Abstract
Background and Purpose
Over the last decade there has been a substantial increase in efforts to better understand how targeted physical activity and exercise interventions can be used to minimize secondary consequences arising from neurological damage in both adult and pediatric populations. This paper overviews contemporary research that address mediators of functional and neuroplastic adaptations to physical activity and exercise, and seeks to highlight the important role that physical therapists can play to increase participation and improve well-being in adults and children with neurological disorders. We further highlight potential strategies to foster translation of evidence-based findings for use by clinicians and consumers.
Summary of Key Points
Engagement in physical activity can serve as a powerful promoter of health and well-being in adults and youth with neurologic disease, and may have the potential to alter the course of disease processes. Physical therapists can play a key role in promoting fitness and wellness by encouraging active living, providing early diagnosis of disease and prescribing targeted activity interventions to improve fitness and participation and helping individuals overcome personal and environmental barriers to an active lifestyle.
Recommendations for Clinical Practice
Physical therapists must adopt a model of rehabilitation that emphasizes secondary prevention in adults and youth with neurological diseases. Physical therapists can play a unique role in developing forward-thinking approaches in using innovative health and wellness strategies to promote positive changes in activity and exercise behaviors.
Health promotion and secondary prevention in adult neurologic populations
Dr. Robert Butler, founding director of the National Institute on Aging, National Institutes of Health has said “if exercise could be packaged into a pill it would be the single most widely prescribed and beneficial medicine in the nation”. Over the past decade, there has been substantial research aimed at understanding the beneficial effects of physical activity and exercise in the general population, as well as in adults and children with a range of diseases and disorders. In adults with chronic diseases such as diabetes and heart disease exercise interventions have similar, if not potentially better, outcomes compared to drug interventions.1 Aerobic exercise and multi-modal exercise interventions are well known for their effect on cardiorespiratory fitness, muscle strength, depression and cognition2. There is also increasing acceptance of the potential that exercise may have in achieving disease modification in neurological conditions due to its positive influence on brain function and health3,4. In adults with neurological conditions, exercise and physical activity have been shown to have the potential both to decrease risk of disease onset,5–7 and to improve motor and cognitive function and quality of life.8–16 The concept of “living well” with chronic diseases has never been as possible as it is today.
Neuroplasticity and disease progression
Early intervention is key to successful implementation of lifestyle strategies such as exercise. An important component of this implementation requires therapists to understand the natural course of diseases. Figure 117 illustrates the clinical progression of Huntington's disease (HD), a neurodegenerative disease predominantly affecting the medium spiny neurons of the striatum and resulting in a triad of mobility, cognitive, and behavioral symptoms. The neurodegenerative pathway is similar to many other diseases, however it has the advantage of being a genetic disease with 100% penetrance, meaning that individuals can know with complete certainty if they have the gene that causes HD. Disease progression starts at the earliest presymptomatic stage, where overt clinical signs are not yet present but degenerative changes in the brain are evident up to 10 years before age of onset18, progressing to prodromal stage, where neuronal loss occurs but overt clinical signs may be subtle, followed by early, moderate and advanced clinical stages, where significant impairments and activity limitations emerge and worsen over time.
Figure 1.
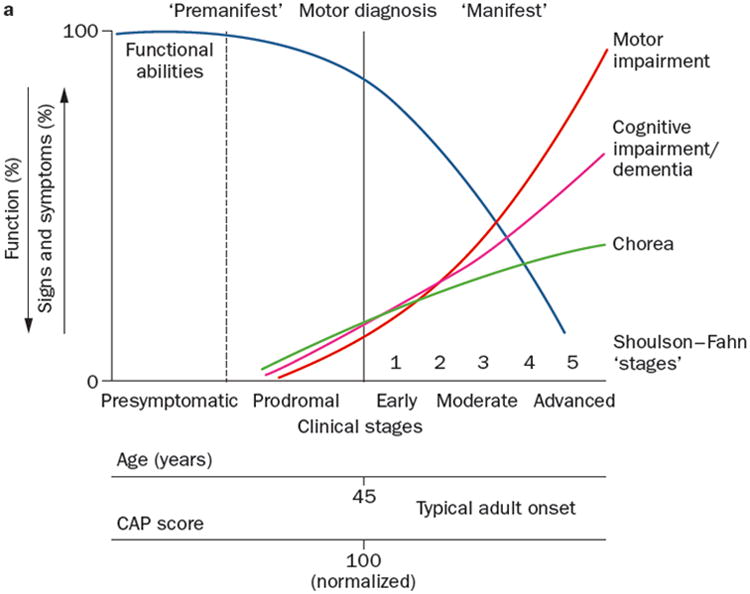
Progression of symptoms of Huntington's disease across the clinical stages of presyptomatic, prodromal (just prior to motor diagnosis), and early, moderate and advanced disease stages. Ross et al. Nat Rev Neurol. 2014;10(4):204-16
While primary prevention of neurodegenerative diseases is most urgently needed, delaying disease onset, even by several years, or slowing disease progression can significantly impact functional abilities and quality of life. Intervention in the earliest stages of the disease, either at or prior to diagnosis, may have the greatest potential to slow progression of neurodegenerative diseases such as Parkinson disease (PD), multiple sclerosis (MS) and Huntington disease (HD).
Physical activity
Physical activity refers to movements of the body that use energy, and can encompass a range of everyday activities including walking, gardening, and climbing stairs, but also includes specific forms of sport or exercise, such as playing soccer, running on a treadmill, or doing Pilates or yoga. There is increasing evidence that physical activity has the potential to prevent or delay the onset of certain neurodegenerative diseases such as Alzheimer's disease (AD), PD and dementia.5–7 Physical activity levels may help to drive compensatory neural networks in individuals with neurological diseases and disorders,19–21 that may in turn compensate for the failing brain and alter disease trajectories. Physical activity has been shown to reduce the risk of developing Alzheimer's disease by up to 45%.6 Furthermore, a study of over 1,700 elderly individuals reported an incidence of dementia of 13.0 per 1000 person-years for those who exercised three or more times per week (≥15 min/session of walking, cycling, swimming, aerobics, strength training, stretching, or other activities) compared with 19.7 per 1,000 person-years for those who exercised fewer than three times per week.22 Similarly in PD, studies have provided preliminary evidence that low levels of physical activity and exercise may contribute to an increased lifetime risk of PD.5,23
Despite the evidence in support of exercise and physical activity in adult neurologic populations, exercise uptake is not consistent11,24,25 and is limited by significant barriers.26–28 These include individual safety and fear of falling, low outcome expectation from exercise, lack of time to exercise, and the location at which exercising takes place.26–28 The American College of Sports Medicine (ACSM) current guidelines recommend the four key components of aerobic, resistance, flexibility and neuromotor for maintaining cardiorespiratory, musculoskeletal and neuromotor fitness in apparently healthy adults.29 The ACSM recommends moderate intensity cardiorespiratory exercise training for at least 30 minutes or more per day on 5 days per week and for a total of 150 minutes, vigorous-intensity cardiorespiratory exercise training for at least 20 minutes per day on 3 days per week (≥75 min/wk), or a combination of moderate and vigorous intensity exercise to achieve a total energy expenditure of 500–1000 MET min per week. Resistance exercises should be performed for each of the major muscle groups 2-3 days per week; neuromotor exercise involving balance, agility, coordination, and flexibility exercises of each the major muscle–tendon groups (a total of 60 s per exercise) should be performed 2 days per week. While there are no specific guidelines for individuals with neurologic conditions, the evidence in support of aerobic10,13,15,30–34 and strengthening8,35,36 exercises in these populations suggests that these recommendations can and should be applied to patients with neurological conditions.
