Abstract
FOXC1 mutations underlie Axenfeld-Rieger syndrome, an autosomal dominant disorder that is characterized by a spectrum of ocular and nonocular phenotypes and results in an increased susceptibility to glaucoma. Proteins interacting with FOXC1 were identified in human nonpigmented ciliary epithelial cells. Here we demonstrate that FOXC1 interacts with the actin-binding protein filamin A (FLNA). In A7 melanoma cells possessing elevated levels of nuclear FLNA, FOXC1 is unable to activate transcription and is partitioned to an HP1α, heterochromatin-rich region of the nucleus. This inhibition is mediated through an interaction between FOXC1 and the homeodomain protein PBX1a. In addition, we demonstrate that efficient nuclear and subnuclear localization of PBX1 is mediated by FLNA. Together, these data reveal a mechanism by which structural proteins such as FLNA can influence the activity of a developmentally and pathologically important transcription factor such as FOXC1. Given the resemblance of the skeletal phenotypes caused by FOXC1 loss-of-function mutations and FLNA gain-of-function mutations, this inhibitory activity of FLNA on FOXC1 may contribute to the pathogenesis of FLNA-linked skeletal disorders.
Stringent control of transcription is essential for the correct spatiotemporal regulation of cell determination and differentiation events that are necessary for proper development of all metazoan organisms. It is therefore not surprising that mutations in transcription factor genes often manifest as deleterious developmental abnormalities. The Forkhead Box (FOX) transcription factor gene family has been implicated in the proper formation of tissues derived from a number of lineages (19). Moreover, a growing number of disease-causing mutations have been attributed to human FOX genes (6, 19).
The FOXC1 transcription factor gene is key to the formation of tissues derived from neural crest and mesenchymal mesoderm cell lineages (13, 14, 35). Mutations in FOXC1 underlie Axenfeld-Rieger malformations, an autosomal dominant eye disorder resulting in numerous gross malformations of the anterior segment of the eye and an increased susceptibility to glaucoma (22, 25). The pathogenesis of glaucoma is often associated with elevated intraocular pressure resulting either from an increase of production and secretion of aqueous humor from the nonpigmented ciliary epithelium (NPCE) or from an impaired drainage of aqueous humor through the trabecular meshwork. In addition to ocular findings, patients harboring FOXC1 mutations can present with dental, craniofacial, umbilical, and cardiac anomalies (20). Alterations in FOXC1 gene dosage are also detrimental since patients possessing interstitial duplications and deletions of chromosome 6p25 present with anterior segment dysgenesis (17, 18, 24). The disease-causing FOXC1 mutations can affect FOXC1 function by reducing protein stability, altering its nuclear localization, or impairing its DNA-binding and transactivation activity (29-31). In mice, Foxc1 is integral to the correct formation of ocular structures as well as the axial skeleton, somites, heart and surrounding vasculature, and urogenital system (11, 13-15, 35). Furthermore, a nonsense mutation in Foxc1 is responsible for the naturally occurring congenital hydrocephalus mutant mouse (14).
How FOXC1 functions to control these developmental events remains relatively unknown. Since transcription factors often do not act alone, it is likely that FOXC1 is a component of a larger complex that regulates the initiation of transcription or the remodeling of chromatin. To this end, we sought to identify proteins interacting with FOXC1 in human eye tissues, particularly the NPCE cells that are involved in IOP homeostasis. In this report we demonstrate that FOXC1 interacts with the actin-binding protein filamin A (FLNA). This interaction partitions FOXC1 to HP1α-rich condensed heterochromatin in the nucleus and promotes an inhibitory interaction between FOXC1 and PBX1a, reducing FOXC1 trans activity. These data reveal a mechanism in which cell structural proteins can influence transcription factor activity through changes in their nuclear and subnuclear localization.
MATERIALS AND METHODS
Plasmids and reagents.
For bacterial expression of FOXC1, the full-length FOXC1 cDNA was subcloned into the pET28b (Novagen) bacterial expression vector in frame with the N-terminal 6× histidine epitope. FOXC1 mammalian expression vectors, pcDNA-FOXC1 and pEGFP-FOXC1, were described previously (2). pREP-Filamin was provided by T. Stossel and Y. Ohta. Fragments corresponding to regions of the human FLNA protein were generated by PCR amplification and subcloned into the pCI-HA vector. Details of primer sequences can be provided on request. All vectors were sequenced to confirm that no mutations were introduced into the cDNA. pHP1α-DsRed2 was kindly provided by A. Underhill. PBX1 and NF1-A cDNAs were purchased from Open Biosystems. Filamin antibody (MAB1758) was purchased from Chemicon. Anti-FOXC1, anti-PBX1 and anti-USF1 antibodies were purchased from Santa Cruz Biotechnology. Anti-REP1 antibodies were a kind gift from I. MacDonald.
Cell culture.
HeLa, C2C12, and human NPCE cells were maintained in Dulbecco's minimal essential medium (DMEM) supplemented with 10% fetal bovine serum (FBS). M2 and A7 melanoma cell lines were kindly provided by T. Stossel and Y. Ohta and were maintained in MEM supplemented with 8% newborn calf serum and 2% FBS. G418 (500 μg/ml) was added to media of the A7 cells to maintain expression of the transfected FLNA cDNA. The cells were transfected with Fugene 6 (Roche) reagent with the following Fugene 6-to-DNA ratios: HeLa cells, 3:1; NPCE, M2, and A7 cells, 5:1 (where Fugene 6 is expressed in microliters and DNA is expressed in micrograms).
FOXC1 Ni2+-NTA pulldowns.
Full-length FOXC1 was expressed in bacteria (BL-21 rosetta; Novagen) as a fusion protein with the N-terminal His6 and T7 epitopes and purified with Ni2+-nitrilotriacetic acid (NTA) agarose as described by the manufacturer (Qiagen). After elution with 500 μM imidazole, recombinant FOXC1 was dialyzed overnight against binding buffer (50 mM Na2HPO4 or NaH2PO4 [pH 8.0], 300 mM NaCl, 10 μM imidazole, 0.1 μM phenylmethylsulfonyl fluoride [PMSF]), quantitated via Bradford Assay (BioRad) and rebound to Ni2+-NTA agarose.
