Abstract
Converting C–H bonds directly into carbon-carbon and carbon-heteroatom bonds can significantly improve step-economy in synthesis by providing alternative disconnections to traditional functional group manipulations. In this context, directed C–H activation reactions have been extensively explored for regioselective functionalization1-5. Though applicability can be severely curtailed by distance from the directing group and the shape of the molecule, a number of approaches have been developed to overcome this limitation6-12. For instance, recognition of the distal and geometric relationship between an existing functional group and multiple C–H bonds has recently been exploited to achieve meta-selective C–H activation by use of a covalently attached U-shaped template13-17. However, stoichiometric installation of the template is not feasible in the absence of an appropriate functional group handle. Here we report the design of a catalytic, bifunctional template that binds heterocyclic substrate via reversible coordination instead of covalent linkage, allowing remote site-selective C–H olefination of heterocycles. The two metal centers coordinated to this template play different roles; anchoring substrates to the proximity of catalyst and cleaving the remote C–H bonds respectively. Using this strategy, we demonstrate remote site-selective C–H olefination of heterocyclic substrates which do not have functional group handles for covalently attaching templates.
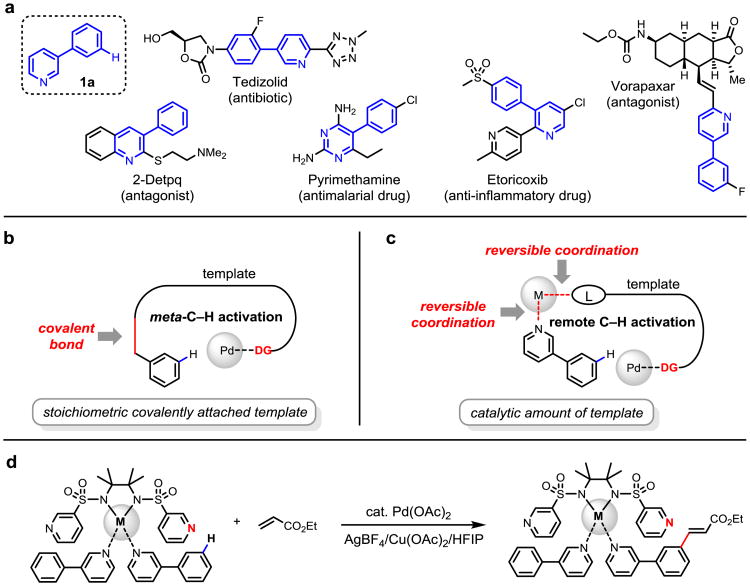
We recently developed U-shaped templates that accommodate a macrocyclic cyclophane transition state13, thereby allowing the activation of meta-C–H bonds (Fig. 1). Despite substantial improvements in the scope and efficiency of this approach, the need for an appropriate functional group handle to covalently attach the stoichiometric template is a limitation. A large number of medicinally important heterocycles are not compatible with our template approach because there are no methods to covalently tether the template to the heterocycle. A prominent example is 3-phenylpyridine motif 1a which is found in more than 6,600 bioactive compounds including a number of pharmaceutical agents according to the PubChem database (Fig. 1a). Coordination of a metal catalyst with the pyridyl nitrogen in these heterocycles typically prohibits transition metal catalysts from reaching distal C–H bonds in these substrates. We wondered whether such reliable non-productive coordination can be harnessed to assemble pre-transition state for C–H activation in supramolecular fashion. A reversible hydrogen bond has recently been employed to anchor an iridium catalyst with appropriate distance and geometry to significantly improve the meta-selectivity in the C–H borylation of benzamides from 66:34 to 96:4 (m:p ratio)18. The high reactivity of the Ir-catalyzed C–H borylation in the absence of directing effect was crucial for this success. Thus far, the exploitation of the hydrogen bonding approach to achieve Pd-catalyzed remote meta-selective C–H activation has not been successful due to lack of reactivity.