Physical therapists must take an active role in implementing strategies to facilitate exercise uptake, ideally from early in the disease process. There has been a substantial number of programs developed in the last 10 years with the aim of increasing levels of physical activity through coaching and behavioral change interventions in individuals with neurological diseases. Programs such as the LIFE program in the UK37, the Blue Prescription for MS in New Zealand38,39, ParkFIT for PD in the Netherlands40,41, and Engage-HD for people with HD in the UK42,43 have used similar models of behavioral change interventions, grounded in theoretical constructs to facilitate uptake and adherence. While these studies are not without their limitations, they provide initial guidance for therapists to begin implementing similar coaching strategies, as well as providing important foundations for future research in this area.
In order for such programs to be widely implemented and translated into clinical practice, detailed information about protocol implementation, therapist training, outcome measures and costs are needed.43 Speelman et al.40 have suggested that specific training for therapists should include education on behavior change theories and concrete and specific examples of exercise goals. Keys to successful physical activity interventions include incorporating personalized programs that consider disease pathophysiology, current stage or movement-system classification,44,45 and the individualized needs and personal preferences of the patient and their family. For a patient with PD, for example, consideration of the stage of disease (e.g. Hoehn and Yahr scale) and motor phenotype (e.g. tremor-predominant, akinetic-rigid, postural-instability with gait-disorder) would affect the intervention focus. Further consideration of work status, home situation and personal preferences for exercise would further shape the intervention strategy. Additional research to understand disease-specific issues, including understanding of comorbidities, impact of cognitive dysfunction and strategies to address lack of motivation and apathy is needed.40
Aerobic and strengthening exercise interventions
In contrast to physical activity interventions, which aim to increase general levels of activity across a range of activity intensities, exercise interventions evaluate programs with a defined prescription of mode, intensity, frequency and duration. Exercise research in dementias16,15, Parkinson disease21, multiple sclerosis10, Alzheimer disease14, mild cognitive impairment46 and Huntington disease12,13 has received considerable attention and yielded positive outcomes in terms of motor symptoms, behavior and quality of life. Importantly, exercise appears to have significant impact on cognitive function as well. In a recent meta-analysis, Groot et al15 reported that aerobic exercise, but not non-aerobic exercise, positively influenced cognitive function, and this effect was independent of the clinical diagnosis (e.g. AD versus non-AD dementia) and the frequency of exercise.
Preliminary research has further indicated that exercise, particularly aerobic exercise, has the potential to drive neuroplasticity changes.47 In PD, for example, studies have suggested that targeted exercise can increase levels of corticomotor excitability,48 weaken the overactive indirect striatal pathway DA-D2R expression levels of brain49 as well as increase levels of brain derived neurotrophic factor.50 Interest in exercise-induced neuroplasticity has largely been driven by animal research. Studies in HD mouse models,6 for example, have demonstrated that sustained wheel running reduced gross anatomical neuropathology associated motor and cognitive decline, and wheel running from an early age delayed the onset of specific motor symptoms.51,52 Continued research to further elucidate the potential mechanisms by which exercise exerts its effects will promote a better understanding of exercise response and inform future trials, which can ultimately promote personalized approaches to management of neurodegenerative diseases.
Intensive motor training
Intensive motor training and targeted, task-specific therapeutic exercise interventions present an exciting, transformative area of research in neurodegenerative diseases.21 This is particularly relevant for those individuals in the early stages of the disease when neuronal dysfunction and other neurobiological abnormalities may still be to some extent reversible.53 Addressing motor impairments in neurodegeneration may provide a long-term beneficial effect in delaying disease progression and maximizing functional abilities over a longer period. Directed motor training paradigms have been successfully implemented in patients with PD.54 Frazzitta et al54–56 conducted a randomized controlled trial to evaluate an inpatient, intensive rehabilitation program in patients with early stage PD, who were also being treated with rasagiline monotherapy. The authors reported a 14% increase in BDNF serum levels, improvements in all secondary outcomes (including Timed Up and Go and 6 minute walk test), and a delay in the need for increasing drug treatment across the course of the disease. Such intensive interventions, provided in the early stages of neurodegenerative diseases, have the potential to be used in combination with disease-modifying drugs, cell replacement therapy or genetic manipulations, when available, to maximize the functional benefits of these interventions by facilitating adaptive neuroplasticity.57,58
A model for secondary prevention in neurological conditions: a paradigm shift
The mounting evidence in support of exercise as a powerful neuroprotective tool in neurological diseases and disorders offers an exciting opportunity for physical therapists to utilize their unique skills to facilitate exercise adherence and uptake throughout the course of a lifespan of an individual with a neurologic disease. With our indepth knowledge of both disease pathophysiology, directed motor training and exercise prescription, physical therapists are uniquely qualified to be in the forefront of delivering exercise and physical activity interventions.
In adult neurologic populations, particularly in neurodegenerative diseases such as PD, HD, and MS, a secondary prevention management strategy is needed to facilitate exercise uptake and adherence from the very earliest time period. Exercise interventions have the potential to delay the onset or slow the progression of disease by specifically targeting neuroprotective mechanisms within the central nervous system, including facilitating synaptogenosis, neurogenesis and neurotransmitter synthesis (e.g. dopamine)21,30,59 (see Figure 2). As such, the traditional role of rehabilitation services to provide an episode of care roughly 6-8 weeks, discharging with a home exercise program and re-evaluating only if there is a significant change or decline in function must be reconsidered.
Figure 2.
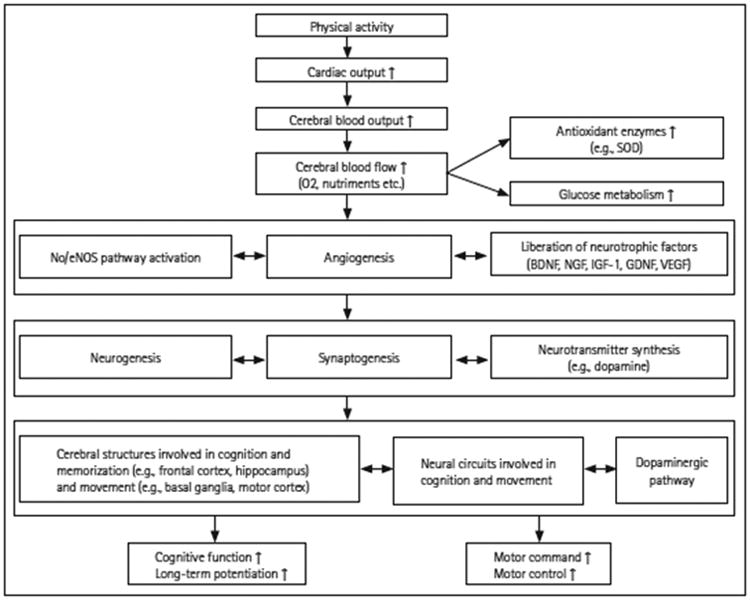
Preventive and neuroprotective mechanisms induced by regular physical exercise on the cognitive and motor functions. BDNF: brain-derived neurotrophic factors, eNOS: endothelial nitric oxide synthases, GDNF: glial cell line-derived neurotrophic factors, IGF-1: insulin-like growth factors, NGF: nerve growth factors, NO: nitric oxide, SOD: superoxide dismutase, VEGF: vascular endothelial growth factor. Reprinted from: Paillard T, Rolland Y, de Souto Barreto P. Protective effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: a narrative review. J Clin Neurol. 2015;11:212-219.
