Abstract
The observation that only 50% of patients with adult asthma manifest atopy indicates that other inflammatory mechanisms are likely involved in producing the characteristic features of this disorder; namely reversible airway obstruction, hyperresponsiveness, and pulmonary inflammation. Our recent discovery that antigen-specific Ig free light chains (LCs) mediate hypersensitivity-like responses suggests that these molecules may be of import in the pathophysiology of asthma. Using a murine experimental model of nonatopic asthma, we now have shown that an LC antagonist, the 9-mer peptide F991, can abrogate the development of airway obstruction, hyperresponsiveness, and pulmonary inflammation. Further, passive immunization with antigen-specific LCs and subsequent airway challenge can elicit a mast cell-dependent reaction leading to acute bronchoconstriction. These findings, and the demonstration that the concentration of free κ LCs in the sera of patients with adult asthma were significantly increased (as compared with age-matched nonasthmatic individuals), provide previously undescribed insight into the pathogenesis of asthma. In addition, the ability to inhibit pharmacologically LC-induced mast cell activation provides a therapeutic means to prevent or ameliorate the adverse bronchopulmonary manifestations of this incapacitating disorder.
Keywords: mast cells, lung
Asthma is a relatively common disorder manifested by airway inflammation that leads to bronchoconstriction and respiratory symptoms. Approximately 50% of adults with this disorder have atopic manifestations that involve IgE-mediated tissue sensitization with a T helper cell 2 response and eosinophilia (1, 2). However, that an equal percentage of patients with asthma are not atopic, as evidenced by negative skin-prick test as well as normal serum IgE levels and eosinophil counts (1, 2), indicates that other causative factors may be involved in the etiology of this disease. Furthermore, standardized comparisons across populations or time show only a weak and inconsistent association between the prevalence of adult asthma and the prevalence of atopy (1).
Mast cells have been implicated as essential elements in the asthmatic process (3–5). Indeed, mast cell numbers are increased in the lungs of individuals with atopic as well as nonatopic variants of adult asthma, and the extent of degranulation of these cells is directly related to disease severity (6, 7). Although antigen-specific mast cell activation results from crosslinking of the high-affinity IgE receptor, FcεRI, this effect can also occur in the absence of IgE antibodies (8–12); furthermore, anti-IgE antibody therapy has been of limited benefit in asthma (13–18).
Our recent discovery that Ig free light chains (LCs) mediate antigen-specific mast cell-dependent hypersensitivity-like responses suggests that these molecules could be involved in the pathophysiology of adult asthma (19). We showed that mice, passively sensitized with antigen-specific LCs, when challenged by cutaneous exposure to the antigen, developed an allergic reaction characterized by mast cell-dependent plasma extravasation and tissue swelling (19).
Based on this observation, we have investigated the role of LC-induced hypersensitivity and mast cell activation in a murine experimental model that has clear similarities of adult asthma (11). The results of our in vivo studies have provided further evidence that implicate LC molecules in asthma pathogenesis; additionally, we have shown that inhibition of LC-induced mast cell activation by chemical means was therapeutically effective in preventing or reducing bronchoconstriction, airway inflammation and hyperresponsiveness. Furthermore, we have demonstrated significant rises in κ LC in sera from both atopic and nonatopic adult asthma patients compared with healthy controls.
Methods
Mice. BALB/c and mast cell-deficient WBB6F1 W/Wv and control littermates WBB6F1 +/+ mice were obtained from the Central Animal Laboratory (Utrecht, The Netherlands) and The Jackson Laboratory, respectively. Utrecht University's Animal Care Committee approved all in vivo experimental protocols.
Antigen-Specific LCs. Antigen-specific LCs were isolated from trinitrophenol (TNP)-(1B7-11, American Type Culture Collection) and oxazolone (OXA)-(NQ10/12.5)-specific IgG (kindly provided by C. Milstein, Medical Research Council, Laboratory of Molecular Biology, Cambridge, U.K.) and purified as described (19, 20). Recombinant LCs were produced by PCR cloning of cDNA 1B7-11 in a pGEX vector (Amersham Pharmacia Biosciences). Fusion proteins were expressed in Escherichia coli and purified by using affinity chromatography (19).
Active Immunization and Airway Challenge. Mice were immunized topically on days 0 and 1 with either 100 μl of 0.5% dinitrofluorobenzene (DNFB) (Sigma) or vehicle control and intranasally challenged with 50 μl of 0.6% dinitrobenzene sulfonic acid (DNBS, a water-soluble form of DNFB) (Sigma) on day 5, as described (11).
Passive Immunization and Airway Challenge. BALB/c mice received single i.v. injections of TNP-specific IgG1, OXA- or TNP-specific LCs isolated from whole IgG molecules, or (GST) recombinant-derived TNP-specific LCs (2 or 5 μg in 50 μl of sterile saline). Control animals were given injections of sterile saline or recombinant GST. The animals were challenged 30 min later by intranasal application of a 50-μl solution containing 0.6% trinitrobenzene sulfonic acid (TNBS) (Sigma), PBS, or OXA coupled with BSA (OXA-BSA, 1% in 25 μl).
Similar studies were performed in mast cell-deficient mice WBB6F1 W/Wv mice, their respective normal littermates (WBB6F1 +/+), and those in which the mast cells were reconstituted 12 wk before passive immunization by injection into the tail vein of 2.5 × 106 bone marrow-derived mast cells (BMMC) cultured from bone marrow of WBB6F1 +/+ mice and BMMC→W/Wv mice (11).
