Abstract
History suggests β agonists, the cognate ligand of the β2adrenoceptor, have been used as bronchodilators for around 5000 years, and β agonists remain today the frontline treatment for asthma and Chronic Obstructive Pulmonary Disease (COPD). The β agonists used clinically today are the products of significant expenditure and over a hundred year's intensive research aimed at minimising side effects and enhancing therapeutic usefulness. The respiratory physician now has a therapeutic toolbox of long acting β agonists to prophylactically manage bronchoconstriction, and short acting β agonists to relieve acute exacerbations. Despite constituting the cornerstone of asthma and COPD therapy, these drugs are not perfect; significant safety issues have led to a black box warning advising that long acting β agonists should not be used alone in patients with asthma. In addition there are a significant proportion of patients whose asthma remains uncontrolled. In this chapter we discuss the evolution of β agonist use and how the understanding of β agonist actions on their principal target tissue, airway smooth muscle, has led to greater understanding of how these drugs can be further modified and improved in the future. Research into the genetics of the β2adrenoceptor will also be discussed, as will the implications of individual DNA profiles on the clinical outcomes of β agonist use (pharmacogenetics). Finally we comment on what the future may hold for the use of β agonists in respiratory disease.
Introduction
β agonists constitute the frontline treatment for both asthma and COPD. They exert their bronchodilatory effects via β2 adrenoceptors (β2ARs) located on airway smooth muscle (ASM) cells. Activation of these receptors results in ASM and thus airway relaxation via the molecular processes outlined later in the “Mechanisms of Action” section of this chapter and also shown in figure 1. In addition to the receptors expressed on ASM cells, β2ARs are also found on a number of other cell types within the lungs including epithelial cells, submucosal glands, vascular endothelium, vascular smooth muscle and inflammatory cells including mast cells, macrophages and eosinophils (Barnes, 2004).
Figure 1. The classic β2AR signalling pathway.
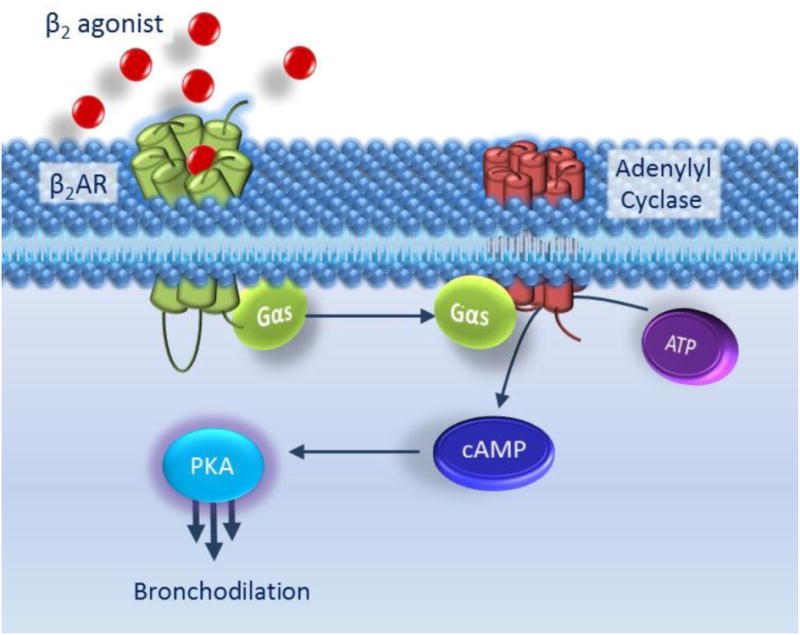
Binding of β2-agonist to β2AR induces a conformational change allowing the α-subunit of the G-protein to dissociate and bind to adenylyl cyclase. Adenylyl cyclase is thus activated and catalyses the formation of cyclic AMP (cAMP) from ATP. cAMP molecules bind to PKA which induces the dissociation of the catalytic and regulatory subunitsfrom each other. Once released, the PKA catalytic subunits phosphorylate and hence activate myriad cellular targets which results in airway smooth muscle relaxation and hence bronchodilation.
The β2AR is a member of the G-protein coupled receptor (GPCR) family and was in fact the first GPCR to be cloned (Dixon et al., 1986). In common with all GPCRs, it is composed of seven transmembrane spanning domains and has an intracellular C-terminus and an extracellular N-terminus. GPCRs have long been overrepresented as targets for drug therapy with an estimated 30-50% of medicines acting via GPCRs either directly or indirectly (Garland, 2013). Both a historical and ongoing challenge in all drug development is of course ensuring selectivity of beneficial over unwanted effects. The β-agonist story is no different and many of the major side effects related to these drugs are due to cross-activation of β1ARS, with activation of these leading to anxiety, tachycardia, tremor and sweating. As we will describe later, there is increasing awareness of not only the selectivity for the β2AR but within β2AR-mediated signalling pathways the need to select for “good” vs “bad” effects. There is a third βAR subtype, the β3AR, however these are located predominantly in adipose tissue and do not contribute to the adverse effects profile.
The history of β agonist use in respiratory disease is a fascinating one and the reader is directed to a number of excellent reviews and books for further reading (Barnes, 2006, Chu and Drazen, 2005, Jackson, 2009). It is thought that β agonist-mediated airway relaxation was first used around 5000 years ago in Chinese medicine when the ephedrine-containing plant ma huang was used to alleviate respiratory conditions. Ephedrine activates the β2AR pathway indirectly via heightening activity of noradrenaline at βARs. However, in western medicine it was not until the early twentieth century that ephedrine-mediated bronchodilatory effects were described (Melland 1910 and as reviewed by Chu and Drazen(Chu and Drazen, 2005)). Throughout the twentieth century further research and enlightenment led to increased use of β agonists in respiratory disease particularly following the introduction of the first pure (but nonselective between β1AR and β2ARs) β agonist, isoprenaline (isoproterenol in the US), in the 1940s. Isoprenaline became the most commonly used inhaled treatment for asthma in the next twenty years. Indeed, in just the ten years following its availability as a metered dose inhaler in 1956, usage increased fourfold (Jackson, 2009). However in the 1960s an epidemic of deaths across six countries, likely due to usage of a higher dose form of isoprenaline led to the realisation that more refined therapies were required. The first β2AR-selective agonist, salbutamol, was synthesised in 1968 by a team at Glaxo and, in addition to reduction of the side effects associated with non-selective β agonist, isoprenaline, it was also superior in terms of duration of effect (Brittain et al., 1968, Cullum et al., 1969). However, it still remains relatively short acting, and hence is considered a short acting beta agonist or SABA. The same team at Glaxo proceeded to further modify salbutamol producing salmeterol, capable of exerting its bronchodilatory effect for up to 12 hours: this class of drugs was called long acting beta agonists, or LABAs for short. The subsequently developed LABA formoterol was also shown to produce effects for 12h and more recently the discovery of even longer acting β2AR agonists such as indacaterol, has allowed for once daily dosing: these agents have therefore been called ultra LABAs. Other drugs in these classes are discussed further below.
