Review of critical non-TH17 sources of IL-17A and associated cytokines during inflammation in mucosal tissues, with a focus on leukocytes of the gastrointestinal tract.
Keywords: IL-17A, IL-17F, IL-22, IL-17RA, mucosal, inflammation
Abstract
In the 2 decades since its discovery, IL-17A has become appreciated for mounting robust, protective responses against bacterial and fungal pathogens. When improperly regulated, however, IL-17A can play a profoundly pathogenic role in perpetuating inflammation and has been linked to a wide variety of debilitating diseases. IL-17A is often present in a composite milieu that includes cytokines produced by TH17 cells (i.e., IL-17F, IL-21, IL-22, and IL-26) or associated with other T cell lineages (e.g., IFN-γ). These combinatorial effects add mechanistic complexity and more importantly, contribute differentially to disease outcome. Whereas TH17 cells are among the best-understood cell types that secrete IL-17A, they are frequently neither the earliest nor dominant producers. Indeed, non-TH17 cell sources of IL-17A can dramatically alter the course and severity of inflammatory episodes. The dissection of the temporal regulation of TH17-associated cytokines and the resulting net signaling outcomes will be critical toward understanding the increasingly intricate role of IL-17A and TH17-associated cytokines in disease, informing our therapeutic decisions. Herein, we discuss important non-TH17 cell sources of IL-17A and other TH17-associated cytokines relevant to inflammatory events in mucosal tissues.
Introduction
The discovery of IL-17A and its role in disease marked a paradigm shift in our understanding of how inflammation is regulated. Originally remarkable for its high homology to Herpesvirus saimiri gene 13 [1], it was evident early on that IL-17A is multifunctional, with wide-ranging effects, including the modulation of NF-κB activation and T cell proliferation [2]. Studies describing the proinflammatory nature of IL-17A soon followed, and the role of IL-17A in the pathology associated with diseases, such as arthritis [3, 4] and psoriasis [5, 6], is now well established. However, recent studies to understand the role of IL-17A in complex diseases of the mucosa, such as colitis, have demonstrated that IL-17A can also serve in a host-protective capacity [7]. Therefore, like many other cytokines, the function of IL-17A appears to be more complex and context dependent than originally appreciated.
IL-17A is primarily produced by immune cells at mucosal sites during inflammatory events. As such, IL-17A is often only 1 component of a complex cytokine microenvironment. There are several cytokines that are often secreted together with IL-17A, including TNF-α, IFN-γ, and TH17-associated IL-17F, IL-21, and IL-22 and in humans, IL-26. Moreover, recent evidence suggests that some populations of IL-17A-expressing cells that coexpress IFN-γ are primarily pathogenic in nature [8]. Whereas cytokine functions are often deduced via reductionist methods, a movement toward understanding the role of IL-17A and its associated cytokines within the framework of the complete cytokine milieu appears to be the logical next step toward understanding the role of TH17-associated cytokines in inflammation.
TH17-ASSOCIATED CYTOKINES
IL-17A and IL-17F
The IL-17 family has 6 members, enumerated IL-17A–IL-17F [9]. Human IL-17A and IL-17F share 40–50% homology at the amino acid level [10, 11], the highest homology between individual members of the IL-17 family, and are adjacently located on chromosome 6 in a head-to-tail fashion [10]. IL-17A and IL-17F can individually homodimerize or form heterodimers of ∼35 kDa [12], both of which bind to a heterodimeric complex containing IL-17RA and IL-17RC [13]. IL-17RA is nearly ubiquitously expressed on a variety of cells within the mucosa, and context-dependent regulation of IL-17RC provides additional signaling capabilities.
Currently, several antibodies targeting IL-17A or IL-17RA are under development, including Ixekizumab (Eli Lilly, Indianapolis, IN, USA), Brodalumab (Amgen, Thousand Oaks, CA, USA), and Secukinumab (Novartis Pharma AG, Basel, Switzerland). All 3 aforementioned drugs are currently showing promise in the treatment of psoriasis [14, 15]. Notably, Secukinumab recently underwent a clinical trial for the treatment of moderate-to-severe Crohn’s disease. Beyond failing to improve Crohn’s disease, treatment with Secukinumab exacerbated the disease in a subset of patients [16]; moreover, Secukinumab was associated with increased infections, including those caused by fungal pathogens [16]. These findings are consistent with the tissue-protective function of IL-17A observed in mouse models of colitis by several groups, including our own [7, 17, 18]. As IL-17A-mediated contributions to intestinal disease have also been observed in mice [19, 20], taken together, these data suggest that the role of IL-17A is complex and perhaps context dependent.
The elimination of IL-17F was shown to be protective in a murine model of colitis by use of dextran sodium sulfate, suggesting that it plays a pathogenic role in intestinal inflammation [18], and the elimination of IL-17A and IL-17F signaling was protective in a T cell-mediated model of colitis [7, 21]. These data suggest that targeting IL-17F may prove more efficacious than targeting IL-17A for the treatment of some mucosal inflammatory disorders, yet therapeutics selectively targeting IL-17F are currently unavailable. New studies determining the utility of targeting multiple IL-17 family members during human disease are needed. Given the complexity in IL-17A/F signal integration, additional microenvironment-specific factors will likely contribute to the efficacy of strategies blocking IL-17 family proteins in patient subpopulations. To appreciate the complexity of the inflammatory milieu in which IL-17A et al. function, going forward, we will consider the roles and cellular producers of disease-relevant, TH17-associated cytokines.
IL-21
IL-21 is a member of the IL-2 family of cytokines and is located near IL-2 on chromosome 4 in humans [22]. Originally identified as a factor produced by activated T cells that could drive NK cell proliferation and maturation [23], IL-21 appears to be secreted predominantly by TH17 cells, TFH cells, and NKT cells (reviewed in ref. [24]). IL-21R is composed of an IL-21-specific component paired with the common γ-chain [23, 25, 26]. This receptor is found on cells of lymphoid origin, including T cells, where IL-21 signaling may act in an autocrine or paracrine fashion to promote TH17 responses [27]. IL-21 signaling in mucosal diseases appears to be largely pathogenic, especially within the intestinal tract; for example, IL-21−/− mice are more resistant to chemically induced colitis [28] and the development of CRC [29]. Given the significant association of IL-21 to mucosal disease pathogenesis, therapeutics designed to target the IL-21 signaling pathway are of great clinical interest [30].
IL-22
With 25% homology to IL-10 in humans, IL-22 falls within the larger IL-10 cytokine family [31]. IL-22 is located on chromosome 12 in humans (chromosome 10 in mice), in close proximity to the genes for IFN-γ and IL-26 [32]. One subunit of the IL-22R , the common β-chain receptor IL-10R2, is shared among IL-10, IL-22, and IL-26. The second subunit of the IL-22R, IL-22RA1, also comprises part of the receptors for IL-20 and IL-24 [33]. An agonist of IL-22, termed IL-22BP or IL-22RA2, is a soluble receptor, structurally related to IL-22RA1, that inhibits binding of IL-22 to the membrane-bound IL-22R [34, 35]. IL-22 was discovered originally as a cytokine produced by T cells [36, 37]; T cells and NK1.1+ cells are the primary cell types in mucosal tissues implicated in IL-22 production [38]. IL-22-responding cells, however, are of nonhematopoietic lineage and include epithelial cells in mucosal tissues (reviewed in ref. [33]). In addition to stimulating the production of antimicrobial peptides, IL-22 promotes epithelial cell proliferation, thereby maintaining epithelial barrier function (reviewed in ref. [39]); indeed, IL-22 can be protective in murine models of intestinal inflammation [38, 40] and influenza [41, 42]. Consistent with its role in epithelial cell proliferation, IL-22 levels are positively correlated with tumor development, such as in colon cancer [43, 44]. IL-22 can also be proinflammatory at the mucosa, where it contributes to lung pathology following fungal infection [45], chemically induced lung injury [46], or colitis in select models [47]. The proinflammatory roles of IL-22 are not well understood but may be linked to the induction of chemokines [48, 49].
IL-26
Like IL-22, IL-26 is part of the IL-10 cytokine family. Originally coined AK155, IL-26 is located on human chromosome 12 [50] and although expressed in teleosts [51], is not found in rodents [52, 53]. IL-26R is composed of the common β−chain receptor IL-10R2 paired with the α-chain receptor for IL-26, IL-20R1 [54, 55]. In epithelial cells, including intestinal epithelial cells [56], signaling through IL-26R leads to activation of STAT1, STAT3 [54, 55], ERK, stress-activated protein kinase /JNK, and Akt [56]. In addition to being expressed by TH17 cells, IL-26 is expressed by ILCs. IL-26 has been linked to intestinal inflammation, including Crohn’s disease and ulcerative colitis [56, 57], where it appears to be antiproliferative and to induce proinflammatory cytokine production [56].
NON-TH17 CELL SOURCES OF TH17-ASSOCIATED CYTOKINES
During maturation, helper CD4+ T cells differentiate into 1 of several effector types, defined primarily by their cytokine and transcription factor expression profiles. Aside from TH17 populations, the best-characterized lineages are TH1, TH2, and Tregs (discussed below); TH1 cells are defined by their expression of the T-box transcription factor 21 (T-bet) and secretion of IFN-γ, whereas TH2 cells are defined by expression of GATA-3 and secretion of IL-4, IL-5, and IL-13. More recently, exciting new studies have defined additional subsets, including TH9 and TH22 cells, which produce IL-9 and IL-22, respectively [58, 59].
The TH1/TH2 paradigm in disease, especially regarding the TH1-driven pathology, was altered dramatically with the elucidation of IL-23 function [60] and the discovery of TH17 cells [61, 62]. TH17 cells are CD4+ T cells that express the transcription factors RORγt and RORα [63] and can secrete IL-17A alone or in combination with IL-17F, IL-21, and IL-22 and in humans, IL-26 [64–66]. Antigen stimulation, in the presence of select cytokines, can induce naïve CD4+ cells in peripheral lymphoid organs to become TH17 cells, currently termed iTH17 cells. Interestingly, a recently identified, thymically derived population of TH17 cells, coined nTH17 cells [67], may differ in select developmental requirements and behave more like an innate cell population [66]. The role of TH17 cells during disease is complex; TH17 cells can be pathogenic (i.e., rheumatoid arthritis [68]) or protective (i.e., bacterial/fungal infection or in models of colitis [7]), and their contribution to disease pathogenesis may be overt (i.e., psoriasis [69]) or nuanced (i.e., uveitis [70]). Ongoing research to define molecular differences between nTH17 and iTH17 cells may clarify differing data on the precise role of TH17 cells in disease but is unlikely to obviate the clear importance that TH17 cells play in the immune response.
