SUMMARY
The drug isoniazid (INH) is a key component of global tuberculosis (TB) control programmes. It is estimated, however, that 16.1% of TB disease cases in Former Soviet Union countries and 7.5% of cases outside of those settings have non-multidrug resistant (MDR) INH resistance. Resistance has been linked to poorer treatment outcomes, post-treatment relapse and death, at least for specific sites of disease. Multiple genetic loci are associated with phenotypic resistance, but the relationship between genotype and phenotype is complex. This restricts the use of rapid sequencing techniques as part of the diagnostic process to determine the most appropriate treatment regimens for patients. The burden of resistance also influences the usefulness of INH preventative therapy (IPT). Despite seven decades of the use of INH our knowledge in key areas- such as the epidemiology of resistant strains, their clinical consequences, and their exact role in fuelling the MDR TB epidemic- is limited. The importance of non-MDR INH resistance needs to be re-evaluated both globally and by national TB control programmes.
Keywords: monoresistance, epidemiology, public health
INTRODUCTION
In 2013 the Director of the World Health Organization’s (WHO) Global Tuberculosis (TB) Programme described drug resistant TB as a ‘ticking time bomb’. A need for ‘visionary political leadership’ was identified.1 Research and public health action in this area has been dominated by multidrug resistant (MDR; resistance to both rifampicin (RMP) and isoniazid (INH)) and extensively drug resistant (XDR; MDR plus resistance to a fluoroquinolone and one or more of three second-line injectables) TB.
INH, first synthesised in 1912 in Prague,2 is an effective first-line drug for the treatment of active TB disease.3 A prodrug, INH is activated by the catalase-peroxidase KatG of Mycobacterium tuberculosis (M. tb). Following this, it binds InhA, an enoyl-acyl carrier protein reductase and so blocks fatty (mycolic) acid synthesis, a key component of the bacterial cell wall. In rapidly dividing bacteria INH is bactericidal, in slower dividing bacteria bacteriostatic. The drug is thought to provide a high initial kill at the start of active TB treatment, after which RMP largely takes over in terms of bactericidal activity and RMP and pyrazinamide (PZA) act as sterilising drugs.4 From its earliest use as monotherapy for TB disease in the 1950s, rapid and frequent development of resistance to INH was reported. Such observations regarding INH and other drugs emphasised the need for combination regimens. INH, streptomycin (STM) and p-aminosalicylic acid (PAS) thus became the standard regimen for many years before the development of the current short course of two months of INH, RMP, PZA and ethambutol (EMB), followed by four months of INH and RMP.4–6 The 1950s also saw the first studies of INH as a treatment for latent TB infections (LTBI),7 for which it is now a standard mono- or combination therapy.8;9
Resistance to INH has been associated with death in TB meningitis patients, where its role in treatment is even more crucial as the only bactericidal agent to easily traverse the blood-brain barrier.10 Additionally, a systematic review and meta-regression of trial data has indicated that initial INH resistance increases the incidence rates of treatment failure and relapse.11 Given its relatively cheap price and low rates of adverse events,3 it is beneficial to both health services and patients to be able to use INH. It is thus important to control the spread of primary INH resistance and prevent the acquisition of secondary resistance.
In this paper, we pose ourselves- and our audience- a single question: is non-MDR INH resistance of clinical concern? Our answer depends upon a host of considerations- the burden of INH resistance globally and regionally, the extent to which INH resistance hinders treatment of active disease, the relationship between INH resistance and MDR- which we describe in the following sections, before concluding with how resistance can be prevented and controlled, our perspectives on the implications of neglecting non-MDR INH resistance, and the gaps and opportunities for public health and research.
GLOBAL BURDEN OF INH RESISTANCE
In 2011, Jenkins et al. produced the first analysis of global INH resistance data reported to the WHO.12 They found that, from 1994–2009, 131 unique settings (including countries and sub-national regions) submitted such data at least once. This covered 56% of the world’s population, meaning that for nearly half of the global population data were not reported at local or national levels (a key knowledge gap- see Table 1). Of the submitted nationwide data, the former Soviet Union countries (FSU) reported the highest percentages of TB cases with INH resistance: 44.9% had some form of INH resistance (including mono-resistance and MDR TB) and 16.1% had non-MDR INH resistance without concurrent RMP resistance. Across the rest of the world, excluding the FSU, 13.9% of TB cases had some form of INH resistance (including mono-resistance and MDR TB) and 7.5% INH resistance without RMP resistance. Between 1994 and 2013, the WHO estimated that 9.5% of global TB cases had INH resistance without RMP resistance.13 The percentage of paediatric TB disease with INH resistance reflects the percentage observed among new adult cases.14 Around 12% of paediatric TB cases globally are estimated to have some form of INH resistance, amounting to 120,000 new child cases annually. Additionally, Dodd et al. have estimated that there are 166,000 new INH (without RMP) resistant infections in children per year.15 Given that there are specific recommendations for the use of LTBI regimens, including INH preventative therapy (IPT), in young children such estimates are significant.9
Table 1.
Summary of knowledge gaps for isoniazid resistant tuberculosis
| Area | Missing information | Potential data sources |
|---|---|---|
|
| ||
| Prevalence of phenotypic INH resistance | 44% of the world’s population is not covered by prevalence data that could be included at the time of Jenkins et al.12 Many reported estimates are old. Temporal trend data is often missing. | (Repeated) cross-sectional studies, surveillance data |
| Phenotypic versus genotypic resistance | How do specific resistance-associated mutations relate to phenotypic resistance? | Cross-sectional microbiological studies |
| Relative prevalence of resistance mutations | How are the different INH resistance-causing mutations distributed globally? Does this differ within specific population groups e.g. populations deemed at high risk of MDR disease? | Systematic review of available literature, cross-sectional studies |
| Treatment outcomes in active disease | How do phenotypic resistance (measured in different ways)21 and genotypic resistance influence treatment outcomes and the likelihood of relapse? | Systematic review of available literature |
| Treatment regimens for active disease* | Are regimens with an increased dose of INH effective in instances of low-level phenotypic resistance? What are the best regimens in children? At what resistance prevalence threshold should recommendations to use specific regimens be made? | Randomised controlled trials, mathematical modelling, health economics |
| Progression to MDR | What is the absolute risk of INH resistant strains becoming MDR during treatment? How does this compare to drug sensitive disease? How does this relate to treatment regimen? | Systematic review of available literature, cohort studies |
| LTBI treatment regimens | How effective are currently recommended LTBI treatment regimens for INH resistant infection? Are other regimens required, including for children? At what population-level of INH resistance is it best to avoid IPT? | Randomised controlled trials, mathematical modelling, health economics |
The ATS,102 NICE87 and WHO5 all have their own recommendations on this topic. ATS have recently updated their guidance on the treatment of drug sensitive disease, but at the time of writing have not released new guidelines for treating drug resistant disease. ATS- American Thoracic Society, INH- isoniazid, IPT- INH preventative therapy, LTBI- latent tuberculosis infection, MDR- multidrug resistance, NICE- National Institute of Health and Care Excellence, UK, WHO- World Health Organization
Time trend data is important to identify changes in the prevalence of INH resistance (Table 1). Jenkins et al. found that only 51 of the 131 settings above reported three or more temporal data points and both upward and downward trends were observed, with no clear global pattern.12 Given the relevance of INH resistance for people living with HIV (since they are targeted for IPT),16 the authors separately examined countries with estimated adult HIV seroprevalences of at least 2%. In those countries, 7.3% of cases had some form of INH resistance. Of concern, the only high HIV burden country with data sufficient to analyse time trends (Botswana), had seen an increase in INH resistance. New data from the South African drug resistance survey of 2012–14 (which are presented nationally and by province) also indicate increasing prevalence.17
RESISTANCE MUTATIONS
Phenotypic INH resistance is associated with a number of mutations (at the time of writing 22 are documented by the TB Drug Resistance Mutation Database),18;19 making creating a minimal predictive mutation set for clinical use complex.20 Lack of clarity about the association between specific mutations, phenotypic resistance, and treatment outcomes hinders genotyping being used to make rapid treatment decisions.21;22 inhA mutations are generally associated with lower phenotypic resistance than katG mutations,23;24 but even within the same gene different mutations can cause differing levels of phenotypic resistance. For example, in vitro katG H270R mutations result in greater resistance levels than A162E.24 Beyond the role of single point mutations a strain’s genetic background contributes to the relationship between the genotype of resistance loci and phenotypic resistance,25 as does the presence of compensatory mutations e.g. those in the ahpC gene.26 It is important to note that inhA promoter mutations also affect susceptibility to ethionamide.27
The distribution of different INH resistance mutations has been less well mapped globally than general prevalence data, but estimates from an international collection of over 5,000 strains (bearing in mind issues due to clustering) suggest that 79% of non-MDR INH resistant isolates have the katG S315T mutation (Manson et al., currently under review). Information on the distribution of mutations in non-MDR INH resistant TB is also individually available from various settings e.g. China (49% of isolates found to have the katG S315T mutation),28 Ethiopia (60% katG),29 Switzerland (57% katG S315T),25 plus pan-country studies e.g. Georghiou et al. (although this includes MDR strains).30 Given that some mutations are less strongly linked to high level phenotypic resistance (and thus theoretically poor treatment outcomes with INH-containing regimens) than others, such data are critically important for global planning (Table 1).
