Abstract
Cerebral ischemia/reperfusion (I/R) triggers a cascade of uncontrolled cellular processes that perturb cell homeostasis. The arctic ground squirrel (AGS), a seasonal hibernator resists brain damage following cerebral I/R caused by cardiac arrest and resuscitation. However, it remains unclear if tolerance to I/R injury in AGS depends on the hibernation season. Moreover, it is also not clear if events such as depletion of ATP, acidosis and glutamate efflux that are associated with anoxic depolarization are attenuated in AGS.
Here, we employ a novel microperfusion technique to test the hypothesis that tolerance to I/R injury modeled in an acute hippocampal slice preparation in AGS is independent of the hibernation season. Acute hippocampal slices were harvested from summer euthermic AGS, hibernating AGS and interbout euthermic AGS. Slices were subjected to oxygen glucose deprivation (OGD), an in vitro model of I/R injury to determine cell death marked by lactate dehydrogenase (LDH) release. ATP was assayed using ENLITEN ATP assay. Glutamate and aspartate efflux was measured using capillary electrophoresis. For acidosis, slices were subjected to pH 6.4 or ischemic shift solution (ISS). Acute hippocampal slices from rat was used as a positive control, susceptible to I/R injury.
Our results indicate that when tissue temperature is maintained at 36°C, hibernation season has no influence on OGD-induced cell death in AGS hippocampal slices. Our data also shows that tolerance to OGD in AGS hippocampal slices occurs despite loss of ATP, and glutamate release and persists during conditions that mimic acidosis and ionic shifts characteristic of cerebral I/R.
Keywords: Ischemia, Hibernation, Hippocampal brain slices, In vitro, oxygen glucose deprivation, Lactate dehydrogenase release
Graphical abstract
Arctic ground squirrel (AGS) hippocampal slices tolerate oxygen glucose deprivation (OGD) injury independent of hibernation season (summer versus winter) and state (hibernating versus interbout arousal). AGS tolerate OGD (green arrow) better than ischemic susceptible Sprague-Dawley rat (red arrow) despite loss of ATP, glutamate release, and acidosis. These findings point towards as yet undefined mechanisms of tolerance at the tissue level that are unique to AGS.
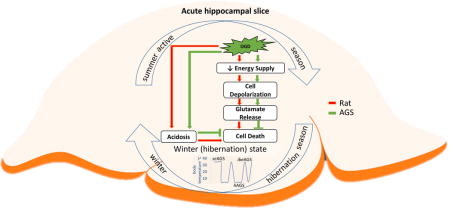
Introduction
Brain tissue is highly vulnerable to ischemic injury in almost all species wherein occlusion of cerebral arteries causes either a transient or a permanent decrease in blood flow to the brain. This in part leads to tissue hypoxia (reduced O2) wherein the tissue is unable to extract adequate O2, the partial pressure of O2 within the tissue falls leading to a reduction in mitochondrial respiration and oxidative metabolism. In addition to hypoxia, decreased blood flow limits delivery of nutrients such as glucose and removal of metabolic waste such as lactate. This cascade of events results in irreversible neurological damage and leads to high rates of disability and death; nearly half of the survivors never regain functional independence (Go et al. 2014).
Arctic ground squirrels (AGS), Urocitellus parryii, resist cerebral ischemia injury. AGS is an obligate hibernating species that expends up to several weeks at a time in a highly regulated and reversible state of prolonged torpor. During torpor metabolic rate, core body temperature (Tb), heart rate, and blood flow fall to ischemic-like levels without any evidence of ischemic injury (Ma et al. 2005, Weltzin et al. 2006, Frerichs et al. 1994). Previous work provides strong evidence that following cerebral I/R caused by cardiac arrest or mimicked by in vitro preparations, AGS have the innate ability to tolerate cerebral ischemia/reperfusion (Dave et al. 2009, Ross et al. 2006, Christian et al. 2008). Ion homeostasis assessed by anoxic depolarization is delayed in AGS brain by 1.23 minutes compared to rat (Dave et al. 2009). Nonetheless, AGS hippocampus resists up to 2h of OGD (Christian et al. 2008), despite anoxic depolarization that occurs within ~ 6.6 min after onset of OGD (Dave et al. 2009).
Cerebral I/R initiates a cascade of events that causes excitatory release of glutamate leading to detrimental effect (Benveniste et al. 1984). However, it remains unclear, if glutamate is released in AGS after anoxic depolarization. Moreover, the contribution of hibernating season or state to ischemia tolerance in mammalian hibernators remains a matter of debate. Some studies suggest that the hibernation state increases tolerance to injury (Zhou et al. 2001, Frerichs & Hallenbeck 1998), and others show that tolerance is lost or decreased in animals during the summer season (Lindell et al. 2005, Kurtz et al. 2006). By contrast, other studies suggest that tolerance to modeled ischemia persists outside of the hibernation season (Dave et al. 2006, Ross et al. 2006, Christian et al. 2008).
In view of these observations, one goal of this study was to assess the influence of hibernating state (i.e., hibernating or not hibernating) and hibernation season (i.e., winter versus summer) on resistance to OGD in AGS brain tissue. A second goal was to identify if events such as depletion of ATP, acidosis and glutamate efflux that are associated with anoxic depolarization are attenuated in AGS. Here, we tested the hypothesis that tolerance to I/R injury modeled in an acute hippocampal slice preparation in AGS is independent of the hibernation season and persists even after glutamate efflux. In this study, we specifically focused on acute hippocampal slices because prior results show that hippocampus plays an important role in both memory and learning and is more vulnerable to ischemic insults than other regions of the brain (Erfani et al. 2015, Ouyang et al. 2007)
Our results show that at the tissue level when tissue temperature is maintained at 36°C, hibernation season or state has no influence on OGD-induced cell death in AGS. Moreover, AGS tolerate OGD better than ischemic susceptible Sprague-Dawley rat despite loss of ATP, excitatory amino acid release, and acidosis. In addition, tolerance persists under conditions that mimic acidosis and ischemic shifts characteristic of cerebral I/R in vivo.
Methods
Animals Groups and Determination of Hibernation Status
Arctic ground squirrel (AGS, Urocitellus parryii) and Sprague-Dawley rats were used for these experiments. All procedures were performed in accordance with University of Alaska Fairbanks Institutional Animal Care and use Committee (IACUC) and performed in accordance with the guidelines of the 8th edition of the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health. Research was conducted in accordance with ARRIVE guidelines for reporting animal research (Kilkenny et al. 2010). AGS resist brain damage caused by global cerebral ischemia in vivo (Dave et al. 2006, Dave et al. 2009). The degree of ischemia resistance demonstrated by AGS is greater than other models of tolerance such as ischemia preconditioning. Moreover, unlike short-lived ischemia preconditioning that can be demonstrated in most species, ischemia resistance in AGS is chronic. Thus, AGS offer a unique and robust model of resistance to ischemia that is unparalleled by any other species and cannot be mimicked by other known manipulations. AGS of both sexes were trapped during mid-July in the northern foothills of the Brooks Range, Alaska, 40 miles south of the Toolik Field Station of the University of Alaska Fairbanks (68°38 N, 149°38 W; elevate on 809 m) and were transported to Fairbanks, AK under permit obtained from The Alaska Department of Fish and Game. AGS were housed individually in facilities with an ambient temperature (Ta) of 16–18°C and a 12:12-h light-dark cycle and fed rodent chow, sunflower seeds, and fresh carrots and apples ad libitum until mid-September, when they were moved to a cold chamber set to a Ta of 2°C and a 4:20-h light-dark cycle. Male Sprague-Dawley rats (3–4 months of age at the time of the experiment) were purchased from Simonsen Laboratories (Gilroy, CA) and were transported by air to the University of Alaska Fairbanks. Rats were housed in groups of two to four at 20–21°C, with a 12:12-h light-dark cycle, and were fed rodent chow ad libitum.
