ABSTRACT
Annexin A1 (ANXA1) is a Ca2+-binding protein overexpressed in the invasive stages of prostate cancer (PCa) development; however, its role in this tumor metastatization is largely unknown. Moreover, hypoxic conditions in solid tumors have been related to poor prognosis in PCa patients. We have previously demonstrated that ANXA1 is implicated in the acquisition of chemo-resistant features in DU145 PCa cells conferring them a mesenchymal/metastatic phenotype. In this study, we have investigated the mechanisms by which ANXA1 regulates metastatic behavior in LNCaP, DU145 and PC3 cells exposed to hypoxia. ANXA1 was differentially expressed by PCa cell lines in normoxia whereas hypoxic stimuli resulted in a significant increase of protein expression. Additionally, in low oxygen conditions ANXA1 was extensively secreted out-side the cells where its binding to formyl peptide receptors (FPRs) induced cell invasion. Loss and gain of function experiments performed by using the RNA interfering siANXA1 and an ANXA1 over-expressing plasmid (MF-ANXA1), also confirmed the leading role of the protein in modulating LNCaP, DU145 and PC3 cell invasiveness. Finally, ANXA1 played a crucial role in the regulation of cytoskeletal dynamics underlying metastatization process, such as the loss of adhesion molecules and the occurrence of the epithelial to mesenchymal transition (EMT). ANXA1 expression increased inversely to epithelial markers such as E-cadherin and cytokeratins 8 and 18 (CKs) and proportionally to mesenchymal ones such as vimentin, ezrin and moesin. Our results indicated that ANXA1 may be a key mediator of hypoxia-related metastasis-associated processes in PCa.
KEYWORDS: annexin A1, cell invasion, epithelial to mesenchymal transition, ERM complex, hypoxia, intermediate filaments
Introduction
Prostate cancer (PCa) is one of the most widespread tumors and the sixth leading cause of cancer-related death in men of Western industrialized countries.1 PCa onset and development are generally androgen-dependent but in the course of time the tumor tends to convert into a castration-resistant disease that remains largely incurable.2 This transition to castration resistant PCa (CRPC) is supposed to originate by several genetic mutations underlying activation of oncogenes and/or inactivation of tumor suppressor genes,3 however, many of the mechanisms essential for PCa progression remain to be clearly defined.
A number of evidences are accumulating that the tumor microenvironment strongly contributes to the malignant transformation of cancer cells and that hypoxia is one of the environmental feature essential to progression of solid tumors, including PCa. It has been well known that, because of a size increase, the inner areas of the tumor mass become gradually hypoxic until enough blood vessels are formed. Hypoxic conditions within tumors purportedly result in increased phosphorylation levels of some kinases such as extracellular signal-regulated kinase (ERK) and p38, which in turn lead to improved hypoxia inducible factor 1 (HIF1) stability and activity.4 Once stabilized and activated, HIF1 dimerizes in the nucleus where it binds to Hypoxia Responsive Elements (HREs). In this way, it regulates genes generally associated with a poor prognosis,5,6 and with decisive roles in some stages of tumor development, including cancer cell spreading and invasion and metastasis formation.6 One of the proteins upregulated in low oxygen conditions by HIF-1 overexpression is annexin A1 (ANXA1).7
ANXA1 is a 37 kDa protein belonging annexin superfamily of proteins and able to bind membrane phospholipids in a Ca2+-dependent manner. This protein is involved in a wide range of physio-pathological processes, including cancer development.8,9,10
ANXA1 dysregulation in PCa has been reported by numerous studies with controversial results. It has been shown that overall ANXA1 expression is unaffected11,12 or downregulated in in situ PCa13,2 whereas cancer microarray databases from Oncomine (publicly available at http://www.oncomine.org) show an increase of the protein expression in the more metastatic PCa stages.14,15 We have previously demonstrated that ANXA1 is able to confer and maintain a more aggressive phenotype in chemo-resistant PCa cells.9 Here we have investigated the mechanism(s) by which ANXA1 promotes PCa progression, utilizing a well characterized in vitro experimental model of cancer development based on the comparison of the 3 human LNCaP, DU145 and PC3 PCa cells. These cell lines differ in their phenotypes and malignancy levels and reflect the androgen-independent progression of PCa: more interestingly, they show dissimilar expression profiles of ANXA1 protein.16 Thus, we have analyzed the effects of short- and long-term hypoxic conditions on ANXA1 expression and on the metastatic-associated functional behaviors of transiently ANXA1 knock down and overexpression in PCa cells.
We show that ANXA1 expression strongly increased during hypoxia and that the protein played a central role in some of the hypoxia-related cytoskeletal events underlying the acquisition of an improved metastatic potential in PCa cells. Moreover, the protein conferred a high aggressive phenotype to all PCa cell lines acting intracellularly and extracellularly as pro-metastatic factor.
Results
ANXA1 expression and localization in PCa cell lines
LNCaP, DU145 and PC3 PCa cell lines show many differences about their genotype, as RB1 and p53 mutations, and phenotype, as in adhesion, migration and invasion capacities.17 Thus, we initially focused to describe ANXA1 expression in these PCa cells. As shown in Figure 1A, RT-PCR analysis revealed that DU145 and PC3 expressed a large amount of ANXA1 whereas LNCaP had low protein expression.
Figure 1.
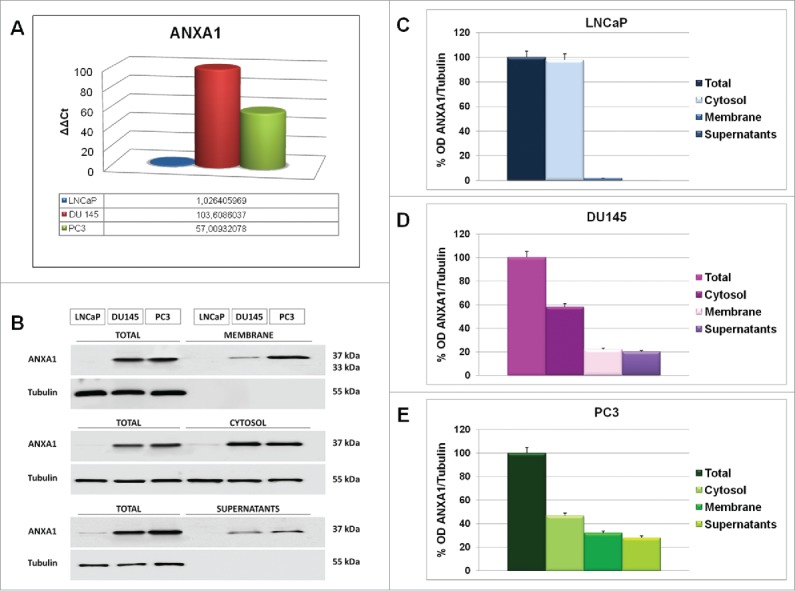
(A) RT-PCR for ANXA1 mRNA expression in LNCaP, DU145 and PC3 cells, measured on levels of HPRT in the same experimental models. (B) Western blotting showing the expression of ANXA1 in total, membrane, cytosolic and supernatant cell compartments from LNCAP, DU145 and PC3 lines. (C, D, E) Densitometry for total, membrane, cytosol and extracellular ANXA1 relative expression in LNCaP (C), DU145 (D) and PC3 (E) cell lines. The protein bands were normalized on tubulin levels. The blots were exposed to Las4000 (GE Healthcare Life Sciences) and the relative intensities of bands were determined using ImageQuant software (GE Healthcare Life Sciences).The data are representative of 5 experiments with similar results.
ANXA1 can be cytoplasmic, membrane associated and/or secreted in extracellular environment.18,9 Hence, we analyzed sub-cellular protein expression by Western blotting. In particular, we obtained membrane, cytosol and supernatant protein extracts as described in Materials and Methods section. In DU145 and PC3 cells we found ANXA1 signal in both cytosol and membrane extracts (Fig. 1, panels B-E). Additionally, ANXA1 was detected in DU145 and PC3 cell supernatants suggesting active secretion of the protein in the extracellular environments. A weak ANXA1 signal was obtained from LNCaP total and cytosolic extracts (Fig. 1, panels B-E) confirming RT-PCR results.