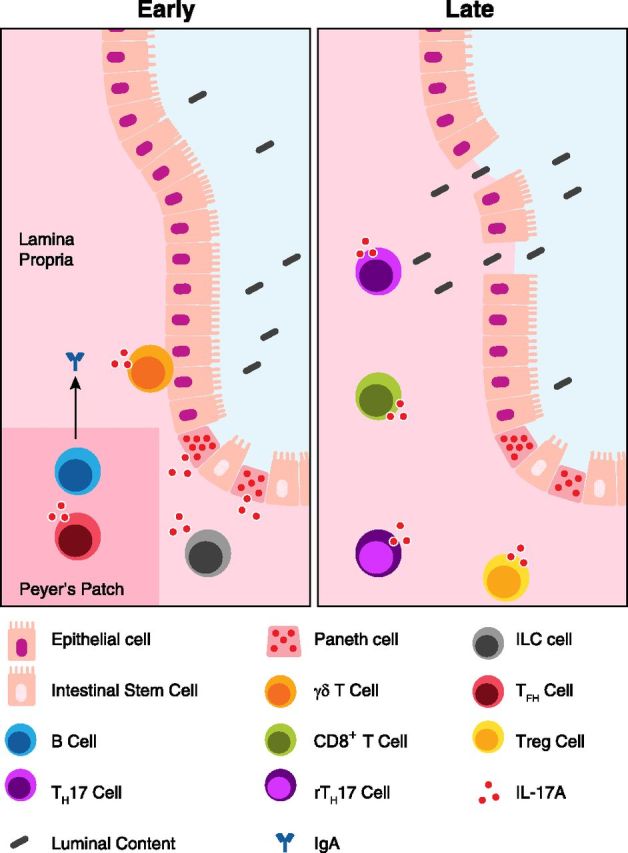
Whereas TH17 cells may be one of the best understood IL-17A-secreting cell populations, there are many additional cell types that produce 1 or more TH17-associated cytokines (Table 1). Remarkably, IL-17 producers are not solely of the T cell lineage, and examination of these cell populations suggests additional important functions for IL-17A. For instance, several secretory cell types, such as neutrophils and Paneth cells, have been reported to produce IL-17A, which hints at a vital role for IL-17A early in the immune/inflammatory response (Fig. 1). More broadly, the wide array of cell populations involved in the secretion of TH17-associated cytokines speaks to the overall importance of these cytokines in the progression of the immune response. A number of recent developments in the elucidation of cellular sources of TH17-associated cytokines warrant attention and careful consideration. Toward that end, we will next review important findings that describe and evaluate cellular sources of TH17-associated cytokines in the mucosa.
TABLE 1.
TH17-associated cytokine production by non-TH17 cells and effects at the mucosa
| Cytokine | Producers | Receptor | Effects |
|---|---|---|---|
| IL-17A | rTH17, Tregs, CD8+ T cell, γδ T cell, TFH, NKT cell, ILC, neutrophil, Paneth cell, macrophage | IL-17RA/IL-17RC | Neutrophil recruitment [71, 72] Antimicrobial peptide production (e.g., S100A8, S100A9 [73]) Cytokine and chemokine production (e.g., G-CSF, IL-6, CXCL1 [74, 75]) Inhibits TH1 polarization [7] |
| IL-17F | γδ T cell | IL-17RA/IL-17RC | Neutrophil recruitment [76] Cytokine and chemokine production (e.g., G-CSF, IL-6, CXCL1 [74, 75]) Lymphocyte and macrophage recruitment [18] Mucus hyperplasia in the lung [18] |
| IL-21 | CD8+ T cell, TFH, NKT cell, neutrophil | IL-21R/γc | Cell proliferation, especially NK cells [22] Enhances TH17 responses [27] B cell class-switching [77] Regulatory B cell function [78] |
| IL-22 | rTH17, CD8+ T cell,γδ T cell, NKT cell, ILC, neutrophil, macrophage | IL-22RA1/IL-10R2 | Antimicrobial peptide production (e.g., S100A8, S100A9, Reg3γ [73, 79]) Epithelial cell proliferation/transformation [43] Enhances mucosal barrier function [79] Inhibits epithelial apoptosis [46] |
| IL-26 | ILCs | IL-20R1/IL-10R2 | Cytokine production (e.g., IL-8, IL-10 [55, 56]) Decreased epithelial proliferation [56] |
Reg3γ, Regenerating islet-derived protein 3 γ.
Figure 1. IL-17A production in the small intestine. The healthy small intestinal mucosa serves the dual function of nutrient exchange and immune surveillance. Early in an immune/inflammatory response, IL-17 appears to be generated by several leukocyte and stromal populations. Prolonged inflammation leads to the breakdown of the epithelial barrier, allowing luminal content to enter the lamina propria. The ensuing inflammatory response leads to recruitment of additional immune cells and "late" IL-17A production. IL-17A binds the heterodimeric receptor IL-17RA/IL-17RC that is ubiquitously expressed on many cells within the mucosa, including epithelial cells. IL-17A production by TFH cells may also support IgA production by neighboring B cells within lymphoid structures, such as Peyer’s patches, leading to more efficient transport of IgA into the lumen of the gut by the polymeric IgR.
CD4+ Tregs
Foxp3+ and Foxp3− Tregs have been shown to produce TH17-associated cytokines. Foxp3+IL-17A-producing T cells have been observed in vitro and in vivo [80–83], including in IBD and CRC [84–86]. Accordingly, in vitro experiments and in vivo transfer experiments suggest that Foxp3+ Tregs can up-regulate RORγt and IL-17A under inflammatory conditions. Further investigation into the stability of Treg/TH17 subsets is necessary; remarkable findings from Zhou et al. [87] showed that Foxp3+ Tregs can be converted into effector T cells, whereas Rubtsov et al. [88] reported that Foxp3+ Tregs are stable, even under inflammatory conditions. This is in line with subsequent data showing that Foxp3 can also be transiently up-regulated by activated T cells [89], which might explain the different outcomes of these studies.
The origin of Foxp3+IL-17A-producing T cells under physiologic conditions is still a matter of debate. There are few studies analyzing the function of Foxp3+IL-17A-producing T cells in vivo. One study suggests that IL-17A-producing Foxp3+ T cells might initiate tumorigenesis, which is, in part, a result of IL-17A production [84]. Foxp3+IL-17A+ T cells seem to be suppressive, at least in vitro [90], although their immunosuppressive function might be partially impaired by the expression of RORγt [86]. Clearly, further studies will be essential to clarify the function of Foxp3+IL-17A-producing T cells in vivo.
Another subset of Tregs has been described as Foxp3−IL-10+IL-17A+ TH cells and are currently referred to as rTH17 cells, which have been found in vitro and in vivo [80, 91, 92] and are generated in the small intestine. In contrast to TH17 cells, rTH17 cells have anti-inflammatory properties in mouse models of colitis and multiple sclerosis, which is, at least partially, dependent on IL-10 [80]. rTH17 cells produce IL-22 and IL-17A in comparable amounts with conventional TH17 cells, suggesting that rTH17 cells might play an important role during the termination of an immune response and in tissue repair. However, it is currently unclear if this cell type is a stable TH cell subset or an intermediate step between TH17 and Foxp3−IL-10+ Tregs (referred to as a type 1 Treg cell), which can modify IL-10 and IL-17A production, based on the environment. Further studies are essential to clarify the function, origin, and stability of rTH17 cells.
CD8+ T cells
CD8+ T cells can produce TH17-related cytokines in the mucosa, where they can mediate pathogenic and nonpathogenic effects. Within the lung, CD8+ T cells can secrete IL-17A, IL-21, and IL-22 following challenge with influenza [93], and IL-17F-producing CD8+ cells have been detected in patients with COPD [94]. Moreover, increased numbers of IL-17A-producing CD8+ T cells have been observed in patients with asthma [95] and following lung transplantation [96], supporting the conclusions drawn from murine studies.
Both TC17 and TNC17 cells have been described. In vitro, TC17 cells can be differentiated with TGF-β and IL-6, similar to TH17 cells; moreover, generation of IL-17A-producing CD8+ T cells may require TH17 cells [97]. It has been suggested that IL-23 is required for the generation of TC17 but not TNC17 cells and also for subsequent IL-22 production. Functionally, TC17 cells have been linked to several diseases of the mucosa. In one model of spontaneous colitis, TC17 cells appear to mediate pathogenesis through production of IL-17A and IFN-γ in an IL-6-dependent fashion [98]. TC17 cells also confer host protection; in an influenza model, the adoptive transfer of TC17 cells promotes survival [93], and TC17 cells are vital for the vaccine immune response against fungal pathogens [99].
CD8+ T cells, in the context of antitumor responses, is a fascinating field as a result of their potential use as an immunotherapeutic [100]. However, whether IL-17A secretion by cytotoxic CD8+ T cells leads to more efficient tumor clearance may depend on the presence of other cells of the innate and adaptive immune systems, such as TH17 cells or MDSCs [101]. In a lung model of melanoma metastasis, IL-17A, produced directly by CD8+ T cells [102] or by TH17 cells that indirectly recruited and activated CD8+ T cells [103], led to more efficient tumor clearance. Conversely, the number of TC17 cells in patients with gastric cancer positively correlates with tumor progression and decreased survival, possibly as a result of recruitment of MDSCs that down-regulate the tumor-suppressive capacity of the TC17 cells [104]. Thus, additional studies aimed at understanding how IL-17A-producing CD8+ T cells function as intratumoral lymphocytes will be invaluable in moving forward with CD8+ T cells as therapeutics.
A subset of CD8+ cells found in the lung and intestine are MAITs. As the name suggests, MAITs are an innate T cell population that expresses an essentially invariant TCR-α chain [105]. MAITs recognize vitamin B metabolites [106] presented by MR1, a class I-related MHC molecule found on APCs, such as B cells. Given that mammals cannot produce vitamin B and that MAITs require commensals for development [107], it appears likely that MAITs are important during early microbial recognition in the mucosa (reviewed in ref. [108]). Human CD8+ MAITs have been shown to produce IL-17A and IL-22 [109] and express CD161, a transmembrane protein suggested to be present on all IL-17A-expressing cells [110]. Moreover, the number of CD8+ MAITs is enhanced greatly during colonic inflammation [111].
γδ T cells
An immediate producer of IL-17A during an immune response, γδ T cells can be vital to host protection [112]. Predominantly found at mucosal sites, such as the intestinal epithelia, γδ T cells constitute the vast majority of the population of local T cells [113]. In addition to expressing CCR6, RORγt, IL-1R [114], and IL-23R [115], γδ T cells may be CD8+ in the mucosa or lack CD4 and CD8 molecules altogether. Following exposure to IL-23, in combination with IL-1β or IL-18, γδ T cells can be stimulated to produce IL-17A in a TCR-independent mechanism [116]. The role of γδ T cells in early IL-17A production has now been well documented (recently reviewed in ref. [117]), and recent evidence suggests that γδ T cells may additionally produce IL-17F [118].
Murine studies have demonstrated that γδ T cells in the lung contribute to protection from Staphylococcus aureus-mediated pneumonia [119] and appear to be critical for the resolution of allergic lung inflammation [120, 121], among other diseases. In response to Mycobacterium tuberculosis, γδ T cells in the lung produce IL-17A [122], which leads to granuloma formation [123]. The role of IL-17A-producing γδ T cells in murine lung inflammation appears to recapitulate closely that observed during human disease, as there is a significant increase in IL-17A-secreting γδ T cells in patients with active tuberculosis [124], and this correlates with granuloma formation [125].
Within the intestinal tract, γδ T cells are found within the epithelial layer (intraepithelial lymphocytes) and the lamina propria [126], where they can produce IL-17A in combination with other cytokines [126, 127]. Intestinal γδ T cells can be regulated by internal factors, such as the transcription factor PU.1 [128], or by external factors, such as Tregs [129, 130]. The role of γδ T cells in inflammatory conditions of the intestine is an area of active research; given their proximity to gut microbiota and ability to produce IL-17A rapidly, it is not surprising that γδ T cells have been linked to dysregulation of intestinal homeostasis. Intriguingly, a recent report revealed a positive association between tumor-infiltrating, IL-17A-producing γδ T cells and tumor progression in patients with CRC, potentially through the recruitment of immunosuppressive MDSCs [131]. In addition to the direct effects following immediate production of IL-17A [130, 132], IL-17A-secreting γδ T cells may contribute to enhancing TH17 responses in the colon [118].
Following exposure to select cytokines, γδ T cells can also produce IL-21 and IL-22, as in the case of IL-1 and IL-23 stimulation in a model of experimental autoimmune encephalomyelitis [133]. In a study of pulmonary fibrosis, γδ T cells were shown to be protective through the production of IL-22 [134]. However, the role of γδ T cells in production of IL-21 and IL-22 at mucosal surfaces has not yet been studied extensively. γδ T cells may require the AHR for production of IL-22 [115] and in contrast to αβ T cells, may not require IRF4 for IL-17A or IL-22 production [135].
