effect of prolonged exposure therapy was not statistically significant. All treatment groups also showed a large reduction in percentage of days drinking, with the greatest effect observed in the naltrexone + supportive counseling group | Foa et al. (2013) |
F, female; M, male; RCT, randomized control trial.
Sertraline and paroxetine are the only FDA-approved medications for the treatment of PTSD. Not surprisingly, the first clinical studies that focused on individuals with PTSD and comorbid AUD examined the utility of these medications in treating this dual diagnosis. The first study was a small open label trial that examined the efficacy of a 12-week sertraline treatment regimen in nine subjects with both disorders. Sertraline significantly reduced PTSD symptoms and also decreased the average number of drinks and increased abstinence days (Brady et al. 1995). These findings prompted a larger randomized clinical trial of 94 individuals with alcohol dependence and PTSD who received sertraline or placebo for 12 weeks. However, in this study, sertraline only modestly improved PTSD symptoms vs. placebo and only a subset of subjects, those with early-onset PTSD and less severe alcohol dependence, showed a significant reduction in drinking measures with sertraline (Brady et al. 2005).
Another double-blinded, placebo-controlled trial compared the efficacy of the other FDA-approved PTSD medication, paroxetine, vs. the NE reuptake inhibitor desipramine, in a mostly veteran population with a PTSD–AUD dual diagnosis (Petrakis et al. 2012). This study also tested the adjunctive efficacy of naltrexone, an FDA-approved medication for AUD. Both paroxetine and desipramine significantly reduced PTSD symptoms, with similar efficacy. However, subjects receiving desipramine had higher retention rates and better alcohol-use outcomes (% heavy drinking days, drinks/week). These findings provided some initial evidence that uptake inhibitors targeting the NE system might offer some advantage over currently approved PTSD medications in patients with comorbid AUD. Notably, naltrexone had no effect on any of the drinking outcomes in this study, underscoring the idea that the neural substrates underlying this dual diagnosis may be less responsive to drug treatments used to treat either PTSD or AUD alone. These findings, along with the earlier sertraline study, also challenge the notion that antidepressants are devoid of any therapeutic value in the treatment of AUD (Brady & Sinha 2005; Pettinati 2001, 2004).
A retrospective study of 50 veterans diagnosed with AUD, of which 90% also suffered from PTSD, was conducted to evaluate the potential utility of the atypical antipsychotic quetiapine, which was prescribed to improve sleep disturbances, in the treatment of alcohol dependence (Monnelly et al. 2004). Outcome measures included total days abstinent, number of hospitalizations for detoxification and days to first relapse over 1 year of clinical treatment. Relative to the control group, veterans treated with quetiapine showed a signifi-cant increase in the number of abstinent days and a decrease in the number of hospitalizations, with a trend for an increase in the number of days to relapse. However, this study did not compare PTSD symptoms between these groups.
Several other studies were designed primarily to assess the efficacy of AUD medications in comorbid populations. The largest of these was a randomized trial that examined the effect of a 12-week treatment with two FDA-approved medications for AUD, naltrexone and disulfiram, in 245 veterans with AUD along with at least one psychological diagnosis, of which 93 subjects were diagnosed with PTSD (Petrakis et al. 2006). This study had four groups: naltrexone alone, placebo alone, disulfiram + naltrexone and disulfiram + placebo and the primary outcome measures were measures of alcohol use, alcohol craving and PTSD symptoms. The main findings of this study were that, across the entire sample, both treatments were better than placebo at decreasing alcohol-use measures, with a high rate of abstinence noted across the active phase of this study. There was however, no signifi-cant benefit gained by combining naltrexone and disulfiram. Notably, the subgroup with comorbid PTSD did report higher overall craving than subjects with other psychiatric disorders but this group still responded favorably to either medication. Interestingly, only subjects who abstained completely from alcohol use during the study showed any improvement in PTSD symptoms and, within this group, disulfiram was the most effective treatment.
The last few years have seen an increase in the number of clinical trials focused on the treatment of comorbid PTSD and AUD. A small double-blind, placebo-controlled pilot study of mostly male VA patients reported significant improvement of PTSD symptoms and a decrease in alcohol use and craving following a 12-week treatment with topiramate. This drug is an FDA-approved anticonvulsant that interacts with numerous neural targets, including voltage-gated sodium channels as well as GABAergic and glutamatergic synapses (Braga et al. 2009; Shank et al. 2000), and has shown potential efficacy in the treatment of PTSD (Watts et al. 2013; Yeh et al. 2011) and AUD (Guglielmo et al. 2015) alone. This drug was generally well tolerated but did produce transient cognitive impairment that recovered by the end of the 12-week treatment period. Prazosin has shown promise in the treatment of both PTSD (Raskind et al. 2007; Taylor et al. 2006) and AUD (Simpson et al. 2009) and two recent studies explored its efficacy in patients diagnosed with both disorders. The first study was a small 6-week pilot trial in 30 individuals with comorbid PTSD and AUD (Simpson et al. 2015) and the other was a larger study of 96 veterans with both disorders that used a 13-week treatment period (Petrakis et al. 2016). Both studies employed a randomized, double-blind, placebo-controlled design. Despite the prior evidence that prazosin significantly improved PTSD symptoms in non-alcohol-dependent patients, neither study found an improvement in these symptoms in these comorbid populations. Moreover, only the pilot study reported a significant effect of prazosin on alcohol-use measures.
Another recent study tested the neurokinin 1 receptor (NK1R) antagonist, aprepitant, in 53 patients with PTSD and AUD using a similar randomized, double-blind, placebo-controlled design (Kwako et al. 2015a). The NK1R is the preferred receptor for substance P, is expressed in brain regions such as the amygdala and hippocampus that regulate stress responsivity and prior clinical and preclinical evidence indicated that blockade of these receptors may have antidepressant effects and alleviate alcohol craving and reinstatement (George et al. 2008; Kramer et al. 1998; Schank et al. 2011, 2012). Despite these earlier findings, this study found no significant effect of aprepitant on PTSD symptoms or behavioral or neuroendocrine response to stress and alcohol cues. This study did report a significant effect of the drug on activation of vmPFC in response to aversive visual stimuli. Because the vmPFC is hyporesponsive to aversive stimuli in PTSD patients (Liberzon & Sripada 2008), the authors speculated that aprepitant might be useful in facilitating behavioral exposure therapy that is used to promote extinction of maladaptive stress responses in these patients.
Interestingly, a relatively large single-blinded, randomized clinical trial recently compared the efficacy of naltrexone with an evidence-based behavioral treatment for PTSD (prolonged exposure therapy) in 154 individuals with comorbid PTSD and AUD (Foa et al. 2013). This trial compared four groups that received either prolonged exposure therapy + naltrexone, prolonged exposure therapy + placebo, supportive counseling + naltrexone or supportive counseling + placebo. This study was notable in the duration of the treatments, with the behavioral therapy consisting of 12 weekly 90-min sessions followed by 6 biweekly sessions and the naltrexone treatments extending for 6 months. All four treatment groups showed large improvements in alcohol-drinking outcome measures, with naltrexone having the greatest overall effect. Percentage of days drinking increased in all groups 6 months after the end of treatment, but the exposure therapy + naltrexone group exhibited the smallest increases. All four groups also showed significant improvement in PTSD symptoms.
Finally, several studies have specifically examined the efficacy of behavioral therapies in comorbid patients. A recent meta-analysis that included patients with PTSD and any comorbid SUD concluded that these individuals may benefit from individual trauma-focused psychological intervention when combined with a behavioral intervention for substance use (Roberts et al. 2015). This study found little evidence to support the use of non-trauma focused individual or group-based interventions in this population.
Gaps in knowledge and solutions for future work
What do we not know?
A review of the literature on the neurobiology of PTSD and AUD returns many results on the neurobiology of PTSD, and the neurobiology of AUD, but very little on the neurobiology of the comorbid condition, which we know is frequently seen in the clinic. Both PTSD and AUD are likely to coexist in a feed-forward process whereby PTSD symptoms drive excessive alcohol drinking, and alcohol abuse worsens PTSD symptoms. Furthermore, at least in combat veterans, this interaction likely needs to be studied and understood in the context of coexisting traumatic brain injury, which can have debilitating consequences for the affected individual, and which may be because of physical insult as much as the secondary cascade of symptoms that follow (Shively et al. 2016).
Future work in humans with comorbid PTSD and AUD, and also work in animal models designed to concurrently model aspects of each of these two diagnoses, are critical for understanding the interaction effects of traumatic stress and alcohol, which is not information that can be garnered from examining literature on the neurobiology of each disorder separately. In this review, we have attempted to provide an unbiased survey of the literature, but even here we have been largely confined to describing neural systems highlighted in the PTSD-alone and AUD-alone literature, although there is no denying the high degree of overlap in the neuropathology that defines each of these two disorders. For lack of options, treatment targets and strategies in clinical trials aimed at reducing PTSD and AUD symptoms in comorbid individuals have been guided by points of intersection in two separate literatures on the neurobiology of PTSD and the neurobiology of AUD. The high failure rate of these clinical trials, noted in detail above, perhaps provides the strongest argument that our approach needs to change ‘from the ground up’ by tweaking animal models to examine the comorbid condition in the same sets of animals. To further complicate matters, clinical trials may actually be excluding individuals with comorbid diagnoses (Hoertel et al. 2014). Also unknown are the dose-dependent effects of alcohol on the PTSD brain, i.e. (1) do moderate and excessive levels of alcohol drinking after PTSD have similar but graded effects, or qualitatively different effects? and (2) is moderate drinking, unlike excessive drinking, safe for the individual diagnosed with PTSD? It would also be critical to know whether specific patterns (i.e. binge) of alcohol intake/exposure after traumatic stress are particularly impactful on PTSD neurobiology.
It is important to understand whether specific subpopulations of humans and animals are particularly susceptible to traumatic stress-induced escalation of alcohol drinking. The limited clinical and preclinical literature on this topic suggest that the answer is yes, but we do not know what are the likely predisposing factors that ‘stack the deck’ against specific individuals exposed to traumatic stress. For example, only some humans and animals exposed to traumatic stress go on to develop PTSD or PTSD-like behaviors, but it is not known whether these animals have genetic, epigenetic, neurobiological or other preexisting differences from those individuals that are resilient to the detrimental effects of traumatic stress. Furthermore, we do not know whether the predisposition toward post-stress escalation of alcohol drinking simply maps onto the organism's overall stress response, or whether this particular predisposition has different predictors than other traumatic stress effects. A logical extension of this question is whether, among comorbid affected individuals, there are subpopulations that can be expected to respond more or less positively to specific treatment strategies. Another important consideration is whether males and females exhibit traumatic stress reactivity and post-stress escalation of alcohol drinking that is similar quantitatively and/or qualitatively, especially considering noted sex differences in the incidence of PTSD and AUD.
It appears there may be critical periods during which individuals are particularly susceptible to traumatic stress-induced escalation of alcohol drinking, at least under certain experimental conditions. For example, our group has shown that social isolation stress during adolescence but not during adulthood produces long-term increases in alcohol drinking at time points far removed from the stress itself (McCool & Chappell 2009; Skelly et al. 2015), and this effect is seen in males but not in females (Butler et al. 2014). Very little is known about the effect of preadolescent traumatic stress on future escalation of alcohol drinking and the neurobiology of this potential effect, especially when interindividual differences in stress reactivity are accounted for. We also do not know the duration of traumatic stress effects on escalation of alcohol drinking, whether duration is dictated by sex and/or whether duration is dictated by when in the life span the stress occurs. Finally, we do not know whether the neurobiological effects of traumatic stress resolve with time in individuals who are able to remain abstinent for a critical period of time post-trauma, information that would have potentially huge impact on management of PTSD patients.
Post-traumatic stress disorder can be conceptualized in terms of stress sensitization and fear-learning deficits, the latter of which is often examined in terms of fear extinction deficits, enhanced fear renewal or fear reinstatement and/or fear over-generalization. One consideration that we have largely glossed over in this review is the potential impact of alcohol, especially in excessive quantities, on fear-learning deficits. These types of effects would potentially contribute to the self-perpetuating and cyclical nature of PTSD and AUD. We do know that in humans, prestress alcohol exposure has dose-dependent effects on subsequent intrusive memories related to the stress (Bisby et al. 2009), and from animal studies that (1) acute alcohol facilitates reconsolidation of a fear memory (Nomura & Matsuki 2008), (2) subchronic alcohol injections facilitate expression of a previously learned fear (Quinones-Laracuente et al. 2015), (3) alcohol-dependent animals withdrawn from alcohol exhibit higher expression of a newly conditioned fear and resistance to extinction of that fear (Bertotto et al. 2006, but see Stephens et al. 2001) and (4) alcohol dependence impairs subsequent fear extinction (Holmes et al. 2012). Acute studies have focused on low-to-moderate alcohol doses (<1.5 g/kg), largely because high doses of alcohol are amnestic (i.e. blackouts; Jellinek 1946; Lee 2009). Therefore, with the exception of the latter two findings described above, most work on this topic has not aimed to model AUD or chronic high-dose alcohol exposure, and none of these studies examined the contributions of escalated alcohol drinking to observed fear-learning deficits.