ANXA1 expression increases in hypoxic conditions
A growing body of evidences indicates that cancer cells surviving to hypoxic insults are arguably responsible for cancer progression, metastasis and resistance to therapies.19 These hypoxia-exposed cancer cells express HIF1-α that in turn regulates the transcription of several genes with a crucial role in cytoskeletal dynamics, cell adhesion and motility such as EMT-related proteins,20 some Ezrin/Radixin/Moesin (ERM) family members,21 Intermediate Filament proteins (IFs)22 and Mitogen-Activated Protein Kinases (MAPKs).23
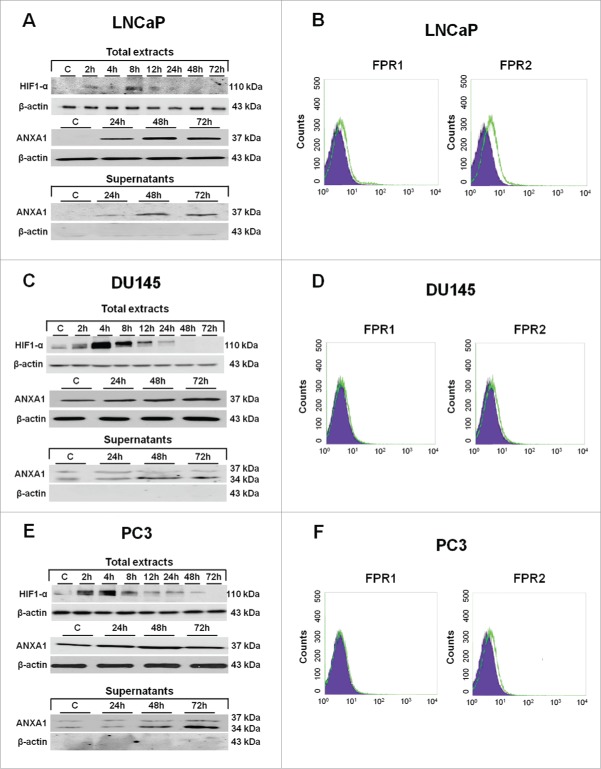
Exploiting the differential expression of ANXA1 protein in LNCaP, DU145 and PC3, we next analyzed ANXA1 expression profiles in these cell lines maintained or not in short- and long-term hypoxic conditions. Hypoxia was induced as described in Materials and Methods section. The expression of HIF1-α and of some of its target proteins was monitored by Western blot (Fig. 2, panels A, C, E) and immunofluorescence analysis (Fig. 2, panels B, D, F).
Figure 2.
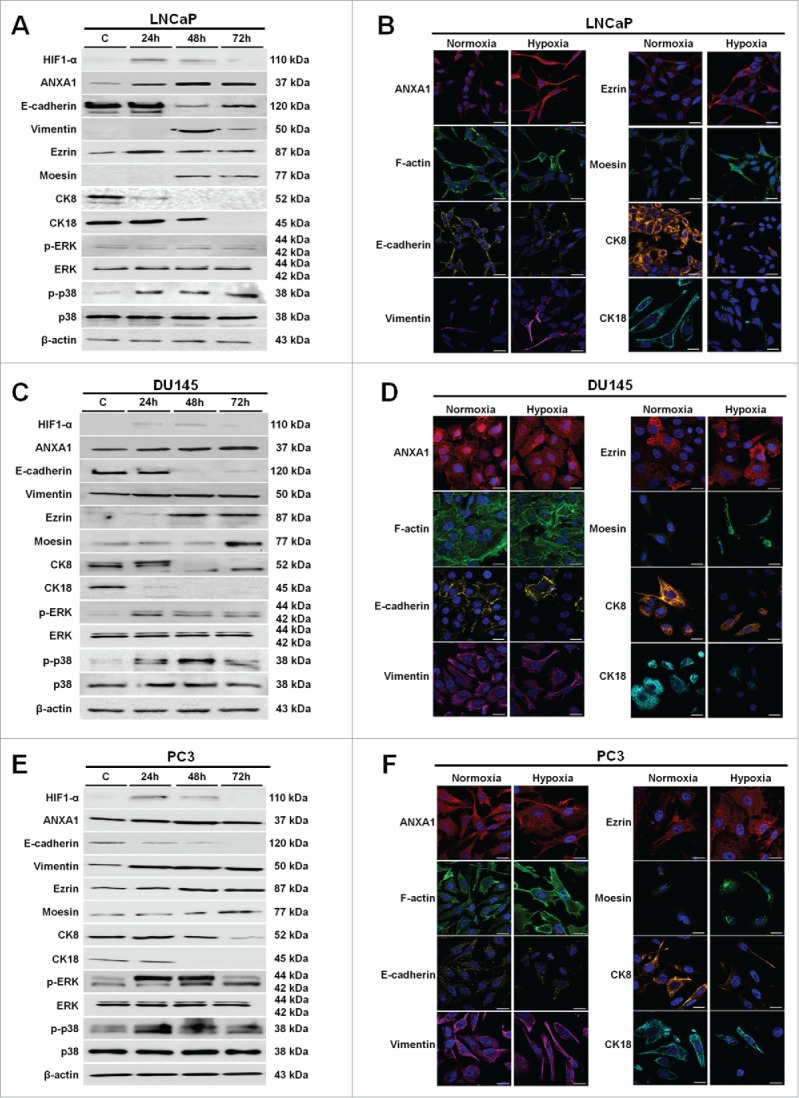
Western blot using antibodies against HIF1-α, ANXA1, E-cadherin, vimentin, ezrin, moesin, CK8, CK18, p-ERK, ERK, p-p38, p38 on protein extracts from LNCaP (A), DU145 (C) and PC3 (E) cells. Protein normalization was performed on β-actin levels. Immunofluorescence analysis to detect: ANXA1, F-actin, E-cadherin, vimentin, ezrin, moesin, CK8 and CK18 on LNCaP (B), DU145 (D) and PC3 (F) cells in both normoxia and hypoxia conditions. Immunofluorescence images refer to 48 hours of low oxygen treatment. Nuclei were stained with DAPI. Magnification 63x. Bar = 10 μm. The data are representative of 3 experiments with similar results.
The phosphorylation increase of p38 and ERK kinases has been reported to underlie significant enhance of HIF1-α stability and activity.24 Therefore, we evaluated the activation status of these proteins in our experimental low oxygen conditions. As reported in Figure 2 (panels A, C, E), hypoxia induced the phosphorylation of p38 kinase in all considered PCa cell lines whereas only in DU145 and PC3 cells we observed the activation of ERK. Thus, we assessed HIF1-α expression profiles in hypoxia exposed PCa cells up to 72 hours of treatment. As shown, HIF1-α expression increased from 24 hours of treatment in all PCa cells. Interestingly, in parallel to HIF1-α augment we observed ANXA1 overexpression in LNCaP, DU145 and PC3 cells (Fig. 2, panels A-F) (Fig. S1, panels A-C).
Numerous studies have demonstrated that tumor metastasis is associated with EMT, a biological process in which epithelial cells lose cell-cell contact, E-cadherin expression and acquire mesenchymal features, resulting in enhanced motility, invasiveness and vimentin expression.25,26 The signaling pathways that rule EMT in tumor progression are triggered by several factors including hypoxic stimuli. Here, we found a significant downregulation of the epithelial marker E-cadherin and a substantial increase of the mesenchymal marker vimentin together with its overall cytosolic re-organization (Fig. 2, panels A, C, E; Fig. S2, panels A-F) confirming the occurrence of a hypoxia-induced EMT switch. Immunofluorescence analysis corroborated the Western Blot results (Fig. 2, panels B, D, F) and showed the loss of cortical actin and the gain of numerous stress fibers indicating an overall cytoskeletal re-organization. Further investigation showed as well a considerable downregulation of the epithelial cytokeratins 8 and 18 (CK8, CK18). These proteins are 2 of the mainly expressed cytokeratins within normal human prostate epithelium and typically indicate a low-aggressive phenotype of PCa cells and a good prognosis in patients (Fig. 2, panels A-F; Fig. S3, panels A-F).27
ERM protein members such as ezrin and moesin have been frequently described to have a role in the progression of many types of human malignancies since their overexpression alters cell shape, adhesion, and motility and correlates with the metastatic behavior of many human cancers.28 Therefore, we hypothesized a modulation of ERM protein expression in LNCaP, DU145 and PC3 PCa cell lines during low oxygen stress adaptation. Our results showed an intriguing increase of both ezrin and moesin proteins starting from 24 hours of hypoxic treatment in PCa cells (Fig. 2, panels A-F; Fig. S4, panels A-F).