TFH cells
TFH is a subset of T cells that is important in mediating B cell activation in germinal center reactions. Found in secondary lymphoid structures, such as lymph nodes and Peyer’s patches, TFH cells express CXCL5, allowing them to home to the B cell-rich follicles. Engagement of programmed death 1 (PD-1) on TFH cells in the presence of IL-12 induces the expression of IL-21 in TFH [136, 137]. In combination with TGF-β1, IL-21 production by TFH drives B cell class-switching to an IgA-expressing plasmablast [77]. Moreover, a recent report suggests that TFH may also secrete IL-17A to assist B cells in IgA production in Peyer’s patches; notably, these cells appear to be derived from TH17 cells that homed to Peyer’s patches and subsequently differentiated [138].
NKT cells
NKT cells bridge the boundary between the innate and adaptive immune system. NKT cells express an αβ TCR that can recognize the lipid-restricted CD1 family of presentation molecules (recently reviewed in ref. [139]) and also express the NK cell marker NK1.1. One subtype of the NKT cell is termed iNKT cells as a result of their expression of select TCR-αβ chains. Of these, a subset can also express RORγt and secrete TH17-associated cytokines and is sometimes referred to as NKT17 cells. These TH17-like iNKT cells appear to require TGF-β [140], IL-15 [141], and E4BP [141] for development but do not require IRF4 [135]. The transcription factor TH-inducing POZ-Kruppel factor (TH-POK) has been shown to repress formation of this lineage [142, 143]. iNKT cells can be stimulated to produce IL-17A following IL-1β and IL-23 exposure [116]. Originally discovered in the lung, NKT17 cells have been shown to contribute significantly to neutrophilia during Rous sarcoma virus [141] or Klebsiella infection [144], demonstrating that IL-17A, produced by NKT17 cells, can promote lung inflammation. Consistent with the role of NKT cells in producing IL-17A in the murine lung, there is an increase in IL-17A-producing, NKT-like cells in human lung transplant recipients [145] and in patients with cystic fibrosis [146]. Interestingly, a recent study investigating environmental antigens suggested that NKT cells can produce IL-17A following activation by house dust extracts, increasing the number of known antigens that can stimulate NKT cells [147].
NKT cells have been demonstrated to secrete IL-21 following stimulation with anti-CD3 and anti-CD28 or bacillus Calmette-Guérin [148, 149]. A recently described subset of iNKT cells, termed NKTFH cells, expresses Bcl-6 and secretes IL-21 in the germinal center. This enables NKTFH cells to help B cells, leading to affinity maturation but not a memory response [150, 151]. Similarly to IL-21, a role for iNKT cell-generated IL-22 has been established in splenocytes [152]; moreover, following influenza infection, IL-22 secreted by iNKT cells is protective against epithelial damage [153], which is consistent with the known role of IL-22 in tissue protection [33]. As previous studies describing NKT cell-generated IL-21 and IL-22 predominantly analyzed splenic cells, interesting questions regarding the presence or absence of similar populations in mucosal tissues remain.
ILCs
A significant portion of TH17-associated cytokines generated in response to danger signals is thought to come from large numbers of ILCs that populate tissues at the host-pathogen interface [154]. Broadly, ILCs are defined by their resemblance to cells arising from a lymphoid lineage and the absence of lineage markers or antigen receptors resulting from rearranged genes (recently reviewed in ref. [155]). All ILCs require Id2 for development [156–158] but can be subdivided based on their transcriptional and cytokine profiles, as in the recently coined ILC3 populations, which are categorized by their resemblance to the cytokine expression profiles of the TH subsets [159]. ILC3s, which include LTi cells and NCR+ ILC3, require IL-7 for development [157, 160], express RORγt [161, 162], and can produce the TH17-associated cytokines IL-17A and/or IL-22. More surprisingly, the transcription factor associated with development and homeostasis of TH2 and ILC2 cells, GATA-3, was demonstrated recently also to be important in the development of ILC3s [163]. Whereas the significance of ILCs, including LTi cells and NCR+ ILCs in the innate immune response, is well documented, the exact nature of the different cell types, their lineage, and their individual roles in disease are highly active areas of research.
LTi cells were the 1st ILC3 member identified [164] and need the AHR [165], thymocyte selection-associated HMG box factor (TOX) [166], and receptor activator for NF-κB ligand [167], among others, for development. Capable of producing IL-17A and IL-22 [168], LTi cells may be important in driving TH17-associated innate immune responses. LTi cells can be subdivided based on expression of CD4 [154], although only CD4+ LTi cells appear to be important for protection against the murine enteric pathogen Citrobacter rodentium [169]. Beyond being critical for innate immune responses following challenge, LTi cells are also important in the induction and development of postnatal but not prenatal lymphoid tissue [165].
Besides expressing RORγt, a subset of the ILC3s displays the NCR NKp46 and therefore is called NCR+ ILC3s (alternatively NKp46 ILCs, NK22, NCR22, NKR-LTi, and ILC22 [159]). In addition to the previously appreciated requirement for AHR in development of NCR+ ILCs, a T-bet and Notch signaling-dependent mechanism has been identified recently for NCR+ ILCs [170]. NCR+ ILC3s can be derived from CD4− LTi cells in vitro [170], which suggests that ILC3s may be highly plastic in nature. NCR+ ILC3s have been found in the skin and intestinal lamina propria of the mouse [171] and in human mucosa and lymphoid tissue [172, 173]. NCR+ ILC3s in the intestine can be divided further into NK1.1lo versus NK1.1+ populations, with NK1.1lo cells as potent producers of IL-22 [171]. IL-22-secreting NCR+ ILCs appear to play an important role in protection against intestinal pathogens, such as C. rodentium [170]. Notably, IL-22 secretion does not require NCR [174], which is consistent with the secretion of IL-22 by non-NCR-expressing cells.
In addition to CD4+LTi cells and NCR+ ILC3s, a population of CD4−NCR− ILC3s appears to accumulate during colonic inflammation. These cells express RORγt and can produce IL-17A, IL-22, and IFN-γ following exposure to IL-23 [175]. Ultimately, colonic inflammation can lead to the initiation of CRC, and ILC3-mediated production of IL-22 and to a lesser extent, IL-17A [176] can drive this progression [177]. However, the precise definition of CD4−NCR− ILC3s remains unclear.
In humans, CD56+NCR+ ILC3s within mucosal-associated lymphoid tissue can concomitantly express IL-22 and IL-26 [57]. IL-26 has been linked to IBD [178]; CD3−CD56+ ILCs, in the lamina propria of humans with ulcerative colitis or Crohn’s disease, secrete IL-26, an effect that can be enhanced with ex vivo IL-23 stimulation [179]. Whether IL-26 production is ultimately tissue protective or a significant source of tissue damage during intestinal inflammation remains unclear.
Neutrophils
Neutrophils constitute a major cell type involved in mediating mucosal TH17 responses. Surprisingly, human neutrophils express IL-17RA [71, 180]; however, they do not constitutively express IL-17RC and thus, may not bind IL-17A or IL-17F directly [181]. Moreover, neutrophils likely do not express IL-21R [182] or IL-22R. TH17 cells secrete chemokines (e.g., CXCL8, G-CSF [71, 181, 183]) that attract neutrophils to sites of inflammation. Thus, the recruitment of neutrophils in a TH17-like immune response is often conceptualized in terms of chemokine gradients [181, 184].
In murine models of inflammation, neutrophils have been implicated in IL-17A generation through direct production, as in the recent, elegant studies out of the Pearlman lab [185], or by promoting the recruitment of IL-17A-expressing cells, such as TH17 cells [181, 186]. In the lung, murine neutrophils can produce IL-17A mRNA and/or protein following LPS challenge [187] or during infection with inhalational anthrax [188], Cryptococcus infection [186], and Aspergillus fumigatus [189]. Importantly, neutrophilic production of IL-17A in the anthrax and Aspergillus models contributes significantly toward survival to an otherwise lethal challenge. It is currently unclear whether murine model systems fully recapitulate human disease, however. Whereas IL-17A production is associated with neutrophilic influx, as in the case of COPD [190], it is more difficult to define accurately the salient producers in human tissues. Thus, future work to measure directly neutrophilic production of IL-17A will be of key interest to the field.
Data implicating neutrophils in production of IL-21 during mucosal inflammation are limited but have been demonstrated during pediatric celiac disease [191]. Notably, a recently discovered population of neutrophils in the spleen, termed B cell helper neutrophils, secrete IL-21 to induce B cell diversification [192], suggesting that neutrophil production of IL-21 at mucosal surfaces is simply awaiting discovery. Finally, neutrophils can be important producers of IL-22 following colonic injury in an IL-23-dependent fashion and therefore, may be crucial for re-establishing the epithelial barrier [193].
Paneth cells
A specialized epithelial cell found in crypt bases of the small intestine, Paneth cells are typically appreciated for their role in production of antimicrobial peptides and degradative enzymes, such as lysozyme. However, there is growing evidence that Paneth cells may be an important, innate source of IL-17A. First detected in a model of TNF-induced shock, Paneth cell expression of IL-17A was associated with acute inflammation of the small bowel and correlated with increased IL-6 levels in the serum [194]. More recently, ablation of Paneth cells in a model of intestinal ischemia-reperfusion injury drastically reduced levels of serum and intestinal IL-17A and associated pathology. Notably, IL-17A appears to be constitutively present in Paneth cell granules [194], and IL-17A was found at the base of small intestine crypts concomitant with Paneth cell degranulation, 3 h after oral administration of the TLR9 ligand CpG-oligodeoxynucleotide [195]. Therefore, the presence of IL-17A in Paneth cell granules and subsequent Paneth cell degranulation during an inflammatory response may constitute an underappreciated, immediate, and rapid source of IL-17A. How IL-17A present in the intestinal lumen contributes to TH17-like immune or inflammatory responses remains to be seen.
Macrophages
Although not primary producers of TH17-associated cytokines, macrophages appear capable of producing IL-17A and IL-22 at the mucosa under select circumstances. In 1 study, following LPS exposure, macrophages could produce IL-17A and IL-22 but only in the absence of IL-10/IL-10R signaling [196]. Moreover, in a model of pulmonary infection during obesity, interstitial but not alveolar macrophages could produce IL-17A [197]. Macrophages could also produce IL-17A in response to infection with Mycobacterium avium [198] and in patients with chronic rhinosinusitis with nasal polyps [199]. Finally, alveolar macrophages can produce IL-22 in response to a model of pulmonary inflammation [200]. Future work, examining the role of macrophages in the generation of TH17-associated cytokines, may be especially interesting in mucosal sites, such as the lung, given the different macrophage populations that are resident within or that can home to the lung during inflammation.
CONCLUDING REMARKS
Whereas IL-17A is sometimes viewed as synonymous with TH17 cells, it is a pleiotropic cytokine that can be generated by many different cell types, including cells of the innate immune system that can respond quickly to microbial challenge. Beyond IL-17A, the other TH17-associated cytokines (i.e., IL-17F, IL-21, IL-22, and IL-26) have important individual contributions to disease severity and resolution that require a complete understanding of the cytokine milieu present in specific conditions. Moreover, there are likely additional cell populations involved in production of TH17-associated cytokines at the mucosa that have currently only been well characterized at nonmucosal sites (i.e., mast cell-mediated production of IL-17A in inflamed joints [201]). Considering the extended time course during which TH17-associated cytokines can be produced (from disease initiation to resolution), the understanding of the cellular sources of TH17-associated cytokines will be useful toward the generation of elegantly designed therapies to modulate inflammation.