Another important avenue of work is to understand whether there are genetic, biological or symptomatic predictors of successful treatment response in people diagnosed with comorbid PTSD and AUD. For example, several studies have shown that behavioral responses (e.g. stress- and cue-elicited craving) and neurobiological measures (e.g. vmPFC and rostral ACC hyperactivation) are predictors of relapse in alcoholics (Blaine et al. 2015; Seo et al. 2013). However, whether these measures are also related to relapse risk in patients with comorbid PTSD is largely unexplored. It is also not known definitively whether drugs that work for PTSD or AUD alone are likely to work for the comorbid condition, but the clinical trials described above suggest that this is not likely to be the case. For individuals with a dual diagnosis, it is not clear whether successful treatment of one of the two disorders reduces symptoms associated with the other disorder. One major challenge is that, particularly in the case of AUD, patients often do not seek treatment until they have misused alcohol for many years. It seems likely that the neurobiological deficits that manifest following a prolonged history of excessive alcohol exposure may potentially render the brain more resistant to AUD treatments. As with other CNS disorders that are often not diagnosed until the underlying neuropathology has progressed to a stage that is resistant to treatment, such as Alzheimer's or Parkinson's disease, early diagnosis and treatment may represent a promising avenue for improving patient outcomes. Given that the dual diagnosis of PTSD and AUD exacerbates the symptoms of both disorders, early detection may be particularly important in the case of the comorbid condition.
Strategies for future work on neurobiology and treatment of comorbid PTSD and AUD
There will not be a single silver bullet for improving the validity of comorbid animal models, design of comorbid experiments and success rates of clinical trials testing drugs aimed to treat comorbid PTSD and AUD. However, adherence to a few basic principles in future work may improve the quality, quantity and translational potential of data in this realm.
First, the parallelism between human and animal studies examining comorbid PTSD and AUD, and reciprocal communication between the investigators performing these two types of studies will be critical for moving the field forward. Better yet, scientific alliances provide a huge advantage in which results from animal studies are translated to human laboratory studies and clinical trials, and results from human experiments and trials are reverse translated to improve and hone the animal models being used by basic scientists. To detect the reciprocal effects of high stress reactivity and excessive alcohol drinking on each other and stress/alcohol interaction effects on other variables, it is critical in both humans and animals that the interaction of PTSD and AUD be studied or modeled in the same individual. Funding opportunities with a focus on this language and detection of these effects will go a long way to increasing the quantity of studies with this specific focus, which are generally regarded by scientists and grant reviewers as ‘too complicated,’ especially in basic science animal models, but nevertheless represent closer approximations of the human condition. Another aspect of the human condition that should be more prominently integrated into animal models of PTSD–AUD comorbidity is the study of subpopulation (or even individual) differences in the biological and behavioral responses to stress, including but not limited to subsequent escalation of alcohol drinking, and dose–response biological sensitivity to alcohol. Finally, it is critical that animal studies aiming to model comorbid PTSD and AUD measure, or at least aim to measure, the same outcomes. Different researchers weigh different aspects of validity as more or less important (Belzung & Lemoine 2011), but it is generally considered important that animal models exhibit high levels of construct validity, generally defined as the accuracy with which an animal model measures what it is intended to measure, and predictive validity, generally defined as the ability to make consistent predictions about a human condition based on an animal's performance in a model of that human condition. There is little doubt that future basic science work on comorbid PTSD and AUD will become more sophisticated and translational, and we are hopeful that this will improve the precision and success of human laboratory experiments and clinical trials aimed at treating these highly comorbid and highly debilitating disorders.
Acknowledgments
This work was supported by NIH grants AA023305 (N.W.G.) and AA017531, AA010422, AA121099 (J.L.W.).
References
- Aalto S, Ingman K, Alakurtti K, Kaasinen V, Virkkala J, Nagren K, Rinne JO, Scheinin H. Intravenous ethanol increases dopamine release in the ventral striatum in humans: PET study using bolus-plus-infusion administration of [(11)C]raclopride. J Cereb Blood Flow Metab. 2015;35:424–431. doi: 10.1038/jcbfm.2014.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Admon R, Lubin G, Rosenblatt JD, Stern O, Kahn I, Assaf M, Hendler T. Imbalanced neural responsivity to risk and reward indicates stress vulnerability in humans. Cereb Cortex. 2013a;23:28–35. doi: 10.1093/cercor/bhr369. [DOI] [PubMed] [Google Scholar]
- Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn Sci. 2013b;17:337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Advani T, Hensler JG, Koek W. Effect of early rearing conditions on alcohol drinking and 5-HT1A receptor function in C57BL/6J mice. Int J Neuropsychopharmacol. 2007;10:595–607. doi: 10.1017/S1461145706007401. [DOI] [PubMed] [Google Scholar]
- Agartz I, Momenan R, Rawlings RR, Kerich MJ, Hommer DW. Hippocampal volume in patients with alcohol dependence. Arch Gen Psychiatry. 1999;56:356–363. doi: 10.1001/archpsyc.56.4.356. [DOI] [PubMed] [Google Scholar]
- Aikins DE, Jackson ED, Christensen A, Walderhaug E, Afroz S, Neumeister A. Differential conditioned fear response predicts duloxetine treatment outcome in male veterans with PTSD: a pilot study. Psychiatry Res. 2011;188:453–455. doi: 10.1016/j.psychres.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Almli LM, Srivastava A, Fani N, Kerley K, Mercer KB, Feng H, Bradley B, Ressler KJ. Follow-up and extension of a prior genome-wide association study of posttraumatic stress disorder: gene x environment associations and structural magnetic resonance imaging in a highly traumatized African-American civilian population. Biol Psychiatry. 2014;76:e3–e4. doi: 10.1016/j.biopsych.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almonte AG, Ewin SE, Carter ES, Weiner JL. Adolescent social isolation enhances excitatory synaptic activity and reduces long-term potentiation in the rat hippocampus. Alcohol Clin Exp Res. 2016;40:247A. [Google Scholar]
- Andersen SL, Teicher MH. Desperately driven and no brakes: developmental stress exposure and subsequent risk for substance abuse. Neurosci Biobehav Rev. 2009;33:516–524. doi: 10.1016/j.neubiorev.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EB, Grossrubatscher I, Frank L. Dynamic hippocampal circuits support learning- and memory-guided behaviors. Cold Spring Harb Symp Quant Biol. 2014;79:51–58. doi: 10.1101/sqb.2014.79.024760. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Lopez MF, Becker HC. Forced swim stress increases ethanol consumption in C57BL/6J mice with a history of chronic intermittent ethanol exposure. Psychopharmacology (Berl) 2016;233:2035–2043. doi: 10.1007/s00213-016-4257-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atwoli L, Stein DJ, Koenen KC, McLaughlin KA. Epidemiology of posttraumatic stress disorder: prevalence, correlates and consequences. Curr Opin Psychiatry. 2015;28:307–311. doi: 10.1097/YCO.0000000000000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU. BNST neurocircuitry in humans. Neuroimage. 2014;91:311–323. doi: 10.1016/j.neuroimage.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avery SN, Clauss JA, Blackford JU. The human BNST: functional role in anxiety and addiction. Neuropsychopharmacology. 2016;41:126–141. doi: 10.1038/npp.2015.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batki SL, Pennington DL, Lasher B, Neylan TC, Metzler T, Waldrop A, Delucchi K, Herbst E. Topiramate treatment of alcohol use disorder in veterans with posttraumatic stress disorder: a randomized controlled pilot trial. Alcohol Clin Exp Res. 2014;38:2169–2177. doi: 10.1111/acer.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batman AM, Miles MF. Translating alcohol research: opportunities and challenges. Alcohol Res. 2015;37:7–14. [PMC free article] [PubMed] [Google Scholar]
- Bayard M, McIntyre J, Hill KR, Woodside J., Jr. Alcohol withdrawal syndrome. Am Fam Physician. 2004;69:1443–1450. [PubMed] [Google Scholar]
- Beck A, Schlagenhauf F, Wustenberg T, Hein J, Kienast T, Kahnt T, Schmack K, Hagele C, Knutson B, Heinz A, Wrase J. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biol Psychiatry. 2009;66:734–742. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Res. 2012;34:448–458. [PMC free article] [PubMed] [Google Scholar]
- Behar D. Flashbacks and posttraumatic stress symptoms in combat veterans. Compr Psychiatry. 1987;28:459–466. doi: 10.1016/0010-440x(87)90011-3. [DOI] [PubMed] [Google Scholar]
- Belzung C, Lemoine M. Criteria of validity for animal models of psychiatric disorders: focus on anxiety disorders and depression. Biol Mood Anxiety Disord. 2011;1:9. doi: 10.1186/2045-5380-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology (Berl) 2008;199:457–480. doi: 10.1007/s00213-008-1099-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Schmeichel BE, Espana RA. Noradrenergic modulation of wakefulness/arousal. Sleep Med Rev. 2012;16:187–197. doi: 10.1016/j.smrv.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertotto ME, Bustos SG, Molina VA, Martijena ID. Influence of ethanol withdrawal on fear memory: effect of D-cycloserine. Neuroscience. 2006;142:979–990. doi: 10.1016/j.neuroscience.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Besemer F, Pereira AM, Smit JW. Alcohol-induced Cushing syndrome. Hypercortisolism caused by alcohol abuse. Neth J Med. 2011;69:318–323. [PubMed] [Google Scholar]
- Birn RM, Shackman AJ, Oler JA, Williams LE, McFarlin DR, Rogers GM, Shelton SE, Alexander AL, Pine DS, Slattery MJ, Davidson RJ, Fox AS, Kalin NH. Evolutionarily conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol Psychiatry. 2014;19:915–922. doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisby JA, Brewin CR, Leitz JR, Valerie Curran H. Acute effects of alcohol on the development of intrusive memories. Psychopharmacology (Berl) 2009;204:655–666. doi: 10.1007/s00213-009-1496-5. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Smith AR, Hommer DW. Striatal sensitivity to reward deliveries and omissions in substance dependent patients. Neuroimage. 2008;42:1609–1621. doi: 10.1016/j.neuroimage.2008.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Seo D, Sinha R. Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addict Biol. 2015 doi: 10.1111/adb.12320. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard EB, Kolb LC, Prins A, Gates S, McCoy GC. Changes in plasma norepinephrine to combat-related stimuli among Vietnam veterans with posttraumatic stress disorder. J Nerv Ment Dis. 1991;179:371–373. doi: 10.1097/00005053-199106000-00012. [DOI] [PubMed] [Google Scholar]
- Blanchard EB, Hickling EJ, Buckley TC, Taylor AE, Vollmer A, Loos WR. Psychophysiology of posttraumatic stress disorder related to motor vehicle accidents: replication and extension. J Consult Clin Psychol. 1996;64:742–751. doi: 10.1037//0022-006x.64.4.742. [DOI] [PubMed] [Google Scholar]
- Blanco C, Xu Y, Brady K, Perez-Fuentes G, Okuda M, Wang S. Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National Epidemiological Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2013;132:630–638. doi: 10.1016/j.drugalcdep.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Assaad JM, Pihl RO, Benkelfat C, Leyton M, Diksic M, Tremblay RE, Dagher A. Alcohol promotes dopamine release in the human nucleus accumbens. Synapse. 2003;49:226–231. doi: 10.1002/syn.10226. [DOI] [PubMed] [Google Scholar]
- Bossini L, Tavanti M, Calossi S, Lombardelli A, Polizzotto NR, Galli R, Vatti G, Pieraccini F, Castrogiovanni P. Magnetic resonance imaging volumes of the hippocampus in drug-naive patients with post-traumatic stress disorder without comorbidity conditions. J Psychiatr Res. 2008;42:752–762. doi: 10.1016/j.jpsychires.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Cameron HA, Holmes A. Chronic swim stress alters sensitivity to acute behavioral effects of ethanol in mice. Physiol Behav. 2007;91:77–86. doi: 10.1016/j.physbeh.2007.01.024. [DOI] [PubMed] [Google Scholar]
- Boyce-Rustay JM, Janos AL, Holmes A. Effects of chronic swim stress on EtOH-related behaviors in C57BL/6J, DBA/2J and BALB/cByJ mice. Behav Brain Res. 2008;186:133–137. doi: 10.1016/j.bbr.2007.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Sinha R. Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. Am J Psychiatry. 2005;162:1483–1493. doi: 10.1176/appi.ajp.162.8.1483. [DOI] [PubMed] [Google Scholar]