THE INFLUENCE OF RESISTANCE ON TREATMENT OUTCOMES
A high burden of non-MDR INH resistance is concerning in terms of TB control if the relative and absolute likelihood of negative treatment outcomes is substantially higher for INH resistant versus drug sensitive disease.
An early review of British Medical Research Council trials of different active TB treatment regimens published in 1986 was optimistic on this front, contrasting ‘the high success rate of short-course regimens in the presence of initial resistance to isoniazid and streptomycin’ to ‘the response of the few patients with initial rifampicin resistance’ (some of whom were MDR).31 Results differed in a more recent and expansive systematic review and meta-regression of trial data.11 The authors found that, after controlling for the different components of treatment regimens, initial INH resistance increased incidence rates of treatment failure and relapse versus a baseline of pan-sensitive strains (incidence rate ratio 10.9 [95% confidence interval 5.9–20] and 1.8 [1.2–2.6], respectively). Some observational studies from a variety of settings (with and without adjustment for treatment regimen and other confounders) have found similar results, including the previously cited study examining deaths in TB meningitis patients.10;32;33 Other studies have not found an association between resistance and negative outcomes.34;35 A large retrospective cohort of patients receiving short course chemotherapy from six countries was also less clear cut, showing an association between INH resistance and the risk of treatment failure in retreatment cases and weaker statistical evidence among new cases.36
Differing levels of phenotypic resistance might be expected to influence the success of INH-containing regimen. Indeed, as stated by Van Deun et al. ‘[b]ecause of the large therapeutic range of isoniazid, a fraction of patients may still benefit from the drug because the high concentration achievable in tuberculosis lesions may overcome low-level resistance’.37 Many studies comparing treatment outcomes in individuals with high and low level phenotypic resistance have not reported differences, although analyses are frequently not adequately statistically adjusted and the methodology for determining resistance will also have been influential.21;33;38–40 Published data on the influence of genotype are conflicting. In Vietnam, an analysis without adjustment for treatment regimen suggested that katG but not inhA mutations are associated with unfavourable treatment outcomes, and both mutations with relapse in new patients.41 In an Indian cohort where patients were all prescribed the same regimen katG, but not inhA mutations, were associated with poor treatment outcomes in an unadjusted analysis (and certain inhA mutations were more associated with cure than others).42 Other analyses have indicated that there is no difference in treatment outcomes by mutation, although again are often not appropriately adjusted.29;40;43
On balance, therefore, the precise link between INH resistance and treatment outcomes is unclear, although resistance is likely to be detrimental at least for certain sites of disease and without adjusted treatment regimens. Further work is required in this area (Table 1).
TAILORING TREATMENT REGIMENS IN THE PRESENCE OF RESISTANCE
If INH resistant TB has a greater likelihood of negative treatment outcomes than drug sensitive disease then specific effective regimens are required. Substituting for INH is clearly not ideal, given its low cost and rate of adverse events. Global guidance does, however, frequently reflect the need for adjusted regimens, albeit without a common consensus on the best approach to take (Table 2). A common theme of guidelines is the acknowledgment of knowledge gaps requiring further research (Table 1).
Table 2.
Global guidance on treating isoniazid resistant tuberculosis disease in adults
| Issuer of guidance | Treatment regimen(s) recommended | Reference(s) |
|---|---|---|
|
| ||
| WHO | Two sets of guidance, one on the basis of background levels of INH resistance and non-availability of DST before the continuation phase of treatment, the other when individual-level DST is available. | 5;103 |
| Where background levels are deemed ‘high’ among new TB patients and INH susceptibility testing results are not available before the continuation phase two months of INH, RMP, PZA and EMB followed by four months of INH, RMP and EMB are recommended. The threshold for ‘high’ levels is not defined.46 | ||
| In the presence of individual-level drug susceptibility results, recommendations are made depending upon the non-MDR INH resistance pattern found. For example, six to nine months of RIF, PZA and EMB (plus or minus a fluoroquinolone) for INH-monoresistant or INH and STM-resistant disease. | ||
| ATS | Six month regimen of RMP, PZA and EMB (plus a fluoroquinolone for extensive disease). | 102 |
| NICE | Nine month regimen (10 months where disease is extensive) of two months of RMP, PZA and EMB, then seven months of RMP and EMB. | 87 |
ATS- American Thoracic Society, DST- drug sensitivity testing, EMB- ethambutol, INH-isoniazid, NICE- National Institute of Health and Care Excellence, UK, PZA- pyrazinamide, RMP-rifampicin, STM- streptomycin, WHO- World Health Organization
In a recent systematic review and network meta-analysis by Stagg et al. of randomised controlled trials (RCTs) of different treatment regimens for non-MDR INH resistant TB, 59 studies were found for inclusion.44 A regimen category of RMP-containing regimens using fewer than three effective drugs at four months, in which RMP was protected by another effective drug at six months, and RMP was taken for six months was used as the baseline for a network meta-analysis (this included the WHO population level recommendation [Table 1]). Extending the duration of RMP to more than six months and increasing the number of effective drugs at four months to three or more lowered the odds of unfavourable versus favourable outcomes in a fixed-effects model (odds ratio 0.31 [95% credibility interval 0.12–0.81]). This was the only regimen category where the credibility interval did not cross the null, however, in a random-effects model all estimates did so. In both models, this regimen category (RMP containing, three or more effective drugs at four months, RMP protected by another effective drug at six months, RMP taken for more than six months) and two others (RMP containing, fewer than three effective drugs at four months, RMP taken for six months; RMP containing, fewer than three effective drugs at four months, RMP taken for more than six months) consistently ranked in the top three out of the 11 included, albeit with much uncertainty.
Menzies et al. also reviewed RCTs for the treatment of INH monoresistant TB in a paper published in 2009,45 with the aim of assessing the effectiveness of the 2008 WHO ‘retreatment’ regimen (two months of STM INH RMP PZA EMB followed by one month of INH RMP PZA EMB and then five months of INH RMP EMB) in patients with INH resistant disease. Despite the two reviews having very different inclusion and exclusion criteria the findings were similar, with the Menzies et al. review concluding that a RMP duration of two months or less, having few drugs in the intensive phase, and having therapy delivered twice weekly throughout increased both treatment failure and relapse rates, with additional factors influencing one or other measure.