Given the relatively recent discoveries of the TH17-associated family of cytokines, it is not surprising that this is an area brimming with cutting-edge research. However, many questions still remain. How do rTH17 cells arise, and are they a separate lineage or represent an intermediate cell type? Can we modulate IL-17A production in CD8+ T cells to tailor their cytotoxic behavior in the context of an anti-tumor response? How does early production of IL-17A from innate-like immune cells alter the clinical course of mucosal disease compared with late production of IL-17A by the adaptive response? Determining the answers to these questions may provide additional tools for a broad spectrum of diseases ranging from allergic inflammation to tumor progression.
ACKNOWLEDGMENTS
The authors thank Dr. Jonathan Harton for critically reading the manuscript and the Center for Immunology and Microbial Disease at Albany Medical Center for funding this effort.
Glossary
- AHR
aryl hydrocarbon receptor
- COPD
chronic obstructive pulmonary disease
- CRC
colorectal cancer
- Foxp3
forkhead box p3
- IBD
inflammatory bowel disease
- ILC2/3
innate lymphoid cell groups 2/3
- iNKT
invariant NKT cell
- IRF
IFN regulatory factor
- iTH17
induced TH17
- LTi
lymphoid tissue-inducer cell
- MAIT
mucosal-associated invariant T cell
- MDSC
myeloid-derived suppressor cell
- NCR
natural cytotoxicity receptor
- NKTFH
follicular helper NKT cell
- nTH17
natural TH17
- ROR
retinoic acid-related orphan receptor
- rTH17
regulatory TH17 cell
- TC17
cytotoxic IL-17A-producing CD8+ T cell
- TFH
follicular helper T cell
- TNC17
noncytotoxic IL-17A-producing CD8+ T cell
- Treg
regulatory T cell
AUTHORSHIP
K.O.B-S., T.W., S.H., and W.O. wrote and edited the review. T.W., K.O.B-S., and W.O. generated the illustration.
DISCLOSURES
The authors declare no conflict of interest.
REFERENCES
- 1.Rouvier E., Luciani M. F., Mattéi M. G., Denizot F., Golstein P. (1993) CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 150, 5445–5456. [PubMed] [Google Scholar]
- 2.Yao Z., Fanslow W. C., Seldin M. F., Rousseau A. M., Painter S. L., Comeau M. R., Cohen J. I., Spriggs M. K. (1995) Herpesvirus saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity 3, 811–821. [DOI] [PubMed] [Google Scholar]
- 3.Nakae S., Saijo S., Horai R., Sudo K., Mori S., Iwakura Y. (2003) IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA 100, 5986–5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fossiez F., Djossou O., Chomarat P., Flores-Romo L., Ait-Yahia S., Maat C., Pin J. J., Garrone P., Garcia E., Saeland S., Blanchard D., Gaillard C., Das Mahapatra B., Rouvier E., Golstein P., Banchereau J., Lebecque S. (1996) T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teunissen M. B., Koomen C. W., de Waal Malefyt R., Wierenga E. A., Bos J. D. (1998) Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J. Invest. Dermatol. 111, 645–649. [DOI] [PubMed] [Google Scholar]
- 6.Miossec P., Kolls J. K. (2012) Targeting IL-17 and TH17 cells in chronic inflammation. Nat. Rev. Drug Discov. 11, 763–776. [DOI] [PubMed] [Google Scholar]
- 7.O’Connor W. Jr., Kamanaka M., Booth C. J., Town T., Nakae S., Iwakura Y., Kolls J. K., Flavell R. A. (2009) A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 10, 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y., Godec J., Ben-Aissa K., Cui K., Zhao K., Pucsek A. B., Lee Y. K., Weaver C. T., Yagi R., Lazarevic V. (2014) The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells. Immunity 40, 355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moseley T. A., Haudenschild D. R., Rose L., Reddi A. H. (2003) Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 14, 155–174. [DOI] [PubMed] [Google Scholar]
- 10.Hymowitz S. G., Filvaroff E. H., Yin J. P., Lee J., Cai L., Risser P., Maruoka M., Mao W., Foster J., Kelley R. F., Pan G., Gurney A. L., de Vos A. M., Starovasnik M. A. (2001) IL-17s adopt a cystine knot fold: structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 20, 5332–5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starnes T., Robertson M. J., Sledge G., Kelich S., Nakshatri H., Broxmeyer H. E., Hromas R. (2001) Cutting edge: IL-17F, a novel cytokine selectively expressed in activated T cells and monocytes, regulates angiogenesis and endothelial cell cytokine production. J. Immunol. 167, 4137–4140. [DOI] [PubMed] [Google Scholar]
- 12.Wright J. F., Guo Y., Quazi A., Luxenberg D. P., Bennett F., Ross J. F., Qiu Y., Whitters M. J., Tomkinson K. N., Dunussi-Joannopoulos K., Carreno B. M., Collins M., Wolfman N. M. (2007) Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J. Biol. Chem. 282, 13447–13455. [DOI] [PubMed] [Google Scholar]
- 13.Wright J. F., Bennett F., Li B., Brooks J., Luxenberg D. P., Whitters M. J., Tomkinson K. N., Fitz L. J., Wolfman N. M., Collins M., Dunussi-Joannopoulos K., Chatterjee-Kishore M., Carreno B. M. (2008) The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J. Immunol. 181, 2799–2805. [DOI] [PubMed] [Google Scholar]
- 14.Spuls P. I., Hooft L. (2012) Brodalumab and ixekizumab, anti-interleukin-17-receptor antibodies for psoriasis: a critical appraisal. Br. J. Dermatol. 167, 710–713, discussion 714–715. [DOI] [PubMed] [Google Scholar]
- 15.Hueber W., Patel D. D., Dryja T., Wright A. M., Koroleva I., Bruin G., Antoni C., Draelos Z., Gold M. H., Durez P., Tak P. P., Gomez-Reino J. J., Foster C. S., Kim R. Y., Samson C. M., Falk N. S., Chu D. S., Callanan D., Nguyen Q. D., Rose K., Haider A., Di Padova F.; Psoriasis Study Group; Rheumatoid Arthritis Study Group; Uveitis Study Group (2010) Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl. Med. 2, 52ra72. [DOI] [PubMed] [Google Scholar]
- 16.Hueber W., Sands B. E., Lewitzky S., Vandemeulebroecke M., Reinisch W., Higgins P. D., Wehkamp J., Feagan B. G., Yao M. D., Karczewski M., Karczewski J., Pezous N., Bek S., Bruin G., Mellgard B., Berger C., Londei M., Bertolino A. P., Tougas G., Travis S. P.; Secukinumab in Crohn’s Disease Study Group (2012) Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ogawa A., Andoh A., Araki Y., Bamba T., Fujiyama Y. (2004) Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin. Immunol. 110, 55–62. [DOI] [PubMed] [Google Scholar]
- 18.Yang X. O., Chang S. H., Park H., Nurieva R., Shah B., Acero L., Wang Y. H., Schluns K. S., Broaddus R. R., Zhu Z., Dong C. (2008) Regulation of inflammatory responses by IL-17F. J. Exp. Med. 205, 1063–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Z., Zheng M., Bindas J., Schwarzenberger P., Kolls J. K. (2006) Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm. Bowel Dis. 12, 382–388. [DOI] [PubMed] [Google Scholar]
- 20.Ito R., Kita M., Shin-Ya M., Kishida T., Urano A., Takada R., Sakagami J., Imanishi J., Iwakura Y., Okanoue T., Yoshikawa T., Kataoka K., Mazda O. (2008) Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem. Biophys. Res. Commun. 377, 12–16. [DOI] [PubMed] [Google Scholar]
- 21.Wedebye Schmidt E. G., Larsen H. L., Kristensen N. N., Poulsen S. S., Lynge Pedersen A. M., Claesson M. H., Pedersen A. E. (2013) TH17 cell induction and effects of IL-17A and IL-17F blockade in experimental colitis. Inflamm. Bowel Dis. 19, 1567–1576. [DOI] [PubMed] [Google Scholar]
- 22.Parrish-Novak J., Foster D. C., Holly R. D., Clegg C. H. (2002) Interleukin-21 and the IL-21 receptor: novel effectors of NK and T cell responses. J. Leukoc. Biol. 72, 856–863. [PubMed] [Google Scholar]
- 23.Parrish-Novak J., Dillon S. R., Nelson A., Hammond A., Sprecher C., Gross J. A., Johnston J., Madden K., Xu W., West J., Schrader S., Burkhead S., Heipel M., Brandt C., Kuijper J. L., Kramer J., Conklin D., Presnell S. R., Berry J., Shiota F., Bort S., Hambly K., Mudri S., Clegg C., Moore M., Grant F. J., Lofton-Day C., Gilbert T., Rayond F., Ching A., Yao L., Smith D., Webster P., Whitmore T., Maurer M., Kaushansky K., Holly R. D., Foster D. (2000) Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408, 57–63. [DOI] [PubMed] [Google Scholar]
- 24.Morrison P. J., Ballantyne S. J., Kullberg M. C. (2011) Interleukin-23 and T helper 17-type responses in intestinal inflammation: from cytokines to T-cell plasticity. Immunology 133, 397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozaki K., Kikly K., Michalovich D., Young P. R., Leonard W. J. (2000) Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc. Natl. Acad. Sci. USA 97, 11439–11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asao H., Okuyama C., Kumaki S., Ishii N., Tsuchiya S., Foster D., Sugamura K. (2001) Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 167, 1–5. [DOI] [PubMed] [Google Scholar]
- 27.Wei L., Laurence A., Elias K. M., O’Shea J. J. (2007) IL-21 is produced by Th17 cells and drives IL-17 production in a STAT3-dependent manner. J. Biol. Chem. 282, 34605–34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Caprioli F., Sarra M., Caruso R., Stolfi C., Fina D., Sica G., MacDonald T. T., Pallone F., Monteleone G. (2008) Autocrine regulation of IL-21 production in human T lymphocytes. J. Immunol. 180, 1800–1807. [DOI] [PubMed] [Google Scholar]
- 29.Stolfi C., Rizzo A., Franzè E., Rotondi A., Fantini M. C., Sarra M., Caruso R., Monteleone I., Sileri P., Franceschilli L., Caprioli F., Ferrero S., MacDonald T. T., Pallone F., Monteleone G. (2011) Involvement of interleukin-21 in the regulation of colitis-associated colon cancer. J. Exp. Med. 208, 2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maurer M. F., Garrigues U., Jaspers S. R., Meengs B., Rixon M. W., Stevens B. L., Lewis K. B., Julien S. H., Bukowski T. R., Wolf A. C., Hamacher N. B., Snavely M., Dillon S. R. (2012) Generation and characterization of human anti-human IL-21 neutralizing monoclonal antibodies. MAbs 4, 69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dumoutier L., Van Roost E., Colau D., Renauld J. C. (2000) Human interleukin-10-related T cell-derived inducible factor: molecular cloning and functional characterization as an hepatocyte-stimulating factor. Proc. Natl. Acad. Sci. USA 97, 10144–10149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumoutier L., Van Roost E., Ameye G., Michaux L., Renauld J. C. (2000) IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun. 1, 488–494. [DOI] [PubMed] [Google Scholar]
- 33.Witte E., Witte K., Warszawska K., Sabat R., Wolk K. (2010) Interleukin-22: a cytokine produced by T, NK and NKT cell subsets, with importance in the innate immune defense and tissue protection. Cytokine Growth Factor Rev. 21, 365–379. [DOI] [PubMed] [Google Scholar]
- 34.Dumoutier L., Lejeune D., Colau D., Renauld J. C. (2001) Cloning and characterization of IL-22 binding protein, a natural antagonist of IL-10-related T cell-derived inducible factor/IL-22. J. Immunol. 166, 7090–7095. [DOI] [PubMed] [Google Scholar]
