Abstract
The tumor antigens simian virus 40 small t antigen (ST) and polyomavirus small and medium T antigens mediate cell transformation in part by binding to the structural A subunit of protein phosphatase 2A (PP2A). The replacement of B subunits by tumor antigens inhibits PP2A activity and prolongs phosphorylation-dependent signaling. Here we show that ST mediates PP2A A/C heterodimer transfer onto the ligand-activated androgen receptor (AR). Transfer by ST is strictly dependent on the agonist-activated conformation of AR, occurs within minutes of the addition of androgen to cells, and can occur in either the cytoplasm or the nucleus. The binding of ST changes the conformation of the A subunit, and ST rapidly dissociates from the complex upon PP2A A/C heterodimer binding to AR. PP2A is transferred onto the carboxyl-terminal half of AR, and the phosphatase activity is directed to five phosphoserines in the amino-terminal activation function region 1, with a corresponding reduction in transactivation. Thus, ST functions as a transfer factor to specify PP2A targeting in the cell and modulates the transcriptional activity of AR.
The nuclear receptor (NR) superfamily of ligand-activated transcription factors directs the expression of genes that control a wide range of processes, including development, metabolism, and reproductive biology (24). NRs such as the androgen receptor (AR) reside initially in the cytoplasm, where they bind their cognate ligand, undergo chaperone-assisted conformational changes, engage with the nuclear import machinery, and translocate to the nucleus (8). NRs undergo sequence-specific binding to response element DNAs and recruit regulatory proteins to enhancers and promoters of target genes en route to assembling preinitiation complexes, attracting RNA polymerase II, and initiating transcription (14). There is recent evidence that NR recruitment of regulatory proteins needed for transcription occurs in an ordered and cyclical fashion (25).
Members of the NR superfamily have a modular structure consisting of an N-terminal domain, a central DNA binding domain (DBD), a flexible hinge region, and a C-terminal ligand-binding domain (LBD) (10). The AR makes contact with regulatory proteins through subdomains with activation functions in the N-terminal domain activation function region 1 (AF-1) and the C-terminal LBD (AF-2). The AR DBD mediates binding to androgen response element DNA, which contains two palindromic hexanucleotide half-sites separated by a three-nucleotide spacer. The AR LBD forms the ligand-binding pocket, provides binding sites for heat shock proteins, and mediates intramolecular interactions with the N-terminal domain (17, 31).
Ligand binding is the primary mechanism for controlling the transactivation of NRs, and for some steroid hormone receptors, including AR, ligand binding controls subcellular localization as well (2). There is, however, a growing body of evidence that NR activity can be regulated by posttranslational modification. The phosphorylation of NRs can regulate ligand sensitivity, DNA binding, cofactor interactions, nuclear transport, and degradation (22, 35, 46). With a few exceptions, little is known about the kinases that directly phosphorylate NRs in vivo, and even less is known about the phosphatases responsible for NR dephosphorylation. The signal transduction pathways that regulate AR action have attracted interest in the context of ligand-independent mechanisms for transcriptional activation in advanced prostate cancer (18). Mitogen-activated protein (MAP) kinase signaling initiated by the addition of a growth factor or by activating mutations in Ras promotes AR-dependent gene expression and tumor growth in model systems (1, 5, 6). The candidate targets of MAP kinase signaling that modulate AR-dependent gene expression include AR itself and the p160 coactivators that function as molecular scaffolds for preinitiation complex assembly. The AR is phosphorylated on at least six sites in response to androgen binding, and alanine mutations in certain of these sites reduce the androgen-dependent transactivation of reporter genes (13, 50).
One of the major serine/threonine phosphatases in the cell is protein phosphatase 2A (PP2A). The PP2A core enzyme is a heterodimer consisting of a catalytic C subunit tightly bound to a structural A subunit, which binds reversibly to a B subunit to form the PP2A holoenzyme (19). There is considerable isoform diversity in PP2A subunits, particularly the B subunits, which are composed of more than a dozen proteins and mediate holoenzyme targeting to intracellular locations and distinct protein substrates (40, 42). PP2A regulation is critical for growth control because it dephosphorylates kinases and cell cycle components that regulate proliferation. PP2A is also a target of DNA tumor viruses (38). The simian virus 40 (SV40) small t antigen (ST) and polyomavirus middle T and ST can each bind directly to the PP2A A/C heterodimer and reduce its activity toward intracellular substrates. SV40 ST and B subunits have overlapping binding sites on the A subunit, resulting in B-subunit replacement and a corresponding loss of PP2A targeting in the cell (29, 33). SV40 ST can also reduce the phosphatase activity of the PP2A A/C heterodimer for certain protein substrates, probably by perturbing access to the C subunit (47). The negative regulation of PP2A by ST is thought to be important for anchorage-independent growth in transformed cells and for tumorigenesis in animal models (16, 26, 30, 48).
Against the backdrop of studies showing that ST reduces PP2A activity, we made the unexpected finding that ST increases the PP2A activity toward the AR. We found that ST mediates the transfer of the PP2A A/C heterodimer onto the AR, resulting in the dephosphorylation of five phosphoserines in the N-terminal AF-1 and a reduction in AR-dependent transactivation. The PP2A transfer reaction is correlated with ST-induced conformational changes in the A subunit of PP2A, requires the agonist conformation in AR, and is accompanied by a rapid dissociation of ST from the AR-PP2A complex.
MATERIALS AND METHODS
Cell culture and transfection.
Cell lines were obtained from the American Type Culture Collection (Manassas, Va.), and tissue culture reagents were purchased from Gibco/Invitrogen (Carlsbad, Calif.). For transcription and imaging assays, PC-3 and LNCaP cells were grown overnight in RPMI medium 1640 (free of phenol red) supplemented with charcoal- and dextran-treated fetal bovine serum (HyClone, Logan, Utah). Cos7, CV-1, and PC-3 cells were transfected by the use of Fugene 6 (Roche, Indianapolis, Ind.), and LNCaP cells were transfected by the use of Lipofectin (Invitrogen). AR expression levels obtained by transfection were severalfold higher (Cos7, HEK293, and HEK293T cells) or slightly lower (CV-1, PC-3, and DU145 cells) than the expression level of endogenous AR in LNCaP cells.
Purification of AR complexes.
LNCaP cells expressing endogenous AR or other cell types transfected with AR plasmids were grown to near confluence, washed once with ice-cold phosphate-buffered saline, scraped into phosphate-buffered saline containing 1 mM phenylmethylsulfonyl fluoride, and collected by centrifugation (1,500 × g for 5 min). The cell pellets were resuspended in 3 volumes of Triton lysis buffer (0.5% Triton X-100, 20 mM Tris-HCl [pH 7.5], 150 mM NaCl, 5 mM EDTA, 2 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, and 5 μg each of aprotinin, leupeptin, and pepstatin A/ml), and the samples were incubated for 20 min on ice. The lysates were then clarified by centrifugation (18,000 × g for 15 min), and the supernatants were diluted fivefold with Triton lysis buffer. For immunoprecipitation, the purified anti-AR monoclonal antibody AR441 (28) was prebound to protein G-agarose beads (2 μg of antibody per 10 μl of packed beads) by incubation for 4 h followed by five washes with Triton lysis buffer. Antibody-conjugated beads were mixed with lysates (10 μl of antibody-conjugated beads per 0.5 ml of diluted lysate) for 4 h at 4°C with end-over-end rotation. After five washes with Triton lysis buffer, AR complexes were eluted by incubating the beads with a synthetic peptide (STEDTAEYSPFKGGYTK) (20 μg/ml) containing the AR441 epitope for 1 h at room temperature. For some experiments, AR complexes were released from the beads by incubation in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer and heating to 95°C. AR complexes were analyzed by SDS-PAGE and silver staining performed according to standard methods and by mass spectrometry.
Mass spectrometry analysis of AR complexes.
Gel bands were excised and processed by standard methods for mass spectrometry (MS). Gel pieces were washed with 100 mM ammonium bicarbonate, pH 8.0, reduced in 10 mM dithiothreitol, and alkylated with 50 mM iodoacetamide. The gel pieces were then dehydrated, reswollen in a minimal volume of 100 mM ammonium bicarbonate buffer containing 12.5 ng of trypsin/ml, and allowed to react overnight at room temperature. Peptides were extracted with 100 μl of 50% acetonitrile containing 5.0% formic acid for 15 min (two cycles), followed by a further extraction with 100% acetonitrile. The extracted peptide solutions were then concentrated to ∼1.0 μl. The samples were diluted to 20 μl with 0.1% acetic acid for mass spectrometry analysis. Each sample was loaded into 360-μm-outer-diameter by 75-μm-inner-diameter microcapillary-fused silica tubing packed with a C18 irregular 5- to 20-μm-diameter resin. After sample loading, the preparative column was washed with 0.1% acetic acid for 15 min to remove buffer salts. The preparative column was then connected to a 360-μm-outer-diameter by 50-μm-inner-diameter analytical column packed with a C18 regular 5-μm-diameter resin constructed with an integrated electrospray emitter tip. Samples were eluted with a gradient (Agilent 1100 Series HPLC) directly into a Finnigan LCQ quadrupole ion-trap mass spectrometer (ThermoQuest, San Jose, Calif.) at a flow rate of 60 nl/min. The nanoflow high-performance liquid chromatography gradient used was 0 to 60% acetonitrile in 0.1% acetic acid for 60 min. The ion-trap mass spectrometer was operated in the data-dependent mode, in which an initial MS scan recorded the mass-to-charge (m/z) ratios of parent ions over the mass range of 300 to 2,000 Da. The five most abundant ions were then selected for subsequent collisionally activated dissociation (CAD), and tandem MS (MS/MS) spectra were recorded. MS/MS data from samples purified from Cos7 cells were searched against a database consisting of all primate proteins. MS/MS data from samples purified from HEK293 cells were searched against a human database. All searches were conducted with the Sequest program and validated manually when appropriate.
