Abstract
Behavioural impairment post-stroke is a consequence of structural damage and altered functional network dynamics. Hypoperfusion of intact neural tissue is frequently observed in acute stroke, indicating reduced functional capacity of regions outside the lesion. However, cerebral blood flow (CBF) is rarely investigated in chronic stroke. This study investigated CBF in individuals with chronic Wernicke's aphasia (WA) and examined the relationship between lesion, CBF and neuropsychological impairment.
Arterial spin labelling CBF imaging and structural MRIs were collected in 12 individuals with chronic WA and 13 age-matched control participants. Joint independent component analysis (jICA) investigated the relationship between structural lesion and hypoperfusion. Partial correlations explored the relationship between lesion, hypoperfusion and language measures.
Joint ICA revealed significant differences between the control and WA groups reflecting a large area of structural lesion in the left posterior hemisphere and an associated area of hypoperfusion extending into grey matter surrounding the lesion. Small regions of remote cortical hypoperfusion were observed, ipsilateral and contralateral to the lesion. Significant correlations were observed between the neuropsychological measures (naming, repetition, reading and semantic association) and the jICA component of interest in the WA group. Additional ROI analyses found a relationship between perfusion surrounding the core lesion and the same neuropsychological measures.
This study found that core language impairments in chronic WA are associated with a combination of structural lesion and abnormal perfusion in non-lesioned tissue. This indicates that post-stroke impairments are due to a wider disruption of neural function than observable on structural T1w MRI.
Keywords: Diaschisis, Wernicke's aphasia, Language comprehension, Cerebral blood flow, Lesion-symptom mapping
1. Introduction
The impact of a neural lesion extends beyond the site at which the lesion occurs (Feeney & Baron, 1986). Cognitive functions are supported through the integration of highly interconnected cortical and subcortical regions and, therefore, lesions to isolated network components can cause widespread dysfunction. As such, accounting for behavioural profile/impairment after stroke requires both the site of lesion as well as the functional status of remaining network components to be considered. In this study we investigate the relationship between stroke damage and the functional potential of wider neural regions in a group of individuals with chronic Wernicke's aphasia (WA) by exploring associations between structural lesion and residual cerebral blood flow (CBF). We further examine the relationship between these imaging profiles and neuropsychological impairment.
Within the first hours of an acute stroke, patients show regions of hypoperfusion, which often extend beyond the limits of the observable structural lesion (Neumann-Haefelin et al., 1999). This hypoperfusion can occur in the form of ischaemic penumbra, surrounding the area of core infarction (Croquelois, Wintermark, Reichhart, Meuli, & Bogousslavsky, 2003), or remote from the area of infarction, termed focal diaschisis (Carrera and Tononi, 2014, Feeney and Baron, 1986). Diaschisis most commonly occurs between cortical and subcortical regions; lesions to subcortical areas lead to cortical dysfunction and cortical lesions can lead to subcortical alterations (Bowler et al., 1995, Feeney and Baron, 1986, Hillis et al., 2002, Price et al., 2001). Remote cortico–cortico hypoperfusion/hypometabolism is only rarely reported but is mostly transcallosal (Andrews, 1991, Carrera and Tononi, 2014). CBF supplies oxygen and glucose required for neuronal function and is associated with neuronal activity through neurovascular coupling (Girouard & Iadecola, 2006). Therefore, regional hypoperfusion indirectly indicates reduced functional capacity of associated neural tissue. These hypoperfusion patterns in the acute phase indicate that behavioural impairment emerges as a consequence of infarcted tissue and functional depression of intact tissue.
WA is an acquired language impairment which occurs at both the acute and chronic phases following lesions to the left posterior temporal lobe and inferior parietal lobe (Ogar et al., 2011, Robson et al., 2014b). WA is characterised by severely impaired language comprehension and repetition in the context of fluent speech and relatively well preserved mobility. This impairment profile is underpinned by a spectrum of neuropsychological impairments including verbal short term memory (Robson, Sage, & Lambon Ralph, 2012), acoustic (Robson, Grube, Lambon Ralph, Griffiths, & Sage, 2013), phonological (Baker, Blumstein, & Goodglass, 1981) and semantic (Cohen et al., 1980, De Renzi et al., 1972) processing impairments. The WA comprehension impairment at the acute phase has been associated with hypoperfusion of the left posterior superior temporal and inferior parietal regions (Hillis et al., 2001, Jodzio et al., 2003) with re-perfusion of these regions leading to improvements of language comprehension (Hillis et al., 2001). At the chronic stage, structural imaging investigations have found similar relationships between structural lesion and language comprehension impairments of the WA type, specifically following lesions to mid-posterior middle temporal areas (Dronkers, Wilkins, Van Valin, Redfern, & Jaeger, 2004).
Hypoperfusion investigations of WA at the chronic stage have not matched those undertaken at the acute phase. This may be because diaschisis and penumbral regions are frequently observed to re-perfuse or evolve to lesion (Binkofski et al., 1996, Butcher et al., 2005, Neumann-Haefelin et al., 1999, Seitz et al., 1999, Toni et al., 1997). There is, however, some limited evidence to suggest that hypoperfusion of structurally intact tissue can occur in chronic stroke (Barber et al., 2001, Brumm et al., 2010, Raynaud et al., 1987) and this has implications for understanding the mechanisms of recovery and source of impairments post-stroke.
In this study we investigated whether hypoperfusion of intact tissue, outside the lesion site, could be identified in chronic WA and explored the relationship between hypoperfusion and a range of neuropsychological symptoms observed in WA. Arterial spin labelling (ASL), a non-invasive measure of CBF, and structural T1-weighted MRI data were collected in a group of 12 individuals with chronic WA. Statistical analysis used joint independent component analysis (ICA). Joint ICA is a multivariate analysis which combines multiple imaging modalities, enabling patterns across modalities to be detected (Abel et al., 2015, Calhoun et al., 2009). This analysis enabled the identification of regions of hypoperfusion statistically related to areas of structural lesion. Correlational analyses then investigated the relationship between CBF and lesion distribution and neuropsychological profile.
2. Materials and methods
Ethical approval was provided by the North-West NRES committee, UK. Twelve individuals with Wernicke's aphasia (WA, two female) were recruited from local National Health Service Speech Therapy services. Thirteen age-matched controls (two female) with no neurological history were recruited from a panel of research volunteers (Table 1).
Table 1.
Demographic information presented for WA and control participants.
| WA | Age | Sex | TPO | BDAE percentile |
Control | Age | Sex | ||
|---|---|---|---|---|---|---|---|---|---|
| Comprehension | Repetition | Fluency | |||||||
| CH | 77 | M | 17 m | 40 | 45 | 100 | AM | 58 | M |
| CW | 70 | M | 3 y | 45 | 40 | 100 | BH | 67 | M |
| DL | 73 | M | 9 m | 3 | <1 | 63 | BR | 76 | M |
| DM | 75 | M | 16 m | 13 | <1 | 57 | DW | 72 | M |
| DMC | 67 | M | 10 m | 3 | <1 | 47 | EC | 78 | F |
| DR | 76 | M | 7 m | 2 | <1 | 47 | GP | 78 | M |
| EL | 61 | M | 15 m | 14 | 10 | 75 | HE | 76 | M |
| LB | 80 | F | 7 y | 5 | 5 | 68 | KE | 52 | F |
| LS | 66 | M | 10 m | 5 | 25 | 70 | KW | 69 | M |
| MC | 73 | F | 13 m | 10 | 10 | 83 | ML | 66 | M |
| NM | 59 | M | 11 m | 17 | 10 | 100 | NJ | 78 | M |
| RD | 87 | M | 17 m | 10 | 5 | 80 | PD | 60 | M |
| TT | 61 | M | |||||||
BDAE = Boston Diagnostic Aphasia Examination – Short Form (Goodglass et al., 2001). BDAE criteria for WA is comprehension <47th centile, repetition <60th centile and fluency >45th centile. TPO = time post onset.
