Abstract
Background
Several recent papers have called into question the deleterious effects of high animal fat diets due to mixed results from epidemiologic studies and the lack of clinical trial evidence in meta-analyses of dietary intervention trials. We were interested in examining the theoretical effects of substituting plant-based fats from different types of margarine for animal based fat from butter on the risk of atherosclerosis-related cardiovascular disease (CVD).
Methods
We prospectively studied 71,410 women, aged 50–79 years, and evaluated their risk for clinical myocardial infarction (MI), total coronary heart disease (CHD), ischemic stroke and atherosclerosis-related CVD with an average of 13.2 years of follow-up. Butter and margarine intakes were obtained at baseline and Year 3 by means of a validated food frequency questionnaire. Cox proportional hazards regression using a cumulative average diet method was used to estimate the theoretical effect of substituting 1 teaspoon/day of three types of margarine for the same amount of butter.
Results
Substituting butter or stick margarine with tub margarine was associated with lower risk of MI (HRs=0.95 and 0.91). Subgroup analyses, which evaluated these substitutions among participants with a single source of spreadable fat, showed stronger associations for MI (HRs=0.92 and 0.87). Outcomes of total CHD, ischemic stroke, and atherosclerosis-related CVD showed wide confidence intervals but the same trends as the MI results.
Conclusions
This theoretical dietary substitution analysis suggests that substituting butter and stick margarine with tub margarine when spreadable fats are eaten may be associated with reduced risk of myocardial infarction.
Keywords: diet, butter, margarine, cardiovascular disease, substitution
INTRODUCTION
Diets low in saturated fats and trans fats are recommended by both the American Heart Association and the European Food Safety Authority based on data1,2 suggesting that diets rich in saturated fats and trans fats are associated with high risk for coronary heart disease, both through effects on lipoprotein metabolism and effects on endothelial function, membrane fluidity, and inflammation.3,4 These recommendations rely largely on observational studies of an increased risk of coronary heart disease in participants with diets high in saturated fats and trans fats5–11 and on feeding studies that demonstrate reduced total and low-density lipoprotein (LDL) cholesterol and increased high-density lipoprotein (HDL) cholesterol levels when substituting saturated fatty acids (SFA) and trans fatty acids (TFA) with polyunsaturated fatty acids (PUFA) and monounsaturated fatty acids (MUFA).12–14 One potentially practical way to reduce SFA and TFA in the diet is to substitute butter, which is high in SFA, and stick margarine, which is high in TFA, with tub margarine.15,16 This is because of the differences in fatty acid profiles of butter (SFA 51%, MUFA 21%, PUFA 3%, TFA 3%), stick margarine (SFA 15%, MUFA 39%, PUFA 24%, TFA 15%–21%), tub margarine (SFA 14%, MUFA 36%, PUFA 27%, TFA 5%), and low-fat margarine (SFA 3%–12%, MUFA 7%–30%, PUFA 8%–26%, TFA 1%–17%).17–19
Some recent publications have questioned these recommendations based on the heterogeneity of results of prospective observational studies and randomized control trials, which have failed to support these dietary guidelines that encourage low consumption of SFA and high consumption of PUFA.5,20,21 These negative studies focused on the heterogeneity of effects of specific SFA with some demonstrating an increased risk of coronary heart disease (CHD) and some showing a protective effect, and other showing no effect at all. One explanation of the difficulty finding consistent diet-heart associations in observational studies is the misclassification bias from self-reported dietary assessments.22 Most previous studies have focused on the associations of SFA, MUFA, PUFA, and TFA, rather than the food sources of these fats, with cardiovascular disease (CVD) risk.5–11 Previous feeding studies that measured the substitution association between butter and margarine on CVD risk have utilized lipoprotein surrogates and not incident cardiovascular events.23,24 No epidemiologic studies to date have examined the theoretical effects of substituting butter with different types of margarine on the risk of developing CVD. Dietary substitution methods focusing on specific foods related to similar serving size have been developed, thus making spreadable fats substitution analysis possible.25,26 We were therefore interested in testing the long-term association of this diet substitution on the risk of myocardial infarction (MI), total CHD, ischemic stroke and atherosclerosis-related CVD.
SUBJECTS AND METHODS
Study Population
The Women’s Health Initiative (WHI) enrolled 93,676 postmenopausal women into its observational study at 40 clinical center, from 1994 to 1998. Details of the study design, recruitment, baseline questionnaires and examinations performed have been published previously.27–29 All women at each of the 40 clinical centers provided written informed consent which were approved by their respective institutional review boards. Among the 93,676 women, those who had a self-reported MI, coronary artery bypass graft (CABG), percutaneous transluminal coronary angioplasty or stroke at baseline were excluded to create a disease free cohort. Additionally, we excluded baseline cancer and diabetes as they may lead to changes in diet, and therefore reported diet may not reflect long-term dietary exposures. Women with implausible energy intake (<600 kcal/d or >5000 kcal/d) were also excluded. This resulted in the inclusion of 71,410 women in this analysis. Additionally, we divided the whole population into three groups according to their usage of spreadable fats. Those who neither used butter nor margarine comprised the non-user group; those who used only one type of fats (butter or a specific type of margarine) were in the single-source group; and those who used more than one type of fats were in the multiple-source group. The population selection process is shown in Figure 1.
FIGURE 1.
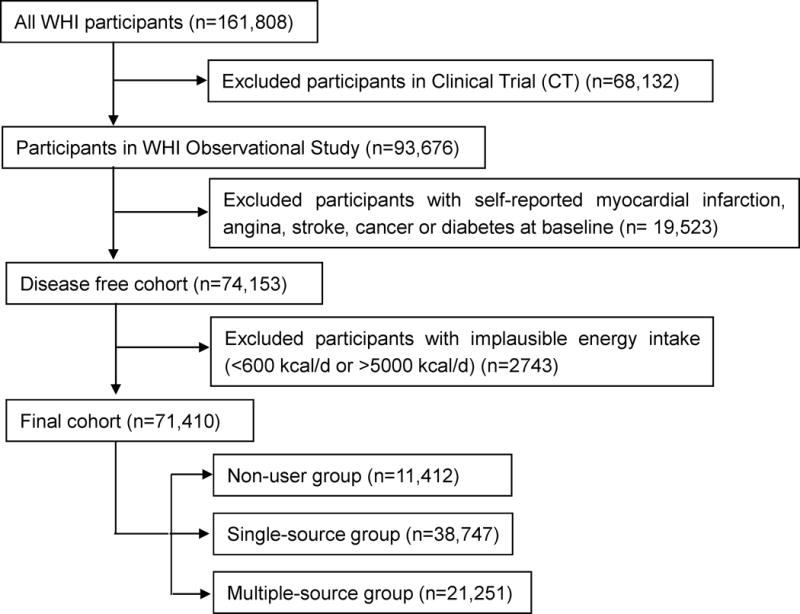
Population selection process. WHI: Women’s Health Initiative
Exposures
The primary exposure of interests were the intakes of butter, stick margarine, tub margarine and low-fat margarine, which were estimated from food frequency questionnaires (FFQs) at baseline and Year 3. The FFQ was based on instruments used in the WHI feasibility studies27,28 and the original National Cancer Institute/Block FFQ.28,29 Four questions in the FFQ asked participants to indicate one or two types of fat that they usually used in food preparation or added after cooking. The questions were: What kinds of fat did you usually use to deep fry, pan fry, or sauté foods? What kinds of fat did you usually add when cooking beans, rice, vegetables, and potatoes? What kinds of fat did you usually add after cooking vegetables, beans, rice, and potatoes? What kinds of fat did you usually use on breads, muffins, tortillas, and rolls? Choices included stick margarine, tub margarine, butter, shortening, olive or canola oil, other oils (vegetable, corn, peanut, safflower), non-stick spray, and didn’t add fat. Correspondingly, the frequency and portion size of fats were measured. Teaspoon (tsp) was used to measure the portion size of spreadable fats (1 tsp=4.73 grams). For those women who only chose one type of fat, the intake was directly calculated from the product of frequency and portion size. For those women who chose two types of fats, we applied a weight of 0.5 for each type of fat that was chosen and calculated the intake of a certain kind of fat by multiplying the product of frequency and portion size by 0.5. The total intake of a certain type of fat was the sum of these four questions.
