CEACAM4 contains a functional immunoreceptor tyrosinebased activation motif, which is tyrosine-phosphorylated, and initiates efficient phagocytosis of attached bacteria.
Keywords: ITAM, Src kinase, tyrosine phosphorylation
Abstract
Human granulocytes express several glycoproteins of the CEACAM family. One family member, CEACAM3, operates as a single-chain phagocytic receptor, initiating the detection, internalization, and destruction of a limited set of gram-negative bacteria. In contrast, the function of CEACAM4, a closely related protein, is completely unknown. This is mainly a result of a lack of a specific ligand for CEACAM4. By generating chimeric proteins containing the extracellular bacteria-binding domain of CEACAM3 and the transmembrane and cytoplasmic part of CEACAM4 (CEACAM3/4) we demonstrate that this chimeric receptor can trigger efficient phagocytosis of attached particles. Uptake of CEACAM3/4-bound bacteria requires the intact ITAM of CEACAM4, and this motif is phosphorylated by Src family PTKs upon receptor clustering. Furthermore, SH2 domains derived from Src PTKs, PI3K, and the adapter molecule Nck are recruited and associate directly with the phosphorylated CEACAM4 ITAM. Deletion of this sequence motif or inhibition of Src PTKs blocks CEACAM4-mediated uptake. Together, our results suggest that this orphan receptor of the CEACAM family has phagocytic function and prompt efforts to identify CEACAM4 ligands.
Introduction
The innate immune system provides early detection and elimination of potentially harmful microbes. It has become clear that evolutionary conserved, germ-line-encoded factors respond to molecular patterns generally associated with microorganisms [1]. One important family of pattern recognition molecules is the TLRs that are not only expressed by hematopoietic cells but also found on cells outside of the immune system, including epithelial or endothelial cells [2]. TLRs allow immune and nonimmune cells to generate danger signals, which direct innate and acquired immune defenses to infected locations within the body. Another set of recognition molecules allows specialized cells of the innate immune system not only to recognize but also to phagocytose and eliminate a broad range of microbes. These include scavenger receptors, C-type lectins, and Siglecs that are found on professional phagocytes and bind to carbohydrate and protein structures found on the surface of diverse parasites, bacteria, and fungi [3, 4]. Besides these broadly responsive systems, it has become clear that innate immune cells also express additional germ-line-encoded receptors that are tailored toward particular subsets of host-associated pathogens. An example is found in a group of mammalian receptors of the Ig family, the CEACAMs [5]. As a result of multiple gene-duplication and -conversion events, the CEACAM family displays extraordinary diversity among the different mammalian lineages [6]. This diversity becomes most evident when comparing the number of CEACAM genes encoded in published genomes [7]. In humans, there are 12 members of the CEACAM family that are expressed in several tissues [5, 8]. Some family members, such as CEACAM1, CEA, CEACAM6, or CEACAM7, are expressed exclusively on epithelia or found on epithelial cells and a variety of additional cell types. In contrast, several members are restricted to hematopoietic cells; e.g., CEACAM3, CEACAM4, and CEACAM8 have only been reported from human myeloid cells. In particular, CEACAM3 (initially termed CGM1 or CD66d) is known to mediate the opsonin-independent recognition and phagocytosis of a limited set of human-restricted bacterial pathogens. For example, strains of Ngo OpaCEA proteins are recognized by this glycoprotein [9–11]. Therefore, CEACAM3 functions as a single-chain phagocytic receptor that provides the innate immune system with a means to detect and eliminate specifically CEACAM-binding bacteria [12]. CEACAM3 is characterized by a cytoplasmic domain containing an ITAM-like sequence [11, 13]. Previous work has demonstrated that this motif is critical to provide CEACAM3 with efficient phagocytic and bactericidal properties [14, 15].
Interestingly, the human genome encodes an additional CEACAM family member with a domain structure closely resembling CEACAM3 [16]. During the revision of the CEA family nomenclature, this CEACAM3-related protein that had been designated initially W236 or CGM7 [16, 17], has been renamed into CEACAM4 [18], which seems to be expressed exclusively by myeloid cells, as its cDNA has been cloned initially from a human leukocyte cDNA library [16], and its mRNA has not been detected in a variety of different epithelial, neuronal, or mesenchymal tumor cells [19]. Similar to CEACAM3, CEACAM4 has a single IgV-like extracellular domain, and its cytoplasmic part contains an ITAM sequence [20]. However, the physiologic function of CEACAM4 is unclear as a result of the lack of known ligands for this membrane protein.
Here, we provide evidence that CEACAM4 can function as a phagocytic receptor. By domain swapping with the bacteria-binding extracellular domain of CEACAM3, the intact transmembrane and intracellular domains of CEACAM4 mediate efficient uptake of Ngo OpaCEA into transfected cells. Bacterial internalization is accompanied by tyrosine phosphorylation of CEACAM4 and depends on the integrity of the cytoplasmic ITAM sequence. Upon Src family kinase-mediated phosphorylation, tyrosine residues of the CEACAM4 ITAM associate with SH2 domain-containing cytoplasmic proteins involved in signaling processes during phagocytosis. Our results suggest that the orphan receptor CEACAM4 serves a phagocytic function in human granulocytes, and they provide impetus to identify CEACAM4 ligands.
MATERIALS AND METHODS
Cell culture and differentiation of HL60 cells
HEK-293T cells were cultured in DMEM containing 10% calf serum. Human promyelocytic leukemia HL60 cells were grown in RPMI-1640 medium, supplemented with 10% FBS (10% FCS). Cells were grown at 37°C in 5% CO2 and subcultured every 2–3 d. For in vitro differentiation, HL60 cells at a density of 5 × 105–1 × 106 cells/ml were cultured in the presence of 1 µM all-trans retinoic acid for 6 d with the medium replaced once after 3 d.
Bacteria
Ngo OpaCEA (strain N309) or Ngo Opa− (strain N302) variants [21] and Neisseria meningitidis H44/76 (serotype B strain N687) [22] were kindly provided by Thomas Meyer (Max-Planck-Institut für Infektionsbiologie, Berlin, Germany). Neisseriae were grown on GC agar plates (Difco BRL, Paisley, United Kingdom), supplemented with vitamins at 37°C, 5% CO2, and subcultured every day by use of a binocular microscope. Moraxella catarrhalis (strain 9143) and Haemophilus aegyptius (strain 21187) were obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen (Braunschweig, Germany). Moraxella and Haemophilus were grown on brain heart infusion agar plates at 37°C, 5% CO2. For infection, overnight grown bacteria were taken from GC agar plates, suspended in PBS, and CFUs were estimated by OD550 readings, according to a standard curve. In some cases, bacteria were labeled before infection with 0.2 µg/ml Pacific Blue or CFSE (Invitrogen, Carlsbad, CA, USA) in PBS for 15 min at 37°C under dark and washed 3 times with PBS before use.
rDNA constructs
Mammalian expression plasmids encoding GFP- and mKate-tagged versions of CEACAM3 have been described previously [15, 23]. Constructs, encoding GFP; mKate; v-Src; the SH2 domains of human Slp-76, c-Yes, and Nck2; as well as the N-terminal SH2 domain of PI3KR3, fused to mKate, have been described [15, 23, 24]. The purified rGST-fusion proteins, encoding the SH2 domains of human Hck, Lck, c-Yes, Tec, Syk (the isolated Syk-N and Syk-C SH2 domains), PI3KR3 (the isolated PI3KR3-N and PI3KR3-C SH2 domains), Vav, Nck1, Nck2, Grb2, Grb14, and Slp76, have been used before [24, 25].
