Abstract
Background:
The purpose of this systematic review and meta-analysis of randomized controlled trials (RCTs) was to evaluate whether intravenous steroids would result in reduced acute pain and postoperative nausea and vomiting (PONV) among patients undergoing total knee arthroplasty (TKA).
Methods:
Electronic databases, including PubMed, Embase, Web of Science, and the Cochrane Library, were searched to identify articles published from database inception to July 2016. RCTs that compared the effects of intravenous steroids with the effects of placebo among patients undergoing TKA were included in this meta-analysis. The primary outcomes were visual analogue scale (VAS) scores after 12, 24, and 48 hours of rest and PONV incidence. The secondary outcomes were blood glucose levels and incidence of infection. We calculated the risk ratio (RR) with its corresponding 95% confidence interval (CI) for dichotomous outcomes and the mean difference (MD) with its corresponding 95% CI for continuous outcomes.
Results:
Seven clinical trials involving 434 patients were included in the final meta-analysis. The pooled results indicated that intravenous steroids were associated with decreased VAS scores after 24 hours (MD = −10.21, 95%CI = −18.80 to −1.63, P = .020) and 48 hours (MD = −2.60, 95%CI = −4.70 to −0.50, P = .015) of rest. Moreover, intravenous steroids were also associated with decreased risk of nausea (RR = 0.58, 95% CI 0.44–0.77, P = .000) and vomiting (RR = 0.46, 95% CI = 0.24–0.88, P = .019). However, intravenous steroids were also associated with increased blood glucose levels. No significant difference in the risk of infection was identified between the 2 groups.
Conclusion:
Intravenous steroids may be associated with decreased pain intensity and decreased risk of nausea and vomiting during the early period following TKA. However, evidence supporting its use is limited by the low quality of and variations in dosing regimens between the included RCTs. Thus, more high-quality RCTs are needed to identify the optimal drug administration intervals for maximal pain control.
Keywords: meta-analysis, steroids, total knee replacement
1. Introduction
Total knee arthroplasty (TKA) has been identified as one of the most effective surgeries for knee arthritis.[1] The number of primary TKA procedures is expected to reach 3.48 million in the United States in 2030, demonstrating an 8-fold increase from 2005.[2] However, many patients experience moderate to severe pain during the early postoperative period, as the surgery involves extensive bone resection.[1,3,4] Furthermore, many patients suffer from postoperative nausea and vomiting (PONV) due to requiring rescue anesthesia and undergoing surgery.[5–7] PONV is often cited as the most common adverse event after anesthesia, with an incidence of 20% to 83% after major orthopedic surgery.[5,8] Severe pain and PONV may be associated with increased length of hospital stay and patient dissatisfaction. At present, no gold standard protocols for the reduction of pain intensity without increasing PONV have been identified. Previous studies have reported that postsurgical serum levels of cytokine interleukin-6 (IL-6) and C-reactive protein (CRP) may be elevated.[9,10] Steroids may be associated with decreased levels of IL-6 and CRP and may, thus, relieve the pain associated with numerous procedures.[11] Several studies have compared the efficacy of adjuvant steroids as a component of multimodal anesthesia after TKA. However, previous results should be interpreted cautiously due to a lack of robustness in and heterogeneity across these studies. The purpose of this systematic review and meta-analysis of randomized controlled trials (RCTs) was to perform an in-depth analysis of the efficacy and safety of steroids by examining their impact on pain control and reduction of PONV after TKA.
2. Materials and methods
This systematic review was registered in PROSPERO (PROSPERO 2016: CRD42016049244) and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.
2.1. Search strategy
Two reviewers (L-ZX and L-L) searched electronic databases, including PubMed, Embase, Web of Science, and the Cochrane Library, since inception to July 26th, 2016 to identified relevant articles. A combination of exploded medical subject headings and appropriate or corresponding terms were used to improve the specificity and sensitivity of the search, including TKA, total knee replacement, TKA, TKR, “Arthroplasty, Replacement, Knee” [Mesh], steroid, glucocorticoid, dexamethasone, and “Steroids” [Mesh]. Boolean operators (AND and OR) were employed. Only published articles that had been originally written in English or translated into English were considered. In addition, the references of relevant reviews and included studies were searched to identify articles potentially missing from the internet databases. No animal experiments or direct human trials were involved in this meta-analysis, and, as such, neither a special ethics review nor ethical approval was necessary.
2.2. Eligibility criteria and study selection
-
(1)
Participants, patients diagnosed with osteoarthritis or rheumatoid arthritis undergoing primary TKA.
-
(2)
Intervention, perioperative intravenous steroids for pain control.
-
(3)
Comparison, placebo or intravenous saline.
-
(4)
Outcomes, visual analogue scale (VAS) scores after 12, 24, and 48 hours of rest, incidence rates of PONV and infection, and blood glucose levels.
-
(5)
Study design, only RCTs.
The included studies were required to report at least 1 outcome. All searches were limited to human subjects, and no language restriction was employed. Eligible studies were assessed independently by 2 authors. In cases of disagreement, the 2 authors reached consensus through discussion.
2.3. Data extraction
A standard data extraction form was designed using Microsoft Excel, and data were independently extracted from the included studies by 2 reviewers (L-ZX and Y-LW). The following data were extracted: author, publication year, sample size, age, gender, doses, and intervals at which steroids were administered, type of anesthesia, follow-up strategy, and outcomes. The primary endpoints included the following: VAS pain score after 12, 24, and 48 hours of rest (0 indicated “no pain” and 10 indicated the “worst imaginable pain”); and incidence of nausea and vomiting. The secondary outcomes included length of hospital stay, total blood loss, incidence of infection, and blood glucose levels upon arrival at the postanesthesia care unit (PACU) and on postoperative day 1. When necessary, we contacted the corresponding authors of the included studies to confirm whether the data met our inclusion criteria. The 10-point VAS score was converted to a 100-point VAS score. Data in other forms (ie, median, interquartile range, and mean ± 95% confidence interval [CI]) were converted to mean ± SD according to the Cochrane Handbook guidelines.[12] We extracted data that were not reported numerically from the published figures using “GetData Graph Digitizer” software. The data were extracted independently by 2 reviewers (BC and Y-LZ). In cases of disagreement, a consensus was reached by consulting a senior reviewer or through discussion.