- 35.Kotenko S. V., Izotova L. S., Mirochnitchenko O. V., Esterova E., Dickensheets H., Donnelly R. P., Pestka S. (2001) Identification, cloning, and characterization of a novel soluble receptor that binds IL-22 and neutralizes its activity. J. Immunol. 166, 7096–7103. [DOI] [PubMed] [Google Scholar]
- 36.Dumoutier L., Louahed J., Renauld J. C. (2000) Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J. Immunol. 164, 1814–1819. [DOI] [PubMed] [Google Scholar]
- 37.Xie M. H., Aggarwal S., Ho W. H., Foster J., Zhang Z., Stinson J., Wood W. I., Goddard A. D., Gurney A. L. (2000) Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J. Biol. Chem. 275, 31335–31339. [DOI] [PubMed] [Google Scholar]
- 38.Zenewicz L. A., Yancopoulos G. D., Valenzuela D. M., Murphy A. J., Stevens S., Flavell R. A. (2008) Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity 29, 947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sonnenberg G. F., Fouser L. A., Artis D. (2011) Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12, 383–390.21502992 [Google Scholar]
- 40.Sugimoto K., Ogawa A., Mizoguchi E., Shimomura Y., Andoh A., Bhan A. K., Blumberg R. S., Xavier R. J., Mizoguchi A. (2008) IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. J. Clin. Invest. 118, 534–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pociask D. A., Scheller E. V., Mandalapu S., McHugh K. J., Enelow R. I., Fattman C. L., Kolls J. K., Alcorn J. F. (2013) IL-22 is essential for lung epithelial repair following influenza infection. Am. J. Pathol. 182, 1286–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar P., Thakar M. S., Ouyang W., Malarkannan S. (2013) IL-22 from conventional NK cells is epithelial regenerative and inflammation protective during influenza infection. Mucosal Immunol. 6, 69–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huber S., Gagliani N., Zenewicz L. A., Huber F. J., Bosurgi L., Hu B., Hedl M., Zhang W., O’Connor W. Jr., Murphy A. J., Valenzuela D. M., Yancopoulos G. D., Booth C. J., Cho J. H., Ouyang W., Abraham C., Flavell R. A. (2012) IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thompson C. L., Plummer S. J., Tucker T. C., Casey G., Li L. (2010) Interleukin-22 genetic polymorphisms and risk of colon cancer. Cancer Causes Control 21, 1165–1170. [DOI] [PubMed] [Google Scholar]
- 45.Lilly L. M., Gessner M. A., Dunaway C. W., Metz A. E., Schwiebert L., Weaver C. T., Brown G. D., Steele C. (2012) The β-glucan receptor dectin-1 promotes lung immunopathology during fungal allergy via IL-22. J. Immunol. 189, 3653–3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sonnenberg G. F., Nair M. G., Kirn T. J., Zaph C., Fouser L. A., Artis D. (2010) Pathological versus protective functions of IL-22 in airway inflammation are regulated by IL-17A. J. Exp. Med. 207, 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kamanaka M., Huber S., Zenewicz L. A., Gagliani N., Rathinam C., O’Connor W. Jr., Wan Y. Y., Nakae S., Iwakura Y., Hao L., Flavell R. A. (2011) Memory/effector (CD45RB(lo)) CD4 T cells are controlled directly by IL-10 and cause IL-22-dependent intestinal pathology. J. Exp. Med. 208, 1027–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Besnard A. G., Sabat R., Dumoutier L., Renauld J. C., Willart M., Lambrecht B., Teixeira M. M., Charron S., Fick L., Erard F., Warszawska K., Wolk K., Quesniaux V., Ryffel B., Togbe D. (2011) Dual role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A. Am. J. Respir. Crit. Care Med. 183, 1153–1163. [DOI] [PubMed] [Google Scholar]
- 49.Andoh A., Zhang Z., Inatomi O., Fujino S., Deguchi Y., Araki Y., Tsujikawa T., Kitoh K., Kim-Mitsuyama S., Takayanagi A., Shimizu N., Fujiyama Y. (2005) Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology 129, 969–984. [DOI] [PubMed] [Google Scholar]
- 50.Knappe A., Hör S., Wittmann S., Fickenscher H. (2000) Induction of a novel cellular homolog of interleukin-10, AK155, by transformation of T lymphocytes with herpesvirus saimiri. J. Virol. 74, 3881–3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Igawa D., Sakai M., Savan R. (2006) An unexpected discovery of two interferon gamma-like genes along with interleukin (IL)-22 and -26 from teleost: IL-22 and -26 genes have been described for the first time outside mammals. Mol. Immunol. 43, 999–1009. [DOI] [PubMed] [Google Scholar]
- 52.She X., Cheng Z., Zöllner S., Church D. M., Eichler E. E. (2008) Mouse segmental duplication and copy number variation. Nat. Genet. 40, 909–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schoenborn J. R., Dorschner M. O., Sekimata M., Santer D. M., Shnyreva M., Fitzpatrick D. R., Stamatoyannopoulos J. A., Wilson C. B. (2007) Comprehensive epigenetic profiling identifies multiple distal regulatory elements directing transcription of the gene encoding interferon-gamma. Nat. Immunol. 8, 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sheikh F., Baurin V. V., Lewis-Antes A., Shah N. K., Smirnov S. V., Anantha S., Dickensheets H., Dumoutier L., Renauld J. C., Zdanov A., Donnelly R. P., Kotenko S. V. (2004) Cutting edge: IL-26 signals through a novel receptor complex composed of IL-20 receptor 1 and IL-10 receptor 2. J. Immunol. 172, 2006–2010. [DOI] [PubMed] [Google Scholar]
- 55.Hör S., Pirzer H., Dumoutier L., Bauer F., Wittmann S., Sticht H., Renauld J. C., de Waal Malefyt R., Fickenscher H. (2004) The T-cell lymphokine interleukin-26 targets epithelial cells through the interleukin-20 receptor 1 and interleukin-10 receptor 2 chains. J. Biol. Chem. 279, 33343–33351. [DOI] [PubMed] [Google Scholar]
- 56.Dambacher J., Beigel F., Zitzmann K., De Toni E. N., Göke B., Diepolder H. M., Auernhammer C. J., Brand S. (2009) The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut 58, 1207–1217. [DOI] [PubMed] [Google Scholar]
- 57.Cella M., Fuchs A., Vermi W., Facchetti F., Otero K., Lennerz J. K., Doherty J. M., Mills J. C., Colonna M. (2009) A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaplan M. H. (2013) Th9 cells: differentiation and disease. Immunol. Rev. 252, 104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rutz S., Ouyang W. (2011) Regulation of interleukin-10 and interleukin-22 expression in T helper cells. Curr. Opin. Immunol. 23, 605–612. [DOI] [PubMed] [Google Scholar]
- 60.Cua D. J., Sherlock J., Chen Y., Murphy C. A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., Zurawski S., Wiekowski M., Lira S. A., Gorman D., Kastelein R. A., Sedgwick J. D. (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748. [DOI] [PubMed] [Google Scholar]
- 61.Park H., Li Z., Yang X. O., Chang S. H., Nurieva R., Wang Y. H., Wang Y., Hood L., Zhu Z., Tian Q., Dong C. (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6, 1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harrington L. E., Hatton R. D., Mangan P. R., Turner H., Murphy T. L., Murphy K. M., Weaver C. T. (2005) Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6, 1123–1132. [DOI] [PubMed] [Google Scholar]
- 63.Yang X. O., Pappu B. P., Nurieva R., Akimzhanov A., Kang H. S., Chung Y., Ma L., Shah B., Panopoulos A. D., Schluns K. S., Watowich S. S., Tian Q., Jetten A. M., Dong C. (2008) T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity 28, 29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Harrington L. E., Mangan P. R., Weaver C. T. (2006) Expanding the effector CD4 T-cell repertoire: the Th17 lineage. Curr. Opin. Immunol. 18, 349–356. [DOI] [PubMed] [Google Scholar]
- 65.Korn T., Bettelli E., Oukka M., Kuchroo V. K. (2009) IL-17 and Th17 cells. Annu. Rev. Immunol. 27, 485–517. [DOI] [PubMed] [Google Scholar]
- 66.Zúñiga L. A., Jain R., Haines C., Cua D. J. (2013) Th17 cell development: from the cradle to the grave. Immunol. Rev. 252, 78–88. [DOI] [PubMed] [Google Scholar]
- 67.Marks B. R., Nowyhed H. N., Choi J. Y., Poholek A. C., Odegard J. M., Flavell R. A., Craft J. (2009) Thymic self-reactivity selects natural interleukin 17-producing T cells that can regulate peripheral inflammation. Nat. Immunol. 10, 1125–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirota K., Hashimoto M., Yoshitomi H., Tanaka S., Nomura T., Yamaguchi T., Iwakura Y., Sakaguchi N., Sakaguchi S. (2007) T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 204, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Cesare A., Di Meglio P., Nestle F. O. (2009) The IL-23/Th17 axis in the immunopathogenesis of psoriasis. J. Invest. Dermatol. 129, 1339–1350. [DOI] [PubMed] [Google Scholar]
- 70.Ke Y., Liu K., Huang G. Q., Cui Y., Kaplan H. J., Shao H., Sun D. (2009) Anti-inflammatory role of IL-17 in experimental autoimmune uveitis. J. Immunol. 182, 3183–3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ye P., Rodriguez F. H., Kanaly S., Stocking K. L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., Shellito J. E., Bagby G. J., Nelson S., Charrier K., Peschon J. J., Kolls J. K. (2001) Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Laan M., Cui Z. H., Hoshino H., Lötvall J., Sjöstrand M., Gruenert D. C., Skoogh B. E., Lindén A. (1999) Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J. Immunol. 162, 2347–2352. [PubMed] [Google Scholar]
- 73.Liang S. C., Tan X. Y., Luxenberg D. P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L. A. (2006) Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203, 2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuestner R. E., Taft D. W., Haran A., Brandt C. S., Brender T., Lum K., Harder B., Okada S., Ostrander C. D., Kreindler J. L., Aujla S. J., Reardon B., Moore M., Shea P., Schreckhise R., Bukowski T. R., Presnell S., Guerra-Lewis P., Parrish-Novak J., Ellsworth J. L., Jaspers S., Lewis K. E., Appleby M., Kolls J. K., Rixon M., West J. W., Gao Z., Levin S. D. (2007) Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol. 179, 5462–5473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang S. H., Dong C. (2007) A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 17, 435–440. [DOI] [PubMed] [Google Scholar]
- 76.Liang S. C., Long A. J., Bennett F., Whitters M. J., Karim R., Collins M., Goldman S. J., Dunussi-Joannopoulos K., Williams C. M., Wright J. F., Fouser L. A. (2007) An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 179, 7791–7799. [DOI] [PubMed] [Google Scholar]