The human monocytic MCSF receptor promoter cloned into a luciferase reporter plasmid (pMCSFR) and its mutated version (pMCSFR mut), as well as the PU.1 expression vector, were described before [26, 27]. The primer sequences used for cloning procedures are summarized in Supplemental Table 1. The human CEACAM3 and CEACAM4 promoters were amplified from human genomic DNA cloned in pBAC e3.6 vectors [28], which were obtained from the BACPAC Resource Center (Children's Hospital Oakland Research Institute, Oakland, CA, USA). With the use of pBAC e3.6 clone RP11-343B1 as a template, the CEACAM3 promoter region, including a 5′-located KpnI site, was amplified with primers promoter-CC3-sense and promoter-CC3-anti-NheI. The CEACAM4 promoter region was amplified from pBAC e3.6 clone RP11-976L23 with primers promoter-CC4-sense KpnI and promoter-CC4-anti-NheI. The promoter sequences were cloned via KpnI/NheI restriction sites into the luciferase reporter vector pGL4.10 (Promega, Mannheim, Germany). The human CEACAM4 cDNA, originally cloned by Kuroki et al. [16], was kindly provided by Wolfgang Zimmermann (Ludwig-Maximilians-Universität, München, Germany). The CEACAM4 coding sequence was amplified with primers CEA4-IF-sense and CEA4-IF-w/oSTOP-anti and inserted into pDNR-Dual by use of the In-Fusion Dry-Down PCR Cloning Kit (Clontech, Mountain View, CA, USA). Via Cre-mediated recombination, the CEACAM4 cDNA was transferred into the mammalian expression vector pLPS-3′EGFP (Clontech), allowing expression of CEACAM4-GFP-fusion protein (CEACAM4-GFP). To generate a chimera of the CEACAM4 transmembrane and cytoplasmic domain fused to the amino-terminal IgV-like domain of CEACAM3, the coding sequence of CEACAM3 was amplified with primers CEACAM3-IF-sense and CEA3-NT(-CEA4-TM-cyto)-antisense. In parallel, the CEACAM4 cDNA was amplified with primers CEA4-TM-cyto-sense and CEA4-IF-w/oSTOP-antisense. The 2 resulting PCR fragments were fused by soeingPCR by use of CEACAM3-IF-sense and CEA4-IF-w/oSTOP-antisense primers. The soeingPCR product was cloned into pDNR-Dual via the In-Fusion Dry-Down PCR Cloning Kit to yield the WT CEACAM3/4 chimera (CEACAM3/4 WT). Furthermore, to construct CEACAM3/4 chimera lacking the cytoplasmic domain, we used pDNR-Dual CEACAM3/4 WT for PCR amplification with primers CEACAM3-IF-sense and CEA4-ΔCT-IF-antisense. The resulting PCR fragments were cloned into pDNR-Dual to yield the CEACAM3/4-ΔCT. Point mutations by substitution of tyrosine residues located in the amino acid positions 222 and 233 of chimera CEACAM3/4 WT were introduced sequentially by site-directed mutagenesis by use of primer pairs: chimCC3/4-Y222F-sense and chimCC3/4-Y222F-antisense, as well as chimCC3/4-Y233F-sense and chimCC3/4-Y233F-antisense to generate the double-mutant CEACAM3/4 Y222/233F. Subsequently, all of the constructs were sequence verified and transferred into pLPS-3′mKate or pLPS-3′EGFP by Cre/Lox recombination, as described previously [29].
Antibodies and reagents
mAb D14HD11 (cross-reactive with CEACAM3) was from GENOVAC (Freiburg, Germany). mAb against GFP (clone JL-8) was from BD Biosciences (Palo Alto, CA, USA), mAb against v-Src (clone EC10) and against pTyr (clone 4G10) were from Upstate Biotechnology (Lake Placid, NY, USA), and mAb against GST (clone B-14) was from Santa Cruz Biotechnology (Santa Cruz, CA. USA). Src inhibitor PP2 was obtained from Calbiochem (La Jolla, CA, USA). The mAb anti-Opa protein antibody (clone 4B12/C11) was a generous gift of Marc Achtman (MPI für Infektionsbiologie, Berlin, Germany). A rabbit polyclonal antibody was generated against formaldehyde-fixed Neisseria gonorrheae and N. meningitidis (IG-511) by Immunoglobe (Himmelstadt, Germany). A rabbit polyclonal antibody against rmKate was custom generated and affinity purified (Animal Research Facility, Universität Konstanz, Germany). mAb against tubulin (clone E-7) was purified from hybridoma cell supernatants obtained from Developmental Studies Hybridoma Bank (University of Iowa, Iowa City, IA, USA). Protein A/G sepharose was obtained from Santa Cruz Biotechnology.
Detection of human CEACAM3 and CEACAM4 mRNA by qRT-PCR analysis
Total RNA from differentiated or nondifferentiated HL60 cells and CEACAM-transfected or nontransfected HEK-293 cells were extracted (Qiagen RNeasy Mini kit; Qiagen, Hilden, Germany) and DNase digested (RNase-free DNase; Qiagen). cDNA was synthesized from 2 μg total RNA in a 20 μl reaction by use of the Moloney murine leukemia virus RT and Oligo-dT primers. As a control for chromosomal DNA contamination, RNA was used directly for PCR amplification. The expression of human CEACAMs was assessed by use of predesigned TaqMan assays for CEACAM3 (ID: Hs00926316_m1) or CEACAM4 (assay ID: Hs00156509_m1) from Life Technologies (Carlsbad, CA, USA). PCR amplification was performed by use of TaqMan Fast Advanced Master Mix (Life Technologies). Primers for GAPDH (5′- GAAGGTGAAGGTCGGAGTCA -3′ and 5′- TTGAGGTCAATGAAGGGGTC -3′) were used to amplify GAPDH cDNA in the presence of SYBR Green (Sigma-Aldrich, St. Louis, MO, USA). All reactions were subjected to 40 PCR cycles (Mastercycler realplex; Eppendorf, Hamburg, Germany) with 95°C for 10 s, 60°C for 20 s, and 72°C for 15 s. Quantification of PCR data was done by the comparative Ct method [30] and shown as N-fold changes of CEACAM mRNA levels.
Transfection of cells, cell lysis, immunoprecipitation, and Western blotting
HEK-293 cells were transfected by the standard calcium phosphate coprecipitation method. Cells were used in infection experiments, 48 h after the transfection. Cell lysis in modified radioimmunoprecipitation assay buffer and Western blotting was performed as described previously [11, 31]. For immunoprecipitations, whole-cell lysates were incubated with 3 µg polyclonal rabbit anti-GFP or anti-mKate antibody overnight, followed by 1 h incubation with protein A/G sepharose, all at 4°C. After 3 washes with Triton buffer, precipitates were taken up in SDS sample buffer and analyzed by Western blotting.
Detection of CEACAM-binding by bacterial pull-down assays
Expression of the soluble amino-terminal domains of human CEACAMs in HEK-293 cells and binding studies with different microorganisms were performed as described previously [32, 33]. Bacteria were added to CEACAM N-domain-containing cell-culture supernatant in a total volume of 1 ml and incubated for 1 h. After incubation, the bacteria were washed twice with PBS and boiled in SDS sample buffer before SDS-PAGE and Western blotting with a GFP-directed mAb.
Gentamicin protection assay
Gentamicin protection assays were conducted as described previously [11]. Cells (4 × 105) were seeded in 24-well plates coated with fibronectin (4 µg/ml) and poly-L-lysine (10 µg/ml). A MOI of 30 bacteria/cell was used routinely, and after 1 h of infection, extracellular bacteria were killed by 45 min incubation with 50 µg/ml gentamicin in DMEM. Then, cells were lysed with 1% saponin in PBS for 15 min. The samples were diluted with PBS, and the number of viable bacteria was determined by plating suitable dilutions on GC agar. For inhibition studies, cells were treated with the Src inhibitor PP2 (Calbiochem), 15 min before infection.
Bacterial adherence assay
Cells were seeded and infected as described for gentamicin protection assays. After the infection, cells were gently washed before they were lysed by addition of 1% saponin in PBS for 15 min. Total cell-associated bacteria were suspended by vigorous pipetting, and CFUs were determined by plating of serial dilutions on GC agar.
GST-pull down and probing of peptide spot membranes
For GST-pull downs, 4 µg purified GST or GST-fusion protein, attached to glutathione-sepharose beads (Amersham Biosciences), was added to WCLs of transfected HEK-293 cells. In some cases, HEK-293 cells were cotransfected with a v-Src-encoding plasmid to ensure maximal tyrosine phosphorylation of CEACAM4. Samples were incubated for 4 h at 4°C under constant rotation. After 4 washes with PBS containing 0.01% Tween, precipitates were boiled in 2× SDS sample buffer before SDS-PAGE and Western blot analysis. Generation and probing of peptide spot membranes were conducted as described previously by use of 20 µg GST-SH2 domains or GST alone [25].
Preparation and use of SH2 domain microarrays
Protein domain microarrays containing GST-tagged rSH2 domains derived from various human proteins were prepared on aldehyde-modified glass slides as reported previously [24]. Arrays were incubated overnight at 4°C with cell lysates from CEACAM4-GFP and v-Src expressing cells containing maximally tyrosine phosphorylated CEACAM4 (p-CEACAM4) or CEACAM4-GFP-only-expressing cells (CEACAM4). After incubation, the arrays were washed and probed with anti-GFP mAb for 1 h at room temperature, followed by Cy3-labeled secondary antibody. After washing, the slides were dried by centrifugation. Binding signals for a particular SH2 domain obtained for unphosphorylated CEACAM4 were subtracted from the signals obtained for p-CEACAM4. The resulting intensities were normalized to the amount of array-bound SH2 domains, as measured by probing arrays with anti-GST antibody, allowing comparison in binding strength between different SH2 domains.