- Brady KT, Sonne SC, Roberts JM. Sertraline treatment of comorbid posttraumatic stress disorder and alcohol dependence. J Clin Psychiatry. 1995;56:502–505. [PubMed] [Google Scholar]
- Brady KT, Sonne S, Anton RF, Randall CL, Back SE, Simpson K. Sertraline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2005;29:395–401. doi: 10.1097/01.alc.0000156129.98265.57. [DOI] [PubMed] [Google Scholar]
- Braga MF, Aroniadou-Anderjaska V, Li H, Rogawski MA. Topiramate reduces excitability in the basolateral amygdala by selectively inhibiting GluK1 (GluR5) kainate receptors on interneurons and positively modulating GABAA receptors on principal neurons. J Pharmacol Exp Ther. 2009;330:558–566. doi: 10.1124/jpet.109.153908. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues Clin Neurosci. 2006;8:445–461. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am J Psychiatry. 1996;153:369–375. doi: 10.1176/ajp.153.3.369. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Ng CK, Staib LH, Salomon RM, Bronen RA, Duncan J, Southwick SM, Krystal JH, Rich D, Zubal G, Dey H, Soufer R, Charney DS. Positron emission tomography measurement of cerebral metabolic correlates of yohimbine administration in combat-related posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:246–254. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:1044–1048. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour--review of data from preclinical research. Acta Psychiatr Scand Suppl. 2005;427:14–21. doi: 10.1111/j.1600-0447.2005.00540.x. [DOI] [PubMed] [Google Scholar]
- Buhler M, Mann K. Alcohol and the human brain: a systematic review of different neuroimaging methods. Alcohol Clin Exp Res. 2011;35:1771–1793. doi: 10.1111/j.1530-0277.2011.01540.x. [DOI] [PubMed] [Google Scholar]
- Butler TR, Carter E, Weiner JL. Adolescent social isolation does not lead to persistent increases in anxiety- like behavior or ethanol intake in female Long-Evans rats. Alcohol Clin Exp Res. 2014;38:2199–2207. doi: 10.1111/acer.12476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoon GG, Tye KM. Resolving the neural circuits of anxiety. Nat Neurosci. 2015;18:1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmassi C, Akiskal HS, Bessonov D, Massimetti G, Calderani E, Stratta P, Rossi A, Dell'Osso L. Gender differences in DSM-5 versus DSM-IV-TR PTSD prevalence and criteria comparison among 512 survivors to the L'Aquila earthquake. J Affect Disord. 2014;160:55–61. doi: 10.1016/j.jad.2014.02.028. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Weems CF, Eliez S, Patwardhan A, Brown W, Ray RD, Reiss AL. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry. 2001;50:943–951. doi: 10.1016/s0006-3223(01)01218-5. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcohol Clin Exp Res. 2013;37(Suppl. 1):E394–E403. doi: 10.1111/j.1530-0277.2012.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chilcoat HD, Breslau N. Investigations of causal pathways between PTSD and drug use disorders. Addict Behav. 1998;23:827–840. doi: 10.1016/s0306-4603(98)00069-0. [DOI] [PubMed] [Google Scholar]
- Clarke TK, Treutlein J, Zimmermann US, Kiefer F, Skowronek MH, Rietschel M, Mann K, Schumann G. HPA-axis activity in alcoholism: examples for a gene-environment interaction. Addict Biol. 2008;13:1–14. doi: 10.1111/j.1369-1600.2007.00084.x. [DOI] [PubMed] [Google Scholar]
- Cohen H, Zohar J, Gidron Y, Matar MA, Belkind D, Loewenthal U, Kozlovsky N, Kaplan Z. Blunted HPA axis response to stress influences susceptibility to posttraumatic stress response in rats. Biol Psychiatry. 2006;59:1208–1218. doi: 10.1016/j.biopsych.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, Grant BF. Lifetime comorbidity of DSM-IV mood and anxiety disorders and specific drug use disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2006;67:247–257. doi: 10.4088/jcp.v67n0211. [DOI] [PubMed] [Google Scholar]
- Cottler LB, Compton WM, 3rd, Mager D, Spitznagel EL, Janca A. Posttraumatic stress disorder among substance users from the general population. Am J Psychiatry. 1992;149:664–670. doi: 10.1176/ajp.149.5.664. [DOI] [PubMed] [Google Scholar]
- Crocq MA, Crocq L. From shell shock and war neurosis to posttraumatic stress disorder: a history of psychotraumatology. Dialogues Clin Neurosci. 2000;2:47–55. doi: 10.31887/DCNS.2000.2.1/macrocq. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft AP, Brooks SP, Cole J, Little HJ. Social defeat increases alcohol preference of C57BL/10 strain mice; effect prevented by a CCKB antagonist. Psychopharmacology (Berl) 2005;183:163–170. doi: 10.1007/s00213-005-0165-6. [DOI] [PubMed] [Google Scholar]
- Daskalakis NP, Lehrner A, Yehuda R. Endocrine aspects of post-traumatic stress disorder and implications for diagnosis and treatment. Endocrinol Metab Clin North Am. 2013;42:503–513. doi: 10.1016/j.ecl.2013.05.004. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Birmaher B, Ryan ND. Urinary catecholamine excretion in childhood overanxious and post-traumatic stress disorders. Ann N Y Acad Sci. 1997;821:451–455. doi: 10.1111/j.1749-6632.1997.tb48303.x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Clark DB, Casey BJ, Giedd JN, Boring AM, Frustaci K, Ryan ND. A.E. Bennett Research Award. Developmental traumatology. Part II: brain development. Biol Psychiatry. 1999;45:1271–1284. doi: 10.1016/s0006-3223(99)00045-1. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, Iyengar S, Beers SR, Hall J, Moritz G. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemo-graphically matched study. Biol Psychiatry. 2002;52:1066–1078. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- Deatherage G. Effects of housing density on alcohol intake in the rat. Physiol Behav. 1972;9:55–57. doi: 10.1016/0031-9384(72)90264-8. [DOI] [PubMed] [Google Scholar]
- Debell F, Fear NT, Head M, Batt-Rawden S, Greenberg N, Wessely S, Goodwin L. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc Psychiatry Psychiatr Epidemiol. 2014;49:1401–1425. doi: 10.1007/s00127-014-0855-7. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol Psychiatry. 2000;48:940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- Delahanty DL, Nugent NR, Christopher NC, Walsh M. Initial urinary epinephrine and cortisol levels predict acute PTSD symptoms in child trauma victims. Psychoneuroendocrinology. 2005;30:121–128. doi: 10.1016/j.psyneuen.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Delfs JM, Zhu Y, Druhan JP, Aston-Jones GS. Origin of noradrenergic afferents to the shell subregion of the nucleus accumbens: anterograde and retrograde tract-tracing studies in the rat. Brain Res. 1998;806:127–140. doi: 10.1016/s0006-8993(98)00672-6. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Olsson A, Phelps EA. Extending animal models of fear conditioning to humans. Biol Psychol. 2006;73:39–48. doi: 10.1016/j.biopsycho.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Driessen M, Schulte S, Luedecke C, Schaefer I, Sutmann F, Ohlmeier M, Kemper U, Koesters G, Chodzinski C, Schneider U, Broese T, Dette C, Havemann-Reinicke U, Group TR-S Trauma and PTSD in patients with alcohol, drug, or dual dependence: a multi-center study. Alcohol Clin Exp Res. 2008;32:481–488. doi: 10.1111/j.1530-0277.2007.00591.x. [DOI] [PubMed] [Google Scholar]
- Edwards S, Baynes BB, Carmichael CY, Zamora-Martinez ER, Barrus M, Koob GF, Gilpin NW. Traumatic stress reactivity promotes excessive alcohol drinking and alters the balance of prefrontal cortex-amygdala activity. Transl Psychiatry. 2013;3:e296. doi: 10.1038/tp.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Whiteside EA, Johnson RH. Plasma catecholamine responses to change of posture in alcoholics during withdrawal and after continued abstinence from alcohol. Clin Sci. 1985;68:71–78. doi: 10.1042/cs0680071. [DOI] [PubMed] [Google Scholar]
- Elman I, Ariely D, Mazar N, Aharon I, Lasko NB, Macklin ML, Orr SP, Lukas SE, Pitman RK. Probing reward function in post-traumatic stress disorder with beautiful facial images. Psychiatry Res. 2005;135:179–183. doi: 10.1016/j.psychres.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional neuroimaging of reward circuitry responsivity to monetary gains and losses in posttraumatic stress disorder. Biol Psychiatry. 2009;66:1083–1090. doi: 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enman NM, Zhang Y, Unterwald EM. Connecting the pathology of posttraumatic stress and substance use disorders: monoamines and neuropeptides. Pharmacol Biochem Behav. 2014;117:61–69. doi: 10.1016/j.pbb.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JN, Saunders BE, Kilpatrick DG, Resnick HS. PTSD as a mediator between childhood rape and alcohol use in adult women. Child Abuse Negl. 1998;22:223–234. doi: 10.1016/s0145-2134(97)00133-6. [DOI] [PubMed] [Google Scholar]
- Erol A, Karpyak VM. Sex and gender-related differences in alcohol use and its consequences: contemporary knowledge and future research considerations. Drug Alcohol Depend. 2015;156:1–13. doi: 10.1016/j.drugalcdep.2015.08.023. [DOI] [PubMed] [Google Scholar]
- van Erp AM, Miczek KA. Persistent suppression of ethanol self-administration by brief social stress in rats and increased startle response as index of withdrawal. Physiol Behav. 2001;73:301–311. doi: 10.1016/s0031-9384(01)00458-9. [DOI] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476–1488. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evren C, Sar V, Dalbudak E, Cetin R, Durkaya M, Evren B, Celik S. Lifetime PTSD and quality of life among alcohol-dependent men: impact of childhood emotional abuse and dissociation. Psychiatry Res. 2011;186:85–90. doi: 10.1016/j.psychres.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Fabricius K, Helboe L, Fink-Jensen A, Wortwein G, Steiniger-Brach B, Sotty F. Increased dopaminergic activity in socially isolated rats: an electrophysiological study. Neurosci Lett. 2010;482:117–122. doi: 10.1016/j.neulet.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Famularo R, Kinscherff R, Fenton T. Propranolol treatment for childhood posttraumatic stress disorder, acute type. A pilot study. Am J Dis Child. 1988;142:1244–1247. doi: 10.1001/archpedi.1988.02150110122036. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Cardenas VA, Goldmann H, Tolou-Shams M, Meyerhoff DJ. Cortical gray matter loss in treatment-naive alcohol dependent individuals. Alcohol Clin Exp Res. 2002;26:558–564. [PMC free article] [PubMed] [Google Scholar]
- Felmingham K, Kemp A, Williams L, Das P, Hughes G, Peduto A, Bryant R. Changes in anterior cingulate and amygdala after cognitive behavior therapy of posttraumatic stress disorder. Psychol Sci. 2007;18:127–129. doi: 10.1111/j.1467-9280.2007.01860.x. [DOI] [PubMed] [Google Scholar]
- Felmingham KL, Falconer EM, Williams L, Kemp AH, Allen A, Peduto A, Bryant RA. Reduced amygdala and ventral striatal activity to happy faces in PTSD is associated with emotional numbing. PLoS One. 2014;9:e103653. doi: 10.1371/journal.pone.0103653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PJ. Elevated norepinephrine may be a unifying etiological factor in the abuse of a broad range of substances: alcohol, nicotine, marijuana, heroin, cocaine, and caffeine. Subst Abuse. 2013;7:171–183. doi: 10.4137/SART.S13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flagel SB, Waselus M, Clinton SM, Watson SJ, Akil H. Antecedents and consequences of drug abuse in rats selectively bred for high and low response to novelty. Neuropharmacology. 2014;76(Pt. B):425–436. doi: 10.1016/j.neuropharm.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Jr., Oslin D, O'Brien CP, Imms P, Riggs DS, Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial. JAMA. 2013;310:488–495. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Fonzo GA, Simmons AN, Thorp SR, Norman SB, Paulus MP, Stein MB. Exaggerated and disconnected insular-amygdalar blood oxygenation level-dependent response to threat-related emotional faces in women with intimate-partner violence posttraumatic stress disorder. Biol Psychiatry. 2010;68:433–441. doi: 10.1016/j.biopsych.2010.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox HC, Anderson GM, Tuit K, Hansen J, Kimmerling A, Siedlarz KM, Morgan PT, Sinha R. Prazosin effects on stress-and cue-induced craving and stress response in alcohol-dependent individuals: preliminary findings. Alcohol Clin Exp Res. 2012;36:351–360. doi: 10.1111/j.1530-0277.2011.01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Tromp do.P.M., Fudge JL, Kalin NH. Extending the amygdala in theories of threat processing. Trends Neurosci. 2015;38:319–329. doi: 10.1016/j.tins.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin CL, Zimmerman M. Posttraumatic stress disorder and major depressive disorder: investigating the role of overlapping symptoms in diagnostic comorbidity. J Nerv Ment Dis. 2001;189:548–551. doi: 10.1097/00005053-200108000-00008. [DOI] [PubMed] [Google Scholar]
- Funk D, Harding S, Juzytsch W, Le AD. Effects of unconditioned and conditioned social defeat on alcohol self-administration and reinstatement of alcohol seeking in rats. Psychopharmacology (Berl) 2005;183:341–349. doi: 10.1007/s00213-005-0194-1. [DOI] [PubMed] [Google Scholar]