2.4. Quality assessment
A quality assessment was performed using the Cochrane risk of bias tool. This tool assesses the following 5 domains: random sequence generation, allocation concealment, blinding to participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and the baseline imbalance bias. These domains were categorized as having a low risk of bias, a high risk of bias, or an unclear risk of bias. Studies with a high risk of bias in 1 or more domains were categorized as having a high risk of bias. Studies with a low risk of bias in all domains were categorized as having a low risk of bias. Otherwise, the studies were categorized as at an unclear risk of bias.
2.5. Quality of evidence assessment
The quality of the evidence supporting the results was evaluated using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology,[13] and summary tables were constructed using GRADEpro 3.6. The 5 assessed domains using this tool were the risk of bias, inconsistency, indirectness, imprecision, and publication bias.[13,14] The results were categorized as having a high, moderate, low, or very low quality to assess their importance. Two reviewers (L-ZX and Y-LW) independently performed this evaluation. When consensus could not be reached, a 3rd reviewer (L-ZX) was consulted.
2.6. Statistical analyses
Stata 12.0 (Stata Corp., College Station, TX) was used to perform the meta-analyses. The mean difference (MD) and its corresponding 95% CI were utilized to assess the following continuous variable outcomes: VAS pain score at 12, 24, and 48 hours of rest and blood glucose level. The dichotomous outcomes (the incidence of PONV and infection) are presented as risk ratios (RRs) with a 95% CIs.
Heterogeneity across studies was assessed based on I2 values. An I2 value <50% indicated a low level of heterogeneity across studies, and a fixed-effects model was used for these variables. Otherwise, a random-effects model was used for the purposes of this meta-analysis. To further analyze the level of heterogeneity across studies, sensitivity and subgroup analyses were performed to determine the source of heterogeneity. Egger linear regression test and funnel plots were used to evaluate publication bias. The relationship between steroid dosage and the incidence of nausea was explored using SPSS software. A correlation coefficient (r) was generated to evaluate the relationship between the steroids dosage and the incidence of nausea.
3. Results
3.1. Search results and quality assessment
A total of 375 studies were identified during the initial search (PubMed = 121, Embase = 98, Web of Science = 90, and Cochrane Library = 66), of which 154 were excluded due to duplication and 213 were excluded because they did not meet the eligibility criteria based on a review of their titles and abstracts. After reviewing the full texts for the remaining 8 studies, 1 study[15] was excluded because it assessed the application of steroids for joint arthroplasty. Finally, 7 clinical trials involving 434 patients were included in this meta-analysis.[16–22] The study selection methodology is described in Fig. 1. Overall, 222 and 221 patients were included in the steroids group and control group, respectively. The participants were all elderly patients (aged 57–72 years old) undergoing TKA. The ratio of males to females was equal in each of the included studies. The types of intraoperative anesthesia included general anesthesia, spinal anesthesia, and spinal-epidural anesthesia. The dose of steroids utilized in each study was converted to the equivalent dose of dexamethasone and ranged from 4 to 25 mg. All of the general characteristics are exhibited in Table 1. The quality assessment is shown in Figs. 2 and 3. Only 2 studies did not describe random sequence generation; these studies were assessed as having an unclear risk of bias.[19,20] Only 1 study was assessed as having an unclear risk of bias due to incomplete outcome data.[17] All of the remaining studies were determined to have a low risk of bias. We rated 2 (28.6%) RCTs as having an unclear risk in the random sequence generation domain, and 1 (14.3%) RCT was rated as having unclear risk in the incomplete outcome data domain.
Figure 1.
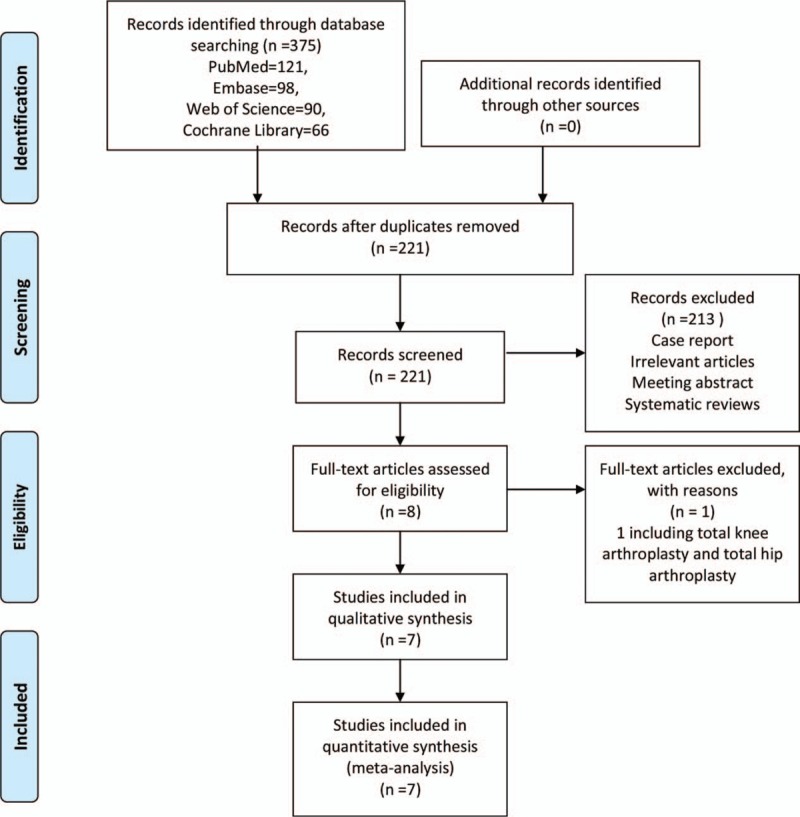
Study selection flowchart.
Table 1.
The general characteristics of the included studies.
