Abstract
The natural disease course of chronic obstructive pulmonary disease (COPD) is often punctuated by exacerbations: acute events of symptom worsening associated with significant morbidity and healthcare resource utilization; reduced quality of life; and increased risk of hospitalization and death. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommend that patients at risk of exacerbations (GOLD Groups C and D) receive a long-acting muscarinic antagonist (LAMA) or a long-acting β2-agonist (LABA)/LAMA combination, respectively, as preferred initial treatments. The latter recommendation is based on recent trial evidence demonstrating the superior efficacy of a fixed-dose LABA/LAMA over an inhaled corticosteroid (ICS)/LABA in exacerbation prevention. ICS in combination with a LABA is also indicated for prevention of exacerbations, but the use of ICS is associated with an increased risk of adverse events such as pneumonia, and offers limited benefits beyond those provided by LABA or LAMA monotherapy. In this review, we examine evidence from a number of pivotal studies of LABAs and LAMAs, administered as monotherapy or as part of dual or triple combination therapy, with a specific focus on their effect on exacerbations. We also discuss a new proposed treatment paradigm for the management of COPD that takes into account this recent evidence and adopts a more cautious approach to the use of ICS. In alignment with GOLD 2017, we suggest that ICS should be reserved for patients with concomitant asthma or in whom exacerbations persist despite treatment with LABA/LAMA.
Keywords: Dual bronchodilation, ICS, LABA, LAMA, Treatment guidelines, Triple therapy
Background
The natural trajectory of chronic obstructive pulmonary disease (COPD) is punctuated by exacerbations, defined as an acute worsening of symptoms that results in additional therapy [1, 2]. In many cases, exacerbations are triggered by respiratory tract infections (predominantly viral, but also bacterial) and environmental factors such as air pollution, yet in approximately one third of cases, the cause remains unknown [3].
COPD exacerbations have a marked negative effect on both the patient and underlying disease processes, and can result in hospitalization and readmission, an increased risk of death [4] and a significant reduction in health status [5, 6]. Exacerbations are also associated with long-term decline in lung function and a high socioeconomic cost [7–10]. Thus, optimizing the prevention and management of COPD exacerbations is an important clinical issue.
A key step towards meeting this goal is to identify patients at greatest risk of exacerbation. The ‘frequent exacerbator’ phenotype (≥2 exacerbations/year) describes patients who are particularly susceptible to exacerbations, in contrast to infrequent exacerbators [11, 12]. The exacerbator phenotype, which remains relatively stable over time [12], has a complex pathophysiology and is prevalent across all disease severities, but is more common in patients with worse lung function [12].
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) provides treatment recommendations for patients who are at high risk of exacerbation: a long-acting muscarinic antagonist (LAMA) or a long-acting β2-agonist (LABA)/LAMA combination are recommended as primary choice treatment for these patients. Although the GOLD strategy document was developed with an international audience in mind, some countries and regions (e.g. Spain, UK, South America, and Canada) follow guidance outlined in their own recommendations as well [13–16].
Inhaled corticosteroids (ICS) are recommended for patients at high risk of exacerbation with concomitant asthma, or who experience further exacerbations following initial bronchodilator treatment [2]. However, there is widespread evidence of inappropriate use of ICS in patients at low risk of exacerbation [17, 18]. Considering the known risks associated with long-term ICS use, only patients carefully considered as suitable candidates should receive treatment with the appropriate ICS and associated dose, such that treatment benefit will outweigh any potential risk [19].
Strong evidence supports the favorable efficacy and safety profile of dual bronchodilators. Compared with bronchodilator monotherapy and ICS/LABA, LABA/LAMAs improve lung function [20–29] and reduce exacerbation risk [23, 24, 28, 29]. The safety profile of LABA/LAMA combinations is generally similar to that of placebo or individual monocomponents [22, 30–33]. However, LABA/LAMA use is still relatively limited and further experience with these agents is needed [34].
In this review, we will examine the efficacy of various COPD treatments in terms of exacerbation prevention, with particular focus on bronchodilators, and discuss a new proposed treatment paradigm for reducing exacerbation risk in patients with COPD.
Efficacy Of Bronchodilators In Prevention Of Exacerbations
Non-pharmacological intervention
Smoking cessation is the most effective initial strategy for reducing COPD disease progression, particularly exacerbation risk [2, 35]. Smoking cessation significantly reduces the progressive decline in lung function [36], and substantially lowers the risk of mortality [37], however a significant number of COPD patients continue to smoke [38]. Although smoking cessation results in a reduced rate of decline in lung function, disease progression may persist [39].
Influenza vaccination can reduce serious illness and death in patients with COPD [40–43].
Furthermore, a significant reduction in exacerbation rate has also been observed with vaccination versus placebo [40]. Findings from a population-based study suggested that COPD patients, especially the elderly, had a decreased risk of ischemic heart disease when receiving the influenza vaccine over many years [44].
Pulmonary rehabilitation (PR) reduces hyperinflation by promoting lung deflation and better lung ventilation, which is linked to an improved health status and exercise capacity [45]. PR also provides benefits that extend beyond the initial training period, such as: improved survival; improved recovery following hospitalization for exacerbation; reduced perceived intensity of breathlessness; and reduced anxiety and depression [2, 46, 47]. PR programs can significantly reduce the frequency of exacerbations and hospitalization, and the proportion of patients classified as ‘frequent exacerbator’ [48]. Non-pharmacological interventions, such as surgical modes or bronchoscopic modes of lung volume reduction, are also associated with a reduction of exacerbation; however, these interventions are limited to a small number of patients [49].
Encouraging patients to increase their levels of daily activity is also recommended, due to the general beneficial effects of physical exercise [2] and the reported links between lower levels of physical activity in patients with COPD and an increased risk of hospitalization [50, 51] and readmission [52].
Pharmacological interventions
It must be recognized that many studies presented here were not designed to test for the relative efficacy of treatments in exacerbation prevention, making it difficult to draw conclusions on their effects in at-risk populations. However, the following studies were powered to test for differences in exacerbation rate, and recruited patients at high risk of exacerbation, thus, enriching study populations: POET (1 year) [53]; SPARK (64 weeks) [24]; FLAME (52 weeks) [29]; and INSPIRE (2 years) [54].
Single bronchodilation versus placebo in the prevention of exacerbations
The two main classes of bronchodilators are β2-agonists and muscarinic receptor antagonists. Both classes of bronchodilators improve patients’ ability to breathe by relaxing airway smooth muscle, thereby reducing respiratory muscle activity and dynamic hyperinflation, and improving ventilatory mechanics [55–57]. Exacerbations are mainly triggered by infections associated with small airway inflammation [3], however environmental conditions, such as pollution, may also initiate or amplify these events [2]. The mechanisms by which bronchodilators prevent exacerbations are unclear, yet are thought to include decreased hyperinflation and mechanical stress, decreased mucus production and enhanced mucociliary clearance, the improvement of symptom severity and fluctuation, and potential anti-inflammatory properties [58]. Compared with placebo, the use of tiotropium 18 μg once daily (q.d.) was associated with sustained reductions in lung hyperinflation and inspiratory capacity both at rest and during exercise, contributing to improvements in exertional dyspnea and increased exercise endurance in patients with COPD [57]. Treatment with formoterol 12 μg twice daily (b.i.d.) significantly enhanced mucus clearance compared with tiotropium 18 μg q.d. in patients with mild-to-moderate COPD [59]. AUGMENT demonstrated that treatment with both formoterol 12 μg b.i.d. and aclidinium 400 μg b.i.d. significantly improved dyspnea and health status compared with placebo in patients with moderate-to-severe COPD [60]. Compared with formoterol 12 μg b.i.d., tiotropium 18 μg q.d. demonstrated a superior anti-inflammatory activity profile, significantly reducing the production of superoxide and pro-inflammatory mediators in COPD patients [61].
Traditionally, much of the evidence for the efficacy of LAMAs in exacerbation prevention has come from studies with tiotropium. More recently, there have been similar findings with glycopyrronium, aclidinium and umeclidinium, as well as the LABAs salmeterol and indacaterol. Table 1 summarizes the findings from major trials of these agents with regards to exacerbation prevention, although it should be noted that exacerbations were studied as a secondary endpoint in the majority of cases.
Table 1.
Overview of key COPD clinical trials comparing single and dual bronchodilator therapies with placebo
| Study title | Study design | Duration | Patient population | Treatment arms | N | Exacerbation definition | Key exacerbation results (Comparator vs placebo) |
|---|---|---|---|---|---|---|---|
|
Single BD (LAMA) vs PBO
Tiotropium | |||||||
| Casaburi et al. (2002) [62] | MC, R, DB, PC | 1 year | FEV1 ≤ 65% predicted and ≤ 70% FVC | TIO 18 μg q.d. PBO (3:2) |
550 371 |
Complex of respiratory events (cough, wheezing, dyspnea or sputum production) lasting >3 days (generally treated with AB ± oral CS) | • ≥1 exac: 36% vs 42% (14% reduction with TIO; p < 0.05) • Increased time to first exac with TIO vs PBO (p = 0.011) • Fewer exac events/pt/yr: 0.76 vs 0.95 (20% reduction with TIO; p = 0.045) • Fewer hospitalizations for exac: 0.086 vs 0.161 events/pt/yr (47% reduction; p = 0.019) • Fewer patients hospitalized for exac: 5.5% vs 9.4% (41% reduction with TIO; p < 0.05) |
| Brusasco et al. (2003) [63] Combined analysis of NCT02172287/NCT02173691 |
2 x MC, R, DB, DD, PG, PC | 6 months | FEV1 ≤ 65% predicted and ≤ 70% FVC | TIO 18 μg q.d. PBO SALM 50 μg b.i.d.a (1:1:1) |
402 400 405 |
Complex of respiratory symptoms (new onset or increase in one or more of cough, sputum, dyspnea, wheeze, chest discomfort) lasting at least 3 days and usually associated with therapeutic intervention | • Delayed time to first exac with TIO vs PBO (p ≤ 0.01) • Fewer exac/pt/yr: 1.07 vs 1.49 (28% reduction with TIO vs PBO; p = 0.025) • Exac days/pt/yr: 17.2 vs 25 (31% reduction with TIO vs PBO; p = 0.025) • No significant differences between TIO and PBO in hospital admissions, days in hospital or unscheduled physician visits for exac |
| Niewoehner et al. (2005) [64] NCT00274547 |
MC, R, DB, PG, PC | 6 months | Moderate-to-severe COPD (FEV1 ≤ 60% predicted and ≤ 70% FVC) | TIO 18 μg q.d. PBO (1:1) |
914 915 |
Complex of respiratory symptoms (increase or new-onset) of more than one of cough, sputum, wheezing, dyspnea, or chest tightness with a duration of ≥3 days requiring treatment with AB or systemic CS, hospitalization or both | • ≥1 exac: 27.9% vs 32.3% (OR 0.81; 95% CI 0.66, 0.99; p = 0.037) • ≥1 hospitalization for exac: 7.0% vs 9.5% (OR 0.72; 95% CI 0.51, 1.01; p = 0.056, NS) • Extended time to first exac (HR 0.83; 95% CI 0.70, 0.98; p = 0.028) • Reductions (events/pt/yr) in TIO vs PBO: o Frequency of exac: 0.85 vs 1.05 (p = 0.031) o Exac days: 12.6 vs 16.0 (p = 0.019) o Unscheduled medical visits: 0.39 vs 0.49 (p = 0.019) o Hospitalizations for exac: 0.18 vs 0.25 (p = 0.047) |
| Dusser et al. (2006) [65] MISTRAL |
MC, R, DB, PG, PC | 1 year | FEV1 30–65% predicted and FEV1/FVC ≤ 0.7 | TIO 18 μg q.d. PBO (1:1) |
500 510 |
Onset of at least one clinical descriptor (worsening dyspnea, cough or sputum production; appearance of purulent sputum; fever [>38 ºC]; appearance of new chest radiograph abnormality) lasting ≥2 days and requiring dose increase of β2-agonists, AB, CS or BD | • ≥1 exac: 49.9% vs 60.3% (17% reduction with TIO; p < 0.01) • Fewer exac/pt/yr: 1.57 vs 2.41 (35% reduction with TIO; p < 0.001) • TIO reduced exac days by 37% vs PBO (p < 0.001) and delayed time to first exac by ~100 days (p < 0.001) • ≥1 moderate-to-severe exac: 42.5% vs 53.4% (30% reduction with TIO, p < 0.001) |
| Powrie et al. (2007) [66] NCT00405236 |
SC, R, DB, PC | 1 year | FEV1 < 80% predicted; FEV1/FVC < 0.7 | TIO 18 μg q.d. PBO (1:1) |
69 73 |
Presence for ≥2 consecutive days of increase in any two major symptoms (dyspnea, sputum purulence, sputum volume) or increase in one major and one minor symptom (wheeze, sore throat, cough, symptoms of a common cold) | • ≥1 exac: 43% vs 64% (p = 0.01) • Fewer exac/yr: 1.17 vs 2.46 (52% reduction with TIO; p = 0.001) • Time to first exac: 236 vs 157 days (p = 0.0092) • Fewer exac days: 17.3 vs 34.5 (p = 0.002) • No treatment differences for number of treated exac or hospitalizations for exac |
| Chan et al. (2007) [125] SAFE |
MC, R, DB, PG, PC | 1 year | Moderate-to- severe COPD (FEV1 ≤ 65% predicted and FEV1/FVC ≤ 0.7) | TIO 18 μg q.d. PBO (2:1) |
608 305 |
Complex of respiratory symptoms (new-onset or increase in at least one of cough, sputum, sputum purulence, dyspnea, wheeze, chest discomfort) lasting ≥3 days and requiring treatment with AB ± systemic CS | • No statistically significant differences between TIO and PBO: o ≥1 exac: 44.1% vs 41.0% o ≥1 hospitalization for exac: 8.4% vs 8.2% o Exac/pt/yr: 0.88 vs 0.92 o Exac days/pt yr: 16.13 vs 16.19 o Hospitalizations for exac: 0.13 vs 0.15 o Hospitalization days for exac: 1.14 vs 1.16 |
| Tonnel et al. (2008) [67] TIPHON |
MC, R, DB, PG, PC | 9 months | Mild, moderate or severe COPD (FEV1 20–70% predicted FEV1/FVC ≤ 0.7b) | TIO 18 μg q.d. PBO (1:1) |
266 288 |
Worsening of COPD (from stable state beyond normal day-to-day variation) that was acute in onset and necessitated a change in regular medication | • ≥1 exac: 38.0% vs 45.1% (p = 0.10; NS) • Fewer exac/yr: 1.05 vs 1.83 (43% reduction with TIO; p = 0.03) • Fewer exac days/yr: 10.5 vs 20.6 (49% reduction with TIO; p = 0.02) • Delayed time to first exac: 201 days vs 181 days (p = 0.0081) |
| Tashkin et al. (2008) [68] NCT00144339 UPLIFT |
MC, R, DB, PG, PC | 4 years | Moderate-to-very severe COPD (FEV1 ≤ 70% predicted and ≤ 70% FVC) | TIO 18 μg q.d. PBO (1:1) |