Antagonist Studies. The LC antagonist F991, a 9-mer peptide (AHWSGHCCL), was synthesized (19) by Fmoc chemistry (Ansynth, Roosendaal, The Netherlands) and administered intranasally or i.p. (200 μg in 50 μl or 50 μg in 100 μl of sterile saline, respectively). Intraperitoneal pretreatment with F991 according the described regimen inhibited dose-dependently DNFB-induced cutaneous hyperresponsiveness (data not shown).
Measurement of Acute Bronchoconstriction. Bronchoconstriction was measured in unrestrained conscious mice by using a whole-body plethysmographic chamber (Buxco Electronics, Sharon, CT) (11). After intranasal challenge, maximal Penh readings were taken 2½, 5, 7½, 10, 15, and 20 min later.
In Vivo Mast Cell Activation. Mouse mast cell protease 1 (mMCP-1) activity in blood samples taken from mice 30 min after intranasal challenge were measured by ELISA (Moredun Scientific, Midlothian, U.K.) (11). For microscopic studies, tracheal samples obtained 1 h postchallenge were fixed in Karnovsky solution, and semithin sections (10 per animal) were stained with toluidine blue. Mast cells were scored for their degranulation light microscopically. For ultrastructural studies, ultrathin sections were stained with aqueous uranyl acetate plus Reynolds lead citrate and examined by transmission electron microscopy by using a Philips 201 Transmission Electron Microscope (RIVM, Bilthoven, The Netherlands).
Mucosal Exudation. The bronchial airway lumen exudate was assessed by using Evans blue dye, as described (21). The amount of mucosal leakage in the lavage fluid (μl per lung) was determined by dividing the Evans blue content in the total lavage fluid by that in 1 ml of plasma.
Leukocyte Accumulation in BAL Fluid. Mice were lavaged 24 h after the DNBS challenge and the leukocyte count determined by hemocytometry (11, 22). The percentage of neutrophils, eosinophils, and mononuclear cells (i.e., lymphocytes, macrophages, and monocytes) was estimated by examination of air-dried preparations fixed and stained with hematoxylin/eosin (Diff-Quik, Merz & Dade, Dubingen, Switzerland).
Tracheal Reactivity in Vitro. Assays were performed 24 and 48 h after challenge, as described (11, 22). Isolated nine-ring sections of tracheas were mounted in 10-ml organ baths containing oxygenated Krebs' solution (37°C). Isometrically, cumulative concentration-response curves for carbachol (concentration range: 10-8 to 10-4 M) were determined in vehicle- or DNFB-immunized and DNBS-challenged mice.
Serum-Free κ and λ LC Assays. The concentration of free κ and λ LCs in serum obtained from (age-matched) normal and adult asthmatic individuals (Table 1) was measured by ELISA by using mAbs specific for nonheavy chain bound LCs, as described (23).
Table 1. Characteristics of male individuals aged 18–55 years in combination with atopy defined as one or more positive skin test (wheal ≥5 mm).
| Asthma
|
Unaffected
|
|||
|---|---|---|---|---|
| Characteristics | Nonatopic | Atopic | Nonatopic | Atopic |
| Number | 14 | 17 | 34 | 15 |
| Mean age, years | 30.7 | 35.4 | 36.6 | 31.4 |
| Median FEV1 (I) [range] | 3.6 [2.1–5.5] | 3.4 [1.7–5.5] | 4.3 [2.7–6.7] | 4.4 [2.9–5.9] |
| Median FEV1 % predicted [range] | 83.7 [49–107] | 80.5 [19–155] | 101.5 [77–138] | 99.4 [81–122] |
| More than 9% predicted reversible* (%) [n] | 57.1 [8] | 70.5 [12] | 8.8 [3] | 13.2 [2] |
| C20 ≤ 32 mg/ml (%) [n] | 85.7 [12] | 100 [17] | 0 | 0 |
| Ig E ≥ 120 (%) [n] | 14.3 [2] | 58.8 [10] | 17.6 [6] | 40.0 [6] |
| Ig E ≥ 100 (%) [n] | 14.3 [2] | 64.7 [11] | 20.5 [7] | 46.6 [7] |
FEV1, forced expiratory volume in the first second; PC20, provocative concentration of histamine causing 20% drop in FEV1.; 30-sec methods, i.e. healthy is <32 mg/ml.
Reversibility of airway obstruction after inhalation of 400 μg of salbutamol
Specimens were appropriately diluted to obtain three data points within the linear portion of the standard curves and measured in a double-blind way. Asthmatic patients were all nonsmokers, hyperresponsive to histamine, and had reversible airway obstruction. Healthy controls, matched for age and gender, were all nonsmokers, were not hyperresponsive to histamine, and had normal lung function.
