Abstract
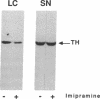
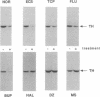
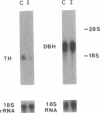
Regulation of tyrosine hydroxylase expression by antidepressant treatments was investigated in the locus coeruleus (LC), the major noradrenergic nucleus in brain. Rats were treated chronically with various antidepressants, and tyrosine hydroxylase levels were measured in the LC by immunoblot analysis. Representatives of all major classes of antidepressant medication-including imipramine, nortriptyline, tranylcypromine, fluvoxamine, fluoxetine, bupropion, iprindole, and electroconvulsive seizures-were found to decrease levels of tyrosine hydroxylase immunoreactivity by 40-70% in the LC. Decreased levels of enzyme immunoreactivity were shown to be associated with equivalent decreases in enzyme mRNA levels. Antidepressant regulation of LC tyrosine hydroxylase appeared specific to these compounds, inasmuch as chronic treatment of rats with representatives of other classes of psychotropic drugs, including haloperidol, diazepam, clonidine, cocaine, and morphine, failed to decrease levels of this protein. The results demonstrate that chronic antidepressants dramatically downregulate the expression of tyrosine hydroxylase in the LC and raise the possibility that such regulation of the enzyme represents an adaptive response of LC neurons to antidepressants that mediates some of their therapeutic actions in depression and/or other psychiatric disturbances.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abercrombie E. D., Jacobs B. L. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J Neurosci. 1987 Sep;7(9):2837–2843. doi: 10.1523/JNEUROSCI.07-09-02837.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson A. L., Zigmond M. J. Short and long term changes in tyrosine hydroxylase activity in rat brain after subtotal destruction of central noradrenergic neurons. J Neurosci. 1981 May;1(5):493–504. doi: 10.1523/JNEUROSCI.01-05-00493.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanian G. K. Tolerance of locus coeruleus neurones to morphine and suppression of withdrawal response by clonidine. Nature. 1978 Nov 9;276(5684):186–188. doi: 10.1038/276186a0. [DOI] [PubMed] [Google Scholar]
- Biguet N. F., Buda M., Lamouroux A., Samolyk D., Mallet J. Time course of the changes of TH mRNA in rat brain and adrenal medulla after a single injection of reserpine. EMBO J. 1986 Feb;5(2):287–291. doi: 10.1002/j.1460-2075.1986.tb04211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black I. B., Adler J. E., Dreyfus C. F., Friedman W. F., LaGamma E. F., Roach A. H. Biochemistry of information storage in the nervous system. Science. 1987 Jun 5;236(4806):1263–1268. doi: 10.1126/science.2884727. [DOI] [PubMed] [Google Scholar]
- Blier P., de Montigny C. Serotoninergic but not noradrenergic neurons in rat central nervous system adapt to long-term treatment with monoamine oxidase inhibitors. Neuroscience. 1985 Dec;16(4):949–955. doi: 10.1016/0306-4522(85)90107-1. [DOI] [PubMed] [Google Scholar]
- Bobker D. H., Williams J. T. Serotonin agonists inhibit synaptic potentials in the rat locus ceruleus in vitro via 5-hydroxytryptamine1A and 5-hydroxytryptamine1B receptors. J Pharmacol Exp Ther. 1989 Jul;250(1):37–43. [PubMed] [Google Scholar]
- Broquet P., Baubichon-Cortay H., George P., Peschard M. J., Louisot P. Effect of desipramine on a glycoprotein sialyltransferase activity in C6 cultured glioma cells. J Neurochem. 1990 Feb;54(2):388–394. doi: 10.1111/j.1471-4159.1990.tb01885.x. [DOI] [PubMed] [Google Scholar]