- Gan JO, Bowline E, Lourenco FS, Pickel VM. Adolescent social isolation enhances the plasmalemmal density of NMDA NR1 subunits in dendritic spines of principal neurons in the basolateral amygdala of adult mice. Neuroscience. 2014;258:174–183. doi: 10.1016/j.neuroscience.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, Liberzon I. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci. 2014;34:13435–13443. doi: 10.1523/JNEUROSCI.4287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Chandler LJ. The plasticity of extinction: contribution of the prefrontal cortex in treating addiction through inhibitory learning. Front Psychiatry. 2013;4:46. doi: 10.3389/fpsyt.2013.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates MA, Holowka DW, Vasterling JJ, Keane TM, Marx BP, Rosen RC. Posttraumatic stress disorder in veterans and military personnel: epidemiology, screening, and case recognition. Psychol Serv. 2012;9:361–382. doi: 10.1037/a0027649. [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo TC, Yeh PH, Hardin D, Banys P, Meyerhoff DJ. Chronic cigarette smoking modulates injury and short-term recovery of the medial temporal lobe in alcoholics. Psychiatry Res. 2008;162:133–145. doi: 10.1016/j.pscychresns.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George DT, Gilman J, Hersh J, Thorsell A, Herion D, Geyer C, Peng X, Kielbasa W, Rawlings R, Brandt JE, Gehlert DR, Tauscher JT, Hunt SP, Hommer D, Heilig M. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319:1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr., Loosen PT, Ebert MH, Ekhator NN, Burns D, Nicholson WE, Orth DN. Concentrations of corticotropin-releasing hormone, norepinephrine, MHPG, 5-hydroxyindoleacetic acid, and tryptophan in the cerebrospinal fluid of alcoholic patients: serial sampling studies. Neuroendocrinology. 1994;60:635–642. doi: 10.1159/000126807. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr., Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE, Jr., Kasckow JW. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158:1227–1230. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry. 2004;55:263–272. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Neurobiology of alcohol dependence: focus on motivational mechanisms. Alcohol Res Health. 2008;31:185–195. [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Koob GF. Effects of beta-adrenoceptor antagonists on alcohol drinking by alcohol-dependent rats. Psychopharmacology (Berl) 2010;212:431–439. doi: 10.1007/s00213-010-1967-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Lovallo WR, Fox PT. Reduced amygdala activation in young adults at high risk of alcoholism: studies from the Oklahoma family health patterns project. Biol Psychiatry. 2007;61:1306–1309. doi: 10.1016/j.biopsych.2006.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 2011;12:652–669. doi: 10.1038/nrn3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Bradley B, Ressler KJ, Powers A. Associations between posttraumatic stress disorder, emotion dysregulation, and alcohol dependence symptoms among inner city females. J Clin Psychol. 2016 doi: 10.1002/jclp.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiat. 2015a;72:757–766. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant MM, Wood K, Sreenivasan K, Wheelock M, White D, Thomas J, Knight DC, Deshpande G. Influence of early life stress on intra- and extra-amygdaloid causal connectivity. Neuropsychopharmacology. 2015b;40:1782–1793. doi: 10.1038/npp.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groote Veldman R, Meinders AE. On the mechanism of alcohol-induced pseudo-Cushing's syndrome. Endocr Rev. 1996;17:262–268. doi: 10.1210/edrv-17-3-262. [DOI] [PubMed] [Google Scholar]
- Guglielmo R, Martinotti G, Quatrale M, Ioime L, Kadilli I, Di Nicola M, Janiri L. Topiramate in alcohol use disorders: review and update. CNS Drugs. 2015;29:383–395. doi: 10.1007/s40263-015-0244-0. [DOI] [PubMed] [Google Scholar]
- Guina J, Welton RS, Broderick PJ, Correll TL, Peirson RP. DSM-5 criteria and its implications for diagnosing PTSD in military service members and veterans. Curr Psychiatry Rep. 2016;18:43. doi: 10.1007/s11920-016-0686-1. [DOI] [PubMed] [Google Scholar]
- Hall FS, Huang S, Fong GW, Pert A, Linnoila M. Effects of isolation-rearing on voluntary consumption of ethanol, sucrose and saccharin solutions in Fawn Hooded and Wistar rats. Psychopharmacology (Berl) 1998;139:210–216. doi: 10.1007/s002130050706. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD, Shirtcliff EA, Pollak SD, Davidson RJ. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry. 2015;77:314–323. doi: 10.1016/j.biopsych.2014.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon RJ, Riggs PD. Clonidine for posttraumatic stress disorder in preschool children. J Am Acad Child Adolesc Psychiatry. 1996;35:1247–1249. doi: 10.1097/00004583-199609000-00022. [DOI] [PubMed] [Google Scholar]
- Hasin DS, O'Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170:834–851. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges DW, Allen S, Tate DF, Thatcher GW, Miller MJ, Rice SA, Cleavinger HB, Sood S, Bigler ED. Reduced hippocampal volume in alcohol and substance naive Vietnam combat veterans with posttraumatic stress disorder. Cogn Behav Neurol. 2003;16:219–224. doi: 10.1097/00146965-200312000-00003. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Herman JP, Cullinan WE, Ziegler DR, Tasker JG. Role of the paraventricular nucleus microenvironment in stress integration. Eur J Neurosci. 2002;16:381–385. doi: 10.1046/j.1460-9568.2002.02133.x. [DOI] [PubMed] [Google Scholar]
- Hien DA, Cohen LR, Miele GM, Litt LC, Capstick C. Promising treatments for women with comorbid PTSD and substance use disorders. Am J Psychiatry. 2004;161:1426–1432. doi: 10.1176/appi.ajp.161.8.1426. [DOI] [PubMed] [Google Scholar]
- Hoefer ME, Pennington DL, Durazzo TC, Mon A, Abe C, Truran D, Hutchison KE, Meyerhoff DJ. Genetic and behavioral determinants of hippocampal volume recovery during abstinence from alcohol. Alcohol. 2014;48:631–638. doi: 10.1016/j.alcohol.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoertel N, Falissard B, Humphreys K, Gorwood P, Seigneurie AS, Limosin F. Do clinical trials of treatment of alcohol dependence adequately enroll participants with co-occurring independent mood and anxiety disorders? An analysis of data from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). J Clin Psychiatry. 2014;75:231–237. doi: 10.4088/JCP.13m08424. [DOI] [PubMed] [Google Scholar]
- Hoexter MQ, Fadel G, Felicio AC, Calzavara MB, Batista IR, Reis MA, Shih MC, Pitman RK, Andreoli SB, Mello MF, Mari JJ, Bressan RA. Higher striatal dopamine transporter density in PTSD: an in vivo SPECT study with [(99m)Tc]TRODAT-1. Psychopharmacology (Berl) 2012;224:337–345. doi: 10.1007/s00213-012-2755-4. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Holmes A, Fitzgerald PJ, MacPherson KP, DeBrouse L, Colacicco G, Flynn SM, Masneuf S, Pleil KE, Li C, Marcinkiewcz CA, Kash TL, Gunduz-Cinar O, Camp M. Chronic alcohol remodels prefrontal neurons and disrupts NMDAR-mediated fear extinction encoding. Nat Neurosci. 2012;15:1359–1361. doi: 10.1038/nn.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW, Bjork JM, Gilman JM. Imaging brain response to reward in addictive disorders. Ann N Y Acad Sci. 2011;1216:50–61. doi: 10.1111/j.1749-6632.2010.05898.x. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Pitman RK, Su Z, Heyman GM, Lasko NB, Macklin ML, Orr SP, Lukas SE, Elman I. Probing reward function in posttraumatic stress disorder: expectancy and satisfaction with monetary gains and losses. J Psychiatr Res. 2008;42:802–807. doi: 10.1016/j.jpsychires.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horrigan JP. Guanfacine for PTSD nightmares. J Am Acad Child Adolesc Psychiatry. 1996;35:975–976. doi: 10.1097/00004583-199608000-00006. [DOI] [PubMed] [Google Scholar]
- Horrigan JP, Barnhill LJ. The suppression of nightmares with guanfacine. J Clin Psychiatry. 1996;57:371. [PubMed] [Google Scholar]
- Hoskins M, Pearce J, Bethell A, Dankova L, Barbui C, Tol WA, van Ommeren M, de Jong J, Seedat S, Chen H, Bisson JI. Pharmacotherapy for post-traumatic stress disorder: systematic review and meta-analysis. Br J Psychiatry. 2015;206:93–100. doi: 10.1192/bjp.bp.114.148551. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Holly EN, DeBold JF, Miczek KA. Social stress-escalated intermittent alcohol drinking: modulation by CRF-R1 in the ventral tegmental area and accumbal dopamine in mice. Psychopharmacology (Berl) 2016;233:681–690. doi: 10.1007/s00213-015-4144-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder WJ, Joyce PR, Wells JE, Evans MJ, Ellis MJ, Mattioli L, Donald RA. The acute effects of oral ethanol on the hypothalamic-pituitary-adrenal axis in normal human subjects. Clin Endocrinol (Oxf) 1995;42:65–71. doi: 10.1111/j.1365-2265.1995.tb02600.x. [DOI] [PubMed] [Google Scholar]
- Ipser 2015.
- Ipser JC, Wilson D, Akindipe TO, Sager C, Stein DJ. Pharmacotherapy for anxiety and comorbid alcohol use disorders. Cochrane Database Syst Rev. 2015;1:CD007505. doi: 10.1002/14651858.CD007505.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin MR, Ziegler M. Sleep deprivation potentiates activation of cardiovascular and catecholamine responses in abstinent alcoholics. Hypertension. 2005;45:252–257. doi: 10.1161/01.HYP.0000153517.44295.07. [DOI] [PubMed] [Google Scholar]
- Itoga CA, Roltsch Hellard EA, Whitaker AM, Lu YL, Schreiber AL, Baynes BB, Baiamonte BA, Richardson HN, Gilpin NW. Traumatic stress promotes hyperalgesia via corticotropin-releasing factor-1 receptor (CRFR1) signaling in central amygdala. Neuropsychopharmacology. 2016;41:2463–2472. doi: 10.1038/npp.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoi K, Sugimoto N. The brainstem noradrenergic systems in stress, anxiety and depression. J Neuroendocrinol. 2010;22:355–361. doi: 10.1111/j.1365-2826.2010.01988.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. Am J Psychiatry. 2001;158:1184–1190. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Jacobson IG, Ryan MA, Hooper TI, Smith TC, Amoroso PJ, Boyko EJ, Gackstetter GD, Wells TS, Bell NS. Alcohol use and alcohol-related problems before and after military combat deployment. JAMA. 2008;300:663–675. doi: 10.1001/jama.300.6.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinek EM. Phases in the drinking history of alcoholics: analysis of a survey conducted by the official organ of Alcoholics Anonymous. Q J Stud Alcohol. 1946;7:1–88. [PubMed] [Google Scholar]
- Jenkins JS, Connolly J. Adrenocortical response to ethanol in man. Br Med J. 1968;2:804–805. doi: 10.1136/bmj.2.5608.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji D, Gilpin NW, Richardson HN, Rivier CL, Koob GF. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav Pharmacol. 2008;19:1–12. doi: 10.1097/FBP.0b013e3282f3cf70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Maren S. Prefrontal-hippocampal interactions in memory and emotion. Front Syst Neurosci. 2015;9:170. doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin C, Qi R, Yin Y, Hu X, Duan L, Xu Q, Zhang Z, Zhong Y, Feng B, Xiang H, Gong Q, Liu Y, Lu G, Li L. Abnormalities in whole-brain functional connectivity observed in treatment-naive post-traumatic stress disorder patients following an earthquake. Psychol Med. 2014;44:1927–1936. doi: 10.1017/S003329171300250X. [DOI] [PubMed] [Google Scholar]
- Jones BE, Yang TZ. The efferent projections from the reticular formation and the locus coeruleus studied by anterograde and retrograde axonal transport in the rat. J Comp Neurol. 1985;242:56–92. doi: 10.1002/cne.902420105. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez B, Han MH. Diversity of dopaminergic neural circuits in response to drug exposure. Neuropsychopharmacology. 2016;41:2424–2446. doi: 10.1038/npp.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Locke JL, McCool BA, Weiner JL, Jones SR. Social isolation rearing increases nucleus accumbens dopamine and norepinephrine responses to acute ethanol in adulthood. Alcohol Clin Exp Res. 2014;38:2770–2779. doi: 10.1111/acer.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Alexander NJ, McCool BA, Weiner JL, Jones SR. Chronic social isolation during adolescence augments catecholamine response to acute ethanol in the basolateral amygdala. Synapse. 2015;69:385–395. doi: 10.1002/syn.21826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Weiner JL, Jones SR. Early-life social isolation stress increases kappa opioid receptor responsiveness and downregulates the dopamine system. Neuropsychopharmacology. 2016;41:2263–2274. doi: 10.1038/npp.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl A, Schaefer M, Malta LS, Dorfel D, Rohleder N, Werner A. A meta-analysis of structural brain abnormalities in PTSD. Neurosci Biobehav Rev. 2006;30:1004–1031. doi: 10.1016/j.neubiorev.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Keding TJ, Herringa RJ. Abnormal structure of fear circuitry in pediatric post-traumatic stress disorder. Neuropsychopharmacology. 2015;40:537–545. doi: 10.1038/npp.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keding TJ, Herringa RJ. Paradoxical prefrontal-amygdala recruitment to angry and happy expressions in pediatric posttraumatic stress disorder. Neuropsychopharmacology. 2016;41:2903–2912. doi: 10.1038/npp.2016.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC. Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Arch Gen Psychiatry. 1997;54:313–321. doi: 10.1001/archpsyc.1997.01830160031005. [DOI] [PubMed] [Google Scholar]