The intakes of spreadable fats at baseline and Year 3 were computed separately. We used the cumulative average diet method to evaluate the associations of higher levels of spreadable fats consumptions with each outcome and the theoretical effects of substituting equal servings of spreadable fats.9,30 In this method, disease incidence within the first 3 years was related to butter and margarine intakes from the baseline FFQs only, while the disease incidence following the first 3 years was related to the average intake between the baseline and Year 3 FFQs. For those who failed to complete the FFQs in Year 3 (n=8980), baseline FFQs were used. We performed a sensitivity analysis by using the baseline diet only method, in which disease incidence was related to butter and margarine intakes from the baseline FFQs only.
Outcomes
All outcomes were identified annually by medical record review of self-reported hospitalizations and ascertained by the trained physician adjudicator.31 The WHI algorithm for classifying hospitalized MI includes elements of the medical history, electrocardiogram (ECG) readings, and results of cardiac enzyme determinations, and is adapted from standardized criteria.31 Total CHD is defined as the first occurrence of MI, CABG, percutaneous transluminal coronary angioplasty, and CHD death. CHD death was based on medical record review of hospital, emergency room records and emergency medical service records at the time of death, previous hospitalizations for CHD, death certificates, autopsy, coroners and next of kin reports.31 Ischemic stroke was confirmed by medical record documentation of rapid onset of a neurologic deficit consistent with stroke and lasting at least 24 hours or until death. Atherosclerotic related CVD included MI, incident CHD, CHD related death and ischemic stroke.
Potential Confounders
Information on demographic and social economic status (SES), lifestyle factors and CHD risk factors were obtained by self-report at baseline. Demographic and SES included age, region, race/ethnicity and income. Lifestyle factors were defined as physical activity, body mass index (BMI) and smoking. CHD risk factor included family history of MI, postmenopausal hormone use, aspirin use, hysterectomy, treated hypercholesterolemia and hypertension. Age was treated as a continuous variable. For analytic purposes, income was categorized as <$20,000, $20,000 to $74,999, and ≥ $75,000 per year. Physical activity was measured by total physical activity score (MET-h/week) which was computed from a series of questions related to walking and exercise at strenuous levels, and physical activity at moderate- and low-intensity levels.32 BMI was categorized as normal or underweight (<25 kg/m2), overweight (25–<30 kg/m2), and obesity (≥30 kg/m2), or treated as continuous variable. Smoking was categorized as never, past, or current. Family history of MI was defined as first relatives having a MI. Postmenopausal hormone use was categorized as never, past, or current. Treated hypercholesterolemia was defined as taking cholesterol-lowering medication. Hypertension was defined by self-reported hypertension and taking antihypertensive medication or systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg. For those categorical variables with more than 1% missing data, including income, family history of MI and smoking, we created a missing category for each of them. For missing continuous variables and those categorical variables with less than 1% missing values, we excluded them in multivariable analysis.
Potential dietary confounders were total energy intake and the Alternative Healthy Eating Index (AHEI), which were obtained from the baseline and Year 3 FFQs using cumulative average diet method as previously described.9 AHEI, as a measure of dietary quality, was calculated from a series of items from the FFQ, including vegetables, fruits, whole grains, sugar-sweetened beverage, nuts, legumes, red meat, TFA%, long-chain fats, PUFA%, sodium, and alcohol intake, using the standard methodology as previously described.33 To prevent over adjustment, we didn’t evaluate AHEI and nutrient density measures of SFA, TFA or PUFA in our models as potential confounders, but adjusted for AHEI or food groups (red meat, fruits, vegetables, and whole grains) in sensitivity analyses.
Statistical Methods
We calculated the mean (SD) intakes of spreadable fats by baseline characteristics (continuous variables were categorized as under median and above median). Person-years of follow up were calculated from the date of the return of baseline FFQ to the following, whichever came first: the first occurrence of each event, loss to follow-up or the end of the study follow-up (September 30, 2013). Cox proportional hazards regression using the cumulative average diet method was used.9,30 In multivariable analyses, we regarded demographic and SES, lifestyle factors, dietary factors, and CHD risk factors as potential confounders. The fully adjusted model included all of the above variables. We found no evidence of violation of the proportional hazard assumption on the basis of Schoenfeld residuals or the Wald test for an interaction between the exposure of interest and follow-up time.
Dietary substitution analyses
In order to estimate the theoretical effects of substituting 1 tsp /day of three types of margarine for the same amount of butter on the risk of CVD, we included butter, stick margarine, tub margarine and low-fat margarine in one multivariable model. Difference in regression coefficients was used to estimate the hazard ratio (HR) for each substitution association. For example, the difference between the regression coefficients for 1 tsp of tub margarine and 1 tsp of butter was used to define the effect of substituting tub margarine for butter, and stick margarine and low fat margarine were treated as covariates in the model. The 95% confidence interval (CI) was derived from the variances and covariance matrix of the coefficients.26 When examining the association of substituting tub margarine and low-fat margarine for butter and stick margarine on CVD risk, the sum of tub and low-fat margarine as well as the sum of butter and stick margarine were included in one model. Butter and the sum of three types of margarine were included in one model to obtain the association of substituting total margarine for butter. The association of substituting butter and stick margarine with tub margarine were determined by including tub margarine and the sum of butter and stick margarine intake in one model. The substitution associations were measured overall, as well as among the single-source group and multiple-source group. Further substitution analysis was performed by stratifying all participants according to tertiles of dietary total fat.
Associations of increased spreadable fat consumptions with each outcome
The associations between increased spreadable fats and four outcomes were measured using 1) unadjusted; 2) demographic and SES factor adjusted; 3) demographic and lifestyle factor adjusted; 4) demographic and dietary factor adjusted; 5) demographic, lifestyle, and dietary factors adjusted; and 6) fully adjusted models. The associations were also examined among participants using single source of spreadable fats.
All data were analyzed with the Statistics Analysis Systems software package (version 9.3; SAS Institute, Inc., Cary, NC).
