Abstract
Eltrombopag is a small, non-peptide thrombopoietin mimetic that has been approved for increasing platelet count not only in immune thrombocytopenia and Hepatitis C virus-related thrombocytopenia, but also in aplastic anemia. Moreover, this drug is under investigation for increasing platelet counts in myelodysplastic syndromes. Despite current clinical practice, the mechanisms governing eltrombopag’s impact on human hematopoiesis are largely unknown, in part due to the impossibility of using traditional in vivo models. To investigate eltrombopag’s impact on megakaryocyte functions, we employed our established in vitro model for studying hematopoietic stem cell differentiation combined with our latest 3-dimensional silk-based bone marrow tissue model. Results demonstrated that eltrombopag favors human megakaryocyte differentiation and platelet production in a dose-dependent manner. These effects are accompanied by increased phosphorylation of AKT and ERK1/2 signaling molecules, which have been proven to be crucial in regulating physiologic thrombopoiesis. These data further clarify the different mechanisms of action of eltrombopag when compared to romiplostim, which, as we have shown, induces the proliferation of immature megakaryocytes rather than platelet production, due to the unbalanced activation of AKT and ERK1/2 signaling molecules. In conclusion, our research clarifies the underlying mechanisms that govern the action of eltrombopag on megakaryocyte functions and its relevance in clinical practice.
Introduction
Hematopoiesis occurs in a complex microenvironment within the bone marrow, which provides an ideal habitat for the production of mature blood cells from the multipotent, self-renewing hematopoietic stem cells (HSCs).1,2 The failure of HSCs to guarantee the physiologic homeostasis of one or more progenitors for circulating blood cells leads to pathologic conditions, such as aplastic anemia (AA) or myelodysplastic syndromes (MDS), characterized by peripheral pancytopenia of heterogeneous severity.3,4 A selective cytopenia of blood platelets, namely thrombocytopenia, may occur because of mutations in genes relevant for the functions of maturing megakaryocytic progenitors, as in inherited thrombocytopenias (IT).5 However, thrombocytopenia may also be secondary to viral infections or autoimmune diseases.6,7 It is well known that thrombopoietin, through binding to its receptor (c-Mpl), which is expressed by HSCs and megakaryocytes, is a critical regulator of both HSC homeostasis and megakaryopoiesis.8 The opportunity to synthesize in vitro molecules able to mimic the physiologic effect of thrombopoietin on platelet production recently opened new perspectives for the treatment of thrombocytopenic states.9 Among the small, non-peptide c-Mpl agonists,10,11 eltrombopag has been successfully employed to stimulate platelet production in patients suffering from IT caused by mutations of the MYH9 gene, immune thrombocytopenia (ITP), and thrombocytopenia due to hepatitis C infection.12–14 Moreover, improvement of thrombocytopenia has been obtained in patients with acute myeloid leukemia (AML) and MDS.15,16 Interestingly, promising clinical results have also demonstrated multi-lineage responses and the maintenance of normalized hematopoiesis in several patients suffering from AA,17–19 suggesting that eltrombopag may exert a beneficial effect on HSCs recovery by yet unexplored ways. Unfortunately, the use of traditional animal models for studies on the mechanisms of action of eltrombopag is not possible due to its selective activity only in humans and chimpanzees.20 The management of both thrombocytopenias and hematologic malignancies is challenging. Therefore, further improvement of the current knowledge strongly depends on the possibility to create laboratory assays in order to understand the effects of eltrombopag on human cells, both in physiologic and pathologic conditions.21 To this end, we have established a translational study made up of two complementary approaches. The first, based on the development of a culture system for the study of the basic mechanisms of the action of eltrombopag on human HSCs, focuses on the evaluation of the ability to promote megakaryopoiesis and platelet formation. The second, dedicated to the implementation of this knowledge into our recently established silk-based bone marrow model, integrates important physical and physiological elements characterizing the hematopoietic niche, conducive to evaluating platelet production.22 Together our studies demonstrate that eltrombopag significantly increases the activation of all the major c-Mpl downstream signaling pathways in a dose-dependent manner, and that this is paralleled by the differentiation of human HSCs, which results in an increased output of mature megakaryocytes which show an improved ability to form proplatelets and release platelets. Furthermore, we propose a novel mechanism explaining such effects on thrombopoiesis through the activation of AKT and ERK1/2 signaling molecules.
Methods
Cell culture
Human cord blood was collected following normal pregnancies and deliveries upon the informed consent of the parents; peripheral blood samples were collected from healthy volunteers after written informed consent. All samples were processed in accordance with the ethical committee of the IRCCS Policlinico San Matteo Foundation and the principles of the Declaration of Helsinki. Hematopoietic progenitor cells from cord and peripheral blood were separated by immunomagnetic bead selection, as previously described,23–25 and cultured in StemSpan medium (Stem Cell Technologies, Vancouver, Canada) supplemented with 1% L-glutamine, 1% penicillin-streptomycin, 10 ng/ml interleukin (IL)-6 and IL-11 (PeproTech, London, UK) and 50, 100, 200, 500 or 2000 ng/ml of eltrombopag (kindly supplied by GlaxoSmithKline), at 37°C in a 5% CO2 fully humidified atmosphere for 13 days. Instead of eltrombopag, 10 ng/ml recombinant human thrombopoietin (rHuTPO, PeproTech) was always used as the standard control to ensure normal megakaryocyte differentiation and functions. Eltrombopag and all cytokines were dissolved in sterile distilled deionized water.
Immunofluorescence analysis
At the end of the culture, a sample of 105 cells was cytospun on glass coverslips, fixed with 4% paraformaldehyde (PFA) for 20 minutes at room temperature (RT), blocked with 5% bovine serum albumin (BSA, Sigma, Milan, Italy) for 30 minutes, at RT, and then stained with the mouse monoclonal (clone SZ21) antibody against CD61 (1:100, Immunotech, Marseille, France), for 1 hour at RT, and the Alexa Fluor secondary antibody (1:500, Invitrogen, Milan, Italy) for 2 hours at RT. Nuclear counterstaining was performed using Hoechst 33258 (100 ng/ml, Sigma Aldrich, Milan, Italy) for 3 minutes at RT. Specimens were mounted in ProLong Gold Antifade Reagent (Invitrogen). Negative controls were routinely performed by omitting the primary antibody. Immunofluorescence images were acquired by an Olympus BX51 microscope (Olympus, Deutschland GmbH, Hamburg, Germany).