In vitro reconstitution of PP2A transfer onto AR.
The ST-dependent PP2A transfer reaction was reconstituted by use of the purified PP2A heterodimer (Upstate Biotech, Charlottesville, Va.) and a recombinant PP2A A subunit. The PP2A A subunit (human α isoform) was expressed with an N-terminal His6 tag in Escherichia coli BL21(DE3)/pLysS bacteria (Novagen, Madison, Wis.) and purified by standard methods. SV40 ST was expressed in bacteria and purified as described previously (43). For the in vitro transfer reaction, AR complexes from androgen-treated cells were immobilized on AR441 antibody-protein G beads (10 μl) and incubated with the PP2A heterodimer (0.2 U) or the recombinant His6-A subunit (2 μg) in the absence or presence of recombinant SV40 ST (1 μg). The reactions contained bovine serum albumin (0.1 mg/ml) in a total volume of 150 μl in Triton lysis buffer and were incubated overnight at 4°C. After five washes with Triton lysis buffer, the bound fractions were resolved by SDS-PAGE and analyzed by immunoblotting.
Transcription assays.
Transcription was measured by use of a dual-luciferase reporter assay system (Promega). Cells were seeded onto six-well plates (106 cells per well), grown overnight, and transfected with the indicated plasmids according to protocols appropriate for each cell type. Each well received a total of 1 μg of DNA, which typically included 500 ng of reporter plasmids, 100 ng of an AR plasmid, 50 ng of a small t plasmid, and the balance as the empty vector pCDNA3.0. The reporters were prostate-specific antigen (PSA)-firefly luciferase, Vit6-firefly luciferase, and cytomegalovirus-Renilla luciferase. After 24 h of transfection, the medium was changed and the cells were treated with AR ligands for an additional 24 h. The ligands used were 5-α-dihydrotesterone, androstenedione (ASD), Flutamide (all from Sigma, St. Louis, Mo.), the synthetic androgen methyltrienolone (R1881) (Perkin-Elmer), and Casodex (Astra Zeneca). Luciferase activities in lysates were measured on a Berthold LB 953 luminometer. Firefly luciferase activities were normalized to Renilla luciferase activities (plotted as F/R), assays were performed in triplicate, and the data are representative of at least three experiments. Immunoblotting was used to establish the fact that any differences in transcription were not due to expression levels.
Antibodies.
AR441 is an anti-peptide monoclonal antibody that recognizes an epitope within the N-terminal domain of AR. Protein G chromatography was used to purify AR441 secreted by the hybridoma AR441/B2/H8 (a gift of D. Edwards). PG-21 (Upstate) is an anti-peptide polyclonal antibody that recognizes the N terminus of AR. Antibodies that recognize phosphorylation sites in AR were generated against the following phosphopeptides: for Ser16, CYPRPPSKTYRG; for Ser94, CQGEDGSPQAHR; for Ser256, CALEHLSPGEQL; for Ser308, CDTAEYSPFKGG; for Ser424, CGPGSGSPSAAA; and for Ser650, CASSTTSPTEET (phosphorylation sites are shown in italics). The phospho-Ser81 antibody has been described previously (3). The ST antibody PAb430 (a gift of M. Imperiale) was used for immunoprecipitation, and PAb108 (American Type Culture Collection) was used for immunoblotting. Four PP2A antibodies were used, including the anti-C subunit mouse monoclonal 1D6 (Upstate), an anti-C subunit rabbit polyclonal (27), the anti-A subunit rat monoclonal MRT-204R (Covance, Richmond, Calif.), and the anti-A subunit goat polyclonal sc-6112 (Santa Cruz, Santa Cruz, Calif.). Immunoblotting was performed with peroxidase-labeled secondary antibodies by enhanced chemiluminescence (Pierce, Rockford, Ill.). Other antibodies used were the monoclonal M2 against the FLAG epitope, the monoclonal DM1A against α-tubulin (Sigma), and the rabbit polyclonal MU014-UC against PSA (BioGenex, San Ramon, Calif.).
RESULTS
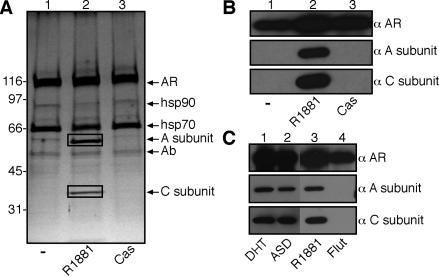
Ligand binding to AR facilitates the recruitment of factors that are needed for translocation into the nucleus and for the assembly of preinitiation complexes during transcription. To identify factors involved in these processes, we used a proteomic approach to define the composition of AR complexes isolated from cells that were treated with androgen agonists and antagonists. The AR was expressed in Cos7 cells by transient transfection, and AR complexes were affinity purified by use of the anti-AR monoclonal antibody AR441 (28). The complexes were eluted with a synthetic peptide corresponding to the monoclonal antibody epitope and analyzed by SDS-PAGE and silver staining. The synthetic androgen (R1881) treatment resulted in the appearance of AR-associated ∼65- and ∼36-kDa polypeptides that were not present in complexes isolated from control cells or from cells treated with the androgen antagonist Casodex (Fig. 1A, lanes 1 to 3). Based on mass spectrometry, the polypeptides unique to the AR-androgen complex were the regulatory (A subunit, 65 kDa) and catalytic (C subunit, 36 kDa) subunits of the serine/threonine PP2A (Table 1). The A and C subunits heterodimerize and form the core PP2A enzyme, which is known to regulate diverse pathways in the cell (19). The PP2A A/C heterodimer associates with different regulatory B subunits, but no B subunits were found in the AR-androgen complex by silver staining or mass spectrometry. Immunoblotting with antibodies for the A and C subunits confirmed that PP2A binds to AR in the presence of R1881, but not in the absence of ligand or the presence of Cas (Fig. 1B, lanes 1 to 3). PP2A binding to AR was also observed in the presence of 5-α-dihydrotestosterone and the adrenal androgen ASD, but not in the presence of the anti-androgen Flutamide (Fig. 1C). Thus, PP2A binding to AR is strictly dependent on the agonist-induced conformation of the LBD.
FIG. 1.
Identification of PP2A as a subunit of an AR complex. (A) Isolation of AR complexes by immunoaffinity purification. Cos7 cells expressing AR were treated with 10 nM R1881 (synthetic androgen) or 30 μM Casodex (Cas) for 1 h. After immunopurification, the complexes were analyzed by SDS-PAGE and silver staining, and the polypeptides were excised and analyzed by mass spectrometry. The polypeptides enclosed in boxes correspond to the A subunit and the C subunit of PP2A. (B) Immunoblotting of AR complexes obtained from Cos7 cells by use of antibodies to the PP2A A subunit and C subunit. (C) Agonist-specific interaction between AR and PP2A. Cos7 cells expressing AR were treated with 1 nM 5-α-dihydrotesterone (DHT), 100 nM ASD, 10 nM R1881, or 10 μM Flutamide (Flut) for 1 h, and the AR complexes were isolated and examined by immunoblotting for the presence of the PP2A A and C subunits.
TABLE 1.
Summary of proteins identified by mass spectral analysis of AR complexes immunoprecipitated from Cos-7 cells after various ligand treatments
| Treatment | Gel band no. | Protein | genInfo identifier | No. of peptides |
|---|---|---|---|---|
| Control | 1 | Androgen receptor | 460281 | 9 |
| 2 | HSP 70.1 | 462325 | 8 | |
| 2 | HSP 70 protein 8 isoform 1 | 5729877 | 7 | |
| R1881 | 1 | Androgen receptor | 460281 | 10 |
| 2 | HSP 70.1 | 462325 | 3 | |
| 2 | HSP 70 protein 8 isoform 1 | 5729877 | 3 | |
| 3 | PP2A catalytic α subunit | 21619480 | 3 | |
| 4 | PP2A regulatory α subunit | 231443 | 6 | |
| Casodex | 1 | Androgen receptor | 460281 | 8 |
| 2 | HSP 70.1 | 462325 | 7 | |
| 2 | HSP 70 protein 8 isoform 1 | 5729877 | 4 |
PP2A binding is specific for AR.