A model of rehabilitation that emphasizes secondary prevention in neurodegenerative diseases, such as that proposed in PD and MS60, is urgently needed. Indeed many therapists already work in such a way intuitively, particularly those who are part of interdisciplinary clinics, however it is not being implemented consistently. Such a model would provide a framework for management over the lifespan of neurodegenerative diseases, starting first with assessment upon diagnosis to establish baseline status and identify key impairments and activity limitations. Therapists would provide ongoing consultation with follow-up visits scheduled regularly, similar to doctor's visits, to facilitate exercise adherence, identify changes in functional abilities and collaborate on setting new goals as needed.60 Therapists may take on a more coaching and advisory role over the course of the disease, importantly incorporating behavioural interventions to facilitate exercise adherence and uptake.
Health promotion and secondary prevention in pediatric neurologic populations
Reduced physical activity levels in youth with neurologic disease (ND) can lead to a cycle of deconditioning, further inactivity, diminished physical function, and reduction in overall health status.61-64 In addition, obesity rates in youth with disabilities are 38% higher compared to age-matched children who are not disabled.65 Overweight and obese youth are also more likely to become obese adults66 and excessive weight gain in young people with ND can increase cardiovascular and metabolic disease risk and lead to a number of secondary conditions, including mobility limitations, fatigue, pain depression, and social isolation.67-75 Because physical activity tracks from childhood and adolescence into adulthood at moderate to high levels,76 young people with ND should be encouraged to develop, engage in, and maintain active living behaviors and limit participation in sedentary behaviors. Efforts to identify the presence of ‘exercise deficit disorder’77 in youth with ND and reduce the onset and progression of early-stage lifestyle disease by creating opportunities to engage in health-producing physical activity should be aggressively promoted to positively impact future health outcomes.
Physical Activity Levels in Youth with ND
Indirect (e.g., surveys, questionnaires) and direct (e.g., StepWatch Activity Monitor (SAM), ActiGraph and RT3 accelerometers, SenseWear Armband, doubly-labelled water) methods of quantifying physical activity have consistently shown that youth with ND are not as physically active as typically-developing peers. Quantitative assessment of physical activity levels and patterns has revealed that children and adolescents with cerebral palsy (CP) and other neuromuscular impairments take fewer daily steps in free-living settings, spend a lower percentage of time being active, engage in a higher percentage of time in low-intensity activity and a lower absolute and percentage of time in moderate-to-vigorous physical activity (MVPA), exhibit a lower relative total energy expenditure, perceive themselves to be less fit, and report a greater number of perceived limitations in the types or amount of physical activities they can perform compared to young people without ND.78-91
A review of electronic databases indicates that children and adolescents with CP are 13% to 53% less active than their peers and physical activity levels are approximately 30% lower compared to existing guidelines.92 In addition, findings from a systematic review demonstrated that in children with CP, increased habitual physical activity is positively associated with higher motor capacity as reflected by Gross Motor Function Classification Scale (GMFCS) levels.93 Results from a limited number of studies have also shown that in youth with CP, (1) fundamental movement skill is positively related to time spent in MVPA, (2) boys are more active than girls, (3) activity levels are higher on weekdays than weekends, and (4) a minimum of six (GMFCS I), five (GMFCS II), and four (GMFCS III) days of activity monitoring is sufficient to acquire reliable step count data, as measured by the SAM. 84-85,87,94
Sedentary Behavior
Sedentary behavior, defined as any waking activity characterized by very low levels of energy expenditure (< 1.5 METS) and performed in a sitting or reclining position,95-96 tracks during childhood and adolescence and into adulthood at moderate levels similar to, or slightly larger than, those observed for physical activity.97 Excessive sedentary behavior, and especially screen-based sedentary activity, is independently associated with adiposity and cardiometabolic disease risk in children and youth.98 No evidence-based guidelines have been established for limiting general sedentary activity, but current recommendations suggest that young people should avoid extended periods (> 2 hours) of screen time.99
A paucity of research exists describing sedentary behavior in youth with ND.64 Analysis of survey and questionnaire data have produced conflicting findings, with one study indicating that adolescents with physical disabilities are two times more likely to watch television and play video and computer games for extended periods on school days80 and another reporting little difference in weekly recreational screen time between youth with CP and typically-developing peers and no association between sedentary activity and gross motor function, age, and activity level.81 In contrast, data from studies employing accelerometry revealed that when compared to able-bodied peers, absolute and relative sedentary time is greater in children with CP and adolescents with CP accumulate more sedentary time and display fewer interruptions in sedentary behaviors.87,100
Health-Related Fitness
Health-related fitness (HRF) is comprised of cardiorespiratory, muscular, morphological, metabolic, and motor components.101 Based on plausible causal pathways, a bidirectional association exists between physical activity and HRF.101 Compared to typically-developing children, clusters of cardiorespiratory (CR), metabolic and biomechanical (MB), and neuromuscular (NM) variables measured in youth with CP (GMFCS I - II) were 25% to 60% below values for age- and sex-matched peers.102 A comprehensive review of published studies indicates that cardiorespiratory endurance, muscle strength, and anaerobic fitness are lower in children with CP (GMFCS I-III) compared to typically-developing youth and responsive to various training regimens featuring different activity modes and dosages, although the extent to which fitness improvements are sustained following training is less clear.103 In an 8-month randomized controlled trial (RCT) of sequential aerobic and anaerobic training in youth with CP (GMFCS I-II), increases of 20% to 38% occurred in aerobic (20%) and anaerobic (25%) capacity, lower-extremity muscle strength (20-23%), and agility (15%), and these gains in HRF were maintained, to some extent, four months after training ended.104
The use of various modes, types, and combinations of activity interventions has led to generally positive effects on HRF in youth with CP.103 In an 8-week RCT, internet-based lifestyle activity intervention for adolescents with CP (GMFCS I-III), a non-significant trend for improvement in physical activity was noted105 and a 6-month physical activity stimulation program consisting of counseling, fitness training, and home-based physiotherapy did not alter physical activity, mobility capacity, walking capacity and functional muscle strength, or fitness in children with CP (GMFCS I-III).106 In contrast, a 6-month lifestyle intervention featuring supervised physical fitness training and counseling sessions was effective in improving cardiopulmonary fitness and body composition in adolescents and young adults with CP (GMFCS I-IV)107 and a 9-week functional circuit training program increased aerobic endurance, walking distance, and ambulation in children with CP (GMFCS I-II).108 Better arm-cranking metabolic economy, higher maximal aerobic power, and increased GMFM scores in youth with spastic diplegia were also detected after 12 weeks of combined aerobic interval and strength training109 and a recent systematic review found that whole-body vibration resulted in better standing function, greater femur bone density, and increased gait speed in children with CP (GMFCS I-IV).110