For immunofluorescence imaging of the silk-based bone marrow system, samples were fixed in 4% PFA for 20 minutes and blocked with 5% BSA for 30 minutes, at RT. Samples were probed with anti-CD61 (1:100) overnight at 4°C, and then immersed in Alexa Fluor secondary antibody (1:500) for 2 hours at RT. Nuclei were stained with Hoechst. Samples were imaged by an Olympus FluoView FV10i confocal laser-scanning microscope (Olympus). Silk fibroin scaffolds were stained with Hoechst in all experiments.26
Analysis of proplatelet formation
For the analysis of proplatelet yields by mature megakaryocytes, 12 mm glass coverslips were coated with 100 μg/ml fibrinogen (Merck Millipore, Milan, Italy) for 2 hours at RT and subsequently blocked with 1% BSA (Sigma) for 1 hour at RT. Megakaryocytes at day 13 of differentiation, either from liquid or 3D cultures, were harvested and plated onto fibrinogen-coated coverslips in 24-well plates, and allowed to adhere for 16 hours at 37°C in a 5% CO2 fully humidified atmosphere. After incubation, cells were fixed in 4% PFA, permeabilized with 0.5% Triton X-100, blocked with 5% BSA and stained with a rabbit anti-β1-tubulin antibody (kindly supplied by Prof. J. Italiano Jr). Megakaryocytes extending proplatelets were identified as cells extending β1-tubulin positive long filamentous structures ending with platelet-sized tips. Immunofluorescence images were acquired as reported above. Phase contrast images were obtained using an Olympus IX53 microscope (Olympus).
Statistics
Values are expressed as the mean plus or minus the standard deviation (mean±SD). A Student’s t-test was performed for paired observations. ANOVA, followed by the Bonferroni post hoc t-test, was performed for grouped observations. Spearman’s rank-order correlation coefficient (rs) was used to measure the association between two variables. A value of at least P<0.05 was considered statistically significant. All experiments were independently replicated at least three times.
A detailed description of silk bone marrow model preparation, western blotting and flow cytometry analysis have been reported in the Online Supplementary Methods.
Results
Eltrombopag supports full megakaryocyte differentiation and maturation
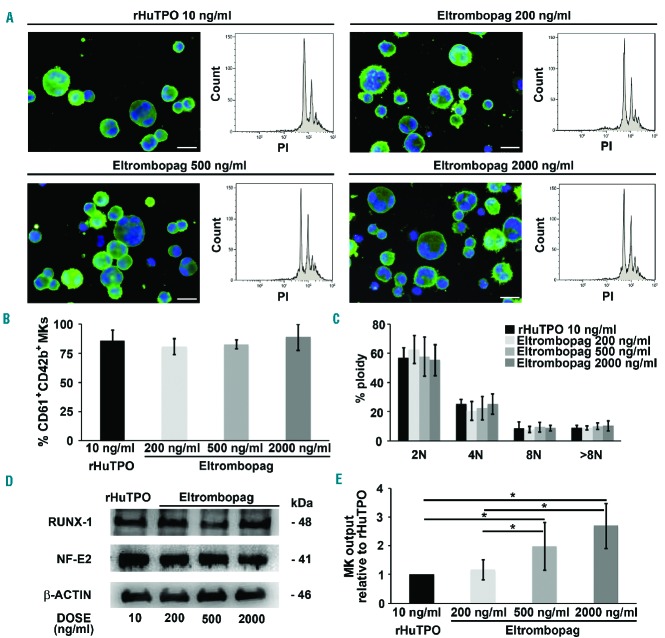
To investigate the effect of eltrombopag in promoting megakaryocyte differentiation, human cord blood-derived HSCs were cultured in the presence of 50, 100, 200, 500 or 2000 ng/ml eltrombopag. As a positive control of normal megakaryocyte differentiation, 10 ng/ml of rHuTPO was utilized, a concentration able to induce optimal megakaryocytic maturation and function, as shown in our previous research.23–25 After 13 days of culture, 50 and 100 ng/ml of eltrombopag failed to promote megakaryocyte differentiation (data not shown). Conversely, when concentrations of eltrombopag, in the same range as those measured in the serum of healthy subjects during oral eltrombopag administration (Cmax 7–10 μg/ml),27,28 were used in culture, megakaryocytes efficiently differentiated from their progenitors. Specifically, starting from 200 to 500 and 2000 ng/ml of eltrombopag, megakaryocyte maturation was observed with more than 90% of mature megakaryocytes displaying increased DNA content, as assessed by both immunofluorescence and flow cytometry analysis (Figure 1A–C). Furthermore, Western blot of protein lysates revealed similar expression levels of RUNX1 and NF-E2 transcription factors with respect to rHuTPO, suggesting comparable induction of late megakaryocyte differentiation (Figure 1D). Despite the similar efficacy with respect to 200 ng/ml eltrombopag in promoting megakaryocyte differentiation, 500 ng/ml and 2000 ng/ml eltrombopag also resulted in an increased absolute number of megakaryocytes. Specifically, megakaryocyte output in the presence of 500 ng/ml and 2000 ng/ml eltrombopag prompted a significant 2- and 3-fold increase, respectively, compared to 200 ng/ml eltrombopag. The data were normalized with respect to 10 ng/ml rHuTPO as the positive control (Figure 1E). Similar results were obtained by culturing human adult peripheral blood progenitor cells with 500 ng/ml eltrombopag, which determined an increase of megakaryocyte output with respect to 10 ng/ml rHuTPO (Online Supplementary Figures S1A and S1B).
Figure 1.
Eltrombopag promotes megakaryocyte differentiation in vitro. Megakaryocytes (MKs) were differentiated from human umbilical cord blood progenitors and cultured for 13 days in the presence of 200, 500 and 2000 ng/mL eltrombopag or 10 ng/mL recombinant human thrombopoietin (rHuTPO) as positive control. (A) Representative immunofluorescence staining of CD61 (green=CD61; blue=nuclei; scale bar=25 μm) and flow cytometry analysis of ploidy levels (PI=propidium iodide) in mature megakaryocytes at the end of the culture. (B) Percentage of CD61+CD42b+ megakaryocytes at the end of the culture. Data are presented as mean±SD (P= not significant (NS)). (C) Statistical analysis of ploidy levels. Data are presented as mean±SD (P=NS). (D) Western blot analysis of megakaryocytic transcription factors RUNX-1 and NF-E2. Samples were probed with ‘anti-β-actin’ antibody to ensure equal loading. (E) Output was calculated as the number of CD61+CD42b+ cells at day 13 of culture with respect to the total starting number of hematopoietic progenitor cells. Histograms show the fold increases of megakaryocyte output in the presence of 200, 500 or 2000 ng/mL eltrombopag with respect to 10 ng/mL rHuTPO. Data are expressed as mean ± SD (*P<0.05).