AGS hibernate only in the winter season so hibernating and interbout arousal groups are from the winter season. Tissue from summer AGS was collected during the summer season. Differences between summer AGS and all other AGS were assessed to determine if season influenced tolerance to cerebral I/R injury. AGS late in a torpor bout were collected after 80–90% of the duration of the previous torpor bout (8–12 days) and were defined as hibernating AGS (hAGS); AGS early after induced arousal from torpor (4h and 20h) were used as interbout euthermy AGS (ibeAGS). Post reproductive summer euthermic animals sampled in July and August 2–3 months after ending hibernation were used as summer euthermic AGS (seAGS)”. Subjects were matched as best as possible given the animal available for study. Detailed characteristics are provided in Supplementary table 1. Trectal and Ttemporalis were recorded at time of tissue collection. Arousal was induced by handling hibernating AGS. During interbout euthermy, tissue was collected 4h after inducing arousal (4h ibeAGS) or 20h after inducing arousal (20h ibeAGS). Ambient temperature and light-dark cycle remained the same for all groups.
Acute Hippocampal slice preparation and in vitro modeled ischemia/reperfusion
Animals were anesthetized using 5% (v/v) isoflurane with medical grade O2 at a constant flow rate of 1.5 L/min. Once unresponsive, the animals were euthanized via rapid decapitation and brains were removed within 2min. The whole brain was then placed in ice chilled, oxygenated HEPES buffered artificial cerebral spinal fluid (HEPES-aCSF) containing 120 mM NaCl, 20 mM NaHCO3, 6.68 mM HEPES acid, 3.3 mM HEPES sodium salt, 5.0 mM KCl, 2.0 mM MgSO4 (pH 7.3 - 7.4) to attenuate edema during the course of slicing and incubation. Rapidly dissected hippocampi were embedded in 2.5 % agar and transverse hippocampal slices. Approximately 10 mm were discarded from both ends and 400 μm thick slices from each hippocampus (~ 50–60 slices) were cut at approximately 2°C in oxygenated HEPES-aCSF using a Vibratome® 1000plus sectioning system (The Vibratome Company, St. Louis, MO). The slices were then transferred to a brain slice keeper (Scientific Systems Design Inc., Mississauga, Ontario, CA) and allowed to recover for 1–1.5 h at room temperature (20–21°C) in HEPES-aCSF bubbled continuously with 95% O2/5% CO2 before transferring to microperfusion chambers.
To address the time course of injury, treatment was applied using an in vitro microperfusion technique described previously. Briefly, after 1–1.5 h recovery as described above, individual slices were transferred gently to microperfusion chambers and lids sealed. The 4–8 parallel chambers were perfused with artificial cerebrospinal fluid (aCSF), pH 7.3 containing 120 mM NaCl, 45 mM NaHCO3, 10 mM glucose, 3.3 mM KCl, 1.2 mM NaH2PO4, 2.4 mM MgSO4, 1.8 mM CaCl2 equilibrated with 95% O2/5% CO2 and submerged in aCSF bath at 36°C (±0.2°C) at a flow rate of 7 μL/min. The osmolarity of these solutions was between 290 and 300 mOsm. Sampling began no sooner than 75 min after submerging the sealed chambers to allow adequate time for stabilization of neurochemical efflux. Perfusates was collected at 15 min intervals and analyzed as indicated.
Treatment and Injury conditions
To model in vivo ischemia/reperfusion (I/R)-induced alterations in the ionic microenvironment, hippocampus slices were perfused with one of the following, (1) aCSF, pH 7.3 as a control solution, (2) OGD, pH 7.3 (glucose-oxygen free aCSF), (3) aCSF, pH 6.4, (4) ionic shift solution (ISS), pH 6.5. aCSF, pH 6.4 containing 120 mM NaCl, 7.03 mM NaHCO3, 10 mM glucose, 3.3 mM KCl, 1.2 mM NaH2PO4, 2.4 mM MgSO4 1.8 mM CaCl2. Ionic shift solution (ISS), pH 6.5 contained 36.5mM NaCl, 11mM Na-gluconate, 65 mM K-gluconate, 38mM NMDG-Cl, 1mM NaH2PO4, 0.13 mM CaCl2, and 2.4 mM MgCl2, 1mM NaHCO3 as described previously (Ming et al. 2006). All aCSF solutions (pH 7.3 and 6.4) were equilibrated with 95% O2 and 5% CO2 whereas the OGD and ISS solutions were equilibrated with 5% CO2 and 95% N2, for a minimum of 1 h until pH stabilized in the desired range. The PO2 in OGD and ISS solution varied from 0–2.9 mmHg with an average for 6 determinations of 1.1 mmHg as measured using a miniature Clark-style electrode (Instech Laboratories, Plymouth Meeting, PA). Fractions were analyzed for cellular injury (LDH release) on the day of collection. Remaining volume was kept at −80°C for subsequent analysis.
Quantification of Cell Death
Lactate dehydrogenase (LDH) concentration in the perfusates was used as a marker of necrotic tissue damage. Perfusates, 50 μL was transferred to 384-well plates and mixed with 50 μL reaction solution provided in the LDH assay kit (Cayman Chemical). Optical density was measured at 490 nm 60 min later, using a microplate reader (BioTek Epoch). Background absorbance at 620nm was subtracted. The LDH release was expressed in arbitrary units per mg of protein (Biorad protein assay kit). The maximal releasable LDH was also obtained by treating the slices with 1% NP-40 lysis buffer (50 mM Tris-HCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% Igepal, 150 mM NaCl, 0.05% Triton X-100) at the end of each experiment.
Determination of ATP
ATP was determined after bath application of OGD or aCSF as described previously (Christian et al. 2008). In brief, slices were incubated in either aCSF or OGD solution and collected at the time point indicated. From each treatment condition, 3 hippocampal slices were transferred to a flat-bottomed microfuge tube containing 200 μL of ice-cold 5% trichloroacetic acid, sonicated (8 pulses), centrifuged at 16,000 g for 1 min at 4°C. Supernatant was then collected and assayed for ATP using ENLITEN ATP assay (Promega, Madison, WI). The pellets were further reconstituted with 200 μL 1M NaOH and protein concentration was analyzed (Bio-Rad protein assay kit, Hercules, CA). ATP activity was represented as a concentration of ATP normalized to total protein.
Capillary electrophoresis determination of glutamate and Aspartate efflux
Glutamate and aspartate were analyzed by capillary electrophoresis. Perfusates were derivatized as described previously (Kirschner et al. 2007, Kirschner et al. 2009) to form highly fluorescent cyanobenz[f]isoindole amino acids (CBI-amino acids). Briefly, 2 μL thawed perfusates was reacted with 2 μL 2 mM naphthalene-2,3-dicarboxaldehyde (NDA) in methanol and 2 μL NaCN (5.5 mM) in 60 mM sodium tetraborate. Cyanide solution contained 3μM D-amino adipic acid as internal standard. Samples were reacted at room temperature for approximately 20 min prior to analysis. CE-LIF was performed on a custom in-house-built instrument using a 420-nm diode laser, 490-nm bandpass filter, and PMT for LIF detection. Samples were injected onto a bare fused silica capillary of dimensions 25 cm × 25 μm (22.5 cm to detection window) for 1–3 sec at 380-mbar vacuum and separated by using positive polarity (20 kV). Separation buffer optimized for analysis of L-glutamate and L-aspartate is a 50mM sodium tetraborate background electrolyte (BGE) adjusted to pH 9.3. BGE additionally contained 1.0 mM β-cyclodextrin to decrease sample run time for glutamate and aspartate analysis. The addition of β-cyclodextrin allowed for baseline resolution of glutamate, aspartate, and the internal standard in less than 2 min. PeakFit software (SeaSolve Software Inc., Framingham, MA) was used to process raw data and quantify peak areas in all experiments based on a Gaussian peak shape for each analyte. Linear calibration curves were constructed for glutamate and aspartate as a function of concentration verses the peak area ratio (analyte area/internal standard area). Efflux of analytes was quantified as a concentration of glutamate or aspartate normalized to total protein for the slice used in the efflux experiment and represented as a fraction of baseline glutamate or aspartate release.