Whilst these longer acting β2 agonists constitute the cornerstone of treatment for people with asthma and COPD, the manner in which their use is recommended differs dramatically. In 2011 the US Food and Drug Administration (FDA) published a warning that, when treating asthma, long acting β2 agonists (LABAs) must only be used in combination with an Inhaled Cortico Steroid (ICS). However for COPD, in at least some countries, LABA monotherapy remains an option for frontline treatment. In addition the ultra-long-acting β agonist indacaterol is only indicated for COPD and not asthma at present. In the next section we will explore the different classes of β agonists available and consider their current clinical use.
Clinical classification of β agonists
As discussed above, β agonists are grouped into three classes namely Short-Acting β Agonists (SABAs), Long-Acting β Agonists (LABAs) and Ultra-Long Acting β Agonists (ultra-LABAs). Table 1 lists the β agonists used clinically. As suggested by the names SABAs have short half-lives and are used as rapid relievers whereas LABAs and ultra-LABAs provide sustained symptomatic relief due to their longer duration of action. As noted above, LABA monotherapy for asthma is contraindicated due to safety concerns. The prolonged duration of action of the LABAs currently used in clinical practice is not thought to be due to a difference in receptor kinetics but rather retention within the cell membrane and hence a continued presence of the drug near to the receptor.
Table One.
Clinically used β2 agonists with their respective times of onset of action, duration of effects, dosing regimen and specificity at the β2 AR (β2/β1). Doses given are for inhalation. Adapted from (Tamm et al., 2012, Baker, 2010, BNF)
| Onset of action | Duration of Effect | Therapeutic Use | Specificity at β2 AR (β2/β1) | |
|---|---|---|---|---|
| SABAs | ||||
| Salbutamol | <5min | 3-6h | 100-200μgAs required (up to 4 times per day) | 27 |
| Terbutaline | <5min | 4-6h | 500μg As required (up to 4 times per day) | 63 |
| LABAs | ||||
| Salmeterol | ∼15min | 12h | 50-100μg Twice daily | 3000 |
| Formoterol | ∼7min | 12h | 12-24μg Twice daily | 150 |
| Olodaterol | ∼5min | 12h | 5μg Once daily | 65 |
| Vilanterol | ∼5min | 12h | 55μg Once daily | 2400 |
| Ultra-LABA | ||||
| Indacaterol | ∼5min | 24h | 150-300μg Once daily | 16 |
SABAs
SABAs (e.g. salbutamol) delivered via metered dose or dry powder inhalers provide almost instant symptomatic relief and are the frontline therapy in asthma to combat bronchoconstriction and acute exacerbations. Their bronchoprotective effect is evident in minutes and remains for 4-6 hours. These drugs are also available for oral administration in some countries however this method is of less therapeutic value with the patient being more prone to systemic side effects and it is thus rarely employed. SABAs are recommended to be used only on an ‘as required’ basis rather than a regular basis in asthma and an escalation of use should prompt clinicians to review patient management. British Thoracic Society (BTS) guidelines currently recommend the use of inhaled SABAs ‘asrequired’ for mild intermittent asthma in adults.
Similar to asthma, for COPD SABAs are recommended as the initial treatment for the relief of breathlessness and exercise limitation (NICE clinical guidelines). As would be predicted in a disease where reversibility is by definition limited, the efficacy in COPD of these agents is less than in asthma.
LABAs
BTS guidelines for adults with asthma recommend an inhaled LABA as the initial add-on therapy in patients already taking a regular inhaled steroid but with inadequate control of disease. Monotherapy with a LABA in asthma is contraindicated. This is due both to LABA monotherapy proving less clinically effective than treatment with ICS but mainly due to the safety issues highlighted in the “Adverse Effects” section of this chapter. If asthma is still persistently poorly controlled even following increased dose of steroids, further add on drugs are advised to be trialled including leukotriene receptor antagonists, slow release theophylline or antimuscarinic agents including the new long acting muscarinic antagonists (LAMAs) such as tiotropium.
LABAs are a frontline treatment for COPD. They are recommended to be offered as maintenance therapy either alone (if FEV1≥50% predicted) or certainly in the UK, more commonly in combination with an ICS (if FEV1<50% predicted) (NICE guidelines). In people with stable COPD and an FEV1≥50% who remain breathless or have exacerbations despite maintenance therapy with a LABA a combination inhaler comprising a LABA and ICS is also recommended. Long acting muscarinic antagonists (LAMAs) can be used interchangeably with LABAs depending on the patients' symptomatic response and preference in addition to the drug's potential to reduce exacerbations, its side effects and cost (NICE guidelines).
Ultra-LABAs
The ultra-LABAindacaterol was given approval by the European Medicines Agency (EMA) in 2009 and by the FDA in 2011 for the maintenance treatment of patients with COPD. Indacaterol is delivered by inhalation as a dry powder and has a fast onset of action due to its rapid absorption. In December 2014 a combination ultra-LABA/LAMA (indacaterol/glycopyrronium bromide) was launched in the UK, also indicated for maintenance bronchodilator treatments for patients with COPD. Also available in other European countries, authorisation of this product in the US is ongoing. No specific NICE guidance currently exists surrounding use of indacaterol as mono- or combination therapy in COPD. Indacaterol has not yet been approved for use in the treatment of asthma however clinical trials to ascertain its suitability for asthma therapy are ongoing.
Mechanisms of Action
The β2AR is the most exhaustively studied GPCR, with respect to both its signalling and its regulation, and is therefore frequently referred to as the “prototypical GPCR.” Although for a time there appeared to be a consensus as to what constituted “canonical” β2AR signalling, recent studies identify a complexity of β2AR signalling that portends a new era of research in β2AR biology and pharmacology.
Results from studies involving numerous cell and cell–free systems have contributed to the description of canonical β2AR (Fig. 1), which also serves as an example of prototypical heterotrimeric G protein signalling. Early studies by Gilman, Lefkowitz, Birnbaum, Bourne, Perkins, and others (reviewed in (Penn and Benovic, 1998)) characterize transmembrane signalling involving GPCR (β2AR), heterotrimeric G protein (Gs for the β2AR) and an effector (adenylyl cyclase downstream of β2AR-Gs). Adenylyl cyclase mediates the hydrolysis of ATP into cAMP, which in turn activates the cAMP-dependent protein kinase (aka PKA (Protein kinase A)). PKA is the first discovered cAMP effector, and has been shown to phosphorylate numerous intracellular substrates to effect various functions in a cell type-dependent manner. This classical signalling paradigm resulting in PKA activation was presumed to be the predominant pathway stimulated by β-agonists in all cell types; the functional consequences of activation of this pathway depended on the specific PKA substrates expressed in a cell and whatever downstream signalling/targets these substrates regulate. Thus, functional diversity of β-agonist signalling was thought to be determined by the stoichiometry of signalling elements, and signalling targets, in a given cell. For example, in airway smooth muscle (ASM) important PKA substrates include various Gq-coupled receptors, Gq, phospholipase C, myosin light chain kinase (MLCK), IP3 receptors, K Ca channels, heat shock protein 20, and phosphorylation of each is believed to antagonize pro-contractile Gq-coupled receptor signalling or directly inhibit mechanisms important to ASM contraction. In addition, phosphorylation of the MAP kinase kinase kinase Raf-1 as well as the transcription factor CREB inhibits mitogenic signalling and pro-mitogenic gene induction in many mesenchymal cell types to regulate cell growth (for an extensive discussion of β2AR signalling and regulation in ASM cells, the reader is referred to (Billington and Penn, 2003, Penn, 2008, Walker et al., 2011a)).