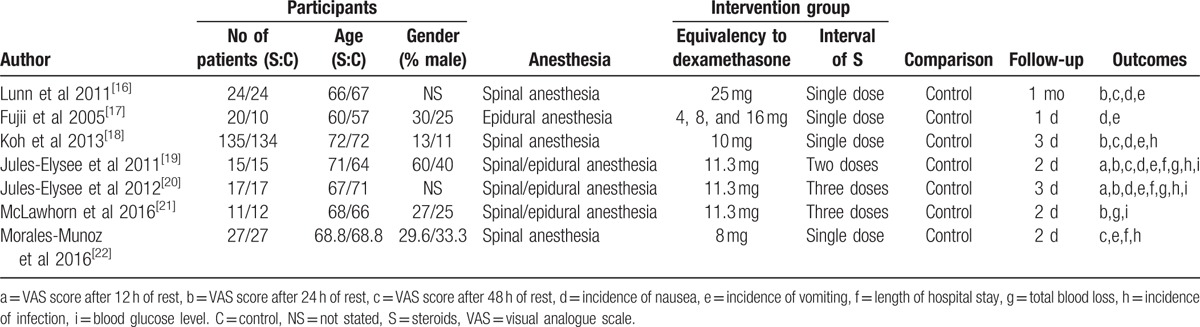
Figure 2.
Summary of the risk of bias.
Figure 3.
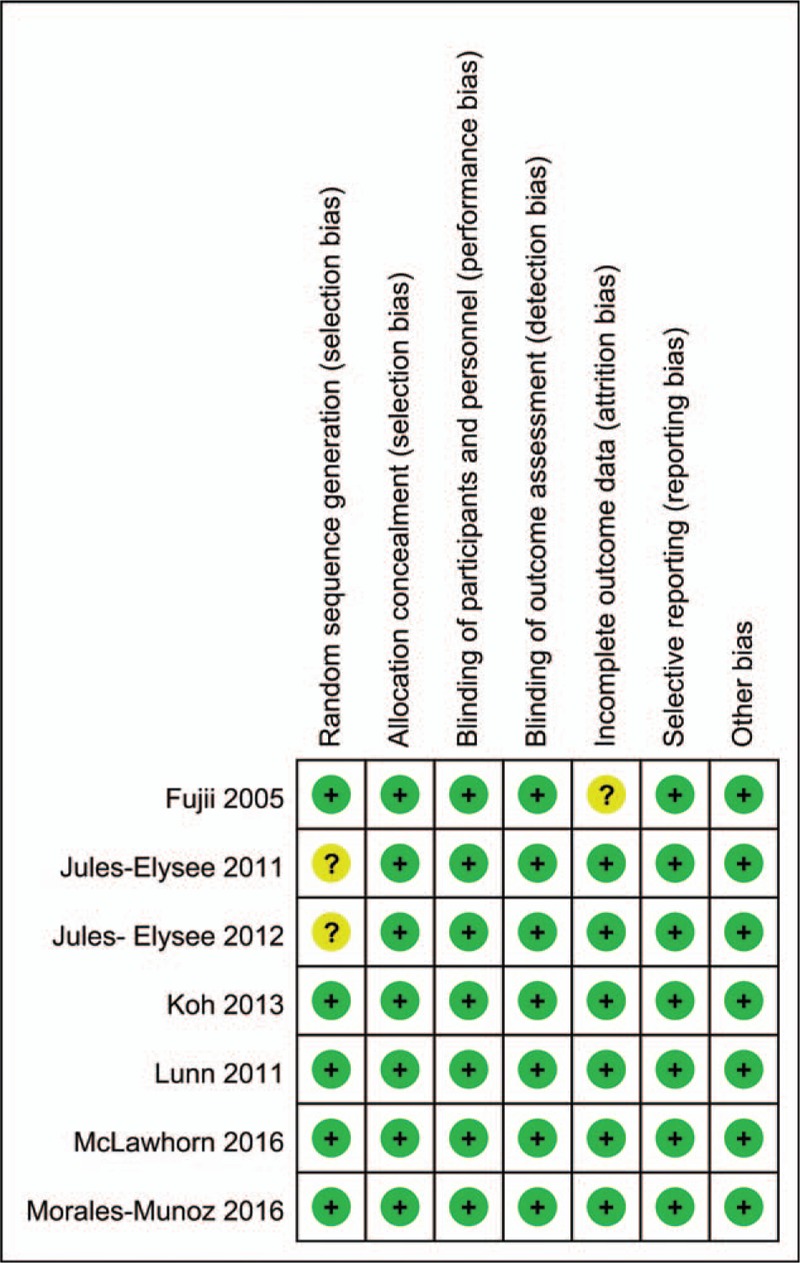
Graph of the risk of bias.
3.2. Quality of evidence assessment
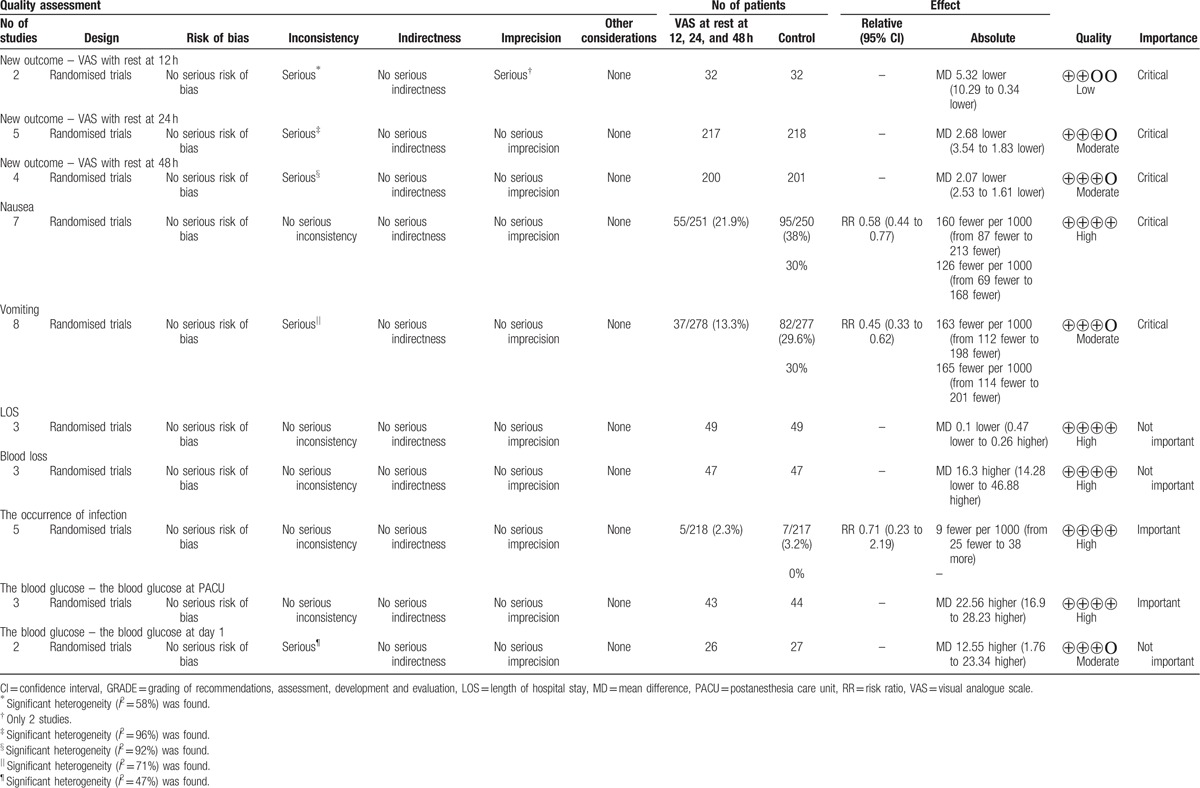
A summary of the quality of the evidence according to the GRADE approach is shown in Table 2. The GRADE level of evidence was determined to be low for VAS after 24 hours of rest, moderate for VAS after 12 and 48 hours of rest, and high for the incidence of nausea and vomiting.
