Abstract
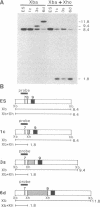
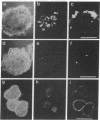
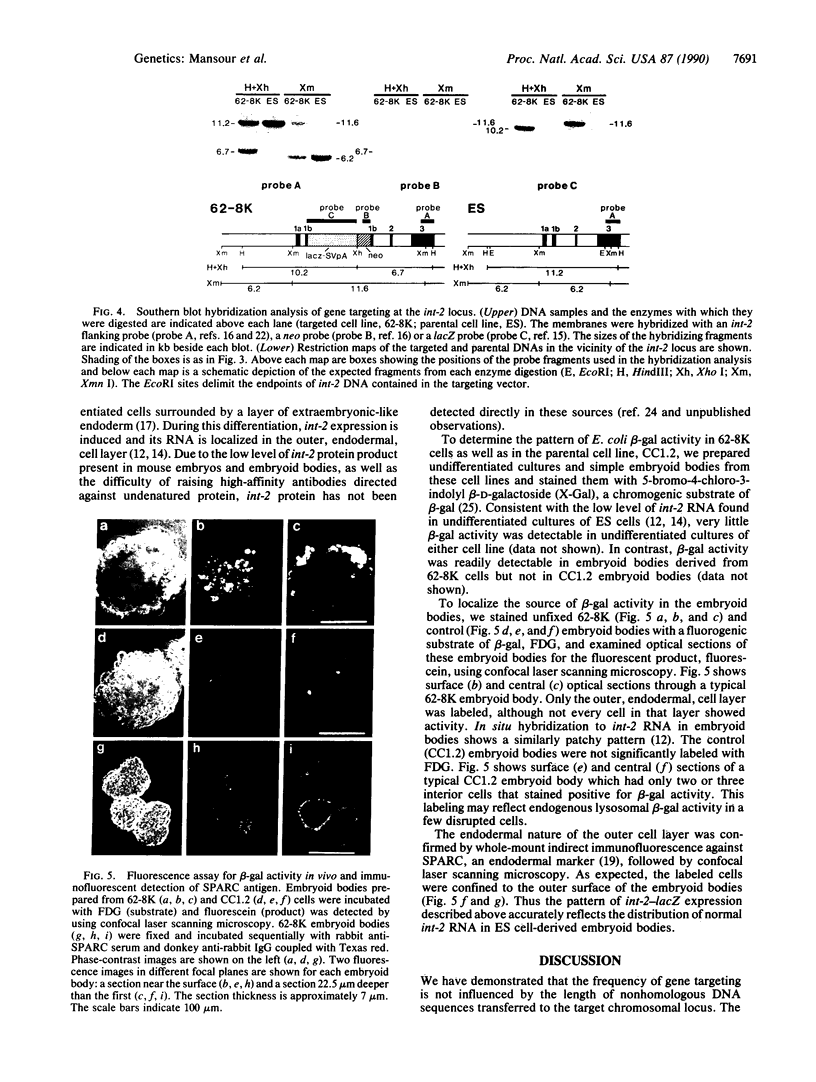
We demonstrate that the frequency of gene targeting is unaffected by the length of nonhomologous DNA transferred to a target chromosomal sequence. A result of this finding is that a much wider spectrum of designed genomic alterations is now feasible. As a first application, we inserted a 5.4-kilobase cassette of nonhomologous DNA into the int-2 locus in mouse embryo-derived stem cells by gene targeting. The inserted DNA contained a lacZ gene positioned to create an in-frame fusion with the int-2 protein-coding region. Upon differentiation of these cells to embryoid bodies, the int-2-lacZ fusion faithfully reproduced the expression pattern of int-2 RNA. This ability to target reporter genes, such as lacZ, to specific mouse loci, combined with the ability to move the tagged gene into different mutant backgrounds, may provide an ideal approach for analyzing interactions among genes that participate in a developmental network.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bollag R. J., Waldman A. S., Liskay R. M. Homologous recombination in mammalian cells. Annu Rev Genet. 1989;23:199–225. doi: 10.1146/annurev.ge.23.120189.001215. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Capecchi M. R. The new mouse genetics: altering the genome by gene targeting. Trends Genet. 1989 Mar;5(3):70–76. doi: 10.1016/0168-9525(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Dent J. A., Polson A. G., Klymkowsky M. W. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development. 1989 Jan;105(1):61–74. doi: 10.1242/dev.105.1.61. [DOI] [PubMed] [Google Scholar]
- Dickson C., Peters G. Potential oncogene product related to growth factors. 1987 Apr 30-May 6Nature. 326(6116):833–833. doi: 10.1038/326833a0. [DOI] [PubMed] [Google Scholar]
- Dickson C., Smith R., Brookes S., Peters G. Tumorigenesis by mouse mammary tumor virus: proviral activation of a cellular gene in the common integration region int-2. Cell. 1984 Jun;37(2):529–536. doi: 10.1016/0092-8674(84)90383-0. [DOI] [PubMed] [Google Scholar]
- Dixon M., Deed R., Acland P., Moore R., Whyte A., Peters G., Dickson C. Detection and characterization of the fibroblast growth factor-related oncoprotein INT-2. Mol Cell Biol. 1989 Nov;9(11):4896–4902. doi: 10.1128/mcb.9.11.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W., Thoma G., Nüsslein-Volhard C. Determination of spatial domains of zygotic gene expression in the Drosophila embryo by the affinity of binding sites for the bicoid morphogen. Nature. 1989 Aug 3;340(6232):363–367. doi: 10.1038/340363a0. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Martin G. R. Cut, paste, and save: new approaches to altering specific genes in mice. Cell. 1989 Jan 27;56(2):145–147. doi: 10.1016/0092-8674(89)90887-8. [DOI] [PubMed] [Google Scholar]