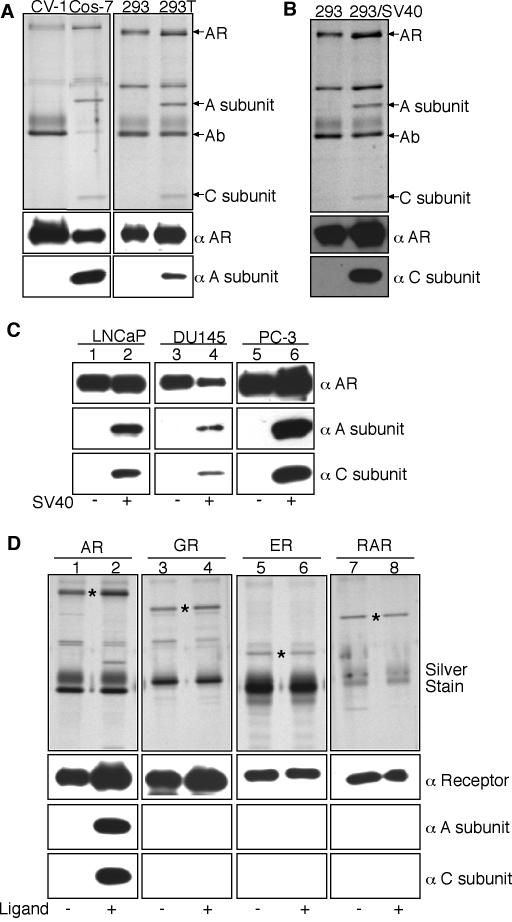
AR-PP2A complexes were recovered by immunoprecipitation from SV40-transformed Cos7 and 293T cells, but not from the untransformed parental lines CV-1 and 293, respectively (Fig. 2A). The interaction between AR and PP2A was not dependent on cell transformation, however, since it was reconstituted in 293 cells by transient transfection of the SV40 early region (Fig. 2B). The SV40 early region also reconstituted the androgen-dependent binding of AR to PP2A in the prostate cancer cell lines LNCaP (from lymph node metastasis), DU145 (from brain metastasis), and PC-3 (from bone metastasis) (Fig. 2C). In SV40-transformed Cos7 cells, PP2A was not transferred onto the glucocorticoid receptor (GR), the estrogen receptor (ER), or the retinoic acid receptor (RAR) (Fig. 2D). While the LBD is structurally and functionally similar in these NRs, the PP2A A/C heterodimer recognizes features that are unique to the agonist-bound form of AR.
FIG. 2.
Requirement for the SV40 early region for PP2A transfer onto AR. (A) PP2A transfer onto AR occurs in SV40-transformed Cos7 and 293T cells. AR was transfected into the cell types indicated, and after the addition of 10 nM R1881 (1 h), the AR complexes were isolated and examined by silver staining and immunoblotting. (B) Transient transfection of 293 cells with the SV40 early region results in PP2A transfer onto androgen-bound AR. (C) Transient transfection of prostate cancer cell lines with the SV40 early region also results in PP2A transfer onto androgen-treated AR. (D) PP2A transfer onto AR in the presence of 10 nM R1881, but not onto GR, ER, and green fluorescent protein-RAR in the presence or absence of their cognate ligands dexamethasone, estradiol, and all-trans retinoic acid, respectively. The positions of AR, GR, ER, and green fluorescent protein-RAR are indicated (*). The amount of AR complexes loaded in immunoblots was one-half that used for silver staining, whereas the amounts of other NRs used in immunoblots were the same as those used for silver staining.
PP2A binding to AR is induced by ST.
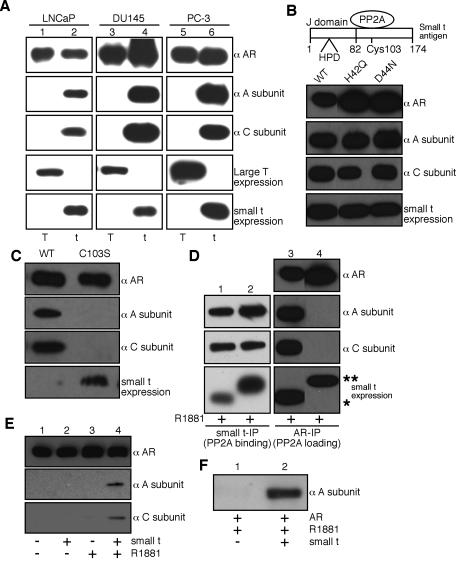
Deletion analysis and immunoprecipitation were used to identify the minimal fragment of the SV40 early region that, when introduced into cells, results in AR binding to PP2A (Fig. 3A and data not shown). The fragment encodes ST, a 174-amino-acid protein known to bind directly to PP2A (29, 47). The expression of ST, not that of the large T antigen, was sufficient to reconstitute the androgen-dependent association of endogenous AR with endogenous PP2A in LNCaP cells (Fig. 3A, lanes 1 and 2). Similarly, ectopically expressed AR in DU145 and PC-3 cells bound to endogenous PP2A in the presence of ST but not the large T antigen. This binding was strictly agonist dependent. SV40-transformed Cos7 and 293T cells express ST, which explains why PP2A is transferred onto androgen-bound ARs expressed in these cell types.
FIG. 3.
ST mediates PP2A transfer onto AR. (A) Transient transfection of small t but not large T antigen (T) in prostate cancer lines is necessary for PP2A transfer onto androgen-treated AR. After transfection, the cells were treated with R1881 (10 nM for 1 h), and the AR complexes were isolated and analyzed by immunoblotting. Note that neither ST nor the large T antigen was detected in AR complexes isolated from control or androgen-treated cells (data not shown). (B) Analysis of J domain function in the PP2A transfer reaction. Mutations that resulted in a loss of function in the J domain did not affect ST-mediated PP2A transfer onto AR. AR and wild-type ST (WT) or the indicated mutant forms of ST were expressed in 293 cells. After androgen treatment, the AR complexes were isolated and examined for the presence of PP2A subunits. (C) PP2A transfer onto AR requires a physical interaction with ST. A mutation in ST (C103S) that disrupted binding to PP2A inhibited the transfer reaction. AR together with wild-type ST (WT) or the C103S mutant was expressed in 293 cells. (D) PP2A binding to ST can be uncoupled from the ST-mediated transfer of PP2A onto AR. The fusion of a 10-amino-acid Myc tag to the C terminus resulted in a form of ST that bound PP2A (lane 2) but failed to mediate the PP2A transfer onto AR (lane 4). The positions of ST (*) and ST-Myc (**) are indicated. (E) Reconstitution of PP2A transfer reaction in vitro with purified components. AR expressed in 293 cells was immobilized on beads and used for transfer reactions with the purified PP2A A/C heterodimer and recombinant ST. (F) Reconstitution of PP2A transfer reaction with recombinant A subunit. The reactions were performed as described for panel E except that a His-tagged A subunit expressed in bacteria was used in place of the PP2A A/C heterodimer.
ST contains an N-terminal J domain, which is an ∼70-amino-acid, four-helix structure found in DNA J-type molecular chaperones (11, 41). J domains function as cochaperones by recruiting and activating the ATPase activity of Hsp70 (heat shock protein 70) proteins (21). Since Hsp70 proteins interact with NRs and were isolated as AR-associated subunits in our purification (Table 1), we tested whether the J domain of ST is involved in AR binding to PP2A. Mutations within the conserved His-Pro-Asp (HPD) sequence that inhibit the J domain function in ST were engineered and coexpressed with AR. STs with mutations in the J domain (H42Q or D44N) mediated the transfer of PP2A onto androgen-activated AR (Fig. 3B). Thus, J domain activity in ST is not required for the transfer reaction. PP2A transfer to AR does, however, require contact between ST and PP2A. A mutation of a cysteine (C103S) that disrupts ST binding to PP2A (26) resulted in a complete loss of PP2A binding to AR, even upon overexpression of the mutant ST (Fig. 3C). We also found that PP2A binding to ST can be uncoupled from PP2A transfer onto the AR. Fusing the Myc epitope to the C terminus of ST prevented the transfer of PP2A from ST to the activated AR without affecting binding to PP2A, as assayed by coimmunoprecipitation (Fig. 3D). The C terminus of ST may be directly involved in the transfer reaction, or the Myc epitope may alter the structure of a region of ST that is important for the transfer reaction.