Data from a trio of studies featuring young and very young children with CP (GMFCS I-IV) have shown that prolonged, moderate-to-intense, land-based treadmill training, performed with or without body support, lasting four to seven weeks, and occurring in either clinic- or home-based settings, produced gains in mobility, gross motor function, and accelerated the onset of walking skills while reducing support needed during walking.111-113 A 10-week RCT featuring underwater treadmill training in youth with CP (GMFCS I-II) also led to faster preferred and maximal walking speeds and more distance covered during a 5-minute overground walk.114
Future Directions
As the use of accelerometers becomes an increasingly common method of quantifying childhood physical activity, it is essential that youth-specific calibration equations and associated cutpoint thresholds for sedentary, light, moderate, and vigorous physical activity are validated against criterion assessments of energy expenditure or activity assessment for the population being evaluated.115-116 This is especially important when using published accelerometer equations and cutpoint values to quantify physical activity behavior in young people with CP, because their increased locomotor energy demands compared to typically-developing youth117-118 may lead to an underestimation of MVPA.93,115 In this regard, recent studies involving ambulatory youth with CP (GMFCS I – III) have demonstrated good concurrent validity between accelerometry data and indirect calorimetry while performing standardized physical activities of varying intensity119 and shown that GMFCS-specific intensity-based cutpoint thresholds produce more accurate assessments of MVPA levels compared to previously published cutpoint values.120
One limitation of using accelerometry to document physical activity levels is that accelerometer-derived energy expenditure values obtained during non step-based activity (e.g., cycling, weight-training, swimming) may be inaccurate.121 Another limitation of accelerometers is their inability to identify the types of activities being performed and the location and context of physical activity participation. One alternative to relying solely on accelerometry to measure physical activity levels is to combine the use of accelerometers with direct observation systems (e.g., SOPLAY, SOPARC, ORSAC)122 or global positioning system devices123 to provide a richer and fuller description of physical activity and enable interventions to be customized to specific domains and related subdomains of childhood activity participation.123
Given the link between fundamental movement skills and physical activity in children with CP,87 efforts should be undertaken to develop motor skill ability and proficiency in rehabilitation and school physical education programs. Longitudinal research examining the influence of age, sex, and GMFCS levels on the natural history of physical activity during childhood and adolescence should also be conducted and greater attention should be focused on measuring physical activity and promoting physical activity participation in preschoolers with ND, especially in light of current trends signaling a higher prevalence of overweight and obesity in children aged 2 to 5 years.66
More studies featuring accelerometry-based monitoring are needed to better characterize habitual sedentary activity of young people with ND, define what is meant by excessive sedentary time, determine the effects of reducing sedentary behaviors on HRF, and describe the physiological and health effects of prolonged sitting. From a measurement perspective, research should be conducted to identify the types of sedentary behavior that children are engaged in and quantify the amount of time spent in sedentary activities, frequency of breaks in daily sedentary behavior, and within- and between-day variability in sedentary behaviors. The impact of substituting light physical activity (e.g., standing124) for sedentary behaviors and the combined influence of reducing sedentary activity and increasing physical activity on HRF in youth with ND is another issue worth exploring.
While cardiorespiratory endurance and muscle strength can be improved in youth with CP, relatively little is known regarding the trainability of anaerobic fitness, a key factor limiting motor ability in youth with this neurological condition.125 Continued emphasis should be placed on documenting the effectiveness of new and innovative exercise interventions on HRF and exploring ways of combining training modes and intervention approaches that are age-appropriate and suitable for children and adolescents with differing levels of gross motor function. In addition, concerted efforts should be made to translate physical activity and exercise interventions which have proven successful in laboratory settings for use by therapists in clinical and community settings. Ongoing evaluation of the clinimetric properties of laboratory- and field-based fitness tests in youth with physical disabilities126 is a high priority that has the potential to lead to standardized testing and evaluation of the impact of activity and exercise on participation in daily life. Further work should also be performed to assess (1) the unique and combined influence of aerobic exercise and strength training on reducing excess body weight and preventing unhealthy changes in body composition, (2) the extent to which HRF tracks from childhood into adolescence and from adolescence into adulthood, and (3) the nature of the dose-response relationship between volume of physical activity and specific HRF components and how this relationship is moderated by factors such as age, sex, and biological maturation.
Summary
Regular engagement in physical activity can serve as a powerful strategy to promote health and well-being in adults and youth with neurologic disease. Accurate and reliable measurement of physical activity and ongoing monitoring, improvement, and maintenance of health-related fitness can help individuals with physical disabilities achieve optimal physical function. Physical therapists must play a key role in promoting fitness and wellness by encouraging active living, providing early diagnosis of disease, and prescribing targeted activity interventions to improve fitness and participation, connecting individuals to community-based activity programs and resources, and helping persons of all ages overcome personal and environmental barriers to a physically-active lifestyle.
Contributor Information
Lori Quinn, Department of Biobehavioral Sciences, Teachers College, Columbia University.
Don Morgan, Department of Health and Human Performance, Middle Tennessee State University.
References