2.1. Neuropsychological assessment
All stroke participants were in the chronic phase of their acquired aphasia (at least 7 months post onset, Table 1) following cerebral infarction. No clinical information was available regarding cardiovascular risk factors or stenosis. No participant with WA presented with pre-stroke decline and all individuals had displayed a degree of post-stroke recovery. Participants were screened with the Boston Diagnostic Aphasia Examination – Short Form (Goodglass, Kaplan, & Barresi, 2001) which confirmed diagnosis of WA (Table 1). The majority of individuals presented with severe single word auditory comprehension and repetition impairments. The presence of jargon in spontaneous speech was not a WA inclusion criterion for the current study. However, five of the twelve participants did present with jargon speech (DL, DM, DMC, DR and LS), these were the more severely impaired individuals (see Table 2). The remaining participants produced phonological non-word errors during neuropsychological testing and in discourse; however, spontaneous speech was not affected to a sufficient extent to be considered jargon. Further neuropsychological testing examined cognitive-semantic skills and phonological skills. Non-verbal semantic performance was assessed with the Pyramids and Palm Trees semantic association test (three-picture version) (Howard & Patterson, 1992). Additionally, the Raven's Coloured Progressive Matrices (Raven, 1962), a test of non-verbal reasoning, was administered. Phonological skills were examined with 80-item reading and repetition tests from the PALPA battery (Kay, Coltheart, & Lesser, 1992). A naming assessment from the Cambridge Semantic Battery (Bozeat, Lambon Ralph, Patterson, Garrard, & Hodges, 2000) was included and which requires both semantic and phonological processing. Neuropsychological assessment results are detailed in Table 2 and in the results section. Overall, all individuals presented within the spectrum of WA but with a range of impairment severity on comprehension, production and semantic tasks.
Table 2.
Neuropsychological assessment results.
| WA | Cognitive-semantic tasks |
Phonological tasks |
Naming |
||
|---|---|---|---|---|---|
| PPT |
RCPM |
Reading |
Repetition |
||
| Max. 52 | Max. 33 | Max. 80 | Max. 80 | Max. 64 | |
| CH | 50 | 31 | 19 | 21 | 18 |
| CW | 52 | 29 | 44 | 39 | 41 |
| DL | 32 | 22 | 14 | 1 | 2 |
| DM | 40 | 24 | 1 | 0 | 0 |
| DMC | 39 | 23 | 1 | 0 | 0 |
| DR | 33 | 10 | 0 | 1 | 3 |
| EL | 36 | 27 | 27 | 14 | 24 |
| LB | 42 | 21 | 14 | 7 | 12 |
| LS | 34 | 21 | 0 | 5 | 1 |
| MC | 47 | 30 | 21 | 16 | 20 |
| NM | 52 | 31 | 28 | 43 | 28 |
| RD | 52 | 22 | 4 | 7 | 8 |
PPT = Three picture version of the Pyramids and Palm Trees Test (Howard & Patterson, 1992), a visual semantic association task. RCPM = Raven's Coloured Progressive Matrices (Raven, 1962), a non-verbal reasoning task. Reading and repetition tasks are taken from the Psycholinguistic Assessment of Language Processing in Aphasia (PALPA: Kay et al., 1992), Naming assessment was taken from the Cambridge Sematic Battery (Bozeat et al., 2000). Italicised result indicates participant outside normal limits. No normal cut-off data are available for RCPM.
2.2. WA lesion and CBF profiles
Automated lesion delineation was performed on T1-weighted MRI scans using the Fuzzy Clustering method (Seghier, Ramlackhansingh, Crinion, Leff, & Price, 2008). Lesions extended over the middle cerebral artery territory with peak overlap in the left mid-posterior superior and middle temporal lobe and inferior parietal lobe. Over the group, lesions extended outside these core regions into occipital, middle and anterior temporal, insular and inferior frontal areas (see Fig. 1A). The lesion pattern was mirrored by significant CBF reductions over the left hemisphere (see Fig. 1B). The average CBF to the left and right hemispheres was extracted from the pre-processed smoothed CBF maps in both the WA and control group using the MarsBar software (Brett, Anton, Valabregue, & Poline, 2002). The WA group displayed an average CBF of 29.3 ml/100 ml/min, SD. 7.1 ml/100 ml/min in the left hemisphere and an average of 39.1 ml/100 ml/min, SD. 6.2 ml/100 ml/min in the right hemisphere. The control group displayed an average CBF of 37.8 ml/100 ml/min, SD. 7.0 ml/100 ml/min in the left hemisphere and an average of 39.8 ml/100 ml/min, SD. 6.5 ml/100 ml/min in the right hemisphere. A 2 × 2 ANOVA exploring the effects of group and hemisphere on CBF found a significant main effect of group [F(1,44) = 5.1, p = .029] due to significantly reduced CBF in the WA group compared to the controls, a main effect of hemisphere [F(1,44) = 9.0, p = .004] due to less CBF to the left hemisphere in both the WA and the control group and a borderline hemisphere × group interaction [F(1,44) = 3.5, p = .067] caused by a greater hemisphere CBF difference (left < right) in the WA than the control group. In addition, we extracted the average CBF from both groups from left hemisphere regions which were not affected by lesion in any WA participant (WA: ave. CBF 31.5 ml/100 ml/min, SD. 7.5 ml/100 ml/min; Cont. ave CBF 37.0 ml/100 ml/min, SD. 6.8 ml/100 ml/min); despite not including any area of lesion, the difference between the groups displayed a borderline significant difference [t(23) = 1.9, p = .067].
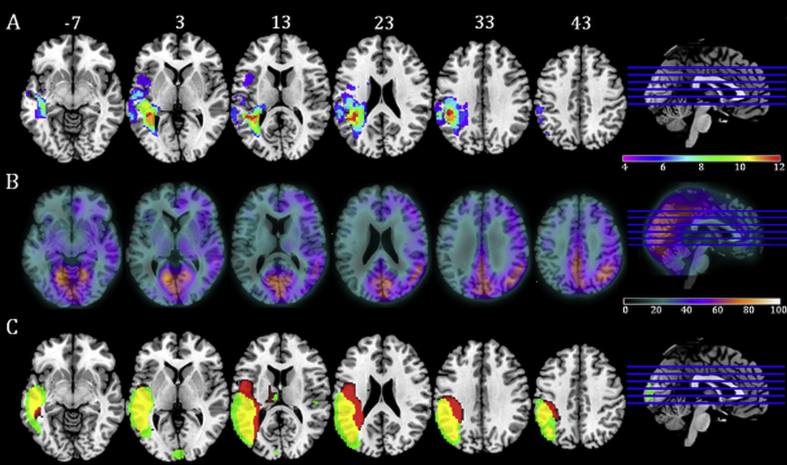
Fig. 1.
Lesion overlap map, CBF map and jICA results for component six. Panel A: Lesion overlap map derived using the fuzzy clustering method (Seghier et al., 2008). Individual participants' lesions were overlaid onto an MNI template brain using the MRIcron software. Map displays voxels in which lesion overlap occurred in a minimum of a third of the WA group. Axial slices are presented with MNI Z coordinate. Panel B: Cerebral Blood Flow map displaying the average CBF (ml/100 ml/min) in the WA group. Panel C: jICA result displaying component six. Map displays regions where presence of lesion (red) correlated with reduction in CBF (green). Overlapping areas of lesion and hypoperfusion are displayed in yellow.
2.3. Neuroimaging
Structural T1-weighted images and ASL images were collected on a 3T Philips Achieva Scanner with an eight-element SENSE head coil. T1-weighted imaging used a 3D inversion recovery sequence to produce a 256 × 256 matrix of 128 transverse slices of 1 mm3 voxels. A multi-timepoint ASL sequence was used with STAR labelling (Edelman et al., 1994), gradient echo EPI readout and the following scan parameters: label thickness 150 mm; label gap 10 mm; TE 21 msec; TR 3000 msec; 4 delay times of 800, 1200, 1600, 2000 msec from label to start of readout; FOV 224 × 224 mm; matrix size 64 × 64; 20 slices of 5 mm thickness with 1 mm gap. Voxel size 3.5 × 3.5 × 6.0 mm3; 20 control/label pairs. Vascular crushing was enabled. An additional calibration scan with TR 10000 msec, no label, was taken to allow quantification. CBF and arterial arrival time (AAT) were calculated from the four time points. AAT is not of central interest in the study, but it is important to correct CBF estimates for regional alterations in AAT that are known to occur in stroke (MacIntosh et al., 2010).