RESULTS
The mean (SD) age for all participants was 63.1 (7.3) years and the average (SD) follow-up person time was 13.2 (4.3) years. Of the 71,410 participants, 288 were lost to follow-up for events. The numbers of events documented during follow-up were 1914 for MI, 4344 for total CHD, 1588 for ischemic stroke, and 5654 atherosclerosis-related CVD. The average (SD) consumptions among users were 6.15 (7.6) g/day for butter, 5.06 (6.6) g/day for stick margarine, 5.87 (6.6) g/day for tub margarine and 4.78 (5.7) g/day low-fat margarine. Among all participants, 84% were butter or margarine users, and 54% used one source of spreadable fat. The Pearson correlation coefficients among different spreadable fats were between −0.097 to 0.00003.
The mean consumptions of spreadable fats by baseline characteristics are shown in Table 1. Women who lived in the Midwest had higher intakes of both butter and margarine compared with other areas. White/non-Hispanic participants were higher in butter and tub margarine intakes; Black participants were higher in stick margarine intake. Women who had lower income had higher intakes of margarine. Physical activity and AHEI were inversely associated with butter, stick, and tub margarine consumptions, while BMI, smoking, total energy, and aspirin use were positively associated with spreadable fat consumptions.
Table 1.
The mean (SD) intakes for butter and margarine by baseline characteristics among all participants. (N=71,410)
| Subjects (%) |
Butter (Tsp/day) |
Stick Margarine (Tsp/day) |
Tub Margarine (Tsp/day) |
Low-fat Margarine (Tsp/day) |
|
|---|---|---|---|---|---|
| Overall means (SD) | 71,410 | 0.42 (1.1) | 0.30 (0.9) | 0.37 (1.0) | 0.28 (0.8) |
|
| |||||
| Spreadable fats consumers (%) | 59,998 (84) | 23,310 (33) | 20,389 (29) | 21,175 (30) | 19,477 (27) |
|
| |||||
| Means among consumers (SD) | 1.30 (1.6) | 1.07 (1.4) | 1.24 (1.4) | 1.01 (1.2) | |
| Demographics | |||||
| Age a | |||||
| < 63 | 33,902 (47) | 0.43 (1.1) | 0.27 (0.8) | 0.36 (1.0) | 0.27 (0.8) |
| ≥ 63 | 37,508 (53) | 0.42 (1.1) | 0.33 (0.9) | 0.37 (1.0) | 0.28 (0.8) |
| Region | |||||
| Northeast | 16,645 (23) | 0.52 (1.2) | 0.30 (0.9) | 0.33 (0.9) | 0.25 (0.7) |
| South | 17,969 (25) | 0.29 (0.9) | 0.31 (0.9) | 0.32 (0.9) | 0.31 (0.8) |
| Midwest | 15,734 (22) | 0.49 (1.2) | 0.37 (1.0) | 0.47 (1.1) | 0.31 (0.8) |
| West | 21,062 (29) | 0.42 (1.1) | 0.25 (0.8) | 0.35 (0.9) | 0.24 (0.7) |
| Race | |||||
| American India/Alaska Native | 245 (0) | 0.33 (0.9) | 0.20 (0.6) | 0.29 (0.7) | 0.33 (0.8) |
| Asian/Pacific Islander | 2,048 (3) | 0.20 (0.6) | 0.14 (0.5) | 0.17 (0.5) | 0.10 (0.3) |
| Black/African American | 4,843 (7) | 0.28 (0.8) | 0.42 (1.0) | 0.33 (0.9) | 0.25 (0.8) |
| Hispanic/Latino | 2,586 (4) | 0.21 (0.7) | 0.19 (0.7) | 0.22 (0.7) | 0.18 (0.6) |
| White, non-Hispanic | 60,708 (85) | 0.45 (1.1) | 0.31 (0.9) | 0.38 (1.0) | 0.29 (0.8) |
| Other | 793 (1) | 0.50 (1.4) | 0.21 (0.7) | 0.28 (0.9) | 0.22 (0.6) |
| Education | |||||
| ≤High school | 20,856 (29) | 0.40 (1.1) | 0.40 (1.0) | 0.46 (1.1) | 0.29 (0.8) |
| Some college | 27,370 (39) | 0.45 (1.1) | 0.29 (0.8) | 0.36 (0.9) | 0.29 (0.8) |
| Post-graduate | 22,617 (32) | 0.42 (1.1) | 0.23 (0.7) | 0.29 (0.8) | 0.25 (0.7) |
| Income | |||||
| <$20,000 | 9,183 (13) | 0.42 (1.2) | 0.39 (1.0) | 0.42 (1.1) | 0.29 (0.8) |
| $20,000–$74,999 | 42,515 (60) | 0.43 (1.1) | 0.31 (0.9) | 0.39 (1.0) | 0.29 (0.8) |
| ≥$75,000 | 14,564 (20) | 0.42 (1.0) | 0.21 (0.7) | 0.28 (0.8) | 0.23 (0.7) |
| Missing | 5,148 (7) | 0.41 (1.1) | 0.33 (0.9) | 0.36 (1.0) | 0.25 (0.7) |
| Lifestyle | |||||
| Physical Activity (MET-h/wk)a | |||||
| < 10.5 | 35,113 (50) | 0.48 (1.2) | 0.37 (1.0) | 0.43 (1.1) | 0.28 (0.8) |
| ≥10.5 | 35,440 (50) | 0.37 (1.0) | 0.24 (0.7) | 0.30 (0.9) | 0.27 (0.7) |
| BMI (Kg/m2) | |||||
| Normal or underweight (<25) | 30,250 (43) | 0.39 (1.0) | 0.27 (0.8) | 0.32 (0.8) | 0.23 (0.7) |
| Overweight (25~<30) | 24,197 (34) | 0.40 (1.1) | 0.30 (0.9) | 0.37 (1.0) | 0.30 (0.8) |
| Obesity (≥30) | 16,150 (23) | 0.52 (1.3) | 0.37 (1.0) | 0.45 (1.1) | 0.34 (0.9) |
| Smoking | |||||