Table 2.
Analysis and quality of the evidence using GRADE; LOS, VAS.
3.3. Primary outcomes
3.3.1. VAS scores after 12, 24, and 48 hours of rest
No significant difference in VAS score at 12 hours post-TKA (MD = −6.59, 95%CI = −15.23–2.05, P = .135, Fig. 4) was identified between the steroid and control groups, and a high level of heterogeneity was observed (I2 = 58.4%, P = .121). Five studies involving 435 patients reported VAS scores after 24 hours of rest.[16,18–20,22] The pooled results indicated that IV steroids were associated with decreased VAS scores at 24 hours (MD = −10.21, 95%CI = −18.80 to −1.63, P = .020, Fig. 4), and a high level of heterogeneity was identified (I2 = 95.9%, P = .000). Four studies[16,18,19,22] assessed VAS scores after 48 hours; the results of these studies indicated that steroids were associated with decreased VAS scores after 48 hours of rest (MD = −2.60, 95%CI = −4.70 to −0.50, P = .015, Fig. 4), and a high level of heterogeneity was observed (I2 = 89.6%, P = .000).
Figure 4.
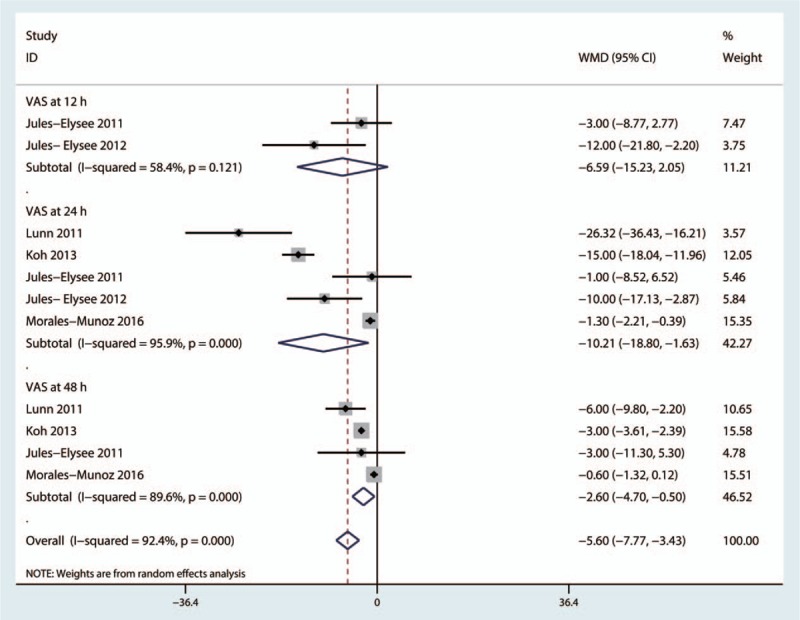
Forest plot comparing visual analogue scale (VAS) scores between the 2 groups after 12, 24, and 48 hours of rest.
Due to the high level of heterogeneity identified across the included studies, a sensitivity analysis was performed to determine the sources of the observed heterogeneity. The results of the sensitivity analysis for the effects of steroids on VAS scores at 12, 24, and 48 hours indicated that neither the direction of the steroid effect nor the level heterogeneity changed (Fig. 5). A subgroup analysis was conducted to determine the effects of high dose and low dose of steroids (higher than 10 mg and less than 10 mg, respectively). These results are presented in Table 3. A funnel plot was generated and Egger test was performed to assess publication bias. The funnel plot (Fig. 6) and Egger test results (Fig. 7) indicated that no publication bias was observed.
Figure 5.
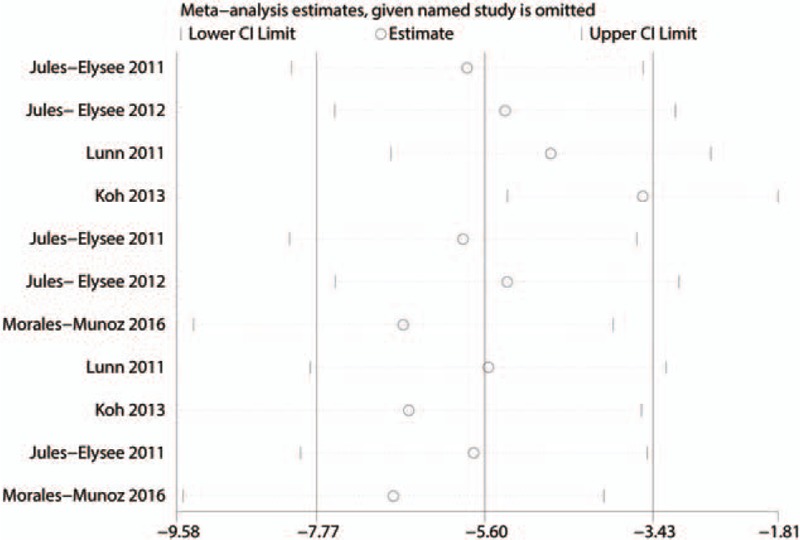
Sensitivity analysis for the differences in visual analogue scale (VAS) scores between the 2 groups after 12, 24, and 48 hours of rest.