- Khoury L, Tang YL, Bradley B, Cubells JF, Ressler KJ. Substance use, childhood traumatic experience, and posttraumatic stress disorder in an urban civilian population. Depress Anxiety. 2010;27:1077–1086. doi: 10.1002/da.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: results from the National Survey of Adolescents. J Consult Clin Psychol. 2003;71:692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Traum Stress. 2013;26:537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick HA, Heller GM. Post-traumatic stress disorder: theory and treatment update. Int J Psychiatry Med. 2014;47:337–346. doi: 10.2190/PM.47.4.h. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Kline A, Weiner MD, Ciccone DS, Interian A, St Hill L, Losonczy M. Increased risk of alcohol dependency in a cohort of National Guard troops with PTSD: a longitudinal study. J Psychiatr Res. 2014;50:18–25. doi: 10.1016/j.jpsychires.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Posttraumatic stress disorder: the role of medial prefrontal cortex and amygdala. Neuroscientist. 2009;15:540–548. doi: 10.1177/1073858409333072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Raymont V, Cheon B, Solomon J, Wassermann EM, Grafman J. Focal brain damage protects against post-traumatic stress disorder in combat veterans. Nat Neurosci. 2008;11:232–237. doi: 10.1038/nn2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, Ibi D, Mizoguchi H, Nagai T, Nitta A, Takuma K, Nabeshima T, Yoneda Y, Yamada K. Behavioral abnormality and pharmacologic response in social isolation-reared mice. Behav Brain Res. 2009;202:114–121. doi: 10.1016/j.bbr.2009.03.028. [DOI] [PubMed] [Google Scholar]
- Koller A, Stein DJ. Pharmacotherapy of posttraumatic stress disorder. Mod Trends Pharmacopsychiatry. 2013;29:154–163. doi: 10.1159/000353540. [DOI] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW, Jr., George O. Addiction as a stress surfeit disorder. Neuropharmacology. 2014;76(Pt. B):370–382. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten TR, Mason JW, Giller EL, Ostroff RB, Harkness L. Sustained urinary norepinephrine and epinephrine elevation in post-traumatic stress disorder. Psychoneuroendocrinology. 1987;12:13–20. doi: 10.1016/0306-4530(87)90017-5. [DOI] [PubMed] [Google Scholar]
- Kozaric-Kovacic D, Ljubin T, Grappe M. Comorbidity of posttraumatic stress disorder and alcohol dependence in displaced persons. Croat Med J. 2000;41:173–178. [PubMed] [Google Scholar]
- Kramer MS, Cutler N, Feighner J, et al. Distinct mechanism for antidepressant activity by blockade of central substance P receptors. Science. 1998;281:1640–1645. doi: 10.1126/science.281.5383.1640. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Neumeister A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 2009;1293:13–23. doi: 10.1016/j.brainres.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Cooney N, Kranzler HR, Charney DS. Specificity of ethanollike effects elicited by serotonergic and noradrenergic mechanisms. Arch Gen Psychiatry. 1994;51:898–911. doi: 10.1001/archpsyc.1994.03950110058008. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Webb E, Cooney NL, Kranzler HR, Southwick SW, Heninger GR, Charney DS. Serotonergic and noradrenergic dysregulation in alcoholism: m-chlorophenylpiperazine and yohimbine effects in recently detoxified alcoholics and healthy comparison subjects. Am J Psychiatry. 1996;153:83–92. doi: 10.1176/ajp.153.1.83. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol Psychiatry. 2013;73:70–74. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, Weiss DS. The National Vietnam Veterans’ Readjustment Study: tables of findings and technical appendices. Contracutal Report of Findings From the National Vietnam Veterans’ Readjustment Study. 1988:1–710. [Google Scholar]
- Kumari A, Singh P, Baghel MS, Thakur MK. Social isolation mediated anxiety like behavior is associated with enhanced expression and regulation of BDNF in the female mouse brain. Physiol Behav. 2016;158:34–42. doi: 10.1016/j.physbeh.2016.02.032. [DOI] [PubMed] [Google Scholar]
- Kwako LE, George DT, Schwandt ML, Spagnolo PA, Momenan R, Hommer DW, Diamond CA, Sinha R, Shaham Y, Heilig M. The neurokinin-1 receptor antagonist aprepitant in co-morbid alcohol dependence and posttraumatic stress disorder: a human experimental study. Psychopharmacology (Berl) 2015a;232:295–304. doi: 10.1007/s00213-014-3665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Schwandt ML, Sells JR, Ramchandani VA, Hommer DW, George DT, Sinha R, Heilig M. Methods for inducing alcohol craving in individuals with co-morbid alcohol dependence and posttraumatic stress disorder: behavioral and physiological outcomes. Addict Biol. 2015b;20:733–746. doi: 10.1111/adb.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin JP, Koek RJ, Schwartz HN, Chen JW, Sultzer DL, Mandelkern MA, Kulick AD, Krahl SE. Deep brain stimulation of the basolateral amygdala for treatment-refractory posttraumatic stress disorder. Biol Psychiatry. 2016;79:e82–e84. doi: 10.1016/j.biopsych.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y. Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology (Berl) 2005;179:366–373. doi: 10.1007/s00213-004-2036-y. [DOI] [PubMed] [Google Scholar]
- Lee H, Roh S, Kim DJ. Alcohol-induced blackout. Int J Environ Res Public Health. 2009;6:2783–2792. doi: 10.3390/ijerph6112783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Rivier C. An initial, three-day-long treatment with alcohol induces a long-lasting phenomenon of selective tolerance in the activity of the rat hypothalamic-pituitary-adrenal axis. J Neurosci. 1997;17:8856–8866. doi: 10.1523/JNEUROSCI.17-22-08856.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. The functional neuroanatomy of PTSD: a critical review. Prog Brain Res. 2008;167:151–169. doi: 10.1016/S0079-6123(07)67011-3. [DOI] [PubMed] [Google Scholar]
- Lindauer RJ, Vlieger EJ, Jalink M, Olff M, Carlier IV, Majoie CB, den Heeten GJ, Gersons BP. Smaller hippocampal volume in Dutch police officers with posttraumatic stress disorder. Biol Psychiatry. 2004;56:356–363. doi: 10.1016/j.biopsych.2004.05.021. [DOI] [PubMed] [Google Scholar]
- Liu JH, You QL, Wei MD, Wang Q, Luo ZY, Lin S, Huang L, Li SJ, Li XW, Gao TM. Social isolation during adolescence strengthens retention of fear memories and facilitates induction of late-phase long-term potentiation. Mol Neurobiol. 2015;52:1421–1429. doi: 10.1007/s12035-014-8917-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP. Stress history increases alcohol intake in relapse: relation to phosphodiesterase 10A. Addict Biol. 2012;17:920–933. doi: 10.1111/j.1369-1600.2012.00460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Doremus-Fitzwater TL, Becker HC. Chronic social isolation and chronic variable stress during early development induce later elevated ethanol intake in adult C57BL/6J mice. Alcohol. 2011;45:355–364. doi: 10.1016/j.alcohol.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Anderson RI, Becker HC. Effect of different stressors on voluntary ethanol intake in ethanol-dependent and nondependent C57BL/6J mice. Alcohol. 2016;51:17–23. doi: 10.1016/j.alcohol.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahan AL, Ressler KJ. Fear conditioning, synaptic plasticity and the amygdala: implications for posttraumatic stress disorder. Trends Neurosci. 2012;35:24–35. doi: 10.1016/j.tins.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makley AT, Falcone RA., Jr. Posttraumatic stress disorder in the pediatric trauma patient. Semin Pediatr Surg. 2010;19:292–299. doi: 10.1053/j.sempedsurg.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Management of Post-Traumatic Stress Working Group 2010.
- Manjoch H, Vainer E, Matar M, Ifergane G, Zohar J, Kaplan Z, Cohen H. Predator-scent stress, ethanol consumption and the opioid system in an animal model of PTSD. Behav Brain Res. 2016;306:91–105. doi: 10.1016/j.bbr.2016.03.009. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Baker DA, Funk D, Le AD, Shaham Y. Stress-induced reinstatement of drug seeking: 20 years of progress. Neuropsychopharmacology. 2016;41:335–356. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek R, Strobel C, Bredy TW, Sah P. The amygdala and medial prefrontal cortex: partners in the fear circuit. J Physiol. 2013;591:2381–2391. doi: 10.1113/jphysiol.2012.248575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden CA, King MV, Fone KC. Influence of social isolation in the rat on serotonergic function and memory--relevance to models of schizophrenia and the role of 5-HT(6) receptors. Neuropharmacology. 2011;61:400–407. doi: 10.1016/j.neuropharm.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Marusak HA, Martin KR, Etkin A, Thomason ME. Childhood trauma exposure disrupts the automatic regulation of emotional processing. Neuropsychopharmacology. 2015;40:1250–1258. doi: 10.1038/npp.2014.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JW, Wang S, Yehuda R, Riney S, Charney DS, South-wick SM. Psychogenic lowering of urinary cortisol levels linked to increased emotional numbing and a shame-depressive syndrome in combat-related posttraumatic stress disorder. Psycho-som Med. 2001;63:387–401. doi: 10.1097/00006842-200105000-00008. [DOI] [PubMed] [Google Scholar]
- Matsuoka Y, Yamawaki S, Inagaki M, Akechi T, Uchitomi Y. A volumetric study of amygdala in cancer survivors with intrusive recollections. Biol Psychiatry. 2003;54:736–743. doi: 10.1016/s0006-3223(02)01907-8. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Morrow AL. Effects of acute and chronic ethanol exposure on spatial cognitive processing and hippocampal function in the rat. Hippocampus. 2000;10:122–130. doi: 10.1002/(SICI)1098-1063(2000)10:1<122::AID-HIPO13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Mayo-Smith MF. Pharmacological management of alcohol withdrawal. A meta-analysis and evidence-based practice guideline. American Society of Addiction Medicine Working Group on Pharmacological Management of Alcohol Withdrawal. JAMA. 1997;278:144–151. doi: 10.1001/jama.278.2.144. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Rodd ZA, Bell RL, Lumeng L, Li TK. The alcohol-preferring (P) and high-alcohol-drinking (HAD) rats--animal models of alcoholism. Alcohol. 2014;48:209–215. doi: 10.1016/j.alcohol.2013.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCool BA, Chappell AM. Early social isolation in male Long-Evans rats alters both appetitive and consummatory behaviors expressed during operant ethanol self-administration. Alcohol Clin Exp Res. 2009;33:273–282. doi: 10.1111/j.1530-0277.2008.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ, Mott DD. Functional neuroanatomy of amygdalohippocampal interconnections and their role in learning and memory. J Neurosci Res. 2016 doi: 10.1002/jnr.23709. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Mechanisms of stress in the brain. Nat Neurosci. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFall ME, Murburg MM, Ko GN, Veith RC. Autonomic responses to stress in Vietnam combat veterans with post-traumatic stress disorder. Biol Psychiatry. 1990;27:1165–1175. doi: 10.1016/0006-3223(90)90053-5. [DOI] [PubMed] [Google Scholar]
- McGovern MP, Lambert-Harris C, Acquilano S, Xie H, Alter-man AI, Weiss RD. A cognitive behavioral therapy for co-occurring substance use and posttraumatic stress disorders. Addict Behav. 2009;34:892–897. doi: 10.1016/j.addbeh.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie-Quirk SD, Miczek KA. Social rank and social separation as determinants of alcohol drinking in squirrel monkeys. Psychopharmacology (Berl) 2008;201:137–145. doi: 10.1007/s00213-008-1256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae AL, Saladin ME, Brady KT, Upadhyaya H, Back SE, Timmerman MA. Stress reactivity: biological and subjective responses to the cold pressor and Trier Social stressors. Hum Psychopharmacol. 2006;21:377–385. doi: 10.1002/hup.778. [DOI] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B. Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry. 1995;38:174–179. doi: 10.1016/0006-3223(94)00238-X. [DOI] [PubMed] [Google Scholar]
- Meyer EM, Long V, Fanselow MS, Spigelman I. Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcohol Clin Exp Res. 2013;37:566–574. doi: 10.1111/acer.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miczek KA, Yap JJ, Covington HE., 3rd Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther. 2008;120:102–128. doi: 10.1016/j.pharmthera.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rosenbaum BL, Simon NM. Neuroscience of fear extinction: implications for assessment and treatment of fear-based and anxiety related disorders. Behav Res Ther. 2014;62:17–23. doi: 10.1016/j.brat.2014.08.006. [DOI] [PubMed] [Google Scholar]
- Mills K. Treatment of comorbid substance dependence and posttraumatic stress disorder. JAMA. 2013;310:482–483. doi: 10.1001/jama.2013.8269. [DOI] [PubMed] [Google Scholar]