NPCE whole-cell extracts (WCE) were prepared by sonicating cells at 2 ml/g in 20 mM HEPES (pH 7.9)-500 mM NaCl-1.5 mM MgCl2-20% glycerol-0.1% Triton X-100-1 mM dithiothreitol-1 mM PMSF followed by centrifugation at 10,000 × g for 15 min at 4°C. NPCE WCE (20 mg) was precleared with 200 μl of Ni2+-NTA agarose for 1 h at 4°C. FOXC1 protein-binding experiments were performed in batch format by adding 250 μg of FOXC1 bound to 100 μl of Ni2+-NTA beads to the precleared NPCE WCE and incubated overnight at 4°C. As a control, unbound Ni2+-NTA agarose beads were added to a separate aliquot of NPCE WCE. Following binding to Ni2+-NTA beads, cell extracts were centrifuged at 1,000 × g for 5 min at 4°C and washed three times for 30 min with 500 μl of low-salt buffer (50 mM NaPO4 [pH 8.0], 100 mM NaCl, 50 μM imidazole, 0.1 μM PMSF) and then five times with high-salt buffer (50 mM NaPO4 [pH 8.0], 300 mM NaCl, 50 μM imidazole, 0.1 μM PMSF). After each wash, the Ni2+-NTA agarose beads were collected by centrifugation at 1,000 × g for 5 min at 4°C. To elute FOXC1 and bound proteins, the beads were incubated with high-salt wash buffer supplemented with 500 μM imidazole. Fractions were precipitated with 100% trichloroacetic acid (10% final volume), resolved on a 10% polyacrylamide gel, and stained with Coomassie Brilliant Blue. Protein bands were isolated from the gel and analyzed by quadrupole time-of-flight mass spectroscopy at the Institute for Biomolecular Design (Edmonton, Alberta, Canada).
Immunoprecipitation.
HeLa cells (106) were transfected with 4 μg of FOXC1 expression vector per 100-mm plate, using Fugene 6 reagent as describe above. For FOXC1 and HA-tagged filamin fragment cotransfections, 2 μg of pcDNA-FOXC1 and 2 μg of each pCI-HA filamin fragment expression vector were used. Two days after transfection, the cells were harvested by sonication in buffer containing 20 mM HEPES (pH 7.9), 500 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 20% (vol/vol) glycerol, 0.1% (vol/vol) Triton X-100, 1 mM dithiethreitol, and 1 mM PMSF (100 μl/100-mm plate) and stored at −80°C. Protein extracts (250 to 300 μg) were added to immunoprecipitation (IP) buffer (50 mM Tris [pH 7.5], 250 mM NaCl, 5 mM EDTA, 10% [vol/vol] glycerol, 2.5% [vol/vol] Ipagel, 0.1 μM PMSF) containing protein G-agarose (Sigma) and precleared for 1 h at 4°C. The precleared lysates were removed from the beads by centrifugation at 1,000 × g for 5 min at 4°C and incubated with 1 μg of anti-FLNA, anti-Xpress, or anti-HA antibodies (Santa Cruz Biotechnology) and incubated overnight at 4°C with gentle rocking. Then 25 μl of protein G-agarose, equilibrated in IP buffer, was added, and the extracts were incubated for a further 2 h. Immunoprecipitates were collected by centrifugation (1,000 × g for 5 min at 4°C), washed four times with IP buffer, and resolved on a 7% (for FLNA immunoblotting) or 10% (for FOXC1 immunoblotting) polyacrylamide gel. For FOXC1 immunodetection, blots were probed with either anti-green fluorescent protein (GFP) (1:3,000), anti-Xpress (1:5,000) or anti-FOXC1 (1:1,000) antibodies. FLNA expression was detected by immunoblotting with MAb1758 (1:5,000), which recognizes the N terminus of human FLNA. Then 10% of the protein extract was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis as the input fraction. For immunoprecipation of endogenous proteins, 750 mg of C2C12, M2, or A7 nuclear extract was precleared with 50 μl of protein G-agarose for 1 h. Precleared lysates were immunoprecipitated overnight with 5 μg of anti-filamin 1 antibody (Abcam) or anti-PBX1 at 4°C. Then 50 μl of protein G-agarose was added, and the lysates were incubated for another 2 h. Protein G beads were washed six times with IP buffer. Immunoprecipitates were loaded onto a 10% polyacrylamide gel and transferred to nitrocellulose. For the input fraction, 10% of the protein content of each nuclear extract was loaded on to the gel. The immunoblot was probed with anti-FOXC1 antibody (C18; Santa Cruz Biotechnology) at a dilution of 1:100 overnight at 4°C.
Immunofluorescence.
NPCE, HeLa, M2, or A7 cells (105) were plated onto coverslips and transfected with 0.5 μg of enhanced green fluorescent protein (EGFP)-FOXC1. At 18 h after transfection the cells were fixed with either 2% paraformaldehyde or methanol, blocked with 5% bovine serum albumin, and incubated with mouse monoclonal filamin antibody MAb1758 (1:500) and then with a cy3-conjugated anti-mouse immunoglobulin G (IgG) secondary antibody (1:500). Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI), and coverslips were mounted with Prolong (Molecular Probes) onto microscope slides. The slides were viewed on a Leica DMRE epifluorescence microscope and captured with a 10-bit mono-QICAM (QImaging) using the Northern Elite version 6.0 software package.
Transactivation assays.
M2 or A7 cells were plated into 24-well tissue culture plates at a density of 2 × 104 cells per well. The cells were cotransfected with 100 ng of FOXC1 or empty expression vector along with 20 ng of 6XFOXC1-BS-Luciferase and 0.2 ng of pRL-CMV per well. They were harvested and assayed for luciferase activity 40 h after transfection. Unless otherwise stated, all transfections were performed in triplicate and repeated at least three times.
EMSA.
Electrophoretic mobility shift assays (EMSAs) were performed as described in reference 29. For supershift assays, 500 ng of anti-Xpress or anti-T7 antibody were added to the reaction mixture prior to the addition of radiolabeled FOXC1 EMSA probe.
Isolation of cytoplasmic and nuclear fractions.
Cytoplasmic and nuclear extracts from HeLa, M2, or A7 cells were isolated as described in reference 16. A 30-μg sample of each nuclear or cytoplasmic fraction was resolved on a 7% polyacrylamide gel and transferred to a nitrocellulose membrane. The membranes were probed with anti-FLNA (1:5,000), anti-REP1 (1:750), or anti-USF1 (1:100) antibody and visualized with the SuperSignal West Pico chemiluminescent substrate (Pierce).
TF-TF interaction arrays.
Transcription factor (TF)-TF interaction arrays were performed as described by the manufacturer (Panomics), except for the following modifications. A 200-ng portion of recombinant His6-FOXC1 protein was added to 30 μg of M2 or A7 nuclear extracts and incubated along with TF-TF interaction array I biotinylated oligonucleotide probe mix overnight at 4°C. The following day, the reaction mixture was incubated with 10 μl of magnetic Ni2+-NTA agarose for 1 h at 4°C. As a negative control, A7 nuclear extracts were incubated with magnetic Ni2+-NTA agarose in the absence of FOXC1. All incubation and wash buffers were supplemented with 50 mM imidazole.