- 1.Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577. doi: 10.1136/bmj.f5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blair SN, Morris JN. Healthy hearts--and the universal benefits of being physically active: physical activity and health. Ann Epidemiol. 2009;19:253–6. doi: 10.1016/j.annepidem.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 3.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci England. 2002:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 4.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:464–72. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Xu Q, Park Y, Huang X, et al. Physical activities and future risk of Parkinson disease. Neurology. 2010;75:341–8. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamer M, Chida Y. Physical activity and risk of neurodegenerative disease: a systematic review of prospective evidence. Psychol Med. 2009;39:3–11. doi: 10.1017/S0033291708003681. [DOI] [PubMed] [Google Scholar]
- 7.Trembath MK, Horton ZA, Tippett L, et al. A retrospective study of the impact of lifestyle on age at onset of Huntington disease. Mov Disord. 2010;25:1444–50. doi: 10.1002/mds.23108. [DOI] [PubMed] [Google Scholar]
- 8.Shulman LM, Katzel LI, Ivey FM, et al. Randomized clinical trial of 3 types of physical exercise for patients with Parkinson disease. JAMA Neurol. 2013;70:183–90. doi: 10.1001/jamaneurol.2013.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodwin V, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: A systematic review and meta-analysis. Mov Disord. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- 10.Latimer-Cheung AE, Pilutti LA, Hicks AL, et al. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: a systematic review to inform guideline development. Arch Phys Med Rehabil. 2013;94:1800–28.e3. doi: 10.1016/j.apmr.2013.04.020. [DOI] [PubMed] [Google Scholar]
- 11.Motl RW, McAuley E, Snook EM. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005;11:459–63. doi: 10.1191/1352458505ms1188oa. [DOI] [PubMed] [Google Scholar]
- 12.Busse M, Quinn L, Debono K, et al. A randomized feasibility study of a 12-week community-based exercise program for people with Huntington's disease. J Neurol Phys Ther. 2013;37:149–58. doi: 10.1097/NPT.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 13.Quinn L, Hamana K, Kelson M, et al. A randomized, controlled trial of a multi-modal exercise intervention in Huntington's disease. Parkinsonism Relat Disord. 2016 doi: 10.1016/j.parkreldis.2016.06.023. in press. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann K, Sobol NA, Frederiksen KS, et al. Moderate-to-High Intensity Physical Exercise in Patients with Alzheimer's Disease: A Randomized Controlled Trial. J Alzheimers Dis. 2015:1–11. doi: 10.3233/JAD-150817. Preprint. [DOI] [PubMed] [Google Scholar]
- 15.Groot C, Hooghiemstra AM, Raijmakers PGHM, et al. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res Rev. 2016;25:13–23. doi: 10.1016/j.arr.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: A meta-analysis. Arch Phys Med Rehabil. 2004;85:1694–704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 17.Ross Ca, Aylward EH, Wild EJ, et al. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat Rev Neurol. 2014;10:204–16. doi: 10.1038/nrneurol.2014.24. [DOI] [PubMed] [Google Scholar]
- 18.Tabrizi SJ, Scahill RI, Durr A, et al. Biological and clinical changes in premanifest and early stage Huntington's disease in the TRACK-HD study: the 12-month longitudinal analysis. Lancet Neurol England. 2011:31–42. doi: 10.1016/S1474-4422(10)70276-3. [DOI] [PubMed] [Google Scholar]
- 19.Nithianantharajah J, Hannan AJ. The neurobiology of brain and cognitive reserve: mental and physical activity as modulators of brain disorders. Prog Neurobiol. 2009;89:369–82. doi: 10.1016/j.pneurobio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Cramer SC, Sur M, Dobkin BH, et al. Harnessing neuroplasticity for clinical applications. Brain. 2011;134:1591–609. doi: 10.1093/brain/awr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petzinger GM, Fisher BE, McEwen S, Beeler Ja, Walsh JP, Jakowec MW. Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson's disease. Lancet Neurol. 2013;12:716–26. doi: 10.1016/S1474-4422(13)70123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larson EB, Wang L, Bowen JD, et al. Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med. 2006;144:73–81. doi: 10.7326/0003-4819-144-2-200601170-00004. [DOI] [PubMed] [Google Scholar]
- 23.Chen H, Zhang SM, Schwarzschild MA, Hernán MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–9. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- 24.Cavanaugh JT, Ellis TD, Earhart GM, Ford MP, Foreman KB, Dibble LE. Capturing ambulatory activity decline in Parkinson's disease. J Neurol Phys Ther. 2012;36:51–7. doi: 10.1097/NPT.0b013e318254ba7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.English C, Healy GN, Coates A, Lewis L, Olds T, Bernhardt J. Sitting and Activity Time in People With Stroke. Phys Ther. 2016;96:193–201. doi: 10.2522/ptj.20140522. [DOI] [PubMed] [Google Scholar]
- 26.Quinn L, Busse M, Khalil H, Richardson S, Rosser A, Morris H. Client and therapist views on exercise programmes for early-mid stage Parkinson's disease and Huntington's disease. Disabil Rehabil. 2010;32:917–28. doi: 10.3109/09638280903362712. [DOI] [PubMed] [Google Scholar]
- 27.Ellis T, Boudreau JK, DeAngelis TR, et al. Barriers to exercise in people with Parkinson disease. Phys Ther. 2013;93:628–36. doi: 10.2522/ptj.20120279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asano M, Duquette P, Andersen R, Lapierre Y, Mayo NE. Exercise barriers and preferences among women and men with multiple sclerosis. Disabil Rehabil. 2013;35:353–61. doi: 10.3109/09638288.2012.742574. [DOI] [PubMed] [Google Scholar]
- 29.Garber CE, Blissmer B, Deschenes MR, et al. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–59. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 30.Paillard T, Rolland Y, de Souto Barreto P. Protective Effects of Physical Exercise in Alzheimer's Disease and Parkinson's Disease: A Narrative Review. J Clin Neurol. 2015;11:212–9. doi: 10.3988/jcn.2015.11.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray DK, Sacheli MA, Eng JJ, Stoessl AJ. The effects of exercise on cognition in Parkinson's disease: a systematic review. Transl Neurodegener. 2014;3:5. doi: 10.1186/2047-9158-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reynolds GO, Otto MW, Ellis TD, Cronin-Golomb A. The Therapeutic Potential of Exercise to Improve Mood, Cognition, and Sleep in Parkinson's Disease. Mov Disord. 2015;31:23–38. doi: 10.1002/mds.26484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonnell MN, Smith AE, Mackintosh SF. Arch Phys Med Rehabil United States: 2011 American Congress of Rehabilitation Medicine. Published by Elsevier Inc; 2011. Aerobic exercise to improve cognitive function in adults with neurological disorders: a systematic review; pp. 1044–52. [DOI] [PubMed] [Google Scholar]
- 34.Hale LA, Smith C, Mulligan H, Treharne GJ. ‘Tell me what you want, what you really really want…’: asking people with multiple sclerosis about enhancing their participation in physical activity. Disabil Rehabil. 2012;34:1887–93. doi: 10.3109/09638288.2012.670037. [DOI] [PubMed] [Google Scholar]
- 35.Prodoehl J, Rafferty MR, David FJ, et al. Two-year exercise program improves physical function in Parkinson's disease: the PRET-PD randomized clinical trial. Neurorehabil Neural Repair. 2015;29:112–22. doi: 10.1177/1545968314539732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dibble LE, Foreman KB, Addison O, Marcus RL, LaStayo PC. Exercise and Medication Effects on Persons With Parkinson Disease Across the Domains of Disability: A Randomized Clinical Trial. J Neurol Phys Ther. 2015;39:85–92. doi: 10.1097/NPT.0000000000000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elsworth C, Winward C, Sackley C, et al. Supported community exercise in people with long-term neurological conditions: a phase II randomized controlled trial. Clin Rehabil. 2011;25:588–98. doi: 10.1177/0269215510392076. [DOI] [PubMed] [Google Scholar]