Statistical Analysis
A priori power analysis (G*Power software, (Faul et al. 2007)) was performed to estimate sample size needed to yield 80% power for detecting a significant (p<0.05) effect of treatment. An expected difference in means of 17.81 and standard deviations of 10.93 and 2.24, taken from rat OGD and seAGS OGD indicates that a sample size of 5 slices will have 80% power for detecting a difference. For experimental group with smaller sample size, no non-significant effect was under powered. No animal was excluded from the study. Slices from animals were randomly assigned to treatment and control groups without prior knowledge to the treatment conditions. The statistical analysis was performed on the data that were normalized to baseline and expressed as fraction of baseline to avoid any variation in the baseline values among the treatment groups. Baseline was calculated as the mean of the two samples preceding onset of treatment (Supplementary table2). Data processing and statistical analyses were performed using Microsoft Office Excel 2010 and Prism6 (GraphPad, San Diego CA, USA). Data were analyzed with one-way or two-way ANOVA with repeated measures. Significant main effects or interactions were followed by t-tests with Bonferroni’s correction. Data are expressed as mean ± SEM, and P < 0.05 was considered statistically significant. Treatment with aCSF and OGD were run in every experiment and combined for the final analysis; as a result, sample size was larger in aCSF and OGD groups than in many of the experimental manipulations.
Results
Tolerance to OGD injury in AGS hippocampus is not dependent on hibernation season
To investigate whether at the tissue level when tissue temperature is normalized to 36°C, AGS are more tolerant to OGD injury than ischemic susceptible rat, hippocampal slices from a cohort of each species were examined. Following 30 min of OGD, slices harvested from rats displayed a significant increase in LDH release relative to control slices (aCSF, n= 25 slices; OGD, n= 30 slices, 15 animals, p<0.0001, 2-way ANOVA, treatment × time). seAGS showed a significant increase in LDH release relative to aCSF treated control slices (aCSF, n= 28 slices; OGD, n= 30 slices, 14 animals, p=0.0001, 2-way ANOVA, treatment × time). When OGD treated slices from rats and seAGS were compared, rat slices displayed a significantly greater degree of LDH efflux than AGS slices (rat, OGD, n= 30 from 15 animals; AGS, OGD, n= 30 from 14 animals, p<0.0001, 2-way ANOVA, species × time) Figure 1(A).
Figure 1. AGS brain tolerates OGD regardless of hibernation season.
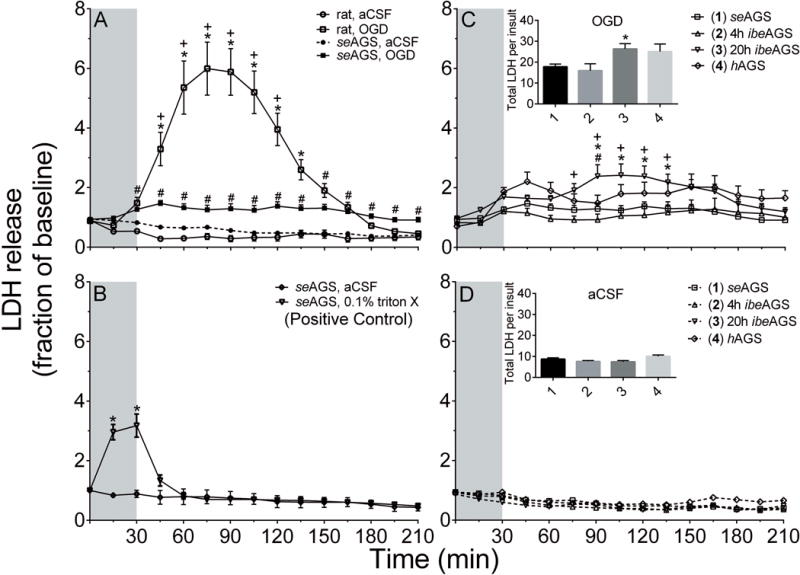
(A) seAGS are better protected from OGD than rats. Slices from rats and seAGS were subjected to 30 min OGD and time course of LDH release was monitored (OGD: rat, n=30 slices; seAGS, n= 30 slices; aCSF: rat, n=25 slices; seAGS, n= 28 slices, *p<0.05 rat aCSF vs rat OGD, +p<0.05 rat vs AGS OGD, #p<0.05 seAGS aCSF vs seAGS OGD). (B) AGS slices exposed to 0.1% Triton-X as a positive control (n= 4 slices from 2 AGS). *p<0.05 vs. seAGS aCSF (C) AGS slices obtained based on hibernation season were subjected to 30 min OGD and time course and total LDH release was monitored. *p<0.05 seAGS vs. 20h ibeAGS, +p<0.05 4h ibeAGS vs. 20h ibeAGS, #p<0.05 hAGS vs. 20h ibeAGS. (D) AGS slices obtained based on hibernation season were subjected to aCSF treatment. Grey bar indicates 30 min treatment period. Data shown are means ± SEM.
Next, to confirm our ability to detect an increase in LDH release in AGS hippocampus we applied TritonX-100, a nonionic detergent to directly disrupt cell membrane as a positive control. Treatment of slices with 0.1% TritonX-100 produced a significant increase in LDH release (aCSF, n= 4 slices; 0.1% TritonX-100, n= 4 slices, 2 animals, p<0.0001, 2-way ANOVA, treatment × time) Figure 1 (B). We further analyzed the total LDH content per slice to ask if the amount of LDH release following OGD is limited by total LDH content. No difference in total LDH content was observed in slices from rat or AGS that were subjected to either aCSF or OGD treatment (Supplementary Figure1). These results suggested that the difference in LDH release between rat and AGS is not due to a difference in total LDH content but is due to a difference in the injury associated with OGD exposure.
Next, to address if tolerance to OGD injury depends on the hibernation season, groups of AGS - seAGS, hAGS and ibeAGS, 4h or 20h after inducing arousal, were matched as best as possible for season, frequency, age, sex and body weight prior to tissue collection. Body weight of seAGS was greater than hAGS and ibeAGS due to the seasonal fattening of this species (Sheriff et al. 2013). Because date of birth is not known for AGS, age is only reported as adult which means greater than one year old. Trectal and Ttemporalis did not differ between seAGS ibeAGS, 4h or 20h after inducing arousal, but were as expected warmer than Trectal and Ttemporalis in hAGS at time of tissue collection (Supplementary Table 1). All tissue was normalized to 36°C prior to experimental manipulations. Results show that OGD-induced a significant increase in total LDH release in the 20h ibeAGS group compared to seAGS (p= 0.01, 1-way ANOVA, n= 30–33 slices, (Figure 1 C insert). Relative to aCSF (Figure 1 C vs 1D insert), OGD induced a 1-fold increase in total LDH release in seAGS, 4h ibeAGS and hAGS slices. By contrast, OGD induced a 3-fold increase in LDH release in 20h ibeAGS. The same pattern was seen when the effect of OGD on LDH was assessed over time. A significant LDH response was observed in slices from 20h ibeAGS during OGD compared to seAGS, 4h ibeAGS and hAGS slices (P< 0.0001, 2-way ANOVA season × time). However, the statistically significant injury observed in slices taken from 20h ibeAGS OGD, was less than the injury observed in slices from rat OGD (p<0.0001, 2-way ANOVA species (rat and 20h ibeAGS) × time) (Figure 1 A vs 1C). These results suggest that 20h into interbout euthermy, AGS hippocampus is the most vulnerable to OGD relative to other times in the hibernation season. However, even at this most vulnerable time point AGS are still less vulnerable to OGD-induced cell death than rat.