2,987 3,006 |
Increase in/new onset of more than one respiratory symptom (cough, sputum, sputum purulence, wheeze, or dyspnea) lasting ≥3 days and requiring treatment with AB or systemic CS | • Exac/pt/yr: 0.73 vs 0.85 (RR 0.86; 95% CI 0.81, 0.91; p < 0.001) • Exac days/pt/yr: 12.11 vs 13.64 (RR 0.89; 95% CI 0.83, 0.95; p = 0.001) • Hospitalizations for exac (no./pt/yr): 0.15 vs 0.16 (RR 0.94; 95% CI 0.82, 1.07; p = NS) • Significantly delayed median time to first exac: 16.7 months (95% CI 14.9, 17.9) vs 12.5 months (95% CI 11.5, 13.8) • Significantly delayed time to first hospitalization for exacc |
| Bateman et al. (2010) [70] NCT00387088 |
MC, R, DB, PG, PC | 48 weeks | FEV1 ≤ 60% predicted and FEV1/FVC ≤ 0.7 | TIO 5 μg q.d.d
PBO (1:1) |
1,989 2,002 |
Complex of respiratory events/symptoms lasting ≥3 days and requiring treatment with AB and/or systemic CS, or prompting a change in regular medication | • ≥1 exac: 35.3% vs 43.1% (HR 0.693; 95% CI 0.625, 0.769; p < 0.0001) • Fewer exac/pt/yr: 0.69 vs 0.87 (RR 0.79; 95% CI 0.72, 0.87; p < 0.0001) • Fewer exac requiring hospitalization (pt/yr: 0.12 vs 0.15 (RR 0.81; 95% CI 0.70, 0.93; p < 0.005) |
| Bateman et al. (2010) [69] Combined analysis of NCT00168844/NCT00168831 |
2 x MC, R, DB, PG, PC | 1 year | Moderate-to-severe COPD (FEV1 ≤ 60% predicted and ≤ 70% FVC) | TIO 5 μg q.d.d
TIO 10 μg q.d.d PBO (1:1) |
670 667 653 |
Respiratory adverse events lasting ≥3 days and requiring treatment with AB ± oral CS ± a significant change in prescribed medication including inhaled BD | • ≥1 exac: 37.2% (TIO 5 μg) and 36.9% (TIO 10 μg) vs 44.1% (PBO) • Exac rate (per pt-yr) o TIO 5 μg vs PBO: OR 0.75 (p < 0.01) o TIO 10 μg vs PBO: OR 0.74 (p < 0.001) • Time to first exac (days): 160 and 178 vs 86 (both p < 0.001) • Hospitalization/pt/yr: 0.12 and 0.16 vs 0.20 (p = NS) |
| Abrahams et al. (2013) [126] NCT00528996 |
MC, R, DB, PG, PC | 24 weeks | FEV1 < 80% predicted and FEV1/FVC ≤ 0.7 | TIO 5 μg q.d.d
PBO (1:1) (BEA2810 50, 100 and 200 μg also assesseda) |
427 429 (Total 2,080) |
Complex of respiratory events/symptoms (increased/new onset of ≥2 of: shortness of breath, sputum production, [volume], purulent sputum, cough, wheeze, chest tightness) related to COPD, with a duration of ≥3 days requiring a change in treatment | • No significant difference between TIO and PBO in risk of COPD exac |
| Glycopyrronium | |||||||
| D’Urzo et al. (2011) [71] NCT01005901 GLOW1 |
MC, R, DB, PG, PC | 26 weeks | Moderate-to-severe COPD (FEV1 ≥ 30% and < 80% predicted; FEV1/FVC < 0.7) | GLY 50 μg q.d. PBO (2:1) |
552 270 |
Increase in ≥2 COPD symptoms or worsening of any one major symptom together with a minor symptom over ≥2 consecutive days. AB ± systemic CS (moderate exac), or hospitalization (severe exac) | • Delayed time to first moderate or severe exac by 31% vs PBO (HR, 0.69; 95% CI 0.500, 0.949; p = 0.023) • Reduced risk of severe exac leading to hospitalization vs PBO (HR, 0.35; 95% CI 0.141, 0.857; p = 0.022) • Reduced the proportion of hospitalizations due to exacs vs PBO: 1.7% vs 4.2% (OR, 0.34; 95% CI 0.129, 0.868; p = 0.024) |
| Kerwin et al. (2012) [72] NCT00929110 GLOW2 |
MC, R, DB, PG, PC | 52 weeks | Moderate-to-severe COPD (FEV1 ≥ 30% and < 80% predicted; FEV1/FVC < 0.7) | GLY 50 μg q.d. PBO OL TIO 18 μg q.d.a (2:1:1) |
529 269 268 |
Increase in ≥2 COPD symptoms or worsening of any one major symptom together with a minor symptom over ≥2 consecutive days. AB ± systemic CS (moderate exac), or hospitalization (severe exac). | • Risk of time to first moderate-to-severe exac reduced by 34% with GLY vs PBO (HR 0.66; 95% CI 0.520, 0.850; p = 0.001) • Rate of moderate-to-severe exac reduced by 34% with GLY vs PBO (RR 0.66; 95% CI 0.496, 0.869; p = 0.003) • Exac requiring treatment with systemic CS or AB significantly reduced with GLY vs PBO (OR 0.61 [p = 0.006] and OR 0.69 [p = 0.026], respectively) |
| Aclidinium | |||||||
| Kerwin et al. (2012) [73] NCT00891462 ACCORD COPD I |
MC, R, DB, PG, PC | 12 weeks | Moderate-to-severe COPD (FEV1 ≥ 30% and < 80% predicted; FEV1/ FVC < 0.7) | ACL 200 μg b.i.d. ACL 400 μg b.i.d. PBO (1:1:1) |
185 190 186 |
Increase in COPD symptoms over ≥2 consecutive days resulting in medical intervention | • Rate of any exac significantly reduced with ACL 400 μg vs PBO (RR 0.52; p = 0.0009) • Trend (not significant) towards reduced rate of moderate-to-severe exac/pt/yr with ACL 200 μg (33%) and ACL 400 μg (34%) vs PBO |
| Jones et al. (2012) [74] NCT01001494 ATTAIN |
MC, R, DB, PG, PC | 24 weeks | Moderate-to-severe COPD (FEV1 < 80% predicted; FEV1/ FVC < 0.7) | ACL 200 μg b.i.d. ACL 400 μg b.i.d. PBO (1:1:1) |
280 272 276 |
Increase in COPD symptoms over ≥2 consecutive days resulting in increased use of short-acting BD ± ICS (mild exac), AB ± systemic CS (moderate exac), or hospitalization (severe exac) | • Rate of any exac significantly reduced with ACL 200 μg and 400 μg vs PBO (RR: 0.72, 95% CI 0.52, 0.99, p < 0.05 and RR 0.67, 95% CI 0.48, 0.94, p < 0.05, respectively) • Trend (not significant) towards reduced rate of moderate or severe exac with ACL vs PBO (RR 0.74 for ACL 200 μg [p = 0.08] and 0.72 for ACL 400 μg [p = 0.06]) |
| Umeclidinium | |||||||
| Donohue et al. (2013) [22] NCT01313650 DB2113373 |
MC, R, DB, PG, PC | 24 weeks | FEV1 ≤ 70% predicted and FEV1/FVC < 0.7 | UMEC/VI 62.5/25 μg q.d.a
UMEC 62.5 μg q.d. VI 25 μg q.d.a PBO (3:3:3:2) |
413 418 421 280 |
Acute worsening of symptoms of COPD requiring emergency treatment, hospitalization or use of additional therapy beyond study drug/rescue salbutamol (e.g. oral CS and AB) | • Reduced risk of exac with UMEC vs PBO (HR 0.6; 95% CI 0.4, 1.0, p < 0.05) |
| Celli et al. (2014) [75] NCT01313637 |
MC, R, DB, PG, PC | 24 weeks | FEV1 ≤ 70% predicted and FEV1/FVC < 0.7 | UMEC/VI 125/25 μg q.d.a
UMEC 125 μg q.d. VI 25 μg q.d.a PBO (3:3:3:2) |
403 407 404 275 |
Acute worsening of symptoms of COPD requiring emergency treatment, hospitalization or use of any therapy beyond study drug/rescue albuterol | • Reduced risk of COPD exac with UMEC vs PBO (HR 0.5; 95% CI 0.3, 0.8, p ≤ 0.006) |
|
Single BD (LABA) vs PBO
Salmeterol | |||||||
| Stockley et al. (2006) [76] | MC, R, DB, PC | 1 year | FEV1 < 70% predicted and established history of exacerbations (≥2 in previous year needing treatment with AB and/or oral CS) | SALM 50 μg b.i.d. PBO (1:1) |
316 318 |
Exacerbations were identified using an event-based definition in which a worsening of symptoms required a change in medication. Exacerbations classed as moderate if required treatment with AB +/- oral CS/increase in ICS dose; severe if required hospital admission | • Mean number of moderate/severe exac/year in ITT population was lower with SALM (0.93) vs PBO (1.18); p = NS • Mean number of moderate/severe exac/year in PP population was significantly lower with SALM (0.58) vs PBO (0.83); p = 0.007 |
| Indacaterol | |||||||
| Dahl et al. (2010) [77] NCT00393458 INVOLVE |
MC, R, DB, DD, PG, PC | 1 year | Moderate-to-severe COPD (FEV1 < 80% and ≥ 30% predicted and FEV1/FVC < 0.7) | IND 300 μg q.d. IND 600 μg q.d. PBO FOR 12 μg b.i.d.a (1:1:1:1) |
437 428 432 435 |
Onset/worsening of more than one respiratory symptom (dyspnea, cough, sputum purulence/volume or wheeze) for >3 consecutive days plus documented proof of intensified treatment (e.g. systemic CS, AB or oxygen) ± hospitalization/ER visit | • Exac: 32.8% (IND 300 μg) and 29.3% (IND 600 μg) vs 36.3% (PBO) • Time to first exac improved with IND 300 μg and 600 μg vs PBO: HR 0.77 (95% CI 0.606, 0.975, p < 0.05) and 0.69 (95% CI 0.538, 0.882, p < 0.05) • RR vs PBO were 0.82 for IND 300 μg (p = NS) and 0.74 (0.74, 95% CI 0.56, 0.97, p < 0.05) for IND 600 μg |
| Donohue et al. (2010) [79] NCT00463567 INHANCE |
MC, R, DB, PC | 26 weeks | Moderate-to-severe COPD | IND 150 μg q.d. IND 300 μg q.d. PBO OL TIO 18 μg q.d.a |
416 416 418 415 |
Onset/worsening of one or more respiratory symptoms (dyspnea, cough, sputum purulence/volume, or wheeze) for ≥3 consecutive days, plus intensified treatment (e.g., systemic CS, AB, oxygen) ± hospitalization/ER visit | • Risk of time to first exac reduced vs PBO for IND 150 μg (HR 0.69; 95% CI 0.51, 0.94; p = 0.019); numerically reduced risk for IND 300 μg (HR 0.74; 95% CI 0.55, 1.01, p = 0.054) • RR of exac vs PBO: 0.67 IND 150 μg (95% CI 0.46, 0.99; p = 0.044); for IND 300 μg (0.75, 95% CI 0.51, 1.08, p = NS) • Rate of exac/yr: 0.50 and 0.53 vs 0.72 for IND 150 μg, 300 μg vs PBO, respectively |
| Chapman et al. (2011) [78] NCT00677807 INDORSE |
MC, R, DB, PC | 26-week extension (52 weeks including core study; see above) | Moderate-to-severe COPD (FEV1 < 80% and ≥ 30% predicted; and FEV1/FVC < 0.7) | IND 150 μg q.d. IND 300 μg q.d. PBO |
420 418 425 |
Onset/worsening of more than one respiratory symptom (dyspnea, cough, sputum purulence/volume, or wheeze) for >3 consecutive days, plus intensified treatment (e.g., systemic CS, AB, oxygen) ± hospitalization/ER visit | • Exac/yr: 0.39 (IND 150 μg; p < 0.05) and 0.38 (IND 300 μg; p = NS) vs 0.54 (PBO) • RR of exac vs PBO: 0.64 IND 150 μg (95% CI 0.43, 0.96, p = 0.029); 0.62 IND 300 μg (95% CI 0.42, 0.92, p = 0.018) • Time to first exac: HR (vs PBO): 0.82 (95% CI 0.51, 1.34) IND 150 μg; 0.86 (95% CI 0.53, 1.39) IND 300 μge |
|
Dual bronchodilation (LAMA/LABA) vs PBO
Aclidinium/formoterol | |||||||
| Singh et al. (2014) [23] NCT01462942 ACLIFORM-COPD |
MC, R, DB, PG, PC/AC | 24 weeks | Moderate-to-severe COPD (FEV1 < 80% and ≥ 30% predicted and FEV1/FVC < 0.7) | A/F 400/12 μg b.i.d. A/F 400/6 μg b.i.d. ACL 400 μg b.i.d.a FOR 12 μg b.i.d.a PBO (2:2:2:2:1) |
385 381 385 384 194 |
HCRU: increase of COPD symptoms during ≥2 consecutive days that require a change in COPD treatment; and EXACT: persistent increase from baseline in total EXACT score of ≥9 points for ≥3 days or ≥12 points for ≥2 days | • HCRU rate: 27% lower with A/F 400/12 μg vs PBO (did not reach significance); RR were 0.73 A/F 400/12 μg and 0.80 A/F 400/6 μg • EXACT rate: significantly lower with A/F 400/12 μg vs PBO (0.71; 95% CI 0.5, 0.9, p < 0.05) • No. pts hospitalized for exac was low and similar between treatments |
| Bateman et al. (2015) [84] Pooled analysis of ACLIFORM-COPD and AUGMENT NCT01462942/NCT01437397 |
2 x MC, R, DB, PG, PC/AC | 24 weeks | Moderate-to-severe COPD (FEV1 < 80% and ≥ 30% predicted and FEV1/FVC < 0.7) | A/F 400/12 μg b.i.d. A/F 400/6 μg b.i.d.f ACL 400 μg b.i.d.a FOR 12 μg b.i.d.a PBO |
723 719 725 723 531 |
HCRU: increase in COPD symptoms during ≥2 consecutive days that required a change in COPD treatment; and EXACT: persistent increase from baseline in total EXACT score of ≥9 points for ≥3 days or ≥12 points for ≥2 days | • HCRU rate: 24% (RR 0.76; p = NS) and 29% (RR 0.71; p < 0.05) reductions in any and in moderate or severe exac, respectively • Increased time to first exac vs PBO: o Any severity (HR 0.72; 95% CI 0.53, 0.97, p < 0.05) o Moderate or severe (HR 0.70; 95% CI 0.51, 0.96, p < 0.05) • Results supported by EXACT data: o RR 0.78 (95% CI 0.65, 0.94, p < 0.001) o Time to first EXACT exac of any severity (HR 0.79; 95% CI 0.65, 0.95, p < 0.05) |
| Umeclidinium/vilanterol | |||||||
| Donohue et al. (2013) [22] NCT01313650 DB2113373 |
MC, R, DB, PG, PC/AC | 24 weeks | FEV1 ≤ 70% predicted and FEV1/FVC < 0.7 | UMEC/VI 62.5/25 μg q.d. UMEC 62.5 μg q.d.a VI 25 μg q.d.a PBO (3:3:3:2) |
413 418 421 280 |
Acute worsening of symptoms of COPD requiring emergency treatment, hospitalization or use of additional therapy beyond study drug/rescue salbutamol (e.g. oral CS and AB) | • Reduced risk of exac with UMEC/VI vs PBO (HR 0.5; 95% CI 0.3, 0.8, p ≤ 0.01) |
| Celli et al. (2014) [75] NCT01313637 |
MC, R, DB, PG, PC/AC | 24 weeks | FEV1 ≤ 70% predicted and FEV1/FVC < 0.7 | UMEC/VI 125/25 μg q.d. UMEC 125 μg q.d.a VI 25 μg q.d.a PBO (3:3:3:2) |
403 407 404 275 |
Acute worsening of symptoms of COPD requiring emergency treatment, hospitalization or use of any therapy beyond study drug/rescue albuterol | • Reduced risk of COPD exac with UMEC/VI vs PBO (HR 0.4; 95% CI 0.2, 0.6, p ≤ 0.006) |
aData not included; bAccording to 1995 American Thoracic Society criteria; cHospitalizations occurred in <50% of patients and therefore a median time to first event could not be calculated; dSoft mist formulation delivered via the Respimat® device; eStudy was not powered to make comparison; fData for aclidinium/formoterol 400/6 μg b.i.d. not reported in publication. AB antibiotics, AC active controlled, ACCORD COPD I AClidinium in Chronic Obstructive Respiratory Disease COPD I, ACL aclidinium; A/F aclidinium/formoterol, AUGMENT Aclidinium/formoterol FUmarate Combination for InvestiGative use in the TreatMENT of Moderate-to-Severe COPD, ATTAIN Aclidinium To Treat Airway obstruction In COPD patieNts, BD bronchodilators, b.i.d. twice daily, CI confidence interval, CS corticosteroids, DB double blind, DD double dummy, ER emergency room, exac exacerbation, EXACT EXAcerbations of Chronic pulmonary disease Tool, FEV 1 forced expiratory volume in 1 s, FVC forced vital capacity, FOR formoterol, GLOW2 GLycopyrronium bromide in COPD airWays clinical Study 2, GLY glycopyrronium, HCRU Healthcare Resource Utilization, HR hazard ratio, ICS inhaled corticosteroids, IND indacaterol, INHANCE INdacaterol [versus tiotropium] to Help Achieve New COPD treatment Excellence, INVOLVE: Indacaterol: Value in COPD: Longer Term Validation of Efficacy and Safety, LABA long-acting β2-agonist, LAMA long-acting muscarinic antagonist, MC multicenter, MISTRAL Mesure de l’Influence de Spiriva® sur les Troubles Respiratoires Aigus à Long terme, NS not statistically significant, OL open label, OR odds ratio, PBO placebo, PC placebo controlled, PG parallel group, PP per protocol; pt patient, q.d. once daily, R randomized, RR relative risk, SALM salmeterol, SC single center, SVC slow vital capacity, TIO tiotropium, UMEC umeclidinium, VI vilanterol, UPLIFT: Understanding Potential Long-Term Impacts on Function with Tiotropium, yr year
Most of the 11 studies comparing tiotropium (5 or 10 μg q.d., via the soft-mist inhaler, or 18 μg q.d. via dry-powder inhaler) with placebo reported significant beneficial effects on various exacerbation-related outcomes. In nine studies, the number of exacerbation events per patient per year was significantly lower with tiotropium than placebo [62–70]. Eight studies reported significant delays in the time to first exacerbation with tiotropium versus placebo [62–69], and in six studies the proportion of patients experiencing one or more exacerbations, and the number of exacerbation days per year, were significantly lower with tiotropium than with placebo [62, 64–70]. Only three studies reported significantly lower hospitalizations due to exacerbation (rates, events or proportions of patients) with tiotropium [62, 64, 70]. Glycopyrronium (50 μg q.d.) [71, 72], aclidinium (200 or 400 μg b.i.d. [73, 74], umeclidinium (62.5 μg and 125 μg q.d.) [22, 75], salmeterol (50 μg b.i.d.) [76] and indacaterol (doses ranging from 150–600 μg q.d.) [77–79] have demonstrated similar beneficial effects compared with placebo.