Hypoxic stress determines significant changes in ANXA1 sub-cellular localization
ANXA1 protein has been frequently detected in several sub-cellular localizations: in the cytosol, at the plasma membrane surface (inner and/or outer membrane side), in the nucleus and in the extracellular environments enclosed or not in different types of vesicles such as micro-particles (MPs).29 Consequently, we have investigated the effects of hypoxia on ANXA1 sub-cellular localization in PCa cell lines. We found a huge secretion of ANXA1 in the extracellular environments starting from 24 hours of incubation in low oxygen conditions. Additionally, we detected the cleaved form (34 kDa) of ANXA1 protein (Fig. 3, panels A, C, E) in both DU145 and PC3 cells. The occurrence of ANXA1 in the extracellular environment has been frequently reported as a way for the protein to exert its pro-oncogenic effects, mainly interacting with FPRs that were identified as its cognate partners and were involved in actin polymerization and cell motility.30 Therefore, we analyzed by flow cytometry (Fig. 3, panels B, D, F) and RT-PCR (Fig. S4, panels A, B) FPR expression in LNCaP, DU145 and PC3 PCa cells. Our results showed that all PCa cell lines express both FPR1 and FPR2 with no significant differences with exception of a major expression of FPR2 in LNCaP cells (Fig. 3, panels B, D, F).
Figure 3.
Western blots showing the increased expression of HIF1-α when LNCaP (A), DU145 (C) and PC3 (E) cells were incubated in hypoxia conditions as described in Materials and Methods section. Total protein extracts from 2 up to 72 hours of hypoxia were analyzed with HIF1-α antibody. Total and supernatants protein extracts at 24, 48 and 72 hours of hypoxia were also analyzed with ANXA1 antibody. Protein normalization was performed on β-actin levels. The data are representative of 3 experiments with similar results. (B) (D) (E) Cell surface expression of FPR1 and FPR2 was analyzed by flow cytometry. The violet area in the plot is relative to human IgG1; FPR signals were showed in green.
Secretion of ANXA1 protein has a crucial role during hypoxia adaptation of PCa cells
Several lines of evidence exist referring that ANXA1-nFPR bond could trigger numerous cellular responses, as the induction of cell motility.31,32 and that the occurrence of this event in cancer cells possibly will result in the acquisition of a very aggressive phenotype characterized by the acquisition of augmented invasion capability and of stem-cell like features.9
Therefore, we evaluated if ANXA1 could have a pro-invasive role in LNCaP, DU145 and PC3 cell lines thanks to its ability to activate FPRs, as previously reported in pancreas and colon carcinomas.18,33 We performed a matrigel invasion assay by stimulating or not LNCaP, DU145 and PC3 cells by administration of Ac2–26 peptide, the N-terminus mimetic peptide of ANXA1, of the natural FPR agonist N-Formylmethionyl-leucyl-phenylalanine (fMLP) peptide30 and of 2 FPR antagonists, in normoxia and hypoxia conditions.
As showed in Figure 4 (panels A-C) when treated with Ac2–26 (1 μM) and fMLP (50 nM), LNCaP (Fig. 4, panel A), DU145 (Fig. 4, panel B) and PC3 (Fig. 4, panel C) cells showed an invasion increase through the coating of matrigel in both normal (data not shown) and low oxygen conditions. In all cases, experimental points were compared with non treated controls, with cells treated by the selective FPR1 antagonist cyclosporin H (CicloH; 500 nM) or the selective FPR2 antagonist WRW4 (10 μM). On the other hand, the rate of invasion decreases when hypoxia exposed PCa cells were treated by Ac2–26 peptide together with FPR antagonists (Fig. 4, panels A-C).
Figure 4.
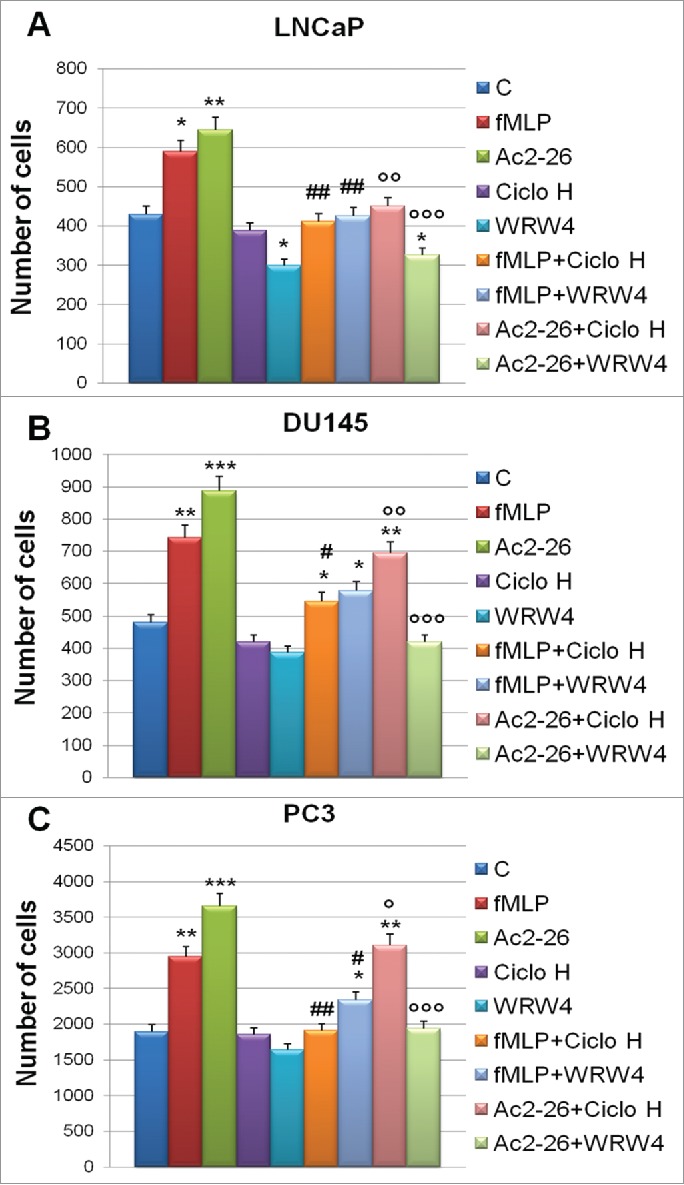
Invasiveness rate of LNCaP (A), DU145 (B) and PC3 (C) cells exposed to hypoxia for 48 hours in the presence of FPRs agonists, as fMLP (50 nM) and Ac2–26 (1 µM), and antagonists as Ciclosporine H (CicloH; 500 nM) and WRW4 (10 µM). Data represent mean cell counts of 12 separate fields per well ± SEM of 5 experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs untreated cells; #p < 0.05, ###p < 0.005 vs fMLP treated cells and °p < 0.05, °°p < 0.01, °°°p < 0.001 vs Ac2–26 treated cells.
Altogether our data strongly support the functional engagement of FPR receptors by ANXA1 in regulating invasion in all considered PCa cells.