Eltrombopag promotes proplatelet formation
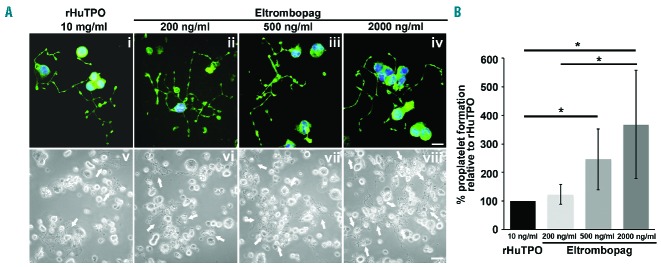
To investigate the ability of megakaryocyte to form platelets, at the end of the culture cells were harvested and plated on fibrinogen, an extracellular matrix component known to support proplatelet formation and branching.23 Proplatelet formation did not occur in the presence of 50 and 100 ng/ml eltrombopag (data not shown), consistent with the lack of megakaryocyte differentiation in these conditions. Conversely, immunofluorescence analysis showed a normal architecture of proplatelet forming megakaryocytes, with cells displaying β1-tubulin positive long filamentous structures and bulbous tips at their terminal ends, in the presence of 200, 500 or 2000 ng/ml eltrombopag, or 10 ng/ml rHuTPO, used as the positive control condition (Figure 2Ai–iv). Importantly, the beneficial effect of eltrombopag on megakaryocyte function was further sustained by the evidence that the increased output observed in response to 500 or 2000 ng/ml was paralleled by a significant increase in the number of cells displaying proplatelet branching, with respect to both 200 ng/ml eltrombopag or 10 ng/ml rHuTPO (Figure 2Av–viii). Specifically, a comparison of the percentage of proplatelet forming megakaryocytes among the different tested conditions highlighted that 500 ng/ml eltrombopag determined a significant 2-fold increase in the percentage of proplatelet forming megakaryocytes, while a 4-fold increase was reached with 2000 ng/ml eltrombopag (Figure 2B). Accordingly, megakaryocytes likewise differentiated from human adult peripheral blood progenitor cells in the presence of 500 ng/ml eltrombopag, showing a significantly increased proplatelet formation compared to those differentiated in the presence of 10 ng/ml rHuTPO (Online Supplementary Figures S1C and S1D).
Figure 2.
Eltrombopag sustains proplatelet formation in vitro. (Ai–iv) Analysis of proplatelet structure was performed by immunofluorescence staining of the megakaryocyte-specific cytoskeleton component β1-tubulin (green=β1-tubulin; blue=nuclei; scale bar=25 μm). In all tested conditions, the representative pictures show similar elongation of proplatelet shafts, with the presence of bulbous tips at the terminal ends of each branch, resembling mature platelets. (Av-viii) Representative light microscopy images of proplatelet formation by human megakaryocytes cultured in the presence of recombinant human thrombopoietin (rHuTPO) or increasing concentrations of eltrombopag (scale bar=50 μm). Arrows indicate proplatelet-forming megakaryocytes. (B) The percentage of proplatelet forming megakaryocytes was calculated as the number of cells displaying long filamentous pseudopods with respect to the total number of round megakaryocytes per analyzed field. Histograms show the fold increase of proplatelet formation in the presence of 200, 500 or 2000 ng/mL eltrombopag with respect to 10 ng/mL rHuTPO, used as optimum standard condition to test proplatelet formation. Data are expressed as mean ± SD (*P<0.05).
Eltrombopag promotes megakaryocyte differentiation in the silk-based 3D bone marrow model
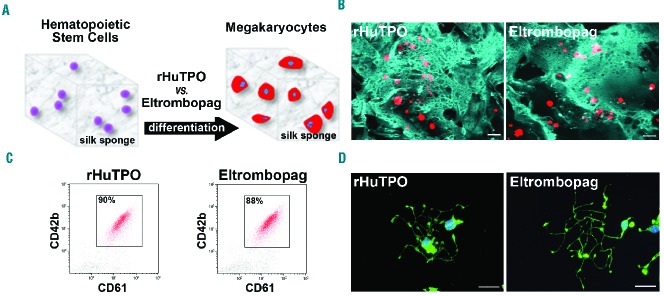
We have recently engineered a silk protein scaffold-based tissue system to mimic the 3D spongy architecture that surrounds the marrow vasculature, in order to support mature megakaryocyte function ex vivo.22 We took advantage of this system to assess HSC differentiation towards the megakaryocyte lineage in the presence of eltrombopag (Figure 3A). Human cord blood-derived HSCs were seeded inside the sponge immediately after isolation and differentiated for two weeks, in the same conditions used for liquid cultures. The silk scaffolds, in the presence of 200 ng/ml of eltrombopag, supported megakaryocyte differentiation, which appeared fully mature as compared to 10 ng/ml of rHuTPO (Figure 3B,C). To confirm this feature, megakaryocytes were recovered from the silk sponge and seeded onto fibrinogen coated coverslips, where they formed normal branched proplatelets displaying β1-tubulin staining (Figure 3D). The same results were obtained with 500 ng/ml and 2000 ng/ml eltrombopag (data not shown).
Figure 3.
Eltrombopag stimulates ex vivo megakaryocyte differentiation within the 3D silk bone marrow model. (A) Aqueous silk solution was mixed with salt particles and dried at room temperature overnight. After leaching out the salt, the resulting porous silk sponge was trimmed and sterilized. CD34+ hematopoietic stem cells from human cord blood were then seeded into the sponges and cultured for 13 days in the presence of 10 ng/mL recombinant human thrombopoietin (rHuTPO) or 200 ng/mL eltrombopag. (B) Confocal microscopy analysis of CD61+ megakaryocytes after 13 days of differentiation within the silk sponges under the different tested conditions (red=CD61; blue=silk; scale bar=100 μm). (C) Flow cytometry analysis of samples from the silk-sponges demonstrated an almost comparable percentage of CD61+CD42b+ cells between rHuTPO and eltrombopag. (D) Representative β1-tubulin staining of proplatelet formation by megakaryocytes collected from the silk sponge scaffold and seeded on fibrinogen. Both rHuTPO and eltrombopag supported normal proplatelet extension (green=β1-tubulin; blue=nuclei; scale bar=20 μm).