In two pivotal studies, GLOW1 (26 weeks) and GLOW2 (1 year), glycopyrronium (50 μg q.d.) significantly prolonged time to first moderate-to-severe exacerbation versus placebo [71, 72]. In GLOW1, glycopyrronium also significantly reduced the risk of severe COPD exacerbations leading to hospitalization and the proportion of hospitalizations due to COPD exacerbations [71]. In GLOW2, glycopyrronium significantly reduced the rate of moderate-to-severe exacerbations and the number of exacerbations requiring treatment with systemic corticosteroids or antibiotics, versus placebo [72].
In ACCORD (12 weeks) and ATTAIN (24 weeks), aclidinium (200 or 400 μg b.i.d.) significantly reduced the rate of exacerbations of any severity and numerically reduced rates of moderate or severe exacerbations per patient per year compared with placebo [73, 74]. Two 24-week studies examining the efficacy of umeclidinium demonstrated significant reductions in the risk of exacerbations versus placebo [22, 75].
Comparison of the efficacy of single bronchodilators in the prevention of exacerbations
Only a few head-to-head studies have examined the relative effects of different bronchodilators on exacerbation outcomes (Table 2).
Table 2.
Overview of key clinical trials comparing single or dual bronchodilator therapies with single bronchodilators
| Study title | Study design | Duration | Patient population | Treatment arms | N | Exacerbation definition | Key exacerbation results |
|---|---|---|---|---|---|---|---|
|
Comparison of single BDs
LAMA vs LAMA | |||||||
| GLOW2 Kerwin et al. (2012) [72] NCT00929110 |
MC, R, DB, DD, PG, PC, OL | 52 weeks | Moderate-to-severe stable COPD (FEV1 ≥ 30% and <80% predicted; FEV1/ FVC < 0.7) | GLY 50 μg q.d. PBOa OL TIO 18 μg q.d. (2:1:1) |
529 269 268 |
N/A | • Time to first moderate or severe exac: comparable risk reduction for GLY and TIO vs PBOb
o 34% risk reduction with GLY vs PBO (HR 0.66; 95% CI 0.520, 0.850, p = 0.001) o 39% risk reduction with TIO vs PBO (HR 0.61; 95% CI 0.456, 0.821, p = 0.001) • Rate of moderate or severe exac: o 34% with GLY vs PBO (RR 0.66; 95% CI 0.496, 0.869, p = 0.003) o P = NS for TIO vs PBO (RR 0.80; 95% CI 0.586, 1.105) |
| LAMA vs LABA or LABA vs LAMA | |||||||
| Vogelmeier et al. (2011) [53] NCT00563381 POET |
MC, R, DB, DD, PG, AC | 1 year | Moderate-to-very-severe COPD (FEV1 ≤ 70% predicted and FEV1/FEV ≤ 0.7) plus a history of exac in the preceding year | TIO 18 μg q.d. SALM 50 μg b.i.d. (1:1) |
3,707 3,669 |
Increase in/onset of more than one symptom of COPD (cough, sputum, dyspnea, wheezing, chest tightness) with at least one lasting ≥3 days and requiring treatment with systemic CS, AB or both (criterion for moderate exac) or hospitalization (criterion for severe exac) | • Time to first exac increased by 42 days with TIO vs SALM (145 days vs 187 days; 17% reduced risk; HR 0.83, 95% CI 0.77, 0.90, p < 0.001) • TIO increased time to first severe exacerbation vs SALM (HR 0.72, 95% CI 0.61, 0.85, p < 0.001) • TIO significantly reduced risk of moderate and severe exac vs SALM by 14% (HR 0.86, 95% CI 0.79, 0.93, p < 0.001) and 28% (HR 0.72, 95% HR, 0.61, 0.85, p < 0.001), respectively • TIO vs SALM reduced the annual rate of moderate exac by 7% (0.54 vs 0.59; RR 0.93, 95% CI 0.86, 1.00, p < 0.05) and severe exac by 27% (0.09 vs 0.13; RR 0.73, 95% CI 0.66, 0.82, p < 0.001) • TIO reduced the risk of exac requiring treatment with CS and or AB (p < 0.001) |
| Decramer et al. (2013) [80] NCT00845728 INVIGORATE |
MC, R, blinded, DD, PG, AC | 52 weeks | Severe COPD (FEV1 30% and < 50% predicted and FEV1/FVC < 0.70 plus and a documented history of ≥ 1 moderate or severe exac in the previous 12 months | IND 150 μg q.d. TIO 18 μg q.d. (1:1) |
1,723 1,721 |
Worsening for ≥2 consecutive days of ≥2 major symptoms (dyspnea, sputum volume or sputum purulence) or worsening of any one major symptom plus one minor symptom (sore throat, colds, fever without other cause, increased cough or increased wheeze) | • Rate of exacc: 0.79 with IND and 0.61 with TIO (non-inferiority not met; RR 1.29, p = NS) • Annual rate of exac higher with IND vs TIO: 0.90 vs 0.73 (RR 1.24; 95% CI 1.12, 1.37, p < 0.0001)d • No treatment difference in rates of exac leading to hospitalization in patients receiving ICS |
|
Comparison of dual vs single BDs
LAMA/LABA vs LAMA or LABA | |||||||
| Aaron et al. (2007) [127] ISRCTN29870041 |
MC, R, DB, PG, PC | 52 weeks | Moderate or severe COPD (FEV1 < 65% predicted and FEV1/FVC < 0.7) | TIO 18 μg q.d. TIO 18 μg q.d. + SALM 50 μg b.i.d. TIO 18 μg q.d. + SFC 50/500 μg b.i.d.a |
156 148 145 |
Sustained worsening of patient’s respiratory condition, from stable state and beyond normal day-to-day variations, requiring a change in regular medicatione | • Pts with ≥1 exac: 64.8% TIO + SALM vs 62.8% with TIO (p = NS) • Exac/pt/yr: 1.75 TIO + SALM vs 1.61 TIO (p = NS) o IRR vs TIO: 1.09 (95% CI 0.84, 1.40) • Time to first exac: 128 days TIO + SALM vs 130 days TIO (p = NS) • No. of hospitalizations for exac: 38 vs 49 (p = NS) |
| Wedzicha et al (2013) [24] NCT01120691 SPARK |
MC, R, DB, PG | 64 weeks | Severe or very severe COPD (FEV1 < 50% predicted and FEV1/FVC < 0.7) plus a documented history of ≥ 1 exac in previous 12 months requiring treatment with systemic CS or AB or both | IND/GLY 110/50 μg q.d. GLY 50 μg q.d. OL TIO 18 μg q.d. (1:1:1) |
741 741 742 |
Presence of two major symptoms (dyspnea, sputum volume, sputum purulence) for ≥2 consecutive days or a worsening of one major symptom together with an increase in any one minor symptom (sore throat, cold, fever without other cause, cough, wheeze) for ≥2 consecutive days | • IND/GLY significantly reduced annualized rate of moderate or severe exac by 12% vs GLY (RR 0.88; 95% CI 0.77, 0,99, p = 0.038) o 10% reduction vs TIO (RR 0.90; 95% CI 0.79, 1.02, p = NS) • Rate of all exac reduced with IND/GLY vs GLY (RR 0.85; 95% CI 0.77, 0.94, p = 0.0012) and vs TIO (RR 0.86; 95% CI 0.78, 0.94, p = 0.0017) |
| Maleki-Yazdi et al. (2014) [128] NCT01777334 ZEP117115 |
MC, R, blinded, DD, PG | 24 weeks | Moderate-to-very-severe COPD (FEV1 ≤ 70% predicted and FEV1/FVC < 0.7) plus mMRC score of ≥ 2 | UMEC/VI 62.5/25 μg q.d. TIO 18 μg q.d. (1:1) |
454 451 |
Acute worsening of COPD symptoms requiring use of any treatment beyond study drug or rescue albuterol/salbutamol | • Time to first exac reduced with UMEC/VI vs TIO (HR 0.5; 95% CI 0.3, 1.0, p = 0.044) |
| Decramer et al. (2014) [85] Study 1 (S1) NCT01316900 DB2113360 Study 2 (S2) NCT01316913 DB2113374 |
2 x MC, R, blinded, DD, PG, AC | 24 weeks | Moderate-to-very-severe COPD (FEV1 ≤ 70% predicted and FEV1/FVC < 0.7) plus mMRC score of ≥ 2 | UMEC + VI 125 + 25 μg q.d. UMEC + VI 62.5 + 25 μg q.d. UMEC 125 μg q.d. VI 25 μg q.d. TIO 18 μg q.d. |
S1 216 S2 217 S1 212 S2 218 S2 222 S1 209 S1 209 S2 215 |
Acute worsening of symptoms of COPD requiring the use of any treatment other than study drug or rescue salbutamol | • No significant differences in risk of exac between UMEC + VI vs UMEC, VI or TIO monotherapies. Time to first exac: o S1: UMEC 125 μg + VI 25 μg (HR 1.0 vs TIO; 0.6 vs VI) o S1: UMEC 62.5 μg + VI 25 μg (HR 1.2 vs TIO; 0.7 vs VI) o S2: UMEC 125 μg + VI 25 μg (HR 1.1 vs TIO; 0.6 vs UMEC 125) o S2: UMEC 62.5 μg + VI 25 μg (HR 1.9 vs TIO; 0.1.0 vs UMEC 125) |
| Buhl et al. (2015) [20] Combined data for NCT01431274 TOnado 1 and NCT01431287 TOnado 2 |
2 x MC, R, DB, AC, PG | 24 weeks | Moderate-to-very-severe COPD (FEV1 < 80% predicted and FEV1/FVC < 0.7) plus mMRC score of ≥ 2 | TIO + OLO 5/5 μg q.d. TIO + OLO 2.5/5 μg q.d. OLO 5 μg q.d. TIO 5 μg q.d. TIO 2.5 μg q.d. |
1,029 1,030 1,038 1,033 1,032 |
N/A | • Trend for improvements in moderate/severe exac with TIO + OLO vs monotherapiesf
• Risk ratios for o TIO + OLO 5/5 μg vs OLO (0.83; p = 0.033); vs TIO 5 μg (0.92; p = NS) o TIO + OLO 2.5/5 μg vs OLO (0.69; p < 0.0001); vs TIO 2.5 μg and 5 μg (both 0.76; p = 0.0021) |
aData not included in this table; bA direct comparison between glycopyrronium and tiotropium was not conducted; cNon-inferiority comparison in per protocol population; dPre-specified superiority comparison in full analysis population; eDefined according to the 2000 Aspen Lung Conference Consensus; fStudies were not designed to assess impact of tiotropium + olodaterol fixed-dose combination on COPD exacerbations. AB antibiotics, AC active controlled; BD bronchodilators, b.i.d., twice daily; CI confidence interval, CS corticosteroids, DB double blind, DD double dummy, exac exacerbation, FEV 1 forced expiratory volume in 1 s, FVC forced vital capacity, GLOW2 GLycopyrronium bromide in COPD airWays clinical Study 2, GLY glycopyrronium, HR hazard ratio, ICS inhaled corticosteroids; IND indacaterol, INVIGORATE indacaterol: providing opportunity to reengage patients with life, IRR incidence rate ratio, LABA long-acting β2-agonist, LAMA long-acting muscarinic antagonist, MC multicenter, mMRC modified Medical Research Council, N/A not available in publication, NS not statistically significant, OL open label, OLO olodaterol, PBO placebo, PC placebo controlled, PG parallel group, POET-COPD Prevention of Exacerbations with Tiotropium in COPD, pt patient, q.d. once daily, R randomized, RR rate ratio, SALM salmeterol, SFC salmeterol fluticasone propionate combination, TIO tiotropium, UMEC umeclidinium, VI vilanterol
The first study to specifically test the efficacy of a LABA versus a LAMA in exacerbation prevention was POET, a randomized, double-blind, double-dummy, parallel-group trial in patients with moderate-to-very-severe COPD and a history of exacerbations. Compared with salmeterol (50 μg b.i.d.), tiotropium (18 μg q.d.) delayed the time to first exacerbation and significantly reduced the risk of exacerbation (187 days versus 145, respectively; hazard ratio [HR] 0.83; p < 0.001). Tiotropium also significantly prolonged the time to first severe exacerbation (HR 0.72; p < 0.001) and reduced the annual rates of severe, and moderate or severe exacerbations versus salmeterol (rate ratios [RR], 0.73 [p < 0.001] and 0.89 [p = 0.002], respectively) [53]. Similar findings were reported in INVIGORATE, where tiotropium (18 μg q.d.) significantly reduced annualized exacerbation rate versus indacaterol (150 μg q.d.) (0.73 versus 0.90; RR 1.24; p < 0.0001) [80].