2.4. Pre-processing
T1-weighted and ASL CBF images were pre-processed with the SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/) and Matlab2013a. T1-weighted images were co-registered and normalised to the SPM T1-weighted template, no lesion masking was used following the normalisation procedure detailed in Crinion et al. (2007). After normalisation, images were segmented in to grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF) maps. This segmentation procedure classifies areas of lesion as CSF, hereafter described as lesion maps. The ASL control/label images were aligned to the first image using the SPM “Align function”. CBF maps were calculated from the mean ASL subtraction images using in-house software and the single-blood-compartment model described by Parkes, Rashid, Chard, and Tofts (2004). A global blood equilibrium magnetisation value was calculated from the calibration scan using voxels within a head mask derived from the T1-weighted image. The relaxation time of blood was fixed at 1600 msec (Stanisz et al., 2005); the labelling efficiency at .9; the blood–brain partition coefficient at .9 (Roberts, Rizi, Lenkinski, & Leigh, 1996) and the bolus width at 1100 msec. The labelling efficiency and bolus width were taken from earlier unpublished studies using this sequence. Two-parameter fits for CBF were performed on a voxel-wise basis. If the fitted value for CBF was negative, it was set to zero; if greater than 250 ml/100 ml/min, it was set to 250 ml/100 ml/min. CBF maps were co-registered and normalised to the SPM8 EPI template. To account for differing volumes of different tissue types in the large ASL voxels, we carried out a partial volume correction on the CBF images. The partial volume correction calculation was carried out for each voxel independently. The CBF measured within the voxel was divided by the sum of probabilities of GM and WM within the voxel (i.e., total tissue), taken from the co-registered segmented T1w image. This correction factor was applied if GM probability + WM probability >.1, as otherwise it can give an unacceptably large value for CBF. Finally, both the lesion and partial volume corrected CBF maps were smoothed with a 12 mm full-width half-maximum Gaussian filter. All the normalised and smoothed images were inspected visually to confirm that the pre-processing procedure had not resulted in image distortion.
2.5. Joint independent component analysis
Independent component analysis (ICA) is a multivariate statistical method which enables the separation and identification of sources underlying complex observable data (Stone, 2005). These sources, or components, are statistically independent and when linearly combined produce the best fit representation of the observable data. When applied to neuroimaging, ICA is able to extract the hidden spatiotemporal components within the data, thought to correspond to networks of functionally or structurally connected regions. Joint ICA (jICA) advances this procedure by allowing multiple modalities of data over multiple groups to be considered simultaneously (Calhoun et al., 2009). This allows the shared information between different modalities to be examined (Rachakonda, Liu, & Calhoun, 2012). Components extracted from a jICA indicate modality specific spatial patterns that carry similar covariance between participants across the modalities, i.e., where information in one modality correlates with information in another modality (Abel et al., 2015, Guo et al., 2012, Specht et al., 2009). For each component each participant has an associated component mixing coefficient, and group comparisons can be made by linear inference statistics on these coefficients.
Joint ICA was employed in the current study in order to identify spatial patterns of CBF related to spatial patterns of lesion and where significant differences in these patterns occur between stroke and control groups. Joint ICA analysis was implemented using the Matlab Fusion ICA Toolbox (FIT; for further methodological details see: http://icatb.sourceforge.net). Images from the two imaging modalities, CBF and lesion, were entered into the analysis for both the WA and control groups alongside binary masks created from an average of the control images GM and WM maps. The analysis estimated and subsequently derived seven z-scaled components using the INFOMAX algorithm. For each component a two-tailed t-test was performed to compare each component's mixing matrix coefficients between the groups. Components of interest were those with a significant group difference of p < .05.
3. Results
3.1. Neuropsychological profile
Participants with WA displayed consistent and severe impairments on neuropsychological tasks involving phonological processing: single word reading, single word repetition and picture naming (Table 2). On these tasks the error rate ranged from almost 100% in the more severely impaired participants (DM, DMC, DR) to approximately 45% in the least severely impaired participant (CH). All individuals displayed a large proportion of phonological errors with semantic, circumlocution and no response errors occurring less frequently. Two thirds of the WA participants displayed impairments in the visual semantic association task (three picture version of the Pyramids and Palm Trees test) and these individuals also displayed lower scores on non-verbal reasoning (Raven's Colour Progressive Matrices). These data are consistent with previous reports of the neuropsychological profile in WA (e.g., Ogar et al., 2011) and confirm a consistent contribution of phonological processing impairment with an additional contribution of semantic processing disruption in a large proportion of the population (Robson et al., 2012).1 This neuropsychological profile is consistent with the lesion distribution (Fig. 1), in which core lesion overlap occurs in auditory-phonological processing regions of the superior temporal and supramarginal gyri with less consistent but still high overlap in surrounding semantic processing regions such as the angular and middle temporal gyri.
3.2. Joint ICA
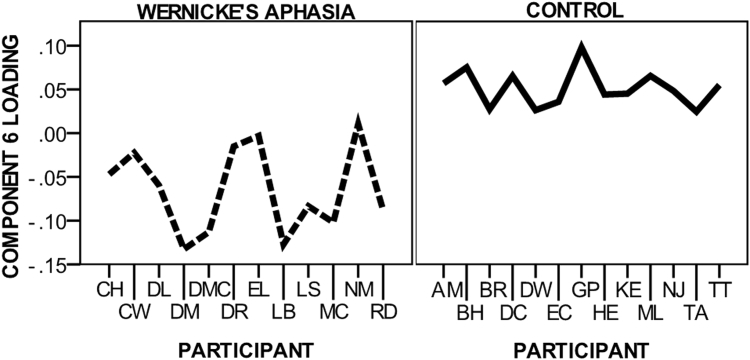
One of the seven jICA components extracted (component 6) was found to have significantly different mixing coefficients between the WA and control groups. Fig. 2 displays component 6 loading parameters for participants in the WA and control groups.
Fig. 2.
Component six mixing matrix loading. Figure displays participant loading for the WA and control groups on component Six.
Joint ICA results for component 6 are displayed in Fig. 1C. Component 6 was associated with a large area of lesion in the left posterior middle-superior temporal lobe, inferior parietal lobe and superior occipital lobe. This lesion pattern was commensurate with the core lesion, i.e., the areas of greatest lesion overlap in the WA group (Fig. 1A). The component captured lesion affecting the WM adjacent to the posterior lateral ventricle extending medially into the lateral dorsal nucleus region of the thalamus and laterally to impact neighbouring GM.
As expected, this pattern of lesion was associated with hypoperfusion in overlapping areas. However, lesion distribution showed greater medial involvement, whereas hypoperfusion was observed in a wider area of GM lateral to and surrounding the core area of lesion. Outside the immediate lesion area two regions of hypoperfusion were observed: (1) A small cluster of voxels in the right middle superior temporal gyrus, anterior to the auditory core and adjacent to the posterior insula and (2) part of the mid lateral cuneus of the left occipital lobe.
Six further jICA components were extracted over which no significant group difference occurred. These components identified regions of lesion associated with only one or two members of the WA group, therefore not reaching group level significance, e.g., frontal lobe lesion extension, as well as components associated with members of both group such as patterns of CBF overlapping with default mode network regions including the posterior cingulate cortex and medial frontal lobe.
3.3. Associations with behaviour
Partial correlations, accounting for time post onset, were performed to identify associations between the mixing coefficient loading of component six and the non-verbal reasoning, semantic, phonological and naming neuropsychological assessment in the WA group. Significant positive correlations were observed between component loading and the phonological assessments (reading and repetition), naming assessment and the semantic association Pyramids and Palm Trees test (repetition, r2 = .677, p = .022; reading aloud r2 = .683, p = .021; naming r2 = .703, p = .016; PPT r2 = .649, p = .031). The mixing coefficient loading for component six was negative for all members of the WA group and positive for all control group members. The correlations indicated that the less negative the WA participants or the more “control-like” in loading, the greater accuracy in behavioural assessment.