We reconstituted the PP2A transfer reaction with immobilized AR, bacterially expressed ST, and the purified PP2A A/C heterodimer, which ruled out the involvement of cellular B subunits or other proteins in the reaction (Fig. 3E). ST was not detected in the AR complex after the in vitro transfer reaction (data not shown), indicating that it dissociates from PP2A during or subsequent to PP2A transfer. The transfer reaction was also reconstituted by use of the bacterially expressed PP2A A subunit, which established that the interaction between AR and PP2A involves direct contact with the A subunit (Fig. 3F). The in vitro transfer reaction, like the in vivo PP2A transfer reaction, was strictly dependent on the androgen-activated form of AR. This observation further emphasizes the critical nature of the AR conformation for accepting PP2A in the transfer reaction. In addition, because the androgen activation of AR (in vivo) and the ST-dependent transfer of PP2A (in vitro) were performed as separate steps, it is unlikely that ST mediates PP2A transfer by trapping AR in an intermediate conformation that occurs during androgen binding. Rather, it seems more likely that the ST-occupied form of the A subunit is targeted to the agonist-specific conformation of AR.
DBD-hinge-LBD region of AR is required for PP2A association.
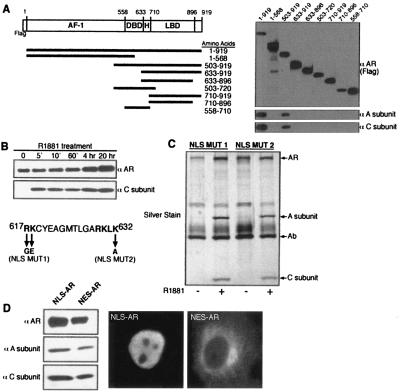
We used a series of AR deletion mutants to map the regions that are necessary for a stable association with PP2A. The minimal fragment of AR that is functional for PP2A binding contains the DBD, the hinge region, and the LBD (Fig. 4A). To examine the kinetics of the transfer reaction, we treated Cos7 cells expressing AR with R1881 over a time course of 5 min to 20 h. PP2A transfer onto AR was observed at the earliest time point (5 min) after the addition of androgen, indicating that the reaction is rapid and possibly occurs in the cytoplasm prior to AR import into the nucleus (Fig. 4B). Mutations in the bipartite nuclear localization signal (NLS) of AR reduce nuclear import and render the AR predominantly cytoplasmic (36). These forms of AR were fully active for PP2A binding (Fig. 4C). In addition, engineered forms of AR that were constitutively nuclear (NLS-AR) or cytoplasmic (nuclear export signal [NES]-AR) were functional for agonist-dependent binding to PP2A (Fig. 4D). Therefore, ST-mediated PP2A transfer to AR can occur in either the cytoplasm or the nucleus.
FIG. 4.
PP2A transfer requires DBD-hinge-LBD region of AR and occurs in the cytoplasm and the nucleus. (A) Deletion analysis to identify the minimal region of AR that can stably bind to PP2A. AR constructs were expressed in Cos7 cells as N-terminal fusions with the FLAG epitope, and an anti-FLAG antibody was used for immunoprecipitation and immunoblotting. (B) PP2A transfer onto AR in vivo is rapid. Cos7 cells expressing AR were treated with R1881 (10 nM) for the indicated times. The PP2A transfer onto AR did not occur if R1881 was added to cell lysates, indicating that the transfer reaction did not occur after cell lysis (not shown). (C) PP2A can be transferred onto NLS-mutated AR that is primarily in the cytosol. (D) PP2A can be transferred onto AR that is constitutively localized to the nucleus or the cytosol. AR was expressed as fusions with the NLS from SV40 (NLS-AR) and with the NES from a protein kinase inhibitor (NES-AR) in Cos7 cells. After R1881 treatment, the AR complexes were isolated and examined by immunoblotting for the presence of the PP2A A and C subunits.
AR is dephosphorylated by PP2A.
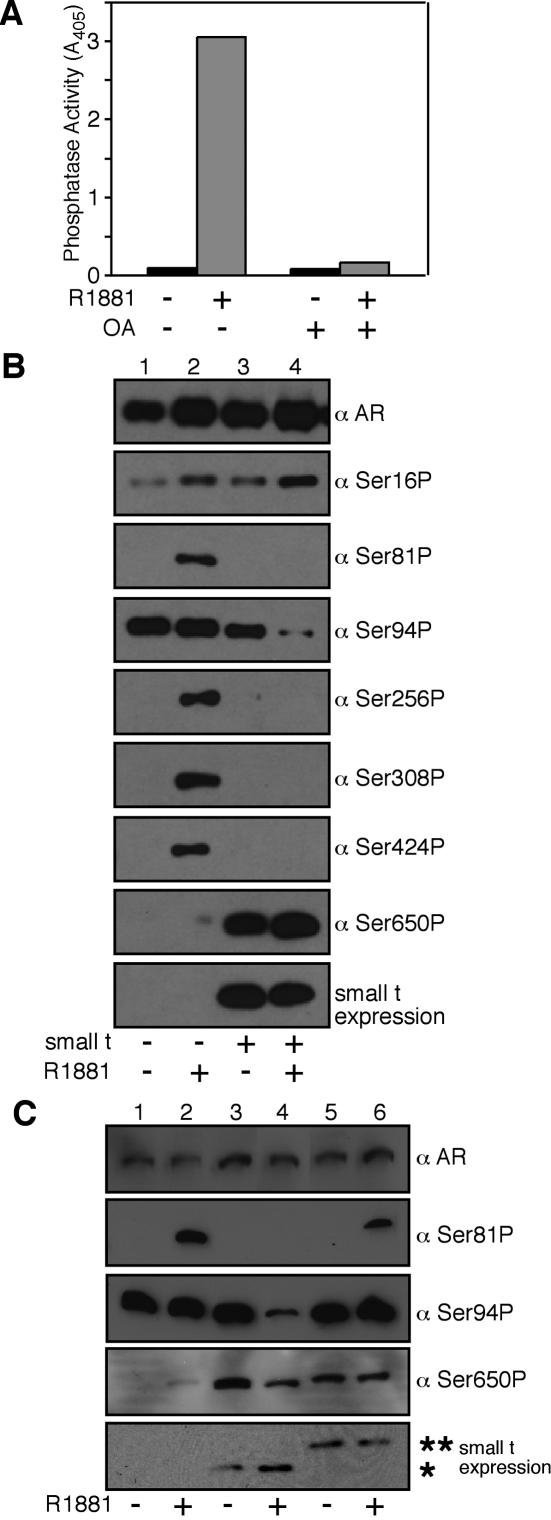
We measured the phosphatase activity of PP2A associated with AR by using the chromogenic substrate p-nitrophenyl phosphate. The R1881 treatment of Cos7 cells expressing AR (Fig. 1A) resulted in the recovery of AR-associated phosphatase activity (Fig. 5A). The phosphatase activity associated with androgen-bound AR was inhibited 98% by 10 nM okadaic acid, a result consistent with our identification of PP2A in the AR complex.
FIG. 5.
PP2A dephosphorylates the AF-1 region of AR. (A) PP2A bound to AR is catalytically active. AR was expressed in Cos7 cells, which were untreated or treated with R1881 to induce PP2A loading in vivo. The AR complexes were eluted and analyzed for phosphatase activity. (B) ST-dependent loading and PP2A-dependent dephosphorylation of five phosphoserines in AF-1 region of AR. AR was expressedin 293 cells in the absence or presence of ST, and the cells were either untreated or treated with R1881 (10 nM for 2 h). The samples were analyzed with a pan-AR antibody (PG-21) and a panel of phosphospecific AR antibodies. (C) Dephosphorylation by PP2A requires loading onto AR. 293 cells expressing AR and wild-type or Myc-tagged forms of ST were untreated or treated with R1881, and the AR complexes were isolated and analyzed by immunoblotting with phosphospecific antibodies. The positions of ST (*) and ST-Myc (**) are indicated.