To date, no direct head-to-head, LAMA versus LAMA studies have been performed. In GLOW2, open-label tiotropium (18 μg q.d.) was included as a reference arm; however, the study was not designed nor powered to test for differences between the two active treatments. Compared with placebo, exacerbation risk was reduced with glycopyrronium (HR 0.66; p = 0.001) and with tiotropium (HR 0.61; p = 0.001), although no formal comparisons between the two treatments were made [72]. In SPARK, which studied the efficacy of indacaterol/glycopyrronium (IND/GLY 110/50 μg q.d.) compared with glycopyrronium (50 μg q.d.) and tiotropium (18 μg q.d.) in patients with severe-to-very severe COPD and an exacerbation history, the rate of moderate or severe exacerbations was similar between glycopyrronium and tiotropium monotherapies (HR 1.03; p = 0.68) [24].
Dual bronchodilation versus placebo in the prevention of exacerbations
The mechanisms underlying interactions between LABAs and LAMAs have not been fully elucidated. However, β2-agonists can amplify muscarinic antagonist-mediated smooth muscle relaxation by modulating cholinergic neurotransmission and decreasing acetylcholine release, and muscarinic antagonists can augment β2-agonist-mediated bronchodilation by reducing the bronchoconstrictor effects of acetylcholine [81]. The complimentary mechanisms of action of LABAs and LAMAs elicit additive effects on lung function, and provide a rationale for combining the two agents to optimize bronchodilation. Mechanisms that most likely involve reduced airway resistance, improved inspiratory capacity and reduced hyperinflation may confer benefits in terms of exacerbations [58].
Numerous dual bronchodilators have been developed with this aim in mind. They include once-daily IND/GLY, umeclidinium/vilanterol (UMEC/VI) and tiotropium/olodaterol (TIO/OLO), and twice-daily aclidinium/formoterol (A/F) and glycopyrrolate/formoterol fumarate (GLY/F). In the USA, IND/GLY has been developed for twice-daily use [82]. Table 1 summarizes data from studies comparing the effects of dual bronchodilators on exacerbations with placebo.
Exacerbation risk was significantly reduced relative to placebo in UMEC/VI (62.5/25 and 125/25 μg) studies [22, 75] whereas the effects of A/F were less consistent across different doses (400/12 and 400/6 μg) and exacerbation assessments [23, 83]. In a prespecified analysis of pooled data from ACLIFORM and AUGMENT, the higher dose of A/F significantly reduced the rate of moderate or severe exacerbations compared with placebo, whether defined according to EXAcerbation of Chronic Pulmonary Disease Tool (EXACT) criteria (RR 0.78; p < 0.01) [84] or healthcare resource utilization (HRU; RR 0.71; p < 0.05) [83]. The higher-dose combination of A/F also prolonged time to first exacerbation of any severity defined according to HRU (HR 0.72; p < 0.05) or EXACT (HR 0.79; p < 0.05) versus placebo ([83]. There are currently no published data comparing IND/GLY or TIO/OLO with placebo [23, 83].
Dual versus single bronchodilation in the prevention of exacerbations
As shown in Table 2, LABA/LAMAs can improve exacerbation outcomes compared with monotherapy, although not all studies were designed for this objective, and the results are variable. In SPARK, an exacerbation study, the annualized rate of moderate or severe exacerbations was significantly lower with IND/GLY versus glycopyrronium (primary endpoint, RR 0.88; p = 0.038), and rates of all exacerbations (mild, moderate or severe) were significantly lower with IND/GLY versus either glycopyrronium (RR 0.85; p = 0.0012) or tiotropium (RR 0.86; p = 0.0017) [24].
By contrast, in a 24-week study not designed for studying exacerbation prevention, UMEC/VI (125/25 μg q.d.) conferred no significant benefit for exacerbation risk compared with individual monotherapies or with tiotropium (18 μg q.d.) in patients with moderate-to-very severe COPD [85]. The results of the ongoing 52-week DYNAGITO trial (NCT02296138), comparing the annualized rate of moderate-to-severe COPD exacerbations (primary endpoint) with TIO/OLO (5/5 μg q.d.) versus tiotropium (5 μg q.d.) in patients with severe-to-very severe COPD, will therefore be of interest [86].
Single bronchodilation versus ICS/LABA combinations in the prevention of exacerbations
Of the six ICS/LABA versus bronchodilator monotherapy studies, only two demonstrated significant benefits on exacerbations (Table 3) [87, 88]. It should be noted that these studies were not all designed to compare the effects of an ICS/LABA with single bronchodilation (but with placebo), and exacerbation endpoints were often secondary or exploratory.
Table 3.
Overview of key clinical trials comparing single or dual bronchodilator therapies with ICS/LABA combination therapy
| Study title | Study design | Duration | Patient population | Treatment arms | N | Exacerbation definition | Key exacerbation results |
|---|---|---|---|---|---|---|---|
|
Single BD vs ICS/LABA
LABA: salmeterol | |||||||
| Calverley et al. (2007) [87] NCT00268216 TORCH |
MC, R, DB, PG, PC, AC | 3 years | FEV1 < 60% predicted and FEV1/ FVC ≤ 0.7 | SALM 50 μg b.i.d. FP 500 μg b.i.d. SFC 500/50 μg b.i.d. PBOa |
1,542 1,551 1,546 1,545 |
Symptomatic deterioration requiring treatment with AB agents, systemic CS, hospitalization or a combination of these |
• Annual rate of moderate or severe exac: 0.97 (SALM), 0.93 (FP), 0.85 (FP/SALM), 1.13 (PBO) • Combination therapy reduced the rate of moderate or severe exac; RR: o FP/SALM vs SALM: 0.88 (95% CI 0.81, 0.95, p = 0.002) o FP/SALM vs FP: 0.91 (95% CI 0.84, 0.99, p = 0.02) • Hospitalization for exac did not differ significantly between FP/SALM and monotherapies |
| Ohar et al. (2014) [129] NCT01110200 ADC113874 |
MC, R, DB, PG, AC | 26 weeks | FEV1 < 70% predicted and FEV1/ FVC < 0.7 plus recent (≤ 14 days) history of exac requiring hospitalization for ≤ 10 days; ER observation for ≥ 24 h during which OCS/ OCS + AB administered; or physician’s office/ER visit of < 24 h with OCS/OCS + AB and 6-month history of exac-related hospitalization | SALM 50 μg b.i.d. SFC 250/50 μg b.i.d. (1:1) |
325 314 |
Worsening for ≥2 documented consecutive days of at least two of: dyspnea, sputum volume, sputum purulence, or at least one of these combined with sore throat, cold symptoms, fever or increased cough or wheeze | • No significant difference between FP/SALM vs SALM in rates of recurrent severe (ratio 0.92; 95% CI 0.58, 1.45) or moderate/severe (ratio 0.82; 95% CI 0.64, 1.06) exac • No difference between FP/SALM vs SALM in time to first moderate/severe exac (HR 0.83; 95% CI 0.63, 1.09) • Annualized exac rates in patient subgroupb lower with FP/SALM (1.54) vs SALM (2.28); ratio 0.68 (95% CI 0.47, 0.97) |
| LABA: formoterol | |||||||
| Calverley et al. (2010) [130] NCT476099 |
MC, R, DB, DD, PG, AC | 48 weeks | Severe stable COPD (FEV1 30–50% predicted and FEV1/FVC ≤ 0.7) plus ≥ 1 exac requiring medical intervention (OCS and/or AB and/or ER visit and/or hospitalization) within 2–12 months before screening and to be clinically stable for 2 months before study entry | FOR 12 μg b.i.d. BDP/FOR 200/12 μg b.i.d. BUD/FOR 400/12 μg b.i.d. (1:1:1) |
239 237 242 |
Need for treatment with OCS and/or AB and/or visit/admission to hospital. | • ≥1 exac and mean rate/pt/yr similar between groups; corresponding data were o BDP/FOR: 27.6% and 0.414 o BUD/FOR: 26.9% and 0.423 o FOR: 28.3% and 0.431 • Hospitalizations for exac: 5.6% for BDP/FOR, 2.9% for BUD/FOR and 3.4% for FOR (p < 0.001 and p = 0.008 vs BDP/FOR, respectively) |
| LABA: vilanterol | |||||||
| Dransfield et al. (2013) [88] Pooled analysis Study 1 NCT01009463 HZC102871 Study 2 NCT01017952 HZC102970 |
2 x MC, R, DB, PG, AC | 1 year | FEV1 ≤ 70% predicted and FEV1/FEV ≤ 0.7 plus a documented history of ≥ 1 exac requiring treatment (systemic/OCS/AB/hospitalization) in the preceding year | VI 25 μg q.d. FF/VI 50/25 μg q.d. FF/VI 100/25 μg q.d. FF/VI 200/25 μg q.d. (1:1:1:1) |
818 820 806 811 |
Worsening symptoms of COPD (≥2 consecutive days) necessitating treatment with OCS or AB or both; severe exac were similar events that necessitated hospital admission | • Mean annual rate of moderate and severe exac was significantly lower with FF/VI vs VI alone; yearly ratios vs VI were o 0.8 (p = 0.0398) FF/VI 50/25 μg o 0.8 (p = 0.0244) FF/VI 100/25 μg o 0.7 (p = 0.0004) FF/VI 200/25 μg • Time to first moderate or severe exac longer with FF/VI 100/25 and 200/25 μg vs VI o HR 0.8 (95% CI, 0.7, 1.0, p = 0.0365) and 0.7 (95% CI, 0.5, 0.8, p = 0.0001), respectively • Exac necessitating treatment with CS significantly lower with FF/VI vs VI alone (p < 0.05 for 100/25 μg and p = 0.0009 for 200/25 μg) |
| Martinez et al. 2016 [89] NCT01313676 SUMMIT (post hoc analysis) |
MC, R, DB, PG, PC | Event-driven, mortalityc | Moderate COPD (FEV1 ≥ 50– ≤ 70% predicted; FEV1/FEV ≤ 0.7) and a history of/multiple risk factors for CV diseased | FF/VI 100/25 μg q.d. FF 100 μg q.d. VI 25 μg q.d. PBOa |
4121 4135 4118 4111 |
Moderate exac: treated with AB and/or systemic CS; severe exac: required hospitalization | • % reduction in moderate/severe exacerbations compared with PBO: 12% (95% CI 4, 19) for FF; 10% (95% CI 2, 18) for VI; and 29% (95% CI 22, 35) for FF/VI • % reduction in exacerbations requiring hospital admissions compared with PBO: 18% (95% CI 3, 31) for FF; 20% (95% CI 5, 32) for VI; and 27% (95% CI 13, 39) for FF/VI • FF/VI reduced the rate of moderate/severe exacerbations by 19% vs FF (95% CI 12, 26, p < 0.001) and by 21% vs VI (95% CI 14, 28, p < 0.001) • FF/VI reduced the % of exacerbations requiring hospital admissions by 11% vs FF (95% CI –6, 25, p = 0.204) and by 9% vs VI (95% CI –8, 25, p = 0.282) |
| LAMA: tiotropium | |||||||
| Wedzicha et al. (2008) [54] NCT00361959 INSPIRE |
MC, R, DB, DD, PG | 2 years | Severe and very severe COPD (FEV1 < 50% predicted) and mMRC score ≥ 2 | TIO 18 μg q.d. SFC 500/50 μg b.i.d. |
665 658 |
Defined by HCRU: episodes that required treatment with OCS and/or AB or hospitalization | • No difference in overall rate between FP/SALM (1.28/yr) and TIO (1.32/yr) • Exac requiring AB with FP/SALM vs TIO: 0.97 vs 0.82/yr (p = 0.028) • Exac requiring systemic CS with FP/SALM vs TIO: 0.69 vs 0.85/yr (p = 0.039) • Hospitalizations: 16% with FP/SALM vs 13% with TIO (p = NS) |
| Study title | Study design | Duration | Patient population | Treatment arms | N | Exacerbation definition | Key exacerbation results |
|
Dual BD vs ICS/LABA
Indacaterol/glycopyrronium | |||||||
| Bateman et al. and Banerji et al. (2014) [90, 131] NCT01315249 ILLUMINATE (post hoc analysis) |
MC, R, DB, DD, PG | 26 weeks | Moderate-to-severe COPD (FEV1 ≥ 40%– < 80% predicted and FEV1/FEV < 0.7) | IND/GLY 110/50 μg q.d. SFC 500/50 μg b.i.d. |
258 264 |
Defined by modified Anthonisen criteriae (increased dyspnea, sputum production and sputum purulence) | • No significant difference between treatments; RR (IND/GLY vs FP/SALM) of moderate/severe exac: 0.80 (95% CI 0.41, 1.56) and all exac: 0.69 (95% CI 0.44, 1.07) • IND/GLY reduced risk of time to first exac by 35% vs FP/SALM (HR 0.65; 95% CI 0.44, 0.96, p = 0.03) |
| Zhong et al. (2015) [28] NCT01709903 LANTERN |
MC, R, DB, DD, PG | 26 weeks | Moderate-to-severe COPD (FEV1 ≥ 30– < 80% predicted and FEV1/FEV < 0.7), mMRC score ≥ 2 and history of ≤ 1 exac in the previous year | IND/GLY 110/50 μg q.d. SFC 500/50 μg b.i.d. |
372 372 |
Worsening of symptoms captured via eDiary; defined by Anthonisen criteriae. Moderate exac: requiring treatment with systemic CS and/or AB; severe exac: requiring hospitalization/ER visit > 24 hours | • Annualized rate of moderate or severe exac significantly lower with IND/GLY vs FP/SALM (31% reduction; p = 0.048) • IND/GLY prolonged time to first moderate or severe exac by 35% (p = 0.028) • In patients with a history of moderate or severe exac, annualized rate |
| Wedzicha et al. (2016) [29] NCT01782326 FLAME |
MC, R, DB, DD, PG, NI | 52 weeks | Moderate-to-very severe COPD (FEV1 ≥ 25– < 60% predicted and FEV1/FEV < 0.7), mMRC score ≥ 2 and documented history of ≥ 1 exac treated with systemic CS and/or AB in previous year | IND/GLY 110/50 μg q.d. SFC 500/50 μg b.i.d. |
1,680 1,682 |
Defined according to Anthonisen criteriae. Categorized as mild (worsening of symptoms for >2 consecutive days but not requiring treatment), moderate (treated with systemic CS and/or AB) or severe (requiring hospital \admission/ER visit of >24 h plus systemic CS and/or AB) | • Annual rate of all exac: IND/GLY (3.59) was non-inferior to FP/SALM (4.03): representing an 11% lower rate (RR, 0.89, 95% CI 0.83, 0.96, p = 0.003) • IND/GLY showed superiority to FP/SALM as the upper limits of the 95% CIs for the primary endpoint RRs were less than 1 • IND/GLY had a longer time to first exac than the FP/SALM group (median, 71 days [95% CI 60, 82] vs. 51 days [95% CI 46, 57]: HR 0.84 (95% CI 0.78, 0.91, representing a 16% lower risk; p < 0.001) |
| Aclidinium/formoterol | |||||||
| Vogelmeier et al. (2015) [132] NCT01908140 AFFIRM |
MC, R, DB, DD, AC | 24 weeks | Symptomatic pts with FEV1 < 80%, FEV1/FVC < 0.7 and CAT ≥ 10 | A/F 400/12 μg b.i.d. SFC 500/50 μg b.i.d. |
468 463 |
Defined by HCRU or identified using EXACT | • ≥1 exac: comparable between treatment groups: o HCRU: 15.8% (A/F) vs 16.6% (FP/SALM); OR 0.95 o EXACT: 37.8% (A/F) vs 39.5% (FP/SALM); OR 0.94 |
| Umeclidinium/vilanterol | |||||||
| Donohue et al. (2015) [26] Study 1 NCT01817764 DB2114930 Study 2 NCT01879410 DB2114951 |
MC, R, DB, DD, PG | 12 weeks | Moderate-to-severe COPD (FEV1 ≥ 30– ≤ 70% predicted), mMRC score ≥ 2, no exacerbations in the previous year | UMEC/VI 62.5/25 μg q.d. SFC 250/50 μg b.i.d. |