Tolerance to injury in AGS hippocampus is not reversed by Ionic shift solution (ISS) or low pH
Ionic homeostasis has been tightly linked to cell injury/death (Bortner & Cidlowski 2004). To simulate the ionic microenvironment in our in vitro slice preparation to mimic the changes in the extracellular milieu that occurs in vivo during cerebral I/R, slices from rat and AGS were subjected to 30 min of an ionic shift solution insult (K+= 65mM; Na+ = 55mM; HCO3− = 2.5mM, Cl− = 77.0mM, pH 6.5) and LDH released was measured. Results show that slices from rat show significant release of LDH as compared to control slices (aCSF, n=3; ISS, n= 3 slices from 4 animals, p< 0.0001, 2-way ANOVA, treatment × time). In contrast, ISS had no influence on slices from seAGS (aCSF, n=3; ISS, n= 3 from 3 animals, p= 0.10, 2-way ANOVA, treatment × time). ISS treated slices from rats also displayed significant increase in LDH release compared to seAGS slices. (p< 0.0001, 2-way ANOVA, species × time) Figure 2 (A). Next, we addressed the role of pH alone in acute hippocampal slices. Rat slices subjected to 30 min low pH 6.3 causes significant LDH release (aCSF, pH 7.4 = 7 slices, 6 animals; aCSF, pH 6.3 = 7 slices, 5 animals, p= 0.001, 2-way ANOVA, treatment × time). In contrast, AGS slices displayed no significant release of LDH with low pH compared to control slices (aCSF, pH 7.4 = 7 slices, 5 animals; aCSF, pH 6.3 = 7 slices, 4 animals). When low pH treated slices from rats and seAGS were compared, rat slices displayed significantly greater LDH efflux than AGS slices (rat aCSF pH 6.3, n = 7 slices, 5 animals; AGS aCSF pH 6.3, n = 7 slices, 4 animals, p<0.0001, 2-way ANOVA, species × time) Figure 2 (B).
Figure 2. AGS brain is tolerant to ionic shift solution (ISS) or low pH injury.
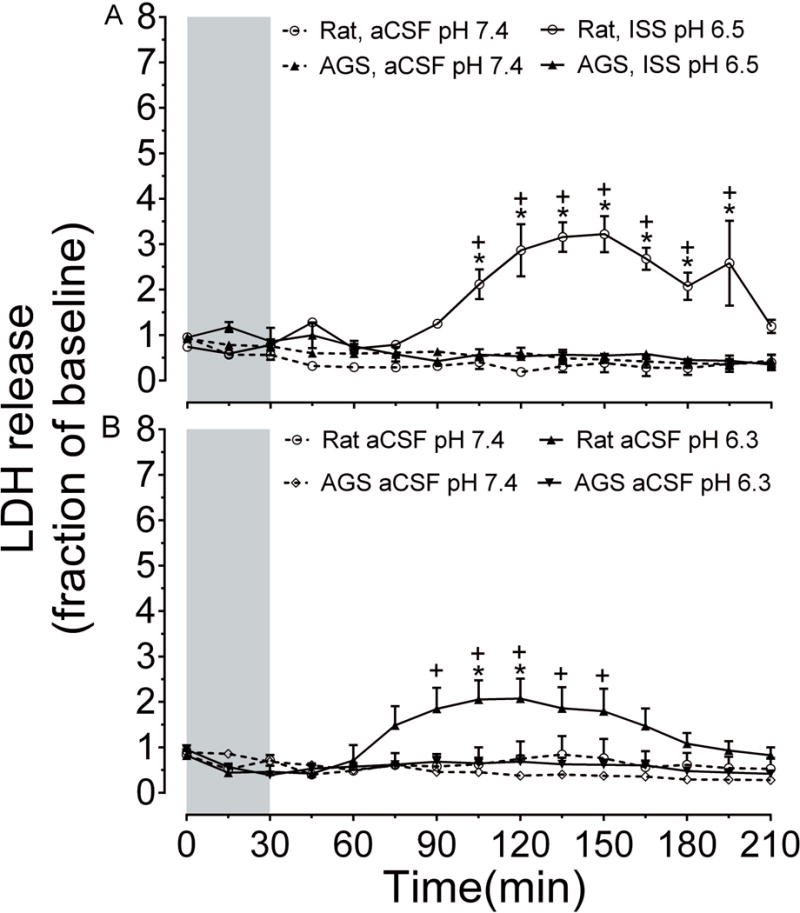
(A) Shows time course of LDH release in acute hippocampal slices from rat and seAGS exposed to ISS, pH 6.3 (n= 3) or aCSF, pH 7.3 (n=3). *p< 0.05 rat aCSF vs. rat ISS (pH 7.4), +p< 0.05 rat ISS vs seAGS ISS (pH 6.3). (B) Shows time course of LDH release exposed to low pH 6.3 (n= 7) or aCSF, pH 7.3 (n=7). *p< 0.05 rat aCSF pH 7.4 vs. rat aCSF pH 6.3, +p< 0.05 rat aCSF pH 6.3 vs seAGS aCSF pH 6.3. Grey bar indicates treatment period. Means ± SEM.
We also explored pH dependent injury over a wider range of pH (pH 7.4, 7.0, 6.6, 6.3) in seAGS. We found that pH did not influence LDH release in seAGS (aCSF pH 7.4, n= 7; aCSF pH 7.0, n= 4; aCSF pH 6.6, n= 5; aCSF pH 6.3, n= 7; 15 animals; p= 0.33, 1-way ANOVA) (Supplementary Figure2). This suggests that AGS hippocampus is less vulnerable to the damaging effects of ISS or low pH than rat.
AGS hippocampus resist OGD injury not because of enhanced energy conservation
In the context of bioenergetics, the brain consumes large amounts of oxygen to maintain ionic gradients across neuronal plasma membranes. However, ischemic injury causes bioenergetic failure leading to depletion in cellular ATP and prevents neurons from maintaining membrane potential, resulting in neuronal depolarization. Here, we asked whether resistance to ischemic injury in AGS is due to preservation of energy homeostasis. We tested this by exposing acute hippocampal slices from seAGS and rat to 30 min of OGD and monitoring ATP levels. Results show that exposure of rat hippocampal slices to 30 min OGD produced a significant depletion in ATP when compared to control slices. Slices with OGD showed no significant signs of ATP restoration even after 3 hours of reperfusion (aCSF, n=4 slices; OGD, n=4, 4 animals, p= 0.0037, 2-way ANOVA, treatment × time; Figure 3 (A). Hippocampal slices from seAGS displayed similar depletion in ATP following OGD when compared to slices exposed to aCSF (aCSF, n= 3 slices, OGD, n= 3 slices, 3 animals, p= 0.0097, 2-way ANOVA, treatment × time). Tendency to restore ATP following reperfusion was similar in rat and AGS slices Figure 3 (B). These results indicate that energy homeostasis is disrupted in AGS in a fashion similar to rat and that tolerance to OGD cannot be explained by enhanced energy homeostasis.
Figure 3. ATP declines in rat and seAGS following OGD.