- Chiodo L. A., Acheson A. L., Zigmond M. J., Stricker E. M. Subtotal destruction of central noradrenergic projections increases the firing rate of locus coeruleus cells. Brain Res. 1983 Mar 28;264(1):123–126. doi: 10.1016/0006-8993(83)91128-9. [DOI] [PubMed] [Google Scholar]
- Chouvet G., Akaoka H., Aston-Jones G. Diminution sélective par la sérotonine de l'excitation des neurones du locus coeruleus évoquée par le glutamate. C R Acad Sci III. 1988;306(11):339–344. [PubMed] [Google Scholar]
- Duman R. S., Strada S. J., Enna S. J. Effect of imipramine and adrenocorticotropin administration on the rat brain norepinephrine-coupled cyclic nucleotide generating system: alterations in alpha and beta adrenergic components. J Pharmacol Exp Ther. 1985 Aug;234(2):409–414. [PubMed] [Google Scholar]
- Engberg G., Elam M., Svensson T. H. Clonidine withdrawal: activation of brain noradrenergic neurons with specifically reduced alpha 2-receptor sensitivity. Life Sci. 1982 Jan 18;30(3):235–243. doi: 10.1016/0024-3205(82)90504-5. [DOI] [PubMed] [Google Scholar]
- Fishman P. H., Finberg J. P. Effect of the tricyclic antidepressant desipramine on beta-adrenergic receptors in cultured rat glioma C6 cells. J Neurochem. 1987 Jul;49(1):282–289. doi: 10.1111/j.1471-4159.1987.tb03427.x. [DOI] [PubMed] [Google Scholar]
- Gold P. W., Goodwin F. K., Chrousos G. P. Clinical and biochemical manifestations of depression. Relation to the neurobiology of stress (1) N Engl J Med. 1988 Aug 11;319(6):348–353. doi: 10.1056/NEJM198808113190606. [DOI] [PubMed] [Google Scholar]
- Guitart X., Nestler E. J. Identification of morphine- and cyclic AMP-regulated phosphoproteins (MARPPs) in the locus coeruleus and other regions of rat brain: regulation by acute and chronic morphine. J Neurosci. 1989 Dec;9(12):4371–4387. doi: 10.1523/JNEUROSCI.09-12-04371.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y. H., Maas J. W., Hu G. H. The time course of noradrenergic pre- and postsynaptic activity during chronic desipramine treatment. Eur J Pharmacol. 1980 Nov 7;68(1):41–47. doi: 10.1016/0014-2999(80)90058-8. [DOI] [PubMed] [Google Scholar]
- Leonard D. G., Ziff E. B., Greene L. A. Identification and characterization of mRNAs regulated by nerve growth factor in PC12 cells. Mol Cell Biol. 1987 Sep;7(9):3156–3167. doi: 10.1128/mcb.7.9.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masserano J. M., Takimoto G. S., Weiner N. Electroconvulsive shock increases tyrosine hydroxylase activity in the brain and adrenal gland of the rat. Science. 1981 Nov 6;214(4521):662–665. doi: 10.1126/science.6117127. [DOI] [PubMed] [Google Scholar]
- McMahon A., Geertman R., Sabban E. L. Rat dopamine beta-hydroxylase: molecular cloning and characterization of the cDNA and regulation of the mRNA by reserpine. J Neurosci Res. 1990 Mar;25(3):395–404. doi: 10.1002/jnr.490250317. [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Tallman J. F. Chronic morphine treatment increases cyclic AMP-dependent protein kinase activity in the rat locus coeruleus. Mol Pharmacol. 1988 Feb;33(2):127–132. [PubMed] [Google Scholar]
- Nestler E. J., Terwilliger R. Z., Duman R. S. Chronic antidepressant administration alters the subcellular distribution of cyclic AMP-dependent protein kinase in rat frontal cortex. J Neurochem. 1989 Nov;53(5):1644–1647. doi: 10.1111/j.1471-4159.1989.tb08564.x. [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Terwilliger R. Z., Walker J. R., Sevarino K. A., Duman R. S. Chronic cocaine treatment decreases levels of the G protein subunits Gi alpha and Go alpha in discrete regions of rat brain. J Neurochem. 1990 Sep;55(3):1079–1082. doi: 10.1111/j.1471-4159.1990.tb04602.x. [DOI] [PubMed] [Google Scholar]