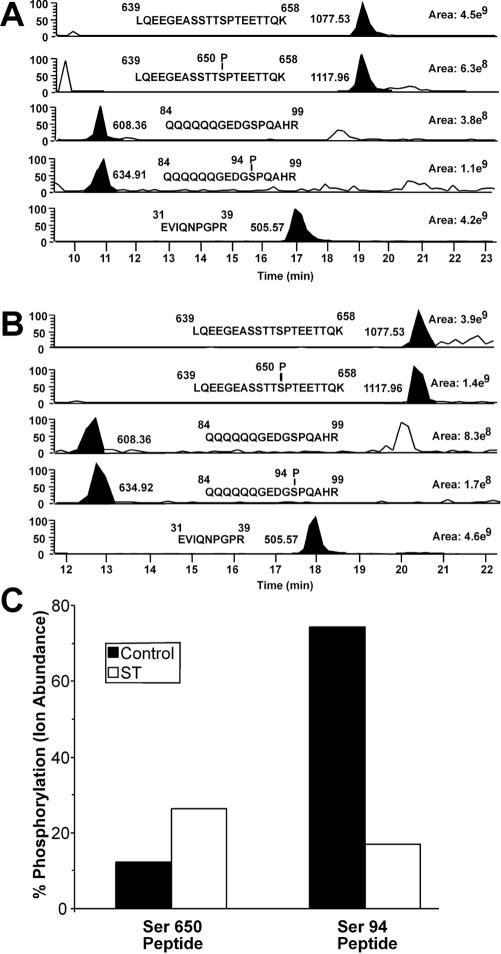
AR undergoes phosphorylation in response to androgen binding, the addition of a growth factor, and the pharmacological activation of kinase pathways (46). To determine whether PP2A dephosphorylates AR, we examined the phosphorylation state following the ST-dependent transfer of PP2A onto AR. The analysis relied on a panel of AR-phosphospecific antibodies generated against six phosphorylation sites in the AF-1 region and one phosphorylation site in the hinge region. AR was expressed in 293 cells in the absence or presence of ST, the cells were treated with R1881, and the phosphorylation state of AR was determined by immunoblotting. In the absence of ST, the androgen induced the phosphorylation of six sites (Ser16, Ser81, Ser256, Ser308, Ser424, and Ser650) (Fig. 5B, lanes 1 and 2). This finding is consistent with data obtained by phosphopeptide mapping and mass spectrometry for other cell types (3, 13). In the presence of ST, AR was dephosphorylated at five sites (Ser81, Ser94, Ser256, Ser308, and Ser424) (Fig. 5B, lanes 3 and 4). The Ser650 site in AR was not dephosphorylated under conditions in which PP2A was bound to AR, and Ser650 displayed a relatively high level of constitutive (androgen-independent) phosphorylation in the presence of ST. To rule out the possibility that ST expression facilitates AR dephosphorylation in vivo through an indirect route that involves the activation of another phosphatase, we expressed the Myc-tagged form of ST and examined the phosphorylation state of several sites in AR (Fig. 5C). Myc-tagged ST bound PP2A but failed to transfer PP2A onto AR and accordingly did not cause the dephosphorylation of AR (Fig. 5C). The correlation between ST- and androgen-dependent PP2A binding to AR and the dephosphorylation of five sites in the AF-1 region of AR provides evidence that PP2A is an AR phosphatase. We used mass spectrometry to characterize the phosphorylation status of Ser94 and Ser650 in ARs prepared from control and ST-expressing cells and obtained results that are consistent with our phospho-antibody detection (Fig. 6).
FIG. 6.
Mass spectrometry analysis of AR phosphopeptides from control and ST-transfected HEK293 cells treated with R1881. (A) Selected ion chromatograms for the nonphosphorylated and phosphorylated forms of two AR peptides containing either Ser650 or Ser94 are shown from the control sample. The abundance of each peptide was determined by calculating the area under the curve for all charge states of each selected ion. The total ion abundance for each peptide in all of its forms was calculated by addition of the ion abundances of both the nonphosphorylated and phosphorylated forms. The selected ion chromatogram for EVIQNPGPR was included as a control to demonstrate the levels of an AR peptide that does not have multiple forms in each sample. (B) Same analysis as in panel A, except that we used samples prepared from cells expressing ST. (C) The differences in phosphorylation of Ser650 and Ser94 between the control and ST-transfected samples are presented as percentages of the total ion abundance for each peptide attributed to the phosphorylated form of that peptide. These numbers were calculated by dividing the ion abundance of the phosphorylated form of each peptide by the total ion abundance of all forms of that peptide from the same sample.
ST represses AR activity.
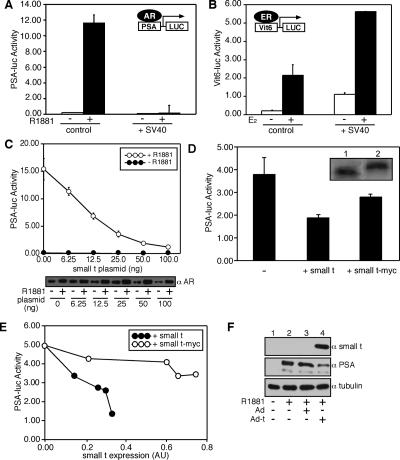
LNCaP cells express endogenous AR and show robust, androgen-dependent transactivation of AR-responsive genes, including PSA. We measured the transactivation of the PSA promoter in LNCaP cells in the absence and presence of the SV40 early region that encodes the ST and large T antigens. Upon SV40 transfection, there was a virtually complete inhibition of AR-dependent transcription from the 6-kB PSA promoter fused to luciferase (Fig. 7A). In contrast, ER-dependent transcription measured in LNCaP cells by use of the Vit6 promoter fused to luciferase was increased (Fig. 7B). The inhibition of AR-dependent transcription in this assay could be reconstituted by use of a plasmid that expressed only ST (Fig. 7C). Our data suggest that the dephosphorylation of AR by ST-dependent PP2A transfer reduces AR-mediated transcription. We tested the PP2A transfer-defective (Myc-tagged) form of ST for its effect on AR-dependent transcription measured from the PSA promoter (Fig. 7D and E). Under conditions in which ST inhibited AR-dependent transcription ∼80%, the expression of twofold more Myc-tagged ST inhibited AR-dependent transcription only ∼20% (Fig. 7E). Thus, the efficient inhibition of AR-dependent transcription in these assays required the PP2A transfer from ST onto AR.
FIG. 7.
PP2A loading and dephosphorylation inhibit AR transcription activity. (A) Effect of SV40 early region on AR-dependent transcription. Androgen-dependent transcription (endogenous AR) was measured in LNCaP cells by use of the 6-kb PSA promoter fused to firefly luciferase (PSA-LUC) in the absence and presence of the SV40 early region. Cells were cultured in phenol red-free medium with charcoal-stripped serum and then further treated with 1 nM R1881 for 24 h to induce transcription. Assays were performed in triplicate, were normalized by the use of cytomegalovirus-Renilla luciferase, and are representative of at least three experiments. (B) Effect of SV40 early region on estrogen receptor-dependent transcription. Estrogen (E2)-dependent transcription (transfected ERα) was measured in LNCaP cells by use of a firefly luciferase reporter based on the vitellogenin promoter (Vit6-LUC). (C) Effect of ST expression on AR-dependent transcription. Increasing concentrations of a plasmid encoding SV40 ST (6.25 to 100 ng per well) were cotransfected with PSA-LUC into LNCaP cells. The samples were analyzed for transcription (top) and AR expression levels (bottom). (D) PP2A loading-dependent and -independent effects on transcription mediated by AR. AR-dependent transcription from the PSA promoter was measured in the presence of wild-type ST and Myc-tagged ST, the latter of which binds PP2A but does not mediate PP2A loading onto AR. (E) Dose-response curves showing ST effects on AR-dependent expression. Relative expression levels of ST (closed circles) and ST fused with a Myc epitope (open circles) were determined by enhanced chemiluminscence, scanning densitometry of X-ray films, and ImageQuant software. (F) ST expression reduces endogenous PSA levels. Lanes 1 and 2, PSA expression in LNCaP cells was inducible by the addition of 1 nM R1881; lanes 3 and 4, LNCaP cells were infected with control adenovirus (Ad) or ST-expressing adenovirus (Ad-t) for 24 h and then treated with 1 nM R1881 for an additional 24 h. PSA levels were analyzed in whole-cell lysates by immunoblotting.
To determine the effect of ST on endogenous gene expression, we infected LNCaP cells with an adenovirus expressing ST or a control adenovirus and examined the PSA levels by immunoblotting. We observed a reduction in PSA levels in cells infected with the virus encoding ST but not in cells infected with the control virus (Fig. 7F).
Mechanism of PP2A transfer.