Differential staining of intracellular bacteria and confocal microscopy
To discriminate between intra- and extracellular bacteria, a differential immunofluorescence staining protocol was applied as described [34]. In brief, transfected HEK-293 cells were seeded on glass coverslips in 24-well plates and infected for 60 min with Ngo OpaCEA at an MOI of 30. Samples were fixed with 4% paraformaldehyde, and after 3 washes with PBS, samples were incubated in blocking buffer (PBS, 10% FCS) for 5 min. Extracellular bacteria were stained with a rabbit polyclonal N. gonorrheae antibody (IG-511) for 1 h, followed by incubation for 45 min with Cy5-coupled goat anti-rabbit antibody in blocking buffer. Following 2 PBS washes, samples were incubated for 20 min with 0.1% Triton X-100 to permeabilize cellular membranes. After 3 further PBS washes and 5 min in blocking buffer, samples were incubated again for 1 h with the rabbit polyclonal anti-N. gonorrheae antibody (IG-511) to detect intracellular and extracellular bacteria. Samples were washed 3 times with PBS, they were treated with blocking buffer for 5 min, and then incubated for 45 min with Cy2-coupled goat anti-rabbit antibody. Following the last 3 PBS washes, samples were embedded in mounting medium (Dako, Glostrup, Denmark).
Fixed samples were analyzed with a Leica TCS SP5 confocal laser-scanning microscope (Leica Microsystems, Wetzlar, Germany). Fluorescence signals of triple-labeled specimens were serially recorded to avoid bleedthrough. Images were digitally processed with ImageJ (U.S. National Institutes of Health, Bethesda, MD, USA) and merged to yield pseudocolored pictures.
RESULTS AND DISCUSSION
CEACAM4 is under the control of a myeloid-specific promoter
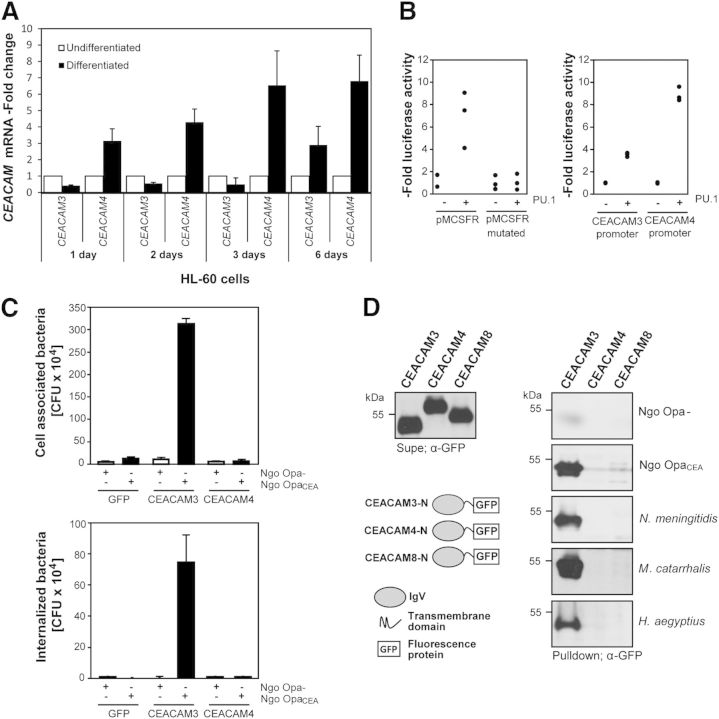
CEACAM3 is expressed by human granulocytes and mediates the opsonin-independent phagocytosis of Ngo OpaCEA [11]. CEACAM4, another CEACAM family member, perfectly matches the domain structure of CEACAM3 [16, 20]. Both proteins are highly homologous in the carboxy-terminal intracellular part (amino acid identity 73%; Supplemental Fig. 1). However, they display less sequence identity in the microbe-binding extracellular IgV-like domain (amino acid identity 49%), in particular, in the CFG face of the Ig fold (Supplemental Fig. 1). Strikingly, these 2 membrane proteins harbor an ITAM-like sequence close to the carboxy-terminus, suggesting that CEACAM4 might function to transduce signals into the cell (Supplemental Fig. 1). CEACAM4 was cloned originally from a pooled human leukocyte cDNA library [16]. This suggests that similar to CEACAM3, CEACAM4 might also be expressed by phagocytic cells. As CEACAM4-specific antibodies are not available, we used a TacMan probe-based qRT-PCR analysis that discriminates between the CEACAM3 and the CEACAM4 cDNA (Supplemental Fig. 1). This assay was used to analyze the presence of the CEACAM4 mRNA in the promyeloid cell line HL60, which can be differentiated in vitro by retinoic acid toward a granulocyte phenotype. Importantly, there was a slight increase in CEACAM3 mRNA and a clear 6- to 7-fold rise in CEACAM4 mRNA in HL60 cells during the course of in vitro differentiation (Fig. 1A). To investigate further the myeloid-specific regulation of these 2 receptors, we performed luciferase reporter assays by use of ∼3 kb fragments derived from the promoter regions of the CEACAM3 or the CEACAM4 gene, respectively. As a comparison, the promoter of the human MCSF receptor was used, which is known to respond to the myeloid transcription factor PU.1 (Fig. 1B) [26, 35]. In agreement with its myeloid-specific expression, WT pMCSFR showed 4- to 9-fold increase in activity in the presence of PU.1, whereas the pMCSFR with a mutated PU.1-binding site (pMCSFR mutated) did not respond. Likewise, PU.1 enhanced CEACAM3 and CEACAM4 promoter activity 4- or 10-fold, respectively, explaining the expression of the encoded receptor proteins in the myeloid lineage (Fig. 1B). These results are in line with findings from unbiased screens in myeloid precursor cells that have identified the CEACAM4 promoter as a target of the promyelocytic leukemia-retinoic acid receptor α oncogene [36] and suggest that CEACAM4 is expressed in human myeloid cells.
Figure 1. CEACAM4 is expressed in human phagocytes but does not recognize CEACAM-binding bacteria. (A) HL60 cells were differentiated in vitro, and CEACAM3 or CEACAM4 RNA levels were quantified by real-time PCR. Samples were normalized according to expression of GAPDH and are shown as -fold increase in mRNA compared with undifferentiated HL60 cells. (B) Luciferase reporter assays with HEK-293 cells transfected with plasmids encoding the M-CSF receptor promoter (pMCSFR), the MCSFR promoter with a mutated PU.1-binding site (pMCSFR mutated), the CEACAM3 promoter, or the CEACAM4 promoter. Plasmids were transfected together with an empty control vector or with an expression plasmid encoding the myeloid transcription factor PU.1, respectively. Each point represents luciferase activity in independent samples and is given as n–fold activity of PU.1-transfected cells compared with the control vector samples. (C) HEK-293 cells were transfected with the empty pLPS-3′-EGFP vector or the same plasmid encoding CEACAM3 or CEACAM4, respectively. Cells were infected for 1 h with Ngo OpaCEA or Ngo Opa− at a MOI of 30. Values represent the mean ± sd (n = 3) of the total cell-associated bacteria (upper) or internalized bacteria (lower). (D) The secreted GFP-fusion proteins of the amino-terminal domains of the indicated CEACAMs were expressed in HEK-293 cells, and cell culture supernatants were analyzed by Western blotting with anti-GFP antibody (Supe; left). The indicated GFP-fusion proteins were incubated with Ngo Opa−, Ngo OpaCEA, N. meningitidis, H. aegyptius, or M. catarrhalis. After washing, bacteria-associated fusion proteins were detected by Western blotting with an anti-GFP mAb (Pulldown; right).
CEACAM4 does not recognize known CEACAM-binding bacteria
Based on the known interaction of several human-restricted pathogens with other members of the CEACAM family [9, 37], we attempted initially to identify CEACAM4 ligands by a candidate approach by use of strains of N. gonorrheae, N. meningitidis, H. aegyptius, and M. catarrhalis with documented binding properties for CEACAM1, CEACAM3, CEA, or CEACAM6 [33, 38–40]. Therefore, human HEK-293 cells were transfected with cDNA encoding GFP, GFP-tagged CEACAM3, or GFP-tagged CEACAM4. To verify the expression of the transfected constructs, cell lysates were probed with GFP-directed antibodies (Supplemental Fig. 2). Next, cells were infected for 1 h with Ngo Opa−, which do not bind to any CEACAM, or an isogenic Ngo OpaCEA at an MOI of 30. Opa protein expression by the used strains was detected by Western blotting (Supplemental Fig. 2). Infected samples were used in bacterial adhesion assays and gentamicin protection assays to measure the cell association and internalization of the bacteria by the transfected cells. As shown before, HEK-293 cells transfected with the control vector did not bind or internalize Ngo Opa− or OpaCEA protein-expressing bacteria (Fig. 1C). In contrast, CEACAM3 expression allowed efficient binding and uptake of pathogens (Fig. 1C). Importantly, CEACAM4-expressing cells did not show enhanced association with Ngo Opa− or OpaCEA protein-expressing bacteria and did not internalize the microorganisms (Fig. 1C). To test directly CEACAM4 binding of different pathogenic bacteria, we used the amino terminal, extracellular domains of CEACAM3, CEACAM4, and CEACAM8 in the form of soluble GFP-fusion proteins in bacterial pull downs. As seen previously [33, 34], Ngo OpaCEA or N. meningitidis, as well as selected strains of H. aegyptius and M. catarrhalis, associated with CEACAM3 but not with CEACAM8 (Fig. 1D). Importantly, none of these strains showed binding to the CEACAM4 extracellular domain (Fig. 1D). This is in line with a previous report by Popp and colleagues[41] , who also did not detect binding of CEACAM4 to different laboratory strains of N. gonorrheae. However, the failure to detect CEACAM4-binding bacteria in this limited set of known CEACAM-binding pathogens does not rule out the possibility that CEACAM4 could function as a receptor for microorganisms.