Table 3.
Subgroup analysis for VAS with rest at 24 and 48 h.
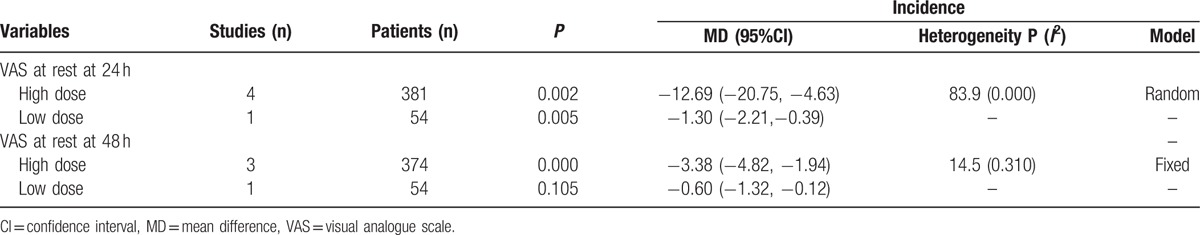
Figure 6.
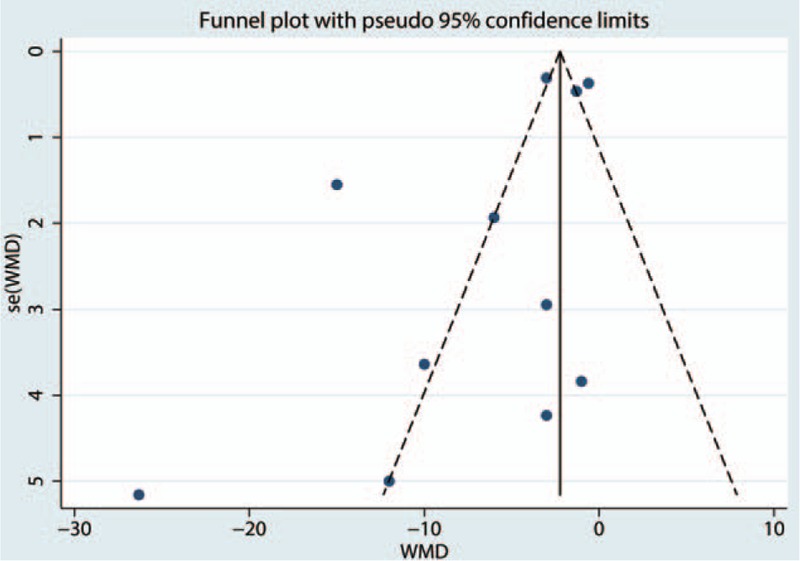
Funnel plot of visual analogue scale (VAS) scores after 12, 24, and 48 hours of rest.
Figure 7.
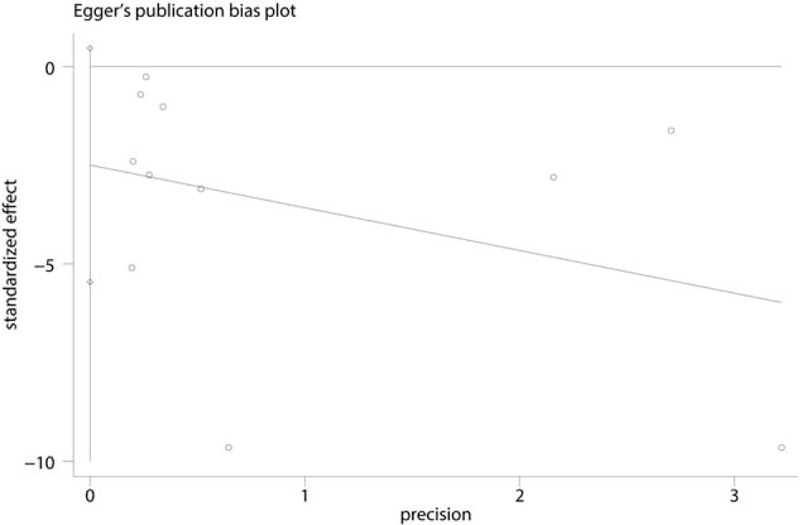
Egger test for visual analogue scale (VAS) scores after 12, 24, and 48 hours of rest.
3.3.2. Nausea
The incidence of postoperative nausea was reported by 5 studies.[16,18–20,22] The results of the meta-analysis indicated that intravenous steroids were associated with decreased risk of nausea (RR = 0.58, 95%CI = 0.44–0.77, P = .000, Fig. 8), and a low level of heterogeneity was identified (I2 = 38.1%, P = .138). A subgroup analysis was performed to compare the effects of a nerve block versus no nerve block. The results (presented in Fig. 9) indicated that patients with a nerve block had less nausea than patients without a nerve block.
Figure 8.
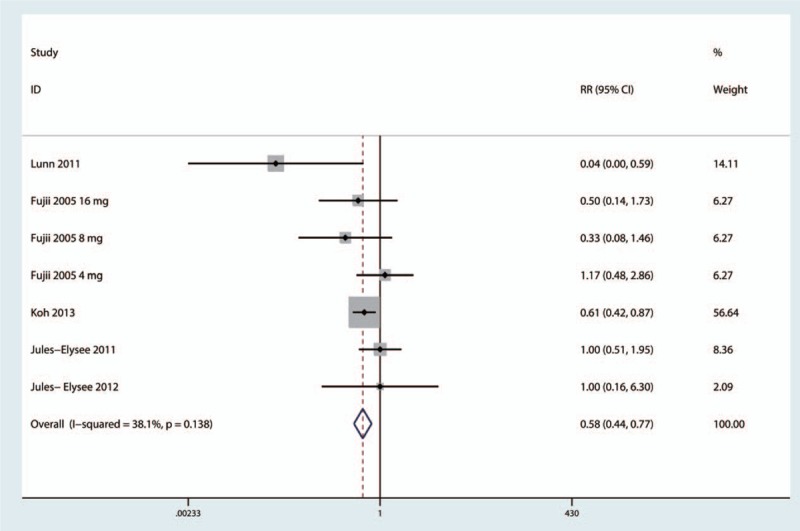
Forest plot comparing the incidence of nausea between the 2 groups.