High-quality data are lacking on the influence of treatment adherence, setting, the use of combination pills, and the presence of different resistance mutations on the efficacy of regimen recommendations. Additionally, neither of the two cited reviews specifically looked at regimens for children. For drug sensitive TB in children without HIV co-infection the WHO recommends a three drug two month intensive phase of INH, RMP and PZA, followed by four months of INH RMP.46 In the presence of INH resistance, or if the child is diagnosed where there is a ‘high background prevalence of isoniazid resistance’ WHO state that EMB should be added during the intensive phase and that ‘[f]or patients with more extensive disease, consideration should be given to the addition of a fluoroquinolone and to prolonging treatment to a minimum of 9 months’. Drug doses are also adjusted.
FROM INH RESISTANCE TO MULTIDRUG RESISTANCE
Aside from the implications of INH resistance on treatment, if non-MDR INH resistance is the key precursor to MDR (as opposed to non-MDR RMP resistance) and the risk of progression from INH resistance to MDR is high enough, then the control of such strains is very important. The relative prevalence of different resistance patterns across settings can be informative here, as can studies of the particular INH resistance mutations commonly observed in MDR strains. At a population level, evidence can also be provided through phylogenetic studies calculating the temporal order in which mutations occur. If we are convinced that INH resistance precedes RMP resistance then the risk/rate of a strain becoming MDR once INH resistant becomes critical. This is calculable through clinical trials and prospective observational studies analysed at the individual level. We examine each line of evidence in turn in the following paragraphs.
Globally, the proportion of RMP resistant strains that are MDR is higher than the equivalent proportion of INH resistant strains. An analysis of aggregate WHO data from 125 settings and several years has estimated that 87% of RMP resistant isolates are MDR.47 By comparison, using available nationwide data from Jenkins et al.,12 we calculated an average (weighted by the population in each country) of 39% of INH resistant strains being MDR. (It should be noted that this estimate relies upon reported data that is two or more decades old in some cases.) Such patterns likely reflect one of three things- the relatively high INH resistance mutation rate as opposed to that for RMP;48 that strains, once RMP resistant, rapidly acquire additional INH resistance; or that INH resistance is generally the first step to MDR.
Given that katG mutations are generally associated with greater phenotypic resistance than inhA mutations, if INH resistance is the first step to MDR it might be assumed that the former will be more common in MDR strains than the latter. Studies in various settings (of which the cited are a few) have demonstrated this to be the case,25;28;29;49–55 (including enrichment of katG mutations in MDR versus non-MDR INH resistant strains20;25;28;29;56). The ‘spectrum’ of mutations observed in MDR strains varies from setting to setting, however, and may be linked to the dose of INH used for treatment54 and the clonal spread of different mutations. The prevalence of different mutations will also reflect relative fitness, which is a complex trait57 that may additionally be related to the speed at which bacteria are growing.58
A systematic review and meta-regression of trial data published by Menzies et al. in 2009 examined the question of whether initial INH resistance is associated with increased rates of additional resistance.11 Incidence rates of acquired drug resistance were found to increase 5.1 times in patients with INH resistant disease versus drug sensitive disease (95% confidence interval 2.3 to 11.0) after treatment regimen was controlled for. This study examined any additional resistance to the drugs received, rather than looking specifically at the transition to MDR, however (Table 1). Although there are RCTs that specifically document RMP resistance arising in INH resistant versus drug sensitive patient populations by regimen both during and after treatment, data are relatively minimal.59–71 Within these RCTs (all of which treated with RMP in all arms) the development of RMP resistance almost exclusively occurred in less than 1% of drug sensitive disease patients across failure and relapse. In most instances this was also true for INH resistant disease. Notable exceptions in the latter population (high risk of progression during treatment [8–31%], but not at relapse) occurred when regimens consisted of INH and RMP alone (plus minimal STM or STM in the presence of STM co-resistance). One RCT in HIV positives documented a much higher risk of developing RMP resistance in drug sensitive patients during both treatment failure and relapse and INH resistant patients during treatment failure, but this may have been because patients were repeatedly re-infected during treatment.70 Although the findings above do not include RCTs where a comparator drug sensitive disease group was missing or where information was not presented by treatment regimen, it does given an indication of a generally low risk of the development of additional resistance. By comparison, observational studies without a comparator drug sensitive disease group have documented highly differing estimates of the likelihood of INH resistant disease progressing to MDR, ranging from <1–10%.38;43;72–74 In both RCTs and observational studies estimates will be highly regimen dependent.
Rapid and cheap whole genome sequencing makes analysing the progressive gain of resistance mutations at the population level using phylogenetic trees an achievable approach.75 A recent study of samples from a particular M. tb clone from KwaZulu-Natal in South Africa indicated that INH resistance (katG) mutations arose approximately 30 years earlier than RMP resistance.76 A previous study from Argentina also placed katG mutations prior to rpoB ones, albeit with a much shorter (3 year) gap and overlapping confidence intervals.77 Other studies using different typing techniques (including phenotyping) at the individual or population level have similarly suggested that INH resistance arises before RMP resistance.78–81 Results at the population level may, however, simply reflect when the different drugs were introduced and the more rapid mutation rate to INH resistance. A recent study across five continents, however, not only indicated that in 96% of MDR strains INH resistance was observed before RMP resistance, but also that this was independent of lineage, where strains were sampled from, and the time when resistance arose i.e. INH resistance predated RMP resistance even after both drugs were in use (Manson et al., currently under review).
Saunders et al. have proposed that INH resistance might precede RMP resistance in the development of MDR because the selective pressure of RMP is smaller than that of INH, thus RMP resistant strains are more likely to be killed by INH than INH resistant strains by RMP.80 The latter strains thus survive and develop additional resistance during substandard treatment. A higher mutation rate in strains with katG mutations in the presence of oxidative stress has also been suggested as a potential explanation, although evidence is lacking.82
HOW CAN WE PREVENT INH RESISTANCE?
The prevention of INH resistance falls into two categories- the need to control the spread of INH resistant strains (primary resistance) and the need to prevent individual patients developing secondary resistance.
The prevention of primary resistance relies upon ensuring that patients with INH resistant TB are rapidly detected and placed on an effective treatment regimen in order to avert transmission. Importantly, modelling work has indicated no evidence that katG S315T (for example) impairs virulence or transmissibility.83 Effective treatment regimens for INH resistant LTBI (which may need to be different to standard LTBI regimens) are also important, including knowing when the population level prevalence of resistance is sufficient to require such regimens to be used nationally as opposed to only in contacts of INH resistant disease cases.
Guidance and studies on the treatment of drug resistant LTBI infections are few and far between, with work focussing on MDR infections. Early reports exist of INH prophylaxis failing in contacts of patients with INH resistant TB, but such studies do not contain good comparison estimates of the failure of prophylaxis in individuals with drug sensitive infections.84–86 Neither the WHO nor (for example) the National Institute for Health and Care Excellence (NICE) in the UK make explicit recommendations regarding the treatment of contacts exposed to INH resistant TB (including for children).9;46;87 The American Thoracic Society (ATS) and Centers for Disease Control and Prevention (CDC), USA recommend a four month regimen of RMP for such individuals (six months for children),88–90 unless they are ‘HIV-infected persons taking some combinations of ART’. This recommendation was based upon a small number of publications.85;91–94 Of note, three to four months of RMP is the only non-INH containing regimen currently recommended for LTBI by the WHO.9 In the absence of clearer evidence from trials and observational studies about whether INH-containing regimens are suitable for INH resistant LTBI (Table 1), data may also be gleaned by comparing the results of studies undertaken in settings of different prevalences of INH resistance.
In order to estimate the critical prevalence of INH resistance before RMP LTBI regimens should replace nine months of INH a modelling study was undertaken in migrant children.95 From a cost/benefit perspective, the regimen switch was recommended for children originating from settings where the prevalence is at least 11%. The study was, however, criticised by other researchers, particularly for its assumptions regarding the relative effectiveness of different LTBI regimens.96
The prevention of secondary resistance largely relies upon ensuring appropriate adherence to treatment, responsive monitoring of patient progress, and ensuring good access to drugs to avoid regimen breaks.97 Higher strength pills (to reduce the number of tablets a patient takes at any one time) and combination pills may improve adherence and ensure adequate dosing. Additionally, the role of IPT in producing INH resistant LTBI has been debated.98;99 Of note, INH resistant disease in this instance would be incorrectly classified as having primary drug resistance.