ANXA1 regulates PCa cell invasion capability in hypoxic conditions
Epithelial cells possess apical-basal polarity and are strictly connected to adjacent cells by cell adhesion molecules and junctions. During embryogenesis, they undertake EMT to generate a differentiated tissue. Differently from epithelial cells, mesenchymal cells have front end to back end polarity and the capability to move from the initial site by digesting the surrounding extracellular matrix (ECM).34
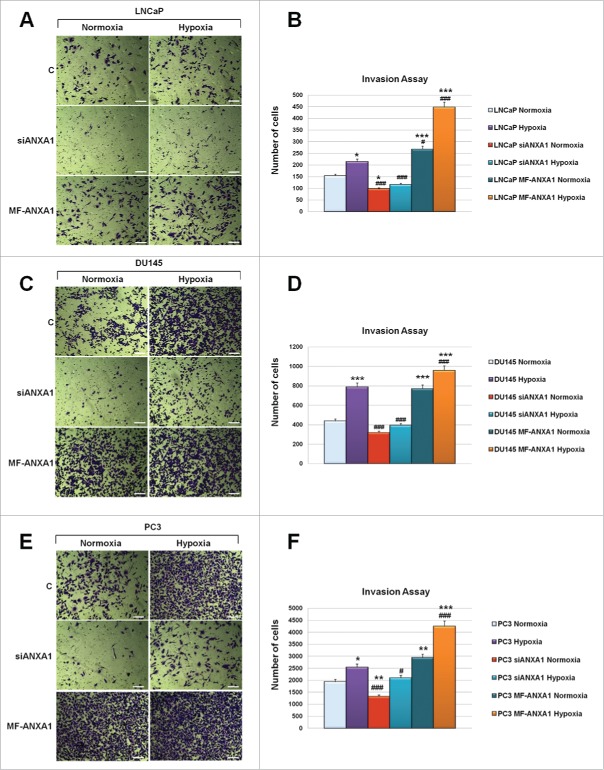
Since our previous results showed a strict correlation between the expression increase of ANXA1 and of some metastatic-related proteins such as vimentin, ezrin and moesin in hypoxia conditions, we next evaluated the effects of ANXA1 loss and gain of function by transfecting LNCaP, DU145 and PC3 cells respectively with siRNAs (siANXA1) and with ANXA1 over-expressing plasmid (MF-ANXA1). As shown in Figure 5, hypoxia induced a significant increase of invasiveness in PCa cells whereas siANXA1 were able to partially (LNCaP) or drastically (DU145 and PC3) reduce invasion (Fig. 5, panels A-F). Interestingly, ANXA1 loss of function strongly decreases LNCaP invasion capability in low oxygen conditions (Fig. 5, panels A, B).
Figure 5.
Invasiveness rate of LNCaP, DU145 and PC3 cells transfected or not with siANXA1 and MF-ANXA1 plasmid, further incubated or not in hypoxia conditions. 48 hours from transfection correspond to invasion assay starting point whereas 48 hours refer to invasion assay ending one. Representative bright field images of invasion assay experimental end points for LNCaP (A), DU145 (C) and PC3 (E) cells. Invasiveness rate was determined by counting stained cells on the lower surface of the filters of LNCaP (B), DU145 (D) and PC3 (F) cells. Data represent mean cell counts of 12 separate fields per well ± SEM of 5 experiments. *p < 0.05, **p < 0.01, ***p < 0.001 vs untreated normoxia; #p < 0.05, ###p < 0.005 vs untreated hypoxia.
On the other hand, a different scenario disclosed when PCa cells were treated to over-express ANXA1 protein (MF-ANXA1). In this case, we observed a significant enhance of PCa cell line invasion capability in both normoxia and hypoxia conditions (Fig. 5, panels A-F).
ANXA1 expression strictly correlates with PCa cell mesenchymal phenotype
As mentioned above, numerous translational studies have suggested a link between loss of epithelial markers, gain of mesenchymal markers, overall re-organization of cytoskeletal proteins and the induction of signaling pathways that promote EMT and metastatization of PCa cells.35
Therefore, we defined by Western Blot analysis the expression profiles of the proteins involved in EMT in normal and hypoxic conditions and their variations following ANXA1 downregulation. Our results showed a significant reversion of EMT process upon ANXA1 knock down in normoxia and hypoxia exposed PCa cells suggested by noteworthy E-cadherin enhancement and vimentin decrease (Fig. 6, panels A-C). The loss of mesenchymal features was also confirmed by enhancement of the 2 epithelial cytokeratins 8 and 18 (Fig. 6, panels A-C). Interestingly, our results showed a strict correlation between ANXA1, ezrin and moesin expression. Specifically, we found a significant reduction of these proteins coincidently with siANXA1 transfection similar to those observed in low oxygen conditions (Fig. 6, panels A-C).
Figure 6.
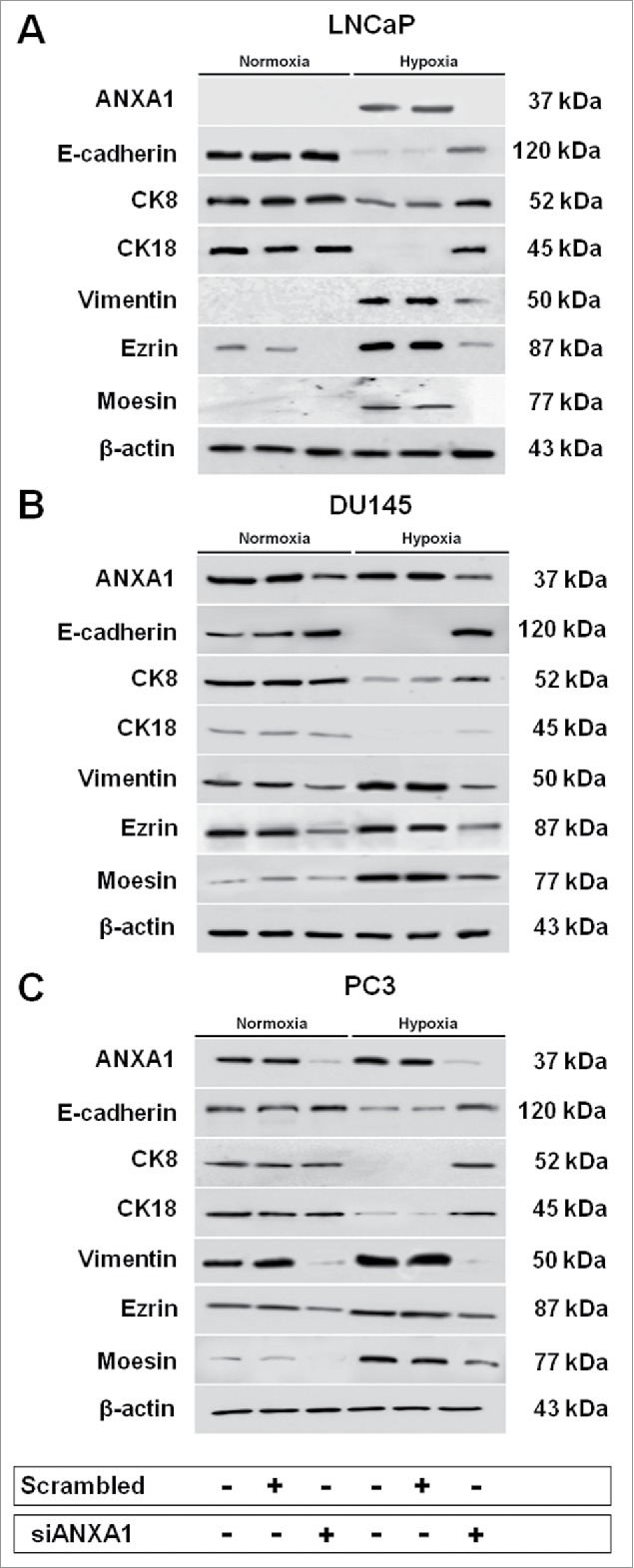
Western blot analysis performed using antibodies against ANXA1, E-cadherin, CK8, CK18, vimentin, ezrin and moesin on protein extracts from LNCaP (A), DU145 (B) and PC3 (C) cells treated or not with scrambled and siANXA1s for 48 hours. After transfection cells were exposed or not to hypoxia for 48 hours. Protein normalization was performed on β-actin levels. The data are representative of 3 experiments with similar results.