- 77.Dullaers M., Li D., Xue Y., Ni L., Gayet I., Morita R., Ueno H., Palucka K. A., Banchereau J., Oh S. (2009) A T cell-dependent mechanism for the induction of human mucosal homing immunoglobulin A-secreting plasmablasts. Immunity 30, 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yoshizaki A., Miyagaki T., DiLillo D. J., Matsushita T., Horikawa M., Kountikov E. I., Spolski R., Poe J. C., Leonard W. J., Tedder T. F. (2012) Regulatory B cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491, 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rendon J. L., Li X., Akhtar S., Choudhry M. A. (2013) Interleukin-22 modulates gut epithelial and immune barrier functions following acute alcohol exposure and burn injury. Shock 39, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Esplugues E., Huber S., Gagliani N., Hauser A. E., Town T., Wan Y. Y., O’Connor W. Jr., Rongvaux A., Van Rooijen N., Haberman A. M., Iwakura Y., Kuchroo V. K., Kolls J. K., Bluestone J. A., Herold K. C., Flavell R. A. (2011) Control of TH17 cells occurs in the small intestine. Nature 475, 514–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L., Kim J., Boussiotis V. A. (2010) IL-1β-mediated signals preferentially drive conversion of regulatory T cells but not conventional T cells into IL-17-producing cells. J. Immunol. 185, 4148–4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Osorio F., LeibundGut-Landmann S., Lochner M., Lahl K., Sparwasser T., Eberl G., Reis e Sousa C. (2008) DC activated via dectin-1 convert Treg into IL-17 producers. Eur. J. Immunol. 38, 3274–3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Radhakrishnan S., Cabrera R., Schenk E. L., Nava-Parada P., Bell M. P., Van Keulen V. P., Marler R. J., Felts S. J., Pease L. R. (2008) Reprogrammed FoxP3+ T regulatory cells become IL-17+ antigen-specific autoimmune effectors in vitro and in vivo. J. Immunol. 181, 3137–3147. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Yang S., Wang B., Guan C., Wu B., Cai C., Wang M., Zhang B., Liu T., Yang P. (2011) Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J. Leukoc. Biol. 89, 85–91. [DOI] [PubMed] [Google Scholar]
- 85.Kryczek I., Wu K., Zhao E., Wei S., Vatan L., Szeliga W., Huang E., Greenson J., Chang A., Roliński J., Radwan P., Fang J., Wang G., Zou W. (2011) IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J. Immunol. 186, 4388–4395. [DOI] [PubMed] [Google Scholar]
- 86.Blatner N. R., Mulcahy M. F., Dennis K. L., Scholtens D., Bentrem D. J., Phillips J. D., Ham S., Sandall B. P., Khan M. W., Mahvi D. M., Halverson A. L., Stryker S. J., Boller A. M., Singal A., Sneed R. K., Sarraj B., Ansari M. J., Oft M., Iwakura Y., Zhou L., Bonertz A., Beckhove P., Gounari F., Khazaie K. (2012) Expression of RORgammat marks a pathogenic regulatory T cell subset in human colon cancer. Sci. Transl. Med. 4, 164ra159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou X., Bailey-Bucktrout S. L., Jeker L. T., Penaranda C., Martínez-Llordella M., Ashby M., Nakayama M., Rosenthal W., Bluestone J. A. (2009) Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 10, 1000–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rubtsov Y. P., Niec R. E., Josefowicz S., Li L., Darce J., Mathis D., Benoist C., Rudensky A. Y. (2010) Stability of the regulatory T cell lineage in vivo. Science 329, 1667–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Miyao T., Floess S., Setoguchi R., Luche H., Fehling H. J., Waldmann H., Huehn J., Hori S. (2012) Plasticity of Foxp3(+) T cells reflects promiscuous Foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity 36, 262–275. [DOI] [PubMed] [Google Scholar]
- 90.Li L., Patsoukis N., Petkova V., Boussiotis V. A. (2012) Runx1 and Runx3 are involved in the generation and function of highly suppressive IL-17-producing T regulatory cells. PLoS ONE 7, e45115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McGeachy M. J., Bak-Jensen K. S., Chen Y., Tato C. M., Blumenschein W., McClanahan T., Cua D. J. (2007) TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397. [DOI] [PubMed] [Google Scholar]
- 92.Huber S., Gagliani N., Esplugues E., O’Connor W. Jr., Huber F. J., Chaudhry A., Kamanaka M., Kobayashi Y., Booth C. J., Rudensky A. Y., Roncarolo M. G., Battaglia M., Flavell R. A. (2011) Th17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity 34, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hamada H., Bassity E., Flies A., Strutt T. M., Garcia-Hernandez Mde. L., McKinstry K. K., Zou T., Swain S. L., Dutton R. W. (2013) Multiple redundant effector mechanisms of CD8+ T cells protect against influenza infection. J. Immunol. 190, 296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang Y., Nadigel J., Boulais N., Bourbeau J., Maltais F., Eidelman D. H., Hamid Q. (2011) CD8 positive T cells express IL-17 in patients with chronic obstructive pulmonary disease. Respir. Res. 12, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Li K., Wang Z., Cao Y., Bunjhoo H., Zhu J., Chen Y., Xiong S., Xu Y., Xiong W. (2013) The study of the ratio and distribution of Th17 cells and Tc17 cells in asthmatic patients and the mouse model. Asian Pac. J. Allergy Immunol. 31, 125–131. [DOI] [PubMed] [Google Scholar]
- 96.Verleden S. E., Vos R., Vandermeulen E., Ruttens D., Vaneylen A., Dupont L. J., Verbeken E. K., Verleden G. M., Van Raemdonck D. E., Vanaudenaerde B. M. (2013) Involvement of interleukin-17 during lymphocytic bronchiolitis in lung transplant patients. J. Heart Lung Transplant. 32, 447–553. [DOI] [PubMed] [Google Scholar]
- 97.Duan M. C., Huang Y., Zhong X. N., Tang H. J. (2012) Th17 cell enhances CD8 T-cell cytotoxicity via IL-21 production in emphysema mice. Mediators Inflamm. 2012, 898053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tajima M., Wakita D., Noguchi D., Chamoto K., Yue Z., Fugo K., Ishigame H., Iwakura Y., Kitamura H., Nishimura T. (2008) IL-6-dependent spontaneous proliferation is required for the induction of colitogenic IL-17-producing CD8+ T cells. J. Exp. Med. 205, 1019–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Nanjappa S. G., Heninger E., Wüthrich M., Gasper D. J., Klein B. S. (2012) Tc17 cells mediate vaccine immunity against lethal fungal pneumonia in immune deficient hosts lacking CD4+ T cells. PLoS Pathog. 8, e1002771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Caserta S., Borger J. G., Zamoyska R. (2012) Central and effector memory CD4 and CD8 T-cell responses to tumor-associated antigens. Crit. Rev. Immunol. 32, 97–126. [DOI] [PubMed] [Google Scholar]
- 101.Martin-Orozco N., Dong C. (2009) The IL-17/IL-23 axis of inflammation in cancer: friend or foe? Curr. Opin. Investig. Drugs 10, 543–549. [PubMed] [Google Scholar]
- 102.Yu Y., Cho H. I., Wang D., Kaosaard K., Anasetti C., Celis E., Yu X. Z. (2013) Adoptive transfer of Tc1 or Tc17 cells elicits antitumor immunity against established melanoma through distinct mechanisms. J. Immunol. 190, 1873–1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Martin-Orozco N., Muranski P., Chung Y., Yang X. O., Yamazaki T., Lu S., Hwu P., Restifo N. P., Overwijk W. W., Dong C. (2009) T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31, 787–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhuang Y., Peng L. S., Zhao Y. L., Shi Y., Mao X. H., Chen W., Pang K. C., Liu X. F., Liu T., Zhang J. Y., Zeng H., Liu K. Y., Guo G., Tong W. D., Shi Y., Tang B., Li N., Yu S., Luo P., Zhang W. J., Lu D. S., Yu P. W., Zou Q. M. (2012) CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology 143, 951–962 e8. [DOI] [PubMed] [Google Scholar]
- 105.Tilloy F., Treiner E., Park S. H., Garcia C., Lemonnier F., de la Salle H., Bendelac A., Bonneville M., Lantz O. (1999) An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J. Exp. Med. 189, 1907–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kjer-Nielsen L., Patel O., Corbett A. J., Le Nours J., Meehan B., Liu L., Bhati M., Chen Z., Kostenko L., Reantragoon R., Williamson N. A., Purcell A. W., Dudek N. L., McConville M. J., O’Hair R. A., Khairallah G. N., Godfrey D. I., Fairlie D. P., Rossjohn J., McCluskey J. (2012) MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723. [DOI] [PubMed] [Google Scholar]
- 107.Treiner E., Duban L., Bahram S., Radosavljevic M., Wanner V., Tilloy F., Affaticati P., Gilfillan S., Lantz O. (2003) Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169. [DOI] [PubMed] [Google Scholar]
- 108.Gapin L. (2014) Check MAIT. J. Immunol. 192, 4475–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dusseaux M., Martin E., Serriari N., Péguillet I., Premel V., Louis D., Milder M., Le Bourhis L., Soudais C., Treiner E., Lantz O. (2011) Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259. [DOI] [PubMed] [Google Scholar]
- 110.Maggi L., Santarlasci V., Capone M., Peired A., Frosali F., Crome S. Q., Querci V., Fambrini M., Liotta F., Levings M. K., Maggi E., Cosmi L., Romagnani S., Annunziato F. (2010) CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur. J. Immunol. 40, 2174–2181. [DOI] [PubMed] [Google Scholar]
- 111.Serriari N. E., Eoche M., Lamotte L., Lion J., Fumery M., Marcelo P., Chatelain D., Barre A., Nguyen-Khac E., Lantz O., Dupas J. L., Treiner E. (2014) Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin. Exp. Immunol. 176, 266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stark M. A., Huo Y., Burcin T. L., Morris M. A., Olson T. S., Ley K. (2005) Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity 22, 285–294. [DOI] [PubMed] [Google Scholar]
- 113.Bonneville M., Janeway C. A. Jr., Ito K., Haser W., Ishida I., Nakanishi N., Tonegawa S. (1988) Intestinal intraepithelial lymphocytes are a distinct set of gamma delta T cells. Nature 336, 479–481. [DOI] [PubMed] [Google Scholar]
- 114.Duan J., Chung H., Troy E., Kasper D. L. (2010) Microbial colonization drives expansion of IL-1 receptor 1-expressing and IL-17-producing gamma/delta T cells. Cell Host Microbe 7, 140–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Martin B., Hirota K., Cua D. J., Stockinger B., Veldhoen M. (2009) Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity 31, 321–330. [DOI] [PubMed] [Google Scholar]
- 116.Dungan L. S., Mills K. H. (2011) Caspase-1-processed IL-1 family cytokines play a vital role in driving innate IL-17. Cytokine 56, 126–132. [DOI] [PubMed] [Google Scholar]
- 117.Sutton C. E., Mielke L. A., Mills K. H. (2012) IL-17-producing γδ T cells and innate lymphoid cells. Eur. J. Immunol. 42, 2221–2231. [DOI] [PubMed] [Google Scholar]
- 118.Do J. S., Visperas A., Dong C., Baldwin W. M. III, Min B. (2011) Cutting edge: generation of colitogenic Th17 CD4 T cells is enhanced by IL-17+ γδ T cells. J. Immunol. 186, 4546–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cheng P., Liu T., Zhou W. Y., Zhuang Y., Peng L. S., Zhang J. Y., Yin Z. N., Mao X. H., Guo G., Shi Y., Zou Q. M. (2012) Role of gamma-delta T cells in host response against Staphylococcus aureus-induced pneumonia. BMC Immunol. 13, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jaffar Z., Ferrini M. E., Shaw P. K., FitzGerald G. A., Roberts K. (2011) Prostaglandin I₂promotes the development of IL-17-producing γδ T cells that associate with the epithelium during allergic lung inflammation. J. Immunol. 187, 5380–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Murdoch J. R., Lloyd C. M. (2010) Resolution of allergic airway inflammation and airway hyperreactivity is mediated by IL-17-producing gammadeltaT cells. Am. J. Respir. Crit. Care Med. 182, 464–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lockhart E., Green A. M., Flynn J. L. (2006) IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 177, 4662–4669. [DOI] [PubMed] [Google Scholar]