RESULTS
To identify proteins interacting with FOXC1 from human ocular tissues, we utilized a FOXC1 affinity pulldown approach. WCE from human NPCE cells were incubated with recombinant His6-FOXC1 protein immobilized to Ni2+-NTA agarose. The FOXC1 protein complexes were washed with an increasing salt concentration gradient and eluted with imidazole. The eluted fraction was resolved by polyacrylamide gel electrophoresis and stained with Coomassie brilliant blue (Fig. 1A). To monitor for proteins binding nonspecifically to the Ni2+-NTA agarose, an equal amount of NPCE cell lysate was subjected to a mock purification regimen with Ni2+-NTA agarose alone. A band corresponding to a protein with an approximate molecular mass of 250 kDa was preferentially present in fractions incubated with FOXC1. This band was excised, and the protein was identified by mass spectrometry as the actin-binding protein FLNA. Since a growing body of evidence has implicated FLNA in nuclear and transcription factor functions (21, 23, 26, 33, 36), we pursued this interaction between FOXC1 and FLNA further by immunoprecipitation experiments. HeLa cells were used for immunoprecipitation since transfection efficiencies in NPCE cells were low and FOXC1 expression levels were below our level of detection (data not shown). FLNA was able to immunoprecipitate FOXC1 when expressed in HeLa cells transfected with plasmids expressing FOXC1 fused to the Xpress epitope (Fig. 1B and C), substantiating the interaction detected by the initial affinity purification experiment. To test for an interaction occurring between endogenous FOXC1 and FLNA, nuclear extracts from undifferentiated C2C12 mouse myoblast cells were used for immunoprecipitation experiments. As demonstrated in Fig. 1D, Foxc1 was expressed in these nuclear extracts. Foxc1 was detected when C2C12 nuclear extracts were immunoprecipitated with FLNA antibodies, indicating that this interaction occurs between endogenous Foxc1 and FlnA in C2C12 cells. The multiple immunoreactive FOXC1 bands detected probably represent phosphorylated forms of FOXC1 (2). To map regions of FOXC1 responsible for FOXC1-FLNA interactions, a panel of Xpress-tagged FOXC1 deletion constructs (2) were transfected into HeLa cells, immunoprecipitated with anti-Xpress antibody, and detected with an FLNA antibody. As indicated in Fig. 1E, full-length FOXC1 was able to immunoprecipitate FLNA and a minor interaction between FOXC1 51-553 and FLNA was also detected, suggesting that activation domain 1 of FOXC1 may be dispensable for FLNA interactions. The lack of any detectable interaction between the remaining FOXC1 fragments and FLNA suggests a conformational requirement for FOXC1 to interact with FLNA. To define the FOXC1-interacting region on FLNA, a series of vectors expressing HA-tagged FLNA fragments spanning the entire protein were cotransfected with FOXC1 into HeLa cells and immunoprecipitated with an HA antibody. FOXC1 was able to interact with FLNA at amino acid residues 571 to 866, 867 to 1154, and 1779 to 2284 (Fig. 1F). These data suggest a complex interaction occurring between multiple regions of FLNA with FOXC1 or a structural requirement of the full-length protein that is lacking with the FLNA fragments.
FIG. 1.
(A) FOXC1 coelutes with the actin-binding protein FLNA. Metal chelate affinity chromatography was used to isolate FOXC1-interacting proteins from human NPCE cells. Full-length FOXC1 expressed in bacteria as a His6 fusion protein was purified and bound to Ni2+-NTA agarose. Proteins from NPCE WCE (20 mg) were incubated with 250 μg of FOXC1 bound to Ni2+-NTA agarose or with an equivalent volume of control beads (Mock). Proteins eluted with 300 mM NaCl containing 500 mM imidazole were resolved by polyacrylamide gel electrophoresis and stained with Coomassie brilliant blue. Protein bands were extracted and identified by mass spectrometry. (B and C) Coimmunoprecipitation of FOXC1 and FLNA from HeLa cells. Xpress-FOXC1 was transfected into HeLa cells, and whole-cell lysates were immunoprecipitated (IP) with anti-FLNA antibodies. FOXC1 was detected with either an anti-FOXC1 antibody (B) or an anti-Xpress antibody (C). To detect FLNA-FOXC1 endogenous interactions, 750 μg of C2C12 nuclear extract was immunoprecipitated with 5μg of α-FLNA antibodies or 5 μg of an irrelevant IgG (D). Immunoblots were subsequently probed with an α-FOXC1 antibody. (E) WCE from HeLa cells transfected with Xpress-tagged FOXC1 deletion constructs were immunoprecipitated with anti-Xpress antibodies and immunoblotted (IB) with α-FLNA. The input fractions were immunoblotted with anti-Xpress. (F) WCE from HeLa cells cotransfected with Xpress-FOXC1 along with HA-tagged FLNA fragments spanning the length of the protein were immunoprecipated with anti-HA antibodies and immunoblotted with anti-Xpress to detect FOXC1. Immunoglobulin bands are denoted by asterisks.
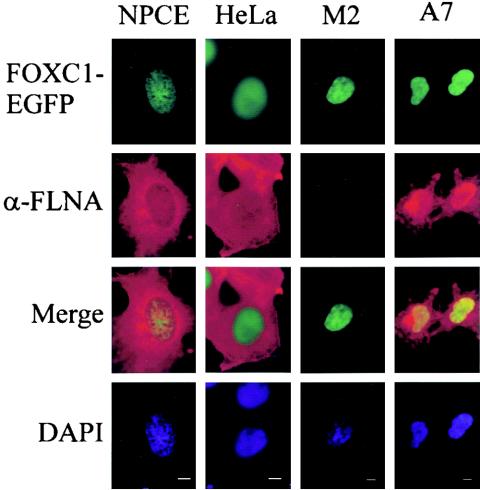
The cellular localization of FLNA was compared with that of FOXC1-EGFP transfected in to NPCE cells. As indicated in Fig. 2, FOXC1-EGFP is localized exclusively to the nucleus. Although FLNA immunofluorescence is detected primarily in the cytoplasm, a discernible signal could be detected in the nuclei of NPCE and HeLa cells. The FLNA immunofluorescence signal displayed a partial overlap with FOXC1 in NPCE cells, suggesting a partial colocalization between these proteins. To examine whether FLNA influenced the nuclear localization of FOXC1, the distribution of FOXC1-EGFP in M2 melanoma cells that lack FLNA, as well as in A7 melanoma cells, which are a subclone of M2 cells where FLNA has been stably transfected, was examined (4). FOXC1-EGFP displayed nuclear localization in both cell types (Fig. 2), indicating that FLNA does not play a role in FOXC1 nuclear import as described for other transcription factors, including members of the SMAD family (33). Interestingly, an elevated nuclear distribution of FLNA was detected in A7 cells compared to NPCE cells (Fig. 2), (see below).