353 and 349 353 and 348 |
Captured only as a safety event. Defined as an acute worsening of COPD symptoms requiring use of AB, systemic CS, and/or emergency treatment or hospitalization |
NCT01817764 • Exac rate was the same in each treatment group: o 3% (UMEC/VI) vs 3% (FP/SALM) NCT01879410 • Exac rate was the same in each treatment group: o 3% (UMEC/VI) vs 3% (FP/SALM) |
| Singh et al. (2015) [92] NCT01822899 |
MC, R, DB, DD, PG | 12 weeks | Moderate-to-severe COPD (FEV1 ≥ 30– ≤ 70% predicted and FEV1/FVC < 0.7), mMRC score ≥ 2, no exacerbations in the previous year | UMEC/VI 62.5/25 μg q.d. SFC 500/50 μg b.i.d. |
358 358 |
Captured only as a safety event. Not defined | • Exac rate was similar between treatment groups: o 2% (UMEC/VI) vs <1% (FP/SALM) |
| Tiotropium/olodaterol | |||||||
| Beeh et al. (2016) [133] NCT01969721 ENERGITO |
MC, R, DB, DD, PG | 12 weeks | Moderate-to-severe COPD (FEV1 ≥ 30– < 80% predicted and FEV1/FEV < 0.7), no exacerbations in the previous 3 months | TIO/OLO 5/5 μg q.d. TIO/OLO 5/2.5 μg q.d. SFC 500/50 μg b.i.d. SFC 250/50 μg b.i.d. |
221 215 219 212 |
Captured only as a safety event as ‘COPD worsening’ | • Exac rate was similar among each of the high- and low-dose groups: o 9.0% (TIO/OLO 5/5 μg) and 8.7% (FP/SALM 500/50 μg) o 5.6% (TIO/OLO 5/2.5 μg) and 4.2% (FP/SALM 250/50 μg) |
aData not included in table; bSubgroup of 373 patients with baseline post-bronchodilator % predicted FEV1 ≥ 30% and history of prior ICS; cPatients expected to contribute 15–44 months of study time; dFor patients aged ≥40 years: any one of established coronary artery disease, established peripheral vascular disease, previous stroke, previous myocardial infarction or diabetes mellitus with target organ disease and for patients aged ≥60 years, any one of those for ≥40 years of age or two of the following: treatment for hypercholesterolemia, hypertension, diabetes mellitus or peripheral vascular disease; eAnthonisen NR et al. Ann Intern Med 1987;106:196–204. AB antibiotics, AC active controlled, A/F aclidinium/formoterol, BD bronchodilator, BDP beclomethasone/formoterol; b.i.d. twice daily, BUD/FF budesonide/formoterol, CAT COPD Assessment Test, CI confidence interval, CS corticosteroids, CV cardiovascular, DB double blind, DD double dummy, ER emergency room, exac exacerbation(s), FEV 1 forced expiratory volume in 1 s, FVC forced vital capacity, EXACT EXAcerbations of Chronic pulmonary disease Tool, FF fluticasone furoate, FOR formoterol, FP fluticasone propionate, GLY glycopyrronium, HCRU healthcare resource utilization, HR hazard ratio, ICS inhaled corticosteroids, IND indacaterol, INSPIRE Investigating New Standards for Prophylaxis in Reducing Exacerbations, LABA long-acting β2-agonist, LAMA long-acting muscarinic antagonist, MC multicenter, mMRC modified Medical Research Council, NI non-inferiority, NS not statistically significant, OCS oral corticosteroids, OLO olodaterol, OR odds ratio, PBO placebo, PC placebo controlled, PG parallel group, q.d. once daily, R randomized, RR rate ratio, SALM salmeterol; SFC. Salmeterol/fluticasone propionate combination, SUMMIT Study to Understand Mortality and Morbidity in COPD, TIO tiotropium, TORCH Towards a Revolution in COPD Health, UMEC umeclidinium, VI vilanterol, yr year
One of the most robust studies was TORCH (3 years), which studied deaths (any cause) as the primary outcome, as well as exacerbation frequency between treatments. In TORCH, salmeterol/fluticasone propionate combination (SFC 50/500 μg b.i.d.) significantly reduced the annual rate of moderate or severe exacerbations compared with salmeterol (50 μg b.i.d.; RR 0.88; p = 0.002) [87].
In a pooled analysis of two 1-year trials, in which the primary endpoint was the yearly rate of moderate and severe exacerbations, fluticasone furoate/vilanterol (FF/VI; 50/25 μg, 100/25 μg and 200/25 μg q.d.) significantly reduced the rate of moderate or severe exacerbations compared with vilanterol (25 μg q.d.; p < 0.05 for all three doses) [88]. FF/VI (100/25 μg and 200/25 μg q.d.) also significantly prolonged time to first moderate or severe exacerbation versus vilanterol monotherapy (HR 0.8; p = 0.0365 and HR 0.7; p = 0.0001, respectively), and significantly reduced the number of exacerbations requiring systemic corticosteroids (p < 0.05 and p = 0.0009, respectively) [88].
In a post-factorial analysis of SUMMIT, FF/VI (100/25 μg q.d.) significantly reduced the rate of moderate or severe exacerbations versus fluticasone (100 μg q.d.; p < 0.001) and versus vilanterol (25 μg q.d.; p < 0.001) [89].
Only INSPIRE has compared an ICS/LABA with LAMA monotherapy in exacerbation prevention [54]. No significant difference was observed between SFC (50/500 μg b.i.d.) and tiotropium (18 μg q.d.) in HRU exacerbation rate (1.28 and 1.32, respectively) in this study [54].
Dual bronchodilation versus ICS/LABA combinations in the prevention of exacerbations
Exacerbation data are available from a number of studies comparing a LABA/LAMA with an ICS/LABA (Table 3). In a post-hoc analysis of ILLUMINATE (26 weeks), IND/GLY (110/50 μg q.d.) significantly reduced the time to first exacerbation (HR 0.65; p = 0.03) versus SFC (50/500 μg b.i.d.), in patients with moderate–to-severe COPD and no exacerbations in the previous year [90]. Likewise, in LANTERN (26 weeks), IND/GLY (110/50 μg q.d.) significantly reduced the rate of moderate or severe exacerbations (RR 0.69; p = 0.048) compared with SFC (50/500 μg b.i.d.), in patients with moderate-to-severe COPD and ≤1 exacerbations in the previous year [28]. In a post-hoc analysis of pooled data from LANTERN and ILLUMINATE, the annualized rate of moderate or severe exacerbations was significantly lower with IND/GLY versus SFC, in both the whole population (p = 0.02) and in subgroups of patients classified as either GOLD Group B (p = 0.16) or GOLD Group D (p = 0.05; patients were classified according to GOLD 2009 and 2010 for ILLUMINATE and LANTERN, respectively). Furthermore, IND/GLY delayed the time to first moderate or severe exacerbation compared with SFC in the overall population and in GOLD Group B and GOLD Group D subgroups [91].
The most recent study comparing a LABA/LAMA with an ICS/LABA was FLAME, which specifically studied the differences in exacerbations between IND/GLY (110/50 μg q.d) and SFC (50/500 μg b.i.d.) as the primary outcome, and included an enriched patient population at high risk of exacerbation (≥1 exacerbation in the previous year) [29]. Compared with SFC, IND/GLY significantly reduced the rate of all COPD exacerbations (RR 0.89; p = 0.003), and the rate of moderate or severe exacerbations (RR 0.83; p < 0.001). Additionally, IND/GLY significantly prolonged the time to first exacerbation (HR 0.84; p < 0.001). The time to first moderate or severe exacerbation (HR 0.78; p < 0.001), and time to the first severe exacerbation (HR 0.81; p = 0.046) were also significantly prolonged with IND/GLY versus SFC. Treatment benefit or time to first exacerbation was detected as early as 4 weeks. Compared with SFC, IND/GLY numerically, but not significantly, reduced the rate of exacerbations in patients with a history of ≥2 exacerbations in the previous year, (19% of the patient population). However, it is worth noting that this was a subgroup analysis and FLAME was not powered to detect treatment differences in subgroups [29].
In a 24-week trial comparing A/F (400/12 μg b.i.d.) with SFC (50/500 μg b.i.d), a similar proportion of patients experienced at least one exacerbation in the A/F and SFC groups, regardless of exacerbation definition (HRU or EXACT criteria) [27]. However, the study was not designed to test exacerbations.
To date, there have been no long-term studies examining the effects of UMEC/VI or TIO/OLO on exacerbations in at-risk patients. Two 12-week studies comparing the efficacy of UMEC/VI (62/25 μg q.d.) and SFC (50/250 μg b.i.d.) captured exacerbations as safety data and did not perform statistical testing [26, 88]. Likewise, in ENERGITO (a 12-week, randomized, double-blind, four-treatment, crossover study comparing the efficacy of TIO/OLO [5/5 μg and 5/2.5 μg q.d.] with SFC [50/500 μg and 50/250 μg b.i.d.]), exacerbations were captured as safety data (reported as an adverse event of ‘COPD worsening’) [92]. Further long-term studies comparing UMEC/VI and TIO/OLO are required.
Triple therapy in the prevention of exacerbations
Evidence for the efficacy of triple therapy (ICS/LABA/LAMA) in exacerbation prevention is currently limited. Nevertheless, in TRILOGY (1 year), the adjusted annual rate of moderate-to-severe exacerbations was significantly reduced following step-up to triple therapy (beclomethasone dipropionate/formoterol fumarate/glycopyrronium bromide [BDP/FF/GB] 100/6/12.5 μg two actuations b.i.d.) compared with continuing on BDP/FF 100/6 μg two actuations b.i.d. (0.41 versus 0.53, respectively; RR 0.77; p = 0.005) [93].
Similarly, TRINITY (1 year) compared the fixed-dose combination [FDC] of BDP/FF/GB (100/6/12.5 μg b.i.d.) with a free combination of the same agents and LAMA monotherapy (tiotropium 18 μg q.d.) in patients with severe–to-very severe COPD. Compared with TIO, BDP/FF/GB FDC significantly reduced the rate of moderate/severe exacerbations (0.46 versus 0.57, respectively; RR 0.80; p = 0.0025). BDP/FF/GB and BDP/FF + TIO showed a similar effect on moderate/severe exacerbations (RR 1.01) [94]. Findings are eagerly anticipated from the ongoing InforMing the PAthway of COPD Treatment (IMPACT) study, comparing the rate of moderate and severe exacerbations between FF/UMEC/VI and FF/VI or UMEC/VI over 52-weeks in symptomatic COPD patients with an exacerbation in the previous 12 months [95].
Other Pharmacological Treatments In The Prevention Of Exacerbations
Mucolytics in the prevention of exacerbations
Mucolytics are oral medicines designed to reduce mucus and sputum viscosity, thereby making it easier for patients to cough up mucus and clear it from the airways [96]. The current GOLD recommendations recognize that regular use of mucolytics may be beneficial in patients not receiving ICS, to reduce exacerbations and improve health status [2].
The antioxidant N-acetylcysteine (NAC; 600 mg/day), is recommended by the American College of Chest Physicians and Canadian Thoracic Society for patients with moderate-to-severe COPD and a history of two or more exacerbations in the previous 2 years [97]. However, NAC 600 mg/day is currently not approved for use in the USA. In a 6-month study, the number of exacerbations was reduced by 41% with standard therapy plus NAC versus standard therapy alone, and fewer patients experienced at least one exacerbation (46 versus 63 patients, respectively) [98]. In PANTHEON (1 year), NAC (600 mg/b.i.d.) significantly reduced the annual rate of exacerbations versus placebo (1.16 versus 1.49 exacerbations per patient-year; p = 0.0011) [99]. By contrast, in BRONCUS (3 year), a randomized, placebo-controlled study, there was no difference between NAC (600 mg/day) and placebo in the number of exacerbations per year (a primary outcome, [1.25 versus 1.29; p = 0.85]), although sub-group analyses suggested that NAC might have reduced exacerbation rate in patients not receiving ICS treatment [100]. In a 2015 meta-analysis of 13 studies (N = 4155), NAC significantly reduced the relative risk of exacerbations in patients with COPD and/or chronic bronchitis. The authors concluded that a dose of 600 mg b.i.d. should be used in patients who have both chronic bronchitis and COPD, whereas the standard 600 mg/day dose should suffice for patients who have chronic bronchitis alone [101]. Accordingly, one study showed that NAC (600 mg b.i.d.) was more effective than placebo in reducing exacerbation risk and prolonging time to first exacerbation in high-risk patients (GOLD Groups C and D, according to the 2011 GOLD recommendations), but not low-risk patients [102].
Two studies have examined the effects of erdosteine on exacerbations. EQUALIFE, an 8-month, randomized, double-blind trial, demonstrated that patients taking erdosteine (300 mg b.i.d.) had significantly fewer exacerbations and spent fewer days in hospital than those on placebo [103]. More recently, Moretti et al demonstrated that 10-day treatment with erdosteine (900 mg/day) was associated with a 39% lower risk of exacerbations in the 2 months post-discharge and a significant delay in time to first exacerbation at post-discharge days 30 (p = 0.009) and 60 (p = 0.075) compared with placebo, in patients hospitalized following acute exacerbation [104]. In a 6-month, randomized trial, a significantly higher proportion of patients experienced no exacerbations when treated with continuous carbocysteine lysine salt monohydrate (SCMC-Lys; 2.7 g q.d.) compared with placebo (p < 0.001) [105]. Similarly, in PEACE (1 year), the annualized rate of exacerbations was significantly lower with carbocysteine (1500 mg/day) than with placebo, representing a 25% reduction in risk (p = 0.004) [106].
Phosphodiesterase-4 inhibitors in the prevention of exacerbations
Phosphodiesterase-4 (PDE-4) inhibitors can inactivate immune and inflammatory cells by blocking the metabolism of cyclic adenosine monophosphate (cAMP) [107]. GOLD recommends addition of the selective, long-acting PDE-4 inhibitor roflumilast to a ICS/LABA/LAMA in GOLD Group D patients who continue to experience exacerbations despite triple therapy, particularly patients with a forced expiratory volume in one second (FEV1) <50% predicted, chronic bronchitis, and ≥1 hospitalization for an exacerbation in the previous year [2].