Discussion
At the begin, PCa growth is typically androgen-dependent, however it tends to convert to androgen-independent disease (CRPC) over time following pharmacological/surgical castration.36 The process by which PCa cells become hormone-resistant is unidentified, but it has been proposed that hormone depletion could result in a selective advantage to androgen-independent cells, which grow, repopulate the tumor and eventually migrate away from the original site to give rise to metastasis.37 The evolution from hormone-dependent to hormone-independent PCa is supposed to be an effect of genetic alterations,38 however numerous evidences are accumulating about the role of tumor microenvironment on the outset of a metastatic disease.39
Hypoxia is an essential feature of the microenvironment of several solid tumors including those of PCa.40,41 One of the key mechanisms mediating the adaptation to low oxygen conditions is the induction of HIF-1, a factor with critical roles in many aspects of cancer biology.6 HIF-1 coordinates the adaptive cellular response to low oxygen microenvironment by activating gene programs regulating metabolism, angiogenesis, cell proliferation, differentiation, and apoptosis, cell migration and invasion. Both initiation and progression of tumorigenesis are promoted by HIF signaling and tumors use several strategies to trigger this pathway.39 Interestingly, the accumulation of HIF-1 has been associated with ANXA1 expression increase.7 The role of this protein in PCa development and progression is still debated, however we have recently showed that the protein plays a significant part in regulating aggressive behaviors of a chemo-resistant sub-line of DU145 PCa cells.9 In the present study, we demonstrated that ANXA1 is able to mediate some of the pro-oncogenic hypoxia-related events in vitro.
We have taken advantage of a widely used experimental model of PCa progression represented by the androgen-dependent cell line LNCaP and by the androgen-independent cell lines DU145 and PC3. Protein expression and localization in PCa cell sub-cellular compartments showed that LNCaP cells were characterized by very low expression of ANXA1 whereas considerable amounts of the protein were observed for DU145 and PC3 cell lines, indicating a correlation between malignant phenotype and protein expression. Moreover, DU145 and PC3 cells displayed ANXA1 translocation onto plasma membrane and a basal level of protein secretion in the extracellular environments.
Hence, we evaluated possible alterations of ANXA1 expression in LNCaP, DU145 and PC3 PCa cell lines exposed to hypoxia. The activation of hypoxia-related pathway was confirmed through the observation of a significant increase of phosphorylation of p38 and ERK kinases, an event which is reported as precursor of HIF1-α activity induction. HIF1-α overexpression is sufficient to induce EMT and invasion in multiple cell types by modulating cytoskeletal dynamics.42,43,44 We observed E-cadherin downregulation, vimentin upregulation and a significant reduction of the 2 epithelial cytokeratins CK8 and CK18 in all PCa cells starting from 24 hours of hypoxic treatment. Interestingly, at the same experimental time points, we also found a significant increase of ezrin. This is a membrane-cytoskeleton-binding protein and a member of the ERM protein family.45 Several studies suggest that ezrin may play a key role in tumor development, invasion, and metastasis, possibly regulating adhesion molecules, cell signal transduction, and signaling to other cell membrane channels in the tumor.28 Together with ezrin, we found a significant expression increase of moesin, another ERM protein which has been equally involved in EMT, metastatization and cancer progression.46,47,28
ANXA1 biological effects could differ according to its intra- or extra-cellular localization.29 Cytosolic ANXA1 for example has been frequently implicated in cytoskeletal organization in several cellular models.48,31,32,9,18,10 ANXA1 localization after hypoxia administration to PCa cell lines showed a noteworthy alteration of its expression profiles. The protein was indeed significantly expressed onto plasma membrane and in the extracellular environments over the time during hypoxia treatment.
The extracellular form of ANXA1 has been described to stimulate cell motility and cancer cell invasion capability, mostly interacting with specific receptors.33,18,9 These have been identified as members of the G-protein coupled FPR family that is involved for the most part in cell motility.30 Our results showed that these receptors are expressed in PCa cell lines and that they are able to mediate PCa cell invasion following stimulation with the ANXA1 mimetic peptide Ac2–26. The alteration of ANXA1 expression and localization profiles by hypoxia suggests an adaptive role of the protein in PCa cells to the adverse tumor microenvironments and implicates a possible mechanism by which cancer cells may acquire more aggressive behavior following exposure to hypoxic conditions. This adaptive role of ANXA1 in PCa progression was to some extent previously observed by Geary et al.,49 which showed how ANXA1 secreted by cancer associated fibroblast (CAF) could contribute to tumor stem cell dynamics and sustain PCa onset and development.
While ANXA1 seems to be inducible by hypoxia in a HIF-1-dependent fashion,7 a functional role for the protein under low oxygen conditions has not been fully investigated. ANXA1 protein has also been reported to promote migration and invasion as a modulator for EMT phenotypic switch.50,9 Our hypothesis was that ANXA1 could induce the acquisition of a mesenchymal phenotype during low oxygen PCa cell adaptation and therefore, to play a crucial role in hypoxia-related invasion capability. Interestingly in LNCaP cells where ANXA1 expression is feeble, the MF-ANXA1 over-expressing plasmid determined a significant increase of invasiveness, by contrast the siRNA-mediated knock down (siANXA1) was able to drastically reduce invasion, mainly the hypoxia-induced one. On the contrary in DU145 and PC3 cell lines where ANXA1 is typically overexpressed, the protein loss significantly reduced invasion capability in both normoxia and hypoxia environment. Even in this case, ANXA1 overexpression acted as pro-invasive stimulus, confirming the initial hypothesis about a pro-metastatic role of the protein in PCa progression.
To identify a potential molecular mechanism by which ANXA1 regulates hypoxia-mediated cell invasion, we analyzed the effects of ANXA1 deregulation on the expression of the epithelial/mesenchymal PCa markers and of 2 ERM proteins, ezrin and moesin. Our results confirmed that ANXA1 is able to mediate EMT switch as it does in other tumors, by inducing the loss of the epithelial E-cadherin, CK8 and CK18 proteins and the overexpression of the mesenchymal marker vimentin. Additionally, we demonstrated for the first time a strict correlation between ezrin, moesin and ANXA1 expression confirming once again, the role of this protein in regulating the expression of cytoskeletal proteins underlying metastatic process.
Taken together, the results from this study suggest that hypoxia may modify the expression and the localization of ANXA1 which in turn promotes hypoxia-related cell invasion by regulating expression and activity of proteins involved in EMT, cell shape, adhesion and motility. Our results about ANXA1 contribution to PCa cell invasion particularly under hypoxic conditions suggest that this protein may act as a key signaling factor in modulating PCa cell characteristics and behavior in response to hypoxia. The present data suggest that ANXA1 may serve as a potential target for therapeutic interventions for metastatic PCa.
Materials and methods
Cell cultures
LNCaP FGC (Fast Growth Clone), DU145 and PC3 cells were purchased from American Type Culture Collection (ATCC) and cultured following provider's instructions (www.lgcstandards-atcc.org). Briefly, cells were cultured in RPMI (Euroclone) containing 10% of heat-inactivated fetal bovine serum (FBS; Lonza) supplemented with antibiotics (10000 U/ml penicillin and 10 mg/ml streptomycin; Lonza) and were grown at 37°C in 5% CO2 - 95% air humidified atmosphere.
RNA isolation and quantitative RT-PCR
mRNA levels of LNCaP, DU145 and PC3 cells were analyzed by Real-time PCR using the Light Cycler 480 II instrument (Roche). 1 µg of total RNA extracted from cells was reverse transcribed into cDNA with Transcriptor First Strand cDNA Synthesis Kit (Roche). cDNAs were processed using Light Cycler 480 Probes Master mix and Real Time Ready Catalog Assay primers (Roche) for ANXA1 (5′-ATCAGCGGTGAGCCCCTATC-3′, 3′-TTCATCCAGGGGCTTTCCTG-5′) and HPRT (5′-GACCAGTCAACAGGGGACAT-3′, 3′-CCTGACCAAGGAAAGCAAAG-5′) following the manufacturer's protocols (www.lifescience.roche.com). Results were analyzed using the Delta-Delta CT method.