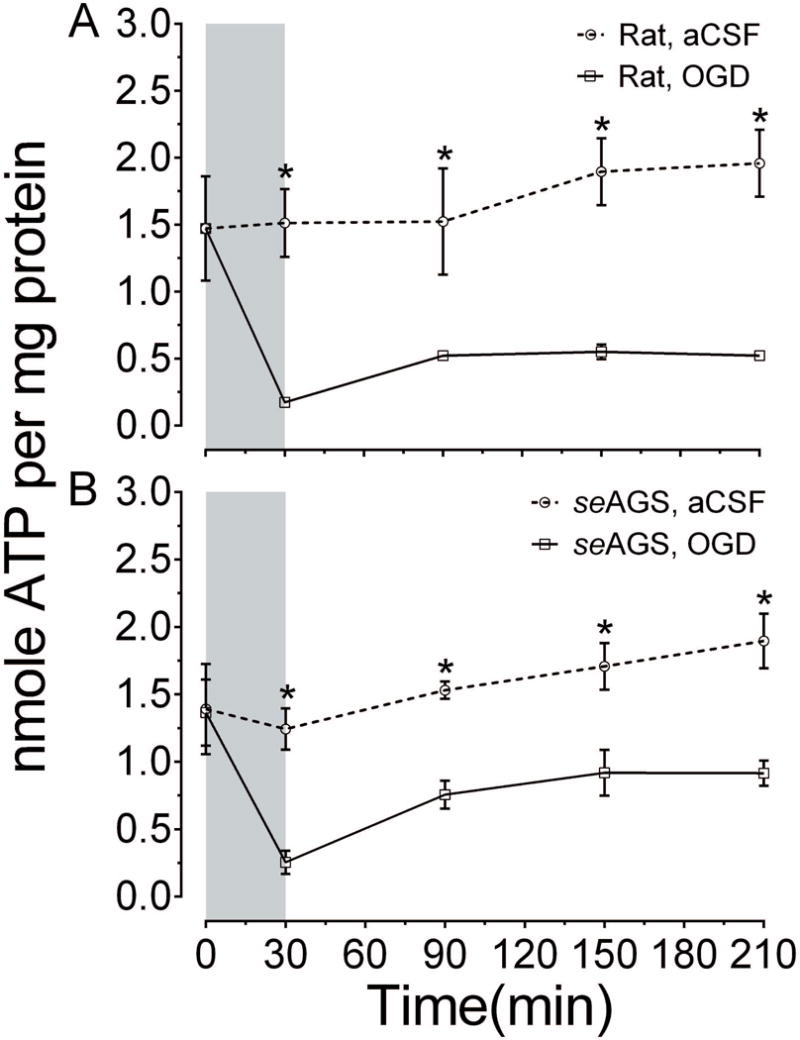
Levels of whole tissue ATP were determined over time following bath application of treatment in slices from (A) rat (aCSF n= 4, OGD n= 4) and (B) seAGS (aCSF n= 3, OGD n= 3) subjected to either 30 min of aCSF or OGD followed by 3 h reperfusion. Grey bar indicates insult period. Data shown are means ± SEM, *p< 0.05 versus aCSF.
Tolerance to OGD in AGS hippocampus is not due to inhibition of excitatory neurotransmitter efflux
We next investigated if tolerance to ischemia in AGS is associated with dampening of excitatory neurotransmitter release, which occurs following ischemia induced depletion of cellular ATP. This was tested by exposing acute hippocampal slices from seAGS and rat to 30 min of OGD and monitoring efflux of glutamate and aspartate using capillary electrophoresis. Results show that rat slices exposed to OGD displayed a 2.3-fold increase in glutamate efflux over basal efflux (n= 16 control slices, 12 animals; n= 17 OGD slices; 12 animals, p<0.0001, 2-way ANOVA, treatment × time) Figure 4 (A), aspartate efflux also increased during OGD in rat (1.7-fold increase over basal efflux, n= 16 control slices, 12 animals; n= 17 OGD slices; 12 animals, p<0.0001, 2-way ANOVA, treatment × time) Figure 4 (C). After the onset of reperfusion, the glutamate levels returned to baseline levels within 60 min, aspartate returned to baseline levels within 45 min. seAGS slices exposed to 30 min OGD displayed both glutamate (3-fold increase over basal level, p<0.0001, 2-way ANOVA, treatment × time) and aspartate efflux (1.3-fold increase over basal level, p<0.0001, 2-way ANOVA, treatment × time; n= 14 slices from 5 seAGS) Figure 4 (B, D). The magnitude and time course of excitatory amino acid efflux in AGS approximates efflux seen in rat hippocampal slices. Despite excitatory neurotransmitter efflux, AGS resist OGD-induced injury suggesting that cerebral I/R injury is modulated in AGS downstream to glutamate or aspartate release.
Figure 4. OGD induces efflux of glutamate and aspartate in both seAGS and rat.
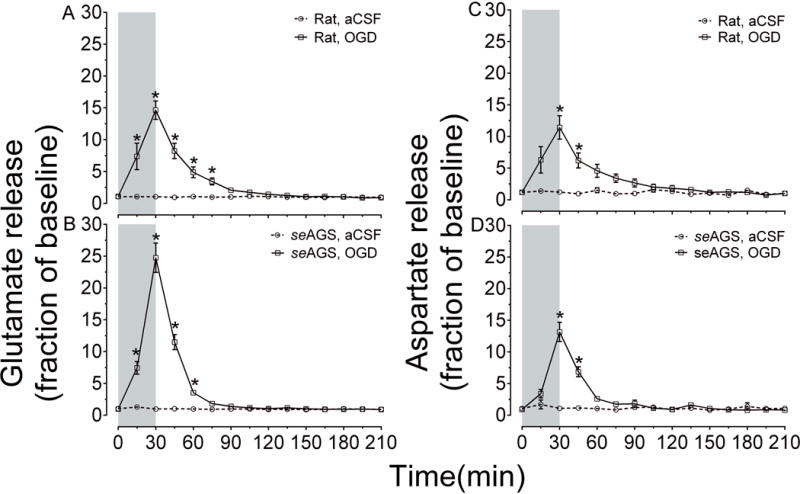
Time-dependent excitatory neurotransmitter (glutamate and aspartate) efflux in rat and seAGS hippocampal slices induced by 30 min OGD insult. (A) and (B) illustrates glutamate efflux, (C) and (D) illustrate aspartate efflux in rat and seAGS hippocampal slices during OGD insult (n=17 slices from 6 rats, n= 14 slices from 5 seAGS). Grey bar indicates insult period. *p<0.05 for OGD versus aCSF group. Data shown are means ± SEM.
Discussion
Using an in vitro microperfusion approach to avoid the influence of cold tissue temperature that is unavoidable in whole animals studies we have identified that the hibernating state or the hibernation season is not necessary for AGS tolerance to cerebral I/R modeled in acute hippocampal slices. The time that AGS are most vulnerable to OGD is 20h into an interbout arousal although even at this most vulnerable period, resistance to OGD in AGS is substantially better than resistance to OGD in rat hippocampal slices. Secondly, our data unequivocally shows that tolerance to OGD in AGS hippocampal slices occurs despite loss of ATP, and excitatory amino acid release and persists during conditions that mimic acidosis (Siesjo 1988) and ionic shifts characteristic of cerebral I/R (Yanagihara & McCall 1982, Hansen & Zeuthen 1981).
The hibernating state or the hibernation season is not necessary for AGS tolerance
This work showing that in AGS the hibernation season or state does not define tolerance to OGD is significant because it is the first set of studies to systematically test the response to OGD in hippocampal tissue throughout the season and hibernation state in AGS. Dave et al., concluded that in AGS, hibernation season was not required for resistance to brain injury following cardiac arrest (Dave et al. 2006), however, studies were only performed during the summer season. (Christian et al. 2008), concluded that hippocampal slices from AGS were least vulnerable to OGD during the summer season. However, the propidium iodide staining used by (Christian et al. 2008) to measure cell death produced high background levels. Background levels of cell death were highest in the summer season and could have masked effects of OGD. (Ross et al. 2006) also showed that the hibernation state did not influence tolerance to OGD in the first 24h of culture. However, as with (Christian et al. 2008) high background cell death measured in cultured slices using propidium iodide may have compromised sensitivity to detect cell death. These results confirm that when tissue temperature is normalized to 36°C both summer and hibernating AGS resist OGD. While vulnerability to OGD is always less in AGS than in rat, vulnerability in AGS does increase slightly towards the end of arousal. Arousal from prolonged torpor is the most stressful aspect of hibernation indicated by increased levels of HIF 1α and inducible nitric oxide synthase (Ma et al. 2005). It is likely that the physiological challenge of rewarming, reanimating and reperfusing the brain contributes to vulnerability to OGD.