CONCLUSION
INH is an important drug for the control of TB that we cannot afford to lose. It is cheap, effective, has a low rate of adverse events, and cannot be substituted by an equally positive alternative. Non-MDR INH resistance is surprisingly prevalent globally, especially in FSU countries. Resistance may increase the likelihood of negative treatment outcomes, post-treatment relapse, and death at least for certain sites of disease and with specific regimen. The incidence of non-MDR INH resistance (which is higher than that of MDR TB) may well affect the effectiveness of IPT at the population level.
Unfortunately, there are many knowledge gaps regarding INH resistant TB (Table 1). The most critical of these is perhaps the exact link between resistance-associated mutations, phenotypic resistance and active TB treatment outcomes, which also influences guidance on treatment regimens for INH resistant disease. Rapid sequencing technologies make genotyping highly attractive as part of a pipeline to rapidly make patient-level treatment decisions, thus these links are crucial. Such technologies will, however, be hindered by the number of mutations associated with INH resistance. Better data on the burden of INH resistance globally is also required in order to ascertain whether IPT policy should be adjusted. Importantly, none of the gaps highlighted would seem complex to fill using pre-existing data sources, or with adequately designed data collection.
Ultimately, decisions as to the importance of INH resistance are relative, and it was interesting to note during the preparation of this piece how interest in the subject has changed over time. When INH was introduced many studies examined INH resistance; this lessened as MDR TB became more of a concern. Interest has been increasing again in recent years as typing techniques have allowed the evolution of MDR to be studied in more detail. The WHO reflected how the relevance of INH resistance is context-specific when writing their treatment guidelines, which depend upon the burden of resistance, stating ‘WHO does not intend to establish thresholds for low, moderate or high levels of prevalence of isoniazid resistance: [National Tuberculosis Programmes] will establish definitions for their own countries.’46 Local decision making about the focus on control efforts also depends upon budgetary limitations (including how much of a country’s resources are currently being spent on MDR disease), the extent to which a country is concerned about further resistance arising, the accessibility of first line drug sensitivity testing, and the availability of alternative regimens for both LTBI and active disease.
Accurate drug sensitivity testing for all patients is critical for global TB control.1 Another critical area that INH resistance has implications for is the use of GeneXpert, an important rapid diagnostic. GeneXpert’s focus on detecting RMP resistance as a proxy for MDR and thus the potential for countries to move away from testing patients for INH resistance is of concern. A reduction in the amount of data available to calculate prevalence estimates for INH resistant disease could be anticipated, as well as patients with non-MDR INH resistance potentially being put at risk of being treated inadequately. If this latter were the case, transmission of INH resistant strains would not only be predicted to increase, but this increase would also not be detected. A modelling study using data from India has suggested a limited role for rapid INH resistance testing on transmission, however.100 It is interesting to note that in Peru, GeneXpert in its current form is not favoured as a diagnostic due to the perceived importance of the country’s burden of INH resistant strains.101
Readers may argue that non-MDR INH resistance has apparently been neglected for many years without too disastrous a consequence, and the fact that the proportion of non-MDR disease cases who fail treatment is low globally despite the current prevalence of INH resistance means that we need not be too concerned. This may well be the case in many settings and, indeed, we do not recommend that INH resistance be given priority over MDR and XDR TB for research funding. Nevertheless, as a stepping stone to MDR, a high or increasing prevalence of INH resistance is concerning in low and high TB-incidence settings alike, and if tracked adequately in the past this may have aided the prevention of the MDR TB epidemic.
At the beginning of this article, we posed a question- to what extent is INH resistance a topic of concern? Our review of the literature suggests that non-MDR INH resistance has been neglected, and that this lack of focus needs to be addressed as an important means of controlling global TB.
Figure 1. Percentage of incident tuberculosis cases with isoniazid resistance but not rifampicin resistance, 1994–2009.
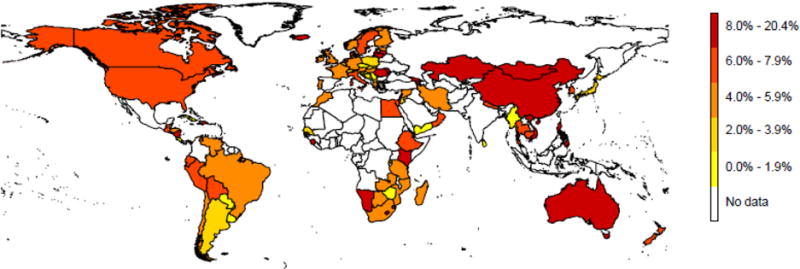
World map showing the percentage of incident tuberculosis disease that was isoniazid resistant, but not multidrug resistant, 1994–2009. National level data only, sourced and analysed as per Jenkins et al.12 Where countries submitted repeated estimates most recent data shown only. White areas did not report national data during the time period in question.
Acknowledgments
The authors wish to acknowledge Professor Andrew Nunn’s valuable comments on an early version of this manuscript.
FUNDING
This report is independent research supported by the National Institute for Health Research (Post Doctoral Fellowship, Dr Helen Stagg, PDF-2014-07-008). The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health. This work was supported by the U.S. National Institutes of Health (US NIH K01AI102944 award to HEJ). The content is solely the responsibility of the authors and does not necessarily represent the views of the U.S. National Institute of Allergy and Infectious Diseases or the U.S. National Institutes of Health.
Footnotes
AUTHOR CONTRIBUTIONS
HRS and HEJ conceived and designed the work, collated the evidence and drafted the original manuscript. All authors interpreted the evidence, revised the manuscript critically for intellectual content, and gave their approval of the manuscript.
CONFLICT OF INTERESTS
HRS declares funding from the National Institute for Health Research (NIHR), UK during the conduct of the study; and, outside of the submitted work, grants and personal fees from Otsuka Pharmaceutical, non-financial support from Sanofi, and other support from the WHO.