- Molander A, Vengeliene V, Heilig M, Wurst W, Deussing JM, Spanagel R. Brain-specific inactivation of the Crhr1 gene inhibits post-dependent and stress-induced alcohol intake, but does not affect relapse-like drinking. Neuropsychopharmacology. 2012;37:1047–1056. doi: 10.1038/npp.2011.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnelly EP, Ciraulo DA, Knapp C, LoCastro J, Sepulveda I. Quetiapine for treatment of alcohol dependence. J Clin Psychopharmacol. 2004;24:532–535. doi: 10.1097/01.jcp.0000138763.23482.2a. [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE. Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci. 1979;2:113–168. doi: 10.1146/annurev.ne.02.030179.000553. [DOI] [PubMed] [Google Scholar]
- Morey RA, Haswell CC, Hooper SR, De Bellis MD. Amygdala, hippocampus, and ventral medial prefrontal cortex volumes differ in maltreated youth with and without chronic posttraumatic stress disorder. Neuropsychopharmacology. 2016;41:791–801. doi: 10.1038/npp.2015.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morilak DA, Barrera G, Echevarria DJ, Garcia AS, Hernandez A, Ma S, Petre CO. Role of brain norepinephrine in the behavioral response to stress. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1214–1224. doi: 10.1016/j.pnpbp.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Philippi CL, Wolf RC, Baskaya MK, Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol Psychiatry. 2015;77:276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulholland PJ. K(Ca)2 channels: novel therapeutic targets for treating alcohol withdrawal and escalation of alcohol consumption. Alcohol. 2012;46:309–315. doi: 10.1016/j.alcohol.2011.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller CA, Geisel O, Banas R, Heinz A. Current pharmacological treatment approaches for alcohol dependence. Expert Opin Pharmacother. 2014;15:471–481. doi: 10.1517/14656566.2014.876008. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, Lumeng L, Li TK. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behav Genet. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Muzyk AJ, Fowler JA, Norwood DK, Chilipko A. Role of alpha2-agonists in the treatment of acute alcohol withdrawal. Ann Pharmacother. 2011;45:649–657. doi: 10.1345/aph.1P575. [DOI] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol Psychiatry. 2012;17:132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myrick H, Anton RF. Treatment of alcohol withdrawal. Alcohol Health Res World. 1998;22:38–43. [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najavits LM, Hien D. Helping vulnerable populations: a comprehensive review of the treatment outcome literature on substance use disorder and PTSD. J Clin Psychol. 2013;69:433–479. doi: 10.1002/jclp.21980. [DOI] [PubMed] [Google Scholar]
- Nawijn L, van Zuiden M, Frijling JL, Koch SB, Veltman DJ, Olff M. Reward functioning in PTSD: a systematic review exploring the mechanisms underlying anhedonia. Neurosci Biobehav Rev. 2015;51:189–204. doi: 10.1016/j.neubiorev.2015.01.019. [DOI] [PubMed] [Google Scholar]
- Nelson CB, Heath AC, Kessler RC. Temporal progression of alcohol dependence symptoms in the U.S. household population: results from the National Comorbidity Survey. J Consult Clin Psychol. 1998;66:474–483. doi: 10.1037//0022-006x.66.3.474. [DOI] [PubMed] [Google Scholar]
- Nikolova YS, Knodt AR, Radtke SR, Hariri AR. Divergent responses of the amygdala and ventral striatum predict stress-related problem drinking in young adults: possible differential markers of affective and impulsive pathways of risk for alcohol use disorder. Mol Psychiatry. 2016;21:348–356. doi: 10.1038/mp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H, Matsuki N. Ethanol enhances reactivated fear memories. Neuropsychopharmacology. 2008;33:2912–2921. doi: 10.1038/npp.2008.13. [DOI] [PubMed] [Google Scholar]
- Noori HR, Helinski S, Spanagel R. Cluster and meta-analyses on factors influencing stress-induced alcohol drinking and relapse in rodents. Addict Biol. 2014;19:225–232. doi: 10.1111/adb.12125. [DOI] [PubMed] [Google Scholar]
- Norman SB, Myers US, Wilkins KC, Goldsmith AA, Hristova V, Huang Z, McCullough KC, Robinson SK. Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology. 2012;62:542–551. doi: 10.1016/j.neuropharm.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KJ, Seiden JA, Klickstein JA, Han X, Hwa LS, DeBold JF, Miczek KA. Social stress and escalated drug self-administration in mice I. Alcohol and corticosterone. Psychopharmacology. 2015;232:991–1001. doi: 10.1007/s00213-014-3733-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt DJ. The psychobiology of posttraumatic stress disorder. J Clin Psychiatry. 2000;61(Suppl. 5):24–29. discussion 30–32. [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, Albrecht DS, Yoder KK, Kareken DA. Beer flavor provokes striatal dopamine release in male drinkers: mediation by family history of alcoholism. Neuropsychopharmacology. 2013;38:1617–1624. doi: 10.1038/npp.2013.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlin BG, Dzemidzic M, Tran SM, Soeurt CM, O'Connor SJ, Yoder KK, Kareken DA. Beer self-administration provokes lateralized nucleus accumbens dopamine release in male heavy drinkers. Psychopharmacology (Berl) 2015;232:861–870. doi: 10.1007/s00213-014-3720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty DC, Chitty KM, Saddiqui S, Bennett MR, Lagopoulos J. A systematic review and meta-analysis of magnetic resonance imaging measurement of structural volumes in posttraumatic stress disorder. Psychiatry Res. 2015;232:1–33. doi: 10.1016/j.pscychresns.2015.01.002. [DOI] [PubMed] [Google Scholar]
- O'Donnell T, Hegadoren KM, Coupland NC. Noradrenergic mechanisms in the pathophysiology of post-traumatic stress disorder. Neuropsychobiology. 2004;50:273–283. doi: 10.1159/000080952. [DOI] [PubMed] [Google Scholar]
- Olds ME, Fobes JL. The central basis of motivation: intracranial self-stimulation studies. Annu Rev Psychol. 1981;32:523–574. doi: 10.1146/annurev.ps.32.020181.002515. [DOI] [PubMed] [Google Scholar]
- Oler JA, Birn RM, Patriat R, Fox AS, Shelton SE, Burghy CA, Stodola DE, Essex MJ, Davidson RJ, Kalin NH. Evidence for coordinated functional activity within the extended amygdala of non-human and human primates. Neuroimage. 2012;61:1059–1066. doi: 10.1016/j.neuroimage.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M, Langeland W, Draijer N, Gersons BP. Gender differences in posttraumatic stress disorder. Psychol Bull. 2007;133:183–204. doi: 10.1037/0033-2909.133.2.183. [DOI] [PubMed] [Google Scholar]
- Orr SP, Lasko NB, Metzger LJ, Pitman RK. Physiologic responses to non-startling tones in Vietnam veterans with post-traumatic stress disorder. Psychiatry Res. 1997a;73:103–107. doi: 10.1016/s0165-1781(97)00110-8. [DOI] [PubMed] [Google Scholar]
- Orr SP, Solomon Z, Peri T, Pitman RK, Shalev AY. Physiologic responses to loud tones in Israeli veterans of the 1973 Yom Kippur War. Biol Psychiatry. 1997b;41:319–326. doi: 10.1016/s0006-3223(95)00671-0. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Pitman RK. Psychophysiology of post-traumatic stress disorder. Psychiatr Clin North Am. 2002;25:271–293. doi: 10.1016/s0193-953x(01)00007-7. [DOI] [PubMed] [Google Scholar]
- Otis JM, Werner CT, Mueller D. Noradrenergic regulation of fear and drug-associated memory reconsolidation. Neuropsychopharmacology. 2015;40:793–803. doi: 10.1038/npp.2014.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peskind ER, Bonner LT, Hoff DJ, Raskind MA. Prazosin reduces trauma-related nightmares in older men with chronic post-traumatic stress disorder. J Geriatr Psychiatry Neurol. 2003;16:165–171. doi: 10.1177/0891988703256050. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16:279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Ralevski E, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid post-traumatic stress disorder. Biol Psychiatry. 2006;60:777–783. doi: 10.1016/j.biopsych.2006.03.074. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Ralevski E, Desai N, Trevisan L, Gueorguieva R, Rounsaville B, Krystal JH. Noradrenergic vs serotonergic antidepressant with or without naltrexone for veterans with PTSD and comorbid alcohol dependence. Neuropsychopharmacology. 2012;37:996–1004. doi: 10.1038/npp.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrakis IL, Desai N, Gueorguieva R, Arias A, O'Brien E, Jane JS, Sevarino K, Southwick S, Ralevski E. Prazosin for veterans with posttraumatic stress disorder and comorbid alcohol dependence: a clinical trial. Alcohol Clin Exp Res. 2016;40:178–186. doi: 10.1111/acer.12926. [DOI] [PubMed] [Google Scholar]
- Pettinati HM. The use of selective serotonin reuptake inhibitors in treating alcoholic subtypes. J Clin Psychiatry. 2001;62(Suppl. 20):26–31. [PubMed] [Google Scholar]
- Pettinati HM. Antidepressant treatment of co-occurring depression and alcohol dependence. Biol Psychiatry. 2004;56:785–792. doi: 10.1016/j.biopsych.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips LJ, McGorry PD, Garner B, Thompson KN, Pantelis C, Wood SJ, Berger G. Stress, the hippocampus and the hypothalamic-pituitary-adrenal axis: implications for the development of psychotic disorders. Aust N Z J Psychiatry. 2006;40:725–741. doi: 10.1080/j.1440-1614.2006.01877.x. [DOI] [PubMed] [Google Scholar]
- Pibiri F, Nelson M, Guidotti A, Costa E, Pinna G. Decreased corticolimbic allopregnanolone expression during social isolation enhances contextual fear: a model relevant for posttraumatic stress disorder. Proc Natl Acad Sci U S A. 2008;105:5567–5572. doi: 10.1073/pnas.0801853105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Psychiatric comorbidity of full and partial posttraumatic stress disorder among older adults in the United States: results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Am J Geriatr Psychiatry. 2012;20:380–390. doi: 10.1097/JGP.0b013e31820d92e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzak RH, Gallezot JD, Ding YS, Henry S, Potenza MN, Southwick SM, Krystal JH, Carson RE, Neumeister A. Association of posttraumatic stress disorder with reduced in vivo norepinephrine transporter availability in the locus coeruleus. JAMA Psychiat. 2013;70:1199–1205. doi: 10.1001/jamapsychiatry.2013.399. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pietrzak RH, Huang Y, Corsi-Travali S, Zheng MQ, Lin SF, Henry S, Potenza MN, Piomelli D, Carson RE, Neumeister A. Cannabinoid type 1 receptor availability in the amygdala mediates threat processing in trauma survivors. Neuropsychopharmacology. 2014a;39:2519–2528. doi: 10.1038/npp.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Pietrzak RH, Naganawa M, Huang Y, Corsi-Travali S, Zheng MQ, Stein MB, Henry S, Lim K, Ropchan J, Lin SF, Carson RE, Neumeister A. Association of in vivo kappa-opioid receptor availability and the transdiagnostic dimensional expression of trauma-related psychopathology. JAMA Psychiat. 2014b;71:1262–1270. doi: 10.1001/jamapsychiatry.2014.1221. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP. Twenty-four hour urinary cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biol Psychiatry. 1990;27:245–247. doi: 10.1016/0006-3223(90)90654-k. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Orr SP, Forgue DF, Altman B, de Jong JB, Herz LR. Psychophysiologic responses to combat imagery of Vietnam veterans with posttraumatic stress disorder versus other anxiety disorders. J Abnorm Psychol. 1990;99:49–54. doi: 10.1037//0021-843x.99.1.49. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP. Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry. 2002;51:189–192. doi: 10.1016/s0006-3223(01)01279-3. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW, Milad MR, Liberzon I. Biological studies of post-traumatic stress disorder. Nat Rev Neurosci. 2012;13:769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter DM, Bell CC. The use of clonidine in post-traumatic stress disorder. J Natl Med Assoc. 1999;91:475–477. [PMC free article] [PubMed] [Google Scholar]
- Quinones-Laracuente K, Hernandez-Rodriguez MY, Bravo-Rivera C, Melendez RI, Quirk GJ. The effect of repeated exposure to ethanol on pre-existing fear memories in rats. Psychopharmacology (Berl) 2015;232:3615–3622. doi: 10.1007/s00213-015-4016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchand R, Schell TL, Karney BR, Osilla KC, Burns RM, Caldarone LB. Disparate prevalence estimates of PTSD among service members who served in Iraq and Afghanistan: possible explanations. J Trauma Stress. 2010;23:59–68. doi: 10.1002/jts.20486. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Troster K, Thomas RG, McFall MM. Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shofer J, O'Connell J, Taylor F, Gross C, Rohde K, McFall ME. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928–934. doi: 10.1016/j.biopsych.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Ratajczak P, Wozniak A, Nowakowska E. Animal models of schizophrenia: developmental preparation in rats. Acta Neurobiol Exp. 2013;73:472–484. doi: 10.55782/ane-2013-1953. [DOI] [PubMed] [Google Scholar]
- Rau V, Fanselow MS. Exposure to a stressor produces a long lasting enhancement of fear learning in rats. Stress. 2009;12:125–133. doi: 10.1080/10253890802137320. [DOI] [PubMed] [Google Scholar]
- Rau AR, Chappell AM, Butler TR, Ariwodola OJ, Weiner JL. Increased basolateral amygdala pyramidal cell excitability may contribute to the anxiogenic phenotype induced by chronic early-life stress. J Neurosci. 2015;35:9730–9740. doi: 10.1523/JNEUROSCI.0384-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Resnick HS, Yehuda R, Acierno R. Acute post-rape plasma cortisol, alcohol use, and PTSD symptom profile among recent rape victims. Ann N Y Acad Sci. 1997;821:433–436. doi: 10.1111/j.1749-6632.1997.tb48298.x. [DOI] [PubMed] [Google Scholar]