- 123.Okamoto Yoshida Y., Umemura M., Yahagi A., O’Brien R. L., Ikuta K., Kishihara K., Hara H., Nakae S., Iwakura Y., Matsuzaki G. (2010) Essential role of IL-17A in the formation of a mycobacterial infection-induced granuloma in the lung. J. Immunol. 184, 4414–4422. [DOI] [PubMed] [Google Scholar]
- 124.Basile J. I., Geffner L. J., Romero M. M., Balboa L., Sabio Y García C., Ritacco V., García A., Cuffré M., Abbate E., López B., Barrera L., Ambroggi M., Alemán M., Sasiain M. C., de la Barrera S. S. (2011) Outbreaks of mycobacterium tuberculosis MDR strains induce high IL-17 T-cell response in patients with MDR tuberculosis that is closely associated with high antigen load. J. Infect. Dis. 204, 1054–1064. [DOI] [PubMed] [Google Scholar]
- 125.Peng M. Y., Wang Z. H., Yao C. Y., Jiang L. N., Jin Q. L., Wang J., Li B. Q. (2008) Interleukin 17-producing gamma delta T cells increased in patients with active pulmonary tuberculosis. Cell. Mol. Immunol. 5, 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Asigbetse K. E., Eigenmann P. A., Frossard C. P. (2010) Intestinal lamina propria TcRgammadelta+ lymphocytes selectively express IL-10 and IL-17. J. Investig. Allergol. Clin. Immunol. 20, 391–401. [PubMed] [Google Scholar]
- 127.Shibata K. (2012) Close link between development and function of gamma-delta T cells. Microbiol. Immunol. 56, 217–227. [DOI] [PubMed] [Google Scholar]
- 128.Jabeen R., Chang H. C., Goswami R., Nutt S. L., Kaplan M. H. (2011) The transcription factor PU.1 regulates γδ T cell homeostasis. PLoS ONE 6, e22189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yurchenko E., Levings M. K., Piccirillo C. A. (2011) CD4+ Foxp3+ regulatory T cells suppress γδ T-cell effector functions in a model of T-cell-induced mucosal inflammation. Eur. J. Immunol. 41, 3455–3466. [DOI] [PubMed] [Google Scholar]
- 130.Park S. G., Mathur R., Long M., Hosh N., Hao L., Hayden M. S., Ghosh S. (2010) T regulatory cells maintain intestinal homeostasis by suppressing γδ T cells. Immunity 33, 791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu P., Wu D., Ni C., Ye J., Chen W., Hu G., Wang Z., Wang C., Zhang Z., Xia W., Chen Z., Wang K., Zhang T., Xu J., Han Y., Zhang T., Wu X., Wang J., Gong W., Zheng S., Qiu F., Yan J., Huang J. (2014) γδT17 cells promote the accumulation and expansion of myeloid-derived suppressor cells in human colorectal cancer. Immunity 40, 785–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bordon Y., Hansell C. A., Sester D. P., Clarke M., Mowat A. M., Nibbs R. J. (2009) The atypical chemokine receptor D6 contributes to the development of experimental colitis. J. Immunol. 182, 5032–5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sutton C. E., Lalor S. J., Sweeney C. M., Brereton C. F., Lavelle E. C., Mills K. H. (2009) Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity 31, 331–341. [DOI] [PubMed] [Google Scholar]
- 134.Simonian P. L., Wehrmann F., Roark C. L., Born W. K., O’Brien R. L., Fontenot A. P. (2010) γδ T cells protect against lung fibrosis via IL-22. J. Exp. Med. 207, 2239–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Raifer H., Mahiny A. J., Bollig N., Petermann F., Hellhund A., Kellner K., Guralnik A., Reinhard K., Bothur E., Huber M., Bauer S., Löhning M., Kiss E. A., Ganal S. C., Diefenbach A., Korn T., Lohoff M. (2012) Unlike αβ T cells, γδ T cells, LTi cells and NKT cells do not require IRF4 for the production of IL-17A and IL-22. Eur. J. Immunol. 42, 3189–3201. [DOI] [PubMed] [Google Scholar]
- 136.Nakayamada S., Kanno Y., Takahashi H., Jankovic D., Lu K. T., Johnson T. A., Sun H. W., Vahedi G., Hakim O., Handon R., Schwartzberg P. L., Hager G. L., O’Shea J. J. (2011) Early Th1 cell differentiation is marked by a Tfh cell-like transition. Immunity 35, 919–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cubas R. A., Mudd J. C., Savoye A. L., Perreau M., van Grevenynghe J., Metcalf T., Connick E., Meditz A., Freeman G. J., Abesada-Terk G. Jr., Jacobson J. M., Brooks A. D., Crotty S., Estes J. D., Pantaleo G., Lederman M. M., Haddad E. K. (2013) Inadequate T follicular cell help impairs B cell immunity during HIV infection. Nat. Med. 19, 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hirota K., Turner J. E., Villa M., Duarte J. H., Demengeot J., Steinmetz O. M., Stockinger B. (2013) Plasticity of Th17 cells in Peyer’s patches is responsible for the induction of T cell-dependent IgA responses. Nat. Immunol. 14, 372–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Girardi E., Zajonc D. M. (2012) Molecular basis of lipid antigen presentation by CD1d and recognition by natural killer T cells. Immunol. Rev. 250, 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Havenar-Daughton C., Li S., Benlagha K., Marie J. C. (2012) Development and function of murine RORγt+ iNKT cells are under TGF-β signaling control. Blood 119, 3486–3494. [DOI] [PubMed] [Google Scholar]
- 141.Watarai H., Sekine-Kondo E., Shigeura T., Motomura Y., Yasuda T., Satoh R., Yoshida H., Kubo M., Kawamoto H., Koseki H., Taniguchi M. (2012) Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 10, e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Enders A., Stankovic S., Teh C., Uldrich A. P., Yabas M., Juelich T., Altin J. A., Frankenreiter S., Bergmann H., Roots C. M., Kyparissoudis K., Goodnow C. C., Godfrey D. I. (2012) ZBTB7B (Th-POK) regulates the development of IL-17-producing CD1d-restricted mouse NKT cells. J. Immunol. 189, 5240–5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Engel I., Zhao M., Kappes D., Taniuchi I., Kronenberg M. (2012) The transcription factor Th-POK negatively regulates Th17 differentiation in Vα14i NKT cells. Blood 120, 4524–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Price A. E., Reinhardt R. L., Liang H. E., Locksley R. M. (2012) Marking and quantifying IL-17A-producing cells in vivo. PLoS ONE 7, e39750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hodge G., Hodge S., Li-Liew C., Reynolds P. N., Holmes M. (2012) Increased natural killer T-like cells are a major source of pro-inflammatory cytokines and granzymes in lung transplant recipients. Respirology 17, 155–163. [DOI] [PubMed] [Google Scholar]
- 146.Tan H. L., Regamey N., Brown S., Bush A., Lloyd C. M., Davies J. C. (2011) The Th17 pathway in cystic fibrosis lung disease. Am. J. Respir. Crit. Care Med. 184, 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Wingender G., Rogers P., Batzer G., Lee M. S., Bai D., Pei B., Khurana A., Kronenberg M., Horner A. A. (2011) Invariant NKT cells are required for airway inflammation induced by environmental antigens. J. Exp. Med. 208, 1151–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Coquet J. M., Kyparissoudis K., Pellicci D. G., Besra G., Berzins S. P., Smyth M. J., Godfrey D. I. (2007) IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J. Immunol. 178, 2827–2834. [DOI] [PubMed] [Google Scholar]
- 149.Harada M., Magara-Koyanagi K., Watarai H., Nagata Y., Ishii Y., Kojo S., Horiguchi S., Okamoto Y., Nakayama T., Suzuki N., Yeh W. C., Akira S., Kitamura H., Ohara O., Seino K., Taniguchi M. (2006) IL-21-induced Bepsilon cell apoptosis mediated by natural killer T cells suppresses IgE responses. J. Exp. Med. 203, 2929–2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.King I. L., Fortier A., Tighe M., Dibble J., Watts G. F., Veerapen N., Haberman A. M., Besra G. S., Mohrs M., Brenner M. B., Leadbetter E. A. (2012) Invariant natural killer T cells direct B cell responses to cognate lipid antigen in an IL-21-dependent manner. Nat. Immunol. 13, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chang P. P., Barral P., Fitch J., Pratama A., Ma C. S., Kallies A., Hogan J. J., Cerundolo V., Tangye S. G., Bittman R., Nutt S. L., Brink R., Godfrey D. I., Batista F. D., Vinuesa C. G. (2012) Identification of Bcl-6-dependent follicular helper NKT cells that provide cognate help for B cell responses. Nat. Immunol. 13, 35–43. [DOI] [PubMed] [Google Scholar]
- 152.Goto M., Murakawa M., Kadoshima-Yamaoka K., Tanaka Y., Nagahira K., Fukuda Y., Nishimura T. (2009) Murine NKT cells produce Th17 cytokine interleukin-22. Cell. Immunol. 254, 81–84. [DOI] [PubMed] [Google Scholar]
- 153.Paget C., Ivanov S., Fontaine J., Renneson J., Blanc F., Pichavant M., Dumoutier L., Ryffel B., Renauld J. C., Gosset P., Gosset P., Si-Tahar M., Faveeuw C., Trottein F. (2012) Interleukin-22 is produced by invariant natural killer T lymphocytes during influenza A virus infection: potential role in protection against lung epithelial damages. J. Biol. Chem. 287, 8816–8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sawa S., Cherrier M., Lochner M., Satoh-Takayama N., Fehling H. J., Langa F., Di Santo J. P., Eberl G. (2010) Lineage relationship analysis of RORgammat+ innate lymphoid cells. Science 330, 665–669. [DOI] [PubMed] [Google Scholar]
- 155.Hwang Y. Y., McKenzie A. N. (2013) Innate lymphoid cells in immunity and disease. Adv. Exp. Med. Biol. 785, 9–26. [DOI] [PubMed] [Google Scholar]
- 156.Yokota Y., Mansouri A., Mori S., Sugawara S., Adachi S., Nishikawa S., Gruss P. (1999) Development of peripheral lymphoid organs and natural killer cells depends on the helix-loop-helix inhibitor Id2. Nature 397, 702–706. [DOI] [PubMed] [Google Scholar]
- 157.Satoh-Takayama N., Lesjean-Pottier S., Vieira P., Sawa S., Eberl G., Vosshenrich C. A., Di Santo J. P. (2010) IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J. Exp. Med. 207, 273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Moro K., Yamada T., Tanabe M., Takeuchi T., Ikawa T., Kawamoto H., Furusawa J., Ohtani M., Fujii H., Koyasu S. (2010) Innate production of T(H)2 cytokines by adipose tissue-associated c-Kit(+)Sca-1(+) lymphoid cells. Nature 463, 540–544. [DOI] [PubMed] [Google Scholar]
- 159.Spits H., Artis D., Colonna M., Diefenbach A., Di Santo J. P., Eberl G., Koyasu S., Locksley R. M., McKenzie A. N., Mebius R. E., Powrie F., Vivier E. (2013) Innate lymphoid cells—a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149. [DOI] [PubMed] [Google Scholar]
- 160.Meier D., Bornmann C., Chappaz S., Schmutz S., Otten L. A., Ceredig R., Acha-Orbea H., Finke D. (2007) Ectopic lymphoid-organ development occurs through interleukin 7-mediated enhanced survival of lymphoid-tissue-inducer cells. Immunity 26, 643–654. [DOI] [PubMed] [Google Scholar]