Tolerance, independent of season or state may be unique to the AGS. Others (Frerichs & Hallenbeck 1998) showed that hibernation state increases tolerance to OGD in 13 lined ground squirrel hippocampus. (Kurtz et al. 2006) demonstrated that hibernation season increases tolerance to I/R in 13 lined ground squirrel in liver and gut. By contrast, the hibernation season is not required for tolerance to ischemia/reperfusion in other organs in AGS (Bogren et al. 2014b, Bogren et al. 2014a). A recent study in AGS, however, did reveal cardioprotective benefit of the hibernation season during cardiopulmonary bypass in vivo with a core body temperature of 18°C (Quinones et al. 2016). Heart may differ from brain and the longer duration (45min) of ischemia studied in the cardiopulmonary bypass procedure may have revealed a seasonal difference not evident in studies of cerebral I/R tolerance in AGS.
Cold tissue temperature is protective (Busto et al. 1987) and confounds whole animal comparisons between hibernating and euthermic ground squirrels. With the acute slice model, it is possible to avoid the influence of cold tissue temperature which is not possible in whole animal models because hibernating animals have a core and brain temperature near 0°C and euthermic animals have a core and brain temperature near 37°C. Seasonal comparisons between summer and winter euthermic animals can be made in whole animals without confounding influence of temperature since both groups have body temperatures near 37°C. Our results are consistent with whole animal studies that show that tolerance to I/R or hypoxia does not require the hibernation season (Dave et al. 2006, Dave et al. 2009, D’Alecy et al. 1990, Bogren et al. 2014b, Bogren et al. 2014a). The present results confirm that AGS do not need to be cold to tolerate cerebral I/R better than rat although cold tissue temperature would likely enhance tolerance as noted by (Frerichs & Hallenbeck 1998).
Our results suggest that the contribution of A1 receptors to seasonal hibernation does not impact cerebral I/R tolerance since season did not play a significant role in tolerance. A1 receptor activation is necessary and sufficient to drive onset of hibernation, however, only in the winter season (Jinka et al. 2011). Sensitivity to A1 adenosine receptor agonists increase in the hibernation season (Olson et al. 2013) and A1 adenosine receptor agonists are neuroprotective (Cunha 2005). However, differences in A1 sensitivity between summer and winter is not relevant to cerebral I/R tolerance since we and others have not found a significant influence of season on tolerance. Indeed, tolerance in AGS does not depend on the hibernation state or season.
The novel microperfusion method developed in our laboratory (Kirschner et al. 2009) for study of cell death in acute, adult slices overcomes the limitations of methods used in prior studies where high baseline cell death compromised interpretation. The microperfusion approach minimizes background cell death by capturing LDH release from the entire slice. This approach is an improvement over other methods where stains such as propidium iodide show cell death only on the surface of the freshly cut slice (Christian et al. 2008, Ross et al. 2006). Moreover, the microperfusion approach, allows us to study cell death in adult AGS tissue that is not available from young animals. In addition, interpretation of organotypic cultures is limited by developmental age of the tissue (Fukuda et al. 1995). The in vitro microperfusion approach in our study might not apply to the in vivo scenario wherein whole animal mechanisms such as pH, buffering capacity and limited inflammatory response contribute to tolerance to ischemia reperfusion injury (Bogren et al. 2014b, Bogren et al. 2014a). Nonetheless, the microperfusion approach isolates processes occurring at the tissue level so that interpretation is not confounded by whole animal physiology. Moreover, the microperfusion approach allows for the concentration of each component in the perfusion fluid to be defined and manipulated and cell death quantified in a time-dependent manner. In this way, pH can be decreased without hypoxia and cell death monitored with temporal resolution relevant to onset and recovery from low pH. Such discrete manipulation of individual components of the extracellular milieu is not possible in vivo where, for example, pH decreases following anoxia. Thus, the microperfusion approach gives superior control over the extracellular environment that could not be achieved in vivo. We acknowledge that the slice preparation differs from the in vivo scenario (Cho et al. 2007, Saeidnia et al. 2015) and in this way serves our objective to study processes at the tissue level that do not depend on cold tissue temperatures and confer tolerance to AGS.
LDH release was used to interpret cell death in our study. LDH release is a standard approach to monitor cell death (Chan et al. 2013) which is released from the cytosol due to membrane disruption and not due to metabolic changes related to OGD. This approach of monitoring cell death overcomes the limitations of methods used in prior studies where high baseline cell death due to acute damage at the slice surface challenged interpretation (Christian et al. 2008, Ross et al. 2006). The microperfusion approach minimizes background cell death by capturing LDH release from the entire slice. This approach overcomes problems with propidium iodide staining. Like LDH release, propidium iodide staining depicts increased membrane permeability, however propidium iodide shows cell death only on the surface of the freshly cut slice.
AGS hippocampus resists OGD as well as ischemic shift solution better than rat
Traditionally, OGD is used to model in vivo ischemic insult, however, OGD excludes other deleterious aspects of the ischemic milieu (Lo 2008, Yao et al. 2007). This artifact of the in vitro preparation could not explain tolerance to OGD because resistance to modeled ischemia was observed in AGS even when the perfusion fluid was modified to mimic loss of ion homeostasis that occurs in vivo. AGS were not vulnerable to ischemic shift solution (ISS) or to acidic media (∼pH 6.4). ISS includes ionic alterations sufficient to induce spreading depression in cortex (Zhou et al. 2010). ISS also mimics tissue acidosis sufficient to activate acid sensing ion channels which increase calcium permeability and leads to cell death during cerebral I/R (Xiong et al. 2004). Previous studies have shown that painted turtle neurons also tolerate prolonged ischemic solution treatment (Pamenter et al. 2012).
AGS hippocampus tolerate OGD despite ATP depletion
Resistance to OGD is evident in AGS even though there is a depletion in ATP, a hallmark of ischemic injury (Sato et al. 1984). By contrast, a balance between ATP-demand and ATP-supply prevails in other anoxia tolerant species such as the painted turtle via a coordinated reduction of ATP demand in the absence of oxygen (Bickler & Buck 2007). In AGS tolerance to OGD resides despite ATP depletion and is not due to restoration of ATP. This finding distinguishes AGS from anoxia tolerant turtle where ATP recovers slowly over time (Pamenter et al. 2012). Depletion of ATP is also seen during OGD in hippocampal slices taken from hibernating AGS although the initial loss of ATP is delayed relative to AGS during interbout arousal (Christian et al. 2008). Here we focused exclusively on seAGS since our first set of experiments found no difference in tolerance to OGD between winter hibernating and summer active AGS. Naked mole-rat show similar depletion of ATP despite remarkable tolerance to anoxia (Larson et al.).
AGS hippocampus tolerate OGD injury even after ischemic depolarization associated excitatory glutamate efflux
The present study also revealed that resistance to OGD in AGS hippocampus is not due to attenuation in excitatory amino acid efflux such as glutamate and aspartate. Ischemia-induced depolarization is delayed in AGS relative to rat in vitro and in vivo. Nonetheless, depolarization is observed within 7 min in acute slices and 3 min during cardiac arrest in vivo (Dave et al. 2009). The 30 min of OGD applied in the present study should have been sufficient to depolarize hippocampal neurons and indeed led to an increase in glutamate and aspartate efflux that was similar to rat and occurred with a time course consistent with in vivo studies (Benveniste et al. 1984, Mitani et al. 1994, Hagberg et al. 1985). While delayed depolarization may contribute to tolerance, additional protective mechanisms are most likely unrelated to glutamate release. Our result is in contrast to anoxia tolerant species such as fresh-water turtle and crucian carp that shows a decrease in tissue level of excitatory neurotransmitter glutamate and aspartate (Nilsson 1990); Downregulation of glutamate release is suggested to be modulated by synergistic activity of K (ATP) (+) channels, adenosine and GABA(A) receptors (Milton et al. 2002, Thompson et al. 2007). Our result suggests that tolerance to OGD in AGS is not due to inhibition of excitatory neurotransmitter release.