The cytoplasmic domain of CEACAM4 is able to induce phagocytosis
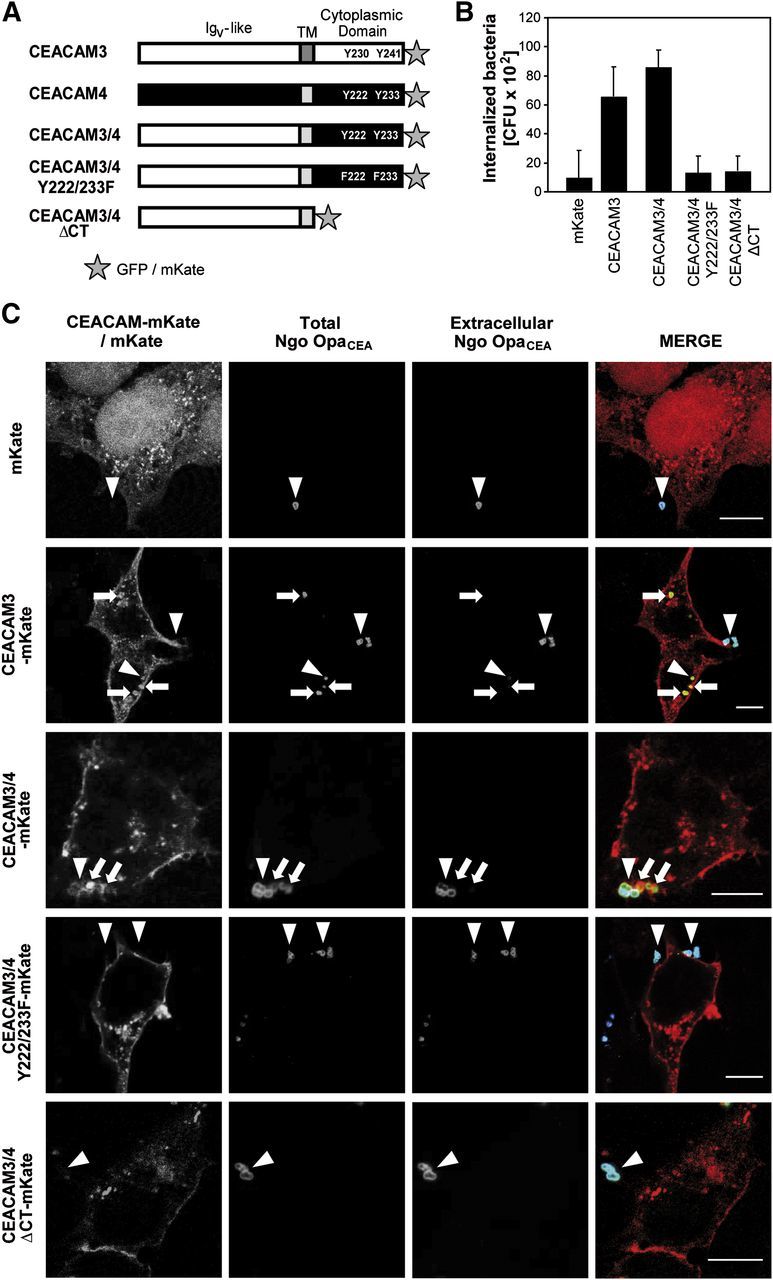
Despite the lack of binding and internalization of known CEACAM-binding bacteria, CEACAM4 could nevertheless have phagocytic activity. To test the functionality of the CEACAM4 cytoplasmic domain in the absence of a known ligand, we constructed chimeric receptors based on the bacteria-binding IgV-like domain of CEACAM3 fused to the transmembrane and cytoplasmic domain of CEACAM4 (CEACAM3/4). In addition to the WT intracellular domain of CEACAM4, we generated a truncated receptor lacking cytoplasmic amino acids (CEACAM3/4 ΔCT) or exchanged the critical tyrosine residues in the CEACAM4 ITAM sequence for phenylalanine (CEACAM3/4 Y222/233F) to abrogate tyrosine phosphorylation (Fig. 2A). To allow detection of the transfected cells, all receptor constructs were fused to a C-terminal mKate or GFP tag (Fig. 2A). HEK-293 cells were transfected with the mKate-tagged constructs or left untransfected, and expression of the chimeric receptor proteins was analyzed by flow cytometry and Western blotting (Supplemental Fig. 3A and B). Transient transfection resulted in equivalent levels of the CEACAM3/4 chimeras, with transfection efficiencies ranging ∼60% of the cell population (Supplemental Fig. 3A). Transfected cells were infected with fluorescein-labeled Ngo OpaCEA for 1 h (MOI 30), and bacterial binding and uptake by the transfected cells were quantified (Supplemental Fig. 3C and Fig. 2B). Importantly, the CEACAM3/4 chimera was able to mediate binding and uptake of the bacteria to a similar extent as CEACAM3 (Supplemental Fig. 3C and Fig. 2B). In contrast, the receptor lacking the cytoplasmic domain (CEACAM3/4 ΔCT), as well as the chimeric receptor with mutated ITAM-like sequence (CEACAM3/4 Y222/233F), showed similar association with bacteria but failed to internalize the microbes (Supplemental Fig. 3C and Fig. 2B). These data demonstrate that the CEACAM4 cytoplasmic domain is able to trigger efficient internalization of bacteria and that this process depends on the tyrosine residues within the ITAM-like sequence.
Figure 2. The cytoplasmic domain of CEACAM4 is able to trigger efficient internalization of bacteria. (A) Schematic representation of CEACAM3 and CEACAM4, as well as the chimeric receptors. CEACAM3-derived regions are indicated by white bars, CEACAM4-derived regions are indicated by black and dark-gray bars. Tyrosine residues and their mutations in the cytoplasmic domain are highlighted. Stars indicate the C-terminal fluorescent protein tag. (B) HEK-293 cells were transfected with vectors encoding mKate or mKate-tagged CEACAM3, CEACAM3/4, CEACAM3/4 Y222/233F, or CEACAM3/4 ΔCT, respectively. Two days later, cells were infected with Ngo OpaCEA (MOI 30) for 1 h, and viable, intracellular bacteria were measured by gentamicin protection assays. Bars represent mean ± sd (n = 3) of the internalized bacteria. (C) HEK-293 cells were transfected as in B and infected with Ngo OpaCEA . Differential staining before and after cell permeabilization allowed the discrimination between extracellular bacteria as well as extra- and intracellular bacteria (total bacteria). Arrows highlight intracellular bacteria, which were only detected in cells expressing CEACAM3 or CEACAM3/4 WT. Arrowheads point to cell-associated, extracellular bacteria. Original scale bars represent 10 µm.