- Glaser R. L., Wolfner M. F., Lis J. T. Spatial and temporal pattern of hsp26 expression during normal development. EMBO J. 1986 Apr;5(4):747–754. doi: 10.1002/j.1460-2075.1986.tb04277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goring D. R., Rossant J., Clapoff S., Breitman M. L., Tsui L. C. In situ detection of beta-galactosidase in lenses of transgenic mice with a gamma-crystallin/lacZ gene. Science. 1987 Jan 23;235(4787):456–458. doi: 10.1126/science.3099390. [DOI] [PubMed] [Google Scholar]
- Goto T., Macdonald P., Maniatis T. Early and late periodic patterns of even skipped expression are controlled by distinct regulatory elements that respond to different spatial cues. Cell. 1989 May 5;57(3):413–422. doi: 10.1016/0092-8674(89)90916-1. [DOI] [PubMed] [Google Scholar]
- Harding K., Hoey T., Warrior R., Levine M. Autoregulatory and gap gene response elements of the even-skipped promoter of Drosophila. EMBO J. 1989 Apr;8(4):1205–1212. doi: 10.1002/j.1460-2075.1989.tb03493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiromi Y., Gehring W. J. Regulation and function of the Drosophila segmentation gene fushi tarazu. Cell. 1987 Sep 11;50(6):963–974. doi: 10.1016/0092-8674(87)90523-x. [DOI] [PubMed] [Google Scholar]
- Jakobovits A., Shackleford G. M., Varmus H. E., Martin G. R. Two proto-oncogenes implicated in mammary carcinogenesis, int-1 and int-2, are independently regulated during mouse development. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7806–7810. doi: 10.1073/pnas.83.20.7806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeMotte P. K., Kuroiwa A., Fessler L. I., Gehring W. J. The homeotic gene Sex Combs Reduced of Drosophila: gene structure and embryonic expression. EMBO J. 1989 Jan;8(1):219–227. doi: 10.1002/j.1460-2075.1989.tb03367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsou A., Liskay R. M. Effect of the molecular nature of mutation on the efficiency of intrachromosomal gene conversion in mouse cells. Genetics. 1987 Dec;117(4):759–769. doi: 10.1093/genetics/117.4.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Martin G. R. Four classes of mRNA are expressed from the mouse int-2 gene, a member of the FGF gene family. EMBO J. 1988 Jul;7(7):2035–2041. doi: 10.1002/j.1460-2075.1988.tb03043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour S. L., Thomas K. R., Capecchi M. R. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988 Nov 24;336(6197):348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- Martin G. R., Wiley L. M., Damjanov I. The development of cystic embryoid bodies in vitro from clonal teratocarcinoma stem cells. Dev Biol. 1977 Dec;61(2):230–244. doi: 10.1016/0012-1606(77)90294-9. [DOI] [PubMed] [Google Scholar]