- 38.Mulligan H, Treharne GJ, Hale LA, Smith C. Combining self-help and professional help to minimize barriers to physical activity in persons with multiple sclerosis: a trial of the ‘Blue Prescription’ approach in New Zealand. J Neurol Phys Ther. 2013;37:51–7. doi: 10.1097/NPT.0b013e318292799e. [DOI] [PubMed] [Google Scholar]
- 39.Hale LA, Mulligan HF, Treharne GJ, Smith CM. The feasibility and short-term benefits of Blue Prescription: a novel intervention to enable physical activity for people with multiple sclerosis. Disabil Rehabil. 2013;35:1213–20. doi: 10.3109/09638288.2012.723787. [DOI] [PubMed] [Google Scholar]
- 40.Speelman A, van Nimwegen M, Bloem B, Munneke M. Evaluation of implementation of the ParkFit program: A multifaceted intervention aimed to promote physical activity in patients with Parkinson's disease. Physiotherapy. 2014;100:134–41. doi: 10.1016/j.physio.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 41.van Nimwegen M, Speelman AD, Overeem S, et al. Promotion of physical activity and fitness in sedentary patients with Parkinson's disease: randomised controlled trial. BMJ. 2013;346:f576. doi: 10.1136/bmj.f576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Busse M, Quinn L, Dawes H, et al. Supporting physical activity engagement in people with Huntington's disease (ENGAGE-HD): study protocol for a randomized controlled feasibility trial. doi: 10.1186/1745-6215-15-487. http://www.trialsjournal.com/content/15/1/487. [DOI] [PMC free article] [PubMed]
- 43.Quinn L, Trubey R, Gobat N, et al. Development and Delivery of a Physical Activity Intervention for People With Huntington Disease: Facilitating Translation to Clinical Practice. J Neurol Phys Ther. 2016;40:1–10. doi: 10.1097/NPT.0000000000000119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheets PL, Sahrmann SA, Norton BJ. Use of movement system diagnoses in the management of patients with neuromuscular conditions: a multiple-patient case report. Phys Ther. 2007:654–69. doi: 10.2522/ptj.20050349. [DOI] [PubMed] [Google Scholar]
- 45.Quinn L, Busse M. Physiotherapy clinical guidelines for Huntington's disease. Neurodegener Dis Manag. 2012;2:21–31. [Google Scholar]
- 46.Baker LD, Frank LL, Foster-Schubert K, et al. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Arch Neurol. 2010;67:71–9. doi: 10.1001/archneurol.2009.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hirsch MA, Iyer SS, Sanjak M. Exercise-induced neuroplasticity in human Parkinson's disease: What is the evidence telling us? Parkinsonism Relat Disord. 2016;22:S78–81. doi: 10.1016/j.parkreldis.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 48.Fisher BE, Wu AD, Salem GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Arch Phys Med Rehabil. 2008;89:1221–9. doi: 10.1016/j.apmr.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fisher BE, Li Q, Nacca A, et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson's disease. Neuroreport. 2013;24:509–14. doi: 10.1097/WNR.0b013e328361dc13. [DOI] [PubMed] [Google Scholar]
- 50.Marusiak J, Żeligowska E, Mencel J, et al. Interval training-induced alleviation of rigidity and hypertonia in patients with Parkinson's disease is accompanied by increased basal serum brain-derived neurotrophic factor. J Rehabil Med. 2015;47:372–5. doi: 10.2340/16501977-1931. [DOI] [PubMed] [Google Scholar]
- 51.Hannan AJ. Environmental enrichment and brain repair: harnessing the therapeutic effects of cognitive stimulation and physical activity to enhance experience-dependent plasticity. Neuropathol Appl Neurobiol. 2014;40:13–25. doi: 10.1111/nan.12102. [DOI] [PubMed] [Google Scholar]
- 52.van Dellen A, Cordery PM, Spires TL, Blakemore C, Hannan AJ. Wheel running from a juvenile age delays onset of specific motor deficits but does not alter protein aggregate density in a mouse model of Huntington's disease. BMC Neurosci. 2008;9:34. doi: 10.1186/1471-2202-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ross CA, Tabrizi SJ. Huntington's disease: from molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10:83–98. doi: 10.1016/S1474-4422(10)70245-3. [DOI] [PubMed] [Google Scholar]
- 54.Frazzitta G, Maestri R, Bertotti G, et al. Intensive rehabilitation treatment in early Parkinson's disease: a randomized pilot study with a 2-year follow-up. Neurorehabil Neural Repair. 2015;29:123–31. doi: 10.1177/1545968314542981. [DOI] [PubMed] [Google Scholar]
- 55.Frazzitta G, Bertotti G, Riboldazzi G, et al. Effectiveness of intensive inpatient rehabilitation treatment on disease progression in parkinsonian patients: a randomized controlled trial with 1-year follow-up. Neurorehabil Neural Repair. 2012;26:144–50. doi: 10.1177/1545968311416990. [DOI] [PubMed] [Google Scholar]
- 56.Frazzitta G, Maestri R, Ghilardi MF, et al. Intensive rehabilitation increases BDNF serum levels in parkinsonian patients: a randomized study. Neurorehabil Neural Repair. 2014;28:163–8. doi: 10.1177/1545968313508474. [DOI] [PubMed] [Google Scholar]
- 57.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–82. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- 58.Warraich Z, Kleim JA. Neural plasticity: the biological substrate for neurorehabilitation. PM R. 2010;2:S208–19. doi: 10.1016/j.pmrj.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Petzinger GM, Holschneider DP, Fisher BE, et al. The Effects of Exercise on Dopamine Neurotransmission in Parkinson's Disease: Targeting Neuroplasticity to Modulate Basal Ganglia Circuitry. Brain Plast. 1:29–39. doi: 10.3233/BPL-150021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellis T, Motl RW, Pt P, Motl RW. Physical activity behavior change in persons with neurologic disorders: overview and examples from Parkinson disease and multiple sclerosis. J Neurol Phys Ther. 2013;37:85–90. doi: 10.1097/NPT.0b013e31829157c0. [DOI] [PubMed] [Google Scholar]
- 61.Durstine JL, Painter P, Franklin BA, Morgan D, Pitetti KH, Roberts SO. Physical activity for the chronically ill and disabled. Sports Med. 2000;30:207–219. doi: 10.2165/00007256-200030030-00005. [DOI] [PubMed] [Google Scholar]
- 62.Damiano DL. Activity, activity, activity: rethinking our physical therapy approach to cerebral palsy. Phys Ther. 2006;86:1534–1540. doi: 10.2522/ptj.20050397. [DOI] [PubMed] [Google Scholar]
- 63.Fowler EC, Kolobe THA, Damiano DL, Thorpe DE, Morgan DW, Brunstrom JE, et al. Promotion of physical fitness and prevention of secondary conditions for children with cerebral palsy: section on pediatrics research summit proceedings. Phys Ther. 2007;87:1495–1510. doi: 10.2522/ptj.20060116. [DOI] [PubMed] [Google Scholar]
- 64.Verschuren O, Darrah J, Novak I, Ketelaar M, Wiart L. Health-enhancing physical activity in children with cerebral palsy: more of the same is not enough. Phys Ther. 2014;94:297–305. doi: 10.2522/ptj.20130214. [DOI] [PubMed] [Google Scholar]
- 65.Wingo BC, Mehta T, Qu P, Vogel LC, Rimmer JH. Exploratory study examining clinical measures of adiposity risk for predicting obesity in adolescents with physical disabilities. Am J Phys Med Rehabil. 2015;94:585–594. doi: 10.1097/PHM.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 66.Morgan D. Right from the start: promotion of health-related physical activity in preschoolers. Kinesiology Review. 2013;2:88–92. [Google Scholar]
- 67.Leech RM, McNaughton SA, Timperio A. The clustering of diet, physical activity and sedentary behavior in children and adolescents: a review. Int J Behav Nutr Phys Act. 2014 doi: 10.1186/1479-5868-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farhat T, Iannotti RJ, Sions-Morton B. Overweight, obesity, youth, and health- risk behaviors. Am J Prev Med. 2010;38:258–267. doi: 10.1016/j.amepre.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Centers for Disease Control and Prevention. Childhood Obesity Facts. [Accessed July 6, 2016]; http://www.cdc.gov/healthyschools/obesity/facts.htm. Updated August 27, 2015.