- Resnik J, Paz R. Fear generalization in the primate amygdala. Nat Neurosci. 2015;18:188–190. doi: 10.1038/nn.3900. [DOI] [PubMed] [Google Scholar]
- Reznikov R, Binko M, Nobrega JN, Hamani C. Deep brain stimulation in animal models of fear, anxiety, and posttraumatic stress disorder. Neuropsychopharmacology. 2016;41:2810–2817. doi: 10.1038/npp.2016.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson HN, Lee SY, O'Dell LE, Koob GF, Rivier CL. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur J Neurosci. 2008;28:1641–1653. doi: 10.1111/j.1460-9568.2008.06455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggs DS, Rukstalis M, Volpicelli JR, Kalmanson D, Foa EB. Demographic and social adjustment characteristics of patients with comorbid posttraumatic stress disorder and alcohol dependence: potential pitfalls to PTSD treatment. Addict Behav. 2003;28:1717–1730. doi: 10.1016/j.addbeh.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Rivier C. Role of hypothalamic corticotropin-releasing factor in mediating alcohol-induced activation of the rat hypothalamic-pituitary-adrenal axis. Front Neuroendocrinol. 2014;35:221–233. doi: 10.1016/j.yfrne.2013.10.005. [DOI] [PubMed] [Google Scholar]
- Roberts NP, Roberts PA, Jones N, Bisson JI. Psychological interventions for post-traumatic stress disorder and comorbid substance use disorder: a systematic review and meta-analysis. Clin Psychol Rev. 2015;38:25–38. doi: 10.1016/j.cpr.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Yager LM, Cogan ES, Saunders BT. On the motivational properties of reward cues: individual differences. Neuropharmacology. 2014;76(Pt. B):450–459. doi: 10.1016/j.neuropharm.2013.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas SM, Bujarski S, Babson KA, Dutton CE, Feldner MT. Understanding PTSD comorbidity and suicidal behavior: associations among histories of alcohol dependence, major depressive disorder, and suicidal ideation and attempts. J Anxiety Disord. 2014;28:318–325. doi: 10.1016/j.janxdis.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer U, Robinson S, Fischmeister FP, Konig D, Oppenauer C, Lueger-Schuster B, Moser E, Kryspin-Exner I, Bauer H. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia. 2008;46:2836–2844. doi: 10.1016/j.neuropsychologia.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Saladin ME, Brady KT, Dansky BS, Kilpatrick DG. Understanding comorbidity between PTSD and substance use disorders: two preliminary investigations. Addict Behav. 1995;20:643–655. doi: 10.1016/0306-4603(95)00024-7. [DOI] [PubMed] [Google Scholar]
- Salonen R, Hietala J, Laihinin A, Lehiloinen P, Leino L, Nagren K, Ruotsalainen U, Oikonen V, Tuokkola T, Nanto V. A PET study on the acute effect of ethanol on striatal D2 dopamine receptors with [11C]raclopride in healthy males. Hum Psychopharmacol. 1997;12:145–152. [Google Scholar]
- Samochowiec J, Samochowiec A, Puls I, Bienkowski P, Schott BH. Genetics of alcohol dependence: a review of clinical studies. Neuropsychobiology. 2014;70:77–94. doi: 10.1159/000364826. [DOI] [PubMed] [Google Scholar]
- Sanna E, Talani G, Obili N, Mascia MP, Mostallino MC, Secci PP, Pisu MG, Biggio F, Utzeri C, Olla P, Biggio G, Follesa P. Voluntary ethanol consumption induced by social isolation reverses the increase of alpha(4)/delta GABA(A) receptor gene expression and function in the hippocampus of C57BL/6J Mice. Front Neurosci. 2011;5:15. doi: 10.3389/fnins.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterthwaite TD, Cook PA, Bruce SE, Conway C, Mikkelsen E, Satchell E, Vandekar SN, Durbin T, Shinohara RT, Sheline YI. Dimensional depression severity in women with major depression and post-traumatic stress disorder correlates with fronto-amygdalar hypoconnectivty. Mol Psychiatry. 2016;21:894–902. doi: 10.1038/mp.2015.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaplen KM, Kaun KR. Reward from bugs to bipeds: a comparative approach to understanding how reward circuits function. J Neurogenet. 2016;30:133–148. doi: 10.1080/01677063.2016.1180385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer I, Najavits LM. Clinical challenges in the treatment of patients with posttraumatic stress disorder and substance abuse. Curr Opin Psychiatry. 2007;20:614–618. doi: 10.1097/YCO.0b013e3282f0ffd9. [DOI] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, Shaham Y, Heilig M. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl) 2011;218:111–119. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Ryabinin AE, Giardino WJ, Ciccocioppo R, Heilig M. Stress-related neuropeptides and addictive behaviors: beyond the usual suspects. Neuron. 2012;76:192–208. doi: 10.1016/j.neuron.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenk S, Gorman K, Amit Z. Age-dependent effects of isolation housing on the self-administration of ethanol in laboratory rats. Alcohol. 1990;7:321–326. doi: 10.1016/0741-8329(90)90090-y. [DOI] [PubMed] [Google Scholar]
- Schreiber AL, Lu YL, Baynes BB, Richardson HN, Gilpin NW. Corticotropin-releasing factor in ventromedial prefrontal cortex mediates avoidance of a traumatic stress-paired context. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.05.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Neylan TC, Lenoci MA, Du AT, Weiss DS, Marmar CR, Weiner MW. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry. 2001;50:952–959. doi: 10.1016/s0006-3223(01)01245-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple WE, Goyer PF, McCormick R, Donovan B, Muzic RF, Jr., Rugle L, McCutcheon K, Lewis C, Liebling D, Kowaliw S, Vapenik K, Semple MA, Flener CR, Schulz SC. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry. 2000;63:65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- Seneviratne C, Johnson BA. Advances in medications and tailoring treatment for alcohol use disorder. Alcohol Res. 2015;37:15–28. [PMC free article] [PubMed] [Google Scholar]
- Seo D, Sinha R. The neurobiology of alcohol craving and relapse. Handb Clin Neurol. 2014;125:355–368. doi: 10.1016/B978-0-444-62619-6.00021-5. [DOI] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, Sinha R. Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiat. 2013;70:727–739. doi: 10.1001/jamapsychiatry.2013.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra M, Pisu MG, Littera M, Papi G, Sanna E, Tuveri F, Usala L, Purdy RH, Biggio G. Social isolation-induced decreases in both the abundance of neuroactive steroids and GABA(A) receptor function in rat brain. J Neurochem. 2000;75:732–740. doi: 10.1046/j.1471-4159.2000.0750732.x. [DOI] [PubMed] [Google Scholar]
- Serra M, Mostallino MC, Talani G, Pisu MG, Carta M, Mura ML, Floris I, Maciocco E, Sanna E, Biggio G. Social isolation-induced increase in alpha and delta subunit gene expression is associated with a greater efficacy of ethanol on steroidogenesis and GABA receptor function. J Neurochem. 2006;98:122–133. doi: 10.1111/j.1471-4159.2006.03850.x. [DOI] [PubMed] [Google Scholar]
- Shank RP, Gardocki JF, Streeter AJ, Maryanoff BE. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(Suppl. 1):S3–S9. [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Ann N Y Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shively SB, Horkayne-Szakaly I, Jones RV, Kelly JP, Armstrong RC, Perl DP. Characterisation of interface astroglial scarring in the human brain after blast exposure: a post-mortem case series. Lancet Neurol. 2016;15:944–953. doi: 10.1016/S1474-4422(16)30057-6. [DOI] [PubMed] [Google Scholar]
- Shorter D, Hsieh J, Kosten TR. Pharmacologic management of comorbid post-traumatic stress disorder and addictions. Am J Addict. 2015;24:705–712. doi: 10.1111/ajad.12306. [DOI] [PubMed] [Google Scholar]
- Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin, for alcohol dependence. Alcohol Clin Exp Res. 2009;33:255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Malte CA, Dietel B, Tell D, Pocock I, Lyons R, Varon D, Raskind M, Saxon AJ. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2015;39:808–817. doi: 10.1111/acer.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. How does stress lead to risk of alcohol relapse? Alcohol Res. 2012;34:432–440. [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Fox HC, Hong KA, Bergquist K, Bhagwagar Z, Siedlarz KM. Enhanced negative emotion and alcohol craving, and altered physiological responses following stress and cue exposure in alcohol dependent individuals. Neuropsychopharmacology. 2009;34:1198–1208. doi: 10.1038/npp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R, Lacadie CM, Constable RT, Seo D. Dynamic neural activity during stress signals resilient coping. Proc Natl Acad Sci U S A. 2016;113:8837–8842. doi: 10.1073/pnas.1600965113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly MJ, Chappell AE, Carter E, Weiner JL. Adolescent social isolation increases anxiety-like behavior and ethanol intake and impairs fear extinction in adulthood: possible role of disrupted noradrenergic signaling. Neuropharmacology. 2015;97:149–159. doi: 10.1016/j.neuropharm.2015.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ME. Bilateral hippocampal volume reduction in adults with post-traumatic stress disorder: a meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Rosenheck R, Petrakis I. Pharmacological treatment of comorbid PTSD and substance use disorder: recent progress. Addict Behav. 2014;39:428–433. doi: 10.1016/j.addbeh.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon SD, Gerrity ET, Muff AM. Efficacy of treatments for posttraumatic stress disorder. An empirical review. JAMA. 1992;268:633–638. [PubMed] [Google Scholar]
- Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiatry. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Krystal JH, Morgan CA, Johnson D, Nagy LM, Nicolaou A, Heninger GR, Charney DS. Abnormal noradrenergic function in posttraumatic stress disorder. Arch Gen Psychiatry. 1993;50:266–274. doi: 10.1001/archpsyc.1993.01820160036003. [DOI] [PubMed] [Google Scholar]
- Spielberg JM, McGlinchey RE, Milberg WP, Salat DH. Brain network disturbance related to posttraumatic stress and traumatic brain injury in veterans. Biol Psychiatry. 2015;78:210–216. doi: 10.1016/j.biopsych.2015.02.013. [DOI] [PubMed] [Google Scholar]
- St Jacques PL, Botzung A, Miles A, Rubin DC. Functional neuroimaging of emotionally intense autobiographical memories in post-traumatic stress disorder. J Psychiatr Res. 2011;45:630–637. doi: 10.1016/j.jpsychires.2010.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starcevic A, Dimitrijevic I, Aksic M, Stijak L, Radonjic V, Aleksic D, Filipovic B. Brain changes in patients with posttraumatic stress disorder and associated alcoholism: MRI based study. Psychiatr Danub. 2015;27:78–83. [PubMed] [Google Scholar]
- Stephens DN, Brown G, Duka T, Ripley TL. Impaired fear conditioning but enhanced seizure sensitivity in rats given repeated experience of withdrawal from alcohol. Eur J Neurosci. 2001;14:2023–2031. doi: 10.1046/j.0953-816x.2001.01824.x. [DOI] [PubMed] [Google Scholar]
- Steuwe C, Daniels JK, Frewen PA, Densmore M, Pannasch S, Beblo T, Reiss J, Lanius RA. Effect of direct eye contact in PTSD related to interpersonal trauma: an fMRI study of activation of an innate alarm system. Soc Cogn Affect Neurosci. 2014;9:88–97. doi: 10.1093/scan/nss105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steuwe C, Daniels JK, Frewen PA, Densmore M, Theberge J, Lanius RA. Effect of direct eye contact in women with PTSD related to interpersonal trauma: psychophysiological interaction analysis of connectivity of an innate alarm system. Psychiatry Res. 2015;232:162–167. doi: 10.1016/j.pscychresns.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Stockhorst U, Antov MI. Modulation of fear extinction by stress, stress hormones and estradiol: a review. Front Behav Neurosci. 2015;9:359. doi: 10.3389/fnbeh.2015.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Witter MP, Lein ES, Moser EI. Functional organization of the hippocampal longitudinal axis. Nat Rev Neurosci. 2014;15:655–669. doi: 10.1038/nrn3785. [DOI] [PubMed] [Google Scholar]
- Strawn JR, Geracioti TD., Jr. Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety. 2008;25:260–271. doi: 10.1002/da.20292. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Knodt AR, Radtke SR, Hariri AR. A neural biomarker of psychological vulnerability to future life stress. Neuron. 2015;85:505–511. doi: 10.1016/j.neuron.2014.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabakoff B, Hoffman PL. The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol Biochem Behav. 2013;113:20–37. doi: 10.1016/j.pbb.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talani G, Licheri V, Masala N, Follesa P, Mostallino MC, Biggio G, Sanna E. Increased voluntary ethanol consumption and changes in hippocampal synaptic plasticity in isolated C57BL/6J mice. Neurochem Res. 2014;39:997–1004. doi: 10.1007/s11064-013-1216-8. [DOI] [PubMed] [Google Scholar]