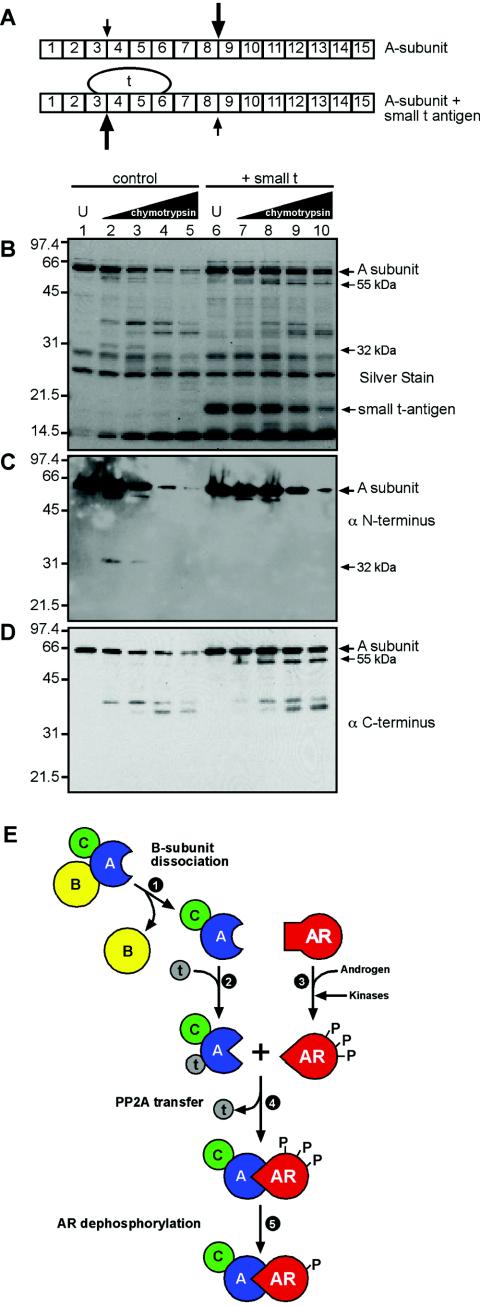
There are two nonmutually exclusive mechanisms that might explain how ST transfers PP2A onto AR. First, ST may transiently bridge the interaction between PP2A and AR and facilitate a hand-off reaction. We do not favor a hand-off reaction as the likely mechanism for PP2A transfer because it would require ST binding to AR, an interaction that has not been observed in our assays. While we cannot formally rule out a hand-off reaction because the ST-AR interaction may be too transient for detection, we have instead explored a mechanism based on ST-induced changes in the PP2A structure. We considered whether a conformational change induced by ST might create an A-subunit surface with structural complementarity to the agonist-bound form of AR. The PP2A A subunit is composed of 15 HEAT repeats (for Huntington, elongation factor, PP2A A-subunit, TOR), and comprehensive deletion and mutagenesis studies have mapped the ST binding site to HEAT repeats 3 to 6 (15, 32, 33) (Fig. 8A). We assayed for ST-induced conformational changes in the PP2A A-subunit structure by limited proteolysis, reasoning that such changes would increase or decrease the accessibility of chymotrypsin cleavage sites within the A subunit. To this end, we used chymotrypsin over a wide concentration range (1:540 to 1:20 [wt/wt]) to digest the recombinant PP2A A subunit and analyzed the products by silver staining and immunoblotting. When digests were performed with a PP2A A subunit that had been preincubated with ST, an ∼55-kDa chymotryptic fragment was detected by silver staining (Fig. 8B, lanes 7 to 10). The ∼55-kDa fragment of the PP2A A subunit was not recognized by an antibody to the extreme N terminus, but it was recognized by an antibody to the extreme C terminus (Fig. 8C and D). These data indicate that the ∼55-kDa fragment was generated by chymotryptic cleavage of a site near HEAT repeat 3 that becomes more accessible upon ST binding to the A subunit (Fig. 8A). ST binding to the A subunit also reduced the accessibility of a chymotryptic site near HEAT repeat 8 that, in the absence of ST, resulted in an ∼32-kDa N-terminal fragment (Fig. 8B and D). Our data show that in the absence of other cellular factors or posttranslational modifications, ST binds and alters the conformation of the A subunit of PP2A.
FIG.8.
ST binding alters conformation of PP2A. (A) Diagram showing the arrangement of the 15 HEAT repeats in the A subunit of PP2A, the ST binding site on HEAT repeats 3 to 6, and the proteolytic cleavage sites that show reduced (small arrows) or enhanced (large arrows) accessibility caused by ST binding. (B) In vitro proteolysis of the A subunit of PP2A. The purified recombinant A subunit was digested with chymotrypsin in the absence and presence of recombinant ST, and the products were analyzed by SDS PAGE and silver staining. U, undigested sample. ST migrates at ∼20 kDa (lanes 6 to 10). (C and D) The products from the digests were probed in parallel with antibodies to epitopes at the extreme N and C termini of the PP2A A subunit. (E) Model of conformation-dependent PP2A loading onto AR. B-subunit dissociation (step 1) is required to reveal the binding site for ST on the A subunit. ST binding to the A/C heterodimer (step 2) induces a conformational change in the A subunit. Androgen binding to AR induces a conformational change (step 3) that, in addition, facilitates recognition and phosphorylation by kinases. The ternary complex of ST and PP2A A/C docks with androgen-bound AR (step 4), releasing ST. PP2A bound to AR dephosphorylates five phosphoserines within the N-terminal AF-1 region (only two are shown). Ser16 within the AF-1 region and Ser650 within the hinge region are not dephosphorylated.
DISCUSSION
The role of ST in cell transformation has been considered primarily in the context of its interaction with PP2A, which alters signal transduction and gene expression (26, 29, 39). The high-affinity binding of ST to the PP2A A/C heterodimer results in the effective displacement of a myriad of B subunits, reduces the pool of the PP2A A/B/C holoenzyme, and alters the activities of cellular substrates that are important for growth regulation. The recent finding that suppression of a PP2A subunit, B56γ, can mimic the transformation effects of ST underscores the critical roles played by B subunits and provides evidence that PP2A holoenzyme targeting to substrates is necessary for growth regulation (4).
Conformation dependence of AR in PP2A transfer reaction.
We determined that the PP2A transfer reaction is strictly dependent on specific conformations elicited by hormone binding to AR and ST binding to the A subunit of the PP2A A/C heterodimer (Fig. 8E). R1881, 5-α-dihydrotestosterone, and androstenedione each can induce the agonist-specific conformation of AR, and all three androgen-related compounds supported PP2A transfer onto AR in vivo in the presence of ST. PP2A was not transferred onto ligand-free AR or AR that contained the anti-androgen agent Casodex or Flutamide or other NRs (GR, ER, and RAR) in the presence of their cognate ligands (Fig. 1 and 2). These results emphasize that the PP2A A subunit-ST complex recognizes structural features that are unique to the agonist-bound conformation of AR. The biochemical and structural features characteristic of the agonist-bound state of AR include enhanced interactions between the N-terminal AF-1 region and the C-terminal LBD and the positioning of helix 12 in the LBD to allow coactivator binding (17, 34). The structural determinants for PP2A binding are not identical to those for coactivator binding, however, since the LBD of AR is not sufficient for the PP2A loading reaction. The minimal fragment of AR that is necessary to reconstitute the PP2A loading reaction spans the DBD-hinge-LBD region. PP2A may bind cooperatively to the LBD and the DBD, or interdomain interactions in AR may be required to stabilize the conformation of the PP2A binding site in the LBD or the DBD.
The N-terminal AF-1 region is not required for PP2A loading despite the fact that it contains the five sites that are dephosphorylated by the C subunit of PP2A. A crystallographic analysis will be required to determine how the AF-1 phosphorylation sites are spatially positioned relative to the DBD-hinge-LBD region where PP2A binds AR. Nonetheless, our finding that PP2A binds to AR through its regulatory A subunit and dephosphorylates five sites is a clear indication of conformational flexibility within the multiprotein complex. Conformational flexibility may be imparted by the proline-rich hinge region of AR or by the A subunit of PP2A. The notion of conformational flexibility within the AR-PP2A complex raises the intriguing possibility that AR might function as a hormone-regulated B subunit that targets PP2A to androgen-responsive promoters and enhancers. Given the plethora of factors, including p160 coactivators, that undergo reversible phosphorylation during transcription and the fact that PP2A can dephosphorylate histone H1 (37), PP2A may join the ranks of protein-modifying enzymes that are selectively recruited to promoters to modulate gene expression.
Conformation dependence of PP2A in transfer reaction.
The conformational changes in AR induced by agonist binding are necessary but not sufficient for PP2A loading, as conformational changes in the A subunit of the PP2A A/C heterodimer also appear to be required (Fig. 8E). Chymotryptic digestion of the recombinant PP2A A subunit showed that ST binding increases the accessibility of a cleavage site near HEAT repeats 3 and 4 and reduces the accessibility of a cleavage site near HEAT repeats 8 and 9. These data support a model whereby ST-induced changes in the A subunit produce a conformation that has structural complementarity to the agonist-activated conformation of AR (Fig. 8E). In this reaction, ST mediates specific targeting of PP2A by directing the A/C heterodimer to AR. Our data validate the long-held hypothesis that ST functions as a viral B subunit by targeting PP2A to a defined substrate. There are, however, two unusual features of ST-dependent PP2A targeting to AR: ST does not appear to directly contact the target protein AR, and ST is released upon PP2A binding to AR (Fig. 8E). We infer from these results that the information that targets PP2A to AR is not carried within ST, but within the conformation of the A subunit in PP2A induced by ST. This contrasts with the conventional view of B-subunit targeting of PP2A holoenzymes that is dependent on the B subunit bridging the interaction between PP2A and its substrates. Given the proclivity of viral proteins to coopt cellular mechanisms, it will be important to test whether PP2A holoenzyme targeting by cellular B subunits involves changes in the A-subunit conformation.
ST represses AR-dependent transcription.