A meta-analysis of 13 studies suggested that roflumilast (500 μg q.d.) was more effective than placebo in reducing the rate of acute exacerbations (p < 0.001) [107]. In REACT, roflumilast (500 μg q.d.) reduced the rate of moderate-to-severe exacerbations by 13.2% versus placebo in patients with severe COPD, chronic bronchitis and at risk of frequent and severe exacerbations, and receiving ICS/LABA treatment with or without tiotropium (Poisson regression analysis, p = 0.0529) [108]. In a post-hoc analysis of RE(2)SPOND (52 weeks), roflumilast (500 μg q.d.) significantly reduced the rate of moderate or severe exacerbations versus placebo in patients with severe-to-very severe COPD and chronic bronchitis, a history of >3 exacerbations and/or ≥1 hospitalizations in the prior year [109]. Roflumilast is indicated as a maintenance treatment (added on to bronchodilator therapy) in adults with severe COPD associated with chronic bronchitis, and a history of frequent exacerbations [110].
Macrolides in the prevention of exacerbations
Macrolides are antibiotics with antimicrobial, anti-inflammatory, and immunomodulating effects. GOLD 2017 recommends the addition of a macrolide to an ICS/LABA/LAMA regimen in GOLD Group D patients who are former smokers and continue to suffer exacerbations despite triple therapy [2]. In COPD, the best studied macrolide is azithromycin. COLUMBUS (1 year) demonstrated that exacerbation rate was significantly reduced with azithromycin (500 mg three times/week), compared with placebo (odds ratio = 0.58, p = 0.001), in patients with COPD who had received treatment for ≥3 exacerbations in the previous year despite optimal inhalation therapy [111]. Similar findings were reported in a 1-year, randomized, controlled trial in patients with COPD at risk for exacerbations, where azithromycin (250 mg q.d.) significantly delayed median time to first exacerbation (266 versus 174 days; p < 0.001), and significantly reduced the frequency of exacerbations (1.48 versus 1.83; p = 0.01) versus placebo [112]. Similar results have been reported in other, smaller studies comparing erythromycin and placebo [113, 114].
Appropriate use of ICS
Much evidence supports the use of ICS in patients with persistent asthma, yet the role of ICS in preventing exacerbations of COPD is less clear [19, 115]. Various methodological issues in trial design and/or statistical analysis affect results and make subsequent study interpretation difficult [116].
However, data are emerging to suggest that raised blood or sputum eosinophil levels could predict a positive response (i.e. a reduction in exacerbations) to ICS/LABA versus LABA monotherapy, or predict any deleterious effects ICS withdrawal may have [117–121].
The potential association between this ‘eosinophilic phenotype’ and a positive response to ICS requires further investigation, as the effect has not been consistent across studies within the same analysis [118], and may only be present in patients with a history of ≥2 exacerbations in addition to raised eosinophil levels [121]. Prospective analysis of FLAME (which excluded patients with a previous diagnosis of asthma and/or a blood eosinophil count >600/mm3), demonstrated that IND/GLY was superior to SFC in reducing the rate of moderate-to-severe exacerbations, regardless of baseline eosinophil levels [29]. Thus, further studies are needed before any recommendations can be made regarding the potential use of ICS in specific sub-populations.
Proposed Treatment Paradigm
Figure 1 shows a proposed treatment paradigm for exacerbation prevention based on the evidence presented and centered on optimal use of bronchodilation, which has previously been published [122–124].
Fig. 1.
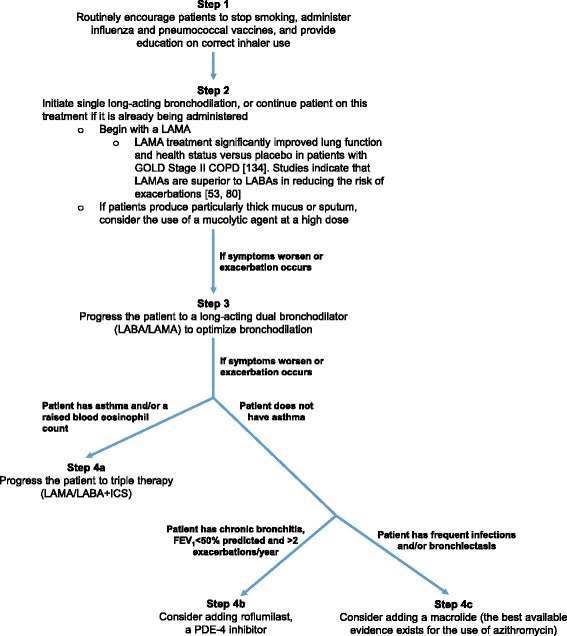
A proposed treatment algorithm for the treatment of chronic obstructive pulmonary disease (COPD). FEV1, forced expiratory volume in 1 s; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroids; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; PDE-4, phosphodiesterase-4 [134]
Conclusions
Not only do COPD exacerbations negatively impact the underlying disease course, but they also have a detrimental effect on patients’ lives, resulting in lung function decline, increased risk of mortality and poor health status. While there has been a tendency to recommend the use of ICS for patients at high risk of exacerbations, ICS are associated with a myriad of side effects such as pneumonia. However, (and as recognized in the updated GOLD strategy document) evidence is emerging that suggests there may be more appropriate treatment strategies for many at risk patients, including a LABA/LAMA combination.
The proposed treatment paradigm for exacerbation prevention is centered on optimizing bronchodilation as an initial pharmacological step, first with a LAMA, and subsequently with a dual LABA/LAMA should symptoms worsen or exacerbation occur. Only if patients continue to suffer exacerbations do we suggest the addition of an ICS or a PDE-4 inhibitor, depending on patient profile/phenotype. It is possible that a subgroup of patients with COPD who have raised blood or sputum eosinophils may respond better than others to ICS, although current data are still preliminary and somewhat contradictory. Future studies are warranted to better define the groups who may benefit from ICS, and to identify the mechanisms by which bronchodilation reduces exacerbations.
Acknowledgements
The authors were assisted in the preparation of the manuscript by David McMinn, a professional medical writer at CircleScience, an Ashfield company, part of UDG Healthcare plc.
Funding
Medical writing support was funded by Novartis Pharma AG (Basel, Switzerland).
Availability of data and materials
Not applicable.
Authors’ contributions
Authors discussed and agreed to the scope of the manuscript and contributed to the development of the manuscript at all stages. All authors read and approved the final manuscript.
Competing interests
Marc Miravitlles has received speaker fees from Almirall, Boehringer Ingelheim, AstraZeneca, Chiesi, GlaxoSmithKline, Menarini, Teva, Grifols and Novartis, and consulting fees from Almirall, Bayer Schering, Boehringer Ingelheim, GlaxoSmithKline, Gebro Pharma, CLS Behring, Cipla, MediImmune, Teva, Takeda, Novartis and Grifols.
Antonio Anzueto has received consultant fees from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, and Novartis.
José R. Jardim has received speaker fees from, Boehringer Ingelheim, AstraZeneca, Chiesi, Teva, ACHE, Grifols, Zambon CLS Behring and Novartis, and consulting fees from Boehringer Ingelheim, Zambon, CLS Behring, Teva, EMS, ACHE, Novartis, Chiesi Farmoquimica and Grifols.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Not applicable.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- A/F
Aclidinium/formoterol
- b.i.d.
Twice daily
- BDP/FF/GB
Beclomethasone dipropionate/formoterol fumarate/glycopyrronium bromide
- CAMP
Cyclic adenosine monophosphate
- COPD
Chronic obstructive pulmonary disease
- EXACT
EXAcerbations of Chronic obstructive pulmonary disease Tool
- FEV1
Forced expiratory volume in 1 second
- FF/VI
Fluticasone furoate/vilanterol
- GLY/F
Glycopyrronium/formoterol
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- HR
Hazard ratio
- HRU
Healthcare resource utilization
- ICS
Inhaled corticosteroids
- IMPACT
InforMing the PAthway of COPD Treatment
- IND/GLY
Indacaterol/Glycopyrronium
- LABA
Long-acting β2-agonist
- LAMA
Long-acting muscarinic antagonist
- NAC
N-acteylcysteine
- OR
Odds ratio
- PDE-4
Phosphodiesterase-4
- PR
Pulmonary rehabilitation
- q.d.
Once daily
- RR
Rate ratio
- SCMC-Lys
Carbocysteine lysine salt monohydrate
- SFC
Salmeterol/Fluticasone propionate combination
- TIO/OLO
Tiotropium/Olodaterol
- UMEC/VI
Umeclidinium/Vilanterol
Contributor Information
Marc Miravitlles, Email: mmiravitlles@vhebron.net.
Antonio Anzueto, Email: anzueto@uthscsa.edu.
José R. Jardim, Email: jardimpneumo@gmail.com
References
- 1.Wedzicha JA, Seemungal TA. COPD exacerbations: defining their cause and prevention. Lancet. 2007;370:786–6. doi: 10.1016/S0140-6736(07)61382-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelmeier CF, Criner GJ, Martínez FJ, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, Chen R, Decramer M, Fabbri LM, Frith P, Halpin DM, López Varela MV, Nishimura M, Roche N, Rodríguez-Roisin R, Sin DD, Singh D, Stockley R, Vestbo J, Wedzicha JA, Agustí A. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch Bronconeumol. 2017;53:128–149. doi: 10.1016/j.arbres.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Sapey E, Stockley RA. COPD exacerbations. 2: aetiology. Thorax. 2006;61:250–8. doi: 10.1136/thx.2005.041822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60:925–31. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seemungal TA, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–22. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 6.Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez-Sala JL, Masa F, Verea H, Murio C, Ros F, Vidal R. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax. 2004;59:387–95. doi: 10.1136/thx.2003.008730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celli BR, Thomas NE, Anderson JA, Ferguson GT, Jenkins CR, Jones PW, Vestbo J, Knobil K, Yates JC, Calverley PM. Effect of pharmacotherapy on rate of decline of lung function in chronic obstructive pulmonary disease: results from the TORCH study. Am J Respir Crit Care Med. 2008;178:332–8. doi: 10.1164/rccm.200712-1869OC. [DOI] [PubMed] [Google Scholar]
- 8.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57:847–52. doi: 10.1136/thorax.57.10.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhamane AD, Moretz C, Zhou Y, Burslem K, Saverno K, Jain G, Renda A, Kaila S. COPD exacerbation frequency and its association with health care resource utilization and costs. Int J Chron Obstruct Pulmon Dis. 2015;10:2609–18. doi: 10.2147/COPD.S90148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miravitlles M, Murio C, Guerrero T, Gisbert R. Pharmacoeconomic evaluation of acute exacerbations of chronic bronchitis and COPD. Chest. 2002;121:1449–55. doi: 10.1378/chest.121.5.1449. [DOI] [PubMed] [Google Scholar]
- 11.Wedzicha JA, Brill SE, Allinson JP, Donaldson GC. Mechanisms and impact of the frequent exacerbator phenotype in chronic obstructive pulmonary disease. BMC Med. 2013;11:181. doi: 10.1186/1741-7015-11-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurst JR, Vestbo J, Anzueto A, Locantore N, Müllerova H, Tal-Singer R, Miller B, Lomas DA, Agusti A, MacNee W, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363:1128–38. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 13.Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, Quintano JA, Trigueros JA, Cosío BG, Casanova C, Riesco JA, Simonet P, Rigau D, Soriano JB, Ancochea J. Spanish COPD guidelines (GesEPOC) 2017. Pharmacological treatment of stable chronic obstructive pulmonary disease. Arch Bronconeumol. 2017;53:324–35. [DOI] [PubMed]
- 14.National Institute for Health and Care Excellence (NICE). Chronic obstructive pulmonary disease. Management of chronic obstructive pulmonary disease in adults in primary and secondary care. NICE clinical guideline 101 (partial update). Updated June 2010. Available at: https://www.nice.org.uk/guidance/cg101. Accessed 5 June 2017.