| Never-smoker | 36,155 (51) | 0.38 (1.0) | 0.32 (0.9) | 0.37 (0.9) | 0.27 (0.7) |
| Past smoker | 30,054 (42) | 0.44 (1.1) | 0.28 (0.8) | 0.35 (1.0) | 0.29 (0.8) |
| Current smoker | 4,219 (6) | 0.67 (1.4) | 0.39 (1.1) | 0.46 (1.2) | 0.25 (0.8) |
| Missing | 982 (1) | 0.43 (1.3) | 0.27 (0.7) | 0.34 (0.9) | 0.27 (0.7) |
| Dietary Factors | |||||
| Dietary quality (AHEI)a | |||||
| < 46.8 | 35,610 (50) | 0.44 (1.1) | 0.39 (1.0) | 0.44 (1.0) | 0.25 (0.7) |
| ≥ 46.8 | 35,780 (50) | 0.41 (1.1) | 0.22 (0.8) | 0.29 (0.9) | 0.31 (0.8) |
| Total energy (kcal/d)a | |||||
| < 1482.4 | 35,706 (50) | 0.24 (0.6) | 0.18 (0.5) | 0.23 (0.6) | 0.21 (0.6) |
| ≥ 1482.4 | 35,704 (50) | 0.60 (1.4) | 0.43 (1.1) | 0.51 (1.2) | 0.34 (0.9) |
| Calibrated total energy (kcal/d)a | |||||
| <2241.8 | 35,302 (50) | 0.36 (0.9) | 0.29 (0.8) | 0.32 (0.8) | 0.24 (0.7) |
| ≥2241.8 | 35,295 (50) | 0.49 (1.2) | 0.32 (0.9) | 0.41 (1.1) | 0.31 (0.9) |
| Total Fat (% of total energy)a | |||||
| <29.3 | 35,811 (50) | 0.15 (0.4) | 0.10 (0.4) | 0.18 (0.5) | 0.26 (0.7) |
| ≥29.3 | 35,599 (50) | 0.70 (1.4) | 0.51 (1.1) | 0.55 (1.2) | 0.30 (0.9) |
| SFA (g/d)a | |||||
| <15.4 | 35,572 (50) | 0.11 (0.3) | 0.13 (0.4) | 0.20 (0.6) | 0.23 (0.6) |
| ≥15.4 | 35,838 (50) | 0.73 (1.5) | 0.47 (1.1) | 0.53 (1.2) | 0.32 (0.9) |
| SFA (%of total energy)a | |||||
| <9.6 | 35,533 (50) | 0.09 (0.3) | 0.14 (0.5) | 0.23 (0.7) | 0.29 (0.7) |
| ≥9.6 | 35,877 (50) | 0.75 (1.5) | 0.47 (1.1) | 0.50 (1.2) | 0.26 (0.8) |
| MUFA (g/d)a | |||||
| <17.7 | 35,655 (50) | 0.18 (0.5) | 0.10 (0.3) | 0.15 (0.4) | 0.21 (0.5) |
| ≥17.7 | 35,755 (50) | 0.67 (1.4) | 0.51 (1.1) | 0.58 (1.3) | 0.34 (0.9) |
| MUFA (%of total energy)a | |||||
| <11.0 | 35,418 (50) | 0.19 (0.5) | 0.09 (0.3) | 0.15 (0.5) | 0.25 (0.6) |
| ≥11.0 | 35,992 (50) | 0.65 (1.4) | 0.51 (1.1) | 0.58 (1.2) | 0.30 (0.9) |
| PUFA (g/d)a | |||||
| <9.8 | 35,972 (50) | 0.24 (0.7) | 0.11 (0.3) | 0.15 (0.4) | 0.19 (0.5) |
| ≥9.8 | 35,483 (50) | 0.61 (1.4) | 0.50 (1.2) | 0.58 (1.3) | 0.37 (1.0) |
| PUFA (%of total energy)a | |||||
| <6.0 | 35,553 (50) | 0.34 (0.9) | 0.11 (0.4) | 0.17 (0.5) | 0.21 (0.6) |
| ≥6.0 | 35,857 (50) | 0.51 (1.2) | 0.50 (1.1) | 0.57 (1.2) | 0.34 (0.9) |
| TFA (g/d)a | |||||
| <3.0 | 35,931 (50) | 0.23 (0.6) | 0.05 (0.2) | 0.06 (0.2) | 0.21 (0.5) |
| ≥3.0 | 35,479 (50) | 0.62 (1.4) | 0.57 (1.2) | 0.67 (1.3) | 0.34 (0.9) |
| TFA (%of total energy)a | |||||
| <1.8 | 34,848 (49) | 0.28 (0.7) | 0.03 (0.1) | 0.04 (0.2) | 0.24 (0.6) |
| ≥1.8 | 36,562 (51) | 0.56 (1.4) | 0.57 (1.2) | 0.67 (1.3) | 0.32 (0.9) |
| CHD risk factors | |||||
| Hypertension | |||||
| No | 56,507 (79) | 0.42 (1.1) | 0.29 (0.8) | 0.36 (0.9) | 0.27 (0.8) |
| Yes | 14,903 (21) | 0.42 (1.1) | 0.35 (0.9) | 0.40 (1.0) | 0.29 (0.8) |
| Treated hypercholesterolemia | |||||
| No | 69,674 (98) | 0.43 (1.1) | 0.31 (0.9) | 0.36 (1.0) | 0.28 (0.8) |
| Yes | 1,736 (2) | 0.24 (0.8) | 0.26 (0.8) | 0.43 (1.1) | 0.30 (0.7) |
| Family history of MI | |||||
| No | 32,789 (46) | 0.44 (1.1) | 0.30 (0.9) | 0.35 (0.9) | 0.26 (0.7) |
| Yes | 34,978 (49) | 0.41 (1.1) | 0.31 (0.9) | 0.38 (1.0) | 0.29 (0.8) |
| Missing | 3,643 (5) | 0.44 (1.2) | 0.34 (0.9) | 0.37 (1.0) | 0.27 (0.7) |
| Postmenopausal hormone use | |||||
| Never | 49,049 (69) | 0.42 (1.1) | 0.32 (0.9) | 0.37 (1.0) | 0.27 (0.8) |
| Past | 6,208 (9) | 0.45 (1.2) | 0.27 (0.8) | 0.38 (1.0) | 0.28 (0.8) |
| Current | 16,123 (23) | 0.42 (1.1) | 0.26 (0.8) | 0.34 (0.9) | 0.28 (0.8) |
| Aspirin use | |||||
| No | 64,609 (90) | 0.42 (1.1) | 0.30 (0.9) | 0.36 (1.0) | 0.28 (0.8) |
| Yes | 6,801 (10) | 0.45 (1.1) | 0.33 (0.9) | 0.40 (1.0) | 0.28 (0.8) |
| Hysterectomy | |||||
| No | 43,693 (61) | 0.44 (1.1) | 0.29 (0.8) | 0.35 (0.9) | 0.26 (0.8) |
| Yes | 27,648 (39) | 0.41 (1.1) | 0.32 (0.9) | 0.40 (1.0) | 0.30 (0.8) |
Continuous variables were categorized into two groups by median.
Abbreviations: AHEI (Alternative Healthy Eating Index), CHD (coronary heart disease), MI (myocardial infarction), MUFA (monounsaturated fatty acid), PUFA (polyunsaturated fatty acid), SFA (saturated fatty acid), TFA (trans fatty acid), Tsp (teaspoon, 1 tsp/day=4.73 grams/day).