- Mason I. J., Taylor A., Williams J. G., Sage H., Hogan B. L. Evidence from molecular cloning that SPARC, a major product of mouse embryo parietal endoderm, is related to an endothelial cell 'culture shock' glycoprotein of Mr 43,000. EMBO J. 1986 Jul;5(7):1465–1472. doi: 10.1002/j.1460-2075.1986.tb04383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. W., Konecki D. S., Brennand J., Caskey C. T. Structure, expression, and mutation of the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2147–2151. doi: 10.1073/pnas.81.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan G. P., Fiering S., Nicolas J. F., Herzenberg L. A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on beta-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2603–2607. doi: 10.1073/pnas.85.8.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters G., Brookes S., Smith R., Dickson C. Tumorigenesis by mouse mammary tumor virus: evidence for a common region for provirus integration in mammary tumors. Cell. 1983 Jun;33(2):369–377. doi: 10.1016/0092-8674(83)90418-x. [DOI] [PubMed] [Google Scholar]
- Rossant J., Joyner A. L. Towards a molecular-genetic analysis of mammalian development. Trends Genet. 1989 Aug;5(8):277–283. doi: 10.1016/0168-9525(89)90102-9. [DOI] [PubMed] [Google Scholar]
- Smith R., Peters G., Dickson C. Multiple RNAs expressed from the int-2 gene in mouse embryonal carcinoma cell lines encode a protein with homology to fibroblast growth factors. EMBO J. 1988 Apr;7(4):1013–1022. doi: 10.1002/j.1460-2075.1988.tb02908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl G., Struhl K., Macdonald P. M. The gradient morphogen bicoid is a concentration-dependent transcriptional activator. Cell. 1989 Jun 30;57(7):1259–1273. doi: 10.1016/0092-8674(89)90062-7. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Capecchi M. R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987 Nov 6;51(3):503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- Thummel C. S., Boulet A. M., Lipshitz H. D. Vectors for Drosophila P-element-mediated transformation and tissue culture transfection. Gene. 1988 Dec 30;74(2):445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Way J. C., Chalfie M. The mec-3 gene of Caenorhabditis elegans requires its own product for maintained expression and is expressed in three neuronal cell types. Genes Dev. 1989 Dec;3(12A):1823–1833. doi: 10.1101/gad.3.12a.1823. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Bhatt S., McMahon A. P. Expression pattern of the FGF-related proto-oncogene int-2 suggests multiple roles in fetal development. Development. 1989 Jan;105(1):131–136. doi: 10.1242/dev.105.1.131. [DOI] [PubMed] [Google Scholar]
- Wilkinson D. G., Peters G., Dickson C., McMahon A. P. Expression of the FGF-related proto-oncogene int-2 during gastrulation and neurulation in the mouse. EMBO J. 1988 Mar;7(3):691–695. doi: 10.1002/j.1460-2075.1988.tb02864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A., Gruss P. Production of chimaeric mice containing embryonic stem (ES) cells carrying a homoeobox Hox 1.1 allele mutated by homologous recombination. Nature. 1989 Mar 9;338(6211):150–153. doi: 10.1038/338150a0. [DOI] [PubMed] [Google Scholar]