Abstract
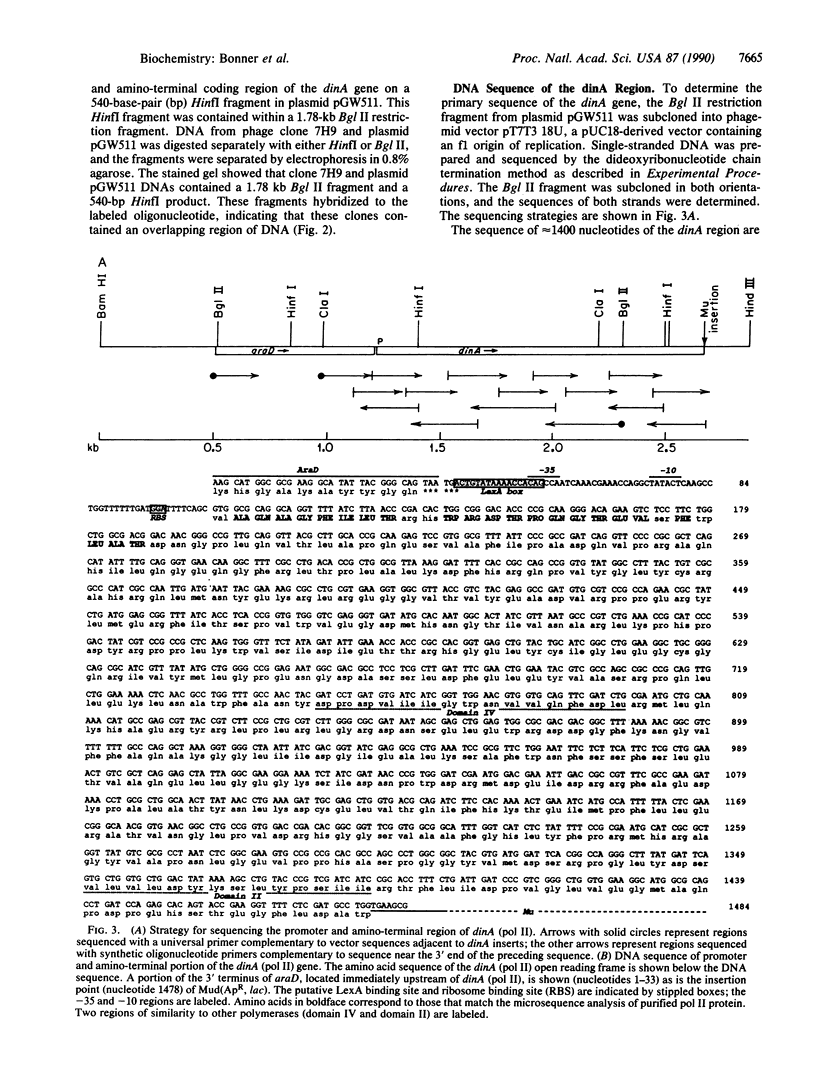
The structural gene for DNA polymerase II was cloned by using a synthetic inosine-containing oligonucleotide probe corresponding to 11 amino acids, which were determined by sequencing the amino terminus of the purified protein. The labeled oligonucleotide hybridized specifically to the lambda clone 7H9 from the Kohara collection as well as to plasmid pGW511 containing the SOS-regulated dinA gene. Approximately 1400 base pairs of dinA sequence were determined. The predicted amino-terminal sequence of dinA demonstrated that this gene encoded DNA polymerase II. Sequence analysis of the upstream region localized a LexA binding site overlapping the -35 region of the dinA promoter, and this promoter element was found to be only two nucleotides downstream from the 3' end of the araD gene. These results demonstrate that the gene order is thr-dinA (pol II)-ara-leu on the Escherichia coli chromosome and that the DNA polymerase II structural gene is transcribed in the same direction as the araBAD operon. Based on the analysis of the predicted protein, we have identified a sequence motif Asp-Xaa-Xaa-Ser-Leu-Tyr-Pro-Ser in DNA polymerase II that is highly conserved among a diverse group of DNA polymerases, which include those from humans, yeast, Herpes and vaccinia viruses, and phages T4 and PRD1. The demonstration that DNA polymerase II is a component of the SOS response in E. coli suggests that it plays an important role in DNA repair and/or mutagenesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner C. A., Randall S. K., Rayssiguier C., Radman M., Eritja R., Kaplan B. E., McEntee K., Goodman M. F. Purification and characterization of an inducible Escherichia coli DNA polymerase capable of insertion and bypass at abasic lesions in DNA. J Biol Chem. 1988 Dec 15;263(35):18946–18952. [PubMed] [Google Scholar]
- Campbell J. L., Shizuya H., Richardson C. C. Mapping of a mutation, polB100, affecting deoxyribonucleic acid polymerase II in Escherichia coli K-12. J Bacteriol. 1974 Aug;119(2):494–499. doi: 10.1128/jb.119.2.494-499.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. L., Soll L., Richardson C. C. Isolation and partial characterization of a mutant of Escherichia coli deficient in DNA polymerase II. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2090–2094. doi: 10.1073/pnas.69.8.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Bryan S. K., Moses R. E. Cloning the polB gene of Escherichia coli and identification of its product. J Biol Chem. 1989 Dec 5;264(34):20591–20595. [PubMed] [Google Scholar]
- Clarke L., Carbon J. Selection of specific clones from colony banks by suppression or complementation tests. Methods Enzymol. 1979;68:396–408. doi: 10.1016/0076-6879(79)68029-1. [DOI] [PubMed] [Google Scholar]