Figure 1. Design of a cooperative bimetallic approach for remote site-selective C–H activation.
a, Model substrate 1a and structurally related pharmaceutical agents. b, Previously developed covalently attached U-shaped template strategy for meta-C-H activation. c, The bimetallic strategy for remote site-selective C–H activation. d, Remote site-selective C–H olefination. DG, directing group; M, metal ion; L, coordinating moieties. HFIP: hexafluoroisopropanol.
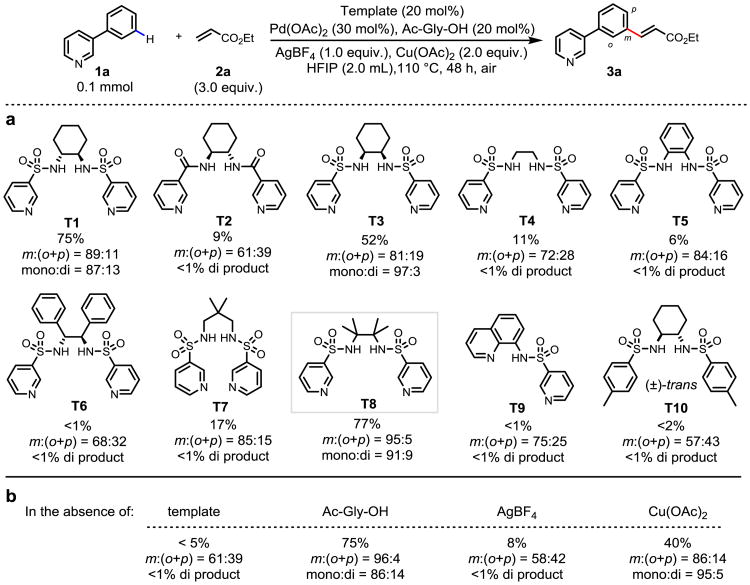
To achieve both the reactivity and meta-selectivity observed using the covalent template approach13, we envision the covalent linkage could be replaced by a reversible metal coordination as shown in Figure 1b. In essence, the often undesired coordination of heterocycles with metal centers is utilized to anchor the substrates to the template reversibly. Following this key design principle, a bifunctional template coordinated with two metal centers19-22 simultaneously is devised to play dual roles: (A) the bis-amide backbone chelates with a metal center that can recruit the substrate via binding to the heterocycles; (B) directing groups on the side arm will direct the palladium catalyst to the specific remote C–H bonds (Fig. 1d). We chose a bis-amide T1 as the backbone for attaching the U-shaped templates due to their ability to chelate with Pd(II) or Cu(II) centers23-24 which will be present in our reaction. Since we have recently demonstrated that a properly positioned C-3 pyridyl group could also function as a U-shaped template in a meta-selective iodination reaction13,17, we choose C-3 pyridine over the nitrile directing group to enhance the coordination with the small amount of Pd(II) catalyst. With these considerations in mind, we synthesized various templates based on the bis-sulfonamide and bis-amide scaffolds containing the directing C-3 pyridine group as pendant side arms. The pyridyl nitrogen atom is positioned at C-3 instead of C-2 to avoid the formation of bisdendate chelation with the sulfonamide or amide. We evaluated these templates using olefination of 1a as the model reaction (Fig. 2). Under the optimized conditions (see Supplementary Information for optimization), olefination of 1a in the presence of 20 mol% template and 30 mol% Pd(OAc)2 gave olefinated products in 75% total yield. Importantly, synthetically useful remote meta-selectivity was obtained (meta:(ortho+para) = 89:11). Replacement of the sulfonamide moieties in T1 with carboxamides (T2) resulted in dramatic decrease in both yield (9%) and m:(o+p) ratio (61:39). Switching from a trans to cis-cyclohexane backbone (T3) also reduced the yield significantly (52%). Although the less rigid acyclic ethylenediamine backbones were not effective (T4-T7), template T8 derived from the sterically hindered 2,3-dimethyl-2,3-butanediamine was found to be the most effective, affording the olefination products in 77% yield and excellent meta-selectivity (95:5). These experimental results indicate the importance of the conformational constrain of the backbone which is consistent with the notion that reactivity and selectivity in remote C–H activation is based on precise recognition of distance and geometry. Notably, mono-selectivity obtained using this bimetallic approach is significantly higher than the previously reported meta-C–H olefinations using covalently attached templates13-15. Presumably, the mono-olefinated product is significantly less reactive due to steric hindrance as the bimetallic assembly is highly sensitive to conformational changes. Replacing T8 with a simplified scaffold (T9) led to loss of reactivity. Template T10 bearing tolyl substituents instead of the C-3 pyridyl groups on the side arm gave 2% of the mono-olefinated products with poor meta-selectivity (m:(o+p) = 57:43), confirming the remote directing effect of this bifunctional template.