- Talani G, Biggio F, Licheri V, Locci V, Biggio G, Sanna E. Isolation rearing reduces neuronal excitability in dentate gyrus granule cells of adolescent C57BL/6J mice: role of GABAergic tonic currents and neurosteroids. Front Cell Neurosci. 2016;10:158. doi: 10.3389/fncel.2016.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor FB, Lowe K, Thompson C, McFall MM, Peskind ER, Kanter ED, Allison N, Williams J, Martin P, Raskind MA. Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry. 2006;59:577–581. doi: 10.1016/j.biopsych.2005.09.023. [DOI] [PubMed] [Google Scholar]
- Thomas JL, Wilk JE, Riviere LA, McGurk D, Castro CA, Hoge CW. Prevalence of mental health problems and functional impairment among active component and National Guard soldiers 3 and 12 months following combat in Iraq. Arch Gen Psychiatry. 2010;67:614–623. doi: 10.1001/archgenpsychiatry.2010.54. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Bacon AK, Randall PK, Brady KT, See RE. An acute psychosocial stressor increases drinking in non-treatment-seeking alcoholics. Psychopharmacology (Berl) 2011;218:19–28. doi: 10.1007/s00213-010-2163-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S, Bacon AK, Sinha R, Uhart M, Adinoff B. Clinical laboratory stressors used to study alcohol-stress relationships. Alcohol Res. 2012;34:459–467. [PMC free article] [PubMed] [Google Scholar]
- Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell Tissue Res. 2013;354:107–118. doi: 10.1007/s00441-013-1636-4. [DOI] [PubMed] [Google Scholar]
- Trimble MD. Post-traumatic stress disorder: history of a concept. In: Figley CR, editor. Trauma and its Wake: The Study and Treatment of Post-Traumatic Stress Disorder. Brunner/Mazel; New York, NY: 1985. [Google Scholar]
- Ullman SE, Filipas HH, Townsend SM, Starzynski LL. Correlates of comorbid PTSD and drinking problems among sexual assault survivors. Addict Behav. 2006;31:128–132. doi: 10.1016/j.addbeh.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Ullman SE, Filipas HH, Townsend SM, Starzynski LL. Correlates of comorbid PTSD and drinking problems among sexual assault survivors. Addict Behav. 2006;31:128–132. doi: 10.1016/j.addbeh.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Umhau JC, Schwandt ML, Usala J, Geyer C, Singley E, George DT, Heilig M. Pharmacologically induced alcohol craving in treatment seeking alcoholics correlates with alcoholism severity, but is insensitive to acamprosate. Neuropsychopharmacology. 2011;36:1178–1186. doi: 10.1038/npp.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, Castillo F, Moadel T, O'Malley SS, Krystal JH, Abi-Dargham A. Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(1)(1)C]raclopride. Biol Psychiatry. 2010;68:689–696. doi: 10.1016/j.biopsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiva G, Ducrocq F, Jezequel K, Averland B, Lestavel P, Brunet A, Marmar CR. Immediate treatment with propranolol decreases posttraumatic stress disorder two months after trauma. Biol Psychiatry. 2003;54:947–949. doi: 10.1016/s0006-3223(03)00412-8. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Zorrilla EP, Roberts AJ, Koob GF. Antagonism of corticotropin-releasing factor attenuates the enhanced responsiveness to stress observed during protracted ethanol abstinence. Alcohol. 2003;29:55–60. doi: 10.1016/s0741-8329(03)00020-x. [DOI] [PubMed] [Google Scholar]
- Vendruscolo LF, Barbier E, Schlosburg JE, Misra KK, Whitfield TW, Jr., Logrip ML, Rivier C, Repunte-Canonigo V, Zorrilla EP, Sanna PP, Heilig M, Koob GF. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J Neurosci. 2012;32:7563–7571. doi: 10.1523/JNEUROSCI.0069-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo LF, Estey D, Goodell V, Macshane LG, Logrip ML, Schlosburg JE, McGinn MA, Zamora-Martinez ER, Belanoff JK, Hunt HJ, Sanna PP, George O, Koob GF, Edwards S, Mason BJ. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J Clin Invest. 2015;125:3193–3197. doi: 10.1172/JCI79828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal G, Canive JM, Calais LA, Toney G, Smith AK. Duloxetine in military posttraumatic stress disorder. Psychopharmacol Bull. 2010;43:26–34. [PubMed] [Google Scholar]
- Vogel S, Klumpers F, Krugers HJ, Fang Z, Oplaat KT, Oitzl MS, Joels M, Fernandez G. Blocking the mineralocorticoid receptor in humans prevents the stress-induced enhancement of centromedial amygdala connectivity with the dorsal striatum. Neuropsychopharmacology. 2015;40:947–956. doi: 10.1038/npp.2014.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6 J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147:719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcohol Clin Exp Res. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Telang F, Baler R. Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain's control circuit. Bioessays. 2010;32:748–755. doi: 10.1002/bies.201000042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic advances from the brain disease model of addiction. N Engl J Med. 2016;374:363–371. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahbeh H, Oken BS. Salivary cortisol lower in posttraumatic stress disorder. J Trauma Stress. 2013;26:241–248. doi: 10.1002/jts.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walderhaug E, Kasserman S, Aikins D, Vojvoda D, Nishimura C, Neumeister A. Effects of duloxetine in treatment-refractory men with posttraumatic stress disorder. Pharmacopsychiatry. 2010;43:45–49. doi: 10.1055/s-0029-1237694. [DOI] [PubMed] [Google Scholar]
- Walker BM, Rasmussen DD, Raskind MA, Koob GF. Alpha1-noradrenergic receptor antagonism blocks dependence-induced increases in responding for ethanol. Alcohol. 2008;42:91–97. doi: 10.1016/j.alcohol.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsteeker JI, Bains JS. A synaptocentric view of the neuroendocrine response to stress. Eur J Neurosci. 2010;32:2011–2021. doi: 10.1111/j.1460-9568.2010.07513.x. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology. 1997;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74:e541–e550. doi: 10.4088/JCP.12r08225. [DOI] [PubMed] [Google Scholar]
- Wei XY, Yang JY, Dong YX, Wu CF. Anxiolytic-like effects of oleamide in group-housed and socially isolated mice. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1189–1195. doi: 10.1016/j.pnpbp.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Weinshenker D, Schroeder JP. There and back again: a tale of norepinephrine and drug addiction. Neuropsychopharmacology. 2007;32:1433–1451. doi: 10.1038/sj.npp.1301263. [DOI] [PubMed] [Google Scholar]
- Weiss SJ. Neurobiological alterations associated with traumatic stress. Perspect Psychiatr Care. 2007;43:114–122. doi: 10.1111/j.1744-6163.2007.00120.x. [DOI] [PubMed] [Google Scholar]
- Whitaker AM, Gilpin NW. Blunted hypothalamo-pituitary adrenal axis response to predator odor predicts high stress reactivity. Physiol Behav. 2015;147:16–22. doi: 10.1016/j.physbeh.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker LR, Degoulet M, Morikawa H. Social deprivation enhances VTA synaptic plasticity and drug-induced contextual learning. Neuron. 2013;77:335–345. doi: 10.1016/j.neuron.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker AM, Farooq MA, Edwards S, Gilpin NW. Post-traumatic stress avoidance is attenuated by corticosterone and associated with brain levels of steroid receptor co-activator-1 in rats. Stress. 2016;19:69–77. doi: 10.3109/10253890.2015.1094689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilk JE, Bliese PD, Kim PY, Thomas JL, McGurk D, Hoge CW. Relationship of combat experiences to alcohol misuse among U.S. soldiers returning from the Iraq war. Drug Alcohol Depend. 2010;108:115–121. doi: 10.1016/j.drugalcdep.2009.12.003. [DOI] [PubMed] [Google Scholar]
- Williams TM, Davies SJ, Taylor LG, Daglish MR, Hammers A, Brooks DJ, Nutt DJ, Lingford-Hughes A. Brain opioid receptor binding in early abstinence from alcohol dependence and relationship to craving: an [11C]diprenorphine PET study. Eur Neuropsychopharmacol. 2009;19:740–748. doi: 10.1016/j.euroneuro.2009.06.007. [DOI] [PubMed] [Google Scholar]
- Wingo AP, Ressler KJ, Bradley B. Resilience characteristics mitigate tendency for harmful alcohol and illicit drug use in adults with a history of childhood abuse: a cross-sectional study of 2024 inner-city men and women. J Psychiatr Res. 2014;51:93–99. doi: 10.1016/j.jpsychires.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wobrock T, Falkai P, Schneider-Axmann T, Frommann N, Wolwer W, Gaebel W. Effects of abstinence on brain morphology in alcoholism: a MRI study. Eur Arch Psychiatry Clin Neurosci. 2009;259:143–150. doi: 10.1007/s00406-008-0846-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RC, Herringa RJ. Prefrontal-amygdala dysregulation to threat in pediatric posttraumatic stress disorder. Neuropsychopharmacology. 2016;41:822–831. doi: 10.1038/npp.2015.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffgramm J. Free choice ethanol intake of laboratory rats under different social conditions. Psychopharmacology (Berl) 1990;101:233–239. doi: 10.1007/BF02244132. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Kaloupek DG, Streeter CC, Kimble MO, Reiss AL, Eliez S, Wald LL, Renshaw PF, Frederick BB, Lane B, Sheikh JI, Stegman WK, Kutter CJ, Stewart LP, Prestel RS, Arsenault NJ. Hippocampal volume, PTSD, and alcoholism in combat veterans. Am J Psychiatry. 2006;163:674–681. doi: 10.1176/ajp.2006.163.4.674. [DOI] [PubMed] [Google Scholar]
- Wrase J, Schlagenhauf F, Kienast T, Wustenberg T, Bermpohl F, Kahnt T, Beck A, Strohle A, Juckel G, Knutson B, Heinz A. Dysfunction of reward processing correlates with alcohol craving in detoxified alcoholics. Neuroimage. 2007;35:787–794. doi: 10.1016/j.neuroimage.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Wrase J, Makris N, Braus DF, Mann K, Smolka MN, Kennedy DN, Caviness VS, Hodge SM, Tang L, Albaugh M, Ziegler DA, Davis OC, Kissling C, Schumann G, Breiter HC, Heinz A. Amygdala volume associated with alcohol abuse relapse and craving. Am J Psychiatry. 2008;165:1179–1184. doi: 10.1176/appi.ajp.2008.07121877. [DOI] [PubMed] [Google Scholar]
- Yeh MS, Mari JJ, Costa MC, Andreoli SB, Bressan RA, Mello MF. A double-blind randomized controlled trial to study the efficacy of topiramate in a civilian sample of PTSD. CNS Neurosci Ther. 2011;17:305–310. doi: 10.1111/j.1755-5949.2010.00188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Southwick SM, Nussbaum G, Wahby V, Giller EL, Jr., Mason JW. Low urinary cortisol excretion in patients with posttraumatic stress disorder. J Nerv Ment Dis. 1990;178:366–369. doi: 10.1097/00005053-199006000-00004. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Kahana B, Schmeidler J, Southwick SM, Wilson S, Giller EL. Impact of cumulative lifetime trauma and recent stress on current posttraumatic stress disorder symptoms in holocaust survivors. Am J Psychiatry. 1995;152:1815–1818. doi: 10.1176/ajp.152.12.1815. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Siever LJ, Teicher MH, Levengood RA, Gerber DK, Schmeidler J, Yang RK. Plasma norepinephrine and 3-methoxy-4-hydroxyphenylglycol concentrations and severity of depression in combat posttraumatic stress disorder and major depressive disorder. Biol Psychiatry. 1998;44:56–63. doi: 10.1016/s0006-3223(98)80007-3. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Kareken DA, Seyoum RA, O'Connor SJ, Wang C, Zheng QH, Mock B, Morris ED. Dopamine D(2) receptor availability is associated with subjective responses to alcohol. Alcohol Clin Exp Res. 2005;29:965–970. doi: 10.1097/01.alc.0000171041.32716.42. [DOI] [PubMed] [Google Scholar]
- Yoder KK, Constantinescu CC, Kareken DA, Normandin MD, Cheng TE, O'Connor SJ, Morris ED. Heterogeneous effects of alcohol on dopamine release in the striatum: a PET study. Alcohol Clin Exp Res. 2007;31:965–973. doi: 10.1111/j.1530-0277.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- Yoon S, Kim JE, Hwang J, Kang I, Jeon S, Jamie Im J, Kim BR, Lee S, Kim GH, Rhim H, Lim SM, Lyoo IK. Recovery from posttraumatic stress requires dynamic and sequential shifts in amygdalar connectivities. Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.136. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Konstantopoulos JK, Weiner JL, Jones SR. Enduring increases in anxiety-like behavior and rapid nucleus accumbens dopamine signaling in socially isolated rats. Eur J Neurosci. 2013;37:1022–1031. doi: 10.1111/ejn.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorgason JT, Calipari ES, Ferris MJ, Karkhanis AN, Fordahl SC, Weiner JL, Jones SR. Social isolation rearing increases dopamine uptake and psychostimulant potency in the striatum. Neuropharmacology. 2016;101:471–479. doi: 10.1016/j.neuropharm.2015.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zafar HM, Pare WP, Tejani-Butt SM. Effect of acute or repeated stress on behavior and brain norepinephrine system in Wistar-Kyoto (WKY) rats. Brain Res Bull. 1997;44:289–295. doi: 10.1016/s0361-9230(97)00140-8. [DOI] [PubMed] [Google Scholar]
- Zhang X, Wang B, Jin J, An S, Zeng Q, Duan Y, Yang L, Ma J, Cao X. Early deprivation reduced anxiety and enhanced memory in adult male rats. Brain Res Bull. 2014;108:44–50. doi: 10.1016/j.brainresbull.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Zindel LR, Kranzler HR. Pharmacotherapy of alcohol use disorders: seventy-five years of progress. J Stud Alcohol Drugs Suppl. 2014;75(Suppl. 17):79–88. doi: 10.15288/jsads.2014.s17.79. [DOI] [PMC free article] [PubMed] [Google Scholar]