Statistical Analysis. All experiments were designed as completely randomized multifactorials with four to seven mice per group. Maximal contraction (Emax) and -log [carbachol] resulting in 50% of maximal response (pD2) values for carbachol-induced tracheal contraction for each animal were calculated separately by nonlinear least-square regression analysis (simplex minimalization) of the measured contractions vs. carbachol concentration, by using the sigmoid concentration–response relationship and including a threshold value. The following data obtained from individual animals were analyzed by one-way ANOVA followed by a post hoc comparison between groups: mMCP-1 content in blood; mucosal exudation values; and pD2 and Emax values for the carbachol-induced tracheal contractility. The group means ± SEM were calculated and the difference considered significant when P < 0.05. The cellular accumulation in BAL fluid was analyzed by using a distribution-free Kruskal–Wallis one-way ANOVA, and the results are expressed as medians (minimum–maximum). The human free LC data were analyzed by using Grubb's test to detect outliers and ANOVA followed by a post hoc comparison between groups. All data manipulation, nonlinear fittings, ANOVA, and post hoc comparisons were made with prism 3.0 (GraphPad, San Diego).
Results
The LC Antagonist F991 Inhibits the Antigen-Induced Early Phase in Actively Immunized Mice. It has been shown that the Tamm–Horsfall protein, a monomeric renal glycoprotein produced by cells in the ascending loop of Henle, has specific binding affinity for free LCs (19, 24). The 9-mer peptide F991, containing the amino acid residues from position 225 to 233 of the Tamm–Horsfall molecule, was able to bind to different free LC preparations, and the binding is at conserved structural elements within the CDR3 (24). F991 has been shown to antagonize cutaneous LC responses (19).
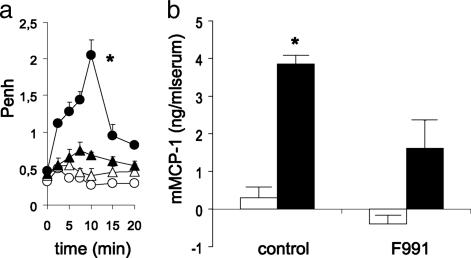
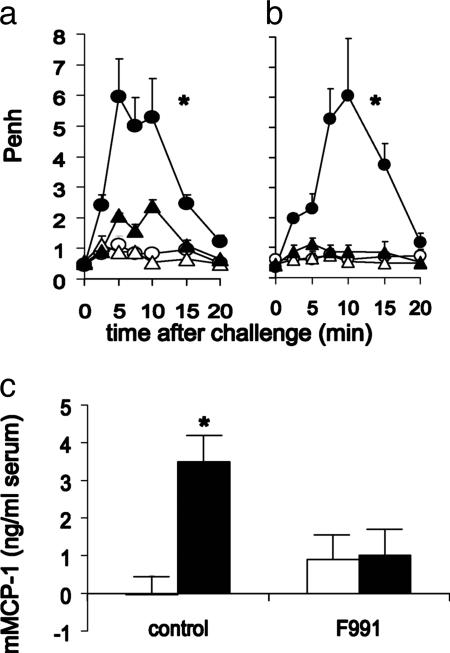
Based on our finding that contact sensitization of BALB/c mice with DNBF resulted in a mast cell-dependent pulmonary hypersensitivity reaction upon intranasal challenge with DNBS (11), we immunized mice with DNFB and found that these animals manifested early signs of bronchoconstriction after exposure to DNBS. This effect was completely blocked when 200-μg amounts of F991 were applied intranasally 30 min before challenge (Fig. 1a). In addition, this treatment also inhibited the DNFB-induced mast cell activation, as assessed by an increase in mMCP-1 serum levels (Fig. 1b).
Fig. 1.
The LC antagonist F991 inhibits antigen-induced early-phase bronchoconstriction and mast cell activation found after intra-airway DNBS challenge of mice actively immunized with DNFB. (a) DNFB- (black circle) but not vehicle- (open circle) immunized mice showed an acute bronchoconstrictive response after challenge. Intranasal application of F991 (200 μg per mouse) 30 min before challenge (black triangle) resulted in a profound reduction of bronchoconstriction in DNFB-immunized mice. Intranasal application of F991 did not influence Penh values of vehicle-immunized mice (open triangle); n = 5–6; *, P < 0.05, for the whole curve. (b) mMCP-1 levels in serum of DNFB-immunized mice (black bars) are enhanced compared with vehicle-immunized (open bars) mice 30 min after challenge. Intranasal application of F991 resulted in inhibition of DNBS-induced increase in mMCP-1 serum levels of DNFB-immunized mice; n = 6; *, P < 0.05.
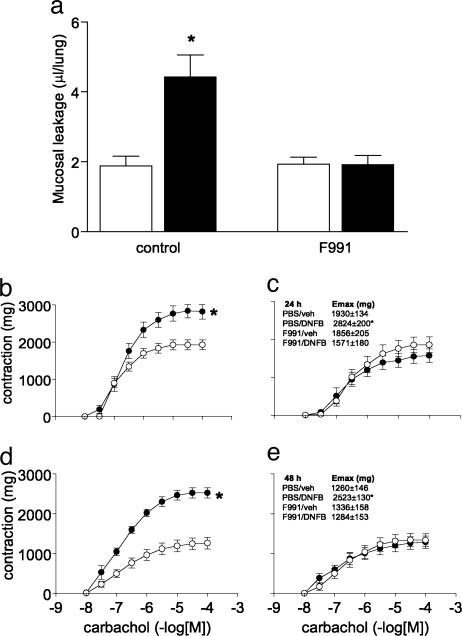
The LC Antagonist F991 Also Inhibits Development of the Antigen-Induced Late Phase in Actively Immunized Mice. The DNFB-induced pulmonary hypersensitivity reaction consists of an early and a late phase, manifested by mucosal exudation, cellular infiltration into BAL fluid, and tracheal hyperreactivity 24–48 h after challenge (11, 21, 22). To determine whether F991 could modulate these delayed responses, mice were injected i.p. with the peptide (50 μg per mouse) 5 min and 24 h before as well as 24 h after the intranasal challenge. This treatment completely inhibited the increased mucosal exudation (Fig. 2a). F991 also prevented the neutrophilic cellular infiltration into the airway lumen (Table 2) and, additionally, blocked the development of tracheal hyperreactivity that characteristically occurs 24–48 h after intranasal DNBS challenge in DNBF-immunized mice (22) (Fig. 2 b–e).