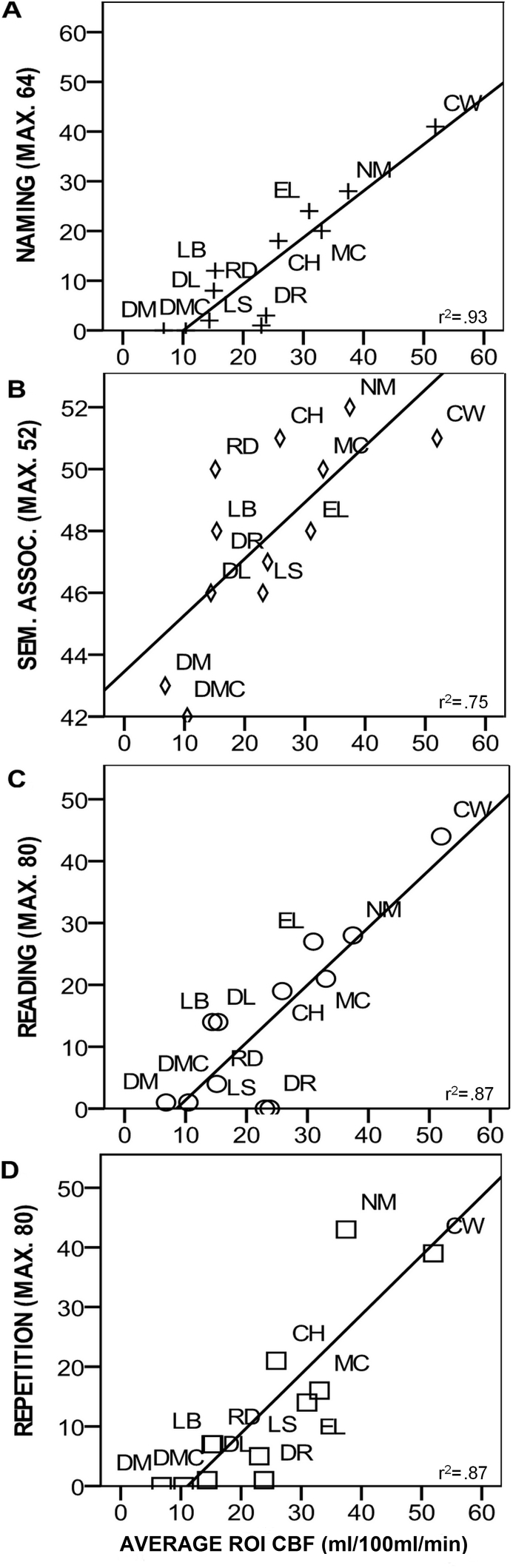
Although the jICA revealed hypoperfusion beyond the core lesion (Fig. 1B), visual inspection of individual lesion maps (Fig. 1A) indicated that lesions overlapped with this area of extended hypoperfusion, to some extent, in a subset of cases. To investigate whether hypoperfusion in this region made an independent contribution to behavioural impairment, ROI analyses were performed. An ROI was derived from the jICA region of extended hypoperfusion (Fig. 1C). Using the MarsBaR toolbox (Brett et al., 2002), average CBF (ml/100 ml/min) and lesion (probability of CSF in CSF segmented images) values over the ROI were extracted from unsmoothed images in the WA group. Partial correlations found significant relationships between the neuropsychological results and CBF but not lesion data (Fig. 3). Independent t-tests using the Fisher-transformation correlation coefficient values indicated significantly different correlation coefficients between each CBF-lesion correlation pair (repetition: CBF r2 = .865, p = .001, lesion r2 = −.13, p = .97; reading CBF r2 = .874, p ≤ .001, lesion r2 = −.167, p = .62; naming CBF r2 = .931, p ≤ .001 lesion r2 = −.091 p = .79; PPT CBF r2 = .75, p = .009, lesion r2 = −.131, p = .70). To support this analysis lesion volume within the ROI was calculated. Lesion volume was taken as the number of voxels of the binary lesion data from the Seghier et al. (2008) automated lesion detection protocol, see above. Within ROI lesion volume was then used as a covariate in partial correlations between CBF and neuropsychological data. All correlations remained significant, with a minimum effect size of r2 > .62. To confirm that these strong correlations were not driven by outliers, the two participants with the greatest residual CBF within the ROI were removed and the initial partial correlations were re-run. Strong effect sizes remained for all correlations (r2 > .63) with correlations remaining significant for naming and repetition. Taken together, these correlation ROI analyses indicated that CBF but not lesion in the region surrounding the core lesion showed a systematic relationship with impairment, with greater CBF corresponding to more preserved performance.
Fig. 3.
Perilesional CBF correlations with neuropsychological results. Scatter plots displaying relationship between CBF (ml/100 ml/min) surrounding core area of lesion and neuropsychological test data. Partial correlation coefficients displayed.
4. Discussion
This study investigated patterns of hypoperfusion associated with chronic temporoparietal lesions and WA. A joint independent component analysis (jICA) was used to identify areas where voxels in resting-state ASL CBF images co-varied significantly with voxels from CSF maps, used as a proxy for lesion, and differed significantly from control participants. Correlations with neuropsychological assessments were then performed to investigate how patterns of structural damage and hypoperfusion related to behavioural language impairment. The results found hypoperfusion extending into perilesional areas which, along with the core structural lesion, was associated with aspects of the language impairment. Follow-up correlations indicated that, outside the area of core lesion, hypoperfusion but not lesion was systematically related to behavioural neuropsychological impairment.
The jICA identified a single component for which there was no numerical overlap in mixing coefficient loading between WA and control groups. This component captured a large area of structural lesion in the left temporoparietal region as well as hypoperfusion in overlapping areas. In addition, extensive hypoperfusion was observed perilesionally, in grey matter lateral, anterior and posterior to the core lesion which primarily affected white matter areas. This indicates that these grey matter regions, although not suffering macrostructural damage, are likely to be functionally limited (Gold & Lauritzen, 2002) or suffering microstructural damage. The average rCBF in this “perilesional” region in the WA group was 25.7 ml/100 ml/min (SD 12.3 ml/100 ml/min), significantly lower [t(21) = 5.95, p < .001] than the average rCBF in the control group, 61.8 ml/100 ml/min (SD 15.9 ml/100 ml/min). Neuronal tissue will die if CBF falls below approximately 20 ml/100 ml/min (Jones et al., 1981). Although the WA group as a whole displayed CBF above this level, a number of individuals displayed CBF below this cut-off (Fig. 3), reflecting the extension of infarction into this region in a subset of participants. Most individuals with WA displayed CBF values within the functional range in this perilesional zone but below that of the controls indicating reduced neuronal function of residual tissue. However, caution must be taken in interpretation of absolute CBF estimates from ASL data due to uncertainty in some of the assumed global variables such as T1 of blood.
The cause of this perilesional hypoperfusion is unclear. It may represent an area of penumbra which has not undergone re-perfusion or evolution to lesion, as typically observed in the acute-subacute phase (Butcher et al., 2005, Neumann-Haefelin et al., 1999). Alternatively, it is credible that the perilesional hypoperfusion observed here has evolved separately following the acute phase through a diaschisis-type mechanism and represents a form of focal diaschisis (Carrera & Tononi, 2014). Under this hypothesis hypoperfusion results from a reduction of coordinated synaptic function because of disconnection and reduced afferent input (Feeney & Baron, 1986). Although acute phase cortico-subcortical diaschisis is common (Bowler et al., 1995, Carrera and Tononi, 2014), lesion-remote cortico–cortico hypoperfusion has been shown to develop over time in humans following stroke (Iglesias et al., 1996, Iglesias et al., 2000). This functional loss could a consequence of numerous neurobiological processes such as Wallerian-like, retrograde or transynaptic degeneration (Iglesias et al., 2000).