Figure 9.
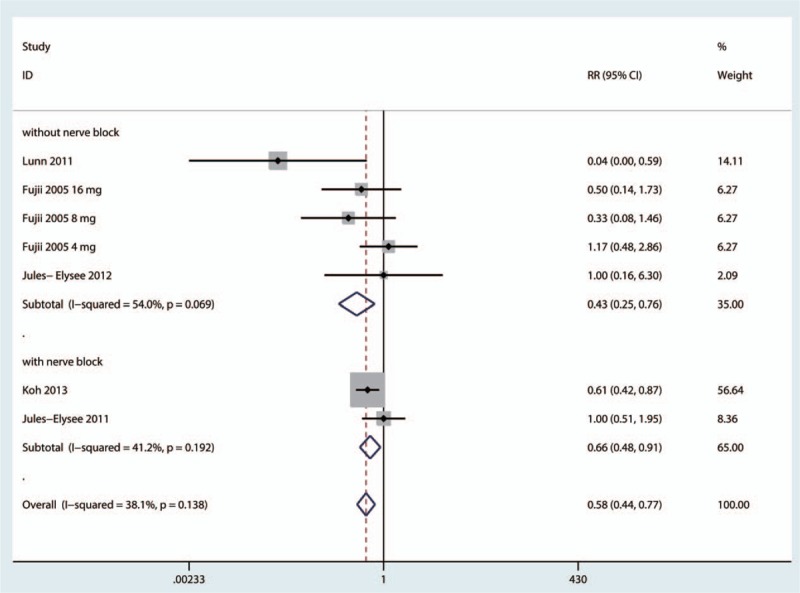
Subgroup analysis of the incidence of nausea.
The dose–effect relationship is shown in Fig. 10. We plotted the equivalent dexamethasone doses on the abscissa against the incidence of nausea as the ordinate to generate a scatterplot. The results indicated that the incidence of nausea tended to drop as the steroids dose increased. In addition, the linear correlation coefficient (r) was also calculated by the Spearman method. A negative correlation was observed between steroids dosage and the incidence of nausea (r = −0.538, P = .056).
Figure 10.
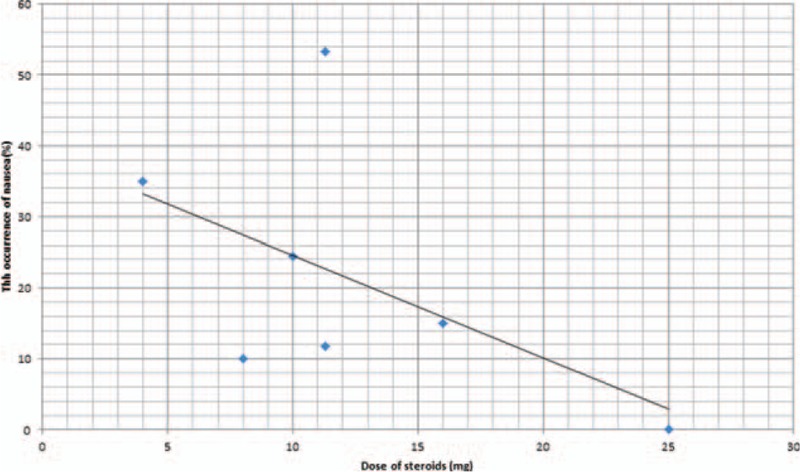
Scatterplot comparing steroids dose with the incidence of nausea.
3.3.3. Vomiting
The pooled results indicated that intravenous steroids may be associated with a statistically significant reduction in the risk of vomiting (RR = 0.46, 95%CI = 0.24–0.88, P = .019, Fig. 11). A high level of heterogeneity was observed across the studies (I2 = 71.0%, P = .001), and thus, a random-effects model was adopted to analyze relevant data.
Figure 11.
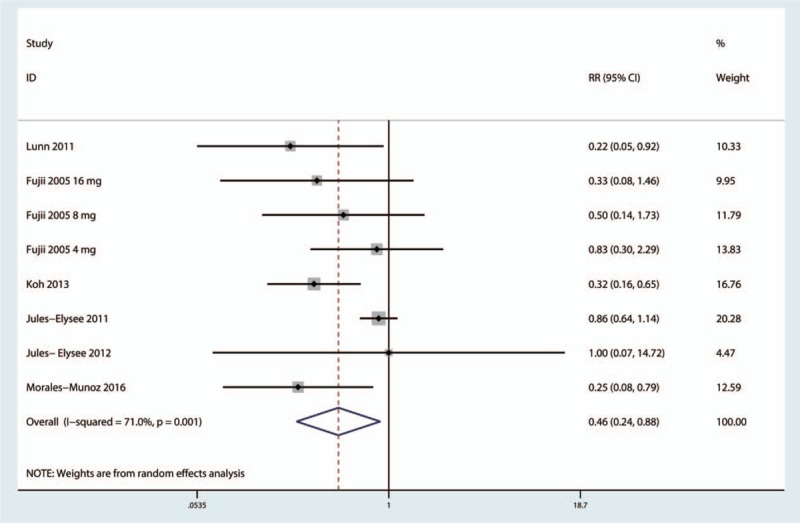
Forest plot comparing the incidence of vomiting between the 2 groups.
3.4. Secondary outcomes
3.4.1. Risk of infection
Five studies[16,18–20,22] reported the incidence of infection, and the pooled results indicated that there was no significant difference between the 2 groups in terms of the risk of infection (RR = 0.71, 95%CI, 0.23–2.19, P = .552, Fig. 12). No heterogeneity was identified (I2 = 0.0%, P = .735).
Figure 12.