Fig. 2.
The LC antagonist F991 inhibits the development of mucosal exudation, cellular infiltration, and tracheal hyperreactivity associated with the late phase of DNFB-induced pulmonary hypersensitivity reaction in mice. (a) Immunization with DNFB (black bars) results in an increase in mucosal exudation 24 h after challenge. Intraperitoneal pretreatment with F991 (50 μg per mouse, 24 h and 5 min before challenge) prevented the development of DNFB/DNBS-induced mucosal exudation in the airway lumen. F991 did not influence basal mucosal exudation found in vehicle-sensitized mice; n = 6; *, P < 0.05. (b and d) Tracheal hyperreactivity is found in DNFB-immunized mice (black circle) compared with vehicle-immunized (open circle) mice 24 and 48 h after challenge. (b and d) Injections with F991 blocked the development of tracheal hyperreactivity normally found 24 and 48 h after challenge (c and e); n = 5–6; *, P < 0.05, for the whole curve.
Table 2. Effect of F991 on leukocyte accumulation in the lung air spaces 24 h after DNBS challenge of vehicle or DNFB-sensitized BALB/c mice.
| Leukocytes in BAL (cells/lung × 104)
|
|||||
|---|---|---|---|---|---|
| n | DNFB | Total cells | PMN | MONO | |
| PBS | 6 | – | 2.20 (1.22–3.15) | 0.03 (0.01–0.05) | 2.16 (1.14–3.10) |
| 6 | + | 2.19 (1.35–2.71) | 0.13* (0.09–0.24) | 2.06 (1.24–2.60) | |
| F991 | 5 | – | 3.10 (2.01–3.45) | 0.06 (0.03–0.12) | 2.89 (2.49–3.41) |
| 6 | + | 2.70 (1.95–3.15) | 0.05 (0.03–0.08) | 2.64 (1.90–3.07) | |
Leukocytes (PMN, polymorphonuclear cells; MONO, mononuclear cells) were determined in BAL fluid of vehicle (–) or DNFB-sensitized (+) mice. Results are expressed as median (minimum – maximum). Significant differences between vehicle- and DNFB-sensitized groups are indicated (*, P < 0.05 vs. vehicle-sensitized mice).
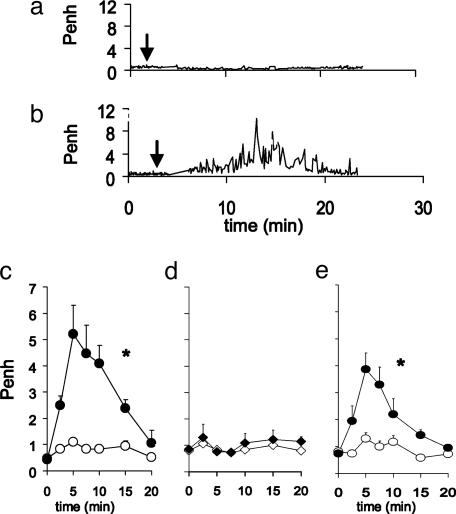
Antigen-Specific LCs Induce in Vivo Bronchoconstriction. To determine whether antigen-specific LCs alone could induce specific pulmonary hypersensitivity, we investigated the effect of intra-airway antigen challenge in LC-immunized mice. Instillation of antigen resulted in profound bronchoconstriction in mice passively immunized with TNP-specific LCs (Fig. 3 a and b). As compared with controls, bronchoconstriction in TNP-specific LC-immunized and antigen-challenged mice was characterized by significant increases in Penh 5–10 min after hapten challenge (maximum Penh: saline/TNBS = 1.11 ± 0.55 and TNP-specific LC/TNBS = 5.95 ± 1.24, P < 0.05) (Fig. 3c). The antigen-specificity of the LC-induced pulmonary response was confirmed in experiments showing that intranasal challenge with the nonrelated antigen OXA-BSA, 30 min after injection of 2 μg TNP-specific LCs, failed to induce bronchoconstriction (Fig. 3d), whereas mice immunized with OXA-specific LCs had a profound bronchospasm upon OXA challenge (data not shown). The possibility that contamination of the LC preparations with intact IgG induced the pulmonary response was excluded by our finding that mice passively immunized with a similar amount of TNP-specific IgG1 (the source of the LCs) failed to result in an airway response after the challenge. Furthermore, mice immunized with recombinant TNP-specific LCs (totally lacking heavy chains) had a similar bronchoconstrictor response after the challenge (Fig. 3e).
Fig. 3.