Conclusion
In agreement with previous results (Dave et al. 2006, Ross et al. 2006, Christian et al. 2008), this study shows that hippocampal slices from AGS are unique in lack of seasonal dependence associated with tolerance to OGD in vitro and cardiac arrest in vivo. Here we show that even though AGS, 20h into an interbout arousal are vulnerable to OGD, the level of susceptibility is negligible as compared to rat. We also conclude that at the tissue level when tissue temperature is normalized to 36°C despite ATP depletion, ionic derangement, tissue acidosis and excitatory neurotransmitter efflux, AGS hippocampus resist OGD injury. Unlike hypoxia and anoxia tolerant species where tolerance is linked to ATP restoration and neurotransmitter release, AGS may have evolved unique adaptations to physiological extremes and subsequently cerebral I/R.
ARRIVE guidelines have been followed:
Yes
-
=>
if No, skip complete sentence
-
=>
if Yes, insert “All experiments were conducted in compliance with the ARRIVE guidelines.”
Supplementary Material
Acknowledgments
This work was supported by the US Army Medical research and Material Command, No 0517800; the National Institute of Neurological Disorder and Stroke, Nos. NS041069-06 and R15NS070779; Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103395.
Abbreviations
- aCSF
artificial cerebral spinal fluid
- I/R
ischemia/reperfusion injury
- OGD
oxygen glucose deprivation
- LDH
Lactate dehydrogenase
- AGS
Arctic ground squirrel
- seAGS
summer euthermic AGS
- ibeAGS
interbout euthermic AGS
- hAGS
hibernating AGS
- ISS
ischemic shift solution
Footnotes
Conflict of interest disclosure
The authors report no conflict of interest. Figure 4 (A–B) was published in preliminary form in book chapter (Drew et al. 2013).
Author Contribution statement:
SB, JTM and KLD contributed to development of study design, performance of research. SB and KLD contributed to writing of manuscript. DLK contributed to performance of research. KLD contributed to financial support. All authors revised the manuscript for important intellectual content and approved the final version.
References
- Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. Journal of neurochemistry. 1984;43:1369–1374. doi: 10.1111/j.1471-4159.1984.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Buck LT. Hypoxia tolerance in reptiles, amphibians, and fishes: life with variable oxygen availability. Annual review of physiology. 2007;69:145–170. doi: 10.1146/annurev.physiol.69.031905.162529. [DOI] [PubMed] [Google Scholar]
- Bogren LK, Murphy CJ, Johnston EL, Sinha N, Serkova NJ, Drew KL. 1H-NMR metabolomic biomarkers of poor outcome after hemorrhagic shock are absent in hibernators. PloS one. 2014a;9:e107493. doi: 10.1371/journal.pone.0107493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogren LK, Olson JM, Carpluk J, Moore JM, Drew KL. Resistance to systemic inflammation and multi organ damage after global ischemia/reperfusion in the arctic ground squirrel. PloS one. 2014b;9:e94225. doi: 10.1371/journal.pone.0094225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortner CD, Cidlowski JA. The role of apoptotic volume decrease and ionic homeostasis in the activation and repression of apoptosis. Pflugers Archiv : European journal of physiology. 2004;448:313–318. doi: 10.1007/s00424-004-1266-5. [DOI] [PubMed] [Google Scholar]
- Busto R, Dietrich WD, Globus MY, Valdes I, Scheinberg P, Ginsberg MD. Small differences in intraischemic brain temperature critically determine the extent of ischemic neuronal injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1987;7:729–738. doi: 10.1038/jcbfm.1987.127. [DOI] [PubMed] [Google Scholar]
- Chan FK, Moriwaki K, De Rosa MJ. Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol Biol. 2013;979:65–70. doi: 10.1007/978-1-62703-290-2_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S, Wood A, Bowlby MR. Brain slices as models for neurodegenerative disease and screening platforms to identify novel therapeutics. Current neuropharmacology. 2007;5:19–33. doi: 10.2174/157015907780077105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian SL, Ross AP, Zhao HW, Kristenson HJ, Zhan X, Rasley BT, Bickler PE, Drew KL. Arctic ground squirrel (Spermophilus parryii) hippocampal neurons tolerate prolonged oxygen-glucose deprivation and maintain baseline ERK1/2 and JNK activation despite drastic ATP loss. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2008;28:1307–1319. doi: 10.1038/jcbfm.2008.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: From A(1) receptor activation to A (2A) receptor blockade. Purinergic Signal. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Alecy LG, Lundy EF, Kluger MJ, Harker CT, LeMay DR, Shlafer M. Beta-hydroxybutyrate and response to hypoxia in the ground squirrel, Spermophilus tridecimlineatus. Comp Biochem Physiol B. 1990;96:189–193. doi: 10.1016/0305-0491(90)90361-v. [DOI] [PubMed] [Google Scholar]
- Dave KR, Anthony Defazio R, Raval AP, Dashkin O, Saul I, Iceman KE, Perez-Pinzon MA, Drew KL. Protein kinase C epsilon activation delays neuronal depolarization during cardiac arrest in the euthermic arctic ground squirrel. Journal of neurochemistry. 2009;110:1170–1179. doi: 10.1111/j.1471-4159.2009.06196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KR, Prado R, Raval AP, Drew KL, Perez-Pinzon MA. The arctic ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke; a journal of cerebral circulation. 2006;37:1261–1265. doi: 10.1161/01.STR.0000217409.60731.38. [DOI] [PubMed] [Google Scholar]
- Drew KL, Zuckerman JA, Shenk PE, Bogren LK, Jinka TR, Moore JT. Hibernation: A Natural Model of Tolerance to Cerebral Ischemia/Reperfusion. In: Gidday MJ, Perez-Pinzon AM, Zhang HJ, editors. Innate Tolerance in the CNS: Translational Neuroprotection by Pre- and Post-Conditioning. Springer New York; New York, NY: 2013. pp. 37–50. [Google Scholar]
- Erfani S, Khaksari M, Oryan S, Shamsaei N, Aboutaleb N, Nikbakht F. Nampt/PBEF/visfatin exerts neuroprotective effects against ischemia/reperfusion injury via modulation of Bax/Bcl-2 ratio and prevention of caspase-3 activation. J Mol Neurosci. 2015;56:237–243. doi: 10.1007/s12031-014-0486-1. [DOI] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior research methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Hallenbeck JM. Hibernation in ground squirrels induces state and species-specific tolerance to hypoxia and aglycemia: an in vitro study in hippocampal slices. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1998;18:168–175. doi: 10.1097/00004647-199802000-00007. [DOI] [PubMed] [Google Scholar]
- Frerichs KU, Kennedy C, Sokoloff L, Hallenbeck JM. Local cerebral blood flow during hibernation, a model of natural tolerance to “cerebral ischemia”. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1994;14:193–205. doi: 10.1038/jcbfm.1994.26. [DOI] [PubMed] [Google Scholar]
- Fukuda A, Czurko A, Hida H, Muramatsu K, Lenard L, Nishino H. Appearance of deteriorated neurons on regionally different time tables in rat brain thin slices maintained in physiological condition. Neuroscience letters. 1995;184:13–16. doi: 10.1016/0304-3940(94)11156-d. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, et al. Heart disease and stroke statistics–2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg H, Lehmann A, Sandberg M, Nystrom B, Jacobson I, Hamberger A. Ischemia-induced shift of inhibitory and excitatory amino acids from intra- to extracellular compartments. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1985;5:413–419. doi: 10.1038/jcbfm.1985.56. [DOI] [PubMed] [Google Scholar]