Eltrombopag sustains human platelet generation ex vivo
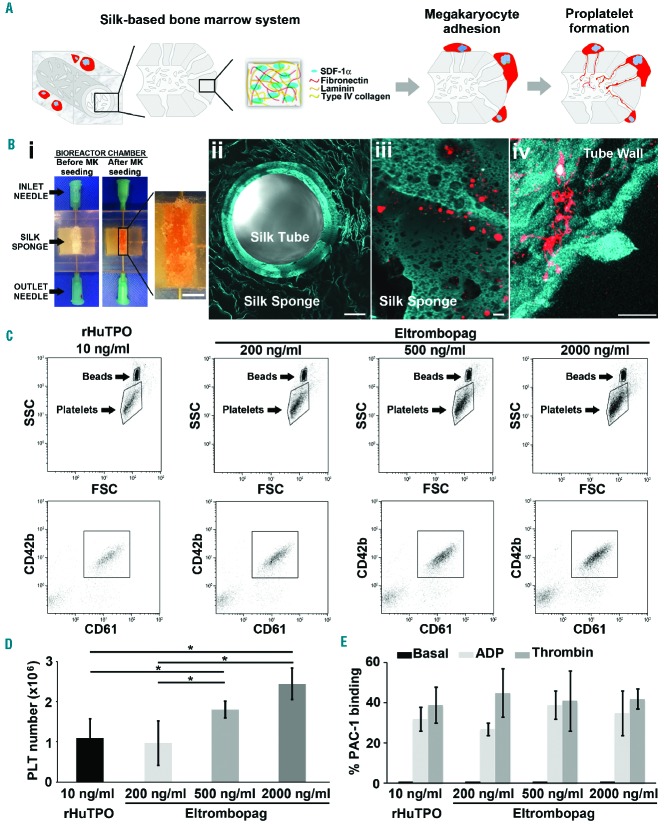
In parallel, silk sponges, previously assembled around a silk microtube functionalized via the entrapment of extracellular matrix components and SDF-1α as a chemoattractant, were perfused at a shear rate of 60 s−1 to allow ex vivo platelet collection (Figure 4A). Confocal microscopy revealed that human mature CD61+ megakaryocytes, contained in the silk sponges, were able to elongate proplatelets through the silk microtube wall (Figure 4B). In addition, media flow mimicking the bloodstream into the bone marrow sinusoids, helped platelet collection into gas-permeable bags containing acid citrate dextrose (ACD) as an anticoagulant. Ex vivo collected platelets were double stained with anti-CD61 and anti-CD42b antibodies, and their number evaluated by a counting bead standard (Figure 4C). The mean number of CD61+CD42b+ platelets collected per 3D tissue perfusion system increased with increasing concentrations of eltrombopag (Figure 4D). We then calculated the Spearman’s rank-order correlation coefficient (rs) to assess the statistical correlation between the percentage of proplatelet formation in vitro and the number of platelets collected in the 3D ex vivo system obtaining a value of rs =0.9002, with a P value of <0.0001. Importantly, analysis of PAC-1 binding demonstrated comparable platelet activation in all tested conditions (Figure 4E).
Figure 4.
Eltrombopag sustains ex vivo platelet release. (A) Silk microtubes were prepared by gel spinning aqueous silk solutions, containing polyethylene oxide porogen, around a wire and functionalized via entrapment of SDF-1α and extracellular matrix components. The resulting microtubes were fitted into the bioreactor chamber and a silk sponge was fabricated around them. Megakaryocytes (MKs) cultured within the system could migrate toward the microtube, adhere and extend proplatelets through the microtube wall to release platelets into the microtube lumen. (Bi) Bioreactor chamber containing the silk microtube-sponge system (scale bar=5 mm). (Bii) Confocal microscopy analysis of the silk microtube-sponge system before megakaryocyte seeding (blue=silk; scale bar=100 μm). (Biii) Mature megakaryocytes cultured into the silk sponge scaffold (red=CD61; blue=silk; scale bar=100 μm). (Biv) Representative megakaryocyte extending proplatelets through the silk microtube wall (red=CD61; blue=silk; scale bar=50 μm). (C) Ex vivo produced platelets (PLTs) were stained with CD61 and CD42b antibodies, and their number calculated by mixing samples with counting beads before flow cytometry analysis. (D) The graph shows the absolute number of CD61+CD42b+ platelets released in the presence of rHuTPO or increasing doses of eltrombopag (mean±SD, *P<0.05). (E) Ex vivo collected platelets revealed increased PAC-1 binding after stimulation with ADP or thrombin (mean±SD). rHuTPO: recombinant human thrombopoietin; SSC: side scatter; FSC: forward scatter; ADP: adenosine diphosphate; SDF-1α: stromal cell-derived factor 1α.
Eltrombopag activates c-Mpl downstream pathways and keeps a balanced activation of AKT and ERK1/2 signaling molecules
Upon its binding to the c-Mpl receptor, thrombopoietin stimulates multiple biochemical signals, including the Janus Kinase (JAK)/signal transducers and activators of transcription 3 (STAT3) and STAT5, the phosphoinositide 3-kinase/AKT (PI3K/AKT), and the extracellular signal-regulated Kinase1/2 (ERK1/2).29 Here, we demonstrated that all these signals are activated upon stimulation of megakaryocytes with eltrombopag (Online Supplementary Figure S2). Among these, the JAK/STAT3 and JAK/STAT5 pathways are known to promote megakaryocyte differentiation, survival, and expansion.30–32 Eltrombopag determined an increase of STAT3 and STAT5 phosphorylation as compared to rHuTPO (Figure 5A–C), consistent with our data demonstrating normal megakaryocyte differentiation, but increased output. Of note, the balance between the activation of AKT and ERK1/2 has been found to be crucial to regulate proplatelet formation. In particular, inhibition of AKT phosphorylation resulted in impaired proplatelet formation,33,34 while over-activation of AKT not associated with a parallel ERK1/2 over-activation led to hyperproliferation of immature megakaryocytes with a defective capacity to form proplatelets.25 In contrast, pathologic over-activation of ERK1/2 only, not paralleled by AKT over-activation, inhibited proplatelet formation.35 Here we demonstrate that eltrombopag sustains parallel AKT and ERK1/2 phosphorylation in a dose-dependent manner (Figure 5A), with a significantly increased activation of both pathways in the presence of 500 and 2000 ng/ml of eltrombopag with respect to 10 ng/ml of rHuTPO (Figure 5D,E). Similar results were obtained by culturing CD34+ hematopoietic progenitors for 1 day with 2000 ng/ml eltrombopag (Figure 6A–E). Consistently, even within 1 hour upon stimulation of both megakaryocytes and hematopoietic progenitors, 2000 ng/ml eltrombopag sustained a marked increase of STAT3, STAT5, AKT and ERK1/2 phosphorylation, compared to 10 ng/ml rHuTPO (Online Supplementary Figures S3A and S3B).