Antigen-specific LCs mediate acute bronchoconstriction after challenge. (a) Typical individual tracings of bronchoconstriction (Penh recordings) of (b) saline or TNP-specific LC-immunized mouse from -5 to 25 min after challenge. Arrows indicate time of challenge. (c) TNP-specific LC- (black circle) but not vehicle- (open circle) immunized mice showed an acute bronchoconstrictive response after challenge. (d) TNP-specific LC-immunized mice (black diamond) that were challenged with OXA-BSA failed to demonstrate bronchoconstriction. (e) Passive immunization with TNP-specific recombinant LC (black circle) resulted in bronchoconstriction after challenge; n = 4–7; *, P < 0.05, for the whole curve.
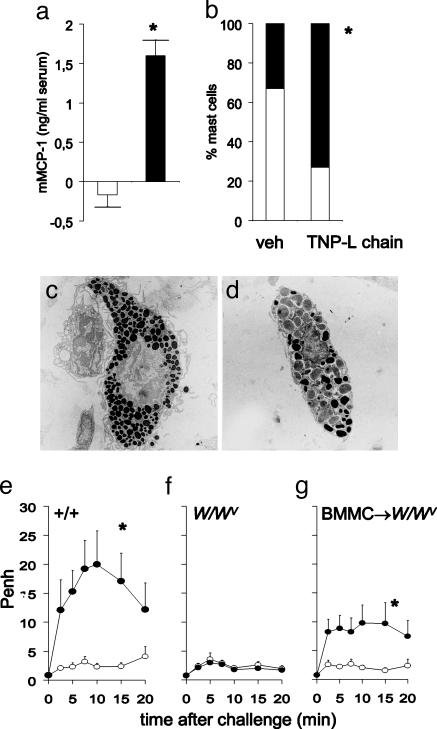
Antigen-Specific LCs Induce in Vivo Pulmonary Mast Cell Activation. In our previous study (11), mast cells were found to play a key role in the development of nonatopic asthma. We now have shown that the acute bronchoconstriction that occurs after intranasal challenge in TNP-specific LC-immunized mice was accompanied by pulmonary mast cell activation (assessed by measuring mMCP-1 serum concentrations) 30 min after intranasal TNBS challenge (Fig. 4a). As expected, there were no significant differences found in mMCP-1 levels in sera from control or TNP-LC immunized mice after intranasal challenge with the nonrelated antigen OXA-BSA [mMCP-1 serum concentrations in vehicle/OXA-BSA and TNP-specific LC/OXA-BSA-immunized animals (n = 6): 0.55 ± 0.69 and 0.55 ± 0.74 ng/ml, respectively].
Fig. 4.
The TNP-specific LC-induced acute bronchoconstriction after challenge is mast cell-dependent. (a) mMCP-1 levels in serum of TNP-specific LC (black bars) are enhanced compared with vehicle-sensitized (open bars) mice 30 min after challenge; n = 6; *, P < 0.05. (b) Mast cells in tracheal sections of mice passively immunized with TNP-specific LC showed degranulation after reexposure to antigen when compared with vehicle-sensitized mice; n = 4; *, P < 0.05 (black, degranulated and white, nondegranulated mast cells). Electron microscopy reveals degranulation characterized by swelling of intracytoplamic granules and decrease of electron density of granules. (c) Tracheal mast cell from vehicle-immunized and challenged mouse. (d) Tracheal mast cell from TNP-specific LC-immunized and challenged mouse. (e) The acute bronchoconstriction found in TNP-specific LC-immunized mice is mast cell-dependent. TNP-specific LC- (black circle) but not vehicle- (open circle) immunized congenic +/+ mice showed an acute bronchoconstrictive response after challenge. (f) TNP-specific LC-injected mast cell-deficient W/Wv mice failed to demonstrate bronchoconstriction after challenge. (g) Selective reconstitution of mast cells in mast cell-deficient mice (BMMC→W/Wv) restored LC-mediated acute bronchoconstriction; n = 5–6; *, P < 0.05, for the whole curve.
Pulmonary mast cell activation was also evidenced by the light and electron microscopic findings of acute degranulation in TNP-specific LC-immunized and TNBS-challenged mice (Fig. 4 b–d). In treated animals, most of the intracytoplasmic granules were swollen and had decreased electron density (Fig. 4d) in contrast to control mice (Fig. 4c).
To investigate the importance of mast cell activation for development of the LC-induced acute bronchoconstriction, we used mast cell-deficient (W/W/v) and reconstituted (BMMC→W/W/v) mice. TNP-specific LC immunization and TNBS challenge resulted in a profound bronchoconstriction in control (+/+) animals (Fig. 4e); however, in contrast to the mast cell-reconstituted animals, after the challenge, the W/Wv mice did not have this response (Fig. 4 f and g).
The LC Antagonist F991 Inhibits the Antigen-Specific Pulmonary Responses in Passively Immunized Mice. To determine whether F991 was able to block the effect of LC immunization, we injected (i.v.) mice with TNP-specific LCs (2 μg per mouse) followed by F991 (200 μg per mouse), and 30 min later the Penh response was monitored after intranasal challenge with TNBS. Injection of LCs together with F991 resulted in a greatly reduced bronchoconstriction, as compared with vehicle/LC-immunized animals (Fig. 5a). The Penh increase in F991-treated mice was only 15–20% of that observed in control animals. In subsequent experiments, we investigated whether F991 could be applied locally to effect site-specific inhibition of LC immunization. After i.v. injection of TNP-specific LCs into mice, a 200-μg dose of F991 was immediately administered intranasally. Treatment with this agent completely blocked TNP-specific LC-induced bronchoconstriction typically found 30 min after TNBS challenge (Fig. 5b). Moreover, intranasal application of F991 resulted in an inhibition of TNBS-induced mast cell activation, as evidenced by a decrease in serum mMCP-1 concentrations in TNP-specific LC-immunized mice (Fig. 5c).