Figure 2. Discovery of a template that enables site-selective remote C–H activation.
a, Template evaluation. b, Control experiments with T8 as template. The yield (percentage under each structure) of the olefinated products, the meta:(ortho + para) ratio of mono-olefinated products and ratio of mono- and di-olefinated products (mono:di) were determined by 1H NMR analysis of the unpurified reaction mixture using CH2Br2 as the internal standard (assisted with GC-MS analysis), the variance is estimated to be within 5%. Ac-Gly-OH: N-acetyl-glycine.
To elucidate the roles of the key components of this reaction, a number of control experiments have been carried out (Fig. 2b). In the absence of T8, the olefination reaction gave less than 5% of the mono-olefinated products with low m:(o+p) ratio (61:39) indicating the essential directing effect from the template. Notably, this remote directing effect controls the site-selectivity based on distance and geometry, which is fundamentally different from previously observed C-2 or C-4 selectivity of pyridines that are governed by electronic and steric effects amplified by the presence of a Lewis acidic metal25,26. The nearly complete loss of reactivity and meta-selectivity by removing the silver salts from the reaction is intriguing, although the formation of a Pd-Ag heterodimer in the transition state could potentially account for this phenomenon27. Notably, other silver salts are less effective (see Supplementary Information). We anticipate both Cu(II) and Pd(II) could coordinate with the bis-amide scaffold and impact this reaction. Indeed, both the yield and meta-selectivity decreased significantly in the absence of Cu(OAc)2. Removal of N-acetyl-protected glycine (Ac-Gly-OH) ligand from the standard conditions only decreased the mono-selectivity, presumably due the steric effect.
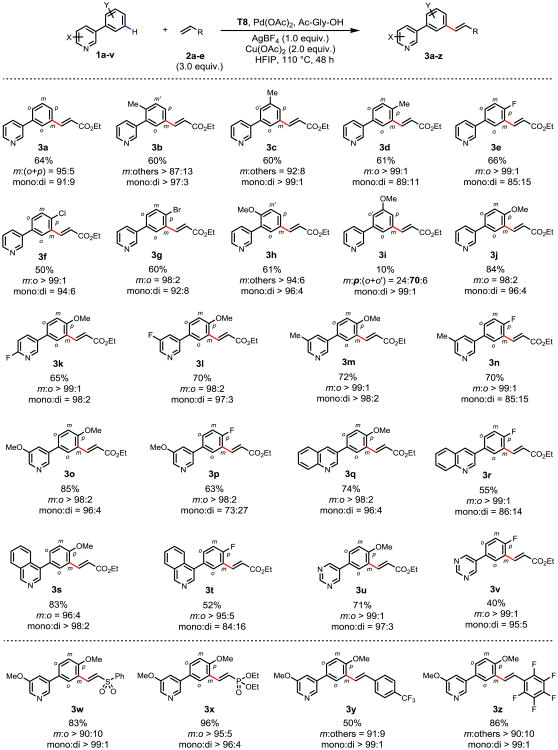
Next, we applied template T8 to the remote meta-selective C-H olefination of 3-phenylpyridine derivatives (Fig. 3). The mono-meta-olefinated 3-phenylpyridine 3a was obtained in 65% isolated yield. Methyl substituents on the ortho, meta and para positions of the phenyl ring were tolerated providing similar yields and mono-selectivity (3b-d). Substrates containing fluorine, chlorine and bromine substituents on the para position of the phenyl ring are olefinated to give the meta-product in 50-66% yields with excellent meta-selectivity and good mono-selectivity (3e-g). An ortho-methoxy group was also tolerated, providing 61% yield and excellent meta-selectivity and mono-selectivity (3h). A