Cytosol and membrane extracts
LNCaP, DU145 and PC3 protein extracts were obtained as previously reported.9 Briefly, cells were harvested and centrifuged for 5 minutes at 600 × g at 4°C. After that, the pellets were resuspended in 4 ml of lysis buffer (Tris HCl 20 mM, pH 7,4; sucrose 250 mM; DTT 1 mM; protease inhibitors, EDTA 1 mM in water), sonicated and centrifuged at 4°C for 10 minutes at 5000 × g. The obtained supernatants were ultra-centrifuged for 1 hour at 100000 × g at 4°C, until to get new supernatants that represent cytosol extracts. The pellets were then resuspended in 250 µl of solubilization buffer (Tris HCl 20 mM, pH 7,4; DTT 1 mM; EDTA 1 mM; Triton X-100 1%, in water) and left overnight on orbital shaker at 4°C. After that, the solution was centrifuged for 30 minutes at 50000 × g at 4°C: the supernatants represent membrane extracts.
Supernatant analysis
Cell growth media were harvested, frozen at −80°C and lyophilized. Dried samples were suspended in lysis buffer containing protease inhibitors and left at 4°C for 30 minutes. After centrifugation, the supernatants containing protein extracts were analyzed by Western blotting.
Hypoxic culture conditions
Hypoxic culture conditions were obtained by incubating cells in tissue culture dishes in a modular incubator chamber (Billups-Rothenberg Inc.) flushed with a gas mixture containing 5% CO2 and 95% N2 at 37°C. Cells were then harvested at different times (from 2 up to 72 hours from treatments) and analyzed as described below.
Western blotting
Protein expression was examined by Western blot, as previously described.48 Proteins were visualized using the chemioluminescence detection system (Amersham) after incubation with primary antibodies against ANXA1 (rabbit polyclonal; 1:10000; 71–3400, Invitrogen), α-tubulin (mouse monoclonal; clone DM1A; 1:1000; Sigma-Aldrich), E-cadherin (mouse monoclonal; clone 36/E-Cadherin; 1:10000; BD Transduction Laboratories), vimentin (mouse monoclonal; clone E-5; 1:1000; Santa Cruz Biotechnologies), HIF1-α (rabbit polyclonal; clone A300–286A; 1:5000; Bethyl Laboratories), Ezrin (mouse monoclonal; clone 3C12; 1:250; Santa Cruz Biotechnologies), Moesin (mouse monoclonal; clone E-10; 1:250; Santa Cruz Biotechnologies), CK8 (mouse monoclonal, clone M20; 1:1000; Santa Cruz Biotechnologies), CK18 (mouse monoclonal, clone C-04; 1:1000; Santa Cruz Biotechnologies), p-ERK (rabbit monoclonal, clone Aw39; 1:1000; Cells Signaling), ERK (mouse monoclonal, clone MK12; Cells Signaling), p-p38 (mouse monoclonal; clone D-8; 1:1000; Santa Cruz Biotechnologies), p38 (rabbit polyclonal; clone C-20; 1:1000; Santa Cruz Biotechnologies), β-actin (mouse monoclonal; clone C-4, 1:1000; Santa Cruz Biotechnologies). The blots were exposed to Las4000 (GE Healthcare Life Sciences) and the relative band intensities were determined using ImageQuant software (GE Healthcare Life Sciences). Results were considered significant if p < 0.01.
Confocal microscopy
LNCaP, DU145 and PC3 cells were fixed in p-formaldehyde (4% v/v in PBS) for 5 minutes. The cells were permeabilized in Triton X-100 (0.5% v/v in PBS) for 5 minutes, and then incubated in goat serum (Lonza; 20% v/v PBS) for 30 minutes, and with rabbit anti-ANXA1 antibody (1:100; Invitrogen), mouse anti-E-cadherin (1:1000; BD Transduction Laboratories), mouse anti-vimentin (1:500; Santa Cruz Biotechnologies), mouse anti-ezrin and anti-moesin (1:100; Santa Cruz Biotechnologies), mouse anti-CK8 and anti-CK18 (1:1000; Santa Cruz Biotechnologies) overnight at 4°C. After two washing steps with PBS, cells were incubated with anti-rabbit and/or anti-mouse AlexaFluor (488 and/or 555; 1:1000; Molecular Probes) for 2 hours at RT and then with FITC-conjugated anti-F-actin (5 µg/ml; Phalloidin-FITC, Sigma) for 30 minutes at RT in the dark. The coverslips were mounted in Mowiol (Mowiol 4–88, Sigma-Aldrich). A Zeiss LSM 710 Laser Scanning Microscope (Carl Zeiss MicroImaging GmbH) was used for data acquisition. To detect nucleus, samples were excited with a 458 nm Ar laser. A 488 nm Ar or a 555 nm He-Ne laser was used to detect emission signals from target stains. Samples were vertically scanned from the bottom of the coverslip with a total depth of 5 µm and a 63X (1.40 NA) Plan-Apochromat oil-immersion objective. Images and scale bars were generated with Zeiss ZEN Confocal Software (Carl Zeiss MicroImaging GmbH) and presented as single stack. Images were processed using ImageJ software (NIH), Adobe Photoshop CS version 5.0, and figures assembled using Microsoft PowerPoint (Microsoft Corporation).
Flow cytometry
LNCaP, DU145 and PC3 cells were harvested at a number of 1×106 and analyzed for FPR1 and FPR2 proteins as previously described.9 Briefly, pellets were incubated on ice for 1 hour in PBS containing a primary antibody against FPR1 (rabbit polyclonal, clone H-230; 1:500, Santa Cruz Biotechnology) or a primary antibody against FPR2 (mouse monoclonal, clone GM-1D6; 1:100, Genovac). Then, cells incubated on ice for 1 hour in 100 µl of PBS containing or not AlexaFluor 488 anti-rabbit (1:500; Molecular Probes) or Alexa-Fluor 488 anti-mouse (1:500; Molecular Probes). The cells were analyzed with Becton Dickinson FACScan flow cytometer using the Cells Quest program.
Matrigel invasion assay
LNCaP, DU145 and PC3 invasiveness was evaluated using the Trans-well Cell Culture (12 mm diameter, 8.0-fim pore size) purchased form Corning Inc. The chambers were coated with Matrigel (Becton Dickinson Labware) that was diluted with 3 volumes of RPMI serum-free and stored at 37°C until its gelation. Cells were plated in 350 µl of RPMI serum-free at a number of 9×104/insert in the upper chamber of the trans-well. 1,4 ml of RPMI with FBS were put in the lower chamber and the trans-well was left for 48 hours at 37°C in 5% CO2 -95% air humidified atmosphere as normoxia or in hypoxia conditions. In the lower chamber were also added fMLP (50 nM, Sigma Aldrich), Ac 2–26 (1 µM, Tocris Bioscience), Ciclosporin H (CicloH; 500 nM, Alexis-Biochemicals) and WRW4 (10 µM, Tocris Bioscience). After that, the medium was discarded, the filters were washed twice with PBS and fixed with 4% p-formaldehyde for 10 minutes, then with 100% methanol for 20 minutes. The filters so fixed, were stained with 0,5% crystal violet prepared from stock crystal violet (powder, Merck Chemicals) by distilled water and 20% methanol for 15 minutes. After that, the filters were washed again in PBS and cleaned with a cotton bud. The number of cells that had migrated to the lower surface was counted in 12 random fields using EVOS light microscope (10X) (Life technologies Corporation).