In contrast to the identification of hypoperfusion in intact grey matter regions, this study also identified regions of structural lesion that were not statistically associated with perfusion changes. These were the most medial regions, adjacent to the lateral ventricle. This pattern is likely to be accounted for by reduced microvascular density (and reduced regional CBF) in white matter in comparison to grey matter (Jensen, Lu, & Inglese, 2006) and the reduced signal-to-noise ratio of the ASL signal in white matter regions (van Gelderen, de Zwart, & Duyn, 2008). This could have led to WM hypoperfusion being missed in the ASL analysis and, therefore, perfusion differences between WA and controls in WM regions not being identified as significant in the jICA statistical analysis. As such, the finding of lesion without corresponding hypoperfusion in the jICA analysis is likely to be an artefact of the ASL method. The perilesional and remote hypoperfusion, see below, identified may have implications for recovery. Although most individuals with acute WA display a strong recovery (Pedersen, Vinter, & Olsen, 2004), WA that persists into the chronic stage is difficult to treat. Although therapeutic input can induce improvement in isolated aspects of the comprehension system (e.g., phonological perception), these improvements often do not generalise to improve comprehension as a whole (Morris et al., 1996, Woolf et al., 2014) except in isolated cases (Tessier, Weill-Chounlamountry, Michelot, & Pradat-Diehl, 2007). The current group of WA participants were not receiving speech and language therapy at the time of participation but had received treatment prior to their involvement. Strong spontaneous recovery in WA has been associated with enhanced functional activation in right superior temporal regions (Leff et al., 2002, Weiller et al., 1995), an area suffering from hypoperfusion in the current group of participants. The hypoperfusion identified in the current study may indicate reduced capacity to engage in local neuroplastic reorganisation processes, potentially contributing to the limited recovery of aphasia symptoms.
The finding of functionally depressed tissue outside the area of core lesion has implications for accounting for the range of neuropsychological impairments observed in WA. Indeed, this study found a relationship between the lesion/hypoperfusion and behavioural impairment. Correlation analyses found that the more “control-like” the WA participants were in their jICA mixing matrix coefficient loading, the better their performance on neuropsychological assessment. Specifically, the greater the perfusion and the less lesion in the identified regions (Fig. 1C) was associated with better naming, reading, repetition and semantic association. This pattern of correlations is interpretable within current neurobiological models of language. The posterior temporal lobe, inferior parietal lobe and temporoparietal occipital junction contain important components of both the phonological network (required for reading, repetition and naming tasks) and the semantic network (required for semantic association and naming tasks). Phonological identification and encoding processes take advantage of the high structural connectivity between auditory and motor regions in the superior temporal and inferior parietal lobe (Ueno, Saito, Rogers, & Lambon Ralph, 2011), the dorsal language stream. Functional imaging shows consistent activation within this stream during phonological tasks and TMS to the same regions disrupts performance in neurologically normal populations (Hartwigsen et al., 2015). The semantic network, while extensive and highly distributed, has components in the left middle temporal lobe and angular gyrus. These areas also respond during functional imaging of semantic processing tasks (Noonan et al., 2013, Seghier et al., 2010), with TMS to these regions impairing performance (Hoffman et al., 2011, Whitney et al., 2011).
Previous lesion-symptom mapping investigations have found similar brain–behaviour relationships to the current study. For example, semantic errors in naming have been associated with lesion in middle temporal regions (Schwartz et al., 2011) whereas phonological errors are associated with lesions to inferior parietal and dorsal stream regions (Schwartz, Faseyitan, Kim, & Coslett, 2012). However, the current study extended these findings by exploring the relationship between CBF surrounding the core lesion and behavioural impairment. Correlation analyses found a highly significant relationship between perilesional CBF which was not found for CSF/lesion data in the same region. These results indicated that perilesional hypoperfusion makes an independent contribution to behavioural performance post stroke over-and-above the structural lesion. However, the lesion symptom mapping approach may inherently capture the CBF-neuropsychology relationships uncovered in the current study, although without explicit visualisation. The WA group was highly homogeneous in that all participants displayed lesions which affected the white matter of the middle-superior temporal lobe or inferior parietal cortex to some degree. It is possible that lesions to this region inevitably lead to reductions in temporoparietal grey matter CBF over the long-term post stroke. As such, the CBF correlations in the present study may represent behaviourally-relevant aspects of lesion and correlations in lesion-symptom mapping methods may capture behaviourally-relevant aspects of CBF.
Although the current data broadly converge with lesion-symptom mapping and neuroimaging evidence, it is important to note that the current study does not aim to identify specific brain–behaviour relationships, rather to investigate the wider relationship between lesion, hypoperfusion and behaviour. Mass univariate lesion-symptom mapping approaches suffer from spatial distortion as a consequence of lesion profiles biased by the constraints of the vascular architecture (Mah, Husain, Rees, & Nachev, 2014). The current study used a multivariate approach designed to identify lesion–hypoperfusion relationships independent of the degree of overlapping damage. However, spatial bias has been reintroduced to this study through the selection of participants with highly overlapping lesion profiles and, as such, this study should not be considered a robust investigation of the neural source of a particular impairment.
The correlations between CBF and neuropsychology scores observed in the ROI analysis were particularly strong. This is likely to be a result of the stringent participant selection criteria employed in this study, where a small number of participants were recruited based on narrowly defined behavioural characteristics. The participants in this study all conformed to the classical definition of WA and displayed qualitatively similar impairment patterns in conversation but with a range of severities. This indicates that the results from the current study may be generalizable within classical WA, however the robustness of the CBF and behavioural relationships may breakdown if more diverse patient populations are explored. A further contribution to the correlation strength could have emerged from age-related reorganisation. Occipito-temporo-parietal regions are through to be particularly vulnerable to neuroanatomical and functional decline in ageing (Davis, Dennis, Daselaar, Fleck, & Cabeza, 2008). Functional imaging has found reduced activation during a range of cognitive tasks in elderly participants in comparison to younger participants (Grossman et al., 2002, Madden et al., 2002, Meulenbroek et al., 2004), including temporal and parietal regions affected by hypoperfusion in the current study.
Evidence for hypoperfusion remote from but causally connected to the lesion, i.e., perfusion changes consistent with the definition of remote focal diaschisis, appeared limited. Only two small regions of hypoperfusion were observed at the group level, the largest in the left cuneus and a small cluster in right mid superior temporal gyrus. The jICA technique identifies regions of consistent lesion-hypoperfusion associations across the group. At the participant level individual patterns of remote hypoperfusion may be present. In the current study the regions of remote hypoperfusion appear limited, however, comparison of neuroimaging techniques in animal models suggest that observed alterations in cerebral perfusion are an underestimation of metabolic changes which, in turn, significantly underestimate alterations in underlying electrical activity (Carmichael et al., 2004, Gold and Lauritzen, 2002). Therefore, even the limited reduction in cerebral perfusion observed in the current study may reflect considerably reduced neuronal activity.
If we are to consider these results as evidence of cortico–cortico focal diaschisis it raises further important questions about why these particular regions were affected and whether replication would reveal a similar pattern of remote hypoperfusion. While there is previous evidence for remote hypometabolism in WA, this been identified in pre-frontal regions (Metter et al., 1989), a pattern not identified in these results. However, evidence exists for structural connectivity, via the inferior fronto-occipital fasciculus, between the area of lesion and the hypoperfused cuneus region identified in the current study (Martino, Brogna, Robles, Vergani, & Duffau, 2010). Similarly, the right superior temporal gyrus is connected to the core lesion via the corpus callosum. However the lesion area also has strong interconnectivity with other cortical areas, including inferior frontal and inferior temporal regions (Cloutman, Binney, Morris, Parker, & Lambon Ralph, 2013), in which no hypoperfusion effect was detected. Therefore, hypoperfusion as a result of white matter structural disconnection is only speculative at this point. Further investigation should include tractography evidence to investigate the association between remote hypoperfusion and structural connectivity to the area of lesion. Secondly, of the two remote regions of hypoperfusion, only hypoperfusion in the right mid-superior temporal gyrus can be easily associated with the behavioural impairment in WA. Superior temporal regions, bilaterally, are involved in processing auditory stimuli, a cognitive skill which is consistently found to be impaired in individuals with WA (Robson et al., 2014a). The affected cuneus region plays a role in low and high level visual processing, a well preserved skill in classical WA (Robson et al., 2012).