- Gough J. A., Murray N. E. Sequence diversity among related genes for recognition of specific targets in DNA molecules. J Mol Biol. 1983 May 5;166(1):1–19. doi: 10.1016/s0022-2836(83)80047-3. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Gefter M., Mindich L. A mutant of Escherichia coli defective in DNA polymerase II activity. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3238–3242. doi: 10.1073/pnas.69.11.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce C. M., Grindley N. D. Method for determining whether a gene of Escherichia coli is essential: application to the polA gene. J Bacteriol. 1984 May;158(2):636–643. doi: 10.1128/jb.158.2.636-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung G. H., Leavitt M. C., Hsieh J. C., Ito J. Bacteriophage PRD1 DNA polymerase: evolution of DNA polymerases. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8287–8291. doi: 10.1073/pnas.84.23.8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. J., Brent R., Ptashne M., Walker G. C. Regulation of damage-inducible genes in Escherichia coli. J Mol Biol. 1982 Sep 25;160(3):445–457. doi: 10.1016/0022-2836(82)90307-2. [DOI] [PubMed] [Google Scholar]
- Kenyon C. J., Walker G. C. DNA-damaging agents stimulate gene expression at specific loci in Escherichia coli. Proc Natl Acad Sci U S A. 1980 May;77(5):2819–2823. doi: 10.1073/pnas.77.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Kornberg T., Gefter M. L. Purification and DNA synthesis in cell-free extracts: properties of DNA polymerase II. Proc Natl Acad Sci U S A. 1971 Apr;68(4):761–764. doi: 10.1073/pnas.68.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Gielow W., Martin R., Hamilton E., Fowler A. The organization of the araBAD operon of Escherichia coli. Gene. 1986;47(2-3):231–244. doi: 10.1016/0378-1119(86)90067-3. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Takeda Y., Nishimura A., Suzuki H., Inouye M., Hirota Y. Synthetic ColE1 plasmids carrying genes for cell division in Escherichia coli. Plasmid. 1977 Nov;1(1):67–77. doi: 10.1016/0147-619x(77)90009-9. [DOI] [PubMed] [Google Scholar]
- Sancar A., Sancar G. B., Rupp W. D., Little J. W., Mount D. W. LexA protein inhibits transcription of the E. coli uvrA gene in vitro. Nature. 1982 Jul 1;298(5869):96–98. doi: 10.1038/298096a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. C. Mutagenesis and inducible responses to deoxyribonucleic acid damage in Escherichia coli. Microbiol Rev. 1984 Mar;48(1):60–93. doi: 10.1128/mr.48.1.60-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T. S., Wong S. W., Korn D. Human DNA polymerase alpha: predicted functional domains and relationships with viral DNA polymerases. FASEB J. 1989 Jan;3(1):14–21. doi: 10.1096/fasebj.3.1.2642867. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]