Figure 5.
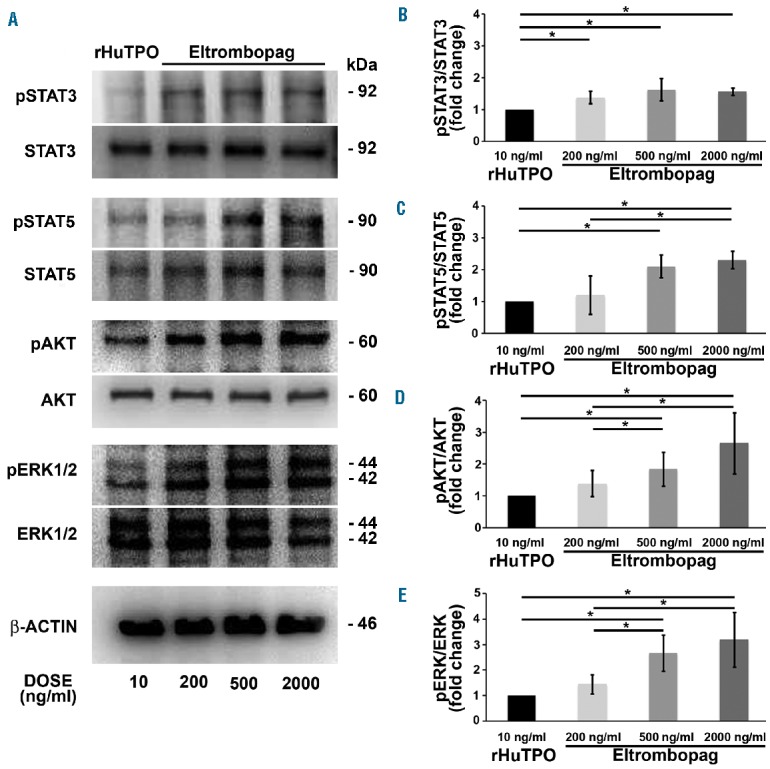
Eltrombopag activates all main biochemical pathways downstream of c-Mpl in in vitro differentiated megakaryocytes. (A) Lysates from megakaryocyte cultures in the presence of 10 ng/mL recombinant human thrombopoietin (rHuTPO), or 200, 500 and 2000 ng/mL eltrombopag, were obtained at day 13 of differentiation. Samples were probed for phosphorylated STAT3 (pSTAT3), phosphorylated STAT5 (pSTA5), phosphorylated AKT (pAKT) and phosphorylated ERK1/2 (pERK1/2). Total STAT3, STAT5, AKT, ERK1/2 and β-actin were revealed to ensure equal loading. (B–D) Densitometric analysis demonstrated sustained activation by eltrombopag of all signaling molecules in a dose-dependent manner, with a significant difference with respect to 10 mg/mL rHuTPO, as standard control condition (*P<0.05).
Figure 6.
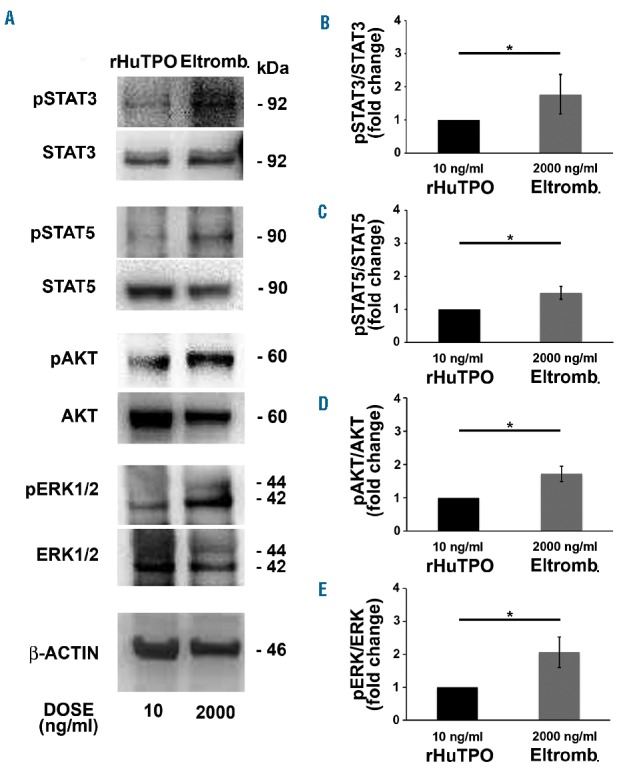
c-Mpl downstream signaling molecules are highly activated in hematopoietic progenitors in the presence of eltrombopag. (A) Lysates from hematopoietic progenitors in the presence of 10 ng/mL recombinant human thrombopoietin (rHuTPO) or 2000 ng/mL eltrombopag (Eltromb.) were obtained after 1 day of culture. Samples were probed for phosphorylated STAT3 (pSTAT3), phosphorylated STAT5 (pSTA5), phosphorylated AKT (pAKT) and phosphorylated ERK1/2 (pERK1/2). Total STAT3, STAT5, AKT, ERK1/2 and β-actin were revealed to ensure equal loading. (B–D) Densitometric analysis demonstrated significant over-activation of all signaling molecules in the presence of 2000 ng/mL eltrombopag with respect to 10 mg/mL rHuTPO, as standard control condition (*P<0.05).