Although some instances of cAMP-independent β2AR signalling had been identified (Kume et al., 1994), cAMP-dependent PKA actions were for years presumed to mediate most of the functional consequences of β2AR activation. However, in 1998 the Bos laboratory discovered the cAMP effector Epac, which was able to activate the small GTPase Rap1 in the presence of PKA inhibition (Kawasaki et al., 1998). Epac1 and Epac2 were determined to be GTPase-activating proteins of Rap1, and subsequent studies identified various cAMP-dependent/PKA-independent functions, attributable to Epac, in various cells (reviewed in (Roscioni et al., 2008)).
The discovery of Epac has led to questioning of the widely held assumption that β2AR actions in a given cell type are entirely PKA-dependent. In many instances, the functional consequences of β-agonists or other agonists of Gs-coupled receptors in a given cell have been dogmatically ascribed to PKA signalling, despite the lack of any direct evidence in such cells. This lack of direct evidence stems from difficulties in selectively inhibiting PKA in intact cells or tissue; all existing small molecular inhibitors lack specificity, and genetic ablation of catalytic PKA is lethal (reviewed in (Morgan et al., 2014, Penn et al., 1999)). Consequently, a role for PKA was often asserted when agents classically known to induce intracellular cAMP (e.g., Gs-coupled receptor agonists, or forskolin (which activates adenylyl cyclase downstream of GPCRs)) to activate PKA could generate a similar functional effect to the agent/receptor in question.
This logic, and not direct evidence, was for years employed to assert a role for PKA in mediating bronchorelaxation and growth inhibition of ASM. In 2011 Zieba et al. (Zieba et al., 2011) demonstrated that Epac-selective cAMP analogues could relax contracted smooth muscle, including ASM, presumably via inhibition of RhoA activity. Moreover, in rat aortic smooth cells, β-agonist induced Rap1 activity that could be inhibited by Epac1 knockdown. This study raised the intriguing possibly that Epac is a novel therapeutic target for obstructive lung diseases, and questioned long held beliefs regarding the mechanisms of bronchodilatory actions of β-agonists. However, a recent study by Morgan et al. (Morgan et al., 2014), employing molecular means of selective PKA inhibition demonstrated a clear, dominant role of PKA in mediating the relaxant effect of β-agonists in both cell- and tissue- based models of ASM contraction. This study, in addition to a prior study ascribing the anti-mitogenic effect of Gs-coupled receptors in ASM to PKA(Yan et al., 2011), suggests that indeed PKA is the main effector through which β-agonist promotes its therapeutic actions on ASM, yet leaves open the possibility that Epac targeting has therapeutic utility.
To further complicate the story of β2AR signalling, an increasing body of evidence accumulated over the last 15+ years demonstrates the ability of the β2AR to signal independent of G protein activation. Most of these studies (recently reviewed in (Kenakin, 2011, Reiter et al., 2012, Walker et al., 2011a)) have focused on the ability of arrestin proteins to function as a scaffold capable of coordinating signalling complexes and to initiate signaling events often distinct, and sometimes antithetical, to those mediated by G proteins (see Figure 2). Arrestins were originally discovered as GPCR-interacting proteins that function to both uncouple GPCRs from G proteins and to mediate GPCR internalization (for recycling or lysosomal degradation)(Kang et al., 2014, Shenoy and Lefkowitz, 2011). Prompted by the discovery of arrestin-dependent signalling, investigation into qualitative signaling or biased agonism has exploded based on the underlying assumption that G protein-dependent and –independent signalling events can be linked with distinct functional outcomes, thus allowing a great range of function diversity (and possibly greater therapeutic utility) among GPCR ligands.
Figure 2. The pros and cons of β2AR activation.
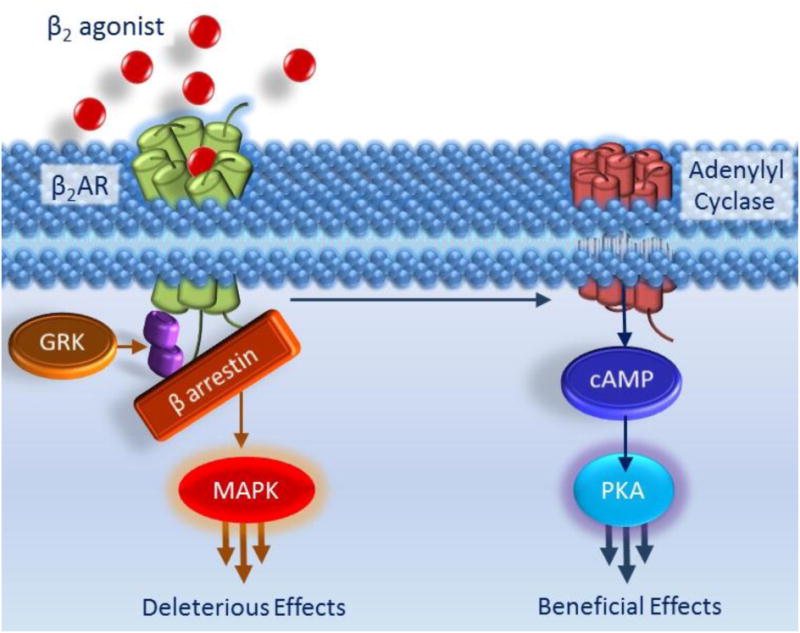
As shown in Figure 1, activation of the β2AR induces bronchorelaxation (i.e. a beneficial effect) via its activation of the adenylyl cyclase-cAMP-PKA pathway. In this Figure, parallel deleterious effects are highlighted whereby the activation of β2ARs are controlled by βarrestins. As shown in the left hand side pathway in this figure, following exposure to β2 agonists, β2ARs are phosphorylated by G Protein Coupled Receptor Kinases (GRKs) and rapidly desensitized meaning that regardless of continued β2 agonist presence, cAMP production is diminished. GRK regulates β2AR activity in part by uncoupling the β2AR from the Gα subunit of the G protein but also by promoting the binding of βarrestin molecules to the β2AR. βarrestin physically blocks further β2AR and Gα subunit interaction and hence further prevents the beneficial, pro-relaxant signalling pathway shown in figure 1 and also, in shortened form, on the right hand side of this diagram. However, the GRK- and βarrestin-mediated effects on cAMP signalling are not the only deleterious effects. βarrestin acts as a scaffold protein bringing together other molecules and initiating signalling via pathways not involving G-proteins for example the MAPK pathway.