Fig. 5.
The LC antagonist F991 inhibits bronchoconstriction and mast cell activation found after challenge of mice immunized with TNP-specific LCs. TNP-specific LC- (black circle) but not vehicle- (open circle) immunized mice showed an acute bronchoconstrictive response after challenge. (a) Simultaneous injection of F991 (200 μg per mouse) and TNP-specific LCs (black triangle) resulted in a profound reduction of bronchoconstriction. (b) Intranasal administration of F991 (200 μg per mouse) (black triangle) completely inhibited the TNP-specific LC-induced bronchoconstriction. i.v. or intranasal application of F991 did not influence Penh values of vehicle-immunized mice (open triangle); n = 5–6; *, P < 0.05, for the whole curve. (c) mMCP-1 levels in serum of TNP-specific LCs (black bars) are enhanced compared with vehicle-sensitized (open bars) mice 30 min after challenge. Intranasal application of F991 (200 μg per mouse) resulted in inhibition of increases in mMCP-1 serum levels of TNP-specific LC-immunized mice; n = 6; *, P < 0.05.
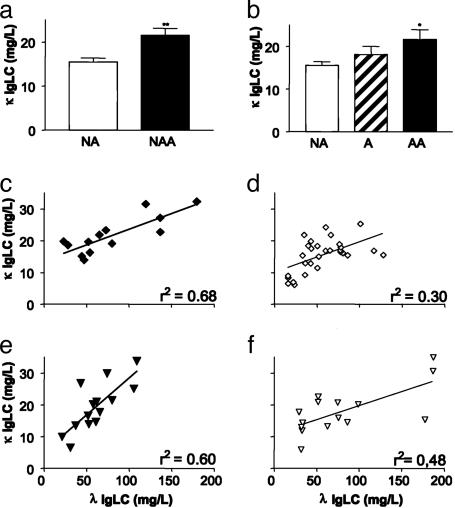
Quantification of Serum LC Levels in Asthma Patients and Healthy Individuals. Serum samples from 14 nonatopic and 17 atopic adult male asthmatics and 34 nonatopic and 15 atopic males who had no manifestation of asthma were randomly selected from a large specimen bank (see Table 1) for quantitative free κ and λ LC assays. We found that the serum concentration of free κ LCs was significantly increased in nonatopic as well as atopic asthmatics, as compared with nonasthmatic individuals (Fig. 6 a and b). In contrast, the serum free λ LC contents in the asthma groups were not significantly different compared with the nonasthmatic. However, nonatopic asthmatics (Fig. 6c), and to a lesser extent atopic asthmatics (Fig. 6e), revealed a significantly higher correlation between λ and κ levels than healthy controls (Fig. 6 d and f).
Fig. 6.
Concentrations of κ and λ LC in serum of nonatopic and atopic adult asthma patients and nonatopic and atopic nonasthmatic subjects. (a) κ LC serum levels in nonatopic adult asthmatics (NAA; n = 14, black bar) compared with nonatopic healthy controls (NA; n = 34, open bar); **, P = 0.001. (b) κ LC serum levels in atopic adult asthmatics (AA; n = 17, black bar) compared with atopic nonasthmatics (A; n = 15, hatched bar) and nonatopic healthy controls (NA; n = 34, open bar); *, P = 0.023 compared with nonatopic healthy controls. (c) Correlation of λ LC vs. κ LC of nonatopic asthma patients (black diamond). (d) Correlation of λ LC vs. κ LC of nonatopic healthy individuals (open diamond). (e) Correlation of λ LC vs. κ LC of atopic asthma patients (black triangle). (f) Correlation of λ LC vs. κ LC of atopic nonasthmatic individuals (open triangle). r2, measure of goodness of fit of linear regression.
Discussion
Based on our previous studies, we hypothesized that antigen-specific LCs were crucial humoral factors in the activation of mast cells in nonatopic adult asthma (11, 19). To test this hypothesis, we have used an experimental murine model that has the characteristics of human nonatopic asthma, namely, neutrophil-associated mast cell-dependent airway inflammation, obstruction, and pulmonary hyperreactivity that occur in the absence of a hyperIgE response (10, 11, 21, 25). The LC antagonist F991 completely inhibited both the early and delayed phase of DNFB-induced pulmonary hypersensitivity. F991 has been demonstrated to have binding affinities for free LCs (19, 24). Therefore, we attribute the therapeutic efficacy of this compound to its interaction with free LCs, thus preventing these molecules to effect mast cell degranulation. The role of LCs in asthma pathogenesis was further evidenced by our demonstration that TNP-specific LCs could passively transfer a hypersensitivity-like pulmonary response. LC-immunized animals that subsequently received an airway challenge developed antigen-specific and mast cell-dependent acute bronchoconstriction. This effect also could be completely abrogated by pretreatment with the LC-antagonist F991.