CEACAM4-initiated signals trigger internalization of bacteria
To verify further that the CEACAM3/4 chimera is able to mediate bacterial uptake, we investigated infected cells by confocal laser-scanning microscopy. Accordingly, HEK-293 cells were transfected with the indicated mKate-tagged constructs or mKate alone, and 2 days later, the cells were infected for 1 h with Ngo OpaCEA. Samples were fixed, and extracellular bacteria were labeled with a polyclonal anti-gonococcal antiserum and Cy5-coupled goat anti-rabbit antibodies. Next, samples were permeabilized, and bacteria were again stained with a polyclonal anti-gonococcal antiserum. In this case, a Cy2-coupled goat anti-rabbit antibody was chosen as a secondary reagent, allowing discrimination between intracellular (labeled with Cy2 only) and extracellular (labeled with Cy2 and Cy5) bacteria. As expected, mKate-transfected cells did not harbor intracellular bacteria, and only very few bacteria were associated with the eukaryotic cells (Fig. 2C). CEACAM3-transfected cells, in contrast, showed increased numbers of cell-associated bacteria and contained intracellular bacteria (Fig. 2C). Likewise, OpaCEA protein-expressing bacteria were detected inside cells transfected with CEACAM3/4, demonstrating that the cytoplasmic domain of CEACAM4 is able to trigger bacterial phagocytosis. The chimeric receptor colocalized with cell-associated bacteria, in agreement with the notion that OpaCEA protein-expressing gonococci bind to the extracellular, CEACAM3-derived IgV-like domain (Fig. 2C). Importantly, neither cells expressing CEACAM3/4 ΔCT (lacking the CEACAM4 cytoplasmic domain) nor cells expressing CEACAM3/4 Y222/233F (with compromised CEACAM4 ITAM-like sequence) supported internalization of OpaCEA protein-expressing bacteria, whereas bacteria were associated with the cell membrane (Fig. 2C). These results support the findings obtained by gentamicin protection assays and provide evidence that the CEACAM4 cytoplasmic domain can initiate the internalization of receptor-bound microorganisms. Similar to other phagocytic receptors, such as the FcγR, Dectin-1, or CEACAM3, the phagocytic properties of the chimeric receptor depend on a tyrosine-based motif in the cytoplasmic domain of CEACAM4.
The ITAM-like sequence of CEACAM4 is tyrosine phosphorylated and mediates interaction with several SH2 domain-containing proteins
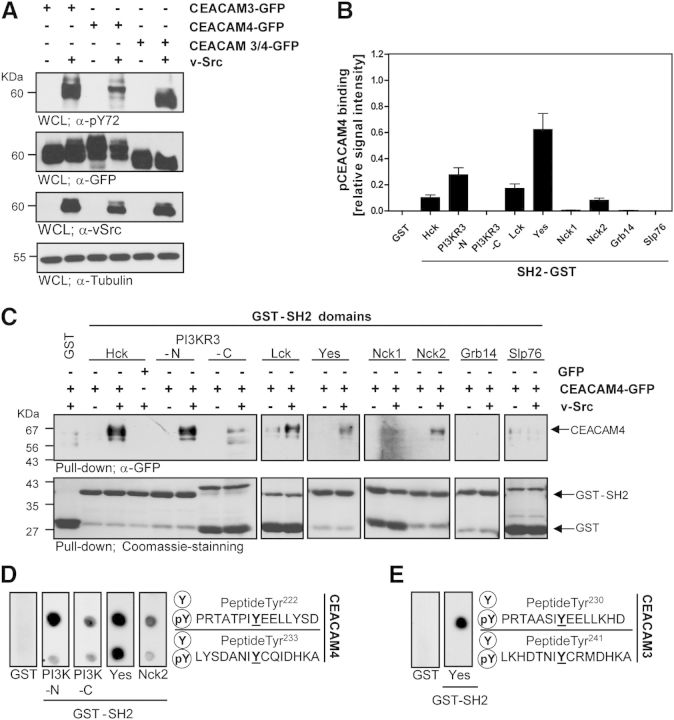
As the ITAM-like sequence derived from CEACAM4 was instrumental to drive phagocytosis by the CEACAM3/4-fusion protein, we wondered if this motif of CEACAM4 becomes tyrosine phosphorylated. Previously, CEACAM3 has been shown to be a substrate of Src family PTKs expressed by human granulocytes [11, 13, 25]. In vitro, the CEACAM3 ITAM-like motif is phosphorylated efficiently by active v-Src [15, 23]. Therefore, we transfected HEK-293 cells with constructs encoding GFP-tagged CEACAM3, CEACAM4, or the CEACAM3/4 chimera, together with or without a v-Src expression plasmid. Western blotting with a phosphotyrosine-specific mAb confirmed that CEACAM3 is tyrosine phosphorylated upon coexpression with v-Src (Fig. 3A). Likewise, CEACAM3/4 and CEACAM4 were tyrosine phosphorylated in HEK-293 cells in the presence of v-Src (Fig. 3A). Western blotting of the lysates with antibodies directed against GFP or against v-Src confirmed comparable expression levels of the transfected constructs (Fig. 3A).
Figure 3. CEACAM4 is tyrosine phosphorylated and binds directly to specific SH2 domains. (A) HEK-293 cells were transfected with plasmids encoding GFP-tagged CEACAM3, CEACAM4, or CEACAM3/4 WT. As indicated, cells were cotransfected with v-Src. Phosphorylated CEACAMs were detected with an anti-pTyr mAb (top). Equivalent expression of the receptors (2nd panel), v-Src (3rd panel), and protein content (bottom panel) was verified by Western blotting of WCLs with mAb against GFP, v-Src, or tubulin, respectively. (B) HEK-293 cells were transfected with CEACAM4-GFP, with or without v-Src as in A. Lysates containing p-CEACAM4 or CEACAM4 were prepared and used to probe protein domain microarrays encompassing the indicated SH2 domains spotted in the form of GST-fusion proteins. p-CEACAM4 or CEACAM4-GFP, bound to individual spots, were detected by anti-GFP antibodies and quantified. Extent of p-CEACAM4 binding versus CEACAM4 binding was calculated, and the values were normalized to the amount of immobilized GST-SH2 domains at individual spots, as detected by an anti-GST mAb. Bars represent the mean ± sd of 4 determinations. (C) Lysates as in B were used in GST pull-down assays with the indicated SH2 domains fused to GST or with GST alone. Precipitates were probed with an anti-GFP mAb for the presence of CEACAM4 (upper). Equal amounts of the GST-fusion proteins used in the pull down were verified by Coomassie staining of the membrane (lower). (D) Peptide spot membranes harboring synthetic 15-mer peptides surrounding the indicated tyrosine residues of the CEACAM4 cytoplasmic domain in the unphosphorylated (Y) or the tyrosine-phosphorylated (pY) form were probed with GST-PI3K-N-SH2, GST-PI3K-C-SH2, GST-c-Yes-SH2, GST-Nck2-SH2, or GST only. Bound GST-fusion proteins were detected with anti-GST mAb. (E) Spot membranes harboring the indicated 15-mer peptides derived from the CEACAM3 cytoplasmic domain were probed with GST or GST-c-Yes-SH2 as in D.
The phosphorylated ITAM-like sequence might connect stimulated CEACAM4 with downstream signaling molecules to drive phagocytosis. To screen for potential SH2 domain-containing binding partners, we used a custom-made SH2 domain microarray [24]. The microarray contained a variety of purified, rGST-fused SH2 domains and was probed with lysates containing unphosphorylated or phosphorylated CEACAM4, generated by coexpression of v-Src. Western blotting demonstrated that the used lysates contained equivalent levels of CEACAM4-GFP or GFP, yet CEACAM4-GFP was only tyrosine phosphorylated upon coexpression of v-Src (Supplemental Fig. 3D). Detection of CEACAM4 bound to the array with an anti-GFP antibody revealed that phosphorylated CEACAM4 associated with the SH2 domains of several Src family PTKs (Hck, Lck, and Yes), the amino-terminal SH2 domain of the PI3K regulatory domain γ (PI3KR3-N), and the Nck2 SH2 domain, whereas no binding was seen for the SH2 domains of the adapter proteins Grb2, Grb14, or Slp76 (Fig. 3B). To confirm these potential binding interactions, classic GST pull-down assays were performed, and the precipitates were probed with anti-GFP antibodies to detect SH2 domain-bound CEACAM4. In agreement with the results of the protein domain microarray, only phosphorylated CEACAM4-GFP but not the unphosphorylated receptor associated with the SH2 domains of Src PTKs, with the amino-terminal SH2 domain of PI3K, and with the Nck2 SH2 domain, whereas no binding was seen for the adapter proteins Grb14 and Slp76 (Fig. 3C). GST alone was not able to pull down phosphorylated CEACAM4 from the lysates (Fig. 3C). To ascertain that the receptor can interact directly with the different SH2 domains and to identify the involved tyrosine residues, we used synthetic peptides spanning the membrane-proximal (Tyr222) or the membrane-distal (Tyr233) tyrosine residue of the CEACAM4 ITAM-like sequence (Fig. 3D). The corresponding peptides were synthesized in the unphosphorylated or in the phosphorylated form on nitrocellulose filters [42] and probed with the purified SH2 domains or GST alone. Importantly, whereas GST was unable to bind, the purified SH2 domains of Yes, Nck2, and the amino-terminal SH2 domain of PI3K associated with synthetic CEACAM4 phospho-peptides, indicating that binding of these SH2 domains is based on a direct protein–protein interaction (Fig. 3D). In line with the GST pull-down analyses, binding did not occur for the unphosphorylated peptides (Fig. 3D). Interestingly, both pTyr residues of the CEACAM4 ITAM sequence served as binding sites for multiple SH2 domains (Fig. 3D). These results demonstrate that the phosphorylated ITAM sequence of CEACAM4 is able to associate directly with a set of SH2 domain-containing proteins, including Src family PTKs, the adapter protein Nck2, as well as PI3KR3 and that this interaction is supported by both tyrosine residues (pY222 and pY233) of the CEACAM4 ITAM sequence. This situation is clearly distinct from CEACAM3, where SH2 domain-mediated binding of protein and lipid kinases or adaptor proteins has only been reported for the membrane proximal tyrosine residue (CEACAM3 pTyr-230) [15, 24, 25]. Indeed, similar to the previous observations, the SH2 domain of the Src PTK Yes only associated with phosphorylated Tyr230 of CEACAM3 (Fig. 3E).