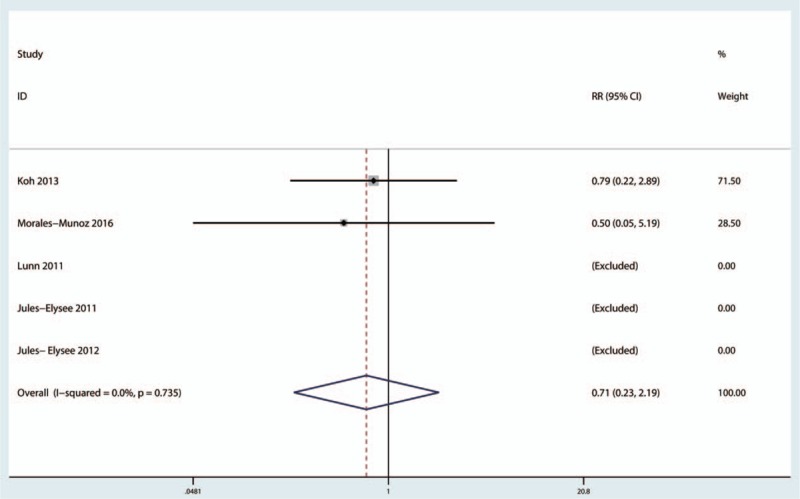
Forest plot comparing the incidence of infection between the 2 groups.
3.4.2. Blood glucose level
Three studies reported the patients’ blood glucose levels. These results indicated that blood glucose levels were elevated upon arrival to the PACU (MD = 22.56, 95%CI = 16.90–28.23, P = .000, Fig. 13), and no heterogeneity was observed (I2 = 0.0%, P = .400). Blood glucose levels were also elevated on postoperative day 1 (MD = 12.55, 95%CI = 1.76–23.34, P = .023, Fig. 13), and a low level of heterogeneity was observed (I2 = 36.6%, P = .178).
Figure 13.
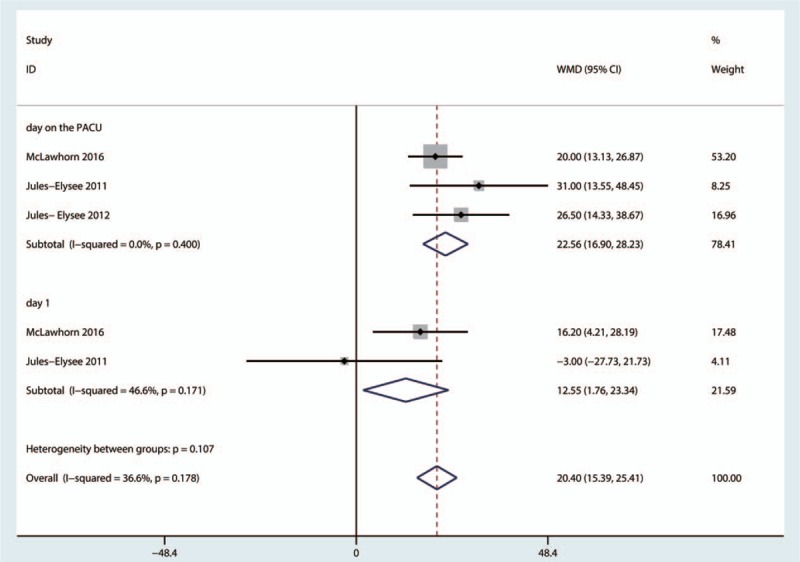
Forest plot comparing blood glucose levels upon arrival at the postanesthesia care unit (PACU) and on postoperative day 1 between the 2 groups.
4. Discussion
This is the first systematic review and meta-analysis to evaluate the efficacy and safety of steroids for reducing postoperative pain and decreasing the incidence of PONV after TKA. The pooled results indicated that the use of intravenous steroids was associated with lower pain scores after 24 and 48 hours of rest and decreased PONV risk after TKA. The use of steroids was not associated with a significant increase in the risk of infection. However, steroid use was associated with increased blood glucose levels upon PACU admission and on postoperative day 1. The GRADE level of evidence was low for VAS scores after 24 hours of rest, moderate for VAS scores after 12 and 48 hours of rest, and high for the incidence of nausea and vomiting. A major strength of this meta-analysis was the application of a comprehensive search strategy and the use of rigorous statistical methods. Electronic databases, including PubMed, Embase, Web of Science, and the Cochrane Library, were manually searched.
The results of this study indicated that the administration of steroids may be associated with pain relief at 24 and 48 hours post-TKA. No statistically significant difference in VAS scores at 12 hours post-TKA was observed. These results suggested that the incorporation of steroids into a multimodal analgesic regimen may provide synergistic analgesia effects. The mechanism by which steroids reduce VAS scores may be modulation of nociceptive input into the spinal cord, which occurs due to the inflammatory process resulting from surgery.[23] Another reason for this reduction may be that steroids suppress the expression of CRP, which is involved in the nociceptive process.[24] The pain that occurs between 6 and 24 hours after surgeries, including TKA, may be more severe than that occurring during other periods.[25] The subgroup analysis indicated that higher doses of steroids might be more effective than lower doses. However, 1 study has examined the effectiveness of low doses of steroids for the alleviation of post-TKA pain, and more clinical trials are required to compare the clinical pain scores associated with high and low doses of steroids. Only 1 study has compared VAS scores in combination with mobilization between the 2 groups; as such, insufficient data were available for the purposes of this meta-analysis.[16] However, this study indicated that the administration of preoperative intravenous steroids was also associated with decreased VAS scores with mobilization at 24 and 48 hours.
Steroids may also decrease the risk of PONV. When applied as a component of multimodal anesthesia, intravenous steroids may be associated with decreased morphine doses, and thus the risk of morphine-related complications (PONV) may be decreased. Lunn et al[16] found that oxycodone used decreased from 0 to 24 hours after treatment with steroids. Koh et al[18] also found that intravenous steroids were associated with a decreased morphine use relative to the placebo group. Furthermore, treatment with steroids may be associated with decreased IL-6 levels, which may be correlated with morbidity and mortality.[26,27] Henzi et al[28] found that the inclusion of steroids as a component of multimodal anesthesia was associated with decreased risk of nausea and vomiting after surgery. The pooled results of this meta-analysis indicated that steroids were associated with increased level of blood glucose levels but did not increase the risk of infection after TKA.
Our meta-analysis has several limitations: the doses of steroids utilized differed across studies, which may have affected the precision of the results; 2 studies included patients undergoing bilateral TKA, which may have caused the observed heterogeneity across studies; and we cannot completely exclude the presence of publication bias because a limited number of studies were included in this meta-analysis.