siRNA and overexpression plasmid transfection
siRNA sequences against ANXA1 were purchased from IDT (Integrated DNA Technologies Inc.) and used at a final concentration of 5 nM. siRNA Oligo-Scrambled (Santa Cruz Biotechnology) was used as control at the same concentration. 2 µg of a pcDNA3.1 plasmid with c-Myc tagged on the N terminus and FLAG tagged on the C terminus (MF-ANXA1) to over-express ANXA1 was transfected into cells. MF-ANXA1 plasmid was generated as reported previously51 and kindly gifted by Prof. Mauro Perretti (William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, London, UK). pcDNA3.1 empty vector was used as control. siRNAs and plasmids were transfected using Lipofectamine 2000 Reagent (Life technologies Corporation), according to the manufacturer's instructions. Cells were harvested after 48 hours from transfection.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- [1].Haas GP, Delongchamps N, Brawley OW, Wang CY, de la Roza G. The worldwide epidemiology of prostate cancer: perspectives from autopsy studies. Can J Urol 2008; 15:3866-71; PMID:18304396 [PMC free article] [PubMed] [Google Scholar]
- [2].Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, Asangani IA, et al.. The mutational landscape of lethal castration-resistant prostate cancer. Nature 2012; 487:239-43; PMID:22722839; https://doi.org/ 10.1038/nature11125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vaarala MH, Porvari K, Kyllönen A, Vihko P. Differentially expressed genes in two LNCaP prostate cancer cell lines reflecting changes during prostate cancer progression. Lab Invest 2000; 80:1259-68; PMID:10950117; https://doi.org/ 10.1038/labinvest.3780134 [DOI] [PubMed] [Google Scholar]
- [4].Kietzmann T, Mennerich D, Dimova EY. Hypoxia-Inducible Factors (HIFs) and phosphorylation: impact on stability, localization, and transactivity. Front Cell Dev Biol 2016; 4:11; PMID:26942179; https://doi.org/ 10.3389/fcell.2016.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fraga A, Ribeiro R, Príncipe P, Lopes C, Medeiros R. Hypoxia and prostate cancer aggressiveness: a tale with many endings. Clin Genitourin Cancer 2015; 13:295-301; PMID:26007708; https://doi.org/ 10.1016/j.clgc.2015.03.006 [DOI] [PubMed] [Google Scholar]
- [6].Bae KM, Dai Y, Vieweg J, Siemann DW. Hypoxia regulates SOX2 expression to promote prostate cancer cell invasion and sphere formation. Am J Cancer Res 2016; 6:1078-88; PMID:27294000 [PMC free article] [PubMed] [Google Scholar]
- [7].Liao SH, Zhao XY, Han YH, Zhang J, Wang LS, Xia L, Zhao KW, Zheng Y, Guo M, Chen GQ. Proteomics-based identification of two novel direct targets of hypoxia-inducible factor-1 and their potential roles in migration/invasion of cancer cells. Proteomics 2009; 9:3901-12; PMID:19637235; https://doi.org/ 10.1002/pmic.200800922 [DOI] [PubMed] [Google Scholar]
- [8].Yi M, Schnitzer JE. Impaired tumor growth, metastasis, angiogenesis and wound healing in annexin A1-null mice. Proc Natl Acad Sci USA 2009; 106:17886-91; PMID:19805119; https://doi.org/ 10.1073/pnas.0901324106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bizzarro V, Belvedere R, Milone MR, Pucci B, Lombardi R, Bruzzese F, Popolo A, Parente L, Budillon A, Petrella A. Annexin A1 is involved in the acquisition and maintenance of a stem cell-like/aggressive phenotype in prostate cancer cells with acquired resistance to zoledronic acid. Oncotarget 2015; 6:25076-92; PMID:26312765; https://doi.org/ 10.18632/oncotarget.4725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Belvedere R, Bizzarro V, Forte G, Dal Piaz F, Parente L, Petrella A. Annexin A1 contributes to pancreatic cancer cell phenotype, behaviour and metastatic potential independently of Formyl Peptide Receptor pathway. Sci Rep 2016; 6:29660; PMID:27412958; https://doi.org/ 10.1038/srep29660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].LaTulippe E, Satagopan J, Smith A, Scher H, Scardino P, Reuter V, Gerald WL. Comprehensive gene expression analysis of prostate cancer reveals distinct transcriptional programs associated with metastatic disease. Cancer Res 2002; 62:4499-4506; PMID:12154061 [PubMed] [Google Scholar]
- [12].Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C, Tamayo P, Renshaw AA, D'Amico AV, Richie JP, et al.. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell 2002; 1:203-9; PMID:12086878; https://doi.org/ 10.1016/S1535-6108(02)00030-2 [DOI] [PubMed] [Google Scholar]
- [13].Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, et al.. Integrative genomic profiling of human prostate cancer. Cancer Cell 2010; 18:11-22; PMID:20579941; https://doi.org/ 10.1016/j.ccr.2010.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Varambally S, Yu J, Laxman B, Rhodes DR, Mehra R, Tomlins SA, Shah RB, Chandran U, Monzon FA, Becich MJ, Wei JT, et al.. Integrative genomic and proteomic analysis of prostate cancer reveals signatures of metastatic progression. Cancer Cell 2005; 7:1011-9. [DOI] [PubMed] [Google Scholar]
- [15].Setlur SR, Mertz KD, Hoshida Y, Demichelis F, Lupien M, Perner S, Sboner A, Pawitan Y, Andrén O, Johnson LA, et al.. Estrogen-dependent signaling in a molecularly distinct subclass of aggressive prostate cancer. J Natl Cancer Inst 2008; 100:815-25; PMID:18505969; https://doi.org/ 10.1093/jnci/djn150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hasegawa N, Mizutani K, Suzuki T, Deguchi T, Nozawa Y. A comparative study of protein profiling by proteomic analysis in camptothecin-resistant PC3 and camptothecin-sensitive LNCaP human prostate cancer cells. Urol Int 2006; 77:347-54; PMID:17135786; https://doi.org/ 10.1159/000096340 [DOI] [PubMed] [Google Scholar]
- [17].Wu X, Gong S, Roy-Burman P, Lee P, Culig Z. Current mouse and cell models in prostate cancer research. Endocr Relat Cancer 2013; 20:R155-70; PMID:23580590; https://doi.org/ 10.1530/ERC-12-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Belvedere R, Bizzarro V, Popolo A, Dal Piaz F, Vasaturo M, Picardi P, Parente L, Petrella A. Role of intracellular and extracellular annexin A1 in migration and invasion of human pancreatic carcinoma cells. BMC Cancer 2014; 14:961; PMID:25510623; https://doi.org/ 10.1186/1471-2407-14-961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Subarsky P, Hill RP. The hypoxic tumor microenvironment and metastatic progression. Clin Exp Metastasis 2003; 20:237-50; PMID:12741682; https://doi.org/ 10.1023/A:1022939318102 [DOI] [PubMed] [Google Scholar]
- [20].Khan MI, Hamid A, Adhami VM, Lall RK, Mukhtar H. Role of epithelial mesenchymal transition in prostate tumorigenesis. Curr Pharm Des 2015; 21:1240-8; PMID:25506896; https://doi.org/ 10.2174/1381612821666141211120326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang Y, Lin Z, Sun L, Fan S, Huang Z, Zhang D, et al.. Akt/Ezrin Tyr353/NF-kappaB pathway regulates EGF-induced EMT and metastasis in tongue squamous cell carcinoma. Br J Cancer 2014; 110:695-705; PMID:24346284; https://doi.org/ 10.1038/bjc.2013.770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Na N, Chandel NS, Litvan J, Ridge KM. Mitochondrial reactive oxygen species are required for hypoxia-induced degradation of keratin intermediate filaments. FASEB J 2010; 24:799-809; PMID:19897662; https://doi.org/ 10.1096/fj.08-128967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Le QT, Denko NC, Giaccia AJ. Hypoxic gene expression and metastasis. Cancer Metastasis Rev 2004; 23:293-310; PMID:15197330; https://doi.org/ 10.1023/B:CANC.0000031768.89246.d7 [DOI] [PubMed] [Google Scholar]