FIG. 2.
Localization of FLNA to the cytoplasm and nucleus. FOXC1-EGFP was transfected into NPCE, HeLa, M2, and A7 cells. Cells were methanol fixed, and FLNA immunofluoresence was detected with an N-terminal FLNA monclonal antibody and anti-mouse IgG Cy3-conjugated secondary antibody. Nuclei were counterstained with DAPI. Bar, 5 μm.
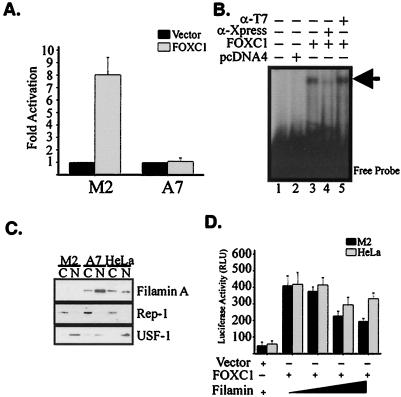
To examine the biological significance of the FOXC1-FLNA interaction, transactivation assays were performed with M2 and A7 cells to monitor the ability of FOXC1 to activate the transcription of a reporter gene containing six FOXC1-binding sites. In M2 cells, FOXC1 was able to activate this reporter eightfold, whereas in A7 cells FOXC1 was unable to activate expression (Fig. 3A), suggesting that FLNA can negatively affect FOXC1 function. EMSAs utilizing an in vivo-derived FOXC1 consensus sequence (5′-GTAAATAAA-3′) demonstrated that FOXC1 was able to bind to its target sequence when expressed in A7 cells and that therefore the deficit in FOXC1 transactivation ability was not due to impaired DNA binding (Fig. 3B). To demonstrate the presence of FOXC1 in the DNA-protein complex, supershift assays were performed. The addition of anti-Xpress antibodies to the FOXC1-DNA-binding reaction was able to prevent FOXC1 from binding to its target, whereas the addition of an irrelevant anti-T7 tag antibody had no affect on FOXC1-DNA binding (Fig. 3B).
FIG. 3.
Impaired FOXC1 transcriptional regulatory activity in the presence of elevated nuclear FLNA levels. (A) M2 and A7 melanoma cells were transfected with Xpress-FOXC1 or empty pcDNA4b vector along with 6XFOXBS-Luc reporter. Data are representative of four independent experiments. Error bars correspond to the standard error of the mean (SEM). (B) FOXC1-DNA-binding activity is not impaired in A7 cells. EMSAs testing the ability of FOXC1 expressed in A7 cells to bind its in vitro consensus binding site (arrow) were performed. Supershifts were performed by adding anti-Xpress antibody to demonstrate the specificity of the FOXC1-DNA complexes or by adding an irrelevant antibody (α-T7). (C) Increased nuclear levels of FLNA in A7 melanoma cells. Nuclear and cytoplasmic fractions were collected from M2, A7, and HeLa cells and immunoblotted with an N-terminal FLNA antibody. Membranes were also probed with antibodies against REP-1 and USF-1 to monitor the integrity of the cytoplasmic and nuclear fractions, respectively. (D) Exogenous FLNA impairs FOXC1 transactivation activity in M2 cells. HeLa and M2 cells were transfected with pcDNA-FOXC1 along with increasing concentrations of FLNA expression vector. Total DNA concentrations were maintained constant by adding empty pcDNA4 vector. Data are representative of two experiments transfected in triplicate. Error bars indicate the SEM.
FLNA is widely expressed in a number of cell types and tissues. In such cells, including HeLa cells, FOXC1 is transcriptionally active (2, 29), yet the presence of FLNA in A7 cells renders FOXC1 inactive. One possible hypothesis is that A7 cells exhibit elevated levels of nuclear FLNA compared with HeLa cells and that such an increase in nuclear FLNA is inhibitory to FOXC1. To test this hypothesis, nuclear and cytoplasmic fractions from HeLa, M2, and A7 cells were isolated and examined for FLNA protein levels. Figure 3C demonstrates that levels of FLNA in the nucleus are indeed elevated in A7 cells compared with HeLa cells. To determine whether the reduced transcriptional activation of FOXC1 was impaired in A7 cells was due to increased levels of FLNA, we cotransfected FOXC1 into M2 and HeLa cells along with different amounts of an FLNA cDNA expression construct. An increase in the amount of FLNA transfected into M2 cells led to a decrease in FOXC1 transactivation ability, indicating the inhibitory influence of FLNA on FOXC1 (Fig. 3D). In HeLa cells, however, an increase in the amount of FLNA had little effect on FOXC1 activity, suggesting that there is a cell-type-specific role for FLNA in regulating FOXC1 activity or that FLNA may not be efficiently localized to the nucleus in all cell types.
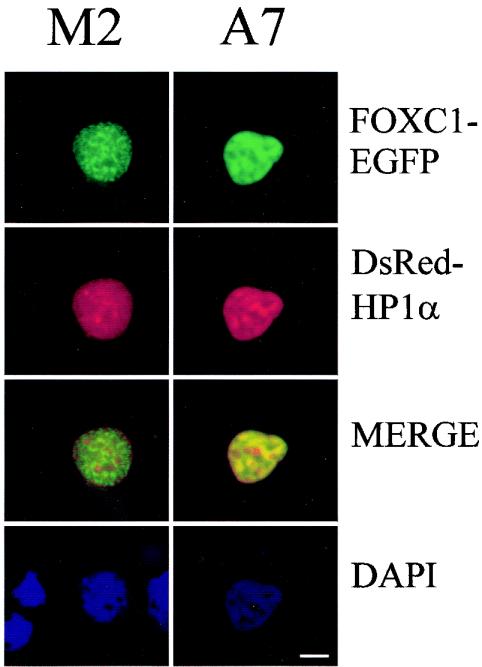
One possible mechanism by which nuclear FLNA acts to inhibit FOXC1 activity is by partitioning FOXC1 to transcriptionally inactive regions of the nucleus, such as heterochromatin. To examine this possibility, FOXC1-EGFP subnuclear distribution was compared with that of the heterochromatin-binding protein HP1α in M2 and A7 cells. FOXC1 displays an overlap with DAPI-rich regions in both M2 and A7 cells (Fig. 4). In M2 cells, HP1α and FOXC1 show little colocalization; however, in A7 cells, FOXC1 and HP1α display a distinct overlap in distribution, suggesting that FLNA partitions FOXC1 to a heterochromatin-rich region of the nucleus. The targeting of FOXC1 to these regions in the A7 cells may prevent FOXC1 from accessing the required cofactors necessary for activation of transcription.
FIG. 4.