One important consideration is that the ASL data were collected during rest. During functioning, the patterns of relative network activation or deactivation are likely to be considerably more complex. Remote diaschisis may be more evident during function, where reduced or absent activity from the area of lesion leads to reduced input/modulation of functionally connected network components. This pattern has been observed in aphasia and termed dynamic diaschisis (Price et al., 2001). However, it is more common to observe lesion-remote activation increases during task-based functional imaging in aphasia. This pattern was observed in the same group of aphasia participants as in the current study during a functional MRI experiment. During two visual semantic tasks, WA participants were found to have increased and more extensive activation in comparison to control participants in bilateral ventral temporal lobe regions (Robson et al., 2014b). The combination of results from this previous study and the current study indicate that, in the same patient group, it is possible to observe both extra-lesional diaschisis and remote functional up-regulation. These results support other functional imaging work in aphasia in indicating that the complex patterns of behaviour cannot be explained by structural lesion alone, but rather a combination of lesion and alterations to network dynamics.
Finally, one methodological consideration important for the interpretation of the current results is the method used to identify lesion. In the current study, masked and segmented CSF maps were used to estimate the probability of lesion in a given region. In the acute phase, T1-weighted MRI imaging is not suitable for identifying lesions as the pathological processes inducing T1-w signal change take time to develop (Davis, Fisher, & Warach, 2003). However, in the chronic phase T1-weighted imaging is the most commonly used research tool for identifying stroke lesions (Rekik, Allassonnière, Carpenter, & Wardlaw, 2012) and is advantageous in that it provides high resolution which facilitates normalisation enabling accurate lesion location to be identified. However, it is T2-weighted imaging that is considered the best approach for identifying lesion volume following the acute phase post-stroke (Davis et al., 2003). Imaging sequences have different sensitivities; T1-weighted MRI, for example, has reduced sensitivity to white matter changes in comparison to FLAIR (Crinion, Holland, Copland, Thompson, & Hillis, 2013) and areas of lesion can display similar intensities to non-lesioned tissue (Hojjatoleslami & Kruggel, 2001). Such factors may have led to an underestimate to the lesion in the current study and, therefore, an overestimate of the perilesional lesion-CBF mismatch. Combining multiple imaging modalities can improve the sensitivity of structural lesion delineation (Lu et al., 2005, Mitra et al., 2014) and should be considered for future investigations of structural damage and hypoperfusion in chronic stroke.
5. Conclusion
This study used a data-driven statistical method to identify regions of hypoperfusion associated with structural lesion in chronic WA. Strong evidence was observed for functionally compromised but structurally intact grey matter regions surrounding the lesion. Additional regions of remote cortical hypoperfusion were also identified which may be indicative of reduced resting-state functional activation in these patients in comparison with a control population. This study indicates that reduced peri-lesional grey matter function may develop secondary to deep middle-superior temporal white matter lesions and contribute to the behavioural impairments in chronic WA.
Funding
This study was supported by a Stroke Association Allied Health Professions Research Bursary (TSAB2008/01), a Stroke Association Senior Research Training Fellowship (TSA SRTF 2012/02) awarded to HR, an MRC clinician scientist fellowship (G0902304) awarded to RZ and an MRC programme grant (MR/J004 146/1) awarded to MALR.
Acknowledgments
Thanks go to all study participants and their family members and carers who facilitated their involvement.
Reviewed 11 July 2016
Action editor Alessandro Tavano
Footnotes
Note that 4 WA participants in the current study also participated in Robson et al. (2012) which may artificially enhance the consistency between these studies.
References
- Abel S., Weiller C., Huber W., Willmes K., Specht K. Therapy-induced brain reorganization patterns in aphasia. Brain. 2015;138:1097–1112. doi: 10.1093/brain/awv022. [DOI] [PubMed] [Google Scholar]
- Andrews R.J. Transhemispheric diaschisis. A review and comment. Stroke. 1991;22:943–949. doi: 10.1161/01.str.22.7.943. [DOI] [PubMed] [Google Scholar]
- Baker E., Blumstein S.E., Goodglass H. Interaction between phonological and semantic factors in auditory comprehension. Neuropsychologia. 1981;19:1–15. doi: 10.1016/0028-3932(81)90039-7. [DOI] [PubMed] [Google Scholar]
- Barber P.A., Consolo H.K., Yang Q., Darby D.G., Desmond P.M., Lichtenstein M. Comparison of MRI perfusion imaging and single photon emission computed tomography in chronic stroke. Cerebrovascular Diseases. 2001;11:128–136. doi: 10.1159/000047624. [DOI] [PubMed] [Google Scholar]
- Binkofski F., Seitz R.J., Arnold S., Classen J., Benecke R., Freund H.J. Thalamic metabolism and corticospinal tract integrity determine motor recovery in stroke. Annals of Neurology. 1996;39:460–470. doi: 10.1002/ana.410390408. [DOI] [PubMed] [Google Scholar]
- Bowler J.V., Wade J.P.H., Jones B.E., Nijran K., Jewkes R.F., Cuming R. Contribution of diaschisis to the clinical deficit in human cerebral infarction. Stroke. 1995;26:1000–1006. doi: 10.1161/01.str.26.6.1000. [DOI] [PubMed] [Google Scholar]
- Bozeat S., Lambon Ralph M.A., Patterson K., Garrard P., Hodges J.R. Non-verbal semantic impairment in semantic dementia. Neuropsychologia. 2000;38:1207–1215. doi: 10.1016/s0028-3932(00)00034-8. [DOI] [PubMed] [Google Scholar]
- Brett M., Anton J.L., Valabregue R., Poline J.B. Region of interest analysis using the MarsBar toolbox for SPM 99. NeuroImage. 2002;16:S497. [Google Scholar]
- Brumm K.P., Perthen J.E., Liu T.T., Haist F., Ayalon L., Love T. An arterial spin labeling investigation of cerebral blood flow deficits in chronic stroke survivors. NeuroImage. 2010;51:995–1005. doi: 10.1016/j.neuroimage.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher K.S., Parsons M., MacGregor L., Barber P.A., Chalk J., Bladin C. Refining the perfusion–diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Liu J., Adalı T. A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage. 2009;45:S163–S172. doi: 10.1016/j.neuroimage.2008.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael S.T., Tatsukawa K., Katsman D., Tsuyuguchi N., Kornblum H.I. Evolution of diaschisis in a focal stroke model. Stroke. 2004;35:758–763. doi: 10.1161/01.STR.0000117235.11156.55. [DOI] [PubMed] [Google Scholar]
- Carrera E., Tononi G. Diaschisis: Past, present, future. Brain. 2014;137:2408–2422. doi: 10.1093/brain/awu101. [DOI] [PubMed] [Google Scholar]
- Cloutman L.L., Binney R.J., Morris D.M., Parker G.J.M., Lambon Ralph M.A. Using in vivo probabilistic tractography to reveal two segregated dorsal ‘language-cognitive’ pathways in the human brain. Brain and Language. 2013;127:230–240. doi: 10.1016/j.bandl.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen R., Kelter S., Woll G. Analytical competence and language impairment in aphasia. Brain and Language. 1980;10:331–351. doi: 10.1016/0093-934x(80)90060-7. [DOI] [PubMed] [Google Scholar]