References
- 1.Abubakar I, Zignol M, Falzon D, Raviglione M, Ditiu L, Masham S, Adetifa I, Ford N, Cox H, Lawn SD, Marais BJ, McHugh TD, Mwaba P, Bates M, Lipman M, Zijenah L, Logan S, McNerney R, Zumla A, Sarda K, Nahid P, Hoelscher M, Pletschette M, Memish ZA, Kim P, Hafner R, Cole S, Migliori GB, Maeurer M, Schito M, Zumla A. Drug-resistant tuberculosis: time for visionary political leadership. Lancet Infect Dis. 2013;13(6):529–39. doi: 10.1016/S1473-3099(13)70030-6. [DOI] [PubMed] [Google Scholar]
- 2.Meyer H, Mally J. Über Hydrazinderivate der Pyridincarbonsäuren. Monatshefte Chemie verwandte Teile anderer Wissenschaften. 1912;33(4):393–414. [Google Scholar]
- 3.Crofton’s Clinical Tuberculosis. International Union Against Tuberculosis and Lung Disease. (Third) 2009 [Google Scholar]
- 4.Mitchison DA. Role of individual drugs in the chemotherapy of tuberculosis. Int J Tuberc Lung Dis. 2000;4(9):796–806. [PubMed] [Google Scholar]
- 5.World Health Organization. Treatment of tuberculosis: guidelines. (4th) 2009 http://www.who.int/tb/publications/2010/9789241547833/en/ Date last updated: 2009. Date last accessed: January 7 2013. [PubMed]
- 6.FOX W, SUTHERLAND I. The clinical significance of positive cultures and of isoniazid-resistant tubercle bacilli during the treatment of pulmonary tuberculosis; report to the Tuberculosis Chemotherapy Trials Committee of the Medical Research Council. Thorax. 1955;10(2):85–98. doi: 10.1136/thx.10.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LINCOLN EM. The effect of antimicrobial therapy on the prognosis of primary tuberculosis in children. Am Rev Tuberc. 1954;69(5):682–9. doi: 10.1164/art.1954.69.5.682. [DOI] [PubMed] [Google Scholar]
- 8.Stagg HR, Zenner D, Harris RJ, Munoz L, Lipman MC, Abubakar I. Treatment of latent tuberculosis infection: a network meta-analysis. Ann Intern Med. 2014;161(6):419–28. doi: 10.7326/M14-1019. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Guidelines on the management of latent tuberculosis infection. 2015 http://www.who.int/tb/publications/ltbi_document_page/en/. Date last updated: 2015. Date last accessed: May 24 2016. [PubMed]
- 10.Vinnard C, Winston CA, Wileyto EP, Macgregor RR, Bisson GP. Isoniazid resistance and death in patients with tuberculous meningitis: retrospective cohort study. BMJ. 2010;341:c4451. doi: 10.1136/bmj.c4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menzies D, Benedetti A, Paydar A, Martin I, Royce S, Pai M, Vernon A, Lienhardt C, Burman W. Effect of duration and intermittency of rifampin on tuberculosis treatment outcomes: a systematic review and meta-analysis. PLoS Med. 2009;6(9):e1000146. doi: 10.1371/journal.pmed.1000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jenkins HE, Zignol M, Cohen T. Quantifying the burden and trends of isoniazid resistant tuberculosis, 1994–2009. PLoS One. 2011;6(7):e22927. doi: 10.1371/journal.pone.0022927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization. Global Tuberculosis Report 2014. World Health Organization; 2014. http://apps.who.int/iris/bitstream/10665/137094/1/9789241564809_eng.pdf. Date last updated: 2014. Date last accessed: February 11 2015. [Google Scholar]
- 14.Yuen CM, Jenkins HE, Rodriguez CA, Keshavjee S, Becerra MC. Global and Regional Burden of Isoniazid-Resistant Tuberculosis. Pediatrics. 2015;136(1):e50–e59. doi: 10.1542/peds.2015-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dodd PJ, Sismanidis C, Seddon JA. Global burden of drug-resistant tuberculosis in children: a mathematical modelling study. Lancet Infect Dis. 2016 doi: 10.1016/S1473-3099(16)30132-3. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization. WHO three I’s meeting. Geneva, Switzerland: 2008. Date last updated: 2008. Date last accessed: August 31 2016. [Google Scholar]
- 17.National Institute for Communicable Disease South Africa. South African Tuberculosis Drug Resistance Survey 2012–14. 2016 http://www.nicd.ac.za/assets/files/K-12750%20NICD%20National%20Survey%20Report_Dev_V11-LR.pdf Date last updated: 2016. Date last accessed: September 7 2016.
- 18.Sandgren A, Strong M, Muthukrishnan P, Weiner BK, Church GM, Murray MB. Tuberculosis drug resistance mutation database. PLoS Med. 2009;6(2):e2. doi: 10.1371/journal.pmed.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandgren A. TB Drug Resistance Mutation Database. doi: 10.1371/journal.pmed.1000002. https://tbdreamdb.ki.se/Data/DrugArea.aspx?AreaID=INH Date last updated: Mar. 26 2014. Date last accessed: Aug. 2 2016. [DOI] [PMC free article] [PubMed]
- 20.Farhat MR, Sultana R, Iartchouk O, Bozeman S, Galagan J, Sisk P, Stolte C, Nebenzahl-Guimaraes H, Jacobson K, Sloutsky A, Kaur D, Posey J, Kreiswirth BN, Kurepina N, Rigouts L, Streicher EM, Victor TC, Warren RM, van SD, Murray M. Genetic Determinants of Drug Resistance in Mycobacterium tuberculosis and Their Diagnostic Value. Am J Respir Crit Care Med. 2016 doi: 10.1164/rccm.201510-2091OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dominguez J, Boettger EC, Cirillo D, Cobelens F, Eisenach KD, Gagneux S, Hillemann D, Horsburgh R, Molina-Moya B, Niemann S, Tortoli E, Whitelaw A, Lange C. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: a TBNET/RESIST-TB consensus statement. Int J Tuberc Lung Dis. 2016;20(1):24–42. doi: 10.5588/ijtld.15.0221. [DOI] [PubMed] [Google Scholar]
- 22.Otto-Knapp R, Vesenbeckh S, Schonfeld N, Bettermann G, Roth A, Bauer T, Russmann H, Mauch H. Isoniazid minimal inhibitory concentrations of tuberculosis strains with katG mutation. Int J Tuberc Lung Dis. 2016;20(9):1275–1278. doi: 10.5588/ijtld.16.0148. [DOI] [PubMed] [Google Scholar]
- 23.Telenti A. Genetics and pulmonary medicine. 5 Genetics of drug resistant tuberculosis. Thorax. 1998;53(9):793–7. doi: 10.1136/thx.53.9.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brossier F, Boudinet M, Jarlier V, Petrella S, Sougakoff W. Comparative study of enzymatic activities of new KatG mutants from low- and high-level isoniazid-resistant clinical isolates of Mycobacterium tuberculosis. Tuberculosis. 2016;100:15–24. doi: 10.1016/j.tube.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Fenner L, Egger M, Bodmer T, Altpeter E, Zwahlen M, Jaton K, Pfyffer GE, Borrell S, Dubuis O, Bruderer T, Siegrist HH, Furrer H, Calmy A, Fehr J, Stalder JM, Ninet B, Bottger EC, Gagneux S. Effect of mutation and genetic background on drug resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56(6):3047–53. doi: 10.1128/AAC.06460-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson TM, Collins DM. ahpC, a gene involved in isoniazid resistance of the Mycobacterium tuberculosis complex. Mol Microbiol. 1996;19(5):1025–34. doi: 10.1046/j.1365-2958.1996.449980.x. [DOI] [PubMed] [Google Scholar]
- 27.Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um KS, Wilson T, Collins D, de LG, Jacobs WR., Jr inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263(5144):227–30. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 28.Hu Y, Hoffner S, Jiang W, Wang W, Xu B. Extensive transmission of isoniazid resistant Mtuberculosis and its association with increased multidrug-resistant TB in two rural counties of eastern China: a molecular epidemiological study. BMC Infect Dis. 2010;10:43. doi: 10.1186/1471-2334-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abate D, Tedla Y, Meressa D, Ameni G. Isoniazid and rifampicin resistance mutations and their effect on second-line anti-tuberculosis treatment. Int J Tuberc Lung Dis. 2014;18(8):946–51. doi: 10.5588/ijtld.13.0926. [DOI] [PubMed] [Google Scholar]