Interestingly, the CEACAM4 tyrosine-based motif (YxxLx(7)YxxI) strictly corresponds to the canonical ITAM signature (YxxL/Ix(6–12)YxxL/I) [43] found in the TCR ζ-chain or the activating FcγRs. In contrast, the membrane distal tyrosine residue (CEACAM3 Tyr-241) in the ITAM-like sequence of CEACAM3 (YxxLx(7)YxxM) deviates from this consensus, and only the membrane proximal tyrosine residue of CEACAM3, Tyr-230, corresponds strictly to the ITAM consensus. In this respect, CEACAM3 shares similarities with the phagocytic receptor Dectin-1, where a single, membrane-proximal, tyrosine-based motif is sufficient for Dectin-1-induced signal transduction [44, 45]. It will be interesting to evaluate in the future which signaling connections are shared among CEACAM3, CEACAM4, and potentially Dectin-1 and which are not. For example, CEACAM3 has the peculiarity to associate directly with Vav, a guanine nucleotide exchange factor for the small GTPase Rac [25]. Via this direct link to Vav, CEACAM3 has a pronounced effect on cellular Rac-GTP levels in response to bacterial binding [11]. The direct association of Vav with the phosphorylated receptor might also explain why CEACAM3-initiated phagocytosis is independent of PI3K activity [15], which is an essential element in phagocytosis via FcγRs [46, 47]. Therefore, it remains to be seen, if and how CEACAM4 is connected to additional cellular components known to mediate signaling downstream of CEACAM3.
The CEACAM4 ITAM recruits specific SH2 domain-containing proteins upon bacterial engagement
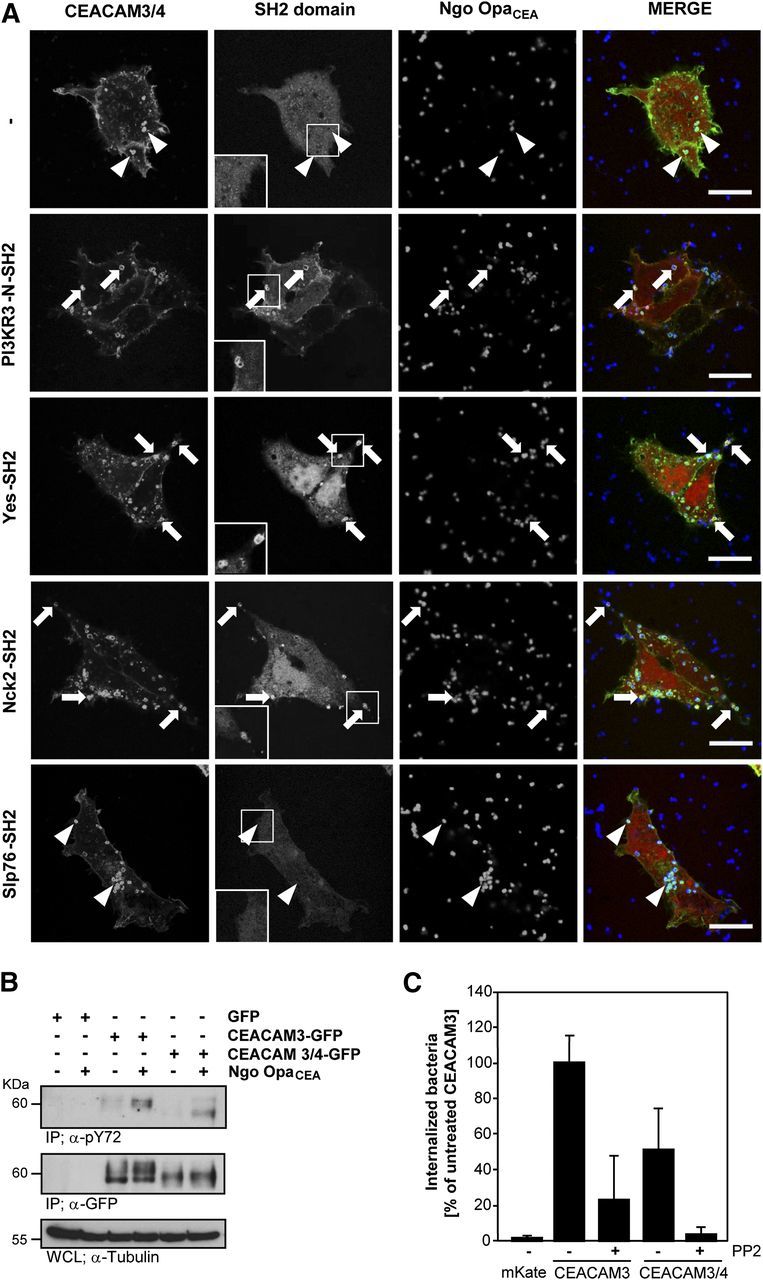
Our biochemical analysis demonstrated association of various SH2 domains with the phosphorylated cytoplasmic domain of CEACAM4 in vitro. To analyze if this interaction also occurs in the context of the intact cell and if it can occur upon bacterial ligation of the receptor, HEK-293 cells were transfected with CEACAM3/4-GFP together with different SH2 domains fused to the red fluorescent protein mKate or with mKate alone. Two days later, the transfected cells were infected with Pacific Blue-labeled Ngo OpaCEA and the bacteria-triggered clustering of the receptor, and the potential recruitment of the SH2 domains was monitored by confocal microscopy. Clearly, no recruitment of mKate alone was observed, when bacteria engaged the chimeric receptor (Fig. 4A). Likewise and in line with the results obtained by GST pull-down assays, the Slp76-SH2 domain was also not recruited to the subcellular contact sites between the bacteria and CEACAM3/4 (Fig. 4A). In striking contrast, a strong, local enrichment of the Yes-SH2 domain, the PI3KR3 amino-terminal SH2 domain, and the Nck2 SH2 domain was observed at the sites, where gonococci colocalized with the receptor (Fig. 4A). These results not only demonstrated that CEACAM-binding bacteria are able to cluster the chimeric receptor locally, but the clear subcellular recruitment of selected SH2 domains also suggested that the clustered receptor is tyrosine phosphorylated and interacts with specific SH2 domains in intact cells.
Figure 4. The CEACAM4 cytoplasmic domain is phosphorylated following bacterial engagement, recruits SH2 domains, and requires Src kinase activity for phagocytosis. (A) HEK-293 cells were cotransfected with CEACAM3/4-GFP together with plasmids encoding mKate or the mKate-tagged SH2 domains derived from PI3KR3, c-Yes, Nck2, or Slp76, respectively. Cells were infected with Pacific Blue-labeled Ngo OpaCEA at an MOI of 30 for 30 min, fixed, and analyzed by confocal microscopy. Bacterial binding to the clustered receptor is indicated by white arrowheads. Specific SH2 domains are recruited to bacteria-engaged, clustered receptors (arrowheads). Insets depict magnifications of selected bacteria CEACAM3/4 clusters in the mKate channel to highlight SH2 domain recruitment. Original scale bars, 10 µm. (B) HEK-293 cells were transfected with vectors encoding GFP, CEACAM3-GFP, or CEACAM3/4-GFP, respectively, and infected or not for 1 h with Ngo OpaCEA. Lysates were used in immunoprecipitations (IP) with a polyclonal anti-GFP-antibody. Precipitates were sequentially probed with an anti-pTyr mAb (top) and an anti-GFP mAb (middle panel). Equivalent protein content in the used WCLs was verified by blotting with anti-tubulin mAb (bottom). (C) HEK-293 cells were transfected with plasmids encoding mKate, CEACAM3-mKate, or CEACAM3/4-mKate. As indicated, cells were infected for 1 h with OpaCEA protein-expressing gonococci at MOI 30. Fifteen minutes before infection, cells were treated or not with the Src kinase inhibitor PP2 (10 µM). Uptake of the bacteria was analyzed by gentamicin protection assays. The bars show mean values ± sd (n = 3) relative to the uptake observed for CEACAM3 in the absence of PP2 treatment.