- 70.Reinehr T, Dobe M, Winkel K, Schaefer A, Hoffmann D. Obesity in disabled children and adolescents. Dtsch Arztebl Int. 2010;107:268–275. doi: 10.3238/arztebl.2010.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rimmer JH, Rowland JL, Yamaki K. Obesity and secondary conditions in adolescents with disabilities: addressing the needs of an underserved population. J Adolesc Health. 2007;41:224–229. doi: 10.1016/j.jadohealth.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 72.Rimmer JH, Wang E, Yamaki K, Davis B. Documenting disparities obesity and disability. Focus (Technical Brief No 24) 2010 [Google Scholar]
- 73.Biro FM, Wien M. Childhood obesity and adult morbidities. Am J Clin Nutr. 2009;91(suppl):1499S–1505S. doi: 10.3945/ajcn.2010.28701B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim Y, Conners RT, Hart PD, Kang YS, Kang M. Association of physical activity and body mass index with metabolic syndrome among US adolescents with disabilities. Disabil Health J. 2013;6:253–259. doi: 10.1016/j.dhjo.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 75.Nelson MD, Widman LM, Abresch RT, Stanhope K, Havel PJ, Styne DM, et al. Metabolic syndrome in adolescents with spinal cord dysfunction. J Spinal Cord Med. 2007;30:S127–S139. doi: 10.1080/10790268.2007.11754591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Telema R. Tracking of physical activity from childhood to adulthood: a review. Obesity Facts. 2009;2:187–195. doi: 10.1159/000222244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Faigenbaum AD, Myer GD. Exercise deficit disorder in youth: play now or pay later. Curr Sports Med Rep. 2012;11:196–200. doi: 10.1249/JSR.0b013e31825da961. [DOI] [PubMed] [Google Scholar]
- 78.Rimmer JA, Rowland JL. Physical activity for youth with disabilities: a critical need in an underserved population. Dev Neurorehabil. 2008;11:141–148. doi: 10.1080/17518420701688649. [DOI] [PubMed] [Google Scholar]
- 79.Longmuir PE, Bar-Or B. Factors influencing the physical activity levels of youths with physical and sensory disabilities. Adapt Phys Activ Q. 2000;17:40–53. [Google Scholar]
- 80.Steele CA, Kalnins IV, Jutai JW, Stevens SE, Bortolussi JA, Biggar WD. Lifestyle health behaviours of 11- to 16-year-old youth with physical disabilities. Health Educ Res. 1996;11:173–186. [Google Scholar]
- 81.Maher CA, Williams MT, Lane AE. Physical and sedentary activity in adolescents with cerebral palsy. Dev Med Child Neurol. 2007;49:450–457. doi: 10.1111/j.1469-8749.2007.00450.x. [DOI] [PubMed] [Google Scholar]
- 82.Bjornson KF, Belza B, Kartin D, Logsdon R, McLaughlin J. Ambulatory physical activity performance in youth with cerebral palsy and youth who are developing typically. Phys Ther. 2007;87:248–257. doi: 10.2522/ptj.20060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bjornson KF, Zhou C, Stevenson R, Christakis D, Song K. Walking activity patterns in youth with cerebral palsy and youth developing typically. Disabil Rehabil. 2014;36:1279–1284. doi: 10.3109/09638288.2013.845254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Stevens SL, Holbrook EA, Fuller DK, Morgan DW. Influence of age on step activity patterns in children with cerebral palsy and typically developing children. Arch Phys Med Rehabil. 2010;91:1891–1896. doi: 10.1016/j.apmr.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mitchell LE, Ziviani J, Boyd R. Habitual physical activity of independently ambulant children and adolescents with cerebral palsy: are they doing enough? Phys Ther. 2015;95:202–211. doi: 10.2522/ptj.20140031. [DOI] [PubMed] [Google Scholar]
- 86.Ryan JM, Forde C, Hussey JM, Gormley J. Comparison of patterns of physical activity and sedentary behavior between children with cerebral palsy and children with typical development. Phys Ther. 2015;95:1609–1616. doi: 10.2522/ptj.20140337. [DOI] [PubMed] [Google Scholar]
- 87.Capio CM, Sit CH, Abernethy B, Masters RS. Fundamental movement skills and physical activity among children with and without cerebral palsy. Res Dev Disabil. 2012;33:1235–1241. doi: 10.1016/j.ridd.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 88.van den Berg-Emons HJG, Saris WHM, de Barbanson DC, Westerterp KR, Huson A, van Baak MA. Daily physical activity of school children with spastic diplegia and of healthy control subjects. J Pediatr. 1995;127:578–584. doi: 10.1016/s0022-3476(95)70115-x. [DOI] [PubMed] [Google Scholar]
- 89.Holtebekk ME, Bernstsen S, Rasmussen M, Jahnsen RB. Physical activity and motor function in children and adolescents with neuromuscular disorders. Pediatr Phys Ther. 2013;25:415–420. doi: 10.1097/PEP.0b013e3182a635f0. [DOI] [PubMed] [Google Scholar]
- 90.Zwier JN, van Schie PEM, Becher JG, Smits DW, Gorter JW, Dallmeijer Physical activity in young children with cerebral palsy. Disabil Rehabil. 2010;32:1501–1508. doi: 10.3109/09638288.2010.497017. [DOI] [PubMed] [Google Scholar]
- 91.Bell KL, Davies PSW. Energy expenditure and physical activity of ambulatory children with cerebral palsy and of typically developing children. Am J Clin Nutr. 2010;92:313–319. doi: 10.3945/ajcn.2010.29388. [DOI] [PubMed] [Google Scholar]
- 92.Carlon Sl, Taylor NF, Dodd KJ, Shields N. Differences in habitual physical activity levels of young people with cerebral palsy and their typically developing peers: a systematic review. Disabil Rehabil. 2013;35:647–655. doi: 10.3109/09638288.2012.715721. [DOI] [PubMed] [Google Scholar]
- 93.Keawutan P, Bell K, Davies PSW, Boyd RN. Systematic review of the relationship between habitual physical activity and motor capacity in children with cerebral palsy. Res Dev Disabil. 2014;35:1301–1309. doi: 10.1016/j.ridd.2014.03.028. [DOI] [PubMed] [Google Scholar]
- 94.Ishikawa S, Kang M, Bjornson KF, Song K. Reliably measuring ambulatory activity levels of children and adolescents with cerebral palsy. Arch Phys Med Rehabil. 2013;94:132–137. doi: 10.1016/j.apmr.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mansoubi M, Pearson N, Clemes SA, Biddle SJH, Bodicoat DH, Tolfrey K, et al. Energy expenditure during common sitting and standing tasks: examining the 1.5 MET definition of sedentary behavior. BMC Public Health. doi: 10.1186/s12889-015-1851-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tremblay M. Letter to the Editor: Standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab. 2012;37:540–542. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 97.Biddle SJH, Pearson N, Ross GM, Braithwaite R. Tracking of sedentary behaviours of young people: a systematic review. Prev Med. 2010;51:345–351. doi: 10.1016/j.ypmed.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 98.Saunders TJ, Chaut JP, Tremblay MS. Sedentary behavior as an emerging risk factor for cardiometabolic diseases in children and youth. Can J Diabetes. 2014;38:53–61. doi: 10.1016/j.jcjd.2013.08.266. [DOI] [PubMed] [Google Scholar]
- 99.National Physical Activity Plan Alliance. 2014 United States Report Card on Physical Activity for Children and Youth. Vol. 8. Columbia SC: 2014. p. 10. [Google Scholar]