- 30.Georghiou SB, Seifert M, Catanzaro D, Garfein RS, Valafar F, Crudu V, Rodrigues C, Victor TC, Catanzaro A, Rodwell TC. Frequency and Distribution of Tuberculosis Resistance-Associated Mutations between Mumbai, Moldova, and Eastern Cape. Antimicrob Agents Chemother. 2016;60(7):3994–4004. doi: 10.1128/AAC.00222-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchison DA, Nunn AJ. Influence of initial drug resistance on the response to short-course chemotherapy of pulmonary tuberculosis. Am Rev Respir Dis. 1986;133(3):423–30. doi: 10.1164/arrd.1986.133.3.423. [DOI] [PubMed] [Google Scholar]
- 32.Deepa D, Achanta S, Jaju J, Rao K, Samyukta R, Claassens M, Kumar AM, Ph V. The impact of isoniazid resistance on the treatment outcomes of smear positive re-treatment tuberculosis patients in the state of Andhra Pradesh, India. PLoS One. 2013;8(10):e76189. doi: 10.1371/journal.pone.0076189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villegas L, Otero L, Sterling TR, Huaman MA, Van der Stuyft P, Gotuzzo E, Seas C. Prevalence, Risk Factors, and Treatment Outcomes of Isoniazid- and Rifampicin-Mono-Resistant Pulmonary Tuberculosis in Lima, Peru. PLoS One. 2016;11(4):e0152933. doi: 10.1371/journal.pone.0152933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cattamanchi A, Dantes RB, Metcalfe JZ, Jarlsberg LG, Grinsdale J, Kawamura LM, Osmond D, Hopewell PC, Nahid P. Clinical characteristics and treatment outcomes of patients with isoniazid-monoresistant tuberculosis. Clin Infect Dis. 2009;48(2):179–85. doi: 10.1086/595689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LoBue PA, Moser KS. Isoniazid- and rifampin-resistant tuberculosis in San Diego County, California, United States, 1993–2002. Int J Tuberc Lung Dis. 2005;9(5):501–6. [PubMed] [Google Scholar]
- 36.Espinal MA, Kim SJ, Suarez PG, Kam KM, Khomenko AG, Migliori GB, Baez J, Kochi A, Dye C, Raviglione MC. Standard short-course chemotherapy for drug-resistant tuberculosis: treatment outcomes in 6 countries. JAMA. 2000;283(19):2537–45. doi: 10.1001/jama.283.19.2537. [DOI] [PubMed] [Google Scholar]
- 37.Van Deun A, Maug AK, Salim MA, Das PK, Sarker MR, Daru P, Rieder HL. Short, highly effective, and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182(5):684–92. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- 38.Chien JY, Chen YT, Wu SG, Lee JJ, Wang JY, Yu CJ. Treatment outcome of patients with isoniazid mono-resistant tuberculosis. Clin Microbiol Infect. 2015;21(1):59–68. doi: 10.1016/j.cmi.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Wang TY, Lin SM, Shie SS, Chou PC, Huang CD, Chung FT, Kuo CH, Chang PJ, Kuo HP. Clinical characteristics and treatment outcomes of patients with low- and high-concentration isoniazid-monoresistant tuberculosis. PLoS One. 2014;9(1):e86316. doi: 10.1371/journal.pone.0086316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bang D, Andersen PH, Andersen AB, Thomsen VO. Isoniazid-resistant tuberculosis in Denmark: mutations, transmission and treatment outcome. J Infect. 2010;60(6):452–7. doi: 10.1016/j.jinf.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 41.Huyen MN, Cobelens FG, Buu TN, Lan NT, Dung NH, Kremer K, Tiemersma EW, van SD. Epidemiology of isoniazid resistance mutations and their effect on tuberculosis treatment outcomes. Antimicrob Agents Chemother. 2013;57(8):3620–7. doi: 10.1128/AAC.00077-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tolani MP, D’souza DT, Mistry NF. Drug resistance mutations and heteroresistance detected using the GenoType MTBDRplus assay and their implication for treatment outcomes in patients from Mumbai, India. BMC Infect Dis. 2012;12:9. doi: 10.1186/1471-2334-12-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobson KR, Theron D, Victor TC, Streicher EM, Warren RM, Murray MB. Treatment outcomes of isoniazid-resistant tuberculosis patients, Western Cape Province, South Africa. Clin Infect Dis. 2011;53(4):369–72. doi: 10.1093/cid/cir406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stagg HR, Harris RJ, Hatherell HA, Obach D, Zhao H, Tsuchiya N, Kranzer K, Nikolayevskyy V, Kim J, Lipman MC, Abubakar I. What are the most efficacious treatment regimens for isoniazid-resistant tuberculosis? A systematic review and network meta-analysis. Thorax. 2016 doi: 10.1136/thoraxjnl-2015-208262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Menzies D, Benedetti A, Paydar A, Royce S, Madhukar P, Burman W, Vernon A, Lienhardt C. Standardized treatment of active tuberculosis in patients with previous treatment and/or with mono-resistance to isoniazid: a systematic review and meta-analysis. PLoS Med. 2009;6(9):e1000150. doi: 10.1371/journal.pmed.1000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization. Guidance for national tuberculosis programmes on the management of tuberculosis in children. Second. Geneva, Switzerland: World Health Organization; 2014. http://apps.who.int/iris/bitstream/10665/112360/1/9789241548748_eng.pdf?ua=1 Date last updated: 2014. Date last accessed: September 1 2016. [PubMed] [Google Scholar]
- 47.Smith SE, Kurbatova EV, Cavanaugh JS, Cegielski JP. Global isoniazid resistance patterns in rifampin-resistant and rifampin-susceptible tuberculosis. Int J Tuberc Lung Dis. 2012;16(2):203–5. doi: 10.5588/ijtld.11.0445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.David HL. Probability distribution of drug-resistant mutants in unselected populations of Mycobacterium tuberculosis. Appl Microbiol. 1970;20(5):810–4. doi: 10.1128/am.20.5.810-814.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casali N, Nikolayevskyy V, Balabanova Y, Harris SR, Ignatyeva O, Kontsevaya I, Corander J, Bryant J, Parkhill J, Nejentsev S, Horstmann RD, Brown T, Drobniewski F. Evolution and transmission of drug-resistant tuberculosis in a Russian population. Nat Genet. 2014;46(3):279–86. doi: 10.1038/ng.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan RC, Hui M, Chan EW, Au TK, Chin ML, Yip CK, AuYeang CK, Yeung CY, Kam KM, Yip PC, Cheng AF. Genetic and phenotypic characterization of drug-resistant Mycobacterium tuberculosis isolates in Hong Kong. J Antimicrob Chemother. 2007;59(5):866–73. doi: 10.1093/jac/dkm054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hazbon MH, Brimacombe M, Bobadillad V, Cavatore M, Guerrero MI, Varma-Basil M, Billman-Jacobe H, Lavender C, Fyfe J, Garcia-Garcia L, Leon CI, Bose M, Chaves F, Murray M, Eisenach KD, Sifuentes-Osornio J, Cave MD, Ponce de LA, Alland D. Population genetics study of isoniazid resistance mutations and evolution of multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2006;50(8):2640–9. doi: 10.1128/AAC.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jagielski T, Grzeszczuk M, Kaminski M, Roeske K, Napiorkowska A, Stachowiak R, Augustynowicz-Kopec E, Zwolska Z, Bielecki J. Identification and analysis of mutations in the katG gene in multidrug-resistant Mycobacterium tuberculosis clinical isolates. Pneumonol Alergol Pol. 2013;81(4):298–307. [PubMed] [Google Scholar]