- 161.Boos M. D., Yokota Y., Eberl G., Kee B. L. (2007) Mature natural killer cell and lymphoid tissue-inducing cell development requires Id2-mediated suppression of E protein activity. J. Exp. Med. 204, 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sanos S. L., Bui V. L., Mortha A., Oberle K., Heners C., Johner C., Diefenbach A. (2009) RORgammat and commensal microflora are required for the differentiation of mucosal interleukin 22-producing NKp46+ cells. Nat. Immunol. 10, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Serafini N., Klein Wolterink R. G., Satoh-Takayama N., Xu W., Vosshenrich C. A., Hendriks R. W., Di Santo J. P. (2014) Gata3 drives development of RORγt+ group 3 innate lymphoid cells. J. Exp. Med. 211, 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mebius R. E., Rennert P., Weissman I. L. (1997) Developing lymph nodes collect CD4+CD3−LTbeta+ cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504. [DOI] [PubMed] [Google Scholar]
- 165.Lee J. S., Cella M., McDonald K. G., Garlanda C., Kennedy G. D., Nukaya M., Mantovani A., Kopan R., Bradfield C. A., Newberry R. D., Colonna M. (2012) AHR drives the development of gut ILC22 cells and postnatal lymphoid tissues via pathways dependent on and independent of Notch. Nat. Immunol. 13, 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aliahmad P., de la Torre B., Kaye J. (2010) Shared dependence on the DNA-binding factor TOX for the development of lymphoid tissue-inducer cell and NK cell lineages. Nat. Immunol. 11, 945–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mueller C. G., Hess E. (2012) Emerging functions of RANKL in lymphoid tissues. Front. Immunol. 3, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Takatori H., Kanno Y., Watford W. T., Tato C. M., Weiss G., Ivanov I. I., Littman D. R., O’Shea J. J. (2009) Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J. Exp. Med. 206, 35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sonnenberg G. F., Monticelli L. A., Elloso M. M., Fouser L. A., Artis D. (2011) CD4(+) lymphoid tissue-inducer cells promote innate immunity in the gut. Immunity 34, 122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rankin L. C., Groom J. R., Chopin M., Herold M. J., Walker J. A., Mielke L. A., McKenzie A. N., Carotta S., Nutt S. L., Belz G. T. (2013) The transcription factor T-bet is essential for the development of NKp46+ innate lymphocytes via the Notch pathway. Nat. Immunol. 14, 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Luci C., Reynders A., Ivanov I. I., Cognet C., Chiche L., Chasson L., Hardwigsen J., Anguiano E., Banchereau J., Chaussabel D., Dalod M., Littman D. R., Vivier E., Tomasello E. (2009) Influence of the transcription factor RORgammat on the development of NKp46+ cell populations in gut and skin. Nat. Immunol. 10, 75–82. [DOI] [PubMed] [Google Scholar]
- 172.Cupedo T., Crellin N. K., Papazian N., Rombouts E. J., Weijer K., Grogan J. L., Fibbe W. E., Cornelissen J. J., Spits H. (2009) Human fetal lymphoid tissue-inducer cells are interleukin 17-producing precursors to RORC+ CD127+ natural killer-like cells. Nat. Immunol. 10, 66–74. [DOI] [PubMed] [Google Scholar]
- 173.Tomasello E., Yessaad N., Gregoire E., Hudspeth K., Luci C., Mavilio D., Hardwigsen J., Vivier E. (2012) Mapping of NKp46(+) cells in healthy human lymphoid and non-lymphoid tissues. Front. Immunol. 3, 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Satoh-Takayama N., Dumoutier L., Lesjean-Pottier S., Ribeiro V. S., Mandelboim O., Renauld J. C., Vosshenrich C. A., Di Santo J. P. (2009) The natural cytotoxicity receptor NKp46 is dispensable for IL-22-mediated innate intestinal immune defense against Citrobacter rodentium. J. Immunol. 183, 6579–6587. [DOI] [PubMed] [Google Scholar]
- 175.Buonocore S., Ahern P. P., Uhlig H. H., Ivanov I. I., Littman D. R., Maloy K. J., Powrie F. (2010) Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chan I. H., Jain R., Tessmer M. S., Gorman D., Mangadu R., Sathe M., Vives F., Moon C., Penaflor E., Turner S., Ayanoglu G., Chang C., Basham B., Mumm J. B., Pierce R. H., Yearley J. H., McClanahan T. K., Phillips J. H., Cua D. J., Bowman E. P., Kastelein R. A., LaFace D. (2014) Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal Immunol. 7, 842–856. [DOI] [PubMed] [Google Scholar]
- 177.Kirchberger S., Royston D. J., Boulard O., Thornton E., Franchini F., Szabady R. L., Harrison O., Powrie F. (2013) Innate lymphoid cells sustain colon cancer through production of interleukin-22 in a mouse model. J. Exp. Med. 210, 917–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Bogaert S., Laukens D., Peeters H., Melis L., Olievier K., Boon N., Verbruggen G., Vandesompele J., Elewaut D., De Vos M. (2010) Differential mucosal expression of Th17-related genes between the inflamed colon and ileum of patients with inflammatory bowel disease. BMC Immunol. 11, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Geremia A., Arancibia-Cárcamo C. V., Fleming M. P., Rust N., Singh B., Mortensen N. J., Travis S. P., Powrie F. (2011) IL-23-responsive innate lymphoid cells are increased in inflammatory bowel disease. J. Exp. Med. 208, 1127–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Dragon S., Saffar A. S., Shan L., Gounni A. S. (2008) IL-17 attenuates the anti-apoptotic effects of GM-CSF in human neutrophils. Mol. Immunol. 45, 160–168. [DOI] [PubMed] [Google Scholar]
- 181.Pelletier M., Maggi L., Micheletti A., Lazzeri E., Tamassia N., Costantini C., Cosmi L., Lunardi C., Annunziato F., Romagnani S., Cassatella M. A. (2010) Evidence for a cross-talk between human neutrophils and Th17 cells. Blood 115, 335–343. [DOI] [PubMed] [Google Scholar]
- 182.Pelletier M., Bouchard A., Girard D. (2004) In vivo and in vitro roles of IL-21 in inflammation. J. Immunol. 173, 7521–7530. [DOI] [PubMed] [Google Scholar]
- 183.Smith E., Stark M. A., Zarbock A., Burcin T. L., Bruce A. C., Vaswani D., Foley P., Ley K. (2008) IL-17A inhibits the expansion of IL-17A-producing T cells in mice through “short-loop” inhibition via IL-17 receptor. J. Immunol. 181, 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Witowski J., Pawlaczyk K., Breborowicz A., Scheuren A., Kuzlan-Pawlaczyk M., Wisniewska J., Polubinska A., Friess H., Gahl G. M., Frei U., Jörres A. (2000) IL-17 stimulates intraperitoneal neutrophil infiltration through the release of GRO alpha chemokine from mesothelial cells. J. Immunol. 165, 5814–5821. [DOI] [PubMed] [Google Scholar]
- 185.Taylor P. R., Roy S., Leal S. M. Jr., Sun Y., Howell S. J., Cobb B. A., Li X., Pearlman E. (2014) Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORγt and dectin-2. Nat. Immunol. 15, 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Wozniak K. L., Kolls J. K., Wormley F. L. Jr. (2012) Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL-17A production by γδ T cells. BMC Immunol. 13, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ferretti S., Bonneau O., Dubois G. R., Jones C. E., Trifilieff A. (2003) IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J. Immunol. 170, 2106–2112. [DOI] [PubMed] [Google Scholar]
- 188.Garraud K., Cleret A., Mathieu J., Fiole D., Gauthier Y., Quesnel-Hellmann A., Tournier J. N. (2012) Differential role of the interleukin-17 axis and neutrophils in resolution of inhalational anthrax. Infect. Immun. 80, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Werner J. L., Gessner M. A., Lilly L. M., Nelson M. P., Metz A. E., Horn D., Dunaway C. W., Deshane J., Chaplin D. D., Weaver C. T., Brown G. D., Steele C. (2011) Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infect. Immun. 79, 3966–3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Eustace A., Smyth L. J., Mitchell L., Williamson K., Plumb J., Singh D. (2011) Identification of cells expressing IL-17A and IL-17F in the lungs of patients with COPD. Chest 139, 1089–1100. [DOI] [PubMed] [Google Scholar]
- 191.Van Leeuwen M. A., Lindenbergh-Kortleve D. J., Raatgeep H. C., de Ruiter L. F., de Krijger R. R., Groeneweg M., Escher J. C., Samsom J. N. (2013) Increased production of interleukin-21, but not interleukin-17A, in the small intestine characterizes pediatric celiac disease. Mucosal Immunol. 6, 1202–1213. [DOI] [PubMed] [Google Scholar]
- 192.Puga I., Cols M., Barra C. M., He B., Cassis L., Gentile M., Comerma L., Chorny A., Shan M., Xu W., Magri G., Knowles D. M., Tam W., Chiu A., Bussel J. B., Serrano S., Lorente J. A., Bellosillo B., Lloreta J., Juanpere N., Alameda F., Baró T., de Heredia C. D., Torán N., Català A., Torrebadell M., Fortuny C., Cusí V., Carreras C., Diaz G. A., Blander J. M., Farber C. M., Silvestri G., Cunningham-Rundles C., Calvillo M., Dufour C., Notarangelo L. D., Lougaris V., Plebani A., Casanova J. L., Ganal S. C., Diefenbach A., Aróstegui J. I., Juan M., Yagüe J., Mahlaoui N., Donadieu J., Chen K., Cerutti A. (2012) B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 13, 170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zindl C. L., Lai J. F., Lee Y. K., Maynard C. L., Harbour S. N., Ouyang W., Chaplin D. D., Weaver C. T. (2013) IL-22-producing neutrophils contribute to antimicrobial defense and restitution of colonic epithelial integrity during colitis. Proc. Natl. Acad. Sci. USA 110, 12768–12773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Takahashi N., Vanlaere I., de Rycke R., Cauwels A., Joosten L. A., Lubberts E., van den Berg W. B., Libert C. (2008) IL-17 produced by Paneth cells drives TNF-induced shock. J. Exp. Med. 205, 1755–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Rumio C., Sommariva M., Sfondrini L., Palazzo M., Morelli D., Viganò L., De Cecco L., Tagliabue E., Balsari A. (2012) Induction of Paneth cell degranulation by orally administered Toll-like receptor ligands. J. Cell. Physiol. 227, 1107–1113. [DOI] [PubMed] [Google Scholar]
- 196.Gu Y., Yang J., Ouyang X., Liu W., Li H., Yang J., Bromberg J., Chen S. H., Mayer L., Unkeless J. C., Xiong H. (2008) Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur. J. Immunol. 38, 1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kasahara D. I., Kim H. Y., Williams A. S., Verbout N. G., Tran J., Si H., Wurmbrand A. P., Jastrab J., Hug C., Umetsu D. T., Shore S. A. (2012) Pulmonary inflammation induced by subacute ozone is augmented in adiponectin-deficient mice: role of IL-17A. J. Immunol. 188, 4558–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Vázquez N., Rekka S., Gliozzi M., Feng C. G., Amarnath S., Orenstein J. M., Wahl S. M. (2012) Modulation of innate host factors by Mycobacterium avium complex in human macrophages includes interleukin 17. J. Infect. Dis. 206, 1206–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jiang X. D., Li G. Y., Li L., Dong Z., Zhu D. D. (2011) The characterization of IL-17A expression in patients with chronic rhinosinusitis with nasal polyps. Am. J. Rhinol. Allergy 25, e171–e175. [DOI] [PubMed] [Google Scholar]
- 200.Hansson M., Silverpil E., Linden A., Glader P. (2013) Interleukin-22 produced by alveolar macrophages during activation of the innate immune response. Inflamm. Res. 62, 561–569. [DOI] [PubMed] [Google Scholar]
- 201.Kenna T. J., Brown M. A. (2013) The role of IL-17-secreting mast cells in inflammatory joint disease. Nat. Rev. Rheumatol. 9, 375–379. [DOI] [PubMed] [Google Scholar]