ST activates promoters for genes, including those for cyclins A and D1 and adenovirus E2 (26, 30, 45). We found that ST represses AR activity and involves two components, a PP2A transfer-dependent component and a PP2A transfer-independent component. The non-transfer-related repression of AR activity may be due to the ST activation of Akt, which phosphorylates and inhibits AR (23, 49). Evidence for the transfer-independent component was obtained by the use of an ST with a C-terminal Myc tag, which bound PP2A but failed to promote the PP2A transfer onto AR. Although ST-Myc can exert a repressive effect on AR-dependent transcription, it does not recapitulate the same level of inhibition observed with the untagged ST, which mediates the PP2A transfer reaction. Binding of the Myc-tagged ST to PP2A may repress AR-dependent transcription by an indirect route such as reducing the pool of active PP2A. The total level of repression of AR-dependent transcription in the presence of untagged ST presumably reflects the sum of transfer-independent and transfer-dependent effects on AR. ST has also been shown to repress the c-fos promoters, although this activity was mapped to the region common to the ST and large T antigens (44).
PP2A dephosphorylates the AF-1 region of AR.
When ST mediates the PP2A transfer onto AR, five of the six phosphoserines in the N-terminal AF-1 region are dephosphorylated. Four of these serines (Ser81, Ser256, Ser308, and Ser424) are phosphorylated in response to androgen binding and in response to compounds that activate protein kinase A (PKA) and PKC signaling (13). The fifth serine (Ser94) is constitutively phosphorylated and changes little upon treatment with the aforementioned reagents. The correlation between PP2A loading, the repression of AR-dependent transcription, and dephosphorylation suggests that a kinase-phosphatase cycle regulates AR activity. Phosphorylation can increase or decrease NR sensitivity to cognate ligands, and phosphorylation can enhance or suppress DNA binding (35). Other steps that may be affected include turnover and cofactor interactions that facilitate preinitiation complex assembly or disassembly.
It is not clear why Ser650 in the hinge region of AR is constitutively phosphorylated in the presence of ST. The phosphorylation of AR Ser650 in the presence of ST may be mediated through PKA, given that compounds that elevate PKA signaling enhance phosphorylation at this site (13). The sequence context (Ser-Pro) raises the possibility that AR Ser650 is phosphorylated by a proline-directed kinase such as one of the MAP kinases, which are known to be hyperactive in the presence of ST (39).
ST and cancer models.
Although human diploid fibroblasts can be immortalized by coexpression of the catalytic subunit of telomerase, oncogenic Ras, and the large T antigen, tumorigenesis requires the coexpression of ST (16, 48). ST has also been shown to play an important role in the tumorigenesis of genetically manipulated prostate cells in mice. Greenberg and coworkers used a prostate-specific promoter to drive the expression of large T and ST in a mouse model termed TRAMP (for transgenic adenocarcinoma mouse prostate). Prostates from TRAMP mice develop prostatic intraepithelial neoplasia and focal adenocarcinoma with 100% frequency, and metastases are observed in bones, lymph nodes, and lungs (12). Matusik and coworkers engineered a related mouse model in which only the large T antigen is expressed (20). Prostates from the large T antigen model develop prostatic intraepithelial neoplasia and focal adenocarcinoma, but unlike the case for TRAMP mice, metastasis to organs does not occur. These data suggest that within the TRAMP model, ST endows prostate cells with a gain-of-function property that is necessary for metastasis or that ST represses a function that usually keeps metastasis in check. Our data for cultured cells point to AR as a potential target of ST in TRAMP mice. The underlying mechanism of ST-dependent metastasis, however, is likely to be complex, given its capacity to modulate gene expression and signal transduction pathways. Thus, it will be important to determine if ST affects AR-dependent gene expression in the TRAMP model and whether this includes genes that are relevant to metastasis. The growing body of evidence showing an association between SV40 and other papovaviruses and human malignancies (9) and the recent demonstration that the human papovavirus BK virus is detected in neoplastic prostate tissues (7) raise the possibility that the BK virus ST may influence AR function in a fashion similar to that described here.
In summary, we identified a novel protein transfer mechanism that is strictly dependent on allosteric complementarity. We showed that ST induces changes in the A subunit of the PP2A heterodimer, which we propose creates structural complementarity to the agonist-induced conformation of AR. The ability of ST to facilitate PP2A transfer onto a transcription factor uncovers a new ST-dependent mechanism for influencing gene expression through dephosphorylation, with potential relevance to tumorigenesis.
Acknowledgments
We thank Dean Edwards and Michael Imperiale for their kind gifts of antibodies. We also thank Wade Gibson, Peggy Shupnik, and Myles Brown for plasmids, Adam Spencer for technical assistance, and Aninyda Dutta for reading the manuscript.
These studies were supported by the NIH.
REFERENCES
- 1.Bakin, R. E., D. Gioeli, R. A. Sikes, E. A. Bissonette, and M. J. Weber. 2003. Constitutive activation of the Ras/mitogen activated protein kinase signaling pathway promotes androgen hypersensitivity in LNCaP prostate epithelial cells. Cancer Res. 63:1981-1989. [PubMed] [Google Scholar]
- 2.Black, B. E., and B. M. Paschal. 2004. Intranuclear organization and function of the androgen receptor. Trends Endocrinol. Metab. 15:411-417. [DOI] [PubMed] [Google Scholar]
- 3.Black, B. E., M. J. Vitto, D. Gioeli, A. Spencer, N. Afshar, M. R. Conaway, M. J. Weber, and B. M. Paschal. 2004. Transient, ligand-dependent arrest of the androgen receptor in subnuclear foci alters phosphorylation and coactivator interactions. Mol. Endocrinol. 18:834-850. [DOI] [PubMed] [Google Scholar]
- 4.Chen, W., R. Possemato, K. T. Campbell, C. A. Plattner, D. C. Pallas, and W. C. Hahn. 2004. Identification of specific PP2A complexes involved in human cell transformation. Cancer Cell 5:127-136. [DOI] [PubMed] [Google Scholar]
- 5.Craft, N., Y. Shostak, M. Carey, and C. L. Sawyers. 1999. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat. Med. 5:280-285. [DOI] [PubMed] [Google Scholar]
- 6.Culig, Z., A. Hobisch, M. V. Cronauer, C. Radmayr, J. Trapman, A. Hittmair, G. Bartsch, and H. Klocker. 1994. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 54:5474-5478. [PubMed] [Google Scholar]
- 7.Das, D., R. B. Shah, and M. J. Imperiale. 2004. Detection and expression of human BK virus sequences in neoplastic prostate tissues. Oncogene 23:7031-7046. [DOI] [PubMed] [Google Scholar]
- 8.Defranco, D. B., A. P. Madan, Y. Tang, U. R. Chandran, N. Xiao, and J. Yang. 1995. Nucleocytoplasmic shuttling of steroid receptors. Vitam. Horm. 51:315-338. [DOI] [PubMed] [Google Scholar]
- 9.Gazdar, A. F., J. S. Butel, and M. S. Carbone. 2002. SV40 and human tumours: myth, association or reality? Nat. Rev. Cancer 2:957-964. [DOI] [PubMed] [Google Scholar]
- 10.Gelmann, E. P. 2002. Molecular biology of the androgen receptor. J. Clin. Oncol. 20:3001-3015. [DOI] [PubMed] [Google Scholar]
- 11.Genevaux, P., F. Lang, F. Schwager, J. V. Vartikar, K. Rundell, J. M. Pipas, C. Georgopoulos, and W. L. Kelley. 2003. Simian virus 40 T antigens and J domains: analysis of Hsp40 cochaperone functions in Escherichia coli. J. Virol. 77:10706-10713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gingrich, J. R., R. J. Barrios, M. W. Kattan, H. S. Nahm, M. J. Finegold, and N. M. Greenberg. 1997. Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 57:4687-4691. [PubMed] [Google Scholar]
- 13.Gioeli, D., S. B. Ficarro, J. J. Kwiek, D. Aaronson, M. Hancock, A. D. Catling, F. M. White, R. E. Christian, R. E. Settlage, J. Shabanowitz, D. F. Hunt, and M. J. Weber. 2002. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J. Biol. Chem. 277:29304-29314. [DOI] [PubMed] [Google Scholar]
- 14.Glass, C. K., and M. G. Rosenfeld. 2000. The coregulator exchange in transcriptional functions of nuclear receptors. Genes Dev. 14:121-141. [PubMed] [Google Scholar]
- 15.Groves, M. R., N. Hanlon, P. Turowski, B. A. Hemmings, and D. Barford. 1999. The structure of the protein phosphatase 2A PR65/A subunit reveals the conformation of its 15 tandemly repeated HEAT motifs. Cell 96:99-110. [DOI] [PubMed] [Google Scholar]