- 53.Luo T, Zhao M, Li X, Xu P, Gui X, Pickerill S, DeRiemer K, Mei J, Gao Q. Selection of mutations to detect multidrug-resistant Mycobacterium tuberculosis strains in Shanghai, China. Antimicrob Agents Chemother. 2010;54(3):1075–81. doi: 10.1128/AAC.00964-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosales-Klintz S, Jureen P, Zalutskayae A, Skrahina A, Xu B, Hu Y, Pineda-Garcia L, Merza MA, Muntean I, Bwanga F, Joloba M, Hoffner SE. Drug resistance-related mutations in multidrug-resistant Mycobacterium tuberculosis isolates from diverse geographical regions. Int J Mycobacteriol. 2012;1(3):124–30. doi: 10.1016/j.ijmyco.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 55.Setarah M, Titov L, Surkova L. High level association of mutation in KatG315 with MDR and XDR clinical isolates of Mycobacterium tuberculosis in Belarus. Acta Microbiologica et Immunologica Hungarica. 2009;56(4) doi: 10.1556/AMicr.56.2009.4.2. [DOI] [PubMed] [Google Scholar]
- 56.Miotto P, Piana F, Penati V, Canducci F, Migliori GB, Cirillo DM. Use of genotype MTBDR assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis clinical strains isolated in Italy. J Clin Microbiol. 2006;44(7):2485–91. doi: 10.1128/JCM.00083-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.O’Sullivan DM, McHugh TD, Gillespie SH. Mapping the fitness of Mycobacterium tuberculosis strains: a complex picture. J Med Microbiol. 2010;59(Pt 12):1533–5. doi: 10.1099/jmm.0.019091-0. [DOI] [PubMed] [Google Scholar]
- 58.Jeeves RE, Marriott AA, Pullan ST, Hatch KA, Allnutt JC, Freire-Martin I, Hendon-Dunn CL, Watson R, Witney AA, Tyler RH, Arnold C, Marsh PD, McHugh TD, Bacon J. Mycobacterium tuberculosis Is Resistant to Isoniazid at a Slow Growth Rate by Single Nucleotide Polymorphisms in katG Codon Ser315. PLoS One. 2015;10(9):e0138253. doi: 10.1371/journal.pone.0138253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Singapore Tuberculosis Service-British Medical Research Council. Five-Year Follow-Up of A Clinical Trial of Three 6-Month Regimens of Chemotherapy Given Intermittently in the Continuation Phase in the Treatment of Pulmonary Tuberculosis. Am Rev Respir Dis. 1988;137(5):1147–50. doi: 10.1164/ajrccm/137.5.1147. [DOI] [PubMed] [Google Scholar]
- 60.Hong Kong Chest Service/British Medical Research Council. Five-year follow-up of a controlled trial of five 6-month regimens of chemotherapy for pulmonary tuberculosis. Am Rev Respir Dis. 1987;136(6):1339–42. doi: 10.1164/ajrccm/136.6.1339. [DOI] [PubMed] [Google Scholar]
- 61.East and Central African/British Medical Research Council. Controlled clinical trial of 4 short-course regimens of chemotherapy (three 6-month and one 8-month) for pulmonary tuberculosis: final report. East and Central African/British Medical Research Council Fifth Collaborative Study. Tubercle. 1986;67(1):5–15. doi: 10.1016/0041-3879(86)90027-9. [DOI] [PubMed] [Google Scholar]
- 62.Singapore Tuberculosis Service British Medical Research Council. Long-Term Follow-Up of A Clinical Trial of Six-Month and Four-Month Regimens of Chemotherapy in the Treatment of Pulmonary Tuberculosis. Am Rev Respir Dis. 1986;133(5):779–83. [PubMed] [Google Scholar]
- 63.Tanzania-British Medical Research Council Study. Controlled Clinical Trial of Two 6-Month Regimens of Chemotherapy in the Treatment of Pulmonary Tuberculosis. Am Rev Respir Dis. 1985;131(5):727–31. doi: 10.1164/arrd.1985.131.5.727. [DOI] [PubMed] [Google Scholar]
- 64.East African/British Medical Research Council. Controlled clinical trial of four short-course regimens of chemotherapy for two durations in the treatment of pulmonary tuberculosis. Second report. Third East African/British Medical Research Council Study. Tubercle. 1980;61(2):59–69. doi: 10.1016/0041-3879(80)90012-4. [DOI] [PubMed] [Google Scholar]
- 65.Zierski M, Bek E, Long MW, Snider DE. Short-Course (6-Month) Cooperative Tuberculosis Study in Poland - Results 18 Months After Completion of Treatment. Am Rev Respir Dis. 1980;122(6):879–89. doi: 10.1164/arrd.1980.122.6.879. [DOI] [PubMed] [Google Scholar]
- 66.Hong Kong Chest Service, British Medical Research Council. Controlled Trial of 6 Month and 8 Month Regimens in the Treatment of Pulmonary Tuberculosis the Results Up to 24 Months. Tubercle. 1979;60(4):201–10. doi: 10.1016/0041-3879(79)90001-1. [DOI] [PubMed] [Google Scholar]
- 67.Girling DJ, Nunn AJ, FOX W, Michison DA. Controlled Trial of Intermittent Regimens of Rifampin Plus Isoniazid for Pulmonary Tuberculosis in Singapore - Results Up to 30 Months. Am Rev Respir Dis. 1977;116(5):807–20. doi: 10.1164/arrd.1977.116.5.807. [DOI] [PubMed] [Google Scholar]
- 68.Algerian Working Group/British Medical Research Council Cooperative Study. Short-course chemotherapy for pulmonary tuberculosis under routine programme conditions: a comparison of regimens of 28 and 36 weeks duration in Algeria. Tubercle. 1991;72(2):88–100. doi: 10.1016/0041-3879(91)90034-p. [DOI] [PubMed] [Google Scholar]
- 69.Mazouni L, Tazir M, Boulahbal F, Chaulet P. Controlled study comparing 3 daily chemotherapy regimens for six months in pulmonary tuberculosis in routine practice in Algiers. Results at 30 months. Rev Mal Respir. 1985;2(4):209–14. [PubMed] [Google Scholar]
- 70.Swaminathan S, Narendran G, Venkatesan P, Iliayas S, Santhanakrishnan R, Menon PA, Padmapriyadarsini C, Ramachandran R, Chinnaiyan P, Suhadev M, Sakthivel R, Narayanan PR. Efficacy of a 6-month versus 9-month intermittent treatment regimen in HIV-infected patients with tuberculosis: A randomized clinical trial. Am J Respir Crit Care Med. 2010;181(7):01. doi: 10.1164/rccm.200903-0439OC. [DOI] [PubMed] [Google Scholar]
- 71.Girling DJ, Chan SL. Controlled trial of 2, 4, and 6 months of pyrazinamide in 6-month, three-times-weekly regimens for smear-positive pulmonary tuberculosis, including an assessment of a combined preparation of isoniazid, rifampin, and pyrazinamide: Results at 30 months. Am Rev Respir Dis. 1991;143(4):700–6. doi: 10.1164/ajrccm/143.4_Pt_1.700. [DOI] [PubMed] [Google Scholar]
- 72.Ruddy MC, Davies AP, Yates MD, Yates S, Balasegaram S, Drabu Y, Patel B, Lozewicz S, Sen S, Bahl M, James E, Lipman M, Duckworth G, Watson JM, Piper M, Drobniewski FA, Maguire H. Outbreak of isoniazid resistant tuberculosis in north London. Thorax. 2004;59(4):279–85. doi: 10.1136/thx.2003.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Van Altena R, van Soolingen D, van der Werf TS. Isoniazid resistant TB and non-compliance. Thorax. 2004;59(12):1098–9. [PMC free article] [PubMed] [Google Scholar]
- 74.Munang ML, Browne C, Khanom S, Evans JT, Smith EG, Hawkey PM, Kunst H, Welch SB, Dedicoat MJ. Tuberculosis microepidemics among dispersed migrants, Birmingham, UK, 2004–2013. Emerg Infect Dis. 2015;21(3):524–7. doi: 10.3201/eid2103.140209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hatherell HA, Colijn C, Stagg HR, Jackson C, Winter JR, Abubakar I. Interpreting whole genome sequencing for investigating tuberculosis transmission: a systematic review. BMC Med. 2016;14:21. doi: 10.1186/s12916-016-0566-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen KA, Abeel T, Manson MA, Desjardins CA, Munsamy V, Shea TP, Walker BJ, Bantubani N, Almeida DV, Alvarado L, Chapman SB, Mvelase NR, Duffy EY, Fitzgerald MG, Govender P, Gujja S, Hamilton S, Howarth C, Larimer JD, Maharaj K, Pearson MD, Priest ME, Zeng Q, Padayatchi N, Grosset J, Young SK, Wortman J, Mlisana KP, O’Donnell MR, Birren BW, Bishai WR, Pym AS, Earl AM. Evolution of Extensively Drug-Resistant Tuberculosis over Four Decades: Whole Genome Sequencing and Dating Analysis of Mycobacterium tuberculosis Isolates from KwaZulu-Natal. PLoS Med. 2015;12(9):e1001880. doi: 10.1371/journal.pmed.1001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Eldholm V, Monteserin J, Rieux A, Lopez B, Sobkowiak B, Ritacco V, Balloux F. Four decades of transmission of a multidrug-resistant Mycobacterium tuberculosis outbreak strain. Nat Commun. 2015;6:7119. doi: 10.1038/ncomms8119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Muller B, Chihota VN, Pillay M, Klopper M, Streicher EM, Coetzee G, Trollip A, Hayes C, Bosman ME, Gey van Pittius NC, Victor TC, Gagneux S, van Helden PD, Warren RM. Programmatically selected multidrug-resistant strains drive the emergence of extensively drug-resistant tuberculosis in South Africa. PLoS One. 2013;8(8):e70919. doi: 10.1371/journal.pone.0070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Victor TC, Streicher EM, Kewley C, Jordaan AM, van der Spuy GD, Bosman M, Louw H, Murray M, Young D, van Helden PD, Warren RM. Spread of an emerging Mycobacterium tuberculosis drug-resistant strain in the western Cape of South Africa. Int J Tuberc Lung Dis. 2007;11(2):195–201. [PubMed] [Google Scholar]