- 100.Obeid J, Balemans ACJ, Noorduyn SG, Gorter JW, Timmons BW. Objectively measured sedentary time in youth with cerebral palsy compared with age-, sex-, and season-matched youth who are developing typically: an explorative study. Phys Ther. 2014;94:1163–1167. doi: 10.2522/ptj.20130333. [DOI] [PubMed] [Google Scholar]
- 101.Dishman RK, Heath GW, Lee IMin. Physical Activity Epidemiology. 2nd. Champaign: Human Kinetics; 2013. Chapter 2: Concepts and Methods in Physical Activity Epidemiology; p. 31. [Google Scholar]
- 102.Garcia CC, Alcocer-Gamboa A, Ruiz MP, Caballero IM, Faigenbaum AD, Esteve-Lanao J, et al. Metabolic, cardiorespiratory, and neuromuscular fitness performance in children with cerebral palsy: a comparison with healthy youth. JER. 2016;12:124–131. doi: 10.12965/jer.1632552.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Maltais DB, Wiart L, Fowler E, Verschuren O, Damiano DL. Health-related physical fitness for children with cerebral palsy. J Child Neurol. 2014;29:1091–1100. doi: 10.1177/0883073814533152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vershuren O, Ketelaar M, Gorter JW, Helders PJM, Uiterwaal CSPM, Takken T. Exercise training program in children and adolescents with cerebral palsy. Arch Pediatr Adoles Med. 2007;161:1075–1081. doi: 10.1001/archpedi.161.11.1075. [DOI] [PubMed] [Google Scholar]
- 105.Maher CA, Williams MT, Olds T, Lane AE. An internet-based physical activity intervention for adolescents with cerebral palsy: a randomized controlled trial. Dev Med Child Neurol. 2010;52:448–455. doi: 10.1111/j.1469-8749.2009.03609.x. [DOI] [PubMed] [Google Scholar]
- 106.Van Wely L, Balemans ACJ, Becher JG, Dallmeijer AJ. Physical activity stimulation program for children with cerebral palsy did not improve physical activity: a randomised trial. J Physiother. 2014;60:40–49. doi: 10.1016/j.jphys.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 107.Slaman J, Roebroeck M, van der Slot W, Twisk J, Wensink A, Stam H, et al. Can a lifestyle intervention improve physical fitness in adolescents and young adults with spastic cerebral palsy? A randomized controlled trial. Arch Phys Med Rehabil. 2014;95:1646–1655. doi: 10.1016/j.apmr.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 108.Gorter H, Holty L, Rameckers EEA, Elvers JWH, Oostendorp RAB. Changes in endurance and walking ability through functional physical training in children with cerebral palsy. Pediatr Phys Ther. 2009;21:31–37. doi: 10.1097/PEP.0b013e318196f563. [DOI] [PubMed] [Google Scholar]
- 109.Unnithan VB, Katsimanis GK, Evangelinou C, Kosmas Kandrali I, Kellis E. Effect of strength and aerobic training in children with cerebral palsy. Med Sci Sports Exerc. 2007;39:1902–1909. doi: 10.1249/mss.0b013e3181453694. [DOI] [PubMed] [Google Scholar]
- 110.Saquetto M, Carvalho V, Silva C, Conceicao C, Gomes-Neto M. The effects of whole body vibration on mobility and balance in children with cerebral palsy: a systematic review with meta-analysis. J Musculoskelet Neuronal. 2015;15:137–144. [PMC free article] [PubMed] [Google Scholar]
- 111.Mattern-Baxter K, Bellamy S, Mansoor JK. Effects of intensive locomotor treadmill training on young children with cerebral palsy. Pediatr Phys Ther. 2009;21:308–319. doi: 10.1097/PEP.0b013e3181bf53d9. [DOI] [PubMed] [Google Scholar]
- 112.Grecco LAC, Zanon N, Sampaio LMM, Oliveira CS. A comparison of treadmill training and overground walking in ambulant children with cerebral palsy: randomized controlled clinical trial. Clin Rehabil. 2013;27:686–696. doi: 10.1177/0269215513476721. [DOI] [PubMed] [Google Scholar]
- 113.Mattern-Baxter K, McNeil S, Mansoor JK. Effects of home-based locomotor treadmill training on gross motor function in young children with cerebral palsy: a quasi-randomized controlled trial. Arch Phys Med Rehabil. 2013;94:2061–2067. doi: 10.1016/j.apmr.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 114.Morgan D, Holbrook E, Stevens SL, Emison K, Fuller D, Damiano D. Impact of underwater treadmill training on walking performance in youth with cerebral palsy. Dev Med Child Neurol. 2012;54(suppl6):43–44. [Google Scholar]
- 115.Ryan J, Wals M, Gormley J. Ability of RT3 accelerometer cut points to detect physical activity intensity in ambulatory children with cerebral palsy. Adapt Phys Activ Q. 2014;31:310–324. doi: 10.1123/apaq.2013-0088. [DOI] [PubMed] [Google Scholar]
- 116.Capio CM, Sit CH, Abernethy B. Physical activity measurement using MTI (Actigraph) among children with cerebral palsy. Arch Phys Med Rehabil. 2010;91:1283–1290. doi: 10.1016/j.apmr.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 117.Morgan D. Locomotor economy. In: Armstrong N, van Mechelen W, editors. Paediatric exercise science and medicine. 2nd. Oxford: Oxford University Press; 2008. pp. 283–295. [Google Scholar]
- 118.Rose J, Morgan D, Gamble J. Energetics of walking. In: Rose J, Gamble J, editors. Human Walking. 3rd. Baltimore: Lippincott, Williams, and Wilkins; 2005. pp. 77–102. [Google Scholar]
- 119.O'Neil ME, Fragala-Pinkham M, Lennon N, George A, Forman J, Trost SG. Reliability and validity of objective measures of physical activity in youth with cerebral palsy who are ambulatory. Phys Ther. 2016;96:37–45. doi: 10.2522/ptj.20140201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Trost SG, Fragala-Pinkham M, Lennon N, O'Neil ME. Decision trees for detection of activity intensity in youth with cerebral palsy. Med Sci Sports Exerc. 2016;48:958–966. doi: 10.1249/MSS.0000000000000842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sallis J. Measuring physical activity: practical approaches for program evaluation in Native American communities. J Public Health Manag Pract. 2010;16:404–410. doi: 10.1097/PHH.0b013e3181d52804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Trost SG. State of the art reviews: measurement of physical activity in children and adolescents. Am J Lifestyle Med. 2007;1:299–314. [Google Scholar]
- 123.Klinker CD, Schipperjin J, Christian H, Kerr J, Ersbøll AK, Troelsen J. Using accelerometers and global positioning system devices to assess gender and age differences in children's school, transport, leisure and home physical activity. Int J Behav Nutr Phys Act. 2014:11. doi: 10.1186/1479-5868-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Verschuren O, Peterson MD, Leferink S, Darrah J. Muscle activation and energy- requirements for varying postures in children and adolescents with cerebral palsy. J Pediatr. 2014;31:310–324. doi: 10.1016/j.jpeds.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Bar-Or O, Rowland TW. Pediatric exercise medicine: from physiologic principles to health care applications. Human Kinetics; Champaign: 2004. p. 275. [Google Scholar]
- 126.Baleman AC, Fragala-Pinkham AM, Lennon N, Thorpe D, Boyd RN, O'Neill ME, et al. Systematic review of the clinimetric properties of laboratory- and field- based aerobic and anaerobic fitness measures in children with cerebral palsy. Arch Phys Med Rehabil. 2013;94:287–301. doi: 10.1016/j.apmr.2012.09.012. [DOI] [PubMed] [Google Scholar]