meta-methoxy group reduced the yield of isolated meta-olefination product to 10% with a complete loss of meta-selectivity (3i). In general, the meta-substitution in substrates interfered with the optimum conformational orientation due to steric effects, consistent with the observed excellent mono-selectivity in contrast to previous meta-selective C-H activation reactions13-15. Para-methoxy substituted substrate gave excellent yield, meta-selectivity and mono-selectivity. Various substituents on the pyridine ring were also examined. Both C-2 and C-3 fluorine on the pyridine ring were well tolerated, providing 65% and 70% yield of the desired products respectively with excellent meta- mono-selectivity (3k, 3l). Electron-donating methyl and methoxy groups on the 3-position of the pyridine ring afforded the desired meta-products in 63-85% yields with excellent meta-selectivity (3m-p). To further explore the scope of this methodology, substrates containing quinoline and isoquinoline were also olefinated to give the meta-products in moderate to good yields (52-83%) and good selectivity (3q-t). 5-phenylpyrimidine substrates, another pharmaceutically important heterocycle family28,29, was also tolerated (3u, 3v). In expanding the scope of the olefin coupling partners, α,β-unsaturated sulfone and phosphonate are reactive, giving good to excellent yields with excellent meta- and mono-selectivity (3w, 3x). Meta-olefination with electron-deficient styrene was also demonstrated (3y, 3z). To investigate whether the template can be recovered, olefination of 1o was also performed on 1 gram scale to give 3o in 70% yield (m:o = 99:1, mono:di = 98:2). The template is readily recovered in 96% by chromatography (see Supplementary Information).
Figure 3. Remote site-selective C–H olefination of heterocycle-containing substrates using a catalytic template.
The percentages under each structure indicate isolated yields of the mono-olefinated meta product. The meta-selectivity of mono-olefinated products and ratio of mono- and di-olefinated products were determined by 1H NMR analysis of the unpurified reaction mixture (assisted with GC-MS analysis), the variance is estimated to be within 5%. Reaction conditions (unless otherwise noted): 1a-v (0.1 mmol), T8 (20 mol%), Pd(OAc)2 (30 mol%), Ac-Gly-OH (20 mol%), AgBF4 (1.0 equiv.), Cu(OAc)2 (2.0 equiv.), 2a-e (3.0 equiv.), HFIP (2.0 mL), 110 °C, 48 h. For 3h-m, 3o, 3q, 3s, 3u, 3w and 3z: T8 (15 mol%), Pd(OAc)2 (20 mol%), Ac-Gly-OH (5 mol%). For 3p, 3t, 3v and 3y: 130 °C. For 3h-j and 3x: 72 h. For 3k, 3q and 3s: 24 h.