- 80.Saunders NJ, Trivedi UH, Thomson ML, Doig C, Laurenson IF, Blaxter ML. Deep resequencing of serial sputum isolates of Mycobacterium tuberculosis during therapeutic failure due to poor compliance reveals stepwise mutation of key resistance genes on an otherwise stable genetic background. J Infect. 2011;62(3):212–7. doi: 10.1016/j.jinf.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 81.Izu A, Cohen T, Degruttola V. Bayesian estimation of mixture models with prespecified elements to compare drug resistance in treatment-naive and experienced tuberculosis cases. PLoS Comput Biol. 2013;9(3):e1002973. doi: 10.1371/journal.pcbi.1002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Sullivan DM, McHugh TD, Gillespie SH. The effect of oxidative stress on the mutation rate of Mycobacterium tuberculosis with impaired catalase/peroxidase function. J Antimicrob Chemother. 2008;62(4):709–12. doi: 10.1093/jac/dkn259. [DOI] [PubMed] [Google Scholar]
- 83.Cohen T, Becerra MC, Murray MB. Isoniazid resistance and the future of drug-resistant tuberculosis. Microb Drug Resist. 2004;10(4):280–5. doi: 10.1089/mdr.2004.10.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fairshter RD, Randazzo GP, Garlin J, Wilson AF. Failure of isoniazid prophylaxis after exposure to isoniazid-resistant tuberculosis. Am Rev Respir Dis. 1975;112(1):37–42. doi: 10.1164/arrd.1975.112.1.37. [DOI] [PubMed] [Google Scholar]
- 85.Polesky A, Farber HW, Gottlieb DJ, Park H, Levinson S, O’Connell JJ, McInnis B, Nieves RL, Bernardo J. Rifampin preventive therapy for tuberculosis in Boston’s homeless. Am J Respir Crit Care Med. 1996;154(5):1473–7. doi: 10.1164/ajrccm.154.5.8912767. [DOI] [PubMed] [Google Scholar]
- 86.Steiner M, Chaves AD, Lyons HA, Steiner P, Portugaleza C. Primary drug-resistant tuberculosis. Report of an outbreak. N Engl J Med. 1970;283(25):1353–8. doi: 10.1056/NEJM197012172832501. [DOI] [PubMed] [Google Scholar]
- 87.National Institute for Health and Care Excellence. Tuberculosis. 2016 http://www.nice.org.uk/guidance/ng33/resources/tuberculosis-prevention-diagnosis-management-and-service-organisation-1837390683589. Date last updated: 2016. Date last accessed: January 14 2016.
- 88.Centers for Disease Control and Prevention. Latent Tuberculosis Infection: A Guide for Primary Health Care Providers. http://www.cdc.gov/tb/publications/ltbi/treatment.htm#specialConsiderations. Date last updated: Apr. 3 2013. Date last accessed: Aug. 3 2016.
- 89.ATS/CDC Statement Committee on Latent Tuberculosis Infection Membership List. Targeted tuberculin testing and treatment of latent tuberculosis infection. American Thoracic Society. MMWR Recomm Rep. 2000;49(RR-6):1–51. [PubMed] [Google Scholar]
- 90.American Thoracic Society, Centers for Disease Control and Prevention. Targeted tuberculin testing and treatment of latent tuberculosis infection. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. This is a Joint Statement of the American Thoracic Society (ATS) and the Centers for Disease Control and Prevention (CDC). This statement was endorsed by the Council of the Infectious Diseases Society of America. (IDSA), September 1999, and the sections of this statement. Am J Respir Crit Care Med. 2000;161(4 Pt 2):S221–S247. doi: 10.1164/ajrccm.161.supplement_3.ats600. [DOI] [PubMed] [Google Scholar]
- 91.Villarino ME, Ridzon R, Weismuller PC, Elcock M, Maxwell RM, Meador J, Smith PJ, Carson ML, Geiter LJ. Rifampin preventive therapy for tuberculosis infection: experience with 157 adolescents. Am J Respir Crit Care Med. 1997;155(5):1735–8. doi: 10.1164/ajrccm.155.5.9154885. [DOI] [PubMed] [Google Scholar]
- 92.Koplan JP, Farer LS. Choice of preventive treatment for isoniazid-resistant tuberculous infection. Use of decision analysis and the Delphi technique. JAMA. 1980;244(24):2736–40. [PubMed] [Google Scholar]
- 93.Bailey WC, Byrd RB, Glassroth JL, Hopewell PC, Reichman LB. Preventive treatment of tuberculosis. Chest. 1985;87(2 Suppl):128S–32S. doi: 10.1378/chest.87.2_supplement.128s. [DOI] [PubMed] [Google Scholar]
- 94.Livengood JR, Sigler TG, Foster LR, Bobst JG, Snider DE., Jr Isoniazid-resistant tuberculosis. A community outbreak and report of a rifampin prophylaxis failure. JAMA. 1985;253(19):2847–9. doi: 10.1001/jama.253.19.2847. [DOI] [PubMed] [Google Scholar]
- 95.Finnell SM, Christenson JC, Downs SM. Latent tuberculosis infection in children: a call for revised treatment guidelines. Pediatrics. 2009;123(3):816–22. doi: 10.1542/peds.2008-0433. [DOI] [PubMed] [Google Scholar]
- 96.Lobato MN, Jereb JA, Castro KG. Do we have evidence for policy changes in the treatment of children with latent tuberculosis infection? Pediatrics. 2009;123(3):902–3. doi: 10.1542/peds.2008-2664. [DOI] [PubMed] [Google Scholar]
- 97.Capstick TG, Laycock D, Lipman MC. Treatment interruptions and inconsistent supply of anti-tuberculosis drugs in the United Kingdom. Int J Tuberc Lung Dis. 2011;15(6):754–60. doi: 10.5588/ijtld.10.0568. [DOI] [PubMed] [Google Scholar]
- 98.Balcells ME, Thomas SL, Godfrey-Faussett P, Grant AD. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis. 2006;12(5):744–51. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.van Halsema CL, Fielding KL, Chihota VN, Russell EC, Lewis JJ, Churchyard GJ, Grant AD. Tuberculosis outcomes and drug susceptibility in individuals exposed to isoniazid preventive therapy in a high HIV prevalence setting. AIDS. 2010;24(7):1051–5. doi: 10.1097/QAD.0b013e32833849df. [DOI] [PubMed] [Google Scholar]
- 100.Denkinger CM, Pai M, Dowdy DW. Do we need to detect isoniazid resistance in addition to rifampicin resistance in diagnostic tests for tuberculosis? PLoS One. 2014;9(1):e84197. doi: 10.1371/journal.pone.0084197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ministerio de Salud. Norma Técnica de Salud 104: para la atención integral de las personas afectadas por tuberculosis. 2013 http://www.minsa.gob.pe/dgsp/observatorio/documentos/infecciones/RM715-2013_MINSA_TB.pdf. Date last accessed: September 14 2016.
- 102.Blumberg HM, Burman WJ, Chaisson RE, Daley CL, Etkind SC, Friedman LN, Fujiwara P, Grzemska M, Hopewell PC, Iseman MD, Jasmer RM, Koppaka V, Menzies RI, O’Brien RJ, Reves RR, Reichman LB, Simone PM, Starke JR, Vernon AA. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 103.World Health Organization. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. 2014 http://www.who.int/tb/publications/pmdt_companionhandbook/en/. Date last updated: 2014. Date last accessed: March 8 2016. [PubMed]