Altered subnuclear localization of FOXC1 in A7 cells. M2 and A7 cells were cotransfected with EGFP-FOXC1 and DsRed-HP1α. Fluorescence was visualized 18 h posttransfection. The cells were fixed in 2% parformaldehyde and permeabilized with Triton X-100. Nuclei were counterstained with DAPI. Bar, 5 μm.
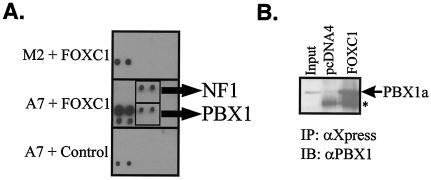
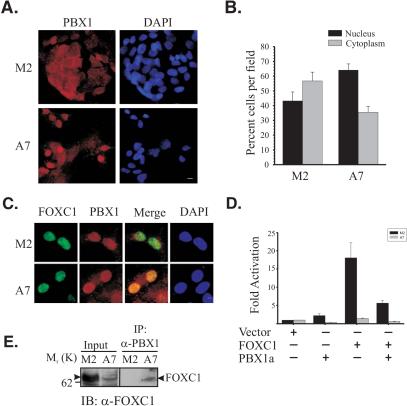
Since FLNA is required for the nuclear import of transcription factors such as the SMAD family and steroid hormone receptors (26, 33), it is possible that factors required for negative regulation of FOXC1 are absent in the nuclei of M2 cells. The presence of FLNA in A7 cells may rescue the nuclear import defect of such factors and impair FOXC1 activity. To search for such factors, a transcription factor-transcription factor interaction array (8) was utilized to identify transcription factors interacting with FOXC1 differentially in A7 versus M2 cells. A signal corresponding to an interaction between FOXC1 and PBX1 was detected only in A7 nuclear extracts incubated with FOXC1 (Fig. 5A). Since the nuclear import and export of PBX1 is regulated (3) and the PBX1a isoform can recruit corepressor complexes (1), this protein was an attractive candidate as an FLNA-regulated, FOXC1-inhibitory partner. Immunoprecipitation experiments confirmed the interaction between FOXC1 and PBX1 (Fig. 5B). Indirect immunofluorescence microscopy revealed that the nuclear distribution of PBX1 was indeed reduced in M2 cells compared to A7 cells (Fig. 6A and B). The relative subnuclear distributions of PBX1 and FOXC1 were also altered in M2 cells compared to A7 cells. In the nuclei of A7 cells, PBX1 immunoreactivity displayed a strong overlap with FOXC1-EGFP, but this was not found in the subset of M2 cells where PBX1 was localized to the nucleus (Fig. 6C). These data suggest an FLNA-dependent role in the nuclear import and subnuclear localization of PBX1. When cotransfected with FOXC1 in M2 or A7 cells, PBX1a could impair the transcriptional regulatory activity of FOXC1 (Fig. 6D), suggesting that PBX1a is a component of the transcriptional inhibitory network acting on FOXC1 in A7 cells. An additional inhibitory candidate, NF1-A, was identified from the transcription factor interaction array screen. NF1-A displayed a marked reduction in its nuclear localization in M2 compared with A7 cells, indicating a requirement for FLNA in the correct nuclear localization (Fig. 7). However, NF1-A did not impair FOXC1 transcriptional activity (Fig. 7). Thus, the correct nuclear localization of many transcription factors may be dependent on FLNA. In melanoma cells, FLNA is required for the efficient nuclear translocation of PBX1a and for the formation of a PBX1a-FOXC1 inhibitory complex, indicating that FLNA can participate in cell-type-specific regulation of FOXC1 activity. Finally, immunoprecipitation of M2 and A7 nuclear extracts with anti-PBX1 antibodies indicates that FOXC1 and PBX1 interact at endogenous levels in the nuclei of A7 cells but not M2 cells (Fig. 6E).
FIG. 5.
PBX1 interacts with FOXC1. (A) Identification of PBX1 as a FOXC1 interaction partner based on the TF-TF interaction array. Nuclear extracts from M2 or A7 cells were incubated with the biotinlylated oligonucleotide probeset for the TF-TF interaction array I (Panomics) followed by incubation with bacterially expressed His6-tagged FOXC1 bound to magnetic Ni2+-NTA agarose or with Ni2+-NTA agarose alone (A7 + Control). The oligonucleotide probes bound to transcription factors interacting with FOXC1 were eluted and hybridized to a membrane containing an array of transcription factor-binding sites. Potential interactions between FOXC1 and PBX and NF1 were identified. Each cis element is probe spotted in duplicate in two rows, with the bottom row diluted 1:10. (B) FOXC1 can immunoprecipitate PBX1. HeLa cells were cotransfected with PBX1 along with pcDNA-FOXC1 or pcDNA4. Extracts were immunoprecipitated (IP) with anti-Xpress antibody immunoblotted (IB) with anti-PBX1. The asterisk indicates the IgG bands.
FIG. 6.
FLNA directs PBX1 nuclear localization and inhibits its activity in melanoma cells. (A) Altered nuclear localization of PBX in M2 versus A7 cells. Indirect immunofluorescence detection of PBX1 in M2 and A7 cells is shown. Bar, 5 μm. (B) Quantitation of nuclear and cytoplasmic localization of PBX1 in M2 and A7 cells. PBX1 localization was scored from at least 10 random fields (>300 cells total) and expressed as percent nuclear and cytoplasmic localization per field. Error bars represent the SEM. (C) PBX1 and FOXC1 colocalize in an FLNA-dependent manner. A comparison of PBX1 subnuclear localization with FOXC1-EGFP in M2 and A7 cells is shown. Bar, 5 μm. (D) Attenuation of FOXC1 activity by PBX1. M2 and A7 cells were cotransfected with pcDNA-FOXC1 and 6XFOXC1-BS-Luc reporter along with PBX1 or an empty pcDNA vector. Data are presented relative to empty pcDNA vector and are representative of three experiments performed in triplicate. Error bars represent the SEM. (E) Interaction between FOXC1 and PBX1 occurs endogenously. M2 and A7 nuclear extracts (750 mg) were immunoprecipitated (IP) with anti-PBX1 antibodies and immunoblotted (IB) with anti-FOXC1 antibodies. Arrowheads indicate FOXC1 bands.
FIG. 7.
NF1-A does not impair FOXC1 activity. (A) M2 or A7 cells were fixed with 2% paraformaldehyde, and NF1 protein localization was monitored by indirect immunofluorescence with an anti-rabbit IgG Alexa 494-conjugated secondary antibody. Nuclei were counterstained with DAPI. Bar, 10 μm. (B) NF1 nuclear localization was scored in 10 random fields (>200 cells) of M2 or A7 cells and scored as percentage nuclear staining only, nuclear staining greater than cytoplasmic, and cytoplasmic staining greater than nuclear. (C) FOXC1 transcriptional activity is unaffected by NF1-A in M2 and A7 cells. M2 and A7 cells were cotransfected with pcDNA-FOXC1 and 6XFOXC1-BS-Luc reporter along with NF1-A or an empty pcDNA vector. Data are presented relative to empty pcDNA vector. Error bars represent the SEM.