Based on the current evidence, steroids are effective in decreasing early pain intensity and PONV incidence among patients prepared for TKA. However, no robust evidence suggests that steroid use was associated with an increased incidence of post-TKA infection. Blood glucose levels were increased upon PACU admission and day 1 after TKA. With respect to the use of steroids as a prophylactic measure for pain management in patients with TKA, additional large-scale RCTs utilizing long periods of follow-up are needed to identify the long-term anesthetic and functional effects of steroids.
Footnotes
Abbreviations: CI = confidence interval, CRP = C-reactive protein, IL-6 = interleukin-6, MD = mean difference, PACU = postanesthesia care unit, PONV = postoperative nausea and vomiting, RCT = randomized controlled trial, RR = risk ratio, TKA = total knee arthroplasty, VAS = visual analogue scale.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Dong CC, Dong SL, He FC. Comparison of adductor canal block and femoral nerve block for postoperative pain in total knee arthroplasty: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780–5. [DOI] [PubMed] [Google Scholar]
- [3].Sun XL, Zhao ZH, Ma JX, et al. Continuous local infiltration analgesia for pain control after total knee arthroplasty: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhai L, Song Z, Liu K. The effect of gabapentin on acute postoperative pain in patients undergoing total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2016;95:e3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].DiIorio TM, Sharkey PF, Hewitt AM, et al. Antiemesis after total joint arthroplasty: does a single preoperative dose of aprepitant reduce nausea and vomiting? Clin Orthop Relat Res 2010;468:2405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Apfel CC, Laara E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999;91:693–700. [DOI] [PubMed] [Google Scholar]
- [7].Fujii Y. Current review of ramosetron in the prevention of postoperative nausea and vomiting. Curr Drug Saf 2011;6:122–7. [DOI] [PubMed] [Google Scholar]
- [8].Fujii Y, Tanaka H. Prevention of nausea and vomiting with ramosetron after total hip replacement. Clin Drug Investig 2003;23:405–9. [DOI] [PubMed] [Google Scholar]
- [9].Hall GM, Peerbhoy D, Shenkin A, et al. Relationship of the functional recovery after hip arthroplasty to the neuroendocrine and inflammatory responses. Br J Anaesth 2001;87:537–42. [DOI] [PubMed] [Google Scholar]
- [10].Smith C, Erasmus PJ, Myburgh KH. Endocrine and immune effects of dexamethasone in unilateral total knee replacement. J Int Med Res 2006;34:603–11. [DOI] [PubMed] [Google Scholar]
- [11].Bjornsson GL, Thorsteinsson L, Gudmundsson KO, et al. Inflammatory cytokines in relation to adrenal response following total hip replacement. Scand J Immunol 2007;65:99–105. [DOI] [PubMed] [Google Scholar]
- [12]. Higgins JPT GS. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011; [ http://www.cochrane-handbook.org]. Accessed 2011. [Google Scholar]
- [13].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guyatt GH, Oxman AD, Kunz R, et al. What is “quality of evidence” and why is it important to clinicians? BMJ 2008;336:995–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Backes JR, Bentley JC, Politi JR, et al. Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial. J Arthroplasty 2013;28:11–7. [DOI] [PubMed] [Google Scholar]
- [16].Lunn TH, Kristensen BB, Andersen LO, et al. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth 2011;106:230–8. [DOI] [PubMed] [Google Scholar]
- [17].Fujii Y, Nakayama M. Effects of dexamethasone in preventing postoperative emetic symptoms after total knee replacement surgery: a prospective, randomized, double-blind, vehicle-controlled trial in adult Japanese patients. Clin Ther 2005;27:740–5. [DOI] [PubMed] [Google Scholar]
- [18].Koh IJ, Chang CB, Lee JH, et al. Preemptive low-dose dexamethasone reduces postoperative emesis and pain after TKA: a randomized controlled study. Clin Orthop Relat Res 2013;471:3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jules-Elysee KM, Lipnitsky JY, Patel N, et al. Use of low-dose steroids in decreasing cytokine release during bilateral total knee replacement. Reg Anesth Pain Med 2011;36:36–40. [DOI] [PubMed] [Google Scholar]
- [20].Jules-Elysee KM, Wilfred SE, Memtsoudis SG, et al. Steroid modulation of cytokine release and desmosine levels in bilateral total knee replacement: a prospective, double-blind, randomized controlled trial. J Bone Joint Surg Am 2012;94:2120–7. [DOI] [PubMed] [Google Scholar]
- [21].McLawhorn AS, Beathe J, YaDeau J, et al. Effects of steroids on thrombogenic markers in patients undergoing unilateral total knee arthroplasty: a prospective, double-blind, randomized controlled trial. J Orthop Res 2015;33:412–6. [DOI] [PubMed] [Google Scholar]
- [22].Morales-Munoz C, Sanchez-Ramos JL, Diaz-Lara MD, et al. Analgesic effect of a single-dose of perineural dexamethasone on ultrasound-guided femoral nerve block after total knee replacement. Rev Esp Anestesiol Reanim 2017;64:19–26. [DOI] [PubMed] [Google Scholar]
- [23].Afman CE, Welge JA, Steward DL. Steroids for post-tonsillectomy pain reduction: meta-analysis of randomized controlled trials. Otolaryngol Head Neck Surg 2006;134:181–6. [DOI] [PubMed] [Google Scholar]
- [24].Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin 2007;45:27–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Valeberg BT, Hovik LH, Gjeilo KH. Relationship between self-reported pain sensitivity and pain after total knee arthroplasty: a prospective study of 71 patients 8 weeks after a standardized fast-track program. J Pain Res 2016;9:625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Damas P, Ledoux D, Nys M, et al. Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg 1992;215:356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Patel RT, Deen KI, Youngs D, et al. Interleukin 6 is a prognostic indicator of outcome in severe intra-abdominal sepsis. Br J Surg 1994;81:1306–8. [DOI] [PubMed] [Google Scholar]
- [28].Henzi I, Walder B, Tramer MR. Dexamethasone for the prevention of postoperative nausea and vomiting: a quantitative systematic review. Anesth Analg 2000;90:186–94. [DOI] [PubMed] [Google Scholar]