- 15.Montes De Oca M, Lopez Varela MV, Acuna A, Schiavi E, Rey MA, Jardim J, Casas A, Tokumoto A, Torres Duque CA, Ramirez-Venegas A, et al. ALAT-2014 Chronic Obstructive Pulmonary Disease (COPD) Clinical Practice Guidelines: questions and answers. Arch Bronconeumol. 2015;51:403–16. doi: 10.1016/j.arbres.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell DE, Hernandez P, Kaplan A, Aaron S, Bourbeau J, Marciniuk D, Balter M, Ford G, Gervais A, Lacasse Y, et al. Canadian Thoracic Society recommendations for management of chronic obstructive pulmonary disease - 2008 update - highlights for primary care. Can Respir J. 2008;15:1A–8A. doi: 10.1155/2008/973062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price D, West D, Brusselle G, Gruffydd-Jones K, Jones R, Miravitlles M, Rossi A, Hutton C, Ashton VL, Stewart R, Bichel K. Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns. Int J Chron Obstruct Pulmon Dis. 2014;9:889–905. doi: 10.2147/COPD.S62750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vestbo J, Vogelmeier C, Small M, Higgins V. Understanding the GOLD 2011 Strategy as applied to a real-world COPD population. Respir Med. 2014;108:729–36. doi: 10.1016/j.rmed.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Price D, Yawn B, Brusselle G, Rossi A. Risk-to-benefit ratio of inhaled corticosteroids in patients with COPD. Prim Care Respir J. 2013;22:92–100. doi: 10.4104/pcrj.2012.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buhl R, Maltais F, Abrahams R, Bjermer L, Derom E, Ferguson G, Fležar M, Hébert J, McGarvey L, Pizzichini E, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2–4) Eur Respir J. 2015;45:969–79. doi: 10.1183/09031936.00136014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bateman ED, Ferguson GT, Barnes N, Gallagher N, Green Y, Henley M, Banerji D. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42:1484–94. doi: 10.1183/09031936.00200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mg in COPD. Respir Med. 2013;107:1538–46. doi: 10.1016/j.rmed.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 23.Singh D, Jones PW, Bateman ED, Korn S, Serra C, Molins E, Caracta C, Gil EG, Leselbaum A. Efficacy and safety of aclidinium bromide/formoterol fumarate fixed-dose combinations compared with individual components and placebo in patients with COPD (ACLIFORM-COPD): a multicentre, randomised study. BMC Pulm Med. 2014;14:178. doi: 10.1186/1471-2466-14-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandstöm T, Taylor AF, D’Andrea P, Arrasate C, Chen H, Banerji D. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1:199–209. doi: 10.1016/S2213-2600(13)70052-3. [DOI] [PubMed] [Google Scholar]
- 25.Vogelmeier CF, Bateman ED, Pallante J, Alagappan VK, D’Andrea P, Chen H, Banerji D. Efficacy and safety of once-daily QVA149 compared with twice-daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. Lancet Respir Med. 2013;1:51–60. doi: 10.1016/S2213-2600(12)70052-8. [DOI] [PubMed] [Google Scholar]
- 26.Donohue JF, Worsley S, Zhu CQ, Hardaker L, Church A. Improvements in lung function with umeclidinium/vilanterol versus fluticasone propionate/salmeterol in patients with moderate-to-severe COPD and infrequent exacerbations. Respir Med. 2015;109:870–81. doi: 10.1016/j.rmed.2015.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Vogelmeier C, Paggiaro PL, Dorca J, Sliwinski P, Mallet M, Kirsten AM, Beier J, Seoane B, Segarra RM, Leselbaum A. Efficacy and safety of aclidinium/formoterol versus salmeterol/fluticasone: a phase 3 COPD study. Eur Respir J. 2016;48:1030–9. doi: 10.1183/13993003.00216-2016. [DOI] [PubMed] [Google Scholar]
- 28.Zhong N, Wang C, Zhou X, Zhang N, Humphries M, Wang C, Thach C, Patalano F, Banerji D. LANTERN: a randomized study of QVA149 versus salmeterol/fluticasone combination in patients with COPD. Int J Chron Obstruct Pulmon Dis. 2015;10:1015–26. doi: 10.2147/COPD.S84436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, Thach C, Fogel R, Patalano F, Vogelmeier C. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374:2222–34. doi: 10.1056/NEJMoa1516385. [DOI] [PubMed] [Google Scholar]
- 30.Welte T, Vogelmeier C, Dahl R, Chapman KR, Rudolf M, Mehta R, D’Andrea P, Chen H, Banerji D. Once-daily QVA149 has a good safety profile in patients with COPD. Eur Respir J. 2013;42(Suppl 57):143s–44s. [Google Scholar]
- 31.Ferguson GT, Barnes N, Mehta R, D’Andrea P, Chen H, Banerji D. Cardio- and cerebro-vascular safety of QVA149: Results from a pooled analysis. Eur Respir J. 2013;42(Suppl 57):878s. [Google Scholar]
- 32.Korn S, Kerwin E, Donohue J, Shreshta P, Leselbaum A, Lei A. Safety of aclidinium bromide/formoterol fumarate fixed-dose combination in COPD: pooled analyses of three Phase III studies. Eur Respir J. 2014;44(Suppl 58):P2416. [Google Scholar]
- 33.Buhl R, Abrahams R, Bjermer L, Derom E, Flezar M, Hebert J, Veale A, Groenke L, Hamilton A, Tetzlaff K, et al. Safety of once-daily tiotropium and olodaterol fixed-dose combination via the Respimat in chronic obstructive pulmonary disease in two 1-year studies. Eur Respir J. 2014;44(Suppl 59):P922. [Google Scholar]
- 34.Barrecheguren M, Monteagudo M, Ferrer J, Borrell E, Llor C, Esquinas C, Miravitlles M. Treatment patterns in COPD patients newly diagnosed in primary care. A population-based study. Respir Med. 2016;111:47–53. doi: 10.1016/j.rmed.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Au DH, Bryson CL, Chien JW, Sun H, Udris EM, Evans LE, Bradley KA. The effects of smoking cessation on the risk of chronic obstructive pulmonary disease exacerbations. J Gen Intern Med. 2009;24:457–63. doi: 10.1007/s11606-009-0907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Petty TL. The history of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:3–14. doi: 10.2147/copd.2006.1.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anthonisen NR, Skeans MA, Wise RA, Manfreda J, Kanner RE, Connett JE, Lung Health Study Research G The effects of a smoking cessation intervention on 14.5-year mortality: a randomized clinical trial. Ann Intern Med. 2005;142:233–9. doi: 10.7326/0003-4819-142-4-200502150-00005. [DOI] [PubMed] [Google Scholar]
- 38.Vozoris NT, Stanbrook MB. Smoking prevalence, behaviours, and cessation among individuals with COPD or asthma. Respir Med. 2011;105:477–84. doi: 10.1016/j.rmed.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 39.Scanlon PD, Connett JE, Waller LA, Altose MD, Bailey WC, Buist AS, Tashkin DP. Lung Health Study Research G. Smoking cessation and lung function in mild-to-moderate chronic obstructive pulmonary disease. The Lung Health Study. Am J Respir Crit Care Med. 2000;161:381–90. doi: 10.1164/ajrccm.161.2.9901044. [DOI] [PubMed] [Google Scholar]
- 40.Poole PJ, Chacko E, Wood-Baker RW, Cates CJ. Influenza vaccine for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2006:CD002733. [DOI] [PubMed]
- 41.Wongsurakiat P, Lertakyamanee J, Maranetra KN, Jongriratanakul S, Sangkaew S. Economic evaluation of influenza vaccination in Thai chronic obstructive pulmonary disease patients. J Med Assoc Thai. 2003;86:497–508. [PubMed] [Google Scholar]
- 42.Nichol KL, Margolis KL, Wuorenma J, Von Sternberg T. The efficacy and cost effectiveness of vaccination against influenza among elderly persons living in the community. N Engl J Med. 1994;331:778–84. doi: 10.1056/NEJM199409223311206. [DOI] [PubMed] [Google Scholar]
- 43.Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NJ. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep. 2009;58:1–52. [PubMed] [Google Scholar]
- 44.Huang CL, Nguyen PA, Kuo PL, Iqbal U, Hsu YH, Jian WS. Influenza vaccination and reduction in risk of ischemic heart disease among chronic obstructive pulmonary elderly. Comput Methods Programs Biomed. 2013;111:507–11. doi: 10.1016/j.cmpb.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 45.Santus P, Radovanovic D, Balzano G, Pecchiari M, Raccanelli R, Sarno N, Di Marco F, Jones PW, Carone M. Improvements in lung diffusion capacity following pulmonary rehabilitation in COPD with and without ventilation inhomogeneity. Respiration. 2016;92:295–307. [DOI] [PubMed]
- 46.McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015;2:CD003793. doi: 10.1002/14651858.CD003793.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puhan MA, Gimeno-Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2011;CD005305. [DOI] [PubMed]
- 48.van Ranst D, Stoop WA, Meijer JW, Otten HJ, van de Port IG. Reduction of exacerbation frequency in patients with COPD after participation in a comprehensive pulmonary rehabilitation program. Int J Chron Obstruct Pulmon Dis. 2014;9:1059–67. doi: 10.2147/COPD.S69574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Washko GR, Fan VS, Ramsey SD, Mohsenifar Z, Martinez F, Make BJ, Sciurba FC, Criner GJ, Minai O, Decamp MM, et al. The effect of lung volume reduction surgery on chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;177:164–69. doi: 10.1164/rccm.200708-1194OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zanoria SJ, ZuWallack R. Directly measured physical activity as a predictor of hospitalizations in patients with chronic obstructive pulmonary disease. Chron Respir Dis. 2013;10:207–13. doi: 10.1177/1479972313505880. [DOI] [PubMed] [Google Scholar]
- 51.Esteban C, Arostegui I, Aburto M, Moraza J, Quintana JM, Aizpiri S, Basualdo LV, Capelastegui A. Influence of changes in physical activity on frequency of hospitalization in chronic obstructive pulmonary disease. Respirology. 2014;19:330–8. doi: 10.1111/resp.12239. [DOI] [PubMed] [Google Scholar]
- 52.Chawla H, Bulathsinghala C, Tejada JP, Wakefield D, ZuWallack R. Physical activity as a predictor of thirty-day hospital readmission after a discharge for a clinical exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11:1203–9. doi: 10.1513/AnnalsATS.201405-198OC. [DOI] [PubMed] [Google Scholar]
- 53.Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten-van Mölken MP, Beeh KM, Rabe KF, Fabbri LM. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093–103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 54.Wedzicha JA, Calverley PM, Seemungal TA. Hagan G, Ansari Z, Stockley RA, INSPIRE investigators. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177:19–26. doi: 10.1164/rccm.200707-973OC. [DOI] [PubMed] [Google Scholar]
- 55.Barnes PJ. Muscarinic receptor subtypes in airways. Life Sci. 1993;52:521–27. doi: 10.1016/0024-3205(93)90310-Y. [DOI] [PubMed] [Google Scholar]
- 56.Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–65. [PubMed] [Google Scholar]
- 57.O’Donnell DE, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23:832–40. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 58.Beeh KM, Burgel PR, Franssen FM, Lopez-Campos JL, Loukides S, Hurst JR, Fležar M, Ulrik CS, Di Marco F, Stolz D, et al. How do dual long-acting bronchodilators prevent exacerbations of chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2016: doi: 10.1164/rccm.201609-201794CI. [DOI] [PubMed]
- 59.Meyer T, Reitmeir P, Brand P, Herpich C, Sommerer K, Schulze A, Scheuch G, Newman S. Effects of formoterol and tiotropium bromide on mucus clearance in patients with COPD. Respir Med. 2011;105:900–6. doi: 10.1016/j.rmed.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 60.D’Urzo AD, Rennard SI, Kerwin EM, Mergel V, Leslbaum AR, Caracta CF. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week, randomized, placebo-controlled AUGMENT COPD study. Respir Res. 2014;15:123. doi: 10.1186/s12931-014-0123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santus P, Buccellati C, Centanni S, Fumagalli F, Busatto P, Blasi F, Sala A. Bronchodilators modulate inflammation in chronic obstructive pulmonary disease subjects. Pharmacol Res. 2012;66:343–48. doi: 10.1016/j.phrs.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Casaburi R, Mahler DA, Jones PW, Wanner A, San PG, ZuWallack RL, Menjoge SS, Serby CW, Witek T., Jr A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19:217–24. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 63.Brusasco V, Hodder R, Miravitlles M, Korducki L, Towse L, Kesten S. Health outcomes following treatment for six months with once daily tiotropium compared with twice daily salmeterol in patients with COPD. Thorax. 2003;58:399–404. doi: 10.1136/thorax.58.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Niewoehner DE, Rice K, Cote C, Paulson D, Cooper JA, Jr, Korducki L, Cassino C, Kesten S. Prevention of exacerbations of chronic obstructive pulmonary disease with tiotropium, a once-daily inhaled anticholinergic bronchodilator: a randomized trial. Ann Intern Med. 2005;143:317–26. doi: 10.7326/0003-4819-143-5-200509060-00007. [DOI] [PubMed] [Google Scholar]
- 65.Dusser D, Bravo ML, Iacono P. The effect of tiotropium on exacerbations and airflow in patients with COPD. Eur Respir J. 2006;27:547–55. doi: 10.1183/09031936.06.00062705. [DOI] [PubMed] [Google Scholar]
- 66.Powrie DJ, Wilkinson TM, Donaldson GC, Jones P, Scrine K, Viel K, Kesten S, Wedzicha JA. Effect of tiotropium on sputum and serum inflammatory markers and exacerbations in COPD. Eur Respir J. 2007;30:472–78. doi: 10.1183/09031936.00023907. [DOI] [PubMed] [Google Scholar]
- 67.Tonnel AB, Perez T, Grosbois JM, Verkindre C, Bravo ML, Brun M. Effect of tiotropium on health-related quality of life as a primary efficacy endpoint in COPD. Int J Chron Obstruct Pulmon Dis. 2008;3:301–10. doi: 10.2147/COPD.S2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–54. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 69.Bateman E, Singh D, Smith D, Disse B, Towse L, Massey D, Blatchford J, Pavia D, Hodder R. Efficacy and safety of tiotropium Respimat SMI in COPD in two 1-year randomized studies. Int J Chron Obstruct Pulmon Dis. 2010;5:197–208. [PMC free article] [PubMed] [Google Scholar]
- 70.Bateman ED, Tashkin D, Siafakas N, Dahl R, Towse L, Massey D, Pavia D, Zhong NS. A one-year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir Med. 2010;104:1460–72. doi: 10.1016/j.rmed.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 71.D’Urzo A, Ferguson GT, van Noord JA, Hirata K, Martin C, Horton R, Lu Y, Banerji D, Overend T. Efficacy and safety of once-daily NVA237 in patients with moderate-to-severe COPD: the GLOW1 trial. Respir Res. 2011;12:156. doi: 10.1186/1465-9921-12-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kerwin E, Hébert J, Gallagher N, Martin C, Overend T, Alagappan VK, Lu Y, Banerji D. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur Respir J. 2012;40:1106–14. doi: 10.1183/09031936.00040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kerwin EM, D’Urzo AD, Gelb AF, Lakkis H, Garcia GE, Caracta CF. Efficacy and safety of a 12-week treatment with twice-daily aclidinium bromide in COPD patients (ACCORD COPD I) COPD. 2012;9:90–101. doi: 10.3109/15412555.2012.661492. [DOI] [PubMed] [Google Scholar]
- 74.Jones PW, Singh D, Bateman ED, Agusti A, Lamarca R, de Miquel G, Segarra R, Caracta C, Garcia GE. Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. Eur Respir J. 2012;40:830–6. doi: 10.1183/09031936.00225511. [DOI] [PubMed] [Google Scholar]
- 75.Celli B, Crater G, Kilbride S, Mehta R, Tabberer M, Kalberg CJ, Church A. Once-daily umeclidinium/vilanterol 125/25 mcg in COPD: a randomized, controlled study. Chest. 2014;145:981–91. doi: 10.1378/chest.13-1579. [DOI] [PubMed] [Google Scholar]
- 76.Stockley RA, Chopra N, Rice L. Addition of salmeterol to existing treatment in patients with COPD: a 12 month study. Thorax. 2006;61:122–8. doi: 10.1136/thx.2004.033266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dahl R, Chung KF, Buhl R, Magnussen H, Nonikov V, Jack D, Bleasdale P, Owen R, Higgins M, Kramer B. Efficacy of a new once-daily long-acting inhaled b2-agonist indacaterol versus twice-daily formoterol in COPD. Thorax. 2010;65:473–9. [DOI] [PubMed]
- 78.Chapman KR, Rennard SI, Dogra A, Owen R, Lassen C, Kramer B, INDORSE study investigators. Long-term safety and efficacy of indacaterol, a long-acting β2-agonist, in subjects with COPD: a randomized, placebo-controlled study. Chest. 2011;140:68–75. [DOI] [PubMed]
- 79.Donohue J, Fogarty C, Lötvall J, Mahler DA, Worth H, Yorgancioglu A, Iqbal A, Swales J, Owen R, Higgins M, Kramer B; INHANCE Study Investigators, et al. Once-daily bronchodilators for chronic obstructive pulmonary disease: indacaterol versus tiotropium. Am J Respir Crit Care Med. 2010;182:155–62. [DOI] [PubMed]
- 80.Decramer ML, Chapman KR, Dahl R, Frith P, Devouassoux G, Fritscher C, Cameron R, Shoaib M, Lawrence D, Young D, et al. Once-daily indacaterol versus tiotropium for patients with severe chronic obstructive pulmonary disease (INVIGORATE): a randomised, blinded, parallel-group study. Lancet Respir Med. 2013;1:524–33. doi: 10.1016/S2213-2600(13)70158-9. [DOI] [PubMed] [Google Scholar]
- 81.Cazzola M, Molimard M. The scientific rationale for combining long-acting β-agonists and muscarinic antagonists in COPD. Pulm Pharmacol Ther. 2010;23:257–67. [DOI] [PubMed]
- 82.Novartis. UTIBRON NEOHALER prescribing information. Revised October 2015. https://www.utibron.com/Utibron-Prescribing-Information.pdf. Accessed 5 June 2017.
- 83.Bateman ED, Chapman KR, Singh D, D’Urzo AD, Molins E, Leselbaum A, Gil EG. Aclidinium bromide and formoterol fumarate as a fixed-dose combination in COPD: pooled analysis of symptoms and exacerbations from two six-month, multicentre, randomised studies (ACLIFORM and AUGMENT) Respir Res. 2015;16:92. doi: 10.1186/s12931-015-0250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Leidy NK, Wilcox TK, Jones PW, Murray L, Winnette R, Howard K, Petrillo J, Powers J, Sethi S. Development of the EXAcerbations of Chronic Obstructive Pulmonary Disease Tool (EXACT): a patient-reported outcome (PRO) measure. Value Health. 2010;13:965–75. doi: 10.1111/j.1524-4733.2010.00772.x. [DOI] [PubMed] [Google Scholar]
- 85.Decramer M, Anzueto A, Kerwin E, Kaelin T, Richard N, Crater G, Tabberer M, Harris S, Church A. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2:472–86. [DOI] [PubMed]
- 86.ClinicalTrials.gov. NCT02296138. Comparing the Efficacy of Tiotropium + Olodaterol (5/5 μg) Fixed Dose Combination (FDC) Over Tiotropium 5 μg in Reducing Moderate to Severe Exacerbations in Patients With Severe to Very Severe Chronic Obstructive Pulmonary Disease. Updated 5 September 2016. https://clinicaltrials.gov/ct2/show/NCT02296138?term = NCT02296138&rank = 1. Accessed 17 Jan 2017.