- Crinion J., Ashburner J., Leff A., Brett M., Price C., Friston K. Spatial normalization of lesioned brains: Performance evaluation and impact on fMRI analyses. NeuroImage. 2007;37:866–875. doi: 10.1016/j.neuroimage.2007.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion J., Holland A.L., Copland D.A., Thompson C.K., Hillis A.E. Neuroimaging in aphasia treatment research: Quantifying brain lesions after stroke. NeuroImage. 2013;73:208–214. doi: 10.1016/j.neuroimage.2012.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croquelois A., Wintermark M., Reichhart M., Meuli R., Bogousslavsky J. Aphasia in hyperacute stroke: Language follows brain penumbra dynamics. Annals of Neurology. 2003;54:321–329. doi: 10.1002/ana.10657. [DOI] [PubMed] [Google Scholar]
- Davis S.W., Dennis N.A., Daselaar S.M., Fleck M.S., Cabeza R. Qué PASA? The posterior–anterior shift in aging. Cerebral Cortex. 2008;18:1201–1209. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S., Fisher M., Warach S. Cambridge University Press; 2003. Magnetic resonance imaging in stroke. [Google Scholar]
- De Renzi E., Faglioni P., Scotti G., Spinnler H. Impairment in associating colour to form, concomitant with aphasia. Brain. 1972;95:293–304. doi: 10.1093/brain/95.2.293. [DOI] [PubMed] [Google Scholar]
- Dronkers N.F., Wilkins D.P., Van Valin R.D., Jr., Redfern B.B., Jaeger J.J. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Edelman R.R., Siewert B., Darby D.G., Thangaraj V., Nobre A.C., Mesulam M.M. Qualitative mapping of cerebral blood flow and functional localization with echo-planar MR imaging and signal targeting with alternating radio frequency. Radiology. 1994;192:513–520. doi: 10.1148/radiology.192.2.8029425. [DOI] [PubMed] [Google Scholar]
- Feeney D.M., Baron J.C. Diaschisis. Stroke. 1986;17:817–830. doi: 10.1161/01.str.17.5.817. [DOI] [PubMed] [Google Scholar]
- van Gelderen P., de Zwart J.A., Duyn J.H. Pitfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magnetic Resonance in Medicine. 2008;59:788–795. doi: 10.1002/mrm.21515. [DOI] [PubMed] [Google Scholar]
- Girouard H., Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. Journal of Applied Physiology. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Gold L., Lauritzen M. Neuronal deactivation explains decreased cerebellar blood flow in response to focal cerebral ischemia or suppressed neocortical function. Proceedings of the National Academy of Sciences. 2002;99:7699–7704. doi: 10.1073/pnas.112012499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodglass H., Kaplan E., Barresi B. 3rd ed. (BDAE). Baltimore Lippincott Williams & Wilkins; 2001. Boston diagnostic aphasia examination. [Google Scholar]
- Grossman M., Cooke A., DeVita C., Alsop D., Detre J., Chen W. Age-related changes in working memory during sentence comprehension: An fMRI study. NeuroImage. 2002;15:302–317. doi: 10.1006/nimg.2001.0971. [DOI] [PubMed] [Google Scholar]
- Guo X., Han Y., Chen K., Wang Y., Yao L. Mapping joint grey and white matter reductions in Alzheimer's disease using joint independent component analysis. Neuroscience Letters. 2012;531:136–141. doi: 10.1016/j.neulet.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwigsen G., Weigel A., Schuschan P., Siebner H.R., Weise D., Classen J. Dissociating parieto-frontal networks for phonological and semantic word decisions: A condition-and-perturb TMS study. Cerebral Cortex. 2016 Jun;26(6):2590–2601. doi: 10.1093/cercor/bhv092. [DOI] [PubMed] [Google Scholar]
- Hillis A.E., Wityk R.J., Barker P.B., Beauchamp N.J., Gailloud P., Murphy K. Subcortical aphasia and neglect in acute stroke: The role of cortical hypoperfusion. Brain. 2002;125:1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- Hillis A.E., Wityk R.J., Tuffiash E., Beauchamp N.J., Jacobs M.A., Barker P.B. Hypoperfusion of Wernicke's area predicts severity of semantic deficit in acute stroke. Annals of Neurology. 2001;50:561–566. doi: 10.1002/ana.1265. [DOI] [PubMed] [Google Scholar]
- Hoffman P., Pobric G., Drakesmith M., Lambon Ralph M.A. Posterior middle temporal gyrus is involved in verbal and non-verbal semantic cognition: Evidence from rTMS. Aphasiology. 2011;26:1119–1130. [Google Scholar]
- Hojjatoleslami S.A., Kruggel F. Segmentation of large brain lesions. IEEE Transactions on Medical Imaging. 2001;20:667. doi: 10.1109/42.932750. [DOI] [PubMed] [Google Scholar]
- Howard D., Patterson K. Thames Valley Test Company; Bury Saint Edmunds: 1992. Pyramids and palm trees: A test of semantic access from pictures and words. [Google Scholar]
- Iglesias S., Marchal G., Rioux P., Beaudouin V., Hauttement J., De La Sayette V. Do changes in oxygen metabolism in the unaffected cerebral hemisphere underlie early neurological recovery after stroke? A positron emission tomography study. Stroke. 1996;27:1192–1199. doi: 10.1161/01.str.27.7.1192. [DOI] [PubMed] [Google Scholar]
- Iglesias S., Marchal G., Viader F., Baron J.-C. Delayed intrahemispheric remote hypometabolism. Cerebrovascular Diseases. 2000;10:391–402. doi: 10.1159/000016096. [DOI] [PubMed] [Google Scholar]
- Jensen J.H., Lu H., Inglese M. Microvessel density estimation in the human brain by means of dynamic contrast-enhanced echo-planar imaging. Magnetic Resonance in Medicine. 2006;56:1145–1150. doi: 10.1002/mrm.21052. [DOI] [PubMed] [Google Scholar]
- Jodzio K., Gasecki D., Drumm D.A., Lass P., Nyka W. Neuroanatomical correlates of the post-stroke aphasias studied with cerebral blood flow SPECT scanning. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research. 2003;9:Mt32–41. [PubMed] [Google Scholar]
- Jones T.H., Morawetz R.B., Crowell R.M., Marcoux F.W., FitzGibbon S.J., DeGirolami U. Thresholds of focal cerebral ischemia in awake monkeys. Journal of Neurosurgery. 1981;54:773–782. doi: 10.3171/jns.1981.54.6.0773. [DOI] [PubMed] [Google Scholar]
- Kay J., Coltheart M., Lesser R. Laurence Erlbaum Associates; Hove, UK: 1992. Psycholinguistic assessments of language processing in aphasia (PALPA) [Google Scholar]
- Leff A., Crinion J., Scott S., Turkheimer F., Howard D., Wise R. A physiological change in the homotopic cortex following left posterior temporal lobe infarction. Annals of Neurology. 2002;51:553–558. doi: 10.1002/ana.10181. [DOI] [PubMed] [Google Scholar]
- Lu M., Mitsias P.D., Ewing J.R., Soltanian-Zadeh H., Bagher-Ebadian H., Zhao Q. Predicting final infarct size using acute and subacute multiparametric MRI measurements in patients with ischemic stroke. Journal of Magnetic Resonance Imaging. 2005;21:495–502. doi: 10.1002/jmri.20313. [DOI] [PubMed] [Google Scholar]
- MacIntosh B.J., Lindsay A.C., Kylintireas I., Kuker W., Günther M., Robson M.D. Multiple inflow pulsed arterial spin-labeling reveals delays in the arterial arrival time in minor stroke and transient ischemic attack. AJNR. American Journal of Neuroradiology. 2010;31:1892–1894. doi: 10.3174/ajnr.A2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden D.J., Turkington T.G., Provenzale J.M., Denny L.L., Langley L.K., Hawk T.C. Aging and attentional guidance during visual search: Functional neuroanatomy by positron emission tomography. Psychology and Aging. 2002;17:24. doi: 10.1037//0882-7974.17.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah Y.-H., Husain M., Rees G., Nachev P. Human brain lesion-deficit inference remapped. Brain. 2014;137:2522. doi: 10.1093/brain/awu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino J., Brogna C., Robles S.G., Vergani F., Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46:691–699. doi: 10.1016/j.cortex.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Metter E.J., Kempler D., Jackson C., Hanson W.R., Mazziotta J.C., Phelps M.E. Cerebral glucose metabolism in Wernicke's, Broca's, and conduction aphasia. Archives of Neurology. 1989;46:27–34. doi: 10.1001/archneur.1989.00520370029014. [DOI] [PubMed] [Google Scholar]