- Hansen AJ, Zeuthen T. Extracellular ion concentrations during spreading depression and ischemia in the rat brain cortex. Acta physiologica Scandinavica. 1981;113:437–445. doi: 10.1111/j.1748-1716.1981.tb06920.x. [DOI] [PubMed] [Google Scholar]
- Jinka TR, Toien O, Drew KL. Season primes the brain in an arctic hibernator to facilitate entrance into torpor mediated by adenosine A(1) receptors. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:10752–10758. doi: 10.1523/JNEUROSCI.1240-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS biology. 2010;8:e1000412. doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner DL, Jaramillo M, Green TK. Enantioseparation and stacking of Cyanobenz[f]isoindole-amino acids by reverse polarity capillary electrophoresis and sulfated beta-cyclodextrin. Analytical chemistry. 2007;79:736–743. doi: 10.1021/ac061725+. [DOI] [PubMed] [Google Scholar]
- Kirschner DL, Wilson AL, Drew KL, Green TK. Simultaneous efflux of endogenous D-ser and L-glu from single acute hippocampus slices during oxygen glucose deprivation. Journal of neuroscience research. 2009;87:2812–2820. doi: 10.1002/jnr.22092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz CC, Lindell SL, Mangino MJ, Carey HV. Hibernation confers resistance to intestinal ischemia-reperfusion injury. American journal of physiology Gastrointestinal and liver physiology. 2006;291:G895–901. doi: 10.1152/ajpgi.00155.2006. [DOI] [PubMed] [Google Scholar]
- Larson J, Peterson BL, Romano M, Park TJ. Buried Alive! Arrested Development and Hypoxia Tolerance in the Naked Mole-Rat. Frontiers in Behavioral Neuroscience [Google Scholar]
- Lindell SL, Klahn SL, Piazza TM, Mangino MJ, Torrealba JR, Southard JH, Carey HV. Natural resistance to liver cold ischemia-reperfusion injury associated with the hibernation phenotype. American journal of physiology Gastrointestinal and liver physiology. 2005;288:G473–480. doi: 10.1152/ajpgi.00223.2004. [DOI] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nature medicine. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Ma YL, Zhu X, Rivera PM, Toien O, Barnes BM, LaManna JC, Smith MA, Drew KL. Absence of cellular stress in brain after hypoxia induced by arousal from hibernation in Arctic ground squirrels. American journal of physiology Regulatory, integrative and comparative physiology. 2005;289:R1297–1306. doi: 10.1152/ajpregu.00260.2005. [DOI] [PubMed] [Google Scholar]
- Milton SL, Thompson JW, Lutz PL. Mechanisms for maintaining extracellular glutamate levels in the anoxic turtle striatum. American journal of physiology Regulatory, integrative and comparative physiology. 2002;282:R1317–1323. doi: 10.1152/ajpregu.00484.2001. [DOI] [PubMed] [Google Scholar]
- Ming Y, Zhang H, Long L, Wang F, Chen J, Zhen X. Modulation of Ca2+ signals by phosphatidylinositol-linked novel D1 dopamine receptor in hippocampal neurons. Journal of neurochemistry. 2006;98:1316–1323. doi: 10.1111/j.1471-4159.2006.03961.x. [DOI] [PubMed] [Google Scholar]
- Mitani A, Andou Y, Matsuda S, Arai T, Sakanaka M, Kataoka K. Origin of ischemia-induced glutamate efflux in the CA1 field of the gerbil hippocampus: an in vivo brain microdialysis study. Journal of neurochemistry. 1994;63:2152–2164. doi: 10.1046/j.1471-4159.1994.63062152.x. [DOI] [PubMed] [Google Scholar]
- Nilsson GE. Long-term anoxia in crucian carp: changes in the levels of amino acid and monoamine neurotransmitters in the brain, catecholamines in chromaffin tissue, and liver glycogen. The Journal of experimental biology. 1990;150:295–320. doi: 10.1242/jeb.150.1.295. [DOI] [PubMed] [Google Scholar]
- Olson JM, Jinka TR, Larson LK, Danielson JJ, Moore JT, Carpluck J, Drew KL. Circannual rhythm in body temperature, torpor, and sensitivity to A(1) adenosine receptor agonist in arctic ground squirrels. Journal of biological rhythms. 2013;28:201–207. doi: 10.1177/0748730413490667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamenter ME, Hogg DW, Gu XQ, Buck LT, Haddad GG. Painted turtle cortex is resistant to an in vitro mimic of the ischemic mammalian penumbra. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2012;32:2033–2043. doi: 10.1038/jcbfm.2012.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinones QJ, Zhang Z, Ma Q, et al. Proteomic Profiling Reveals Adaptive Responses to Surgical Myocardial Ischemia-Reperfusion in Hibernating Arctic Ground Squirrels Compared to Rats. Anesthesiology. 2016;124:1296–1310. doi: 10.1097/ALN.0000000000001113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AP, Christian SL, Zhao HW, Drew KL. Persistent tolerance to oxygen and nutrient deprivation and N-methyl-D-aspartate in cultured hippocampal slices from hibernating Arctic ground squirrel. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2006;26:1148–1156. doi: 10.1038/sj.jcbfm.9600271. [DOI] [PubMed] [Google Scholar]
- Saeidnia S, Manayi A, Abdollahi M. From in vitro Experiments to in vivo and Clinical Studies; Pros and Cons. Current drug discovery technologies. 2015;12:218–224. doi: 10.2174/1570163813666160114093140. [DOI] [PubMed] [Google Scholar]
- Sato M, Paschen W, Pawlik G, Heiss WD. Neurologic deficit and cerebral ATP depletion after temporary focal ischemia in cats. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1984;4:173–177. doi: 10.1038/jcbfm.1984.25. [DOI] [PubMed] [Google Scholar]
- Sheriff MJ, Fridinger RW, Toien O, Barnes BM, Buck CL. Metabolic rate and prehibernation fattening in free-living arctic ground squirrels. Physiological and biochemical zoology : PBZ. 2013;86:515–527. doi: 10.1086/673092. [DOI] [PubMed] [Google Scholar]
- Siesjo BK. Acidosis and ischemic brain damage. Neurochemical pathology. 1988;9:31–88. doi: 10.1007/BF03160355. [DOI] [PubMed] [Google Scholar]
- Thompson JW, Prentice HM, Lutz PL. Regulation of extracellular glutamate levels in the long-term anoxic turtle striatum: coordinated activity of glutamate transporters, adenosine, K (ATP) (+) channels and GABA. Journal of biomedical science. 2007;14:809–817. doi: 10.1007/s11373-007-9190-2. [DOI] [PubMed] [Google Scholar]
- Weltzin MM, Zhao HW, Drew KL, Bucci DJ. Arousal from hibernation alters contextual learning and memory. Behavioural brain research. 2006;167:128–133. doi: 10.1016/j.bbr.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Xiong ZG, Zhu XM, Chu XP, et al. Neuroprotection in ischemia: blocking calcium-permeable acid-sensing ion channels. Cell. 2004;118:687–698. doi: 10.1016/j.cell.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Yanagihara T, McCall JT. Ionic shift in cerebral ischemia. Life sciences. 1982;30:1921–1925. doi: 10.1016/0024-3205(82)90473-8. [DOI] [PubMed] [Google Scholar]
- Yao H, Shu Y, Wang J, Brinkman BC, Haddad GG. Factors influencing cell fate in the infarct rim. Journal of neurochemistry. 2007;100:1224–1233. doi: 10.1111/j.1471-4159.2006.04299.x. [DOI] [PubMed] [Google Scholar]
- Zhou F, Zhu X, Castellani RJ, Stimmelmayr R, Perry G, Smith MA, Drew KL. Hibernation, a model of neuroprotection. The American journal of pathology. 2001;158:2145–2151. doi: 10.1016/S0002-9440(10)64686-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Gordon GR, Feighan D, MacVicar BA. Transient swelling, acidification, and mitochondrial depolarization occurs in neurons but not astrocytes during spreading depression. Cerebral cortex. 2010;20:2614–2624. doi: 10.1093/cercor/bhq018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