Our experimental data have also shown that pulmonary mast cells were activated in vivo after TNP-specific LC immunization and intra-airway TNBS challenge. The studies in mast cell-deficient and mast cell-reconstituted BMMC→W/WV mice confirmed the pivotal role of these cells in pulmonary LC-mediated immune responses. We presume that mast cell activation resulted from crosslinking of receptor-bound LCs, a process that induced degranulation and arachidonic acid metabolite production (19). In studies involving knockout mice, we have shown that γ chain-associated receptors such as FcεRI, FcεRIII, and PIR-A are not involved in LC-induced mast cell activation or bronchoconstriction (ref. 19 and A.D.K., M.K., and F.A.R., unpublished results). Mast cells also have been implicated in regulating the non-IgE immune response involved in skin and lung tissue inflammation (11, 25–28). Specifically, these cells seemingly are involved in asthma pathogenesis (3–5), as evidenced by their increased number in the lungs of patients with nonatopic as well as atopic disease (6, 7).
Our finding that the concentration of κ (but not λ) free LCs is significantly increased in the sera of nonatopic and atopic adult asthma patients, as compared with nonasthmatic age-matched healthy subjects, presumably reflects a polyclonal B cell response. Increased levels of free LCs have also been noted in certain immune disorders such as multiple sclerosis and rheumatoid arthritis (29–31), in which mast cells have been implicated in disease pathogenesis (32–35). In future studies, it remains to be established whether these elevated levels of antigen-specific LCs are causally related to the pathogenesis of the disease. In addition, it will be of great importance to assess whether increases in antigen-specific LCs in sera of asymptomatic individuals can be detected and associated with the future development of asthma.
Conclusion
Our studies have provided evidence that LC-induced hypersensitivity may be of importance in the pathogenesis of asthma, especially in those individuals in whom there is no clear-cut role for a hyper IgE response, i.e., nonatopy. The delineation of a population of patients with LC-induced asthma has potential therapeutic relevance, in that administration of F991 or other LC antagonists may prevent or ameliorate the adverse manifestation in patients with this often incapacitating and occasionally fatal bronchopulmonary disease.
Acknowledgments
We thank C. Vallinga, S. de Jager, and A. Weitenberg for expert technical assistance and L. Wong for performing the electron microscopy studies. This work was supported in part by GlaxoSmithKline Nederland BV (Zeist, The Netherlands) and Fornix Biosciences (Lelystad, The Netherlands), U.S. Public Health Service Grant CA10056 from the National Cancer Institute, and the Aslan Foundation. A.S. is an American Cancer Society Clinical Research Professor.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: mMCP-1, mouse mast cell protease 1; LC, Ig free light chain; DNFB, dinitrofluorobenzene; DNBS, dinitrobenzene sulfonic acid; TNP, trinitrophenol; TNBS, trinitrobenzene sulfonic acid; OXA, oxazolone; OXA-BSA, OXA coupled with BSA; BMMC, bone marrow-derived mast cells; BAL, bronchoalveolar lavage.
See Commentary on page 1267.
References
- 1.Pearce, N., Pekkanen, J. & Beasly, R. (1999) Thorax 54, 268-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Douwes, J., Gibson, P., Pekkanen, J. & Pearce, N. (2002) Thorax 57, 643-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holgate, S. T., Godfrey, R. C. & Church, M. K. (1993) in Allergy, Principles and Practice, eds. Middleton, E., Reed, C. E., Ellis, E. F., Atkinson, N. F., Yuniger, J. W. & Busse, W. W. (Mosby, St. Louis), pp. 267-301.
- 4.Galli, S. J. (1997) J. Exp. Med. 186, 343-347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galli, S. J. & Costa, J. J. (1995) Allergy 50, 851-862. [DOI] [PubMed] [Google Scholar]
- 6.Amin, K., Ludviksdottir, D., Janson, C., Nettelbladt, O., Bjornsson, E., Roomans, G. M., Boman, G., Seveus, L. & Venge, P. (2000) Am. J. Respir. Crit. Care Med. 162, 2295-2301. [DOI] [PubMed] [Google Scholar]