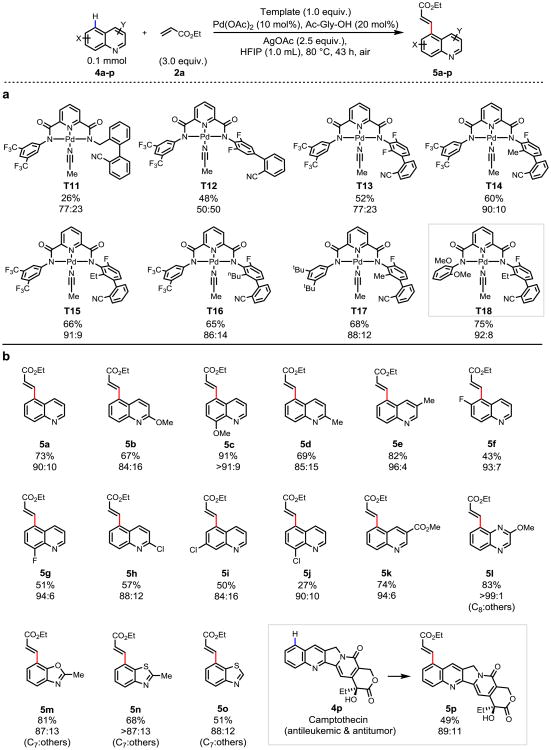
To test the feasibility of extending the design principle of this bimetallic catalysis to different classes of heterocycles, we embarked on site-selective C–H olefination of quinolines which are ubiquitous in drug molecules and natural products28,29. Not surprisingly, the use of T8 gave only trace amount of olefinated products (<2%). Achieving site-selectivity with quinoline, a drastically different molecular scaffold, will require optimization of the templates for precise recognition based on distance and geometry. Considering the broad scope of the covalent nitrile templates for remote C–H activation and ease of synthesis13-16, we prepared various nitrile-based templates capable of anchoring the first metal through tridendate coordination. While the use of catalytic amount of free templates was not effective, pre-assembled complexes T11-18 directed remote C–H activation of quinoline with various degrees of efficiency (Fig. 4). Thus, in the presence of catalytic amount of Pd(OAc)2 and mono-protected amino acid ligand (Ac-Gly-OH), olefination of 4a with the optimized complex T18 gave the olefination product 5a in 75% yield with excellent site-selectivity (C5:others = 92:8). Notably, the complex T18 was recovered in 96% yield by a simple work-up with 4-dimethylaminopyridine (DMAP) followed by the treatment with methanesulfonic acid in acetonitrile (see Supplementary Information), thus rendering T18 as a recyclable reagent for remote C–H activation. A range of quinolines containing both electron-donating (5b-e) and - withdrawing groups (5f-i, 5k) gave the desired olefination product in good yields and selectivity. The 8-chloro retarded the reaction by weakening the coordination of the nitrogen of quinoline ring (5j). This recyclable non-covalent template T18 is also compatible with other heterocycles including quinoxaline, benzoxazole and benzothiazole (5l-o). The potential utility of this template approach is also demonstrated in a late-stage modification of an antileukemic and antitumor alkaloid (+)-camptothecin30 (5p).
Figure 4. Remote site-selective C–H olefination of heterocycles using a non-covalent template.
a, Template evaluation. b, Substrate scope. The percentages under each structure indicate the yields of the isolated olefinated products (unless otherwise noted). The ratio of the major product to other isomers (C5:others, unless otherwise noted) were determined by 1H NMR analysis of the unpurified reaction mixture (assisted with GC-MS analysis), the variance is estimated to be within 5%. For template evaluation (a): The yields were determined by 1H NMR analysis of the unpurified reaction mixture using 1,3,5-trimethoxybenzene as the internal standard. Reaction conditions for template evaluation (a): 4a (0.1 mmol), template (1.0 equiv.), Pd(OAc)2 (15 mol%), Ac-Gly-OH (20 mol%), AgOAc (2.0 equiv.), 2a (3.0 equiv.), HFIP (1.0 mL), 100 °C, 12 h. Reaction conditions for substrate scope (b), (unless otherwise noted): substrates (0.1 mmol), T18 (1.0 equiv.), Pd(OAc)2 (10 mol%), Ac-Gly-OH (20 mol%), AgOAc (2.5 equiv.), 2a (3.0 equiv.), HFIP (1.0 mL), 80 °C, 43 h. For 5b, 5d, 5h and 5p: T14 was used as template. For 5k and 5l: 22 h. For 5m: 20 h. For 5p: 48 h, AgOAc (5.0 equiv.).
Supplementary Material
Acknowledgments
We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS, 1R01 GM102265) for their financial support. We thank Ito Foundation for International Education Exchange (predoctoral fellowship to K.T.).
Footnotes
Data Availability: The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files. Metrical parameters for the structure of templates T1, T8 and products 5l-n (see Supplementary Information) are available free of charge from the Cambridge Crystallographic Data Centre under reference number CCDC 1484666 for T1, CCDC 1484667 for T8, CCDC 1519494 for 5l, CCDC 1519493 for 5m, and CCDC 1519495 for 5n.