Although the relevance of qualitative β2AR signalling in airway biology and disease is unknown, studies of arrestin and PKA function in the airway have led to speculation that G protein/PKA-dependent signalling is therapeutic to inflammatory lung disease such as asthma, whereas arrestin-dependent signalling may be pathological (Penn et al., 2014, Walker et al., 2011a). As noted above, β-agonist-stimulated Gs/PKA signalling in ASM mediates bronchorelaxation through actions on ASM. Early studies by Walker et al demonstrated that β-arrestin2 gene ablation inhibited the development of allergic inflammation in a mouse model (Walker et al., 2003). More recent studies by Bond and colleagues note that “antagonism ” of the β2AR, in the form of either certain β-blockers (Callaerts-Vegh et al., 2004), β2AR gene ablation (Nguyen et al., 2009), or depletion of systemic epinephrine (Thanawala et al., 2013), also thwarted the development of the asthma phenotype (airway inflammation, mucous production, and airway hyperresponsiveness) in mice sensitized and challenged with allergen. Interestingly, the unbiased β-blocker nadolol (which functions as an inverse agonist for both G protein- and arrestin-dependent signaling (Stallaert et al., 2012, Wisler et al., 2007)) was more effective than either (arrestin-activating but G protein-inhibiting) carvedilol or alprenonol, suggesting that all “β-blockers” are not equal, and that their functional effects may be linked to their signalling bias. Interestingly, restoration of systemic β-agonist in (epinephrine-depleted mice) by infusion of formoterol (capable of both G protein and arrestin signalling) fully restored the asthma phenotype in allergen challenged mice (Thanawala et al., 2013). Finally, it is interesting to note that in a pilot study, human asthmatics treated with nadolol exhibited a decrease in airway hyperreactivity in the medium term (Hanania et al., 2007), although in a separate study asthmatics treated with propranol (a β-blocker capable of promoting arrestin signalling) showed no clinical improvement (Short et al., 2013).
Adverse Effects
Despite being used extensively for the treatment of asthma for over half a century, β-agonists have an almost equally long history of adverse effects. Although the reasons for such adverse effects are multiple, with some still unknown, the majority of adverse effects can be attributed to either: 1) a lack of selectivity for the β2AR, resulting in “off-target” effects mediated by either alpha or β1 adrenoceptors; or 2) ill-defined β2AR-mediated effects that appear to involve either β2AR desensitization or exacerbation of airway inflammation and its consequences. Although a thorough discussion of adverse effects associated with β-agonist use is beyond the scope of this review, we will summarize below the current consensus beliefs.
α1AR agonism promotes several adverse effects of therapeutic relevance
Whereas numerous side effects including tachychardia, arrhythmia, tremor, and headache, occurred with the early therapeutic use of nonselective β-agonists such as adrenaline (activating both α and β adrenoceptors) and isoprenaline (activating both β1 and β2ARs), receptor subtype discrimination enabled by the landmark studies of Ahlquist (1948) and Lands et al. (1967) (Ahlquist, 1948, Lands et al., 1967) ultimately resulted in the development of the β2AR -selective salbutamol and terbutaline. Waldeck (Waldeck, 2002) provides an elegant history of the discovery and clinical application of bronchodilatoryβ-agonists and the work that facilitated increasing β2AR subtype selectivity. And although it should be recognized that essentially all currently used SABAs and LABAs used to treat asthma are at least to some extent selective for the β2ARs, there is considerable variability in the degree of selectivity (see Table 1). In addition the sensitivity of patients to experience cardiovascular side effects with drug usage will also depend on individual characteristics including the presence of absence of significant co-morbities such as ischaemic heart disease.
Safety concerns over β agonist use in asthma prompted by mortality and morbidity data are controversial, and the mechanistic basis for increased mortality/morbidity is poorly understood
Mortality and morbidity are the adverse effects that have dominated the discussion of β-agonist safety for the last several decades. A recent review by Ortega and Peters (Ortega and Peters, 2010) provides an excellent history and analysis of the various “epidemics” associated with use of SABAs and LABAs as asthma drugs. As mentioned above, use of the SABA isoprenaline in several countries was associated with increased adverse events and mortality. Asthma-related mortality increased after the release of the SABA fenoterol in New Zealand in 1976, yet subsequently waned after the drug was removed from the market in 1989. In 1990, one of the first prospective trials of β agonist safety reported that regularly scheduled fenoterol therapy resulted in worse asthma control than did as-needed (rescue) fenoterol (Sears et al., 1990).
The safety of LABAs was also questioned shortly after their introduction, first in the Nationwide Surveillance (SNS) Study, a prospective study which suggested a trend towards asthma-related deaths associated with the use of salmeterol (Castle et al., 1993). These results of the SNS Study appeared to be of sufficient concern to prompt numerous retrospective and prospective studies. The critical study that heightened the debate of LABA safety was SMART (Salmeterol Multicentre Asthma Research Trial), a prospective study of salmeterol initiated by Glaxo Smith Kline in 1996. In 2002 an interim analysis of the data demonstrated a 4.4-fold increase in death in those asthmatics receiving salmeterol compared to those receiving placebo (Nelson et al., 2006). A subsequent meta-analysis by Salpeter et al. analyzing results from 19 randomized placebo-control trials (including SMART) reported significantly increased odds ratios for both in life-threatening exacerbations and asthma-related deaths associated with LABA use (Salpeter et al., 2006).
Hotly debated since the SMART study and the Salpeter meta-analysis has been the interpretation of the statistics, and the relevance of the study design, of SMART in addressing the question of LABA safety in people with asthma. Additional prospective clinical studies of LABA safety (reviewed in Ortega and Peters (Ortega and Peters, 2010)) have resulted in conclusions asserting LABA safety in asthmatics. Regardless of the merits of each side of the debate, the major consequence of the SMART study was to cause the US FDA to question the safety the LABAs and issue a black box warning issued for the then available LABAs (salmeterol and formoterol) in the United States. The FDA recommends that labels incorporate the following:
Use of a LABA alone without use of a long-term asthma control medication, such as an inhaled corticosteroid, is contraindicated (absolutely advised against) in the treatment of asthma.
LABAs should not be used in patients whose asthma is adequately controlled on low or medium dose inhaled corticosteroids.
LABAs should only be used as additional therapy for patients with asthma who are currently taking but are not adequately controlled on a long-term asthma control medication, such as an inhaled corticosteroid.
Once asthma control is achieved and maintained, patients should be assessed at regular intervals and step down therapy should begin (e.g., discontinue LABA), if possible without loss of asthma control, and the patient should continue to be treated with a long-term asthma control medication, such as an inhaled corticosteroid.
Pediatric and adolescent patients who require the addition of a LABA to an inhaled corticosteroid should use a combination product containing both an inhaled corticosteroid and a LABA, to ensure adherence with both medications
Partly as a consequence of these concerns, in most European countries guidelines only recommend use of LABAs in conjunction with co-administration of ICS in patients with asthma.
What is the mechanistic basis for increased mortality and morbidity with β2 agonist use in asthmatics? Several possible explanations have been offered but empirical evidence supporting them is largely lacking. The loss of drug effectiveness due to desensitization of β2AR on ASM has been proposed often (reviewed in (Walker et al., 2011b). Indeed, β2AR desensitization as evidenced by diminished beta-agonist stimulated intracellular signalling and function (relaxation, inhibition of ASM proliferation and pro-inflammatory “synthetic” functions) has been seen to occur with chronic β2 agonist treatment in ASM cell, tissue, and in vivo (mouse) models. Moreover, experimental strategies that inhibit mechanisms of β2AR desensitization (involved GRK(Kong et al., 2008, Deshpande et al., 2014) and arrestin molecules (Penn et al., 2001, Deshpande et al., 2008)) can mitigate β2AR desensitization (signalling and function) in these models. These findings are consistent with a loss of bronchoprotective effect (functional desensitization) with chronic β2 agonist use in humans (Bhagat et al., 1995, Cheung et al., 1992, Grove and Lipworth, 1995, Lipworth et al., 1998). Thus the loss of asthma control associated with chronic β2 agonist use causing β2AR desensitization represents one attractive explanation for poorer control of disease in asthmatics taking β2 agonists.