- 7.Carroll, N. G., Mutavdzic, S. & James, A. L. (2002) Thorax 57, 677-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehlhop, P. D. van de Rijn, M., Goldberg, A. B., Brewer, J. P., Kurup, V. P., Martin, T. R. & Oettgen, H. C. (1997) Proc. Natl. Acad. Sci. USA 94, 1344-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hamelmann, E., Cieslewicz, G., Schwarze, J., Ishizuka, T., Joetham, A., Heusser, C. & Gelfand, E. W. (1999) Am. J. Respir. Crit. Care Med. 160, 934-941. [DOI] [PubMed] [Google Scholar]
- 10.Garssen, J., Nijkamp, F. P., Wagenaar, S. S., Zwart, A., Askenase, P. W. & Van Loveren, H. (1989) Immunology 68, 51-58. [PMC free article] [PubMed] [Google Scholar]
- 11.Kraneveld, A. D., van der Kleij, H. P., Kool, M., van Houwelingen, A. H., Weitenberg, A. C., Redegeld, F. A. & Nijkamp, F. P. (2002) J. Immunol. 169, 2044-2053. [DOI] [PubMed] [Google Scholar]
- 12.Oettgen, H. C., Martin, T. R., Wynshaw-Boris, A., Deng, C., Drazen, J. M. & Leder, P. (1994) Nature 370, 367-370. [DOI] [PubMed] [Google Scholar]
- 13.Fahy, J. V., Fleming, H. E., Wong, H. H., Liu, J. T., Su, J. Q., Reimann, J., Fick, R. B., Jr., & Boushey, H. A. (1997) Am. J. Respir. Crit. Care Med. 155, 1828-1834. [DOI] [PubMed] [Google Scholar]
- 14.Boulet, L. P., Chapman, K. R., Cote, J., Kalra, S., Bhagat, R., Swystun, V. A., Laviolette, M., Cleland, L. D., Deschesnes, F., Su, J. Q., et al. (1997) Am. J. Respir. Crit. Care Med. 155, 1835-1840. [DOI] [PubMed] [Google Scholar]
- 15.Fahy, J. V., Cockcroft, D. W., Boulet, L. P., Wong, H. H., Deschesnes, F., Davis, E. E., Ruppel, J., Su, J. Q. & Adelman, D. C. (1999) Am. J. Respir. Crit. Care Med. 160, 1023-1027. [DOI] [PubMed] [Google Scholar]
- 16.Casale, T. B., Bernstein, I. L., Busse, W. W., LaForce, C. F., Tinkelman, D. G., Stoltz, R. R., Dockhorn, R. J., Reimann, J., Su, J. Q., Fick, R. B., Jr., et al. (1997) J. Allergy Clin. Immunol. 100, 110-121. [DOI] [PubMed] [Google Scholar]
- 17.Milgrom, H., Fick, R. B., Jr., Su, J. Q., Reimann, J. D., Bush, R. K., Watrous, M. L. & Metzger, W. J. (1999) N. Engl. J. Med. 341, 1966-1973. [DOI] [PubMed] [Google Scholar]
- 18.Salvi, S. S & Babu, K. S. (2000) N. Engl. J. Med. 342, 1292-1293. [DOI] [PubMed] [Google Scholar]
- 19.Redegeld, F. A., van der Heijden, M. W., Kool, M., Heijdra, B. M., Garssen, J., Kraneveld, A. D., Van Loveren, H., Roholl, P., Saito, T., Verbeek, J. S., et al. (2002) Nat. Med. 8, 694-701. [DOI] [PubMed] [Google Scholar]
- 20.Sun, M., Li, L., Gao, Q. S. & Paul, S. (1994) J. Biol. Chem. 269, 734-738. [PubMed] [Google Scholar]
- 21.Buckley, T. L. & Nijkamp, F. P. (1994) J. Immunol. 153, 4169-4178. [PubMed] [Google Scholar]
- 22.Buckley, T.L. & Nijkamp, F. P. (1994) Am. J. Respir. Crit. Care Med. 149, 400-407. [DOI] [PubMed] [Google Scholar]
- 23.Abe, M., Goto, T., Kosaka, M., Wolfenbarger, D., Weiss, D. T. & Solomon, A. (1998) Clin. Exp. Immunol. 111, 457-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang, Z. Q. & Sanders, P. W. (1997) J. Clin. Invest. 99, 732-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biedermann, T., Kneilling, M., Mailhammer, R., Maier, K., Sander, C. A., Kollias, G., Kunkel, S. L., Hultner, L. & Rocken, M. (2000) J. Exp. Med. 192, 1441-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Askenase, P. W., Bursztajn, S., Gershon, M. D. & Gershon, R. K. (1980) J. Exp. Med. 152, 1358-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Houwelingen, A. H., Kool, M., de Jager, S. C., Redegeld, F. A., van Heuven-Nolsen, D., Kraneveld, A. D. & Nijkamp, F. P. (2002) J. Immunol. 168, 5297-5302. [DOI] [PubMed] [Google Scholar]
- 28.Askenase, P. W., Van Loveren, H., Kraeuter-Kops, S., Ron, Y., Meade, R., Theoharides, T. C., Nordlund, J. J., Scovern, H., Gerhson, M. D. & Ptak, W. (1983) J. Immunol. 131, 2687-2694. [PubMed] [Google Scholar]
- 29.Fagnart, O. C., Sindic, C. J. & Laterre, C. (1988) J. Neuroimmunol. 19, 119-132. [DOI] [PubMed] [Google Scholar]
- 30.Solling, K., Solling, J. & Romer, F. K. (1981) Acta Med. Scand. 209, 473-477. [DOI] [PubMed] [Google Scholar]
- 31.Redegeld, F. A. M. & Nijkamp, F. P. (2003) Trends Immunol. 24, 181-185. [DOI] [PubMed] [Google Scholar]
- 32.Secor, V. H., Secor, W. E., Gutekunst, C. A. & Brown, M. A. (2000) J. Exp. Med. 191, 813-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, D. M., Friend, D. S., Gurish, M. F., Benoist, C., Mathis, D. & Brenner, M. B. (2002) Science 297, 1689-1692. [DOI] [PubMed] [Google Scholar]
- 34.Rozniecki, J. J., Hauser, S. L., Stein, M., Lincoln, R. & Theoharides, T. C. (1995) Ann. Neurol. 37, 63-66. [DOI] [PubMed] [Google Scholar]
- 35.Marone, G. (1998) Clin. Exp. Rheumatol. 16, 245-249. [PubMed] [Google Scholar]