Discussion
Eltrombopag is a non-peptide synthetic thrombopoietin-mimetic that binds to the transmembrane domain of c-Mpl, leading to its activation.10,11 This compound has been approved by the Food and Drug Administration (FDA) for the treatment of ITP and severe AA, and also for increasing platelet counts in subjects with thrombocytopenia due to hepatitis C infection who are undergoing antiviral treatment.12,13,17–19 Moreover, a pivotal study on IT demonstrated that eltrombopag can ameliorate thrombocytopenia in subjects affected by mutations of the MYH9 gene.14 Preliminary data are available concerning the treatment of AML and MDS.15,16,36,37 Despite the wide spectrum of conditions where eltrombopag has been used, little is known about the mechanisms of action of this drug on human HSCs. The results of our study demonstrated that eltrombopag promoted successful in vitro human cord and peripheral blood-derived HSCs differentiation towards the megakaryocytic lineage, as assessed by the maturation of high ploidy megakaryocytes expressing the lineage-specific markers CD61 and CD42b and the transcription factors RUNX-1 and NF-E2. Our data were consistent with recent findings by Jeong et al., demonstrating that eltrombopag can stimulate megakaryocyte differentiation from hematopoietic progenitors isolated from patients with relapsed multiple myeloma and normal controls.38 Interestingly, concentrations of eltrombopag, similar to those observed in patients receiving this drug in vivo,27,28 stimulated the activation of all the main biochemical pathways downstream of the c-Mpl receptor. This activation resulted in the expansion of fully differentiated megakaryocytes with an enhanced ability to extend long branched proplatelets. Taking advantage of our recently developed 3D bone marrow tissue model,22 we also demonstrated that eltrombopag promoted the release of functional platelets under flow conditions mimicking the bloodstream into marrow sinusoids.
The effect of eltrombopag appears to be markedly different from that recently observed in the same experimental conditions with the thrombopoietin mimetic romiplostim,25 a peptibody that binds to the c-Mpl competing with endogenous thrombopoietin for the same binding site.39 Romiplostim promoted the significant proliferation of immature megakaryocytes, displaying an impaired ability to form proplatelets.25 Of note, these different effects reflected different patterns of activation of AKT and ERK1/2 kinases, which are known to be crucial for the regulation of megakaryocyte maturation and the process of platelet production which occurs in healthy conditions.33–35,40–42 In the present study we observed that eltrombopag sustained a concomitant phosphorylation of both AKT and ERK1/2 both in hematopoietic progenitors and mature megakaryocytes, while in our previous work romiplostim was shown to stimulate mainly megakaryocyte proliferation by increasing AKT phosphorylation only.25 Overall, our studies on the effects of eltrombopag and romiplostim on in vitro human megakaryopoiesis suggest that these two drugs increase platelet output by different mechanisms: the former by stimulating increased megakaryocyte maturation and proplatelet formation, the latter by increasing mainly megakaryocyte proliferation without a correspondent parallel stimulation of megakaryocyte maturation (Figure 7). Further studies are required to ascertain whether these differences in vitro also occur in vivo in patients receiving these drugs. Nevertheless, these data provide a rational basis to the observation that ITP patients who do not respond to romiplostim may respond to eltrombopag, and vice versa.43 The peculiarities of the mechanisms of action of eltrombopag could also provide an explanation for the observation that its effect is additive to that of thrombopoietin or romiplostim both in vitro and in vivo.20,44
Figure 7.
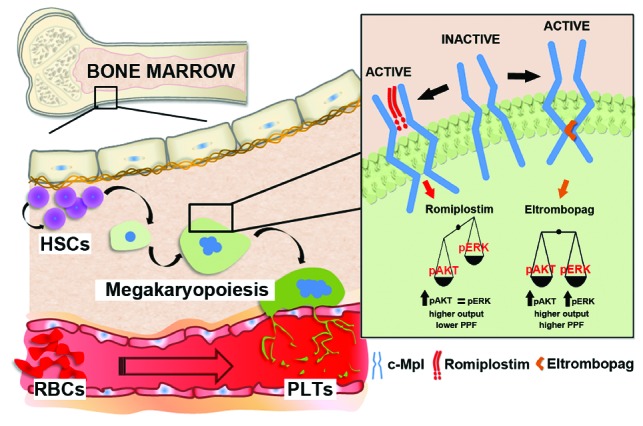
Representative model of different mechanisms of action of eltrombopag and romiplostim. Bone marrow, contained in spongy bones, is a tridimensional network of branching sinusoids surrounding islets of hematopoietic cells. Within this environment hematopoietic stem cells (HSCs) undergo self-renewal as well as differentiation into committed lineages in order to support the physiological homeostasis of all blood cells. Megakaryopoiesis takes place due to the activation of c-Mpl, the thrombopoietin receptor, which promotes HSC commitment and differentiation. Upon c-Mpl binding, eltrombopag promotes higher phosphorylation of both AKT and ERK than recombinant human thrombopoietin (rHuTPO), thus ensuring proper proplatelet formation (PPF). At variance with eltrombopag, romiplostim promotes increased AKT but not ERK activation with respect to rHuTPO, thus promoting megakaryocyte proliferation rather than PPF. PLTs: platelets; RBCs: red blood cells.
It is well known that c-Mpl is expressed not only by hematopoietic progenitors, but also by many malignant hematopoietic cell types, and different in vitro experiments demonstrated that thrombopoietin may sustain the growth of both leukemic cell lines and human leukemic cells.45,46 The concern that thrombopoietin mimetics might favor leukemogenesis has been extensively debated. A rise in blast counts has been seen in patients with MDS receiving romiplostim,47 and a recent retrospective study of a large series of ITP patients indicated an association between the administration of thrombopoietin mimetics and the development of AML.48 On the other hand, recent studies concluded that eltrombopag allowed the formation of normal megakaryocytic colonies without the stimulation of malignant blasts from AML and MDS patients, and also had a strong anti-leukemic effect.37,49 Interestingly, this effect was independent of c-Mpl, but was instead mediated through the modulation of intracellular iron content.49 Finally, Kalota et al. demonstrated that eltrombopag dramatically decreases ROS levels, leading to a disruption of AML intracellular metabolism and rapid cell death.50 Thus, conflicting results were obtained concerning the potential leukemogenic effect of thrombopoietin mimetics, and further studies are required to clarify this important issue.