DISCUSSION
In this report we describe a novel mechanism for the regulation of FOXC1 transcriptional activity mediated through multiple interactions with the actin-binding protein FLNA and with the homeodomain protein PBX1a. There is a growing body of evidence implicating FLNA in the regulation of nuclear functions including transcription, DNA repair, and nuclear import (21, 23, 26, 33, 36). Cells with elevated levels of nuclear FLNA may therefore serve as a transcriptional barrier for FOXC1 activity. How FLNA gets into the nucleus is not known, therefore, we are not certain why A7 cells display elevated nuclear levels of FLNA compared to HeLa cells. Since the levels of exongenous FLNA proteins are in the range of physiologically relevant amounts (4), it is not simply an effect of FLNA overexpression. FLNA is subject to proteolytic digestion in vivo, producing two fragments (180 and 100 kDa) (7). The C-terminal 100-kDa fragment exhibits an increase in its nuclear localization with respect to the full-length protein and can inhibit the transcriptional activation of the androgen receptor by preventing the interaction between the androgen receptor C-terminal activation domain and the intermediary factor 2 coactivator protein (21). This region of FLNA also contains a stretch of basic amino acid residues, which may contribute to its nuclear import. However, an N-terminal FLNA antibody reveals the presence of the full-length FLNA protein in the nucleus by immunoblotting and immunofluorescence (Fig. 2 and 3). Although FOXC1 interacts with a region included in this nuclear C-terminal fragment (residues 1779 to 2284), the mechanisms through which FOXC1 activates transcription by the recruitment of coactivator molecules have yet to be identified. It is therefore unknown whether FLNA binding physically prevents FOXC1 from recruiting such factors.
Alternatively, FLNA can promote the active repression of FOXC1 activity through the association with inhibitory proteins rather than simply the prevention of FOXC1 activation. We demonstrate that FOXC1 can interact with the TALE homeodomain transcription factor PBX1 in A7 and HeLa cells. When coexpressed with FOXC1, PBX1a acts to inhibit FOXC1 transcriptional activation. Alternatively spliced isoforms of PBX1 can act as transcriptional activators or repressors. This dichotomy is achieved through the differential recruitment of coactivator or corepressor complexes (1). The PBX1a isoform possess sequences in its C-terminus that can bind to the corepressor proteins SMRT and NCoR, while the PBX1b isoform lacks this region and is unable to recruit these molecules (1). We are currently examining the nature of the FOXC1-PBX1 interaction to determine whether FOXC1 interacts with both PBX1 isoforms and whether FOXC1-PBX1a complexes recruit the same corepressor molecules.
An additional factor influencing FOXC1 inhibition in A7 cells is the localization of FOXC1 to HP1α-rich nuclear compartments. HP1α binds to trimethylated lysine 9 of histone H3 to demarcate a closed, transcriptionally inactive chromatin state (10). Conceivably, once localized to these regions, FOXC1 is unable to recruit the necessary coactivators required to initiate transcription. It is not known whether the localization of FOXC1 to the HP1α-rich regions is the cause or consequence of FOXC1-PBX1a-mediated inhibition. The inhibition of transcription factors by their partitioning to HP1α-rich regions has also been demonstrated for CEBP (27). The high nuclear FLNA content in A7 cells does not act to generally inhibit all transcription since these cells remain viable and regulation of transforming growth factor β-mediated SMAD transactivation is active in A7 but not M2 cells (33).
We also demonstrate that nuclear localization of PBX1 is at least partially dependent on FLNA. The nuclear and cytoplasmic distribution of PBX1 is subject to complex regulatory mechanisms. Competing nuclear import and export signals can influence PBX1 distribution in a cell-specific manner. PBX1 contains two nuclear localization signals in its homeodomain that are masked by an intramolecular interaction with its N terminus (32). The binding of PBX1 to MEINOX proteins is thought to evoke a conformational change and expose the nuclear localization signals (32). Furthermore, a nuclear export signal located in the N terminus of PBX1 can direct its cytoplasmic accumulation through a CRM1-mediated nuclear export pathway (3). The interplay between nuclear import and export may be influenced by phosphorylation of residues in the PBX1 N-terminal domain since activation of protein kinase A can lead to phosphorylation of PBX1 and enhance its nuclear localization (12). Since FLNA is a key mediator of a number of cell-signaling events, it is possible the FLNA may influence the phosphorylation status of PBX1. Additionally, FLNA may participate in the assembly of PBX1-MEINOX interactions that are required for nuclear import of PBX1. The fact that PBX1 can interact with nonmuscle myosin and promotes the cytoplasmic retention of PBX1 (9) suggests that the actin cytoskeleton may play a profound role in regulating the nuclear import of a number of developmentally important transcription factors, such as PBX1. We are currently investigating whether FLNA directly binds to PBX1 or MEINOX proteins and how it regulates the correct nuclear localization of PBX1.
The regulation of FOXC1 activity by FLNA may have clinical significance. Mutations in FLNA underlie the X-linked disorders otopalatodigital syndrome types I and II, frontometaphyseal dysplasia, and Melnick-Needles syndrome, which affect the craniofacial, skeletal, neurologic, and urogenital systems (28). Interestingly FLNA patients harboring these gain-of-function mutations display similarities to homozygous Foxc1 knockout mice, such as hydrocephalus and malformed sternae. Furthermore, patients harboring 6p deletions that include FOXC1 present with hydrocephalus and skeletal malformations (14). PBX1 is also a key regulator of skeletal formation, since mice deficient in Pbx1 display severe patterning defects of the axial and appendicular skeleton, including structures affected in otopalatodigital syndrome types I and II patients, such as otic vesicles, ribs, and limbs (34). Clearly other roles for FLNA in the cytoplasm may be key in the onset of these disorders. Nonetheless, the phenotypic similarities between FLNA gain-of-function mutations and the Foxc1 or Pbx1 loss-of-function mutations suggest that one effect of FLNA mutations may be dysregulation of FOXC1 and PBX1 activity in chrondrogenic and osteogenic differentiation events.