Supplementary Information is available in the online version of the paper.
Author Contributions Z.Z. developed the catalytic bisdentate template for 3-phenylpyridine and other heterocycles. K.T. developed the non-covalent tridentate template for quinoline and other heterocycles. J.-Q.Y. conceived this concept and prepared this manuscript with feedback from Z.Z. and K.T.
The authors declare no competing financial interests.
Readers are welcome to comment on the online version of this article.
References
- 1.Snieckus V. Directed ortho metalation Tertiary amide and O-carbamate directors in synthetic strategies for polysubstituted aromatics. Chem Rev. 1990;90:879–933. [Google Scholar]
- 2.Flemming JP, Berry MB, Brown JM. Sequential ortho-lithiations; the sulfoxide group as a relay to enable meta-substitution. Org Biomol Chem. 2008;6:1215–1221. doi: 10.1039/b716954j. [DOI] [PubMed] [Google Scholar]
- 3.Daugulis O, Do HQ, Shabashov D. Palladium- and copper-catalyzed arylation of carbon–hydrogen bonds. Acc Chem Res. 2009;42:1074–1086. doi: 10.1021/ar9000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyons TW, Sanford MS. Palladium-catalyzed ligand-directed C–H functionalization reactions. Chem Rev. 2010;110:1147–1169. doi: 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colby DA, Bergman RG, Ellman JA. Rhodium-catalyzed C–C bond formation via heteroatom-directed C–H bond activation. Chem Rev. 2010;110:624–655. doi: 10.1021/cr900005n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breslow R. Biomimetic control of chemical selectivity. Acc Chem Res. 1980;13:170–177. [Google Scholar]
- 7.Das S, Incarvito CD, Crabtree RH, Brudvig GW. Molecular recognition in the selective oxygenation of saturated C–H bonds by a dimanganese catalyst. Science. 2006;312:1941–1943. doi: 10.1126/science.1127899. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Martinez AJ, Kennedy AR, Mulvey RE, O'Hara CT. Directed ortho-meta'-and meta-meta'-dimetalations: A template base approach to deprotonation. Science. 2014;346:834–837. doi: 10.1126/science.1259662. [DOI] [PubMed] [Google Scholar]
- 9.Phipps RJ, Gaunt MJ. A meta-selective copper-catalyzed C–H bond arylation. Science. 2009;323:1593–1597. doi: 10.1126/science.1169975. [DOI] [PubMed] [Google Scholar]
- 10.Saidi O, et al. Ruthenium-catalyzed meta sulfonation of 2-phenylpyridines. J Am Chem Soc. 2011;133:19298–19301. doi: 10.1021/ja208286b. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann N, Ackermann L. Meta-selective C–H bond alkylation with secondary alkyl halides. J Am Chem Soc. 2013;135:5877–5884. doi: 10.1021/ja401466y. [DOI] [PubMed] [Google Scholar]
- 12.Li J, et al. N-acyl amino acid ligands for ruthenium(II)-catalyzed meta-C–H tert-alkylation with removable auxiliaries. J Am Chem Soc. 2015;137:13894–13901. doi: 10.1021/jacs.5b08435. [DOI] [PubMed] [Google Scholar]
- 13.Leow D, Li G, Mei TS, Yu JQ. Activation of remote meta-C–H bonds assisted by an end-on template. Nature. 2012;486:518–522. doi: 10.1038/nature11158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee S, Lee H, Tan KL. Meta-selective C–H functionalization using a nitrile-based directing group and cleavable Si-tether. J Am Chem Soc. 2013;135:18778–18781. doi: 10.1021/ja4107034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang RY, Li G, Yu JQ. Conformation-induced remote meta-C–H activation of amines. Nature. 2014;507:215–220. doi: 10.1038/nature12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bera M, Maji A, Sahoo SK, Maiti D. Palladium(II)-catalyzed meta-C–H olefination: constructing multisubstituted arenes through homo-diolefination and sequential hetero-diolefination. Angew Chem Int Ed. 2015;54:8515–8519. doi: 10.1002/anie.201503112. [DOI] [PubMed] [Google Scholar]