A more recent explanation has emerged that relates to the qualitative signalling properties of β2ARs mentioned above. β2AR signalling can occur via both G protein-dependent and arrestin (G protein-independent) pathways. Murine studies of allergic lung inflammation implicate a pathologic role for arrestins, and β2AR ligands capable of stimulating arrestin signalling, but not those incapable of stimulating arrestin signalling. Those ligands capable of stimulating arrestin signalling thus appear potentially important in promoting allergen-induced inflammation (including mucous production) and AHR. Circulating epinephrine appears to serve this critical permissive function, and specific β2AR ligands (e.g., nadodol) capable of blocking epinephrine activation of the β2AR while themselves not activating arrestin signalling are effective in blocking the development of the allergen-induced asthma phenotype. Which cell types mediate β2AR- and arrestin- dependent inflammation are unclear, although mucin-producing airway epithelia appear to have an important role (Thanawala et al., 2013, Penn et al., 2014). Whether or not exogenous β2 agonists (e.g. SABAs or LABAs when used therapeutically) exacerbate the facilitary pro-inflammatory effects of endogenous epinephrine is unclear, but based on accumulating evidence (reviewed in (Walker et al., 2011b) it does not appear that current therapeutic β2 agonists have anti-inflammatory effects (despite early assertions to the contrary).
Interestingly, these pathogenic effects of β2AR-mediated arrest insignalling are consistent with an early hypothesis attempting to explain the loss of asthma control and safety concerns associated with LABA use (Nelson, 2006): i.e. that beta-agonist fails to address underlying inflammation but effectively bronchodilates via its direct actions on ASM. Ultimately, the failure to reduce (and indeed perhaps exacerbate) inflammation creates the conditions for life-threatening exacerbations.
Thus, as monotherapy β2 agonists may fall short (and lack safety) for effective asthma control in all but patients with very mild disease when as required SABA usage is acceptable. Whether concomitant treatment with ICS addresses the possible neutral or pro-inflammatory effects of β2 agonists is not clear. Of note, the black box warnings exist for combined (LABA+ICS) therapy as well, suggesting that sufficient evidence of ICS addressing the LABA safety concern does not yet exist.
Pharmacogenetics and the β2AR
Pharmacogenetics is a term referring to the study of genetic factors on efficacy and side effect profiles of drugs. The most common type of genetic variation in the human genome is single nucleotide polymorphisms (SNPs) whereby one nucleotide is different at a given position. The prevalence and functional and clinical significance of SNPs in the β2AR have received exhaustive scrutiny over the last few decades. Nine SNPs have been found within the β2AR gene, although only four result in amino acid substitutions because of redundancy in coding for amino acids. The most studied SNP results in a change at the 16th amino acid after the start codon of the β2AR, Gly16Arg. This was first identified in 1993 by Liggett and colleagues (Reihsaus et al., 1993). Since 1993 there have been extensive efforts to identify all the genetic variation present at the locus of the β2AR gene (ADRB2), resulting in the identification of >50 variants within close proximity to the coding region for the gene. The possible functional effects of most of these variants are unknown, although many are likely to have no functional consequences.
Of the four SNPs within the coding region of the gene which alter the amino acid sequence, one, thevaline to methionine 34 substitution (Val34Met) is very rare and does not appear to alter receptor function. Another rare polymorphism is the threonine to isoleucine 164 mutation (Thr164Ile; allelic frequency around 2%). Very few individuals homozygous for this polymorphism have been identified. However, when recombinant approaches in cell based systems are studied, the Thr164Ile variant produces marked alterations in the in vitro behaviour of the receptor. Cells transfected with this form of the receptor display reduced agonist binding to catechol ligands and also show altered receptor trafficking (Green et al., 1993).
In contrast, the Arg16Gly and another nearby variant, glutamine to glutamate 27 (Glu27Gln) substitutions are common in the Caucasian population; allele frequencies at each locus are between 0.3 and 0.7 (Litonjua et al., 2004). In in vitro studies, Gly 16 homozygosity results in increased receptor downregulation and homozygosity of Glu 27 in reduced receptor downregulation following agonist stimulation compared with the control ‘wildtype’ receptor. Because of linkage disequilibrium between the two SNPs, chromosomes carrying the Gly16 variant are more likely to also have Glu 27 (Dewar et al., 1998, Ramsay et al., 1999).
There have been multiple clinical studies addressing the potential contribution of ADRB2 polymorphism to both disease risk and clinical response to treatment. In general, although some small studies have found associations between SNPs at ADRB2 (most frequently the Arg16Gly and/or the Gln27Glu variants) and disease subphenotypes such as bronchial responsiveness, large studies have generally failed to confirm associations (Hall et al., 2006). In keeping with the probable lack of a causal role for ADRB2 polymorphism in asthma itself was the failure to identify a signal at this locus in the large asthma genome wide association studies which have been performed (Moffatt et al., 2010, Wan et al., 2012).
However, there is more debate about the potential contribution of either individual SNPs at this locus, or combination of SNPs (haplotypes) to treatment response. Early studies suggested an association between Arg16Gly and treatment response to regular SABA administration. Although not consistent across all studies, several studies have found that frequent or regular administering of SABAs impairs asthma control in patients with the Arg16Arg genotype (Israel et al., 2004, Israel et al., 2000, Taylor et al., 2000, Basu et al., 2009). Two small retrospective cohort studies also suggested that patients with the Arg16Arg genotype had a worse response to salmeterol than patients with the Gly16Gly genotype (Wechsler et al., 2006, Palmer et al., 2006); however, most larger retrospective and prospective studies have not confirmed these results (Bleecker et al., 2006, Bleecker et al., 2007, Bleecker et al., 2010, Wechsler et al., 2009).
Future Perspectives and summary
β2 agonists continue to have a major role in the treatment of airflow obstruction, and despite the concerns over their safety when administered in patients with asthma as monotherapy, when used in combination with inhaled corticosteroids they have proven effective and have a good overall safety record. Improvements in the way these agents are used clinically, and the development of novel agents with altered profiles offers potential to further refine the use of this class of drugs. Whereas at present the widespread use of genetic profiles to dictate treatment strategies for this class of drugs does not seem warranted, it is conceivable that a better understanding of the pharmacogenetics of this drug class may result in stratified approaches to treatment in the future in at least some conditions. The development of more selective agents with longer durations of action will likely continue, although safety concerns will still need to be assessed with each new drug profile, especially given the signals seen in early clinical studies involving full agonists used at relatively high dosage. Finally, opportunities exist to further modify the profile of β2 agonists to select for biased signalling; whether or not agents with relative selectivity for a given signalling pathway will show clinical benefit over the agents in existing use remains to be explored.