- 87.Calverley PM, Anderson JA. Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J, investigators T. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–89. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 88.Dransfield MT, Bourbeau J, Jones PW, Hanania NA, Mahler DA, Vestbo J, Wachtel A, Martinez FJ, Barnhart F, Sanford L, et al. Once-daily inhaled fluticasone furoate and vilanterol versus vilanterol only for prevention of exacerbations of COPD: two replicate double-blind, parallel-group, randomised controlled trials. Lancet Respir Med. 2013;1:210–23. doi: 10.1016/S2213-2600(13)70040-7. [DOI] [PubMed] [Google Scholar]
- 89.Martinez FJ, Vestbo J, Anderson JA, Brook RD, Celli BR, Cowans NJ, Crim C, Dransfield M, Kilbride S, Yates J, et al. Effect of fluticasone furoate and vilanterol on exacerbations of COPD in patients with moderate airflow obstruction. Am J Respir Crit Care Med. 2017;195:881–8. doi: 10.1164/rccm.201607-1421OC. [DOI] [PubMed] [Google Scholar]
- 90.Banerji D, Fedele M, Chen H. Once-daily dual bronchodilation with QVA149 reduces COPD exacerbations: results from the IGNITE program. Chest. 2014;145(3_Meeting abstracts):403A. doi: 10.1378/chest.1824292. [DOI] [Google Scholar]
- 91.Vogelmeier C, Zhong N, Humphries MJ, Mezzi K, Fogel R, Bader G, Patalano F, Banerji D. Indacaterol/glycopyrronium in symptomatic patients with COPD (GOLD B and GOLD D) versus salmeterol/fluticasone: ILLUMINATE/LANTERN pooled analysis. Int J Chron Obstruct Pulmon Dis. 2016;11:3189–97. doi: 10.2147/COPD.S116786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh D, Worsley S, Zhu CQ, Hardaker L, Church A. Umeclidinium/vilanterol versus fluticasone propionate/salmeterol in COPD: a randomised trial. BMC Pulm Med. 2015;15:91. doi: 10.1186/s12890-015-0092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Singh D, Papi A, Corradi M, Pavlisova I, Montagna I, Francisco C, Cohuet G, Vezzoli S, Scuri M, Vestbo J. Single inhaler triple therapy versus inhaled corticosteroid plus long-acting β2-agonist therapy for chronic obstructive pulmonary disease (TRILOGY): a double-blind, parallel group, randomised controlled trial. Lancet. 2016;388:963–73. [DOI] [PubMed]
- 94.Vestbo J, Papi A, Corradi M, Blazhko V, Montagna I, Francisco C, Cohuet G, Vezzoli S, Scuri M, Singh D. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): a double-blind, parallel group, randomised controlled trial. Lancet. 2017;389:1919–29. [DOI] [PubMed]
- 95.Pascoe SJ, Lipson DA, Locantore N, Barnacle H, Brealey N, Mohindra R, Dransfield MT, Pavord I, Barnes N. A phase III randomised controlled trial of single-dose triple therapy in COPD: the IMPACT protocol. Eur Respir J. 2016;48:320–30. doi: 10.1183/13993003.02165-2015. [DOI] [PubMed] [Google Scholar]
- 96.Poole P, Black PN, Cates CJ. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;8:CD001287. doi: 10.1002/14651858.CD001287.pub4. [DOI] [PubMed] [Google Scholar]
- 97.Criner GJ, Bourbeau J, Diekemper RL, Ouellette DR, Goodridge D, Hernandez P, Curren K, Balter MS, Bhutani M, Camp PG, et al. Prevention of acute exacerbations of COPD: American College of Chest Physicians and Canadian Thoracic Society Guideline. Chest. 2015;147:894–942. doi: 10.1378/chest.14-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Pela R, Calcagni AM, Subiaco S, Isidori P, Tubaldi A, Sanguinetti CM. N-acetylcysteine reduces the exacerbation rate in patients with moderate to severe COPD. Respiration. 1999;66:495–500. doi: 10.1159/000029447. [DOI] [PubMed] [Google Scholar]
- 99.Zheng JP, Wen FQ, Bai CX, Wan HY, Kang J, Chen P, Yao WZ, Ma LJ, Li X, Raiteri L, et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2014;2:187–94. doi: 10.1016/S2213-2600(13)70286-8. [DOI] [PubMed] [Google Scholar]
- 100.Decramer M, Rutten-van Molken M, Dekhuijzen PN, Troosters T, van Herwaarden C, Pellegrino R, van Schayck CP, Olivieri D, Del Donno M, De Backer W, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–60. doi: 10.1016/S0140-6736(05)66456-2. [DOI] [PubMed] [Google Scholar]
- 101.Cazzola M, Calzetta L, Page C, Jardim J, Chuchalin AG, Rogliani P, Gabriella MM. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev. 2015;24:451–61. doi: 10.1183/16000617.00002215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tse HN, Raiteri L, Wong KY, Ng LY, Yee KS, Tseng CZ. Benefits of high-dose N-acetylcysteine to exacerbation-prone patients with COPD. Chest. 2014;146:611–23. doi: 10.1378/chest.13-2784. [DOI] [PubMed] [Google Scholar]
- 103.Moretti M, Bottrighi P, Dallari R, Da Porto R, Dolcetti A, Grandi P, Garuti G, Guffanti E, Roversi P, De Gugliemo M, et al. The effect of long-term treatment with erdosteine on chronic obstructive pulmonary disease: the EQUALIFE Study. Drugs Exp Clin Res. 2004;30:143–52. [PubMed] [Google Scholar]
- 104.Moretti M, Fagnani S. Erdosteine reduces inflammation and time to first exacerbation postdischarge in hospitalized patients with AECOPD. Int J Chron Obstruct Pulmon Dis. 2015;10:2319–25. doi: 10.2147/COPD.S87091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Allegra L, Cordaro CI, Grassi C. Prevention of acute exacerbations of chronic obstructive bronchitis with carbocysteine lysine salt monohydrate: a multicenter, double-blind, placebo-controlled trial. Respiration. 1996;63:174–80. doi: 10.1159/000196540. [DOI] [PubMed] [Google Scholar]
- 106.Zheng JP, Kang J, Huang SG, Chen P, Yao WZ, Yang L, Bai CX, Wang CZ, Wang C, Chen BY, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled study. Lancet. 2008;371:2013–18. doi: 10.1016/S0140-6736(08)60869-7. [DOI] [PubMed] [Google Scholar]
- 107.Luo J, Wang K, Liu D, Liang BM, Liu CT. Can roflumilast, a phosphodiesterase-4 inhibitor, improve clinical outcomes in patients with moderate-to-severe chronic obstructive pulmonary disease? A meta-analysis Respir Res. 2016;17:18. doi: 10.1186/s12931-016-0330-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Martinez FJ, Calverley PM, Goehring UM, Brose M, Fabbri LM, Rabe KF. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet. 2015;385:857–66. doi: 10.1016/S0140-6736(14)62410-7. [DOI] [PubMed] [Google Scholar]
- 109.Martinez FJ, Rabe KF, Sethi S, Pizzichini E, McIvor A, Anzueto A, Alagappan VK, Siddiqui S, Rekeda L, Miller CJ, et al. Effect of roflumilast and inhaled corticosteroid/long-acting β2-agonist on chronic obstructive pulmonary disease exacerbations (RE2SPOND). A randomized clinical trial. Am J Respir Crit Care Med. 2016;194:559–67. [DOI] [PubMed]
- 110.Electronic Medicines Compendium (EMC). DAXAS (roflumilast) 500 micrograms film-coated tablets. Summary of Product Characteristics (SmPC). Updated 1 March 2016. https://www.medicines.org.uk/emc/medicine/23416. Accessed 12 May 2017.
- 111.Uzun S, Djamin RS, Kluytmans JA, Mulder PG, van’t Veer NE, Ermens AA, Pelle AJ, Hoogsteden HC, Aerts JG, van der Eerden MM. Azithromycin maintenance treatment in patients with frequent exacerbations of chronic obstructive pulmonary disease (COLUMBUS): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2014;2:361–8. doi: 10.1016/S2213-2600(14)70019-0. [DOI] [PubMed] [Google Scholar]
- 112.Albert RK, Connett J, Bailey WC, Casaburi R, Cooper JA, Jr, Criner GJ, Curtis JL, Dransfield MT, Han MK, Lazarus SC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365:689–98. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.He ZY, Ou LM, Zhang JQ, Bai J, Liu GN, Li MH, Deng JM, MacNee W, Zhong XN. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration. 2010;80:445–52. doi: 10.1159/000321374. [DOI] [PubMed] [Google Scholar]
- 114.Seemungal TA, Wilkinson TM, Hurst JR, Perera WR, Sapsford RJ, Wedzicha JA. Long-term erythromycin therapy is associated with decreased chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. 2008;178:1139–47. doi: 10.1164/rccm.200801-145OC. [DOI] [PubMed] [Google Scholar]
- 115.Alcázar Navarrete B, Casanova C, Miravitlles M, de Lucas P, Riesco JA, Rodríguez González-Moro JM, Working Group “Consensus document on the appropriate use of inhaled corticosteroids in C. “Correct use of inhaled corticosteroids in chronic obstructive pulmonary disease”: a consensus document. Arch Bronconeumol. 2015;51:193–8. [DOI] [PubMed]
- 116.Suissa S, Ernst P, Vandemheen KL, Aaron SD. Methodological issues in therapeutic trials of COPD. Eur Respir J. 2008;31:927–33. doi: 10.1183/09031936.00098307. [DOI] [PubMed] [Google Scholar]
- 117.Pascoe S, Locantore N, Dransfield MT, Barnes NC, Pavord ID. Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med. 2015;3:435–42. doi: 10.1016/S2213-2600(15)00106-X. [DOI] [PubMed] [Google Scholar]
- 118.Pavord ID, Lettis S, Locantore N, Pascoe S, Jones PW, Wedzicha JA, Barnes NC. Blood eosinophils and inhaled corticosteroid/long-acting β2 agonist efficacy in COPD. Thorax. 2016;71:118–25. [DOI] [PMC free article] [PubMed]
- 119.Watz H, Tetzlaff K, Wouters EF, Kirsten A, Magnussen H, Rodriguez-Roisin R, Vogelmeier C, Fabbri LM, Chanez P, Dahl R, et al. Blood eosinophil count and exacerbations in severe chronic obstructive pulmonary disease after withdrawal of inhaled corticosteroids: a post-hoc analysis of the WISDOM trial. Lancet Respir Med. 2016;4:390–8. doi: 10.1016/S2213-2600(16)00100-4. [DOI] [PubMed] [Google Scholar]
- 120.Siddiqui SH, Pavord I, Barnes N, Lettis S, Guasconi A, Petruzzelli S. Blood eosinophils (EOS) are a biomarker of COPD exacerbation reduction with inhaled corticosteroids (ICS): an across-trials model-based approach. Eur Respir J. 2016;48:OA1763. [Google Scholar]
- 121.Calverley P, Tetzlaff K, Vogelmeier C, Fabbri L, Magnussen H, Wouters E, Mezzanotte W, Disse B, Finnigan H, Asijee GM, Hallmann C, Watz H. Evaluating blood eosinophils and exacerbation history to predict ICS response in COPD. Am J Respir Crit Care Med. 2017. doi:10.1164/rccm.201612-2525LE. [Epub ahead of print]. [DOI] [PubMed]
- 122.Miravitlles M, D’Urzo A, Singh D, Koblizek V. Pharmacological strategies to reduce exacerbation risk in COPD: a narrative review. Respir Res. 2016;17:112. doi: 10.1186/s12931-016-0425-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Miravitlles M, Anzueto A. A new two-step algorithm for the treatment of COPD. Eur Respir J. 2017;49: doi:10.1183/13993003.02200-2016. [DOI] [PubMed]
- 124.Cooper CB, Barjaktarevic I. A new algorithm for the management of COPD. Lancet Respir Med. 2015;3:266–8. doi: 10.1016/S2213-2600(15)00091-0. [DOI] [PubMed] [Google Scholar]
- 125.Chan CK, Maltais F, Sigouin C, Haddon JM, Ford GT. A randomized controlled trial to assess the efficacy of tiotropium in Canadian patients with chronic obstructive pulmonary disease. Can Respir J. 2007;14:465–72. doi: 10.1155/2007/192961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abrahams R, Moroni-Zentgraf P, Ramsdell J, Schmidt H, Joseph E, Karpel J. Safety and efficacy of the once-daily anticholinergic BEA2180 compared with tiotropium in patients with COPD. Respir Med. 2013;107:854–62. doi: 10.1016/j.rmed.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 127.Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, Balter M, O’Donnell D, McIvor A, Sharma S, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone-salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2007;146:545–55. doi: 10.7326/0003-4819-146-8-200704170-00152. [DOI] [PubMed] [Google Scholar]
- 128.Maleki-Yazdi MR, Kaelin T, Richard N, Zvarich M, Church A. Efficacy and safety of umeclidinium/vilanterol 62.5/25 mcg and tiotropium 18 mcg in chronic obstructive pulmonary disease: Results of a 24-week, randomized, controlled trial. Respir Med. 2014;108:1752–60. doi: 10.1016/j.rmed.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 129.Ohar JA, Crater GD, Emmett A, Ferro TJ, Morris AN, Raphiou I, Sriram PS, Dransfield MT. Fluticasone propionate/salmeterol 250/50 μg versus salmeterol 50 μg after chronic obstructive pulmonary disease exacerbation. Respir Res. 2014;15:105. doi: 10.1186/s12931-014-0105-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Calverley PM, Kuna P, Monsó E, Costantini M, Petruzzelli S, Sergio F, Varoli G, Papi A, Brusasco V. Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. Respir Med. 2010;104:1858–68. doi: 10.1016/j.rmed.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 131.Bateman ED, Vogelmeier C, Chen H, Banerji D. Comparison of COPD exacerbations with once-daily QVA149 versus twice-daily salmeterol/fluticasone combination: the ILLUMINATE study. Chest. 2014;145(3_Meeting abstracts):409A. doi: 10.1378/chest.1824338. [DOI] [Google Scholar]
- 132.Vogelmeier C, Paggiaro PL, Dorca J, Sliwinski P, Mallet M, Kirsten A-M, Seoane B, Segarra RM, Leselbaum A, Gil EG. The efficacy and safety of aclidinium/formoterol fixed-dose combination compared with salmeterol/fluticasone in patients with COPD: results from a phase III study. Am J Respir Crit Care Med. 2015;191(1):A3974. [Google Scholar]
- 133.Beeh KM, Derom E, Echave-Sustaeta J, Gronke L, Hamilton A, Zhai D, Bjermer L. The lung function profile of once-daily tiotropium and olodaterol via Respimat® is superior to that of twice-daily salmeterol and fluticasone propionate via Accuhaler® (ENERGITO® study) Int J Chron Obstruct Pulmon Dis. 2016;11:193–205. doi: 10.2147/COPD.S95055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Troosters T, Sciurba FC, Decramer M, Siafakas NM, Klioze SS, Sutradhar SC, Weisman IM, Yunis C. Tiotropium in patients with moderate COPD naive to maintenance therapy: a randomised placebo-controlled trial. NPJ Prim Care Respir Med. 2014;24:14003. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