Src family kinases mediate phosphorylation of the CEACAM4 ITAM, which is required for phagocytosis
To investigate further if tyrosine phosphorylation and the recruitment of particular enzymes are of functional relevance for a potential CEACAM4-mediated phagocytosis, we first analyzed the phosphorylation of the CEACAM3/4 chimera in response to bacterial engagement. Therefore, we infected cells expressing CEACAM3-mKate or CEACAM3/4-mKate for 60 min with Ngo OpaCEA or left the cells uninfected. Upon immunoprecipitation with anti-mKate antibodies, we observed tyrosine phosphorylation of both proteins in response to infection with CEACAM-binding bacteria (Fig. 4B). Additionally, the probing of the precipitates with anti-CEACAM antibodies confirmed that similar amounts of the receptors were precipitated from uninfected and infected cells (Fig. 4B). Together, these results provide evidence that the ITAM of CEACAM4 can become tyrosine phosphorylated upon bacterial engagement and clustering of the receptor. Next, we focused on Src family PTKs, which have been shown to be critical downstream signaling elements in CEACAM3-mediated uptake [10, 14, 48] and applied the Src PTK inhibitor PP2 during in vitro infection with N. gonorrheae. In line with the known role of Src family PTKs, the pharmacological inhibitor severely reduced uptake of OpaCEA protein-expressing gonococci by CEACAM3-expressing HEK-293 cells (Fig. 4C). In a similar manner, PP2 treatment strongly impaired bacterial internalization via the CEACAM3/4 chimera (Fig. 4C). These results demonstrate that Src family PTKs not only associate with the cytoplasmic domain of phosphorylated CEACAM4 but also are of functional relevance for the opsonin-independent uptake, which can be triggered by the CEACAM4 ITAM sequence. Together, our results suggest that tyrosine phosphorylation of CEACAM4 by Src family PTKs allows this receptor to initiate phagocytosis of bound bacteria.
Conclusion
In this study, we have taken advantage of the modular nature of CEACAMs and created chimeric proteins to study the function of the orphan receptor CEACAM4. Until now, the absence of the CEACAM4 gene in rodents and the lack of a known ligand have been the major roadblocks for an in-depth study of this membrane protein. To address this problem, we exchanged the extracellular, amino-terminal IgV-like domain of CEACAM4 with the corresponding domain of CEACAM3. Therefore, the resulting chimeric CEACAM3/4-fusion protein consisted of the bacteria-binding domain of CEACAM3 fused to the transmembrane and cytosolic parts of CEACAM4 and allowed us to probe cellular processes initiated by CEACAM4. With the use of this chimeric protein, we were able to demonstrate that the cytoplasmic ITAM-like sequence of CEACAM4 becomes tyrosine phosphorylated upon ligand-mediated receptor clustering. Furthermore, we identified several potential, direct binding partners of phosphorylated CEACAM4, and we provide evidence that Src family PTKs play a critical role in orchestrating receptor-triggered signaling.
Together, our in vitro data indicate that the orphan receptor CEACAM4 can support efficient phagocytosis of particulate ligands. As a result of the exclusive presence of the CEACAM4 gene in the primate lineage and as a result of the expression of this gene in professional phagocytes, we hypothesize that this receptor provides an interesting, primate-specific contribution to the function and/or regulation of our species’ immune system. The identification of a physiologic ligand, be it an endogenous structure or a human-associated microorganism, could be the next step in elucidating the fascinating biology behind this protein.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by funds from the Deutsche Forschungsgemeinschaft (Ha2568/6-2; to C.R.H.) and the Swiss National Science Foundation (31003A_143739; to M.P.T.). J.D.T. acknowledges support by the German Academic Exchange Service. The authors thank T. F. Meyer (Max-Planck-Institut für Infektionsbiologie) for the Neisseria strains used in this study, W. Zimmermann (Ludwig-Maximilians-Universität) for providing CEACAM cDNA, R. Frank (Leibniz-Institut für Molekulare Pharmakologie, Berlin, Germany) for synthesis of peptide spot membranes, and R. Hohenberger-Bregger and S. Feindler-Boeckh for expert technical assistance.
Glossary
- C
C-terminal domain
- CEA
carcinoembryonic antigen
- CEACAM
carcinoembryonic antigen-related cell adhesion molecule
- EGFP
enhanced GFP
- HEK
human embryonic kidney
- IgV-like
Ig variable-like
- MOI
multiplicity of infection
- N
N-terminal domain
- Ngo
Neisseria gonorrheae
- Ngo Opa−
Ngo lacking Opa protein expression
- p-CEACAM4
tyrosine phosphorylated CEACAM4
- PTK
protein tyrosine kinase
- pTyr
phosphotyrosine
- qRT-PCR
quantitative RT-PCR
- SH2
Src homology 2
- soeingPCR
splicing by overlap extension PCR
- v-Src
viral Src
- WCL
whole-cell lysate
- WT
wild-type
Footnotes
The online version of this paper, found at www.jleukbio.org, includes supplemental information.
AUTHORSHIP
J.D.T., J.A., K. Kopp, P.S., and K. Kuespert performed experimental work. J.D.T., J.A., K. Kopp, M.P.T., and C.R.H. designed experiments and analyzed data. C.R.H. designed the study and wrote the manuscript.
REFERENCES
- 1.Pinheiro V. B., Ellar D. J. (2006) How to kill a mocking bug? Cell. Microbiol. 8, 545–557. [DOI] [PubMed] [Google Scholar]
- 2.Takeda K., Kaisho T., Akira S. (2003) Toll-like receptors. Annu. Rev. Immunol. 21, 335–376. [DOI] [PubMed] [Google Scholar]
- 3.Areschoug T., Gordon S. (2008) Pattern recognition receptors and their role in innate immunity: focus on microbial protein ligands. Contrib. Microbiol. 15, 45–60. [DOI] [PubMed] [Google Scholar]
- 4.Areschoug T., Gordon S. (2009) Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell. Microbiol. 11, 1160–1169. [DOI] [PubMed] [Google Scholar]
- 5.Kuespert K., Pils S., Hauck C. R. (2006) CEACAMs: their role in physiology and pathophysiology. Curr. Opin. Cell Biol. 18, 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kammerer R., Popp T., Härtle S., Singer B. B., Zimmermann W. (2007) Species-specific evolution of immune receptor tyrosine based activation motif-containing CEACAM1-related immune receptors in the dog. BMC Evol. Biol. 7, 196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kammerer R., Zimmermann W. (2010) Coevolution of activating and inhibitory receptors within mammalian carcinoembryonic antigen families. BMC Biol. 8, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammarström S. (1999) The carcinoembryonic antigen (CEA) family: structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 9, 67–81. [DOI] [PubMed] [Google Scholar]
- 9.Chen T., Gotschlich E. C. (1996) CGM1a antigen of neutrophils, a receptor of gonococcal opacity proteins. Proc. Natl. Acad. Sci. USA 93, 14851–14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hauck C. R., Meyer T. F., Lang F., Gulbins E. (1998) CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 17, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitter T., Agerer F., Peterson L., Munzner P., Hauck C. R. (2004) Granulocyte CEACAM3 is a phagocytic receptor of the innate immune system that mediates recognition and elimination of human-specific pathogens. J. Exp. Med. 199, 35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buntru A., Roth A., Nyffenegger-Jann N. J., Hauck C. R. (2012) HemITAM signaling by CEACAM3, a human granulocyte receptor recognizing bacterial pathogens. Arch. Biochem. Biophys. 524, 77–83. [DOI] [PubMed] [Google Scholar]
- 13.McCaw S. E., Schneider J., Liao E. H., Zimmermann W., Gray-Owen S. D. (2003) Immunoreceptor tyrosine-based activation motif phosphorylation during engulfment of Neisseria gonorrhoeae by the neutrophil-restricted CEACAM3 (CD66d) receptor. Mol. Microbiol. 49, 623–637. [DOI] [PubMed] [Google Scholar]
- 14.Schmitter T., Pils S., Weibel S., Agerer F., Buntru A., Kopp K., Hauck C. R. (2007) Opa proteins of pathogenic Neisseriae initiate Src kinase-dependent or lipid raft-mediated uptake via distinct human carcinoembryonic antigen-related cell adhesion molecule isoforms. Infect. Immun. 75, 4116–4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buntru A., Kopp K., Voges M., Frank R., Bachmann V., Hauck C. R. (2011) Phosphatidylinositol 3′-kinase activity is critical for initiating the oxidative burst and bacterial destruction during CEACAM3-mediated phagocytosis. J. Biol. Chem. 286, 9555–9566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroki M., Arakawa F., Matsuo Y., Oikawa S., Misumi Y., Nakazato H., Matsuoka Y. (1991) Molecular cloning of nonspecific cross-reacting antigens in human granulocytes. J. Biol. Chem. 266, 11810–11817. [PubMed] [Google Scholar]