- Nestler E. J., Terwilliger R., Beitner D. Regulation by chronic clonidine of adenylate cyclase and cyclic AMP-dependent protein kinase in the rat locus coeruleus. Life Sci. 1989;45(12):1073–1080. doi: 10.1016/0024-3205(89)90164-1. [DOI] [PubMed] [Google Scholar]
- Nomura S., Duman R. S., Enna S. J. In vivo or in vitro exposure to imipramine reduces alpha 2-adrenoceptor-mediated inhibition of cyclic AMP production in rat brain cerebral cortical slices. Brain Res. 1987 Apr 28;410(1):195–198. doi: 10.1016/s0006-8993(87)80046-x. [DOI] [PubMed] [Google Scholar]
- Perez J., Tinelli D., Brunello N., Racagni G. cAMP-dependent phosphorylation of soluble and crude microtubule fractions of rat cerebral cortex after prolonged desmethylimipramine treatment. Eur J Pharmacol. 1989 Aug 15;172(3):305–316. doi: 10.1016/0922-4106(89)90060-6. [DOI] [PubMed] [Google Scholar]
- Pitts D. K., Marwah J. Chronic cocaine reduces alpha 2-adrenoceptor elicited mydriasis and inhibition of locus coeruleus neurons. Eur J Pharmacol. 1989 Jan 31;160(2):201–209. doi: 10.1016/0014-2999(89)90492-5. [DOI] [PubMed] [Google Scholar]
- Reis D. J., Joh T. H., Ross R. A., Pickel V. M. Reserpine selectively increases tyrosine hydroxylase and dopamine-beta-hydroxylase enzyme protein in central noradrenergic neurons. Brain Res. 1974 Dec 6;81(2):380–386. doi: 10.1016/0006-8993(74)90956-1. [DOI] [PubMed] [Google Scholar]
- Richard F., Faucon-Biguet N., Labatut R., Rollet D., Mallet J., Buda M. Modulation of tyrosine hydroxylase gene expression in rat brain and adrenals by exposure to cold. J Neurosci Res. 1988 May;20(1):32–37. doi: 10.1002/jnr.490200106. [DOI] [PubMed] [Google Scholar]
- Schalling M., Stieg P. E., Lindquist C., Goldstein M., Hökfelt T. Rapid increase in enzyme and peptide mRNA in sympathetic ganglia after electrical stimulation in humans. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4302–4305. doi: 10.1073/pnas.86.11.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal D. S., Kuczenski R., Mandell A. J. Theoretical implications of drug-induced adaptive regulation for a biogenic amine hypothesis of affective disorder. Biol Psychiatry. 1974 Oct;9(2):147–159. [PubMed] [Google Scholar]
- Tank A. W., Lewis E. J., Chikaraishi D. M., Weiner N. Elevation of RNA coding for tyrosine hydroxylase in rat adrenal gland by reserpine treatment and exposure to cold. J Neurochem. 1985 Oct;45(4):1030–1033. doi: 10.1111/j.1471-4159.1985.tb05519.x. [DOI] [PubMed] [Google Scholar]
- Valentino R. J., Curtis A. L., Parris D. G., Wehby R. G. Antidepressant actions on brain noradrenergic neurons. J Pharmacol Exp Ther. 1990 May;253(2):833–840. [PubMed] [Google Scholar]
- Vetulani J., Stawarz R. J., Dingell J. V., Sulser F. A possible common mechanism of action of antidepressant treatments: reduction in the sensitivity of the noradrenergic cyclic AMP gererating system in the rat limbic forebrain. Naunyn Schmiedebergs Arch Pharmacol. 1976 May;293(2):109–114. doi: 10.1007/BF00499215. [DOI] [PubMed] [Google Scholar]
- Wolfe B. B., Harden T. K., Sporn J. R., Molinoff P. B. Presynaptic modulation of beta adrenergic receptors in rat cerebral cortex after treatment with antidepressants. J Pharmacol Exp Ther. 1978 Nov;207(2):446–457. [PubMed] [Google Scholar]
- Zigmond R. E., Schon F., Iversen L. L. Increased tyrosine hydroxylase activity in the locus coeruleus of rat brain stem after reserpine treatment and cold stress. Brain Res. 1974 Apr 26;70(3):547–552. doi: 10.1016/0006-8993(74)90267-4. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Schwarzschild M. A., Rittenhouse A. R. Acute regulation of tyrosine hydroxylase by nerve activity and by neurotransmitters via phosphorylation. Annu Rev Neurosci. 1989;12:415–461. doi: 10.1146/annurev.ne.12.030189.002215. [DOI] [PubMed] [Google Scholar]