Substitution analyses
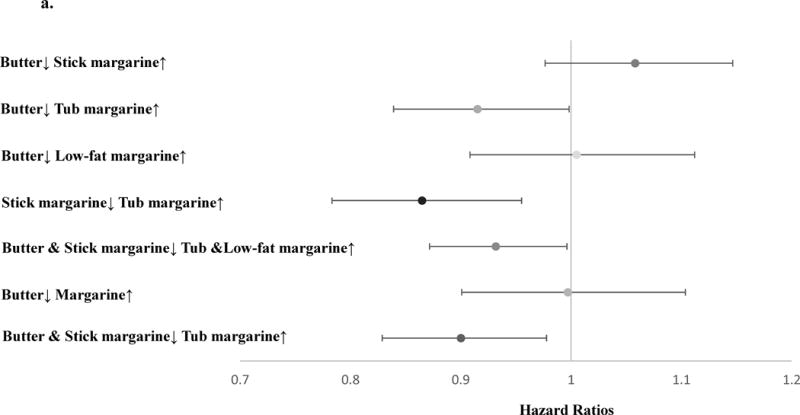
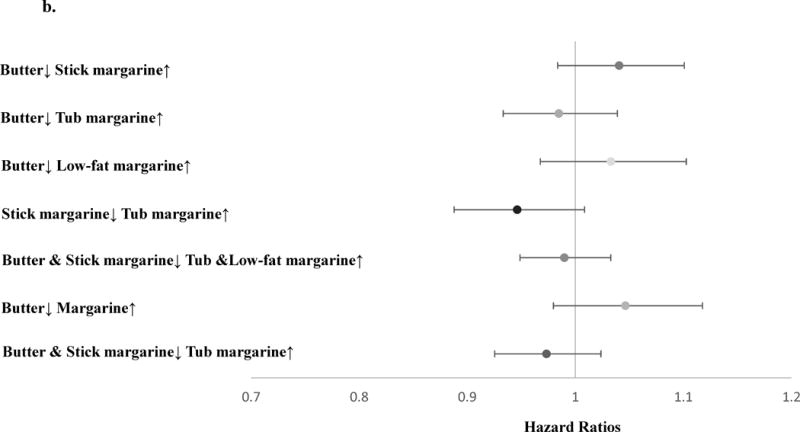
Using the cumulative average diet method in the fully adjusted Cox model, we estimated the theoretical effects of dietary substitution between different types of spreadable fats on the risk of MI, total CHD, ischemic stroke and atherosclerosis-related CVD (Table 2). Substituting stick margarine 1 tsp /day with tub margarine was associated with lower risk of MI (HR=0.91, 95% CI 0.85, 0.99). This substitution gave similar results for total CHD ischemic stroke, and atherosclerosis-related CVD, although these estimates had wide confidence intervals.
Table 2.
The substitution associations (1 tsp/day) among postmenopausal women using fully adjusted modela. (N=69,223)
| MI | Total CHD | Ischemic Stroke | Atherosclerosis-Related CVD | |
|---|---|---|---|---|
|
| ||||
| Events (n) | 1864 | 4229 | 1550 | 5507 |
| Butter↓ Stick margarine↑b | 1.0 (0.97, 1.1) | 1.0 (0.99, 1.1) | 1.1 (0.97, 1.1) | 1.0 (0.99, 1.1) |
| Butter↓ Tub margarine↑ | 0.95 (0.89, 1.0) | 1.0 (0.97, 1.1) | 1.0 (0.93, 1.1) | 1.0 (0.97, 1.0) |
| Butter↓ Low fat margarine↑ | 0.98 (0.90, 1.1) | 1.0 (0.97, 1.1) | 1.0 (0.93, 1.1) | 1.0 (0.97, 1.1) |
| Stick margarine↓ Tub margarine↑ | 0.91 (0.85, 0.99) | 0.97 (0.93, 1.0) | 0.95 (0.88, 1.0) | 0.97 (0.93, 1.0) |
| Butter & Stick margarine↓ Tub & Low fat margarine↑ | 0.95 (0.99, 0.99) | 1.0 (0.97, 1.0) | 0.99 (0.94, 1.1) | 1.0 (0.97, 1.0) |
| Butter↓ Margarine↑ | 1.0 (0.94, 1.1) | 1.1 (1.0, 1.1) | 1.1 (0.97, 1.2) | 1.1 (1.0, 1.1) |
| Butter & Stick margarine↓ Tub margarine↑ | 0.94 (0.88, 0.99) | 1.0 (0.96, 1.0) | 0.99 (0.92, 1.1) | 1.0 (0.96, 1.0) |
The fully adjusted model includes age, region, race/ethnicity, income, physical activity, BMI, smoking, total energy, hypertension, family history of MI, postmenopausal hormone use, aspirin use, and hysterectomy.
↓ and ↑ indicate substituting the spreadable fat with down arrow for equal amount of the fat with up arrow (here in the first row substituting for butter by increasing stick margarine consumption).
Abbreviations: BMI (body mass index), CHD (coronary heart disease), CVD (cardiovascular disease), MI (myocardial infarction), Tsp (teaspoon, 1 tsp/day=4.73 grams/day)
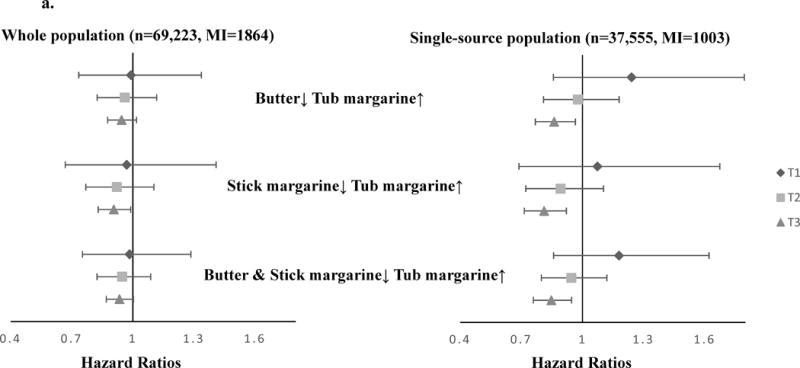
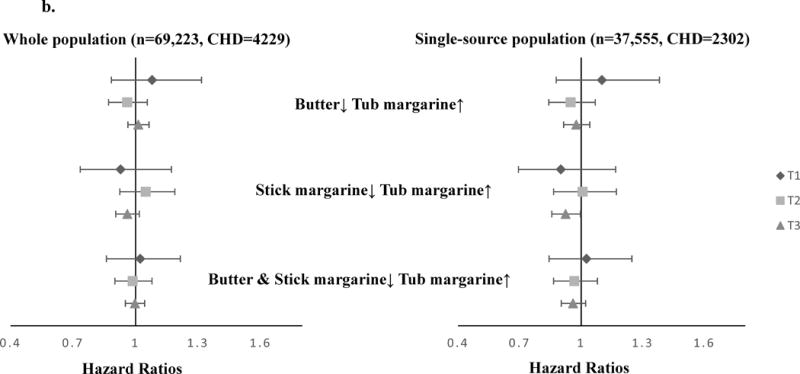
Since the substitution associations may be confounded by fat sources, we measured the substitution associations by fat source groups. Among the single-source group, we found stronger associations with MI when substituting tub margarine for stick margarine (HR=0.87, 95% CI 0.78, 0.96), and substituting tub margarine for butter (HR=0.92, 95% CI 0.84, 0.99) (Figure 2a). A moderate trend towards lower risk was found for total CHD when substituting stick margarine with tub margarine (HR=0.95, 95% CI 0.89, 1.0) (Figure 2b). Among the multiple-source group, the substitution associations were attenuated and close to the null, although similar directions were found for MI. When we combined the two groups together and measured the substitution associations among butter or margarine users, substituting butter and stick margarine with tub margarine was associated with lower risk of MI (HR=0.94, 95% CI 0.88,0.99) (eAppendix 1).
FIGURE 2.
HRs (95% CIs) of MI or total CHD associated with the substitution of margarine with butter using cumulative average diet method and fully adjusted model, among single-source population. a. MI (N=37,555, MI=1003); b. total CHD (N=37,555, CHD=2302).