- 17.Kuroki M., Yamanaka T., Matsuo Y., Oikawa S., Nakazato H., Matsuoka Y. (1995) Immunochemical analysis of carcinoembryonic antigen (CEA)-related antigens differentially localized in intracellular granules of human neutrophils. Immunol. Invest. 24, 829–843. [DOI] [PubMed] [Google Scholar]
- 18.Beauchemin N., Draber P., Dveksler G., Gold P., Gray-Owen S., Grunert F., Hammarström S., Holmes K. V., Karlsson A., Kuroki M., Lin S. H., Lucka L., Najjar S. M., Neumaier M., Obrink B., Shively J. E., Skubitz K. M., Stanners C. P., Thomas P., Thompson J. A., Virji M., von Kleist S., Wagener C., Watt S., Zimmermann W. (1999) Redefined nomenclature for members of the carcinoembryonic antigen family. Exp. Cell Res. 252, 243–249. [DOI] [PubMed] [Google Scholar]
- 19.Thompson J., Mössinger S., Reichardt V., Engels U., Beauchemin N., Kommoss F., von Kleist S., Zimmermann W. (1993) A polymerase-chain-reaction assay for the specific identification of transcripts encoded by individual carcinoembryonic antigen (CEA)-gene-family members. Int. J. Cancer 55, 311–319. [DOI] [PubMed] [Google Scholar]
- 20.Pils S., Gerrard D. T., Meyer A., Hauck C. R. (2008) CEACAM3: an innate immune receptor directed against human-restricted bacterial pathogens. Int. J. Med. Microbiol. 298, 553–560. [DOI] [PubMed] [Google Scholar]
- 21.Kupsch E.-M., Knepper B., Kuroki T., Heuer I., Meyer T. F. (1993) Variable opacity (Opa) outer membrane proteins account for the cell tropisms displayed by Neisseria gonorrhoeae for human leukocytes and epithelial cells. EMBO J. 12, 641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Vries F. P., van Der Ende A., van Putten J. P., Dankert J. (1996) Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect. Immun. 64, 2998–3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pils S., Kopp K., Peterson L., Delgado Tascón J., Nyffenegger-Jann N. J., Hauck C. R. (2012) The adaptor molecule Nck localizes the WAVE complex to promote actin polymerization during CEACAM3-mediated phagocytosis of bacteria. PLoS ONE 7, e32808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kopp K., Buntru A., Pils S., Zimmermann T., Frank R., Zumbusch A., Hauck C. R. (2012) Grb14 is a negative regulator of CEACAM3-mediated phagocytosis of pathogenic bacteria. J. Biol. Chem. 287, 39158–39170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmitter T., Pils S., Sakk V., Frank R., Fischer K. D., Hauck C. R. (2007) The granulocyte receptor carcinoembryonic antigen-related cell adhesion molecule 3 (CEACAM3) directly associates with Vav to promote phagocytosis of human pathogens. J. Immunol. 178, 3797–3805. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D. E., Hetherington C. J., Chen H. M., Tenen D. G. (1994) The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Mol. Cell. Biol. 14, 373–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tschan M. P., Reddy V. A., Ress A., Arvidsson G., Fey M. F., Torbett B. E. (2008) PU.1 binding to the p53 family of tumor suppressors impairs their transcriptional activity. Oncogene 27, 3489–3493. [DOI] [PubMed] [Google Scholar]
- 28.Osoegawa K., Mammoser A. G., Wu C., Frengen E., Zeng C., Catanese J. J., de Jong P. J. (2001) A bacterial artificial chromosome library for sequencing the complete human genome. Genome Res. 11, 483–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Muenzner P., Bachmann V., Zimmermann W., Hentschel J., Hauck C. R. (2010) Human-restricted bacterial pathogens block shedding of epithelial cells by stimulating integrin activation. Science 329, 1197–1201. [DOI] [PubMed] [Google Scholar]
- 30.Schmittgen T. D., Livak K. J. (2008) Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108. [DOI] [PubMed] [Google Scholar]
- 31.Agerer F., Michel A., Ohlsen K., Hauck C. R. (2003) Integrin-mediated invasion of Staphylococcus aureus into human cells requires Src family protein-tyrosine kinases. J. Biol. Chem. 278, 42524–42531. [DOI] [PubMed] [Google Scholar]
- 32.Kuespert K., Hauck C. R. (2009) Characterizing host receptor recognition by individual bacterial pathogens. In Methods in Molecular Biology (Rupp S., Sohn K., Hauser N., eds.), Humana, Totowa, NJ, USA, 57–65. [DOI] [PubMed] [Google Scholar]
- 33.Kuespert K., Weibel S., Hauck C. R. (2007) Profiling of bacterial adhesin—host receptor recognition by soluble immunoglobulin superfamily domains. J. Microbiol. Methods 68, 478–485. [DOI] [PubMed] [Google Scholar]
- 34.Kuespert K., Roth A., Hauck C. R. (2011) Neisseria meningitidis has two independent modes of recognizing its human receptor CEACAM1. PLoS ONE 6, e14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeKoter R. P., Walsh J. C., Singh H. (1998) PU.1 regulates both cytokine-dependent proliferation and differentiation of granulocyte/macrophage progenitors. EMBO J. 17, 4456–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K., Wang P., Shi J., Zhu X., He M., Jia X., Yang X., Qiu F., Jin W., Qian M., Fang H., Mi J., Yang X., Xiao H., Minden M., Du Y., Chen Z., Zhang J. (2010) PML/RARalpha targets promoter regions containing PU.1 consensus and RARE half sites in acute promyelocytic leukemia. Cancer Cell 17, 186–197. [DOI] [PubMed] [Google Scholar]
- 37.Chen T., Grunert F., Medina-Marino A., Gotschlich E. C. (1997) Several carcinoembryonic antigens (CD66) serve as receptors for gonococcal opacity proteins. J. Exp. Med. 185, 1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Virji M., Evans D., Griffith J., Hill D., Serino L., Hadfield A., Watt S. M. (2000) Carcinoembryonic antigens are targeted by diverse strains of typable and non-typable Haemophilus influenzae. Mol. Microbiol. 36, 784–795. [DOI] [PubMed] [Google Scholar]
- 39.Hill D. J., Virji M. (2003) A novel cell-binding mechanism of Moraxella catarrhalis ubiquitous surface protein UspA: specific targeting of the N-domain of carcinoembryonic antigen-related cell adhesion molecules by UspA1. Mol. Microbiol. 48, 117–129. [DOI] [PubMed] [Google Scholar]
- 40.Brooks M. J., Sedillo J. L., Wagner N., Wang W., Attia A. S., Wong H., Laurence C. A., Hansen E. J., Gray-Owen S. D. (2008) Moraxella catarrhalis binding to host cellular receptors is mediated by sequence-specific determinants not conserved among all UspA1 protein variants. Infect. Immun. 76, 5322–5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Popp A., Dehio C., Grunert F., Meyer T. F., Gray-Owen S. D. (1999) Molecular analysis of neisserial Opa protein interactions with the CEA family of receptors: identification of determinants contributing to the differential specificities of binding. Cell. Microbiol. 1, 169–181. [DOI] [PubMed] [Google Scholar]
- 42.Frank R. (1992) SPOT synthesis: an easy technique for the positionally addressable, parallel chemical synthesis on a membrane support. Tetrahedron 48, 9217–9232. [Google Scholar]
- 43.Reth M. (1989) Antigen receptor tail clue. Nature 338, 383–384. [PubMed] [Google Scholar]
- 44.Underhill D. M., Rossnagle E., Lowell C. A., Simmons R. M. (2005) Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood 106, 2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers N. C., Slack E. C., Edwards A. D., Nolte M. A., Schulz O., Schweighoffer E., Williams D. L., Gordon S., Tybulewicz V. L., Brown G. D., Reis e Sousa C. (2005) Syk-dependent cytokine induction by Dectin-1 reveals a novel pattern recognition pathway for C type lectins. Immunity 22, 507–517. [DOI] [PubMed] [Google Scholar]
- 46.Indik Z. K., Park J. G., Hunter S., Schreiber A. D. (1995) The molecular dissection of Fc gamma receptor mediated phagocytosis. Blood 86, 4389–4399. [PubMed] [Google Scholar]
- 47.Araki N., Johnson M. T., Swanson J. A. (1996) A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J. Cell Biol. 135, 1249–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McCaw S. E., Liao E. H., Gray-Owen S. D. (2004) Engulfment of Neisseria gonorrhoeae: revealing distinct processes of bacterial entry by individual carcinoembryonic antigen-related cellular adhesion molecule family receptors. Infect. Immun. 72, 2742–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.