In summary, we report an interaction between the FOXC1 transcription factor and the actin-binding protein FLNA that mediates the inhibition of FOXC1 in melanoma cells through its partitioning of FOXC1 to HP1α-rich, heterochromatic regions of the nucleus. In addition, FLNA is required for the efficient nuclear localization of PBX1 and the formation of a transcriptionally inactive, FOXC1-PBX1a complex. This highlights the fact that proteins traditionally thought of as strictly cytoplasmic structural factors can influence gene regulation. Changes in cell structure that occur during morphogenesis or in response to a mechanical insult, such as elevated intraocular pressure that occurs in the glaucomatous eye, may be transduced to the nucleus by FLNA and result in changes in gene expression either through the correct nuclear shuttling of transcription factors or through the assembly of transcriptional regulatory complexes (Fig. 8). FLNA expression is induced in response to cellular mechanical force (5), which could conceivably increase the nuclear pool of full-length FLNA and/or 100-kDa C-terminal FLNA fragment. This unexpected activity of FLNA can therefore lead to mechanical regulation of developmentally important and glaucoma-associated transcription factors such as FOXC1.
FIG. 8.
Proposed mechanism of transcriptional regulatory activity by FLNA. 1, In the cytoplasm, FLNA binds as a dimer to orthogonal actin filaments (grey bars) to regulate actin cytoskeletal integrity. Full-length FLNA can be localized to the nucleus. Whether it exists as a dimer in the nucleus or binds to nuclear actin is not known. 2, Nuclear import of transcriptional regulatory molecules such as PBX1 (octagon) is regulated by FLNA. Such regulation may be achieved by FLNA association with protein kinases (PK). 3, In response to cell stimuli and cytoskeletal reorganization, FLNA expression can increase, raising the levels of the nuclear FLNA pool. In the nucleus, FLNA acts as a scaffold whereby FOXC1 (oval) and PBX1 (octagon) transcriptional inhibitory complexes are assembled. 4, FOXC1-PBX1 complexes are unable to recruit coactivator complexes (cross) and are targeted to transcriptionally inactive, HP1α-rich heterochromatin regions of the nucleus.
Acknowledgments
We thank D. A. Underhill, D. M. Glerum, R. Rachubinski, and members of the Ocular Genetics Laboratory for critical reading of the manuscript. We thank T. Stossel and Y. Ohta for providing the M2 and A7 cells lines. We also thank May Yu for technical assistance.
This work was supported by operating grants awarded to M.A.W. from the Canadian Institutes for Health Research (CIHR). F.B.B. is supported by a CIHR postdoctoral fellowship. M.A.W. is an AHFMR senior scholar and a CIHR investigator.
REFERENCES
- 1.Asahara, H., S. Dutta, H. Y. Kao, R. M. Evans, and M. Montminy. 1999. Pbx-Hox heterodimers recruit coactivator-corepressor complexes in an isoform-specific manner. Mol. Cell. Biol. 19:8219-8225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berry, F. B., R. A. Saleem, and M. A. Walter. 2002. FOXC1 transcriptional regulation is mediated by N- and C-terminal activation domains and contains a phosphorylated transcriptional inhibitory domain. J. Biol. Chem. 277:10292-10297. [DOI] [PubMed] [Google Scholar]
- 3.Berthelsen, J., C. Kilstrup-Nielsen, F. Blasi, F. Mavilio, and V. Zappavigna. 1999. The subcellular localization of PBX1 and EXD proteins depends on nuclear import and export signals and is modulated by association with PREP1 and HTH. Genes Dev. 13:946-953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cunningham, C. C., J. B. Gorlin, D. J. Kwiatkowski, J. H. Hartwig, P. A. Janmey, H. R. Byers, and T. P. Stossel. 1992. Actin-binding protein requirement for cortical stability and efficient locomotion. Science 255:325-327. [DOI] [PubMed] [Google Scholar]
- 5.D'Addario, M., P. D. Arora, R. P. Ellen, and C. A. McCulloch. 2002. Interaction of p38 and Sp1 in a mechanical force-induced, beta 1 integrin-mediated transcriptional circuit that regulates the actin-binding protein filamin-A. J. Biol. Chem. 277:47541-47550. [DOI] [PubMed] [Google Scholar]
- 6.Erickson, R. P. 2001. Forkhead genes and human disease. J. Appl. Genet. 42:211-221. [PubMed] [Google Scholar]
- 7.Gorlin, J., R. Yamin, S. Egan, M. Stewart, T. Stossel, D. Kwiatkowski, and J. Hartwig. 1990. Human endothelial actin-binding protein (ABP-280, nonmuscle filamin): a molecular leaf spring. J. Cell Biol. 111:1089-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hewetson, A., and B. S. Chilton. 2003. An Sp1-NF-Y/progesterone receptor DNA binding-dependent mechanism regulates progesterone-induced transcriptional activation of the rabbit RUSH/SMARCA3 gene. J. Biol. Chem. 278:40177-40185. [DOI] [PubMed] [Google Scholar]
- 9.Huang, H., M. Paliouras, I. Rambaldi, P. Lasko, and M. Featherstone. 2003. Nonmuscle myosin promotes cytoplasmic localization of PBX. Mol. Cell. Biol. 23:3636-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs, S. A., and S. Khorasanizadeh. 2002. Structure of HP1 chromodomain bound to a lysine 9-methylated histone H3 tail. Science 295:2080-2083. [DOI] [PubMed] [Google Scholar]
- 11.Kidson, S. H., T. Kume, K. Deng, V. Winfrey, and B. L. Hogan. 1999. The forkhead/winged-helix gene, Mf1, is necessary for the normal development of the cornea and formation of the anterior chamber in the mouse eye. Dev. Biol. 211:306-322. [DOI] [PubMed] [Google Scholar]
- 12.Kilstrup-Nielsen, C., M. Alessio, and V. Zappavigna. 2003. PBX1 nuclear export is regulated independently of PBX-MEINOX interaction by PKA phosphorylation of the PBC-B domain. EMBO J. 22:89-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kume, T., K. Deng, and B. L. Hogan. 2000. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127:1387-1395. [DOI] [PubMed] [Google Scholar]
- 14.Kume, T., K. Y. Deng, V. Winfrey, D. B. Gould, M. A. Walter, and B. L. Hogan. 1998. The forkhead/winged helix gene Mf1 is disrupted in the pleiotropic mouse mutation congenital hydrocephalus. Cell 93:985-996. [DOI] [PubMed] [Google Scholar]