- 16.Hahn, W. C., S. K. Dessain, M. W. Brooks, J. E. King, B. Elenbaas, D. M. Sabatini, J. A. DeCaprio, and R. A. Weinberg. 2002. Enumeration of the simian virus 40 early region elements necessary for human cell transformation. Mol. Cell. Biol. 22:2111-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He, B., J. A. Kemppainen, and E. M. Wilson. 2000. FXXLF and WXXLF sequences mediate the NH2-terminal interaction with the ligand binding domain of the androgen receptor. J. Biol. Chem. 275:22986-22994. [DOI] [PubMed] [Google Scholar]
- 18.Huang, H., and D. J. Tindall. 2002. The role of the androgen receptor in prostate cancer. Crit. Rev. Eukaryot. Gene Expr. 12:193-207. [DOI] [PubMed] [Google Scholar]
- 19.Janssens, V., and J. Goris. 2001. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem. J. 353:417-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kasper, S., P. C. Sheppard, Y. Yan, N. Pettigrew, A. D. Borowsky, G. S. Prins, J. G. Dodd, M. L. Duckworth, and R. J. Matusik. 1998. Development, progression, and androgen-dependence of prostate tumors in probasin-large T antigen transgenic mice: a model for prostate cancer. Lab. Investig. 78:319-333. [PubMed] [Google Scholar]
- 21.Kelley, W. L. 1998. The J-domain family and the recruitment of chaperone power. Trends Biochem. Sci. 23:222-227. [DOI] [PubMed] [Google Scholar]
- 22.Lange, C. A. 2004. Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol. Endocrinol. 18:269-278. [DOI] [PubMed] [Google Scholar]
- 23.Lin, H. K., S. Yeh, H. Y. Kang, and C. Chang. 2001. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc. Natl. Acad. Sci. USA 98:7200-7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKenna, N. J., and B. W. O'Malley. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465-474. [DOI] [PubMed] [Google Scholar]
- 25.Metivier, R., G. Penot, M. R. Hubner, G. Reid, H. Brand, M. Kos, and F. Gannon. 2003. Estrogen receptor-alpha directs ordered, cyclical, and combinatorial recruitment of cofactors on a natural target promoter. Cell 115:751-763. [DOI] [PubMed] [Google Scholar]
- 26.Mungre, S., K. Enderle, B. Turk, A. Porras, Y. Q. Wu, M. C. Mumby, and K. Rundell. 1994. Mutations which affect the inhibition of protein phosphatase 2A by simian virus 40 small-t antigen in vitro decrease viral transformation. J. Virol. 68:1675-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murata, K., J. Wu, and D. L. Brautigan. 1997. B cell receptor-associated protein alpha4 displays rapamycin-sensitive binding directly to the catalytic subunit of protein phosphatase 2A. Proc. Natl. Acad. Sci. USA 94:10624-10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nazareth, L. V., D. L. Stenoien, W. E. Bingman III, A. J. James, C. Wu, Y. Zhang, D. P. Edwards, M. Mancini, M. Marcelli, D. J. Lamb, and N. L. Weigel. 1999. A C619Y mutation in the human androgen receptor causes inactivation and mislocalization of the receptor with concomitant sequestration of SRC-1 (steroid receptor coactivator 1). Mol. Endocrinol. 13:2065-2075. [DOI] [PubMed] [Google Scholar]
- 29.Pallas, D. C., L. K. Shahrik, B. L. Martin, S. Jaspers, T. B. Miller, D. L. Brautigan, and T. M. Roberts. 1990. Polyoma small and middle T antigens and SV40 small t antigen form stable complexes with protein phosphatase 2A. Cell 60:167-176. [DOI] [PubMed] [Google Scholar]
- 30.Porras, A., J. Bennett, A. Howe, K. Tokos, N. Bouck, B. Henglein, S. Sathyamangalam, B. Thimmapaya, and K. Rundell. 1996. A novel simian virus 40 early-region domain mediates transactivation of the cyclin A promoter by small-t antigen and is required for transformation in small-t antigen-dependent assays. J. Virol. 70:6902-6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pratt, W. B., M. D. Galigniana, Y. Morishima, and P. J. Murphy. 2004. Role of molecular chaperones in steroid receptor action, p. 41-58. In I. J. McEwan (ed.), The nuclear receptor superfamily, vol. 40. Portland Press, London, United Kingdom. [DOI] [PubMed]
- 32.Ruediger, R., M. Hentz, J. Fait, M. Mumby, and G. Walter. 1994. Molecular model of the A subunit of protein phosphatase 2A: interaction with other subunits and tumor antigens. J. Virol. 68:123-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruediger, R., D. Roeckel, J. Fait, A. Bergqvist, G. Magnusson, and G. Walter. 1992. Identification of binding sites on the regulatory A subunit of protein phosphatase 2A for the catalytic subunit and for tumor antigens of simian virus 40 and polyomavirus. Mol. Cell. Biol. 12:4872-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sack, J. S., K. F. Kish, C. Wang, R. M. Attar, S. E. Kiefer, Y. An, G. Y. Wu, J. E. Scheffler, M. E. Salvati, S. R. Krystek, Jr., R. Weinmann, and H. M. Einspahr. 2001. Crystallographic structures of the ligand-binding domains of the androgen receptor and its T877A mutant complexed with the natural agonist dihydrotestosterone. Proc. Natl. Acad. Sci. USA 98:4904-4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shao, D., and M. A. Lazar. 1999. Modulating nuclear receptor function: may the phos be with you. J. Clin. Investig. 103:1617-1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Simental, J. A., M. Sar, M. V. Lane, F. S. French, and E. M. Wilson. 1991. Transcriptional activation and nuclear targeting signals of the human androgen receptor. J. Biol. Chem. 266:510-518. [PubMed] [Google Scholar]
- 37.Sola, M. M., T. Langan, and P. Cohen. 1991. p34cdc2 phosphorylation sites in histone H1 are dephosphorylated by protein phosphatase 2A1. Biochim. Biophys. Acta 1094:211-216. [DOI] [PubMed] [Google Scholar]
- 38.Sontag, E. 2001. Protein phosphatase 2A: the Trojan horse of cellular signaling. Cell. Signal. 13:7-16. [DOI] [PubMed] [Google Scholar]
- 39.Sontag, E., S. Federov, C. Kamibayashi, D. Robbins, M. Cobb, and M. C. Mumby. 1993. The interaction of SV40 small tumor antigen with protein phosphatase 2A stimulates the Map kinase pathway and induces cell proliferation. Cell 75:887-897. [DOI] [PubMed] [Google Scholar]
- 40.Sontag, E., V. Nunbhakdi-Crai, G. S. Bloom, and M. C. Mumby. 1995. A novel pool of protein phosphatase 2A is associated with microtubules and is regulated during the cell cycle. J. Cell Biol. 128:1131-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Srinivasan, A., A. J. McClellan, J. Vartikar, I. Marks, P. Cantalupo, Y. Li, P. Whyte, K. Rundell, J. L. Brodsky, and J. M. Pipas. 1997. The amino-terminal transforming region of simian virus 40 large T and small t antigens functions as a J domain. Mol. Cell. Biol. 17:4761-4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strack, S., J. A. Zaucha, F. F. Ebner, R. J. Colbran, and B. E. Wadzinski. 1998. Brain protein phosphatase 2A: developmental regulation and distinct cellular and subcellular localization by B subunits. J. Comp. Neurol. 392:515-527. [PubMed] [Google Scholar]
- 43.Turk, B., A. Porras, M. C. Mumby, and K. Rundell. 1993. The simian virus 40 small-t antigen binds two Zn ions. J. Virol. 67:3671-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, W. B., I. Bikel, E. Marsilio, D. Newsome, and D. M. Livingston. 1994. Transrepression of RNA polymerase II promoters by the simian virus 40 small t antigen. J. Virol. 68:6180-6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Watanabe, G., A. Howe, R. J. Lee, C. Albanese, I. W. Shu, A. N. Karnezis, L. Zon, J. Kyriakis, K. Rundell, and R. G. Pestell. 1996. Induction of cyclin D1 by simian virus 40 small tumor antigen. Proc. Natl. Acad. Sci. USA 93:12861-12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weigel, N. L., and Y. Zhang. 1998. Ligand-independent activation of steroid hormone receptors. J. Mol. Med. 76:469-479. [DOI] [PubMed] [Google Scholar]
- 47.Yang, S. I., R. L. Lickteig, R. Estes, K. Rundell, G. Walter, and M. C. Mumby. 1991. Control of protein phosphatase 2A by simian virus 40 small-t antigen. Mol. Cell. Biol. 11:1988-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yu, J., A. Boyapati, and K. Rundell. 2001. Critical role for SV40 small-t antigen in human cell transformation. Virology 290:192-198. [DOI] [PubMed] [Google Scholar]
- 49.Yuan, H., T. Veldman, K. Rundell, and R. Schlegel. 2002. Simian virus 40 small tumor antigen activates AKT and telomerase and induces anchorage-independent growth of human epithelial cells. J. Virol. 76:10685-10691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhou, Z. X., J. A. Kemppainen, and E. M. Wilson. 1995. Identification of three proline-directed phosphorylation sites in the human androgen receptor. Mol. Endocrinol. 9:605-615. [DOI] [PubMed] [Google Scholar]