Acknowledgments
Funding: The authors are funded by grants HL58506 and A110007 (RBP) and MRC grant G1000861(IPH). We thank Dr Shams-un-nisaNaveed and MrVazRaziq for useful discussion.
References
- Ahlquist RP. A study of the adrenotropic receptors. Am J Physiol. 1948;153:586–600. doi: 10.1152/ajplegacy.1948.153.3.586. [DOI] [PubMed] [Google Scholar]
- Baker JG. The selectivity of beta-adrenoceptor agonists at human beta1-, beta2- and beta3-adrenoceptors. Br J Pharmacol. 2010;160:1048–61. doi: 10.1111/j.1476-5381.2010.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes PJ. Distribution of receptor targets in the lung. Proc Am Thorac Soc. 2004;1:345–51. doi: 10.1513/pats.200409-045MS. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Drugs for asthma. Br J Pharmacol. 2006;147(Suppl 1):S297–303. doi: 10.1038/sj.bjp.0706437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu K, Palmer CN, Tavendale R, Lipworth BJ, Mukhopadhyay S. Adrenergic beta(2)-receptor genotype predisposes to exacerbations in steroid-treated asthmatic patients taking frequent albuterol or salmeterol. J Allergy Clin Immunol. 2009;124:1188–94 e3. doi: 10.1016/j.jaci.2009.07.043. [DOI] [PubMed] [Google Scholar]
- Bhagat R, Kalra S, Swystun VA, Cockcroft DW. Rapid onset of tolerance to the bronchoprotective effect of salmeterol. Chest. 1995;108:1235–9. doi: 10.1378/chest.108.5.1235. [DOI] [PubMed] [Google Scholar]
- Billington CK, Penn RB. Signaling and regulation of G protein-coupled receptors in airway smooth muscle. Respir Res. 2003;4:2. [PMC free article] [PubMed] [Google Scholar]
- Bleecker ER, Nelson HS, Kraft M, Corren J, Meyers DA, Yancey SW, Anderson WH, Emmett AH, Ortega HG. Beta2-receptor polymorphisms in patients receiving salmeterol with or without fluticasone propionate. Am J Respir Crit Care Med. 2010;181:676–87. doi: 10.1164/200809-1511OC. [DOI] [PubMed] [Google Scholar]
- Bleecker ER, Postma DS, Lawrance RM, Meyers DA, Ambrose HJ, Goldman M. Effect of ADRB2 polymorphisms on response to longacting beta2-agonist therapy: a pharmacogenetic analysis of two randomised studies. Lancet. 2007;370:2118–25. doi: 10.1016/S0140-6736(07)61906-0. [DOI] [PubMed] [Google Scholar]
- Bleecker ER, Yancey SW, Baitinger LA, Edwards LD, Klotsman M, Anderson WH, Dorinsky PM. Salmeterol response is not affected by beta2-adrenergic receptor genotype in subjects with persistent asthma. J Allergy Clin Immunol. 2006;118:809–16. doi: 10.1016/j.jaci.2006.06.036. [DOI] [PubMed] [Google Scholar]
- BNF. British National Formulary [Google Scholar]
- Brittain RT, Farmer JB, Jack D, Martin LE, Simpson WT. Alpha-[(t-Butylamino)methyl]-4-hydroxy-m-xylene-alpha 1,alpha 3-diol (AH3365): a selective beta-adrenergic stimulant. Nature. 1968;219:862–3. doi: 10.1038/219862a0. [DOI] [PubMed] [Google Scholar]
- Callaerts-Vegh Z, Evans KL, Dudekula N, Cuba D, Knoll BJ, Callaerts PF, Giles H, Shardonofsky FR, Bond RA. Effects of acute and chronic administration of beta-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci U S A. 2004;101:4948–53. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle W, Fuller R, Hall J, Palmer J. Serevent nationwide surveillance study: comparison of salmeterol with salbutamol in asthmatic patients who require regular bronchodilator treatment. BMJ. 1993;306:1034–7. doi: 10.1136/bmj.306.6884.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung D, Timmers MC, Zwinderman AH, Bel EH, Dijkman JH, Sterk PJ. Long-term effects of a long-acting beta 2-adrenoceptor agonist, salmeterol, on airway hyperresponsiveness in patients with mild asthma. N Engl J Med. 1992;327:1198–203. doi: 10.1056/NEJM199210223271703. [DOI] [PubMed] [Google Scholar]
- Chu EK, Drazen JM. Asthma: one hundred years of treatment and onward. Am J Respir Crit Care Med. 2005;171:1202–8. doi: 10.1164/rccm.200502-257OE. [DOI] [PubMed] [Google Scholar]
- Cullum VA, Farmer JB, Jack D, Levy GP. Salbutamol: a new, selective beta-adrenoceptive receptor stimulant. Br J Pharmacol. 1969;35:141–51. doi: 10.1111/j.1476-5381.1969.tb07975.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande DA, Theriot BS, Penn RB, Walker JK. Beta-arrestins specifically constrain beta2-adrenergic receptor signaling and function in airway smooth muscle. FASEB J. 2008;22:2134–41. doi: 10.1096/fj.07-102459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande DA, Yan H, Kong KC, Tiegs BC, Morgan SJ, Pera T, Panettieri RA, Eckhart AD, Penn RB. Exploiting functional domains of GRK2/3 to alter the competitive balance of pro- and anticontractile signaling in airway smooth muscle. Faseb J. 2014;28:956–65. doi: 10.1096/fj.13-240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar JC, Wheatley AP, Venn A, Morrison JF, Britton J, Hall IP. Beta2-adrenoceptor polymorphisms are in linkage disequilibrium, but are not associated with asthma in an adult population. Clin Exp Allergy. 1998;28:442–8. doi: 10.1046/j.1365-2222.1998.00245.x. [DOI] [PubMed] [Google Scholar]
- Dixon RA, Kobilka BK, Strader DJ, Benovic JL, Dohlman HG, Frielle T, Bolanowski MA, Bennett CD, Rands E, Diehl RE, Mumford RA, Slater EE, Sigal IS, Caron MG, Lefkowitz RJ, Strader CD. Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature. 1986;321:75–9. doi: 10.1038/321075a0. [DOI] [PubMed] [Google Scholar]
- Garland SL. Are GPCRs still a source of new targets? J Biomol Screen. 2013;18:947–66. doi: 10.1177/1087057113498418. [DOI] [PubMed] [Google Scholar]
- Green SA, Cole G, Jacinto M, Innis M, Liggett SB. A polymorphism of the human beta 2-adrenergic receptor within the fourth transmembrane domain alters ligand binding and functional properties of the receptor. J Biol Chem. 1993;268:23116–21. [PubMed] [Google Scholar]
- Grove A, Lipworth BJ. Bronchodilator subsensitivity to salbutamol after twice daily salmeterol in asthmatic patients. Lancet. 1995;346:201–6. doi: 10.1016/s0140-6736(95)91265-7. [DOI] [PubMed] [Google Scholar]
- Hall IP, Blakey JD, Al Balushi KA, Wheatley A, Sayers I, Pembrey ME, Ring SM, Mcardle WL, Strachan DP. Beta2-adrenoceptor polymorphisms and asthma from childhood to middle age in the British 1958 birth cohort: a genetic association study. Lancet. 2006;368:771–9. doi: 10.1016/S0140-6736(06)69287-8. [DOI] [PubMed] [Google Scholar]