- 15.Kume, T., H. Jiang, J. M. Topczewska, and B. L. Hogan. 2001. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 15:2470-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lassar, A. B., R. L. Davis, W. E. Wright, T. Kadesch, C. Murre, A. Voronova, D. Baltimore, and H. Weintraub. 1991. Functional activity of myogenic HLH proteins requires hetero-oligomerization with E12/E47-like proteins in vivo. Cell 66:305-315. [DOI] [PubMed] [Google Scholar]
- 17.Lehmann, O. J., N. D. Ebenezer, R. Ekong, L. Ocaka, A. J. Mungall, S. Fraser, J. I. McGill, R. A. Hitchings, P. T. Khaw, J. C. Sowden, S. Povey, M. A. Walter, S. S. Bhattacharya, and T. Jordan. 2002. Ocular developmental abnormalities and glaucoma associated with interstitial 6p25 duplications and deletions. Investig. Ophthalmol. Visual Sci. 43:1843-1849. [PubMed] [Google Scholar]
- 18.Lehmann, O. J., N. D. Ebenezer, T. Jordan, M. Fox, L. Ocaka, A. Payne, B. P. Leroy, B. J. Clark, R. A. Hitchings, S. Povey, P. T. Khaw, and S. S. Bhattacharya. 2000. Chromosomal duplication involving the forkhead transcription factor gene FOXC1 causes iris hypoplasia and glaucoma. Am. J. Hum. Genet. 67:1129-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lehmann, O. J., J. C. Sowden, P. Carlsson, T. Jordan, and S. S. Bhattacharya. 2003. Fox's in development and disease. Trends Genet. 19:339-344. [DOI] [PubMed] [Google Scholar]
- 20.Lines, M. A., K. Kozlowski, and M. A. Walter. 2002. Molecular genetics of Axenfeld-Rieger malformations. Hum. Mol. Genet. 11:1177-1184. [DOI] [PubMed] [Google Scholar]
- 21.Loy, C. J., K. S. Sim, and E. L. Yong. 2003. Filamin-A fragment localizes to the nucleus to regulate androgen receptor and coactivator functions. Proc. Natl. Acad. Sci. USA 100:4562-4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mears, A. J., T. Jordan, F. Mirzayans, S. Dubois, T. Kume, M. Parlee, R. Ritch, B. Koop, W. L. Kuo, C. Collins, J. Marshall, D. B. Gould, W. Pearce, P. Carlsson, S. Enerback, J. Morissette, S. Bhattacharya, B. Hogan, V. Raymond, and M. A. Walter. 1998. Mutations of the forkhead/winged-helix gene, FKHL7, in patients with Axenfeld-Rieger anomaly. Am. J. Hum. Genet. 63:1316-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng, X., Y. Yuan, A. Maestas, and Z. Shen. 2004. Recovery from DNA damage-induced G2 arrest requires the actin-binding protein filamin-A/actin-binding protein 280. J. Biol. Chem. 279:6098-6105. [DOI] [PubMed] [Google Scholar]
- 24.Nishimura, D. Y., C. C. Searby, W. L. Alward, D. Walton, J. E. Craig, D. A. Mackey, K. Kawase, A. B. Kanis, S. R. Patil, E. M. Stone, and V. C. Sheffield. 2001. A spectrum of FOXC1 mutations suggests gene dosage as a mechanism for developmental defects of the anterior chamber of the eye. Am. J. Hum. Genet. 68:364-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishimura, D. Y., R. E. Swiderski, W. L. Alward, C. C. Searby, S. R. Patil, S. R. Bennet, A. B. Kanis, J. M. Gastier, E. M. Stone, and V. C. Sheffield. 1998. The forkhead transcription factor gene FKHL7 is responsible for glaucoma phenotypes which map to 6p25. Nat. Genet. 19:140-147. [DOI] [PubMed] [Google Scholar]
- 26.Ozanne, D. M., M. E. Brady, S. Cook, L. Gaughan, D. E. Neal, and C. N. Robson. 2000. Androgen receptor nuclear translocation is facilitated by the f-actin cross-linking protein filamin. Mol. Endocrinol. 14:1618-1626. [DOI] [PubMed] [Google Scholar]
- 27.Piwien Pilipuk, G., M. D. Galigniana, and J. Schwartz. 2003. Subnuclear localization of C/EBP beta is regulated by growth hormone and dependent on MAPK. J. Biol. Chem. 278:35668-35677. [DOI] [PubMed] [Google Scholar]
- 28.Robertson, S. P., S. R. Twigg, A. J. Sutherland-Smith, V. Biancalana, R. J. Gorlin, D. Horn, S. J. Kenwrick, C. A. Kim, E. Morava, R. Newbury-Ecob, K. H. Orstavik, O. W. Quarrell, C. E. Schwartz, D. J. Shears, M. Suri, J. Kendrick-Jones, and A. O. Wilkie. 2003. Localized mutations in the gene encoding the cytoskeletal protein filamin A cause diverse malformations in humans. Nat. Genet. 33:487-491. [DOI] [PubMed] [Google Scholar]
- 29.Saleem, R. A., S. Banerjee-Basu, F. B. Berry, A. D. Baxevanis, and M. A. Walter. 2001. Analyses of the effects that disease-causing missense mutations have on the structure and function of the winged-helix protein FOXC1. Am. J. Hum. Genet. 68:627-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saleem, R. A., S. Banerjee-Basu, F. B. Berry, A. D. Baxevanis, and M. A. Walter. 2003. Structural and functional analyses of disease-causing missense mutations in the forkhead domain of FOXC1. Hum. Mol. Genet. 12:2993-3005. [DOI] [PubMed] [Google Scholar]
- 31.Saleem, R. A., T. C. Murphy, J. M. Liebmann, and M. A. Walter. 2003. Identification and analysis of a novel mutation in the FOXC1 forkhead domain. Investig. Ophthalmol. Visual Sci. 44:4608-4612. [DOI] [PubMed] [Google Scholar]
- 32.Saleh, M., H. Huang, N. C. Green, and M. S. Featherstone. 2000. A conformational change in PBX1A is necessary for its nuclear localization. Exp. Cell Res. 260:105-115. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki, A., Y. Masuda, Y. Ohta, K. Ikeda, and K. Watanabe. 2001. Filamin associates with Smads and regulates transforming growth factor-beta signaling. J. Biol. Chem. 276:17871-17877. [DOI] [PubMed] [Google Scholar]
- 34.Selleri, L., M. J. Depew, Y. Jacobs, S. K. Chanda, K. Y. Tsang, K. S. Cheah, J. L. Rubenstein, S. O'Gorman, and M. L. Cleary. 2001. Requirement for Pbx1 in skeletal patterning and programming chondrocyte proliferation and differentiation. Development 128:3543-3557. [DOI] [PubMed] [Google Scholar]
- 35.Winnier, G. E., T. Kume, K. Deng, R. Rogers, J. Bundy, C. Raines, M. A. Walter, B. L. Hogan, and S. J. Conway. 1999. Roles for the winged helix transcription factors MF1 and MFH1 in cardiovascular development revealed by nonallelic noncomplementation of null alleles. Dev. Biol. 213:418-431. [DOI] [PubMed] [Google Scholar]
- 36.Yuan, Y., and Z. Shen. 2001. Interaction with BRCA2 suggests a role for filamin-1 (hsFLNa) in DNA damage response. J. Biol. Chem. 276:48318-48324. [DOI] [PubMed] [Google Scholar]