The dots represent the estimated risk associated with 1 tsp/day spreadable fats substitution. The error bars represent 95% CIs of point estimates. The fully adjusted model includes age, region, race/ethnicity, income, physical activity, BMI, smoking, total energy, hypertension, family history of MI, postmenopausal hormone use, aspirin use, and hysterectomy. Abbreviations: HR (Hazard Ratio), MI (myocardial infarction), tsp (teaspoon), BMI (body mass index).
We also considered whether the substitution associations differed between strata (tertiles) of dietary total fat (% of energy intake). The association of substituting stick margarine with tub margarine was slightly stronger among women in the highest tertile of dietary total fat (HR=0.93 for MI) than the overall association (Table 3). However, the substitution associations had wide confidence intervals among women with lower dietary total fat. When limiting the analysis to the single-source population, substituting butter or stick margarine with tub margarine may be associated with lower risk of MI among women in the highest tertile of dietary total fat (HRs=0.85 and 0.81), which was stronger than the association among whole population (Figure 3a). No associations were observed with total CHD in the stratified substitution analysis among the whole population, while substituting stick margarine with tub margarine was found to be associated with lower risk of CHD (HR=0.92, 95% CI 0.86, 0.99) among women in the highest tertile of dietary total fat and only using one type of spreadable fat (Figure 3b).
Table 3.
The substitution associations (1 tsp/day) on MI among postmenopausal women using fully adjusted model, stratified by tertiles of dietary total fat (% energy)a
| Tertile 1 | Tertile 2 | Tertile 3 | Overall | |
|---|---|---|---|---|
| Average dietary fat (% of total energy) | 22 | 29 | 39 | 30 |
|
| ||||
| Events/Participants (n) | 566/23,090 | 640/23,131 | 658/23,001 | 1864/69,223 |
| Butter↓ Stick margarine↑b | 1.0 (0.69, 1.5) | 1.0 (0.87, 1.2) | 1.0 (0.97, 1.1) | 1.0 (0.97, 1.1) |
| Butter↓ Tub margarine↑ | 0.99 (0.73, 1.3) | 0.96 (0.82, 1.1) | 0.94 (0.88, 1.0) | 0.95 (0.89, 1.0) |
| Butter↓ Low fat margarine↑ | 0.98 (0.75, 1.3) | 0.99 (0.85, 1.2) | 0.98 (0.87, 1.1) | 0.98 (0.90, 1.1) |
| Stick margarine↓ Tub margarine↑ | 0.97 (0.67, 1.4) | 0.92 (0.77, 1.1) | 0.91 (0.83, 0.99) | 0.91 (0.85, 0.99) |
| Butter & Stick margarine↓ Tub & Low fat margarine↑ | 0.98 (0.79, 1.2) | 0.96 (0.86, 1.1) | 0.94 (0.88, 1.0) | 0.95 (0.99, 0.99) |
| Butter↓ Margarine↑ | 1.0 (0.75, 1.4) | 1.0 (0.84, 1.2) | 1.0 (0.94, 1.1) | 1.0 (0.94, 1.1) |
| Butter & Stick margarine↓ Tub margarine↑ | 0.98 (0.75, 1.3) | 0.95 (0.82, 1.1) | 0.93 (0.87, 1.0) | 0.94 (0.88, 0.99) |
The fully adjusted model includes age, region, race/ethnicity, income, physical activity, BMI, smoking, total energy, hypertension, family history of MI, postmenopausal hormone use, aspirin use and hysterectomy.
↓ and ↑ indicate substituting the spreadable fat with down arrow for equal amount of the fat with up arrow (here in the first row substituting for butter by increasing stick margarine consumption).
Abbreviations: BMI (body mass index), MI (myocardial infarction), Tsp (teaspoon, 1 tsp/day = 4.73 grams/day).
FIGURE 3.
HRs (95% CIs) of MI or total CHD associated with the substitution of margarine with butter (1 tsp/day) by tertiles of dietary total fat (% energy) in the fully adjusted model, among whole population and single spreadable fat-source population. a. MI; b. total CHD.
The dots represent the estimated risk. The error bars represent 95% CIs of point estimates. The fully adjusted model includes age, region, race/ethnicity, income, physical activity, BMI, smoking, total energy, hypertension, family history of MI, postmenopausal hormone use, aspirin use, and hysterectomy. Abbreviations: HR (Hazard Ratio), MI (myocardial infarction), tsp (teaspoon), BMI (body mass index).
Associations of Higher Butter and Margarine Consumptions with Each Outcome
We evaluated the associations between higher levels of butter or margarine consumption and each outcome, by calculating the HRs and 95% CIs for the risk of each outcome associated with 1 tsp /day higher intake of butter or margarine (eAppendix 2). In demographic- and SES-adjusted models, butter was positively associated with MI, and stick margarine was associated with elevated risk of each outcome. However, these associations were attenuated when lifestyle and CHD risk factors were additionally taken into consideration. When limiting the analysis to the single-source group (eAppendix 3), there was a trend towards higher risk of MI, total CHD and atherosclerotic related CVD at higher levels of stick margarine consumption, and a lower risk of MI at higher levels of tub margarine intake.
Sensitivity Analyses
In the analysis using baseline diet only, we got comparable results to the analysis using cumulative diet method (eAppendix 4). In substitution analyses when we additionally adjusted for AHEI or food groups, the results did not differ from our primary analysis.
DISCUSSION
In this study of postmenopausal women, we evaluated the effects of the theoretical substitution of butter and various types of margarine on the risk of myocardial infarction, coronary heart disease, ischemic stroke and cardiovascular disease. In our substitution analyses, we found a lower risk of MI when stick margarine was substituted by a similar serving of tub margarine (1 tsp/day). Substituting butter with tub margarine was associated with borderline benefit, while substituting butter with stick margarine or low-fat margarine was associated with no benefit. Moderate trends toward lower risk were found for total CHD, ischemic stroke, and atherosclerosis-related CVD when tub margarine was substituted for stick margarine. Substituting stick margarine with tub margarine demonstrated stronger inverse associations than substituting butter with tub margarine. When the substitution analyses were limited to a population consuming a single source of spreadable fat, stronger inverse associations were found for MI and total CHD. The substitution association was also stronger among women with high dietary fat consumption.
Our substitutions are consistent with what has been found when eating patterns and fatty acids substitution associations on lipids have been examined.7,8,10 In a meta-analysis of feeding studies, LDL cholesterol showed no change and HDL cholesterol decreased by 0.02 mmol/l when 10% of energy from butter was replaced by stick margarine; LDL cholesterol decreased by 0.20 mmol/l and HDL cholesterol did not change when butter was replaced by tub margarine for 10% of energy,23 thus showing a protective association with tub margarine. A cross-over feeding study of table spreads providing 8.3% energy as fat found that tub margarine resulted in lower LDL cholesterol levels than butter or stick margarine.34 Most previous observational studies have not measured the substitution associations between butter and margarine, but have focused on the association of SFA, TFA, MUFA and PUFA on CVD with mixed results.6,8,10,11,35 Substituting SFA or TFA with PUFA shows a protective association in most studies.