- Hanania NA, Singh S, El-Wali R, Flashner M, Franklin AE, Garner WJ, Dickey BF, Parra S, Ruoss S, Shardonofsky F, O'connor BJ, Page C, Bond RA. The safety and effects of the beta-blocker, nadolol, in mild asthma: An open-label pilot study. Pulm Pharmacol Ther. 2007 doi: 10.1016/j.pupt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel E, Chinchilli VM, Ford JG, Boushey HA, Cherniack R, Craig TJ, Deykin A, Fagan JK, Fahy JV, Fish J, Kraft M, Kunselman SJ, Lazarus SC, Lemanske RF, Jr, Liggett SB, Martin RJ, Mitra N, Peters SP, Silverman E, Sorkness CA, Szefler SJ, Wechsler ME, Weiss ST, Drazen JM. Use of regularly scheduled albuterol treatment in asthma: genotype-stratified, randomised, placebo-controlled cross-over trial. Lancet. 2004;364:1505–12. doi: 10.1016/S0140-6736(04)17273-5. [DOI] [PubMed] [Google Scholar]
- Israel E, Drazen JM, Liggett SB, Boushey HA, Cherniack RM, Chinchilli VM, Cooper DM, Fahy JV, Fish JE, Ford JG, Kraft M, Kunselman S, Lazarus SC, Lemanske RF, Martin RJ, Mclean DE, Peters SP, Silverman EK, Sorkness CA, Szefler SJ, Weiss ST, Yandava CN. The effect of polymorphisms of the beta(2)-adrenergic receptor on the response to regular use of albuterol in asthma. Am J Respir Crit Care Med. 2000;162:75–80. doi: 10.1164/ajrccm.162.1.9907092. [DOI] [PubMed] [Google Scholar]
- Jackson M. Asthma The Biography. Oxford University Press; 2009. [Google Scholar]
- Kang DS, Tian X, Benovic JL. Role of beta-arrestins and arrestin domain-containing proteins in G protein-coupled receptor trafficking. Curr Opin Cell Biol. 2014;27:63–71. doi: 10.1016/j.ceb.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Functional selectivity and biased receptor signaling. J Pharmacol Exp Ther. 2011;336:296–302. doi: 10.1124/jpet.110.173948. [DOI] [PubMed] [Google Scholar]
- Kong KC, Gandhi U, Martin TJ, Anz CB, Yan H, Misior AM, Pascual RM, Deshpande DA, Penn RB. Endogenous Gs-coupled receptors in smooth muscle exhibit differential susceptibility to GRK2/3-mediated desensitization. Biochemistry. 2008;47:9279–88. doi: 10.1021/bi801056w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume H, Hall IP, Washabau RJ, Tagaki K, Kotlikoff MI. β-adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J Clin Invest. 1994;93:371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands AM, Luduena FP, Buzzo HJ. Differentiation of receptors responsive to isoproterenol. Life Sci. 1967;6:2241–9. doi: 10.1016/0024-3205(67)90031-8. [DOI] [PubMed] [Google Scholar]
- Lipworth B, Tan S, Devlin M, Aiken T, Baker R, Hendrick D. Effects of treatment with formoterol on bronchoprotection against methacholine. Am J Med. 1998;104:431–8. doi: 10.1016/s0002-9343(98)00086-2. [DOI] [PubMed] [Google Scholar]
- Litonjua AA, Silverman EK, Tantisira KG, Sparrow D, Sylvia JS, Weiss ST. Beta 2-adrenergic receptor polymorphisms and haplotypes are associated with airways hyperresponsiveness among nonsmoking men. Chest. 2004;126:66–74. doi: 10.1378/chest.126.1.66. [DOI] [PubMed] [Google Scholar]
- Moffatt MF, Gut IG, Demenais F, Strachan DP, Bouzigon E, Heath S, Von Mutius E, Farrall M, Lathrop M, Cookson WO. A large-scale, consortium-based genomewide association study of asthma. N Engl J Med. 2010;363:1211–21. doi: 10.1056/NEJMoa0906312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan SJ, Deshpande DA, Tiegs BC, Misior AM, Yan H, Hershfeld AV, Rich TC, Panettieri RA, An SS, Penn RB. Beta-agonist-mediated relaxation of airway smooth muscle is PKA-dependent. J Biol Chem. 2014 doi: 10.1074/jbc.M114.557652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HS. Long-acting beta-agonists in adult asthma: Evidence that these drugs are safe. Prim Care Respir J. 2006;15:271–7. doi: 10.1016/j.pcrj.2006.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HS, Weiss ST, Bleecker ER, Yancey SW, Dorinsky PM. The Salmeterol Multicenter Asthma Research Trial: a comparison of usual pharmacotherapy for asthma or usual pharmacotherapy plus salmeterol. Chest. 2006;129:15–26. doi: 10.1378/chest.129.1.15. [DOI] [PubMed] [Google Scholar]
- Nguyen LP, Lin R, Parra S, Omoluabi O, Hanania NA, Tuvim MJ, Knoll BJ, Dickey BF, Bond RA. Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci USA. 2009;106:2435–2440. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega VE, Peters SP. Beta-2 adrenergic agonists: focus on safety and benefits versus risks. Curr Opin Pharmacol. 2010;10:246–53. doi: 10.1016/j.coph.2010.04.009. [DOI] [PubMed] [Google Scholar]
- Palmer CN, Lipworth BJ, Lee S, Ismail T, Macgregor DF, Mukhopadhyay S. Arginine-16 beta2 adrenoceptor genotype predisposes to exacerbations in young asthmatics taking regular salmeterol. Thorax. 2006;61:940–4. doi: 10.1136/thx.2006.059386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn RB. Embracing emerging paradigms of G protein-coupled receptor agonism and signaling to address airway smooth muscle pathobiology in asthma. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:149–169. doi: 10.1007/s00210-008-0263-1. [DOI] [PubMed] [Google Scholar]
- Penn RB, Benovic JL. Regulation of G protein-coupled receptors. In: Conn PM, editor. Handbook of Physiology. Oxford University Press; 1998. [Google Scholar]
- Penn RB, Bond RA, Walker JK. GPCRs and arrestins in airways: implications for asthma. Handb Exp Pharmacol. 2014;219:387–403. doi: 10.1007/978-3-642-41199-1_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn RB, Parent JL, Pronin AN, Panettieri RA, JR, Benovic JL. Pharmacological Inhibition of Protein Kinases in Intact Cells: Antagonism of Beta Adrenergic Receptor Ligand Binding by H-89 Reveals Limitations of Usefulness. J Pharmacol Exp Ther. 1999;288:428–437. [PubMed] [Google Scholar]
- Penn RB, Pascual RM, Kim YM, Mundell SJ, Krymskaya VP, Panettieri RA, Jr, Benovic JL. Arrestin specificity for G protein-coupled receptors in human airway smooth muscle. J Biol Chem. 2001;276:32648–56. doi: 10.1074/jbc.M104143200. [DOI] [PubMed] [Google Scholar]