In conclusion, this study defined the mechanisms of action of the thrombopoietin mimetic eltrombopag on hematopoietic progenitors and human megakaryocytes, and identified relevant differences with respect to those of romiplostim. Importantly, this observation warrants further investigation on outcomes from the HSCs of patients affected by thrombocytopenias and overall bone marrow pathologies. Understanding the efficacy and safety of thrombopoietin mimetics on pathologic samples before treating patients in vivo would represent an important step towards creating more personalized and effective therapies.
Supplementary Material
Acknowledgements
The authors would like to thank Dr. Gianluca Viarengo and Prof. Federica Meloni for technical assistance with the flow cytometry analysis; Dr. Cesare Perotti for supplying human cord blood; Dr. Lorenzo Tozzi and Daniel Smoot for technical assistance in the preparation of gel-spun silk microtubes; Prof. Joseph Italiano for providing β1-tubulin antibody. This paper was supported by the Cariplo Foundation (2012-0529, 2013-0717), ERA-Net for Research Programmes on Rare Diseases (EUPLANE), the Italian Ministry of Health (RF-2010-2310098) and US National Institutes of Health (R01 EB016041-01). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Check the online version for the most updated information on this article, online supplements, and information on authorship & disclosures: www.haematologica.org/content/100/12/1479
References
- 1.Morrison SJ, Scadden DT. The bone marrow niche for haematopoietic stem cells. Nature. 2014;505(7483):327–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Malara A, Abbonante V, Di Buduo CA, Tozzi L, Currao M, Balduini A. The secret life of a megakaryocyte: emerging roles in bone marrow homeostasis control. Cell Mol Life Sci. 2015;72(8):1517–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marsh JC. Bone marrow failure syndromes. Clin Med. 2005;5(4):332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiu R, Gondek L, O’Keefe C, Maciejewski JP. Clonality of the stem cell compartment during evolution of myelodysplastic syndromes and other bone marrow failure syndromes. Leukemia. 2007;21(8):1648–1657. [DOI] [PubMed] [Google Scholar]
- 5.Balduini CL, Pecci A, Noris P. Diagnosis and management of inherited thrombocytopenias. Semin Thromb Hemost. 2013;39(2): 161–171. [DOI] [PubMed] [Google Scholar]
- 6.Nagamine T, Ohtuka T, Takehara K, Arai T, Takagi H, Mori M. Thrombocytopenia associated with hepatitis C viral infection. J Hepatol. 1996;24(2):135–140. [DOI] [PubMed] [Google Scholar]
- 7.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–2393. [DOI] [PubMed] [Google Scholar]
- 8.Hitchcock IS, Kaushansky K. Thrombopoietin from beginning to end. Br J Haematol. 2014;165(2):259–268. [DOI] [PubMed] [Google Scholar]
- 9.Kuter DJ. The biology of thrombopoietin and thrombopoietin receptor agonists. Int J Hematol. 2013;98(1):10–23. [DOI] [PubMed] [Google Scholar]
- 10.Erickson-Miller CL, DeLorme E, Tian SS, et al. Discovery and characterization of a selective, nonpeptidyl thrombopoietin receptor agonist. Exp Hematol. 2005;33(1):85–93. [DOI] [PubMed] [Google Scholar]
- 11.Safonov IG, Heerding DA, Keenan RM, et al. New benzimidazoles as thrombopoietin receptor agonists. Bioorg Med Chem Lett. 2006;16(5):1212–1216. [DOI] [PubMed] [Google Scholar]
- 12.Bussel JB. Update on eltrombopag for ITP. Oncology (Williston Park). 2009;23(13): 1177–1178. [PubMed] [Google Scholar]
- 13.McHutchison JG, Dusheiko G, Shiffman ML, et al. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357 (22):2227–2236. [DOI] [PubMed] [Google Scholar]
- 14.Pecci A, Gresele P, Klersy C, et al. Eltrombopag for the treatment of the inherited thrombocytopenia deriving from MYH9 mutations. Blood. 2010;116(26): 5832–5837. [DOI] [PubMed] [Google Scholar]
- 15.Platzbecker U, Wong RS, Verma A, et al. Safety and tolerability of eltrombopag versus placebo for treatment of thrombocytopenia in patients with advanced myelodysplastic syndromes or acute myeloid leukaemia: a multicentre, randomised, placebo-controlled, double-blind, phase 1/2 trial. Lancet Haematol. 2015;2(10):e417–e426. [DOI] [PubMed] [Google Scholar]
- 16.Santini V, Fenaux P. Treatment of myelodysplastic syndrome with thrombomimetic drugs. Semin Hematol. 2015;52(1):38–45. [DOI] [PubMed] [Google Scholar]
- 17.Olnes MJ, Scheinberg P, Calvo KR, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367(1):11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Desmond R, Townsley DM, Dumitriu B, et al. Eltrombopag restores trilineage hematopoiesis in refractory severe aplastic anemia that can be sustained on discontinuation of drug. Blood. 2014;123(12):1818–1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormack PL. Eltrombopag: a review of its use in patients with severe aplastic anaemia. Drugs. 2015;75(5):525–531. [DOI] [PubMed] [Google Scholar]
- 20.Erickson-Miller CL, Delorme E, Tian SS, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009;27(2):424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balduini A, Di Buduo CA, Kaplan DL. Translational approaches to functional platelet production ex vivo. Thromb Haemost. 2016;115(2):250–256. [DOI] [PubMed] [Google Scholar]
- 22.Di Buduo CA, Wray LS, Tozzi L, et al. Programmable 3D silk bone marrow niche for platelet generation ex vivo and modeling of megakaryopoiesis pathologies. Blood. 2015;125(14):2254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balduini A, Pallotta I, Malara A, et al. Adhesive receptors, extracellular proteins and myosin IIA orchestrate proplatelet formation by human megakaryocytes. J Thromb Haemost. 2008;6(11):1900–1907. [DOI] [PubMed] [Google Scholar]