The potential protective association of tub margarine may be explained by its lower proportion of SFA, TFA, and higher proportion of MUFA and PUFA, compared with butter and stick margarine. These differences in composition were not only present in the 1990s when these dietary data were collected but are still seen in contemporary studies17–19 The reason for lack of associations from low-fat margarine low in SFA and TFA is unclear, but could be explained by its varying composition; this question requires further study.17
We found stronger associations between dietary fat substitution and MI than for other CVD outcomes. One explanation could be the atherogenic effect of SFA and TFA, such that these fatty acids may influence atherothrombotic events associated with MI and CHD more than other CVD outcomes. Another explanation could be the more accurate classification of MI and CHD compared to other CVD related events.
We did not find associations between spreadable fats substitutions and the risk of ischemic stroke. This perhaps could be explained by the different mechanisms for ischemic heart disease and ischemic stroke.36 In the Framingham Study, researchers found that SFA, MUFA, and PUFA were not associated with increased risk of ischemic stroke.36 Similarly, in the WHI Dietary Modification trial, reduction in SFA consumption did not reduce the incidence of ischemic stroke.37 Similar results were also observed in a prospective cohort study showing that dietary fats were not associated with ischemic stroke.38 However, these studies did not examine substitution of SFA with other classes of fatty acids.
When we limited the analyses to a single-source group of spreadable fat, we found stronger associations of substituting butter or stick margarine with tub margarine on MI and total CHD. The single-source findings may be more informative than those in the entire analytic sample due to inaccuracies of measurement when multiple sources of spreadable fats are considered. Considering that the FFQs measured fat intake by asking the source of spreadable fat, a multisource user might well have more trouble truly estimating their frequency of use compared to a single-source user.
Sensitivity Analyses
Compared with the cumulative average method of assessing dietary fat intake, the associations were weaker using the baseline dietary assessment, but were in the same direction. These differences could be explained by the measurement error and changing of eating habits over time. We believe that the cumulative average diet method reflects long-term diet better and is more relevant etiologically than the baseline-only diet information when evaluating CVD risk.9
Strengths and Limitations
There are several strengths of this study. First, our study represents a large, multiethnic and geographically diverse population with a long period of follow-up that has an adequate sample size to evaluate associations of theoretical substitutions of different types of spreadable fats on the risk of CVD. Second, we reduced measurement errors in FFQs by using two measurements, at baseline and at Year 3, and using the cumulative average diet method to calculate spreadable fat intakes, and other dietary confounders. Third, to our knowledge our study is novel, focusing on the potential associations of substituting different types of spreadable fats- margarine for butter on CVD risk, which has a direct public health message. Fourth, the HRs presented were adjusted for multiple potential confounders including dietary quality. To deal with the measurement error of spreadable fats consumption we adjusted for total energy intake using regression calibration equations derived from doubly labeled water studies to give less biased parameter estimates.39 The most important limitation to this study is the potential measurement error associated with butter and margarine intakes by using four self-reported questions. Intake of spreadable fats was queried, with the respondent able to give up to two sources of spreadable fat for each question. We then calculated total consumptions of butter and margarine from these four questions by giving equal weight (0.5) to each type of fat when two were chosen, thus leading to potential misclassification bias of the exposure. Such measurement error would bias the results towards the null, so that the estimates given in the analyses may be conservative. We additionally evaluated a single source of spreadable fats to assess this limitation and found stronger associations. An additional limitation is the fact that we utilized two measurements of diet three years apart which may not be sufficient to get accurate estimates of long-term butter and margarine intakes.
CONCLUSION
This theoretical dietary substitution analysis suggests that substituting butter and stick margarine with tub margarine when spreadable fats are eaten may be associated with reduced risk of myocardial infarction. Future substitution analyses that use multiple dietary measurements of spreadable fats or other whole foods and measure clinically important outcomes are needed.
Supplementary Material
Acknowledgments
WHI investigators
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Elizabeth Nabel, Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA)
Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg, Ruth E. Patterson, Anne McTiernan; (Medical Research Labs, Highland Heights, KY) Evan Stein; (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Aleksandar Rajkovic; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor- UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Yvonne Michael; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael Simon.
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Sources of financial support:
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
References
- 1.European Food Safety Authority. Scientific Opinion on Dietary Reference Values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA Journal. 2010;8:1461. [Google Scholar]
- 2.Eckel RH, Jakicic JM, Ard JD, Miller NH, Hubbard VS, Nonas CA, de Jesus JM, Sacks FM, Lee IM, Smith SC, Jr, Lichtenstein AH, Svetkey LP, Loria CM, Wadden TW, Millen BE, Yanovski SZ. AHA/ACC Guideline on Lifestyle Management to Reduce Cardiovascular Risk: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology. 2013;2013 doi: 10.1016/j.jacc.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Lopez-Garcia E, Schulze MB, Meigs JB, Manson JE, Rifai N, Stampfer MJ, Willett WC, Hu FB. Consumption of trans fatty acids is related to plasma biomarkers of inflammation and endothelial dysfunction. J Nutr. 2005;135:562–6. doi: 10.1093/jn/135.3.562. [DOI] [PubMed] [Google Scholar]
- 4.Roach C, Feller SE, Ward JA, Shaikh SR, Zerouga M, Stillwell W. Comparison of cis and trans fatty acid containing phosphatidylcholines on membrane properties. Biochemistry. 2004;43:6344–51. doi: 10.1021/bi049917r. [DOI] [PubMed] [Google Scholar]
- 5.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. The American journal of clinical nutrition. 2010;91:535–546. doi: 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu FB, Stampfer MJ, Manson JE, Ascherio A, Colditz GA, Speizer FE, Hennekens CH, Willett WC. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. The American journal of clinical nutrition. 1999;70:1001–1008. doi: 10.1093/ajcn/70.6.1001. [DOI] [PubMed] [Google Scholar]
- 7.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fatty acids and risk of coronary heart disease: modulation by replacement nutrients. Current atherosclerosis reports. 2010;12:384–390. doi: 10.1007/s11883-010-0131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu FB, Stampfer MJ, Manson JE, Rimm E, Colditz GA, Rosner BA, Hennekens CH, Willett WC. Dietary fat intake and the risk of coronary heart disease in women. New England Journal of Medicine. 1997;337:1491–1499. doi: 10.1056/NEJM199711203372102. [DOI] [PubMed] [Google Scholar]