- Ramsay CE, Hayden CM, Tiller KJ, Burton PR, Goldblatt J, Lesouef PN. Polymorphisms in the beta2-adrenoreceptor gene are associated with decreased airway responsiveness. Clin Exp Allergy. 1999;29:1195–203. doi: 10.1046/j.1365-2222.1999.00570.x. [DOI] [PubMed] [Google Scholar]
- Reihsaus E, Innis M, Macintyre N, Liggett SB. Mutations in the gene encoding for the beta 2-adrenergic receptor in normal and asthmatic subjects. Am J Respir Cell Mol Biol. 1993;8:334–9. doi: 10.1165/ajrcmb/8.3.334. [DOI] [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscioni SS, Elzinga CR, Schmidt M. Epac: effectors and biological functions. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:345–57. doi: 10.1007/s00210-007-0246-7. [DOI] [PubMed] [Google Scholar]
- Salpeter SR, Buckley NS, Ormiston TM, Salpeter EE. Meta-analysis: effect of long-acting beta-agonists on severe asthma exacerbations and asthma-related deaths. Ann Intern Med. 2006;144:904–12. doi: 10.7326/0003-4819-144-12-200606200-00126. [DOI] [PubMed] [Google Scholar]
- Sears MR, Taylor DR, Print CG, Lake DC, Li QQ, Flannery EM, Yates DM, Lucas MK, Herbison GP. Regular inhaled beta-agonist treatment in bronchial asthma. Lancet. 1990;336:1391–6. doi: 10.1016/0140-6736(90)93098-a. [DOI] [PubMed] [Google Scholar]
- Shenoy SK, Lefkowitz RJ. beta-Arrestin-mediated receptor trafficking and signal transduction. Trends Pharmacol Sci. 2011;32:521–33. doi: 10.1016/j.tips.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short PM, Williamson PA, Anderson WJ, Lipworth BJ. Randomized Placebo-controlled Trial to Evaluate Chronic Dosing Effects of Propranolol in Asthma. Am J Respir Crit Care Med. 2013;187:1308–14. doi: 10.1164/rccm.201212-2206OC. [DOI] [PubMed] [Google Scholar]
- Stallaert W, Dorn JF, Van Der Westhuizen E, Audet M, Bouvier M. Impedance responses reveal beta(2)-adrenergic receptor signaling pluridimensionality and allow classification of ligands with distinct signaling profiles. PLoS One. 2012;7:e29420. doi: 10.1371/journal.pone.0029420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm M, Richards DH, Beghe B, Fabbri L. Inhaled corticosteroid and long-acting beta2-agonist pharmacological profiles: effective asthma therapy in practice. Respir Med. 2012;106(Suppl 1):S9–19. doi: 10.1016/S0954-6111(12)70005-7. [DOI] [PubMed] [Google Scholar]
- Taylor DR, Drazen JM, Herbison GP, Yandava CN, Hancox RJ, Town GI. Asthma exacerbations during long term beta agonist use: influence of beta(2) adrenoceptor polymorphism. Thorax. 2000;55:762–7. doi: 10.1136/thorax.55.9.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanawala VJ, Forkuo GS, Al-Sawalha N, Azzegagh Z, Nguyen LP, Eriksen JL, Tuvim MJ, Lowder TW, Dickey BF, Knoll BJ, Walker JK, Bond RA. beta2-Adrenoceptor agonists are required for development of the asthma phenotype in a murine model. Am J Respir Cell Mol Biol. 2013;48:220–9. doi: 10.1165/rcmb.2012-0364OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck B. Beta-adrenoceptor agonists and asthma--100 years of development. Eur J Pharmacol. 2002;445:1–12. doi: 10.1016/s0014-2999(02)01728-4. [DOI] [PubMed] [Google Scholar]
- Walker JK, Fong AM, Lawson BL, Savov JD, Patel DD, Schwartz DA, Lefkowitz RJ. Beta-arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JK, Penn RB, Hanania NA, Dickey BF, Bond RA. New perspectives regarding beta(2) -adrenoceptor ligands in the treatment of asthma. Br J Pharmacol. 2011a;163:18–28. doi: 10.1111/j.1476-5381.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JK, Penn RB, Hanania NA, Dickey BF, Bond RA. New perspectives regarding beta(2) -adrenoceptor ligands in the treatment of asthma. Br J Pharmacol. 2011b;163:18–28. doi: 10.1111/j.1476-5381.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YI, Shrine NR, Soler Artigas M, Wain LV, Blakey JD, Moffatt MF, Bush A, Chung KF, Cookson WO, Strachan DP, Heaney L, Al-Momani BA, Mansur AH, Manney S, Thomson NC, Chaudhuri R, Brightling CE, Bafadhel M, Singapuri A, Niven R, Simpson A, Holloway JW, Howarth PH, Hui J, Musk AW, James AL, Brown MA, Baltic S, Ferreira MA, Thompson PJ, Tobin MD, Sayers I, Hall IP. Genome-wide association study to identify genetic determinants of severe asthma. Thorax. 2012;67:762–8. doi: 10.1136/thoraxjnl-2011-201262. [DOI] [PubMed] [Google Scholar]
- Wechsler ME, Kunselman SJ, Chinchilli VM, Bleecker E, Boushey HA, Calhoun WJ, Ameredes BT, Castro M, Craig TJ, Denlinger L, Fahy JV, Jarjour N, Kazani S, Kim S, Kraft M, Lazarus SC, Lemanske RF, JR, Markezich A, Martin RJ, Permaul P, Peters SP, Ramsdell J, Sorkness CA, Sutherland ER, Szefler SJ, Walter MJ, Wasserman SI, Israel E. Effect of beta2-adrenergic receptor polymorphism on response to longacting beta2 agonist in asthma (LARGE trial): a genotype-stratified, randomised, placebo-controlled, crossover trial. Lancet. 2009;374:1754–64. doi: 10.1016/S0140-6736(09)61492-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler ME, Lehman E, Lazarus SC, Lemanske RF, JR, Boushey HA, Deykin A, Fahy JV, Sorkness CA, Chinchilli VM, Craig TJ, Dimango E, Kraft M, Leone F, Martin RJ, Peters SP, Szefler SJ, Liu W, Israel E. beta-Adrenergic receptor polymorphisms and response to salmeterol. Am J Respir Crit Care Med. 2006;173:519–26. doi: 10.1164/rccm.200509-1519OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisler JW, Dewire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Deshpande DA, Misior AM, Miles MC, Saxena H, Riemer EC, Pascual RM, Panettieri RA, Penn RB. Anti-mitogenic effects of beta-agonists and PGE2 on airway smooth muscle are PKA dependent. Faseb J. 2011;25:389–397. doi: 10.1096/fj.10-164798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieba BJ, Artamonov MV, Jin L, Momotani K, Ho R, Franke AS, Neppl RL, Stevenson AS, Khromov AS, Chrzanowska-Wodnicka M, Somlyo AV. The cAMP-responsive Rap1 guanine nucleotide exchange factor, Epac, induces smooth muscle relaxation by down-regulation of RhoA activity. J Biol Chem. 2011;286:16681–16692. doi: 10.1074/jbc.M110.205062. [DOI] [PMC free article] [PubMed] [Google Scholar]