- [24].Dimova EY, Michiels C, Kietzmann T. Kinases as upstream regulators of the HIF system: their emerging potential as anti-cancer drug targets. Curr Pharm Des 2009; 15:3867-77; PMID:19671044; https://doi.org/ 10.2174/138161209789649358 [DOI] [PubMed] [Google Scholar]
- [25].Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol 2006; 7:131-42; PMID:16493418; https://doi.org/ 10.1038/nrm1835 [DOI] [PubMed] [Google Scholar]
- [26].Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer 2009; 9:265-73; PMID:19262571; https://doi.org/ 10.1038/nrc2620 [DOI] [PubMed] [Google Scholar]
- [27].Van Leenders GJ, Aalders TW, Hulsbergen-Van De Kaa CA, Ruiter DJ, Schalken JA. Expression of basal cell keratins in human prostate cancer metastases and cell lines. J Pathol 2001; 195:563-70; PMID:11745692; https://doi.org/ 10.1002/path.993 [DOI] [PubMed] [Google Scholar]
- [28].Clucas J, Valderrama F. ERM proteins in cancer progression. J Cell Sci 2014; 127:267-75; PMID:24421310; https://doi.org/ 10.1242/jcs.133108 [DOI] [PubMed] [Google Scholar]
- [29].Boudhraa Z, Bouchon B, Viallard C, D'Incan M, Degoul F. Annexin A1 localization and its relevance to cancer. Clin Sci (Lond) 2016; 130:205-20; PMID:26769657; https://doi.org/ 10.1042/CS20150415 [DOI] [PubMed] [Google Scholar]
- [30].Ye RD, Boulay F, Wang JM, Dahlgren C, Gerard C, Parmentier M, Serhan CN, Murphy PM. International union of basic and clinical pharmacology. LXXIII. Nomenclature for the formyl peptide receptor (FPR) family. Pharmacol Rev 2009; 61:119-61; PMID:19498085; https://doi.org/ 10.1124/pr.109.001578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bizzarro V, Fontanella B, A Carratù, Belvedere R, Marfella R, Parente L, Petrella A. Annexin A1 N-terminal derived peptide Ac2-26 stimulates fibroblast migration in high glucose conditions. PLoS One 2012; 7:e45639; PMID:23029153; https://doi.org/ 10.1371/journal.pone.0045639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bizzarro V, Belvedere R, Dal Piaz F, Parente L, Petrella A. Annexin A1 induces skeletal muscle cell migration acting through formyl peptide receptors. PLoS One 2012; 7:e48246; PMID:23144744; https://doi.org/ 10.1371/journal.pone.0048246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Babbin BA, Lee WY, Parkos CA, Winfree LM. Annexin I regulates SKCO-15 cell invasion by signaling through formyl peptide receptors. J Biol Chem 2006; 281:19588-99; PMID:16675446; https://doi.org/ 10.1074/jbc.M513025200 [DOI] [PubMed] [Google Scholar]
- [34].Jiang YG, Luo Y, He DL, Li X, Zhang LL, Peng T, Li MC, Lin YH. Role of Wnt/beta-catenin signaling pathway in epithelial-mesenchymal transition of human prostate cancer induced by hypoxia-inducible factor-1alpha. Int J Urol 2007; 14:1034-9; PMID:17956532; https://doi.org/ 10.1111/j.1442-2042.2007.01866.x [DOI] [PubMed] [Google Scholar]
- [35].Zhau HE, Odero-Marah V, Lue HW, Nomura T, Wang R, Chu G, Liu ZR, Zhou BP, Huang WC, Chung LW. Epithelial to mesenchymal transition (EMT) in human prostate cancer: lessons learned from ARCaP model. Clin Exp Metastasis 2008; 25:601-10; PMID:18535913; https://doi.org/ 10.1007/s10585-008-9183-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Kirby M, Hirst C, Crawford ED. Characterising the castration-resistant prostate cancer population: a systematic review. Int J Clin Pract 2011; 65:1180-92; PMID:21995694; https://doi.org/ 10.1111/j.1742-1241.2011.02799.x [DOI] [PubMed] [Google Scholar]
- [37].Albertsen PC, Hanley JA, Gleason DF, Barry MJ. Competing risk analysis of men aged 55 to 74 years at diagnosis managed conservatively for clinically localized prostate cancer. JAMA 1998; 280:975-80; PMID:9749479; https://doi.org/ 10.1001/jama.280.11.975 [DOI] [PubMed] [Google Scholar]
- [38].Shore N, Mason M, de Reijke TM. New developments in castrate-resistant prostate cancer. BJU Int 2012; 109:22-32; PMID:22672122; https://doi.org/ 10.1111/j.1464-410X.2012.11217.x [DOI] [PubMed] [Google Scholar]
- [39].Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science 2016; 352:175-80; PMID:27124451; https://doi.org/ 10.1126/science.aaf4405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Brown JM, Giaccia AJ. The unique physiology of solid tumors: opportunities (and problems) for cancer therapy. Cancer Res 1998; 58:1408-16; PMID:9537241 [PubMed] [Google Scholar]
- [41].Movsas B, Chapman JD, Hanlon AL, Horwitz EM, Pinover WH, Greenberg RE, Stobbe C, Hanks GE. Hypoxia in human prostate carcinoma: an Eppendorf PO2 study. Am J Clin Oncol 2001; 24:458-61; PMID:11586096; https://doi.org/ 10.1097/00000421-200110000-00009 [DOI] [PubMed] [Google Scholar]
- [42].Bocca C, Bozzo F, Miglietta A. COX2 inhibitor NS398 reduces HT-29 cell invasiveness by modulating signaling pathways mediated by EGFR and HIF1-α. Anticancer Res 2014; 34:1793-1800; PMID:24692712 [PubMed] [Google Scholar]
- [43].Joseph JV, Conroy S, Pavlov K, Sontakke P, Tomar T, Eggens-Meijer E, Balasubramaniyan V, Wagemakers M, den Dunnen WF, Kruyt FA. Hypoxia enhances migration and invasion in glioblastoma by promoting a mesenchymal shift mediated by the HIF1α-ZEB1 axis. Cancer Lett 2015; 359:107-16; PMID:25592037; https://doi.org/ 10.1016/j.canlet.2015.01.010 [DOI] [PubMed] [Google Scholar]
- [44].Zhou B, Zhan H, Tin L, Liu S, Xu J, Dong Y, Li X, Wu L, Guo W. TUFT1 regulates metastasis of pancreatic cancer through HIF1-Snail pathway induced epithelial-mesenchymal transition. Cancer Lett 2016; 382:11-20; PMID:27566398; https://doi.org/ 10.1016/j.canlet.2016.08.017 [DOI] [PubMed] [Google Scholar]
- [45].Neisch AL, Fehon RG. Ezrin, Radixin and Moesin: key regulators of membrane-cortex interactions and signaling. Curr Opin Cell Biol 2011; 23:377-82; PMID:21592758; https://doi.org/ 10.1016/j.ceb.2011.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wang CC, Liau JY, Lu YS, Chen JW, Yao YT, Lien HC. Differential expression of moesin in breast cancers and its implication in epithelial-mesenchymal transition. Histopathology 2012; 61:78-87; PMID:22439598; https://doi.org/ 10.1111/j.1365-2559.2012.04204.x [DOI] [PubMed] [Google Scholar]
- [47].Chakraborty PK, Zhang Y, Coomes AS, Kim WJ, Stupay R, Lynch LD, Atkinson T, Kim JI, Nie Z, Daaka Y. G protein-coupled receptor kinase GRK5 phosphorylates moesin and regulates metastasis in prostate cancer. Cancer Res 2014; 74:3489-3500; PMID:24755472; https://doi.org/ 10.1158/0008-5472.CAN-13-2708 [DOI] [PubMed] [Google Scholar]
- [48].Bizzarro V, Fontanella B, Franceschelli S, Pirozzi M, Christian H, Parente L, Petrella A. Role of Annexin A1 in mouse myoblast cell differentiation. J Cell Physiol 2010; 224:757-65; PMID:20578244; https://doi.org/ 10.1002/jcp.22178 [DOI] [PubMed] [Google Scholar]
- [49].Geary LA, Nash KA, Adisetiyo H, Liang M, Liao CP, Jeong JH, Zandi E, Roy-Burman P. CAF-secreted annexin A1 induces prostate cancer cells to gain stem cell-like features. Mol Cancer Res 2014; 12:607-21; PMID:24464914; https://doi.org/ 10.1158/1541-7786.MCR-13-0469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].de Graauw M, van Miltenburg MH, Schmidt MK, Pont C, Lalai R, Kartopawiro J, Pardali E, Le Dévédec SE, Smit VT, van der Wal A, et al.. Annexin A1 regulates TGF-beta signaling and promotes metastasis formation of basal-like breast cancer cells. Proc Natl Acad Sci USA 2010; 107:6340-5; PMID:20308542; https://doi.org/ 10.1073/pnas.0913360107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vong L, D'Acquisto F, Pederzoli-Ribeil M, Lavagno L, Flower RJ, Witko-Sarsat V, Perretti M. Annexin 1 cleavage in activated neutrophils: a pivotal role for proteinase 3. J Biol Chem 2007; 282:29998-30004; PMID:17681950; https://doi.org/ 10.1074/jbc.M702876200 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.