- 24.Balduini A, Badalucco S, Pugliano MT, et al. In vitro megakaryocyte differentiation and proplatelet formation in Ph-negative classical myeloproliferative neoplasms: distinct patterns in the different clinical phenotypes. PLoS One. 2011;6(6):e21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Currao M, Balduini CL, Balduini A. High doses of romiplostim induce proliferation and reduce proplatelet formation by human megakaryocytes. PLoS One. 2013;8(1): e54723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Talukdar S, Nguyen QT, Chen AC, Sah RL, Kundu SC. Effect of initial cell seeding density on 3D-engineered silk fibroin scaffolds for articular cartilage tissue engineering. Biomaterials. 2011;32(34):8927–8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng Y, Madatian A, Wire MB, et al. Metabolism and disposition of eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist, in healthy human subjects. Drug Metab Dispos. 2011;39(9):1734–1746. [DOI] [PubMed] [Google Scholar]
- 28.Stasi R, Rhodes E, Benjamin R, et al. The Emergence of Thrombopoietin Receptor Agonists as a Novel Treatment for Immune Thrombocytopenia. Eur Oncol Haematol. 2011;7(1):63–70. [Google Scholar]
- 29.Kaushansky K. The molecular mechanisms that control thrombopoiesis. J Clin Invest. 2005;115(12):3339–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matsumura I, Ishikawa J, Nakajima K, et al. Thrombopoietin-induced differentiation of a human megakaryoblastic leukemia cell line, CMK, involves transcriptional activation of p21(WAF1/Cip1) by STAT5. Mol Cell Biol. 1997;17(5):2933–2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirito K, Watanabe T, Sawada K, Endo H, Ozawa K, Komatsu N. Thrombopoietin regulates Bcl-xL gene expression through Stat5 and phosphatidylinositol 3-kinase activation pathways. J Biol Chem. 2002;277(10):8329–8337. [DOI] [PubMed] [Google Scholar]
- 32.Kirito K, Osawa M, Morita H, et al. A functional role of Stat3 in in vivo megakaryopoiesis. Blood. 2002;99(9):3220–3227. [DOI] [PubMed] [Google Scholar]
- 33.Balduini A, Di Buduo CA, Malara A, et al. Constitutively released adenosine diphosphate regulates proplatelet formation by human megakaryocytes. Haematologica. 2012;97(11):1657–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badalucco S, Di Buduo CA, Campanelli R, et al. Involvement of TGFβ1 in autocrine regulation of proplatelet formation in healthy subjects and patients with primary myelofibrosis. Haematologica. 2013;98(4):514–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bluteau D, Balduini A, Balayn N, et al. Thrombocytopenia-associated mutations in the ANKRD26 regulatory region induce MAPK hyperactivation. J Clin Invest. 2014;124(2):580–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li W, Morrone K, Kambhampati S, Will B, Steidl U, Verma A. Thrombocytopenia in MDS: epidemiology, mechanisms, clinical consequences and novel therapeutic strategies. Leukemia. 2016;30(3):536–544. [DOI] [PubMed] [Google Scholar]
- 37.Will B, Kawahara M, Luciano JP, et al. Effect of the nonpeptide thrombopoietin receptor agonist Eltrombopag on bone marrow cells from patients with acute myeloid leukemia and myelodysplastic syndrome. Blood. 2009;114(18):3899–3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong JY, Levine MS, Abayasekara N, Berliner N, Laubach J, Vanasse GJ. The non-peptide thrombopoietin receptor agonist eltrombopag stimulates megakaryopoiesis in bone marrow cells from patients with relapsed multiple myeloma. J Hematol Oncol. 2015;8:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuter DJ. Milestones in understanding platelet production: a historical overview. Br J Haematol. 2014;165(2):248–258. [DOI] [PubMed] [Google Scholar]
- 40.Mazharian A, Watson SP, Séverin S. Critical role for ERK1/2 in bone marrow and fetal liver-derived primary megakaryocyte differentiation, motility, and proplatelet formation. Exp Hematol. 2009;37(10):1238–1249.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Séverin S, Ghevaert C, Mazharian A. The mitogen-activated protein kinase signaling pathways: role in megakaryocyte differentiation. J Thromb Haemost. 2010;8(1):17–26. [DOI] [PubMed] [Google Scholar]
- 42.Besancenot R, Chaligné R, Tonetti C, et al. A senescence-like cell-cycle arrest occurs during megakaryocytic maturation: implications for physiological and pathological megakaryocytic proliferation. PLoS Biol. 2010;8(9). pii: e1000476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Arena G, Guariglia R, Mansueto G, et al. No cross-resistance after sequential use of romiplostim and eltrombopag in chronic immune thrombocytopenic purpura. Blood. 2013;121(7):1240–1242. [DOI] [PubMed] [Google Scholar]
- 44.Ruiz-Arguelles GJ, Ruiz-Delgado GJ, Velázquez-Sánchez-de-Cima S, Zamora-Ortiz G. Simultaneous romiplostin, eltrombopag, and prednisone were successful in severe thrombocytopenia of Evans syndrome refractory to hydrocortisone, splenectomy, intravenous IgG, and rituximab. Hematology. 2013;18(3):175–177. [DOI] [PubMed] [Google Scholar]
- 45.Drexler HG, Zaborski M, Quentmeier H. Thrombopoietin supports the continuous growth of cytokine-dependent human leukemia cell lines. Leukemia. 1997;11(4): 541–551. [DOI] [PubMed] [Google Scholar]
- 46.Corazza F, Hermans C, D’Hondt S, et al. Circulating thrombopoietin as an in vivo growth factor for blast cells in acute myeloid leukemia. Blood. 2006;107(6):2525–2530. [DOI] [PubMed] [Google Scholar]
- 47.Prica A, Sholzberg M, Buckstein R. Safety and efficacy of thrombopoietin-receptor agonists in myelodysplastic syndromes: a systematic review and meta-analysis of randomized controlled trials. Br J Haematol. 2014;167(5):626–638. [DOI] [PubMed] [Google Scholar]
- 48.Oshima Y, Yuji K, Tanimoto T, Hinomura Y, Tojo A. Association between acute myelogenous leukemia and thrombopoietin receptor agonists in patients with immune thrombocytopenia. Intern Med. 2013;52(19): 2193–2201. [DOI] [PubMed] [Google Scholar]
- 49.Roth M, Will B, Simkin G, et al. Eltrombopag inhibits the proliferation of leukemia cells via reduction of intracellular iron and induction of differentiation. Blood. 2012;120(2):386–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalota A, Selak MA, Garcia-Cid LA, Carroll M. Eltrombopag modulates reactive oxygen species and decreases acute myeloid leukemia cell survival. PLoS One. 2015; 10(4):e0126691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.