- 9.Hu FB, Stampfer MJ, Rimm E, Ascherio A, Rosner BA, Spiegelman D, Willett WC. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. American journal of epidemiology. 1999;149:531–540. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 10.Jakobsen MU, O’Reilly EJ, Heitmann BL, Pereira MA, Bälter K, Fraser GE, Goldbourt U, Hallmans G, Knekt P, Liu S. Major types of dietary fat and risk of coronary heart disease: a pooled analysis of 11 cohort studies. The American journal of clinical nutrition. 2009;89:1425–1432. doi: 10.3945/ajcn.2008.27124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. The American journal of clinical nutrition. 2003;77:1146–1155. doi: 10.1093/ajcn/77.5.1146. [DOI] [PubMed] [Google Scholar]
- 12.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, Miller ER, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. Jama. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 13.Furtado JD, Campos H, Appel LJ, Miller ER, Laranjo N, Carey VJ, Sacks FM. Effect of protein, unsaturated fat, and carbohydrate intakes on plasma apolipoprotein B and VLDL and LDL containing apolipoprotein C-III: results from the OmniHeart Trial. The American journal of clinical nutrition. 2008;87:1623–1630. doi: 10.1093/ajcn/87.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Judd JT, Baer DJ, Clevidence BA, Muesing RA, Chen SC, Weststrate JA, Meijer GW, Wittes J, Lichtenstein AH, Vilella-Bach M. Effects of margarine compared with those of butter on blood lipid profiles related to cardiovascular disease risk factors in normolipemic adults fed controlled diets. The American journal of clinical nutrition. 1998;68:768–777. doi: 10.1093/ajcn/68.4.768. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenstein AH, Appel LJ, Brands M, Carnethon M, Daniels S, Franch HA, Franklin B, Kris-Etherton P, Harris WS, Howard B. Diet and lifestyle recommendations revision 2006 A scientific statement from the American Heart Association nutrition committee. Circulation. 2006;114:82–96. doi: 10.1161/CIRCULATIONAHA.106.176158. [DOI] [PubMed] [Google Scholar]
- 16.Van Horn L, Tian L, Neuhouser ML, Howard BV, Eaton CB, Snetselaar L, Matthan NR, Lichtenstein AH. Dietary Patterns Are Associated with Disease Risk among Participants in the Women’s Health Initiative Observational Study. The Journal of nutrition. 2012;142:284–291. doi: 10.3945/jn.111.145375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.United States Department of Agriculture. USDA National Nutrient Database for Standard Reference. 2014;2015 [Google Scholar]
- 18.United States Department of Agriculture. USDA National Nutrient Database for Standard Reference. 2005;2015 [Google Scholar]
- 19.Chow CK. Fatty acids in foods and their health implications. Third. CRC Press; 2007. [Google Scholar]
- 20.Chowdhury R, Warnakula S, Kunutsor S, Crowe F, Ward HA, Johnson L, Franco OH, Butterworth AS, Forouhi NG, Thompson SG. Association of dietary, circulating, and supplement fatty acids with coronary risk: a systematic review and meta-analysis. Annals of internal medicine. 2014;160:398–406. doi: 10.7326/M13-1788. [DOI] [PubMed] [Google Scholar]
- 21.Harcombe Z, Baker JS, Cooper SM, Davies B, Sculthorpe N, DiNicolantonio JJ, Grace F. Evidence from randomised controlled trials did not support the introduction of dietary fat guidelines in 1977 and 1983: a systematic review and meta-analysis. Open Heart. 2015;2:e000196. doi: 10.1136/openhrt-2014-000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katan MB, Brouwer IA, Clarke R, Geleijnse JM, Mensink RP. Saturated fat and heart disease. The American journal of clinical nutrition. 2010;92:459–460. doi: 10.3945/ajcn.2010.29752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zock PL, Katan MB. Butter, margarine and serum lipoproteins. Atherosclerosis. 1997;131:7–16. doi: 10.1016/s0021-9150(96)06063-7. [DOI] [PubMed] [Google Scholar]
- 24.Jansen S, López-Miranda J, Castro P, López-Segura F, Marín C, Ordovás JM, Paz E, Jiménez-Perepérez J, Fuentes F, Pérez-Jiménez F. Low-fat and high–monounsaturated fatty acid diets decrease plasma cholesterol ester transfer protein concentrations in young, healthy, normolipemic men. The American journal of clinical nutrition. 2000;72:36–41. doi: 10.1093/ajcn/72.1.36. [DOI] [PubMed] [Google Scholar]
- 25.Kelemen LE, Kushi LH, Jacobs DR, Cerhan JR. Associations of dietary protein with disease and mortality in a prospective study of postmenopausal women. American Journal of Epidemiology. 2005;161:239–249. doi: 10.1093/aje/kwi038. [DOI] [PubMed] [Google Scholar]
- 26.Pan A, Malik VS, Schulze MB, Manson JE, Willett WC, Hu FB. Plain-water intake and risk of type 2 diabetes in young and middle-aged women. The American journal of clinical nutrition. 2012;95:1454–1460. doi: 10.3945/ajcn.111.032698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Controlled clinical trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 28.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, Shumaker S, Wang CY, Stein E, Prentice RL. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 29.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–21. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 30.Bertoia ML, Triche EW, Michaud DS, Baylin A, Hogan JW, Neuhouser ML, Freiberg MS, Allison MA, Safford MM, Li W. Long-term alcohol and caffeine intake and risk of sudden cardiac death in women. The American journal of clinical nutrition. 2013;97:1356–1363. doi: 10.3945/ajcn.112.044248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curb JD, Mctiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, Johnson KC, Proulx-Burns L, Pastore L, Criqui M. Outcomes ascertainment and adjudication methods in the Women’s Health Initiative. Annals of epidemiology. 2003;13:S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 32.McTiernan A, Kooperberg C, White E, Wilcox S, Coates R, Adams-Campbell LL, Woods N, Ockene J. Recreational physical activity and the risk of breast cancer in postmenopausal women: the Women’s Health Initiative Cohort Study. Jama. 2003;290:1331–1336. doi: 10.1001/jama.290.10.1331. [DOI] [PubMed] [Google Scholar]
- 33.Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. The Journal of nutrition. 2012;142:1009–1018. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Judd JT, Baer DJ, Clevidence BA, Muesing RA, Chen SC, Weststrate JA, Meijer GW, Wittes J, Lichtenstein AH, Vilella-Bach M, Schaefer EJ. Effects of margarine compared with those of butter on blood lipid profiles related to cardiovascular disease risk factors in normolipemic adults fed controlled diets. Am J Clin Nutr. 1998;68:768–77. doi: 10.1093/ajcn/68.4.768. [DOI] [PubMed] [Google Scholar]
- 35.Livingstone KM, Lovegrove JA, Givens DI. The impact of substituting SFA in dairy products with MUFA or PUFA on CVD risk: evidence from human intervention studies. Nutrition research reviews. 2012;25:193–206. doi: 10.1017/S095442241200011X. [DOI] [PubMed] [Google Scholar]
- 36.Gillman MW, Cupples LA, Millen BE, Ellison RC, Wolf PA. Inverse association of dietary fat with development of ischemic stroke in men. Jama. 1997;278:2145–2150. [PubMed] [Google Scholar]
- 37.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL. Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. Jama. 2006;295:655–666. doi: 10.1001/jama.295.6.655. [DOI] [PubMed] [Google Scholar]
- 38.He K, Merchant A, Rimm EB, Rosner BA, Stampfer MJ, Willett WC, Ascherio A. Dietary fat intake and risk of stroke in male US healthcare professionals: 14 year prospective cohort study. Bmj. 2003;327:777–782. doi: 10.1136/bmj.327.7418.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Prentice RL, Pettinger M, Tinker LF, Huang Y, Thomson CA, Johnson KC, Beasley J, Anderson G, Shikany JM, Chlebowski RT, Neuhouser ML. Regression calibration in nutritional epidemiology: example of fat density and total energy in relationship to postmenopausal breast cancer. Am J Epidemiol. 2013;178:1663–72. doi: 10.1093/aje/kwt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