- Meulenbroek O., Petersson K.M., Voermans N., Weber B., Fernández G. Age differences in neural correlates of route encoding and route recognition. NeuroImage. 2004;22:1503–1514. doi: 10.1016/j.neuroimage.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Mitra J., Bourgeat P., Fripp J., Ghose S., Rose S., Salvado O. Lesion segmentation from multimodal MRI using random forest following ischemic stroke. NeuroImage. 2014;98:324–335. doi: 10.1016/j.neuroimage.2014.04.056. [DOI] [PubMed] [Google Scholar]
- Morris J., Franklin S., Ellis A.W., Turner J.E., Bailey P.J. Remediating a speech perception deficit in an aphasic patient. Aphasiology. 1996;10:137–158. [Google Scholar]
- Neumann-Haefelin T., Wittsack H.-J., Wenserski F., Siebler M., Seitz R.J., Mödder U. Diffusion- and perfusion-weighted MRI: The DWI/PWI mismatch region in acute stroke. Stroke. 1999;30:1591–1597. doi: 10.1161/01.str.30.8.1591. [DOI] [PubMed] [Google Scholar]
- Noonan K.A., Jefferies E., Visser M., Lambon Ralph M.A. Going beyond inferior prefrontal involvement in semantic control: Evidence for the additional contribution of dorsal angular gyrus and posterior middle temporal cortex. Journal of Cognitive Neuroscience. 2013;25:1824–1850. doi: 10.1162/jocn_a_00442. [DOI] [PubMed] [Google Scholar]
- Ogar J.M., Baldo J.V., Wilson S.M., Brambati S.M., Miller B.L., Dronkers N.F. Semantic dementia and persisting Wernicke's aphasia: Linguistic and anatomical profiles. Brain and Language. 2011;117:28–33. doi: 10.1016/j.bandl.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkes L.M., Rashid W., Chard D.T., Tofts P.S. Normal cerebral perfusion measurements using arterial spin labeling: Reproducibility, stability, and age and gender effects. Magnetic Resonance in Medicine. 2004;51:736–743. doi: 10.1002/mrm.20023. [DOI] [PubMed] [Google Scholar]
- Pedersen P.M., Vinter K., Olsen T.S. Aphasia after stroke: Type, severity and prognosis – The Copenhagen aphasia study. Cerebrovascular Diseases. 2004;17:35–43. doi: 10.1159/000073896. [DOI] [PubMed] [Google Scholar]
- Price C.J., Warburton E.A., Moore C.J., Frackowiak R.S.J., Friston K.J. Dynamic diaschisis: Anatomically remote and context-sensitive human brain lesions. Journal of Cognitive Neuroscience. 2001;13:419–429. doi: 10.1162/08989290152001853. [DOI] [PubMed] [Google Scholar]
- Rachakonda S., Liu J., Calhoun V.D. 2012. Fusion ICA toolbox (FIT) manual. [Google Scholar]
- Raven J.C. H. K. Lewis; London: 1962. Coloured progressive matrices sets A, AB, B. [Google Scholar]
- Raynaud C., Rancurel G., Samson Y., Baron J.C., Soucy J.P., Kieffer E. Pathophysiologic study of chronic infarcts with I-123 isopropyl iodo-amphetamine (IMP): The importance of periinfarct area. Stroke. 1987;18:21–29. doi: 10.1161/01.str.18.1.21. [DOI] [PubMed] [Google Scholar]
- Rekik I., Allassonnière S., Carpenter T.K., Wardlaw J.M. Medical image analysis methods in MR/CT-imaged acute-subacute ischemic stroke lesion: Segmentation, prediction and insights into dynamic evolution simulation models. A critical appraisal. NeuroImage: Clinical. 2012;1:164–178. doi: 10.1016/j.nicl.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts D.A., Rizi R., Lenkinski R.E., Leigh J.S. Magnetic resonance imaging of the brain: Blood partition coefficient for water: Application to spin-tagging measurement of perfusion. Journal of Magnetic Resonance Imaging. 1996;6:363–366. doi: 10.1002/jmri.1880060217. [DOI] [PubMed] [Google Scholar]
- Robson H., Cloutman L., Keidel J.L., Sage K., Drakesmith M., Welbourne S. Mismatch negativity (MMN) reveals inefficient auditory ventral stream function in chronic auditory comprehension impairments. Cortex. 2014;59:113–125. doi: 10.1016/j.cortex.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Robson H., Grube M., Lambon Ralph M.A., Griffiths T.D., Sage K. Fundamental deficits of auditory perception in Wernicke's aphasia. Cortex. 2013;49:1808–1822. doi: 10.1016/j.cortex.2012.11.012. [DOI] [PubMed] [Google Scholar]
- Robson H., Sage K., Lambon Ralph M.A. Wernicke's aphasia reflects a combination of acoustic-phonological and semantic control deficits: A case-series comparison of Wernicke's aphasia, semantic dementia and semantic aphasia. Neuropsychologia. 2012;50:266–275. doi: 10.1016/j.neuropsychologia.2011.11.021. [DOI] [PubMed] [Google Scholar]
- Robson H., Zahn R., Keidel J.L., Binney R.J., Sage K., Lambon Ralph M.A. The anterior temporal lobes support residual comprehension in Wernicke's aphasia. Brain. 2014;137:931–943. doi: 10.1093/brain/awt373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.F., Faseyitan O., Kim J., Coslett H.B. The dorsal stream contribution to phonological retrieval in object naming. Brain. 2012;135:3799–3814. doi: 10.1093/brain/aws300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.F., Kimberg D.Y., Walker G.M., Brecher A., Faseyitan O.K., Dell G.S. Neuroanatomical dissociation for taxonomic and thematic knowledge in the human brain. Proceedings of the National Academy of Sciences. 2011;108:8520–8524. doi: 10.1073/pnas.1014935108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Fagan E., Price C.J. Functional subdivisions in the left angular gyrus where the semantic system meets and diverges from the default network. The Journal of Neuroscience. 2010;30:16809–16817. doi: 10.1523/JNEUROSCI.3377-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M.L., Ramlackhansingh A., Crinion J., Leff A.P., Price C.J. Lesion identification using unified segmentation-normalisation models and fuzzy clustering. NeuroImage. 2008;41:1253–1266. doi: 10.1016/j.neuroimage.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz R.J., Azari N.P., Knorr U., Binkofski F., Herzog H., Freund H.-J. The role of diaschisis in stroke recovery. Stroke. 1999;30:1844–1850. doi: 10.1161/01.str.30.9.1844. [DOI] [PubMed] [Google Scholar]
- Specht K., Zahn R., Willmes K., Weis S., Holtel C., Krause B.J. Joint independent component analysis of structural and functional images reveals complex patterns of functional reorganisation in stroke aphasia. NeuroImage. 2009;47:2057–2063. doi: 10.1016/j.neuroimage.2009.06.011. [DOI] [PubMed] [Google Scholar]
- Stanisz G.J., Odrobina E.E., Pun J., Escaravage M., Graham S.J., Bronskill M.J. T1, T2 relaxation and magnetization transfer in tissue at 3T. Magnetic Resonance in Medicine. 2005;54:507–512. doi: 10.1002/mrm.20605. [DOI] [PubMed] [Google Scholar]
- Stone J.V. John Wiley & Sons, Ltd; 2005. Independent component analysis. Encyclopedia of statistics in behavioral science. [Google Scholar]
- Tessier C., Weill-Chounlamountry A., Michelot N., Pradat-Diehl P. Rehabilitation of word deafness due to auditory analysis disorder. Brain Injury. 2007;21:1165–1174. doi: 10.1080/02699050701559186. [DOI] [PubMed] [Google Scholar]
- Toni D., Fiorelli M., Bastianello S., Falcou A., Sette G., Ceschin V. Acute ischemic strokes improving during the first 48 hours of Onset: Predictability, outcome, and possible mechanisms: A comparison with early deteriorating strokes. Stroke. 1997;28:10–14. doi: 10.1161/01.str.28.1.10. [DOI] [PubMed] [Google Scholar]
- Ueno T., Saito S., Rogers T.T., Lambon Ralph M.A. Lichtheim 2: Synthesizing aphasia and the neural basis of language in a neurocomputational model of the dual dorsal-ventral language pathways. Neuron. 2011;72:385–396. doi: 10.1016/j.neuron.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Weiller C., Isensee C., Rijntjes M., Huber W., Müller S., Bier D. Recovery from Wernicke's aphasia: A positron emission tomographic study. Annals of Neurology. 1995;37:723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]
- Whitney C., Kirk M., O'Sullivan J., Lambon Ralph M.A., Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex. 2011;21(5):1066–1075. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf C., Panton A., Rosen S., Best W., Marshall J. Therapy for auditory processing impairment in aphasia: An evaluation of two approaches. Aphasiology. 2014;28:1481–1505. [Google Scholar]