- 17.Chu L, et al. Remote meta-C–H activation using a pyridine-based template: achieving site-selectivity via the recognition of distance and geometry. ACS Cent Sci. 2015;1:394–399. doi: 10.1021/acscentsci.5b00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuninobu Y, Ida H, Nishi M, Kanai M. A meta-selective C–H borylation directed by a secondary interaction between ligand and substrate. Nat Chem. 2015;7:712–717. doi: 10.1038/nchem.2322. [DOI] [PubMed] [Google Scholar]
- 19.Shibasaki M, Yoshikawa N. Lanthanide complexes in multifunctional asymmetric catalysis. Chem Rev. 2002;102:2187–2210. doi: 10.1021/cr010297z. [DOI] [PubMed] [Google Scholar]
- 20.van den Beuken EK, Feringa BL. Bimetallic catalysis by late transition metal complexes. Tetrahedron. 1998;54:12985–13011. [Google Scholar]
- 21.Konsler RG, Karl J, Jacobsen EN. Cooperative asymmetric catalysis with dimeric Salen complexes. J Am Chem Soc. 1998;120:10780–10781. [Google Scholar]
- 22.Park J, Hong S. Cooperative bimetallic catalysis in asymmetric transformations. Chem Soc Rev. 2012;41:6931–6943. doi: 10.1039/c2cs35129c. [DOI] [PubMed] [Google Scholar]
- 23.Alvarado RJ, et al. Structural insights into the coordination and extraction of Pb(II) by disulfonamide ligands derived from o-phenylenediamine. Inorg Chem. 2005;44:7951–7959. doi: 10.1021/ic051103r. [DOI] [PubMed] [Google Scholar]
- 24.Cheng PH, Cheng HY, Lin CC, Peng SM. Oxidations of N,N′-disubstituted o-phenylenediamine in the presence of metal ions and the crystal structure of N,N′-dimethylbenzimidazolium perchlorate, pyridine-bis(o-benzosemiquinonediimine)cobalt(III) chloride and bis(pyridine)(N,N′-bistoluene-p-sulfonyl-o-phenylenediiminato)-copper(II) Inorg Chim Acta. 1990;169:19–21. [Google Scholar]
- 25.Nakao Y, Kanyiva KS, Hiyama T. A strategy for C–H activation of pyridines: direct C-2 selective alkenylation of pyridines by nickel/Lewis acid catalysis. J Am Chem Soc. 2008;130:2448–2449. doi: 10.1021/ja710766j. [DOI] [PubMed] [Google Scholar]
- 26.Tsai CC, et al. Bimetallic nickel aluminum mediated para-selective alkenylation of pyridine: direct observation of η2,η1-pyridine Ni(0)-Al(III) intermediates prior to C–H bond activation. J Am Chem Soc. 2010;132:11887–11889. doi: 10.1021/ja1061246. [DOI] [PubMed] [Google Scholar]
- 27.Yang YF, et al. Palladium-catalyzed meta-selective C–H bond activation with a nitrile-containing template: computational study on mechanism and origins of selectivity. J Am Chem Soc. 2014;136:344–355. doi: 10.1021/ja410485g. [DOI] [PubMed] [Google Scholar]
- 28.Vitaku E, Smith DT, Njardarson JT. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S FDA approved pharmaceuticals. J Med Chem. 2014;57:10257–10274. doi: 10.1021/jm501100b. [DOI] [PubMed] [Google Scholar]
- 29.Taylor RD, MacCoss M, Lawson ADG. Rings in drugs. J Med Chem. 2014;57:5845–5859. doi: 10.1021/jm4017625. [DOI] [PubMed] [Google Scholar]
- 30.Stork G, Schultz AG. The total synthesis of dl-camptothecin. J Am Chem Soc. 1971;93:4074–4075. doi: 10.1021/ja00745a056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.